SUMMARY
Asymmetric segregation of P granules during the first four divisions of the C. elegans embryo is a classic example of cytoplasmic partitioning of germline determinants. It is thought that asymmetric partitioning of P granule components during mitosis is essential to distinguish germline from soma. We have identified a mutant (pptr-1) where P granules become unstable during mitosis and P granule proteins and RNAs are distributed equally to somatic and germline blastomeres. Despite symmetric partitioning of P granule components, pptr-1 mutants segregate a germline that uniquely expresses P granules during post-embryonic development. pptr-1 mutants are fertile, except at high temperatures. Hence, asymmetric inheritance of maternal P granules is not essential to specify germ cell fate. Instead, it may serve to protect the nascent germline from stress.
A general characteristic of germ cells is the presence of cytoplasmic RNA-rich granules called germ granules (1). In C. elegans, germ (P) granules are present in all germ cells except mature sperm, and they segregate asymmetrically with the germline precursors (P blastomeres) during the first embryonic divisions (Fig. 1A) (2). Like embryonic germ granules of other organisms, P granules have been hypothesized to harbor the determinants that specify the germline. However, their function and segregation mechanisms are not fully understood (2, 3).
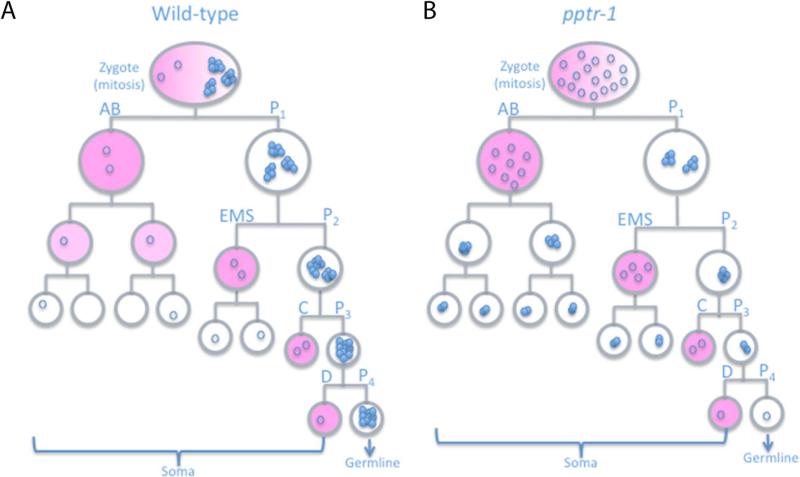
Fig 1. Segregation of the P granule component PGL-1 in C. elegans embryos.
A) Abbreviated embryonic lineage showing the divisions that give rise to somatic (AB, EMS, C and D) and germline (P1-P4) blastomeres. All cells are shown in interphase except for the zygote, which is shown in mitosis. Circles represent PGL-1 molecules: open circles represent PGL-1 diffuse in cytoplasm and closed circles represent PGL-1 assembled into granules visible by microscopy. Pink is MEX-5, which promotes granule disassembly; localized granule assembly ensures that the majority of PGL-1 segregates with the germline (Fig. 2). B) In pptr-1 mutants, PGL-1 granules disassemble at each mitosis and equal numbers of dispersed PGL-1 molecules are segregated to all cells (Fig. 2). During interphase, PGL-1 granules reform in all cells, except in MEX-5-positive somatic blastomeres (pink). Note that somatic PGL-1 granules are not equivalent to true P granules, as they do not contain P granule-associated mRNAs, which are degraded in somatic lineages (Fig. 3).
To monitor P granule dynamics, we used confocal microscopy to image live embryos expressing the P granule protein PGL-1 fused to green fluorescence protein (GFP)(4). We obtained similar results with GFP fusions to two other P granule proteins PGL-3 and GLH-1 (5, 6). In the live movies, we analyzed granule dynamics (number, size and movement) and the overall distribution of each protein by quantifying total (granular + diffuse cytoplasmic) GFP fluorescence (Fig. 1, 2, Sup. Fig. 2A, 2B and Movies 1-3). P granules behaved differently during interphase and mitosis. During interphase, P granules were in a dynamic equilibrium between growing and shrinking phases (Movies 1-3), with a bias for shrinking in the anterior and a bias for growing in the posterior. By the end of interphase, 85% of P granules in the anterior had disappeared completely or crossed over to the posterior (15%; n=41), and the total number of P granules had increased (Sup. Fig. 1A). Although most granules became restricted to the posterior (Fig. 2A), levels of GFP::PGL-1 fluorescence remained equal in the anterior and posterior halves of the zygote during interphase (Fig. 2F), indicating that GFP::PGL-1 was still present in the anterior cytoplasm even though not in discrete granules. During mitosis, P granules grew in size, fused with each other, and decreased in number (Fig. 2A, Sup. Fig. 1 and Movie 1). GFP::PGL-1 fluorescence decreased in the anterior and increased in the posterior, suggesting that GFP::PGL-1 in the anterior cytoplasm was recruited into the posterior granules (Fig. 2F). Using a photoactivatable Dendra::PGL-1 fusion to permanently label a subpopulation of PGL-1, we confirmed that PGL-1 enrichment in the posterior involves redistribution of existing PGL-1 protein from anterior to posterior with no change in total protein levels (Sup. Fig. 2D). Dendra::PGL-1 diffuses throughout the embryo and diffuses fastest in the anterior during mitosis (Sup. Fig. 2E, G). We conclude that enrichment of PGL-1 in the posterior of the zygote does not depend on synthesis or degradation, but correlates with rapid recruitment of cytoplasmic PGL-1 into growing granules during mitosis.
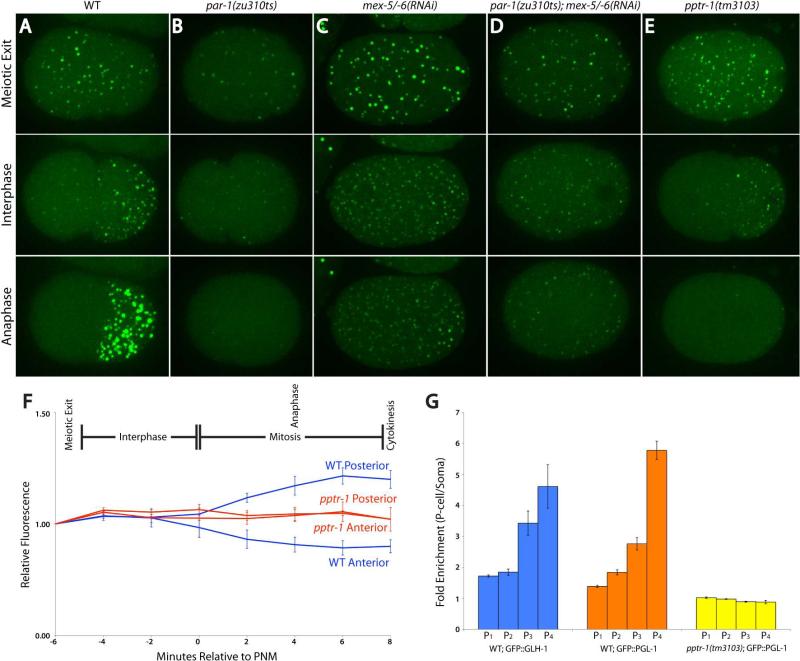
Fig. 2. P granule dynamics require par-1, mex-5/6, and pptr-1.
A-E) Time-lapse images of zygotes expressing GFP::PGL-1. Image are maximum projections of confocal Z-stacks spanning 8µm (~half of embryo depth). P granule numbers are shown in Sup. Fig. 1. MEX-5 and MEX-6 are uniformly distributed in par-1 embryos and PAR-1 localizes to a reduced-size posterior domain in mex-5;mex-6 zygotes (9, 10).
F) GFP::PGL-1 levels over time in wild-type and pptr-1(tm3103) zygotes. Error bars are standard deviation of mean values from 3 zygotes. In wild-type embryos, GFP::PGL-1 fluorescence does not decrease in the anterior during interphase, even though the number of visible granules decreases (Fig. 2A), consistent with granule disassembly. During mitosis, GFP::PGL-1 fluorescence and P granule size increase in the posterior (Sup. Fig. 1), consistent with granule assembly.
G) Fold-enrichment of GFP::PGL-1 and GFP::GLH-1 in each P blastomere over its somatic sister. Enrichment becomes most pronounced with each division in wild-type. No enrichment is observed at any division in pptr-1 mutants. Error bars are standard deviation from values obtained from 3 focal planes in 3 embryos.
P granule asymmetry requires the polarity regulators PAR-1, MEX-5 and MEX-6 (7). PAR-1 is a kinase that segregates with P granules, and MEX-5 and MEX-6 are two redundant RNA binding proteins that segregate opposite P granules in response to PAR-1 asymmetry (8). MEX-5 and MEX-6 are inherited by somatic blastomeres and turned over after 1 or 2 cell divisions (9). We found that PAR-1 and MEX-5/6 promote P granule assembly and disassembly, respectively. In par-1 zygotes, where MEX-5/6 are uniformly distributed, most P granules disassembled completely throughout the zygote (Fig. 2B). Complete disassembly was dependent on MEX-5/6: in par-1;mex-5/6 zygotes and in mex-5/6 zygotes, P granules remained in a dynamic equilibrium between assembly and disassembly throughout the zygote, and P granule number increased (Fig. 2C and D, Sup. Fig. 1A). Consistent with MEX-5/-6 having a direct role in P granule disassembly, we observed mCherry::MEX-5 on shrinking PGL-1::GFP granules in wild-type embryos (Sup. Fig. 1B and Movie 4). In mex-5/6 zygotes, we observed large P granules throughout the zygote at meiotic exit and in a small region in the posterior at mitosis (Fig. 2C), consistent with the localization of PAR-1 in mex-5/6 embryos (10). In contrast, in par-1;mex-5/6 zygotes, P granules were fewer and smaller (Fig. 2D and Sup. Fig. 1). These results suggest that PAR-1 promotes P granule assembly both directly, by an unknown mechanism most active during mitosis, and indirectly by restricting MEX-5/6 to the anterior (also see (7)).
Our observations are consistent with an earlier study (3), which also concluded that P granule asymmetry is driven primarily by localized assembly and disassembly, rather than by granule movement. That study hypothesized that a local change in the concentration threshold for granule assembly might be sufficient to promote disassembly in the anterior, assembly in the posterior, and enrich PGL-1 in the posterior. Our findings, however, indicate that disassembly during interphase and assembly during mitosis are regulated independently, and that preferential segregation of PGL-1 to the germline depends primarily on granule assembly during mitosis.
Consistent with this hypothesis, in an RNAi screen for genes required for GFP::PGL-1 asymmetry, we identified a gene necessary to assemble P granules during mitosis (Methods). pptr-1 encodes a regulatory subunit of the phosphatase PP2A (Methods and (11)). In pptr-1(tm3103) embryos, GFP::PGL-1 granules disassembled during mitosis (Fig. 2E) and equal levels of diffuse GFP::PGL-1 were inherited by somatic and germline blastomeres at each division (Fig. 2F and G, Sup. Fig 3A, and Movie 5). During interphase, GFP::PGL-1 reassembled into granules in the germline daughter, but remained diffusely distributed in the somatic daughter until after the next division (after MEX-5 and MEX-6 have turned over). As a result of equal partitioning, GFP::PGL-1 granules became progressively fewer and smaller with each P blastomere (Fig. 1B, Sup. Fig 3A, B). Staining of fixed pptr-1 embryos with antibodies against core P granule proteins (PGL-1, PGL-3, GLH-1, GLH-2, GLH-4, and the P granule epitope OIC1D4) confirmed that P granules disassemble at each division in pptr-1 mutants (Fig. 3A and Sup. Fig. 4). Granules reformed during interphase in each P blastomere, but were smaller and fewer than in wild-type, consistent with 50% or more loss of P granule components to somatic blastomeres at each division (Fig. 3). Granules also reappeared in somatic cells after the 4-cell stage, in a pattern matching the dynamics of MEX-5/6 turnover (9). By the 100 cell-stage, only small granules remained and these were not enriched in the primordial germ cells Z2 and Z3. We also monitored the distribution of two P granule-associated mRNAs, cey-2 and nos-2. In wild-type embryos, cey-2 and nos-2 mRNAs segregate preferentially with P granules to the germline blastomeres; lower levels inherited by somatic blastomeres are rapidly degraded after division (12, 13). In pptr-1 embryos, cey-2 and nos-2 mRNAs were equally partitioned to somatic and germline blastomeres, but were still degraded in somatic lineages after division (Fig. 3B and Sup. Fig. 3C). Similarly, after the 30-cell stage, PGL-1 remaining in somatic lineages is degraded by the autophagy machinery in wild-type (14) and in pptr-1 embryos (Sup. Fig. 3B). We conclude that in pptr-1 embryos, P granule proteins and RNAs are partitioned equally to somatic and germline blastomeres, but behave differently post mitosis in each cell type. In germline blastomeres, P granules components remained stable and were re-assembled into granules, albeit of diminishing size and number with each division. In somatic blastomeres, P granule RNAs were rapidly degraded; P granule proteins reassembled into granules after 1-2 cell cycles, and were turned over after gastrulation.
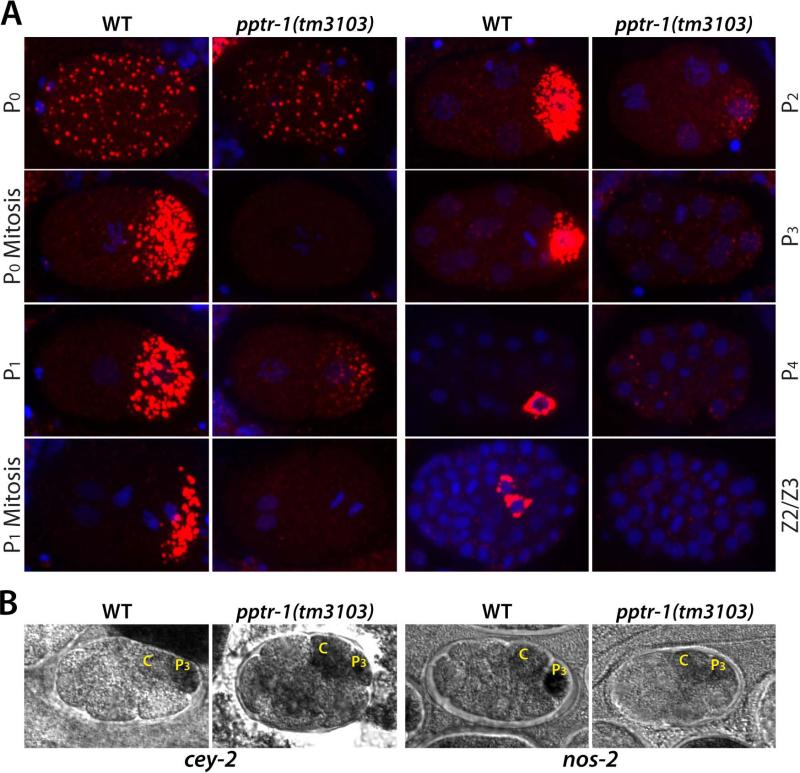
Fig 3. P granule components are segregated equally to germline and somatic blastomeres in pptr-1 mutants.
A) Fixed wild-type and pptr-1 embryos stained with DAPI (blue) and OIC1D4 (red). Images are maximum projections of confocal Z-stacks (spanning entire embryo), except for P4 and Z2/Z3 images, which show single planes.
B) Wild-type and pptr-1 embryos stained for nos-2 and cey-2 RNAs (black) by in situ hybridization. In pptr-1 embryos, nos-2 and cey-2 RNAs are present at equal levels in P3 and C, indicative of symmetric segregation during the P2 division. In pptr-1 embryos, as in wild-type, nos-2 and cey-2 RNAs levels are lower in all other blastomeres, indicative of rapid degradation of these RNAs in somatic lineages.
Segregation of other asymmetric proteins, including PAR-2, PAR-1 and MEX-5, was not affected in pptr-1 mutants (Sup. Fig. 5A). Depletion of mex-5/6 by RNAi in pptr-1 mutants stabilized P granules in the anterior of the zygote and in somatic blastomeres during interphase, but not during mitosis, indicating that MEX-5/6 are active but are not responsible for P granule disassembly during mitosis (Sup. Fig. 5B). PIE-1 is a transcriptional repressor required to silence transcription in the P lineage (15). In the cytoplasm, PIE-1 is enriched on P granules and this association has been proposed to drive PIE-1's preferential segregation into germline blastomeres (16). Consistent with the lack of P granules, in pptr-1 embryos, GFP::PIE-1 was not enriched on granules during mitosis, yet was still asymmetrically segregated (Fig. 4A and Sup. Fig. 5A). We conclude that pptr-1 is required specifically for P granule partitioning, but is not required for the segregation of other germ plasm components, which can segregate independently of P granules.
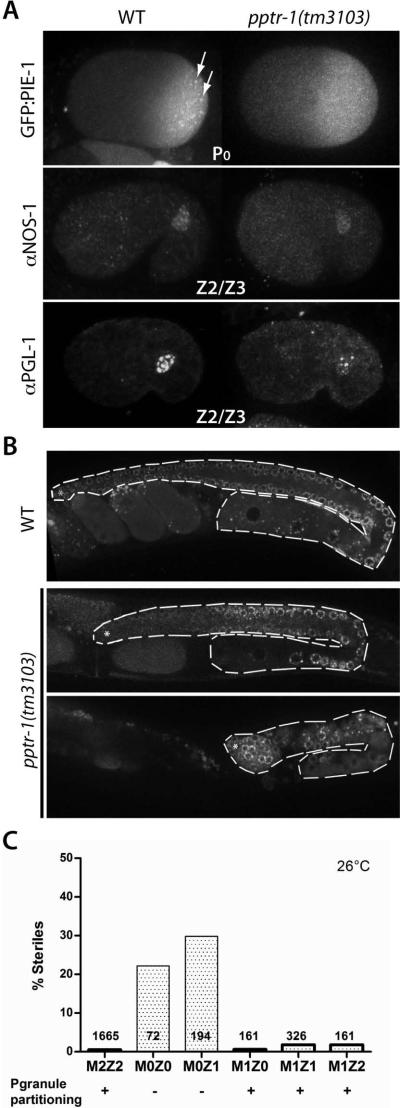
Fig 4. pptr-1 mutants specify a germline, but a minority are sterile at high temperatures.
A) Embryos expressing GFP::PIE-1 or stained with NOS-1 and PGL-1 antibodies. Patterns are identical in wild-type and pptr-1, except that pptr-1 embryos lack GFP::PIE-1 foci seen in wild-type (arrows), and have lower PGL-1 levels.
B) Adult wild-type and pptr-1 hermaphrodites expressing GFP::PGL-1. Gonads are outlined. Most pptr-1 hermaphrodites develop a full gonad with gametes (top panel), but at high temperatures a minority (20%) are sterile, with no gametes (lower panel). Fertile pptr-1 gonads are smaller than wild-type and yield a reduced number of progeny; unlike the P granule defect, however, the brood size defect is partially rescued by zygotic pptr-1 (Sup. Fig. 7).
C) Percentage of sterile hermaphrodites and total numbers scored. Wild-type have 2 maternal and 2 zygotic pptr-1 copies (M2Z2). Mutant pptr-1 hermaphrodites (M0Z0) were crossed with wild-type males to generate M0Z1, which were allowed to self-fertilize to generate M1Z0, M1Z1 and M1Z2. Only M0Z0 and M0Z1 hermaphrodites show significant sterility, demonstrating maternal requirement. pptr-1 is also required maternally for P granule partitioning (Sup. Fig. 3).
If asymmetric partitioning of P granule components is necessary to distinguish germline from somatic blastomeres, then mutants like pptr-1 should show defects in germline specification. Primordial germ cells do not form in par-1, mex-5/6 mutants, and mes-1 mutants (which miss-segregate P granules in the P2 and P3 blastomeres); but these mutants also miss-segregate other factors, notably PIE-1 (17) (18). We found that primordial germ cells form normally in pptr-1 embryos: by mid-embryogenesis, we detected two cells expressing the germline proteins NOS-1 and PGL-1 as is observed in wild-type (13) (4). NOS-1, which is expressed only zygotically, was expressed at the same level in wild-type and pptr-1 embryos (Fig. 4A). In contrast, PGL-1, which is expressed both maternally and zygotically at this stage, was lower in pptr-1 embryos, consistent with mis-segregation of maternal PGL-1 (Fig. 4A). We confirmed that pptr-1 mutants express PGL-1 zygotically in primordial germ cells using a paternally-inherited pgl-1 transgene (Sup. Fig. 5C). Consistent with proper germline specification, at 20°C, 100% of pptr-1 adults (n=5798) were self-fertile with a full germline. At higher temperatures (24°C and 26°C), however, a minority (~20%) of pptr-1 adults were sterile (Fig. 4B and C). Sterile pptr-1 hermaphrodites had underdeveloped gonads with P granule-positive germ cells but no gametes (Fig. 4B). Stunted gonad development at high temperatures is characteristic of mutants lacking maternal PGL or GLH proteins (6, 19), raising the possibility that the sterility of pptr-1 mutants is caused by the P granule partitioning defect. Consistent with this hypothesis, we found that, at 20oC, 15% of pptr-1;pgl-1 double mutants (n= 1191) were sterile, in contrast to the single mutants (1.3% n=1959 pgl-1; 0% n=5798 pptr-1). Furthermore, both the sterility and P granule defects of pptr-1 mutants could be rescued by maternal pptr-1, but not by zygotic pptr-1 (Fig. 4C and Sup. Fig. 6). We conclude that P granule partitioning during embryogenesis is not essential to specify germ cell fate, but is required to promote robust germ cell proliferation and differentiation at high temperatures.
Our findings demonstrate that, in C. elegans, asymmetric partitioning of P granules during division can be uncoupled from the asymmetric partitioning of other germ plasm components (such as PIE-1), and is not essential to distinguish germline from soma. Even after inheriting equal levels of germ granule components, somatic and germline blastomeres maintain distinct fates. Therefore, why are germ granule components partitioned with the germ plasm? Our results suggest that partitioning increases stress resistance in the nascent germline by maximizing the maternal load of germ granule material inherited by primordial germ cells. In this regard, it is interesting that pptr-1 also regulates insulin signaling (10), a pathway important for stress resistance. Many animals (including mammals) do not possess germ plasm and use inductive interactions to specify the germline. In those animals, germ granules are assembled de novo after germ cell specification (20). Our findings suggest that in animals with germ plasm, germ granule assembly is also a consequence, not a cause, of an underlying soma-germline distinction maintained by other factors.
Supplementary Material
Acknowledgments
We thank S. H. Kim, S. Strome, E. Voronina, K. Kemphues, K. Bennett, and C. Eckmann for reagents and advice. We also obtained reagents from the National Bioresource Project for the Nematode (Japan), the Caenorhabditis Genetics Center and the Developmental Studies Hybridoma Bank (USA). This work was supported by NIH grants GM080042 to C.G., HD007276 to J.W., and HD037047 to G.S. G.S. is a Howard Hughes Medical Institute investigator.
References
- 1.Chuma S, Hosokawa M, Tanaka T, Nakatsuji N. Mol. Cell. Endocrinol. 2009 Jul 10;306:17. doi: 10.1016/j.mce.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 2.Updike D, Strome S. J. Androl. 2010 Jan-Feb;31:53. doi: 10.2164/jandrol.109.008292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brangwynne CP, et al. Science. 2009 Jun 26;324:1729. doi: 10.1126/science.1172046. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki I, et al. Cell. 1998;94:635. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- 5.Gruidl ME, et al. Proc Natl Acad Sci U S A. 1996;93:13837. doi: 10.1073/pnas.93.24.13837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawasaki I, et al. Genetics. 2004 Jun;167:645. doi: 10.1534/genetics.103.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheeks RJ, et al. Curr. Biol. 2004 May 25;14:851. doi: 10.1016/j.cub.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 8.Munro E, Bowerman B. Cold Spring Harb Perspect Biol. 2009 Oct;1:a003400. doi: 10.1101/cshperspect.a003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schubert CM, Lin R, de Vries CJ, Plasterk RH, Priess JR. Mol Cell. 2000;5:671. doi: 10.1016/s1097-2765(00)80246-4. [DOI] [PubMed] [Google Scholar]
- 10.Cuenca AA, Schetter A, Aceto D, Kemphues K, Seydoux G. Development. 2003 Apr;130:1255. doi: 10.1242/dev.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padmanabhan S, et al. Cell. 2009 Mar 6;136:939. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seydoux G, Fire A. Development. 1994;120:2823. doi: 10.1242/dev.120.10.2823. [DOI] [PubMed] [Google Scholar]
- 13.Subramaniam K, Seydoux G. Development. 1999;126:4861. doi: 10.1242/dev.126.21.4861. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, et al. Cell. 2009 Jan 23;136:308. [Google Scholar]
- 15.Nakamura A, Seydoux G. Development. 2008 Dec;135:3817. doi: 10.1242/dev.022434. [DOI] [PubMed] [Google Scholar]
- 16.Daniels BR, Perkins EM, Dobrowsky TM, Sun SX, Wirtz D. J. Cell Biol. Feb;17:2009. doi: 10.1083/jcb.200809077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonczy P, Rose LS. WormBook. 2005;1 doi: 10.1895/wormbook.1.30.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strome S. WormBook. 2005;1 doi: 10.1895/wormbook.1.9.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spike C, et al. Genetics. 2008 Apr;178:1973. doi: 10.1534/genetics.107.083469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotaja N, Sassone-Corsi P. Nat Rev Mol Cell Biol. 2007 Jan;8:85. doi: 10.1038/nrm2081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.