Abstract
Liquefaction is an important pathological process that can subsequently lead to cavitation where large numbers of bacilli can be coughed up which in turn causes spread of tuberculosis in humans. Current animal models to study the liquefaction process and to evaluate virulence of mycobacteria are tedious. In this study, we evaluated a rabbit skin model as a rapid model for liquefaction and virulence assessment using M. bovis BCG, M. tuberculosis avirulent strain H37Ra, M. smegmatis, and the H37Ra strains complemented with selected genes from virulent M. tuberculosis strain H37Rv. We found that with prime and/or boosting immunization, all of these live bacteria at enough high number could induce liquefaction, and the boosting induced stronger liquefaction and more severe lesions in shorter time compared with the prime injection. The skin lesions caused by high dose live BCG (5 × 106 CFU) were the most severe followed by live M. tuberculosis H37Ra with M. smegmatis being the least pathogenic. It is of interest to note that none of the above heat-killed mycobacteria induced liquefaction. When H37Ra was complemented with certain wild type genes of H37Rv, some of the complemented H37Ra strains produced more severe skin lesions than H37Ra. These results suggest that the rabbit skin liquefaction model can be a more visual, convenient, rapid and useful model to evaluate virulence of different mycobacteria and to study the mechanisms of liquefaction.
Key words: mycobacteria, tuberculosis, pathology, liquefaction, skin model
Introduction
About one third of world population are latently infected with Mycobacterium tuberculosis, and 5%–10% of them are expected to develop active disease during their lifetime. Due to the emergence of drug-resistant tuberculosis (TB) and the pandemic of HIV infection, TB has once again become a serious threat to public health.1 Despite significant progress, the pathogenesis of TB is not well understood, which limits the effective control of TB.
After M. tuberculosis infection, tissue-damaging delayed-type hypersensitivity (DTH) (mediated by Th1 lymphocytes) develops and results in a solid caseous necrotic center and a peripheral accumulation of partly activated macrophages and lymphocytes, which constitute the TB granuloma. Consequently, some lesions may regress with destruction of bacteria within them, followed by fibrosis and calcification. On the other hand, some lung lesions can go on to become liquefied, which triggers the growth of tubercle bacilli extracellularly in the host, with cavity formation and air-borne spread of the disease. Thus liquefaction is a most important event in the exacerbation and transmission of the disease.2,3 A better understanding of the liquefaction process could lead to prevention of the disease. However, the mechanism of liquefaction is still not understood partly due to the shortage of suitable animal model.3
Rabbits, guinea pigs and mice are commonly used animal models of TB. Dannenberg reviewed the relative merits and historical context of these models in comparison to the human disease. While the mouse model has the advantage of clear genetic background and strong cell-mediated immunity, it does not produce liquefaction. On the other hand, rabbits and guinea pigs could produce liquefaction and cavitation of tuberculosis. Rabbit is a good animal model of human liquefaction and cavitation.2 Dannenberg and colleagues showed that New Zealand White rabbits can be infected with aerosol of hundreds and thousands of bacillary units of bovine-type tubercle bacilli, and found pulmonary cavities were developed in both low and high dose group in 6–33 weeks.4 It also indicated that the cavities formed more quickly in high dose group, which seemed to hasten the progression of the disease.4 In addition, heat-killed bacteria and very low-dose of M. bovis could pre-sensitize the rabbits and increase cavity formation.5,6
In our previous study, we performed a preliminary study in rabbit skin liquefaction model primarily using live M. bovis attenuated strain BCG, M. tuberculosis avirulent strain H37Ra, and M. smegmatis.7 In this study, we further compared the lesions induced by both live and heat-killed different mycobacteria, and validated that the skin lesions caused by live BCG were the most severe followed by live M. tuberculosis H37Ra with fast growing mycobacteria M. smegmatis being the least pathogenic in the rabbit skin model, whereas heat-killed mycobacteria lost the ability to induce liquefaction. Furthermore, using the above validated rabbit skin model we compared the virulence of different H37Ra complemented strains and H37Ra. Our studies suggest that the rabbit skin model can be a useful model to study liquefaction and quickly evaluate the virulence of different mycobacteria and have the potential to be used as a simple model to test inhibitors of liquefaction.8
Results
Differences in skin lesions induced by live and heat-killed BCG, H37Ra and M. smegmatis in the rabbit skin model.
In our previous study, we determined the different doses of live mycobacteria to induce skin lesions in the rabbit skin model.7 High (5 × 106 CFU), intermediate (5 × 104 CFU), and low (5 × 102 CFU) doses of live BCG, H37Ra, M. smegmatis were injected intradermally on each flank of rabbits. Six weeks later, they were boosted with the same dose of the same bacteria close to the first injection site. With the priming and boosting injection, the high dose of each viable bacteria (5 × 106 CFU) could induce liquefaction and ulcer, and the intermediate dose of BCG (5 × 104 CFU) could induce granuloma, while intermediate dose of H37Ra and M. smegmatis and low dose of all three bacteria (5 − 102 CFU) induced no obvious lesions.
In this study, to determine if the lesions produced by mycobacteria depend on the viability of the bacteria, we prepared heat-killed M. tuberculosis H37Ra and M. smegmatis and BCG and injected them into the rabbit skin by prime-boost regimens as above. Live mycobacteria were included as controls for heat-killed mycobacteria in the rabbit skin model. The rabbits were first injected intradermally with live and heat-killed BCG, H37Ra and M. smegmatis (5 × 106) and then boosted with the same bacteria, and the differences of pathology induced by priming and boosting were compared (Table 1 and Figs. 1 and 2). After the prime injection, the injected area of both the live and heat-killed bacteria formed granuloma along with edema and inflammation at the first five days. Then the edema reduced gradually but the granuloma induced by live bacteria liquefied. The liquefaction induced by BCG became obvious at about 10–11 days, followed by live H37Ra at about 12 days and live M. smegmatis at about 13 days. The size of lesions induced by viable BCG was significantly larger than that induced by viable H37Ra or viable M. smegmatis. The liquefied and necrotic material of these lesions discharged its contents by rupturing through the epidermis. All the heat-killed BCG, H37Ra and M. smegmatis produced smaller skin granuloma with no obvious liquefaction compared with live bacteria, and the heat-killed BCG could induce more obvious granuloma than heat-killed H37Ra and M. smegmatis. After 35 days, all the lesions slowly regressed and healed. Six weeks later, these rabbits were reinjected with the same bacteria close to the prime injection site. The time to form granuloma induced by all live and heat-killed bacteria was shortened by 1–2 days at this time compared with prime injection. The granuloma induced by live BCG and live H37Ra developed into obvious liquefaction at about 3–4 days after last injection, and M. smegmatis at about 6–7 days, and then all followed by ulceration. Compared with prime lesions, the size of corresponding boosting lesions was larger and the induced liquefaction was also more severe. Likewise, the size of lesions induced by viable BCG was significantly larger than that induced by viable H37Ra or viable M. smegmatis (Figs. 1 and 2). The heat-killed BCG also produced more obvious granuloma (without liquefaction) than the other two heat-killed mycobacteria (Fig. 2). Beside the lesion size, we also observed that viable BCG induced stronger swelling than viable H37Ra and viable M. smegmatis, with the swelling induced by M. smegmatis being the least obvious. Interestingly, with both priming and boosting, none of the heat-killed mycobacteria induced any obvious liquefaction (Table 1).
Table 1.
Pathologic changes induced by BCG, H37Ra or M. smegmatis in the rabbit skin model
| Prime | Boost | |
| Live BCG 5 × 106 CFU | Granuloma+++ (24–72 h) Liquefaction and ulcer+++, 10–13 days later |
Ulcer++++, 3–7 days later; Liquefaction++++ |
| Live BCG 5 × 104 CFU | Granuloma+ | Granuloma+ |
| Live BCG 5 × 102 CFU | ± | ± |
| Heat-killed BCG 5 × 106 CFU | + | + |
| Live H37Ra 5 × 106 CFU | Granuloma++ (24–72 h) Liquefaction and ulcer ±, 10–13 days later |
Ulcer++, 5–7 days later; Liquefaction++ |
| Live H37Ra 5 × 104 CFU | ± | ± |
| Live H37Ra 5 × 102 CFU | − | − |
| Heat-killed H37Ra 5 × 106 CFU | + | + |
| Live M. smegmatis 5 × 106 CFU | Granuloma+ (24–72 h) Liquefaction and ulcer ±, 10–13 days later |
Ulcer+, 7–9 days later; Liquefaction++ |
| Live M. smegmatis 5 × 104 CFU | ± | ± |
| Live M. smegmatis 5 × 102 CFU | − | − |
| Heat-killed M. smegmatis 5 × 106 CFU | ± | ± |
Symbols: −, no granuloma, liquefaction and ulcer; ±, no obvious granuloma, liquefaction and ulcer; +∼++++, the number of plus represents the degree of pathologic changes.
Figure 1.
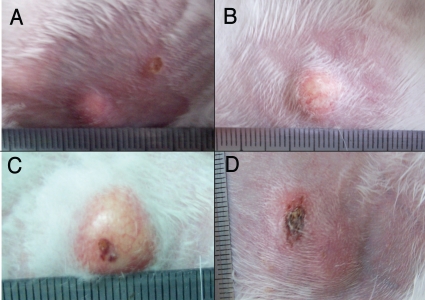
Liquefaction and ulcer lesions in rabbit skin induced by live BCG, M. smegmatis, and H37Ra. The rabbits were injected intradermally with 5 × 106 CFU of BCG, M. smegmatis, and H37Ra, respectively. About 42 days later, they were boosted with the same dose of same bacteria. The lesion liquefied or ulcerated at about 3–7 days after boosting. (A) M. smegmatis induced abscess; (B) H37Ra induced abscess; (C) BCG induced abscess; (D) Ulcerated and scabbed abscess caused by BCG.
Figure 2.
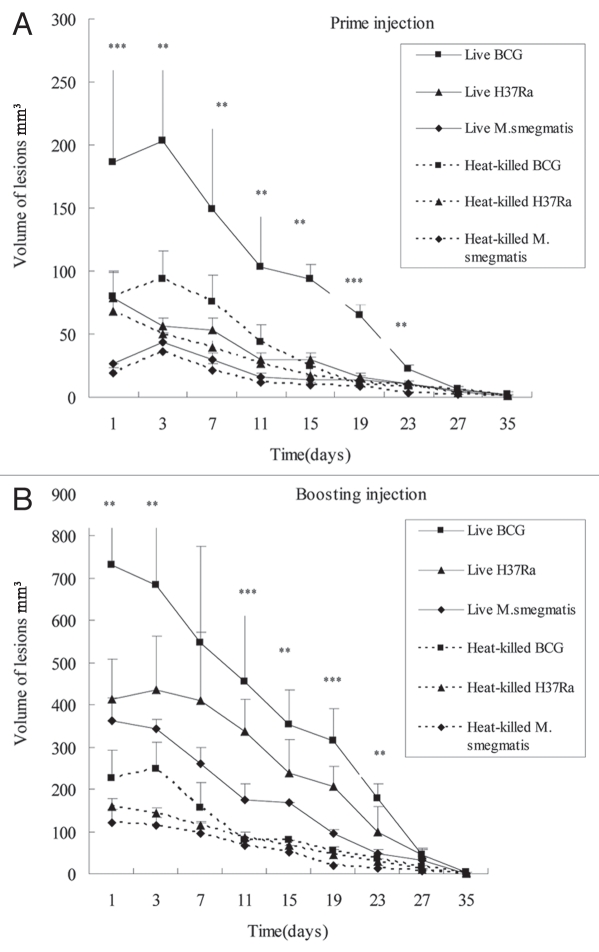
Difference in pathologic changes of skin lesions in rabbits injected with different live and heat-killed mycobacteria by prime-boost strategy. (A) Pathologic changes of the primary skin lesions. The rabbit was injected intradermally with about 5 × 106 CFU of live or heat-killed mycobacteria in 100 µl respectively on both flank. (B) Pathologic changes of the skin lesions induced by boosting with homogeneous mycobacteria. Six weeks after the first injection, the rabbits were boosted with the same bacteria at the same dose close to the first injection site. On both the prime and boosting injection, live BCG induced the most severe inflammation reaction, granuloma, liquefaction and ulceration followed by live H37Ra with M. smegmatis being the least pathogenic. All the heat-killed bacteria induced smaller granuloma without liquefaction than the live bacteria. Each point represents the mean of the lesions from both flanks of three rabbits and its standard error, along with the statistical significance: n = 6, **p < 0.05 BCG vs. H37Ra and M. smegmatis, and ***p < 0.05 BCG vs. H37Ra vs. M. smegmatis.
We analyzed the characteristic lesions induced by viable mycobacteria (BCG, H37Ra and M. smegmatis) by histopathology. The results showed that all three viable bacteria induced similar pathologic changes, with liquefaction in the center and accumulation of lymphocytes, fibroblasts and macrophages surrounding the center.
Effect of live BCG, H37Ra and M. smegmatis boosting on the lesions primed by live BCG.
To investigate if BCG, H37Ra, and M. smegmatis have common components which contribute to the liquefaction and ulceration, rabbit skin was first injected intradermally with live BCG (5 × 106), followed by boosting with live BCG, H37Ra, M. smegmatis (5 × 106), respectively, by injection intradermally close to the site of BCG-primary injection 4 weeks later. Following prior BCG sensitization, boosting with BCG, H37Ra or M. smegmatis all induced liquefaction and ulcer. However, the inflammation reaction and the size of lesions induced by BCG sensitization followed by BCG boost were the strongest and biggest among them, followed by BCG prime and H37Ra boost, while BCG-prime followed by M. smegmatis boosting induced the least reaction.
Detection of mycobacteria in the lesions.
To confirm if the liquefaction was induced by live BCG, H37Ra, M. smegmatis but not by contaminating bacteria, the liquefaction material of representative groups were subjected to acid-fast staining and Gram staining. In all lesions, acid-fast mycobacteria were detected, and no other bacteria were found.
Evaluation of virulence of H37Ra complemented strains in the rabbit skin model.
Since the above findings suggest that the rabbit skin model is useful to distinguish the virulence of BCG, H37Ra and M. smegmatis, we decided to use the rabbit skin model to rapidly evaluate virulence of H37Ra complemented strains based on the mutations found in H37Ra.9 The rabbits were injected by the intradermal route with high (5 × 107 CFU), intermediate (5 × 106 CFU), and low (5 × 105 CFU) doses of H37Ra complemented strains (Table 2) and also the H37Ra strain transformed with the vector pOLYG control,10 and also H37Ra alone as control on both the right and left flanks of rabbits, respectively. Forty-two days later, they were reinjected with the same bacilli at the same dose. It was found that both the high dose (5 × 107 CFU) and intermediate dose (5 × 106 CFU) produced typical tubercles and liquefied, whereas the low dose (5 × 105 CFU) did not cause obvious lesions in rabbit skin. For the primary skin lesions, high dose of all different bacteria induced granuloma 1–2 days after injection, while 1–5 days for intermediate dose, and the injected area formed inflammation with edema. Along with edema decreasing, inflammation peaked about 5–7 days for the high dose and 10–13 days for intermediate dose, and then liquefied. At 35 days, the lesions epithelialized and healed. For the boosting, the inflammation of the high dose peaked about 2–3 days and the intermediate dose need 3–7 days after last reinjection, and these lesions were more severe than primary ones. Shortly after this, the lesions liquefied and discharged its contents, and then slowly regressed. In general, almost all lesions healed at about 35 days (Fig. 3). Irrespective of the particular genes used for complementation, all H37Ra complemented strains and the H37Ra vector control and H37Ra had the same dose lesion relationship and the boosting effect.
Table 2.
Selected genes from virulence strain M. tuberculosis H37Rv and their primers used for complementation of M. tuberculosis H37Ra
| Strain | Gene | Primer | Variation compared with H37Rv | Product | |
| P10 | pstA1 | Rv0930 | 5′-atctctagatgtttaagcagggcaacgtgtcg-3′ | R305* (C914T) | Phosphate ABC transporter, transmembrane protein |
| 5′-tattctagagctaccgttcaccggcctcaaca-3′ | |||||
| P19 | lpdA | Rv3303c | 5′-tacgtctagaatccgttgtggtgtccgaggaa-3′ | promoter** | Flavoprotein disulfide reductase |
| 5′-cgaatctagaagcgtggtcagcgacagttca-3′ | |||||
| P17 | pabB | Rv1005c | 5′-tacgtctagaactggtgttggtgctggcgatag-3′ | promoter (A-56T) | Aminodeoxychorismate synthase component I |
| 5′-cgaatctagacaggaccgcagccagccgat-3′ | |||||
| P15 | mazG | Rv1021 | 5′-tacgtctagaccgaagaacccaaggatgtgcg-3′ | A219E (C656A) | Nucleoside triphosphate pyrophosphohydrolase |
| 5′-cgaatctagagacacttgggattctcgacctcg-3′ | |||||
| P18 | nrdH | Rv3053c | 5′-tacgtctagaattgggtcagacgggtatgg-3′ | promoter Δ-194—180GTGCCACCCCCTAG | Glutaredoxin electron transport protein NrdH |
| 5′-cgaatctagagcaatgacctgtttggggac-3′ | |||||
| P14 | pknH | Rv1266c | 5′-cgctctagacacattcgcatctgtgaccatgaa-3′ | R607Q (G1820A) | Transmembrane serine/threonine-protein kinase H |
| 5′-tcgtctagagatgaagcccagaatcgctacgga-3′ | |||||
| P16 | nadD | Rv2421c | 5′-tacgtctagaatgtgtgtgcaagcagcttcgcc-3′ | promotor (A-44C) | Nicotinic acid mononucleotide |
| 5′-cgaatctagagtaacccgcctgtcgcattttct-3′ | |||||
| P11 | phoP | Rv0757 | 5′-agttctagacggcatcacccaacgcttgtttg-3′ | S219L (C655T) | Two component system response regulator |
| 5′-aattctagagctgcgaccaggcgtacccgtag-3′ | |||||
| P12 | marR | Rv0880 | 5′-ctgtctagaacctcgagcagcacgggtggtag-3′ | R67K (G200A, G201A) | MarR family transcription regulator |
| 5′-gagtctagacggccagatcatccttgagctcg-3′ | |||||
| P20 | ilvD | Rv0189c | 5′-tacgtctagagcacgattacgctgcccctacc-3′ | V285G (T851G) | Dihydroxy-acid dehydratase |
| 5′-cgaatctagagtggtcgcacatcatcgcaggt-3′ | |||||
| P13 | luxR | Rv0890c | 5′-agttctagatacagttgcgcgacgaactctatg-3′ | P866A (C2596G) | LuxR family transcription regulator |
| 5′-atatctagatctccgggaccactgtcatacgag-3′ | |||||
| PolyG | Vector control | Control with empty vector | |||
LpdA promoter GCTGCGGTGCGGGTCATCGTCAAGCTGTGCTT-154—123TGCGCGGCGTGCATCGTCGCCGAGCTGTGTTG.
Figure 3.
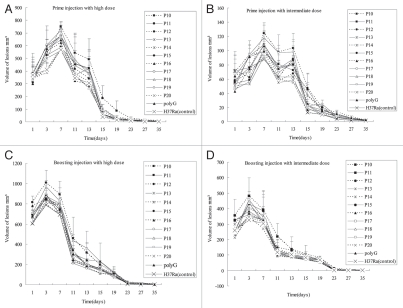
Pathologic changes of rabbit skin lesions produced by different H37Ra complemented strains after prime and boosting immunity. Each point represents the mean with its standard error from four injection sites. Pathologic changes of the primary injection with high dose (A) and intermediate dose (B). High dose (5 × 107 CFU) of bacteria induced granuloma 1–2 days after injection, while 1–5 days for intermediate dose (5 × 106 CFU), and the injected area formed inflammation with edema. Inflammation reached peak about 5–7 days for high dose and 10–13 days for intermediate dose, then liquefied. At 35 days, the lesions epithelialized and healed. Pathologic changes of the boosting infection with high dose (C) and intermediate dose (D). The inflammation of high dose reached peak about 2–3 days and the intermediate dose need 3–7 days after last injection, shortly after this, the lesions liquefied and discharged its contents. These lesions healed at about 35 days. The tubercles caused by P10 was the largest, followed by P19, P17, P15, P18, P14 and others (*p < 0.05 in Table 3), while P14 and P18 induced the most severe liquefaction.
However, the lesions induced by different H37Ra complemented strains differed in size and severity or degree of liquefaction (Fig. 3 and Table 3). H37Ra produced the least lesions, whereas P10, which is H37Ra complemented with pstA1 from H37Rv, caused the largest tubercles, followed by P19 (lpdA), P17 (pabA) and P15 (mazG), P18 (nrdH), P14 (pknH), which all induced obvious bigger tubercle than H37Ra did (*p ≤ 0.05). However, P11 (phoP), P12 (marR family), P13 (luxR/uhpA family), P16 (nadD) and P20 (ilvD) produced similar lesions as the H37Ra transformed with vector pOLYG control and H37Ra. Interestingly, P14 (pknH) and P18 (nrdH) induced the most obvious liquefaction among all groups. The lesions of P10 (pstA1), P11 (phoP), P13 (luxR/uhpA family), P20 (ilvD), and P16 (nadD) were similar in liquefaction to H37Ra vector pOLYG control.
Table 3.
Different H37Ra complemented strains induce varying lesions and liquefaction in the rabbit skin model at different time points
| Prime | Boost | |||
| Volume of lesions (mm3) | Degree of liquefaction (5–7 days) | Volume of lesions (mm3) | Degree of liquefaction (2–3 days) | |
| P10* 5 × 107 CFU | 750.0 ± 34.6 | ++ | 1008.8 ± 123.9 | ++ |
| P19* 5 × 107 CFU | 720.0 ± 48.9 | ++ | 970.0 ± 61.8 | +++ |
| P17* 5 × 107 CFU | 680.0 ± 123.3 | ++ | 896.5 ± 32.5 | ++ |
| P15* 5 × 107 CFU | 677.5 ± 85.0 | ++ | 882.7 ± 62.2 | +++ |
| P18* 5 × 107 CFU | 676.5 ± 55.3 | +++ | 875.0 ± 132.3 | ++++ |
| P14* 5 × 107 CFU | 675.0 ± 115.3 | +++ | 862.5 ± 91.9 | ++++ |
| PolyG 5 × 107 CFU | 578.5 ± 69.1 | ++ | 852.5 ± 34.6 | +++ |
| P16 5 × 107 CFU | 625.0 ± 146.4 | ++ | 845.0 ± 49.4 | +++ |
| P11 5 × 107 CFU | 577.5 ± 31.8 | ++ | 843.8 ± 91.9 | ++ |
| P13 5 × 107 CFU | 562.5 ± 25.0 | + | 812.5 ± 65.0 | +++ |
| P12 5 × 107 CFU | 580.0 ± 77.8 | ++ | 827.5 ± 61.8 | ++ |
| P20 5 × 107 CFU | 575.0 ± 28.9 | + | 791.3 ± 52.6 | ++ |
| H37Ra 5 x 107 CFU | 566.3 ± 79.7 | + | 785.5 ± 90.0 | ++ |
p < 0.05 P10, P14, P15, P17, P18, P19 vs. H37Ra control.
Discussion
Liquefaction is the key deteriorating process in tuberculosis and ultimately leads to cavitation and spread of the bacilli to new hosts. However, the mechanism of liquefaction is still unclear. Accumulating data indicated that liquefaction is related to at least two fundamental factors, host11 and bacteria. It is well-known that only some tuberculosis patients developed liquefaction. Patients co-infected with human immunodeficiency virus (HIV) are less likely to have liquefaction and cavitary disease due to a deficiency in CD4+ T cells, which suggests an important role for the CD4+ T cells in liquefaction.12 In addition, liquefaction may be influenced by the expression of Th2 cytokines such as IL-4 and involves the action of metalloproteinase enzymes in solid caseous material.2,8,13
Liquefaction could be developed in animals such as monkeys and resistant rabbits, but not in mice, which produce strong cell-mediated immunity and develop granuloma lesions and fibrosis following M. tuberculosis infection. Guinea pigs could form granuloma and have caseous necrosis like humans, but could not always develop liquefaction and cavities.3 Converse et al. produced cavitary TB model in New Zealand white rabbits with aerosolized virulent bovine-type tubercle bacilli. Their study showed that pulmonary cavities developed in both low dose (220 to 880 bacillary units) and high dose (3,900 to 5,800 bacillary units) groups.4 Dannenberg found that pulmonary TB in rabbits could be an excellent model for human TB since both species are relatively resistant to TB and readily form liquefied caseous lesions and cavities.2 We initially set up tuberculosis liquefaction model in the rabbit skin with live BCG. We then compared the virulence of M. bovis attenuated strain BCG, M. tuberculosis avirulent strain H37Ra, and M. smegmatis in the rabbit skin model, and found that all of them could induce liquefaction if the dose was high enough. This suggests that the components which could induce liquefaction in rabbit skin might be shared among the three mycobacteria even though their identities remain to be identified. However, there were clear differences in the pathologies produced by these mycobacteria. The inflammation reaction induced by BCG was significantly stronger than that of H37Ra or M. smegmatis. This might be because BCG is more virulent than H37Ra and M. smegmatis. This finding is consistent with previous studies that have shown the order of virulence in other animal models.14,15
We observed that sufficient number of mycobacteria is required to induce lesions in the rabbit skin model. Low dose of H37Ra and M. smegmatis (5 × 102 CFU) almost induced no obvious lesions, and two time injection of low dose of BCG just induced granuloma, while the high dose of live BCG, H37Ra and M. smegmatis (5 × 106 CFU) could induce liquefaction or ulcer. When the bacteria were heat-killed, they lost the ability to induce liquefaction and the granulomas they induced were also smaller than those caused by live mycobacteria. BCG, H37Ra and M. smegmatis could induce nonspecific inflammatory molecules such as MCP-1, IL-8, etc., in 1–3 days,16 so mononuclear cells can be attracted and activated, and the bacteria could be destroyed if the bacterial number is small. The undestroyed bacteria will survive in granuloma or macrophages, and stimulate specific immunity, and enough number of mycobacteria could induce caseous necrosis, liquefaction or ulcer. Surrounding caseous necrosis and liquefaction, mononuclear cell, degenerating epithelioid and multi-nucleated giant (Langhans) cells accumulated. This lesion (called a tubercle) could then be enveloped by fibroblasts. When the mycobacteria were heat-killed, they lost the ability to produce liquefaction lesions, indicating live mycobacteria either survive in the host tissues to stimulate inflammatory responses which are reduced or lost when bacteria are killed.
Using the validated rabbit skin model, we also evaluated the virulence of some H37Ra strains complemented with wild type genes from H37Rv, which were mutated in H37Ra and might be related to virulence attenuation in H37Ra. The complemented H37Ra strain P10, complemented with pstA1, caused most severe inflammatory reaction and induced the largest granuloma, followed by P19 (lpdA), P17 (pabB), P15 (mazG), P18 (nrdH) and P14 (pknH) which produced the most obvious liquefaction among all groups compared with H37Ra. By contrast, the other strains such as H37Ra complemented with vector pOLYG, P11 (phoP), P12 (marR family), P13 (luxR/uhpA family), P16 (nadD), P20 (ilvD) induced similar pathology reaction as H37Ra. From the NCBI database search and literature review, we summarized the function of these complemented genes in Table 2. In H37Ra, compared with H37Rv, the pstA1 gene has a point mutation of C to T change causing Arg at the 913rd nt to a stop codon.9 Complementation of H37Ra with the pstA1 caused the biggest granuloma but not the most obvious liquefaction lesion. This suggests that the gene products involved in producing liquefaction seems to be different from those that induce granuloma. It has been reported that pstA1 is involved in phosphate uptake.17 It is interesting to note that phosphate uptake has been associated with virulence in M. bovis.18 Thus it is quite likely that phosphate uptake is important for in vivo survival and may therefore explain why the pstA1 complemented H37Ra strain produced a more pronounced granuloma than the control H37Ra strain. The gene nrdH of P18 encodes glutaredoxin like protein, which is the hydrogen donor for ribonucleotide reductase. In H37Ra, the nrdH gene has a 14 bp deletion in its putative promoter region compared to that of Rv3053c in H37Rv.9 Since ribonucleotide reductase is essential for DNA biosynthesis and DNA repair which are affected in the presence or absence of oxygen,19 it is likely that defect in nrdH expression in H37Ra due to the promoter region deletion may affect the survival of the bacilli in vivo under low oxygen conditions and thus may be involved in attenuation of virulence in H37Ra. It is interesting to note that when H37Ra is complemented with wild type nrdH containing its own promoter region from H37Rv, the complemented strain caused more severe lesions and more pronounced liquefaction (Table 3). H37Ra has a point mutation of C to T change at the 62nd nt causing amino acid substitution of Arg to Gln at the 21st amino acid of the pknH gene. pknH gene encodes Ser/Thr protein kinase in M. tuberculosis, which controls the expression of a variety of cell wall related enzymes and plays a role in M. tuberculosis virulence.20 Complementation of H37Ra with the wild type pknH gene (P14) led to more pronounced liquefying lesions compared with H37Ra control (Table 3), which is consistent with its involvement in virulence of M. tuberculosis.
PhoP is a well known virulence factor in M. tuberculosis.21 H37Ra has a S219L mutation in the phoP gene,9,22,23 and the PhoP mutation is thought to be involved in virulence attenuation in H37Ra.22–24 However, it is surprising that phoP complemented H37Ra did not seem to be involved in virulence in the rabbit skin model, as it did not show more severe skin lesions than the H37Ra control (Table 3). This is in contrast to nrdH and pknH complemented strains that produced more severe skin lesions (Table 3). It is possible that the same strains can behave differently in terms of virulence in different animal models (i.e., rabbit versus mice or guinea pigs). The rabbit skin model which tests mainly gross lesions differs from virulence testing method based on survival of the bacilli in infected organs and could explain why obvious virulence factors such as phoP in other animal models was not identified as being involved in virulence in the rabbit skin lesion model in the current study.
It is of interest to note that the genes involved in lesion size or inflammation are not necessarily the same as those involved in liquefaction. For example, P10, H37Ra complemented with pstA1 produced the largest lesion size among the different complemented H37Ra strains but did not have stronger ability to produce liquefaction. In contrast, P14 (pknH) and P18 (nrdH) produced both larger lesion size as well as inducing the most severe liquefaction among different H37Ra complemented strains. Further studies are needed to evaluate the virulence of H37Ra complemented strains in other animal models such as mice and guinea pigs and to compare and contrast the findings among these models.
Rabbit skin model of tuberculosis may be a useful model to rapidly assess the virulence of mycobacteria. This conclusion is supported by the clear differences among the pathologies caused by BCG, H37Ra, M. smegmatis, and by the H37Ra complemented strains in this model. Due to the remarkable similarity in pathology between spectrum of rabbit and humans tuberculosis,25 the rabbit model could serve as a more relevant model for vaccine or drug testing.26 As for vaccine efficacy evaluation, we could first immunize the rabbit intradermally with BCG and other candidate vaccines, then assess the protective effect against M. tuberculosis infection in terms of changes in pathology and bacterial load. Once the relation between pathology changes and protection is established, evaluating the vaccine by the rabbit skin model rapidly might become feasible. Recently, Tsenova used a more tedious model of rabbit meningitis to detect the protection of tuberculosis subunit vaccine Mtb72F against H37Rv CNS infection.27 Further studies are needed to compare the rabbit meningitis model and the rabbit skin model and also other animal models in TB vaccine evaluation. In addition, the rabbit skin model could be useful for evaluating compounds that block lipases and proteases involved in the liquefaction process as possible pathology modifying agents for improved treatment of TB.8
In summary, high dose (5 × 106) of live BCG, H37Ra and M. smegmatis could induce liquefaction in rabbit skin, and the inflammation reaction induced by BCG was stronger than that caused by H37Ra and M. smegmatis. When the bacteria were heat-killed, they lost the ability to induce liquefaction. It was demonstrated that the rabbit skin model could tell differences in pathology of complemented H37Ra strains. The rabbit skin model may be a useful model for studying the mechanism of tuberculosis liquefaction, rapidly assessing mycobacterial virulence, and testing new vaccines and inhibitors.
Materials and Methods
Mycobacterial strains.
BCG vaccine Shanghai strain (D2-PB302, a derivative of Copenhagen strain) was obtained from Lanzhou Institute of Biological Products. M. tuberculosis avirulent H37Ra strain was obtained from American Type Culture Collection (ATCC25177), and the fast growing M. smegmatis strain was provided by Dr. Honghai Wang from Fudan University. BCG, H37Ra, H37Ra complemented strains (details of these strains are shown in Table 1) and M. smegmatis were grown in Middlebrook 7H9 liquid medium with ADC (albumin-dextrose-catalase) supplement to log phase. Then the bacteria were harvested and washed by the centrifugal washing method, and at last suspended with 10% glycerol for later use. The colony forming units (CFU) were quantitated on 7H11 agar plates supplemented with ADC and antibiotics, then adjusted to desired concentration for immunization before use. To compare the different pathogenesis of live and killed bacteria, the bacteria were inactivated by heating at 100°C for 10 min to prepare heat-killed mycobacteria.
Complementation of H37Ra.
M. tuberculosis H37Ra and H37Rv were grown in Middlebrook 7H9 broth enriched with albumin-Dextrose-catalase complex and 0.05% Tween 80 at 37°C. The genes for complementation were amplified by PCR from M. tuberculosis H37Rv genomic DNA using the primer pairs as described in Table 1 and cloned into the XbaI site of the hygromycin mycobacteria-E. coli shuttle replicative plasmid vector pOLYG.10 Electrocompetent M. tuberculosis H37Ra cells were prepared from 400 ml of a 10-day-old Middlebrook 7H9 culture. M. tuberculosis H37Ra were harvested by centrifugation at 3,000 g for 20 min at room temperature, washed with sterile distilled H2O at room temperature and resuspended in 1–2 ml of 10% glycerol at room temperature. Mycobacteria (400 µl) were mixed with the respective recombinant plasmids and electroporated using a Bio-Rad gene pulser. After electroporation, M. tuberculosis H37Ra were resuspended in 7H9 medium and incubated overnight at 37°C without shaking. Transformants were selected on Middlebrook 7H11 medium (Difco) supplemented with albumin-dextrose-catalase (Difco) and 50 µg/ml hygromycin. Hygromycin-resistant colonies appearing after 4 weeks were analyzed for the presence of the vector DNA by PCR. Recombinant H37Ra strains were then used for evaluation of changes in virulence in the rabbit skin model as described below.
Animals.
Specific pathogen-free New Zealand White rabbits (female; 2.0–3.0 kg) were purchased from Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences. All animals were housed in standard cage in Animal Center of Lanzhou University, fed commercial rabbit chow and water ad libitum, and then inoculated with live or heat-killed mycobacteria by intradermal route followed by evaluation of skin lesions. Our animal experiments got the approval of Lanzhou University Animal Care and Use Committee.
Infection and observation of rabbits.
Three rabbits were used in each group and each rabbit was injected on both flanks in the experiment of live and heat-killed BCG, H37Ra, and M. smegmatis. Each site was injected intradermally with about 5 × 106 CFU of both live and heat-killed mycobacteria (BCG, H37Ra, M. smegmatis) in 100 µl respectively (previous experiments were done to find this optimum dose). In the experiment of the H37Ra complemented strains, two rabbits were used in each group, and each rabbit was injected intradermally with 5 × 107 CFU, 5 × 106 CFU and 5 × 105 CFU in multiple sites respectively on both flanks with three centimeters interval between each sites. Six weeks later, two centimeters close to the primary injecting site, a second same dose of same bacteria was injected intradermally. The animals were observed, and the resulting lesions including the width and length and thickness were measured (in cubic millimeters) by calipers daily, which provided the volume of the products in mm3.
Removal of skin lesions and acid-fast stain for mycobacteria.
The representative rabbit skin lesions inoculated with each of the three viable bacteria (BCG, H37Ra, M. smegmatis) group were surgically removed for further histological analysis. The liquefied material was subjected to acid fast staining by the Ziehl-Neelsen method to confirm the presence of mycobacteria.
Statistical analysis.
All values are expressed as mean ± SD. SPSS16.0 software was used for statistical analyses. Data in Figure 2 were compared using analysis of variance (ANOVA) and the independent samples t test was used in Table 3. When the values indicated the presence of a significant difference, the LSD test was used. Values of p < 0.05 were considered significant.
Acknowledgements
We are grateful to Dr. Arthur Dannenberg for helpful discussions. This work was funded by the National Major S&T Projects of China (2008zx1000301104). Y.Z. was supported by NIH grant AI44063 and Changjiang Scholars Program.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/11748
References
- 1.Dye C. Global epidemiology of tuberculosis. Lancet. 2006;367:938–940. doi: 10.1016/S0140-6736(06)68384-0. [DOI] [PubMed] [Google Scholar]
- 2.Dannenberg AM., Jr Pathogenesis of pulmonary Mycobacterium bovis infection: basic principles established by the rabbit model. Tuberculosis. 2001;81:87–96. doi: 10.1054/tube.2000.0260. [DOI] [PubMed] [Google Scholar]
- 3.Helke KL, Mankowski JL, Manabe YC. Animal models of cavitation in pulmonary tuberculosis. Tuberculosis. 2006;86:337–348. doi: 10.1016/j.tube.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 4.Converse PJ, Dannenberg AM, Jr, Estep JE, Sugisaki K, Abe Y, Schofield BH, et al. Cavitary tuberculosis produced in rabbits by aerosolized virulent tubercle bacilli. Infect Immun. 1996;64:4776–4787. doi: 10.1128/iai.64.11.4776-4787.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wells W, Lurie MB. Experimental airborne disease: quantitative natureal respiratory contagion of tuberculosis. Am J Hyg. 1941;34:21. [Google Scholar]
- 6.Ratcliffe HL, Wells WF. Tuberculosis of rabbits induced by droplet nuclei infection; initial response to infection. J Exp Med. 1948;87:575–584. doi: 10.1084/jem.87.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang MZ, Shi DZ, Yang AJ, Jiao ZG, Zhu BD, Zhang Y. An examination of a rabbit model of liquefaction induced by mycobactera (in Chinese) J Microbes Infect. 2008;3:208–211. [Google Scholar]
- 8.Dannenberg AM., Jr Liquefaction and cavity formation in pulmonary TB: a simple method in rabbit skin to test inhibitors. Tuberculosis. 2009;89:243–247. doi: 10.1016/j.tube.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Zheng H, Lu L, Wang B, Pu S, Zhang X, Zhu G, et al. Genetic basis of virulence attenuation revealed by comparative genomic analysis of Mycobacterium tuberculosis strain H37Ra versus H37Rv. PLoS One. 2008;3:2375. doi: 10.1371/journal.pone.0002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garbe TR, Barathi J, Barnini S, Zhang Y, Abou-Zeid C, Tang D, et al. Transformation of mycobacterial species using hygromycin resistance as selectable marker. Microbiology. 1994;140:133–138. doi: 10.1099/13500872-140-1-133. [DOI] [PubMed] [Google Scholar]
- 11.Dannenberg AM, Jr, Collins FM. Progressive pulmonary tuberculosis is not due to increasing numbers of viable bacilli in rabbits, mice and guinea pigs, but is due to a continuous host response to mycobacterial products. Tuberculosis. 2001;81:229–242. doi: 10.1054/tube.2001.0287. [DOI] [PubMed] [Google Scholar]
- 12.Brindle RJ, Nunn PP, Githui W, Allen BW, Gathua S, Waiyaki P. Quantitative bacillary response to treatment in HIV-associated pulmonary tuberculosis. Am Rev Respir Dis. 1993;147:958–961. doi: 10.1164/ajrccm/147.4.958. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy JP. The pathogenesis and pathology of bovine tuberculosis with insights from studies of tuberculosis in humans and laboratory animal models. Vet Microbiol. 2006;112:151–161. doi: 10.1016/j.vetmic.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Martin C, Walker B. Attenuated strains of Mycobacterium tuberculosis complex for laboratory and clinical use. Tuberculosis. 2008;88:371–374. doi: 10.1016/j.tube.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006;24:3408–3419. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Sugisaki K, Dannenberg AM, Jr, Abe Y, Tsuruta J, Su WJ, Said W, et al. Nonspecific and immune-specific upregulation of cytokines in rabbit dermal tuberculous (BCG) lesions. J Leukoc Biol. 1998;63:440–450. doi: 10.1002/jlb.63.4.440. [DOI] [PubMed] [Google Scholar]
- 17.Cho S, Mehra V, Thoma-Uszynski S, Stenger S, Serbina N, Mazzaccaro RJ, et al. Antimicrobial activity of MHC class I-restricted CD8+ T cells in human tuberculosis. Proc Natl Acad Sci USA. 2000;97:12210–12215. doi: 10.1073/pnas.210391497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins DM, Kawakami RP, Buddle BM, Wards BJ, de Lisle GW. Different susceptibility of two animal species infected with isogenic mutants of Mycobacterium bovis identifies phoT as having roles in tuberculosis virulence and phosphate transport. Microbiology. 2003;149:3203–3212. doi: 10.1099/mic.0.26469-0. [DOI] [PubMed] [Google Scholar]
- 19.Akyol I. Regulation of the ribonucleotide reductases in Lactococcus lactis subsp. cremoris. Acta Biol Hung. 2007;58:105–114. doi: 10.1556/ABiol.58.2007.1.10. [DOI] [PubMed] [Google Scholar]
- 20.Zheng X, Papavinasasundaram KG, Av-Gay Y. Novel substrates of Mycobacterium tuberculosis PknH Ser/Thr kinase. Biochem Biophys Res Commun. 2007;355:162–168. doi: 10.1016/j.bbrc.2007.01.122. [DOI] [PubMed] [Google Scholar]
- 21.Perez E, Samper S, Bordas Y, Guilhot C, Gicquel B, Martin C. An essential role for phoP in Mycobacterium tuberculosis virulence. Mol Microbiol. 2001;41:179–187. doi: 10.1046/j.1365-2958.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 22.Chesne-Seck ML, Barilone N, Boudou F, Gonzalo Asensio J, Kolattukudy PE, Martin C, et al. A point mutation in the two-component regulator PhoP-PhoR accounts for the absence of polyketide-derived acyltrehaloses but not that of phthiocerol dimycocerosates in Mycobacterium tuberculosis H37Ra. J Bacteriol. 2008;190:1329–1334. doi: 10.1128/JB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee JS, Krause R, Schreiber J, Mollenkopf HJ, Kowall J, Stein R, et al. Mutation in the transcriptional regulator PhoP contributes to avirulence of Mycobacterium tuberculosis H37Ra strain. Cell Host Microbe. 2008;3:97–103. doi: 10.1016/j.chom.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Frigui W, Bottai D, Majlessi L, Monot M, Josselin E, Brodin P, et al. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 2008;4:33. doi: 10.1371/journal.ppat.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lurie MB. Resistance to tuberculosis: Experimental studies in native and acquired defense mechanisms. Cambridge, Mass: Harvard University; 1964. [Google Scholar]
- 26.Basaraba RJ. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis. 2008;88:35–47. doi: 10.1016/S1472-9792(08)70035-0. [DOI] [PubMed] [Google Scholar]
- 27.Tsenova L, Harbacheuski R, Moreira L, Ellison E. Evaluation of the M.tb 72F polyprotein vaccine in a rabbit model of tuberculous meningitis. Infect Immun. 2006;74:2392–2401. doi: 10.1128/IAI.74.4.2392-2401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]