Abstract
The Us3 serine threonine kinases perform multiple roles in alphaherpesvirus infection and can localize to distinct subcellular compartments. Transient expression of Us3 in cells results in two dramatic alterations of the actin cytoskeleton: production of actin-based filamentous processes (FPs); and breakdown of actin stress fibres giving rise to rounded cell morphology. In our recent study on FPs induced by HSV-2 Us3, we noted that FP formation was diminished when HSV-2 Us3 was trapped within the nucleus following treatment of transfected cells with leptomycin B (LMB). This observation suggested that subcellular localization of Us3 could be a determinant of Us3-induced FP formation. Here, we review what is known regarding the effect of subcellular localization of Us3 on FP production and on actin stress fibre breakdown and discuss the potential significance of studies aimed at defining the requirements for subcellular localization of Us3.
Key words: alphaherpesvirus, Us3, serine/threonine kinase, cell-to-cell spread, filamentous processes, subcellular localization
The alphaherpesviruses are a neurotropic subfamily of the Herpesviridae and most of these viruses establish lifelong latent infections in the peripheral nervous systems of their hosts. The alphaherpesviruses include the human pathogens herpes simplex virus types 1 and 2 (HSV) and varicella zoster virus, as well as veterinary pathogens such as the swine pathogen, pseudorabies virus (PRV) and the chicken pathogen, Marek's disease virus.1 All members of the alphaherpesvirus subfamily encode a serine/threonine kinase called Us3 that is not found in the other subfamilies.2 While Us3 is not essential for virus multiplication,3 viruses deleted for Us3 are impaired for growth in cultured cells and show attenuated virulence in a variety of animal model systems.4–18 Many biological functions have been directly ascribed to Us3 including prevention of virus-induced apoptosis,11,19–23 virion maturation16,24–27 and, most pertinent to this communication, cell-to-cell spread of virus infection.7,28 Cell-to-cell spread encompasses mechanisms of intercellular virus transmission that do not involve infection by free virions. These mechanisms include induction of cell-cell fusion resulting in the formation of syncytia, utilization of pre-existing cell-cell connections such as neural synapses and production of unique protrusions from the cell that facilitate virus transmission.29 From a viral pathogenesis standpoint, these mechanisms provide viruses with a means to spread in the presence of a primed immune system, as is the case when alphaherpesviruses reactivate from latency.
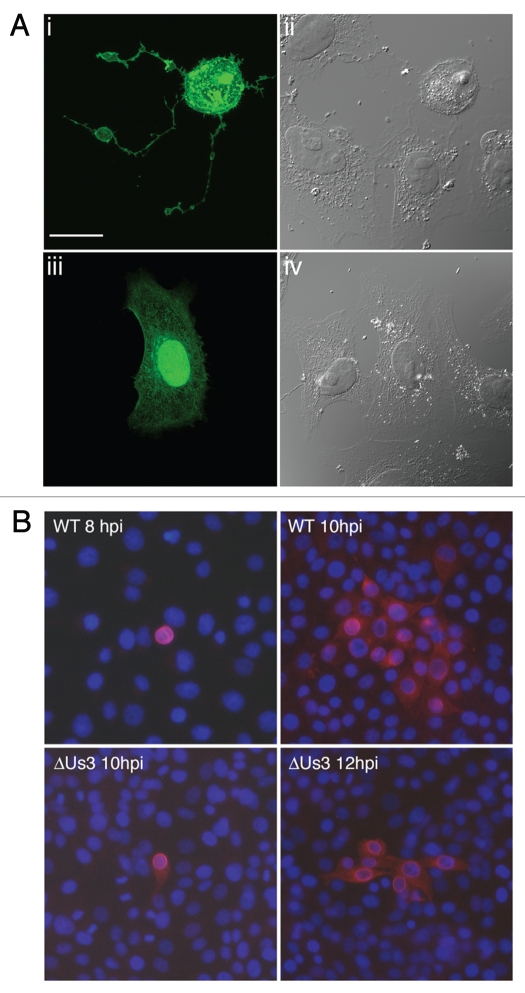
Us3 was first implicated as a determinant of non-syncytial cell-to-cell spread of alphaherpesvirus infection by Demmin and colleagues in studies using PRV.7 These studies were followed by the observation that transient expression of PRV Us3 in cultured cells resulted in the formation of long filamentous processes (FPs) that contained both actin and microtubules.28,30 An example of PRV Us3-induced FP formation is shown in Figure 1A. These tubular structures can extend great distances and are often 100 µm or more in length. We recently reported that transient expression of HSV-2 Us3 in cultured cells also resulted in the induction of similar structures.31 FP formation is frequently observed during alphaherpesvirus infection of cultured cells and, in the case of PRV and HSV-2, has been demonstrated to be dependent upon Us3 kinase activity,31,32 suggesting that FP formation is relevant in virus infection. This notion was solidified by the studies of Favoreel and colleagues, who convincingly demonstrated that PRV virions could travel within these structures from infected cells to neighbouring uninfected cells thereby enhancing virus spread.28 Not surprisingly, reduction in plaque size is a property associated with Us3 null viruses.7 An example of the notable reduction in cell-to-cell spread of PRV in the absence of Us3 is shown in Figure 1B. Very recently, Van den Broeke and co-workers demonstrated that PRV Us3 interacts with and phosphorylates the p21-activated kinases, Pak1 and Pak2, and that Pak1 is specifically required for PRV Us3 induced FP formation.33
Figure 1.
PRV Us3 induces filamentous process (FP) formation and influences cell-to-cell spread of PRV. (A) FPs induced by PRV Us3. Vero cells were transfected with plasmid encoding a PRV Us3-GFP fusion protein (i and ii) or GFP (iii and iv) and fixed at 24 hours post transfection. Fixed specimens were examined by confocal microscopy. Note the long FPs extending from the Us3-transfected cell and also that the Us3-transfected cell has a rounded morphology indicative of actin stress fibre breakdown. Scale bar is 20 µm. (B) Spread of PRV in the absence of Us3. PK15 cell monolayers were infected with wild type (WT) or Us3 null (ΔUs3) PRV at a multiplicity of infection of 0.001. At various times post infection, monolayers were fixed and stained for the early viral protein UL34 to identify infected cells (red) and with Hoechst 33342 to identify nuclei (blue). Note that in the case of infection with ΔUs3 PRV, infection is restricted to cells in immediate proximity to each other.
While the relationship between Us3-induced FPs and cell-to-cell spread of virus in cultured cells is intriguing, it has yet to be established if these structures facilitate to cell-to-cell spread of virus in the infected host. As stated above, Us3 plays a number of roles during virus infection, however which of these roles are relevant in the context of host infection is not known. Furthermore, the comparative studies on HSV-1 and HSV-2 Us3s recently described by Kamakura et al. and Morimoto et al. illustrate that these roles can vary even between Us3s from closely related alphaherpeviruses.4,34 Genetic dissection of these different roles will go a long way towards understanding the specific contributions of Us3 to virulence. While the concept of isolating Us3 mutants defective in one activity but not another is daunting, restricting Us3 localization to a particular subcellular compartment, thereby limiting its access to distinct substrates, is perhaps a more practical idea. The detailed studies of Calton et al. demonstrated that PRV Us3 contains signals allowing it to localize to the nucleus, to mitochondria and to membranes.30 This information provides a good starting point for the development of Us3 variants with restricted localization.
As part of our recent studies on the requirement for HSV-2 Us3 catalytic activity in the induction of FPs, we noted that certain catalytically inactive versions of HSV-2 Us3 (K220M and K220A) appeared to be excluded from the nucleus of transfected cells.31 A K220M HSV-2 Us3 mutant was used in the aforementioned studies of Morimoto et al.4 which raises the possibility that nuclear exclusion of Us3 contributes to the phenotypes described for this viral mutant. The aberrant subcellular localization of our K220M mutant prompted us to investigate the requirements for HSV-2 Us3 nuclear localization and led to the discovery that leptomycin B (LMB), a specific inhibitor of nuclear export mediated by CRM1,35,36 could be used to trap HSV-2 Us3 within the nucleus. This provided the opportunity to investigate the impact of subcellular localization on a function of Us3. We found that HSV-2 Us3-induced FP formation was diminished in the presence of LMB. One interpretation of this result is that Us3 must localize to the cytoplasm in order to induce FP formation. However, given the wide range of cellular molecules that engage the CRM1 machinery,35 it is plausible that LMB inhibits FP formation in a manner that is independent of its effects on Us3 localization. We are currently investigating the requirements for nuclear export of HSV-2 Us3 to enable us to introduce specific mutations that will allow us to test the hypothesis that cytoplasmic Us3 is primarily responsible for FP formation without the use of pharmacological inhibitors.
Another example of the impact of Us3 subcellular localization on its biological function came from studies on the role of PRV Us3 in actin stress fibre disruption. A second profound cytoskeletal alteration that arises upon transient expression of Us3 orthologues is the disruption of actin stress fibres, which results in a rounded cell morphology.25,31,37–39 A correlation between nuclear localization of PRV Us3 and disruption of actin stress fibres has been reported38 and it was shown recently that Pak2 kinase is required for this activity.33 The subcellular compartment where Us3 and Pak2 interact remains to be determined, however, the above findings may suggest that this occurs in the nucleus. It is noteworthy that Pak2 has been shown to contain both nuclear localization and nuclear export signals.40 In keeping with a specific requirement for nuclear Us3 in actin stress fibre breakdown, we are able to detect actin stress fibre breakdown in cells where the majority of HSV-2 Us3 is trapped within the nucleus following LMB treatment (Finnen R, unpublished observations). It will be interesting to determine if, conversely, actin stress fibres remain intact under conditions where Us3 is confined to the cytoplasm.
The discovery and confirmation of additional Us3 substrates, active areas of research by our laboratory and many others, should continue in concert with efforts to define the requirements for subcellular localization of Us3. A thorough understanding of these requirements, be they resident signals within Us3 itself or provided in trans by cellular binding partners, will allow specific manipulation of subcellular localization as a means of studying Us3 function in distinct cellular compartments. This in turn may uncover novel intervention strategies that prevent cell-to-cell spread of virus infection, or other functions of Us3 that contribute to alphaherpesvirus virulence.
Acknowledgements
This work was supported in part by NIH grant AI48626, CFI award 16389 and CIHR operating grant 93804 to B.W.B. We apologize to Us3 researchers whose valuable contributions we were unable to cite owing to space constraints.
Footnotes
Previously published online: www.landesbioscience.com/journals/virulence/article/11980
References
- 1.Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, et al. The order Herpesvirales. Arch Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeoch DJ, Davison AJ. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986;14:1765–1777. doi: 10.1093/nar/14.4.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purves FC, Longnecker RM, Leader DP, Roizman B. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J Virol. 1987;61:2896–2901. doi: 10.1128/jvi.61.9.2896-2901.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morimoto T, Arii J, Tanaka M, Sata T, Akashi H, Yamada M, et al. Differences in the regulatory and functional effects of the Us3 protein kinase activities of herpes simplex virus 1 and 2. J Virol. 2009;83:11624–11634. doi: 10.1128/JVI.00993-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asano S, Honda T, Goshima F, Nishiyama Y, Sugiura Y. US3 protein kinase of herpes simplex virus protects primary afferent neurons from virus-induced apoptosis in ICR mice. Neurosci Lett. 2000;294:105–108. doi: 10.1016/s0304-3940(00)01554-8. [DOI] [PubMed] [Google Scholar]
- 6.Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, et al. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J Gen Virol. 1999;80:51–56. doi: 10.1099/0022-1317-80-1-51. [DOI] [PubMed] [Google Scholar]
- 7.Demmin GL, Clase AC, Randall JA, Enquist LW, Banfield BW. Insertions in the gG gene of pseudorabies virus reduce expression of the upstream Us3 protein and inhibit cell-to-cell spread of virus infection. J Virol. 2001;75:10856–10869. doi: 10.1128/JVI.75.22.10856-10869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimman TG, De Wind N, De Bruin T, de Visser Y, Voermans J. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology. 1994;205:511–518. doi: 10.1006/viro.1994.1672. [DOI] [PubMed] [Google Scholar]
- 9.Klopfleisch R, Klupp BG, Fuchs W, Kopp M, Teifke JP, Mettenleiter TC. Influence of pseudorabies virus proteins on neuroinvasion and neurovirulence in mice. J Virol. 2006;80:5571–5576. doi: 10.1128/JVI.02589-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurachi R, Daikoku T, Tsurumi T, Maeno K, Nishiyama Y, Kurata T. The pathogenicity of a US3 protein kinase-deficient mutant of herpes simplex virus type 2 in mice. Arch Virol. 1993;133:259–273. doi: 10.1007/BF01313767. [DOI] [PubMed] [Google Scholar]
- 11.Leopardi R, Van Sant C, Roizman B. The herpes simplex virus 1 protein kinase US3 is required for protection from apoptosis induced by the virus. Proc Natl Acad Sci USA. 1997;94:7891–7896. doi: 10.1073/pnas.94.15.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffat JF, Zerboni L, Sommer MH, Heineman TC, Cohen JI, Kaneshima H, et al. The ORF47 and ORF66 putative protein kinases of varicella-zoster virus determine tropism for human T cells and skin in the SCID-hu mouse. Proc Natl Acad Sci USA. 1998;95:11969–11974. doi: 10.1073/pnas.95.20.11969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishiyama Y, Yamada Y, Kurachi R, Daikoku T. Construction of a US3 lacZ insertion mutant of herpes simplex virus type 2 and characterization of its phenotype in vitro and in vivo. Virology. 1992;190:256–268. doi: 10.1016/0042-6822(92)91212-d. [DOI] [PubMed] [Google Scholar]
- 14.Olsen LM, Ch'ng TH, Card JP, Enquist LW. Role of pseudorabies virus Us3 protein kinase during neuronal infection. J Virol. 2006;80:6387–6398. doi: 10.1128/JVI.00352-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashima Y, Tamura H, Xuan X, Otsuka H. Identification of the US3 gene product of BHV-1 as a protein kinase and characterization of BHV-1 mutants of the US3 gene. Virus Res. 1999;59:23–34. doi: 10.1016/s0168-1702(98)00119-1. [DOI] [PubMed] [Google Scholar]
- 16.Wagenaar F, Pol JM, Peeters B, Gielkens AL, de Wind N, Kimman TG. The US3-encoded protein kinase from pseudorabies virus affects egress of virions from the nucleus. J Gen Virol. 1995;76:1851–1859. doi: 10.1099/0022-1317-76-7-1851. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto M, Kurachi R, Morishima T, Kito J, Nishiyama Y. Immunohistochemical studies on the transneuronal spread of virulent herpes simplex virus type 2 and its US3 protein kinase-deficient mutant after ocular inoculation. Microbiol Immunol. 1996;40:289–294. doi: 10.1111/j.1348-0421.1996.tb03348.x. [DOI] [PubMed] [Google Scholar]
- 18.Inagaki-Ohara K, Iwasaki T, Watanabe D, Kurata T, Nishiyama Y. Effect of the deletion of US2 and US3 from herpes simplex virus type 2 on immune responses in the murine vagina following intravaginal infection. Vaccine. 2001;20:98–104. doi: 10.1016/s0264-410x(01)00311-5. [DOI] [PubMed] [Google Scholar]
- 19.Geenen K, Favoreel HW, Olsen L, Enquist LW, Nauwynck HJ. The pseudorabies virus US3 protein kinase possesses anti-apoptotic activity that protects cells from apoptosis during infection and after treatment with sorbitol or staurosporine. Virology. 2005;331:144–150. doi: 10.1016/j.virol.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Hata S, Koyama AH, Shiota H, Adachi A, Goshima F, Nishiyama Y. Antiapoptotic activity of herpes simplex virus type 2: the role of US3 protein kinase gene. Microbes Infect. 1999;1:601–607. doi: 10.1016/s1286-4579(99)80059-8. [DOI] [PubMed] [Google Scholar]
- 21.Ogg PD, McDonell PJ, Ryckman BJ, Knudson CM, Roller RJ. The HSV-1 Us3 protein kinase is sufficient to block apoptosis induced by overexpression of a variety of Bcl-2 family members. Virology. 2004;319:212–224. doi: 10.1016/j.virol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Benetti L, Roizman B. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc Natl Acad Sci USA. 2004;101:9411–9416. doi: 10.1073/pnas.0403160101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munger J, Roizman B. The US3 protein kinase of herpes simplex virus 1 mediates the posttranslational modification of BAD and prevents BAD-induced programmed cell death in the absence of other viral proteins. Proc Natl Acad Sci USA. 2001;98:10410–10415. doi: 10.1073/pnas.181344498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds AE, Wills EG, Roller RJ, Ryckman BJ, Baines JD. Ultrastructural localization of the herpes simplex virus type 1 UL31, UL34 and US3 proteins suggests specific roles in primary envelopment and egress of nucleocapsids. J Virol. 2002;76:8939–8952. doi: 10.1128/JVI.76.17.8939-8952.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schumacher D, Tischer BK, Trapp S, Osterrieder N. The protein encoded by the US3 orthologue of Marek's disease virus is required for efficient de-envelopment of perinuclear virions and involved in actin stress fiber breakdown. J Virol. 2005;79:3987–3997. doi: 10.1128/JVI.79.7.3987-3997.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wisner TW, Wright CC, Kato A, Kawaguchi Y, Mou F, Baines JD, et al. Herpesvirus gB-induced fusion between the virion envelope and outer nuclear membrane during virus egress is regulated by the viral US3 kinase. J Virol. 2009;83:3115–3126. doi: 10.1128/JVI.01462-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mou F, Forest T, Baines JD. US3 of herpes simplex virus type 1 encodes a promiscuous protein kinase that phosphorylates and alters localization of lamin A/C in infected cells. J Virol. 2007;81:6459–6470. doi: 10.1128/JVI.00380-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favoreel HW, Van Minnebruggen G, Adriaensen D, Nauwynck HJ. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc Natl Acad Sci USA. 2005;102:8990–8995. doi: 10.1073/pnas.0409099102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sattentau Q. Avoiding the void: cell-to-cell spread of human viruses. Nat Rev Microbiol. 2008;6:815–826. doi: 10.1038/nrmicro1972. [DOI] [PubMed] [Google Scholar]
- 30.Calton CM, Randall JA, Adkins MW, Banfield BW. The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes. 2004;29:131–145. doi: 10.1023/B:VIRU.0000032796.27878.7f. [DOI] [PubMed] [Google Scholar]
- 31.Finnen RL, Roy BB, Zhang H, Banfield BW. Analysis of filamentous process induction and nuclear localization properties of the HSV-2 serine/threonine kinase Us3. Virology. 2010;397:23–33. doi: 10.1016/j.virol.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van den Broeke C, Deruelle M, Nauwynck HJ, Coller KE, Smith GA, Van Doorsselaere J, et al. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology. 2009;385:155–160. doi: 10.1016/j.virol.2008.11.050. [DOI] [PubMed] [Google Scholar]
- 33.Van den Broeke C, Radu M, Deruelle M, Nauwynck H, Hofmann C, Jaffer ZM, et al. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc Natl Acad Sci USA. 2009;106:8707–8712. doi: 10.1073/pnas.0900436106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamakura M, Nawa A, Ushijima Y, Goshima F, Kawaguchi Y, Kikkawa F, et al. Microarray analysis of transcriptional responses to infection by herpes simplex virus types 1 and 2 and their US3-deficient mutants. Microbes Infect. 2008;10:405–413. doi: 10.1016/j.micinf.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 36.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 37.Van Minnebruggen G, Van de Walle GR, Favoreel HW, Nauwynck HJ, Pensaert MB. Temporary disturbance of actin stress fibers in swine kidney cells during pseudorabies virus infection. Vet Microbiol. 2002;86:89–94. doi: 10.1016/s0378-1135(01)00493-x. [DOI] [PubMed] [Google Scholar]
- 38.Van Minnebruggen G, Favoreel HW, Jacobs L, Nauwynck HJ. Pseudorabies virus US3 protein kinase mediates actin stress fiber breakdown. J Virol. 2003;77:9074–9080. doi: 10.1128/JVI.77.16.9074-9080.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murata T, Goshima F, Daikoku T, Takakuwa H, Nishiyama Y. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells. 2000;5:1017–1027. doi: 10.1046/j.1365-2443.2000.00383.x. [DOI] [PubMed] [Google Scholar]
- 40.Jakobi R, McCarthy CC, Koeppel MA, Stringer DK. Caspase-activated PAK-2 is regulated by subcellular targeting and proteasomal degradation. J Biol Chem. 2003;278:38675–38685. doi: 10.1074/jbc.M306494200. [DOI] [PubMed] [Google Scholar]