Abstract
Objectives
To examine the relationship between inflammation and coronary microvascular function in asymptomatic individuals using positron emission tomography (PET) and assessment of coronary flow reserve (CFR).
Background
Coronary microvascular dysfunction is an early precursor of coronary artery disease (CAD) thought to result from endothelial cell activation and inflammation, but data are limited.
Methods
We examined 268 asymptomatic male monozygotic and dizygotic twins. Plasma biomarkers of inflammation and endothelial cell activation included C-reactive protein (CRP), interleukin-6 (IL-6), white blood cell count (WBC), vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). Blood flow quantitation was obtained with [13N] ammonia PET at rest and after adenosine stress. CFR was measured as the ratio of maximum flow to baseline flow at rest; abnormal CFR was defined as a ratio <2.5. A summed stress score for visible perfusion defects was calculated.
Results
In within-pair analyses, all biomarkers, except VCAM-1, were higher in twins with lower CFR than their brothers with higher CFR (p<0.05). This was observed in the entire sample, as well as within pairs discordant for a CFR of <2.5. Associations persisted after adjusting for summed stress score and CAD risk factors. In contrast no biomarker, except IL-6, was related to the summed stress score of visible defects.
Conclusions
Even in asymptomatic subjects, a decrease in coronary microvascular function is accompanied by a systemic inflammatory response, independent of CAD risk factors. Our results, using a controlled twin design, highlight the importance of coronary microvascular function in the early phases of CAD.
Keywords: circulation, imaging, inflammation, coronary disease, endothelium
Coronary microvascular dysfunction refers to abnormal regulation of the coronary microcirculation resulting in reduced myocardial blood flow with increased demand that is not explained by disease in the epicardial coronary arteries.(1) It is thought to result from vasomotor dysregulation or endothelial dysfunction of the small coronary arterioles and represents one of the earliest signs of coronary atherosclerosis.(1,2) Although no technique enables the direct visualization of the coronary microcirculation in humans in vivo, in the absence of coronary artery disease (CAD), coronary microvascular disease manifests as impaired coronary flow reserve (CFR), an index of coronary vasodilator capacity.(2,3)
Among individuals without evidence of CAD and myocardial disease, coronary microvascular dysfunction is typically attributed to traditional coronary risk factors (smoking, hypertension, hyperlipidemia, and diabetes and insulin-resistant states), but the underlying pathophysiology is unclear.(1,4) Functional abnormalities of the microcirculation have been proposed, including endothelial and/or vascular smooth muscle cell dysfunction, as well as extravascular influences, such as autonomic dysfunction.(4) Vascular inflammation and endothelial cell activation have been suggested as plausible pathophysiological mechanisms, since they have also been linked to vascular function in the periphery.(5,6) For the coronary circulation, however, data in humans are limited and inconsistent.(7,8) Part of the difficulties are due to the selected patient samples that have been evaluated using invasive angiographic methods and Doppler technology. Whether a relationship between inflammation and CFR exists in asymptomatic individuals is fundamentally unexplored.
The purpose of our study was to examine the relationship between inflammation and CFR in a controlled study of asymptomatic middle-aged male twins. CFR, measured by positron emission tomography (PET), is currently the most established noninvasive method of assessing microvascular function, and can be used in asymptomatic subjects.(2,3) Because inflammation is a key mechanism through which several risk factors increase CAD risk,(9) and a reduced CFR has been linked to CAD risk factors,(10–15) and with adverse long term prognosis,(16,17) we were interested in examining whether the relationship between inflammation and CFR was independent of CAD risk factors. Twins provide unique opportunities to study the association between inflammation and CFR while controlling for shared environmental or genetic factors, because twin siblings share genes (50%, on average, if dizygotic, and all if monozygotic), maternal factors, and early familial environment.
Methods
Subjects
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical cardiovascular disease using twins.(18,19) Twins were selected from the Vietnam Era Twin (VET) Registry,(20) which includes 7,369 middle-aged male-male twin pairs both of whom served in the United States military during the time of the Vietnam War. THS included 180 twin pairs, 93 monozygotic and 87 dizygotic, who were born between 1946 and 1956. Zygosity information was determined by DNA typing. Two groups of twin pairs were randomly sampled from the VET Registry: one group included twins discordant for a lifetime history of major depression, and in a second group, neither twin had a history of depression. All twins were examined at the Emory University General Clinical Research Center between March 2002 and March 2006; their medical history was updated at the examination. The protocol was approved by the Institutional Review Board at Emory University and informed consent was obtained from all subjects.
Positron Emission Tomography (PET) Protocol
Subjects underwent imaging of myocardial blood flow (MBF) with PET nitrogen 13 [13N] ammonia at rest and following pharmacologic (adenosine) stress during a single imaging session. All twins were admitted to the GCRC in the late morning or early afternoon on the day prior to the PET scan, and stayed overnight in the GCRC facility. They all received a similar low-fat dinner, and then remained fasting until the PET scan was completed the following morning. They were instructed to abstain from smoking and drinking alcoholic or caffeinated beverages and from eating any foods other than what was served to them. All medications were withheld the morning of the PET scan. The imaging protocol was performed by personnel unaware of the twins' medical history or other subject characteristics.
PET scanning was performed in 2-dimensional mode using a CTI ECAT Exact 47 (921) camera (5-mm resolution) (Siemens, Knoxville, TN). Initially, a 2 to 3 mCi dose of [13N] ammonia was injected, and a 4-minute static scan was collected and reconstructed without any corrections to verify subject position. Then, rest and pharmacologic stress (adenosine) ammonia imaging was performed. The rest and stress imaging protocols were identical except that a 4-minute infusion of adenosine (0.14 mg/kg/min) was started 2 minutes prior to the ammonia injection for the stress imaging session. 20 mCi of [13N]ammonia were injected and a 5-minute, 31-frame dynamic acquisition was started (12 frames × 5-seconds, 3 frames × 20-seconds, and 1 frame × 300-seconds). Data were collected in 47 planes 3.375 mm thick covering a range of 16 cm. Immediately after the conclusion of the dynamic sequence, a 15-min gated acquisition was started. Finally, transmission data were collected for 5 minutes in the windowed mode using germanium 68 (68Ge) rods for segmented attenuation correction. The process was repeated, including a second transmission scan, for the stress study. Images were reconstructed with filtered back projection using a Hann filter cutoff at 1 cycle/cm. The electrocardiogram was monitored continuously and blood pressure and heart rate measurements were taken before, during (every minute), and after the adenosine infusion. The rate-pressure product was calculated as the mean systolic blood pressure during adenosine infusion multiplied by the mean heart rate during adenosine infusion divided by 100.
PET Flow Measures
To calculate CFR, measurements of MBF at rest and during adenosine hyperemia were obtained. The last frame of the dynamic sequence was used as a template for sectorial region-of-interest analysis. The input function was generated by drawing a region of interest in the left ventricular chamber on a midventricular slice, and flow was calculated (expressed in milliliters per minute per gram of tissue) using established methodology.(21,22) The left ventricle was sampled radially from 40 different angles, and 40 samples of flow were obtained for each short axis slice. The resulting hundreds of samples were grouped into 20 segments.
Our main outcome was the overall measure of CFR for the entire myocardium (across all 20 regions), defined as the ratio of maximum flow during stress to flow at rest. Secondarily, regional CFR was also calculated (i.e., left anterior descending, left circumflex, and right coronary artery territory). Absolute increase in hyperemic blood flow, calculated as the difference between maximum flow during stress and flow at rest, was also examined.
Myocardial Perfusion Defect Score
In addition to flow measures, we constructed a summation score describing the number and the severity of visible perfusion defects across the 20 segments of acquisition. In each segment, the defect severity was quantified on a 4-point scale and subsequently summed up across the 20 segments yielding a total score. Separate scores were obtained for the rest (summed rest score) and stress (summed stress score) scans. A reversible defect score (summed difference score) was obtained by subtracting the rest score from the stress score. These scores, and a dichotomous indicator of perfusion abnormalities (defined as a summed stress score ≥4(23)), represented secondary perfusion outcomes.
Assessment of Inflammatory Biomarkers
Blood was drawn in the morning prior to the PET scan after an overnight fast. Plasma samples were separated and stored at −80°C until analysis. We measured biomarkers of inflammation and endothelial cell activation, including C-reactive protein (CRP), interleukin-6 (IL-6), white blood cell count (WBC), and two soluble cell adhesion molecules, vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1). CRP was measured with the Beckman Coulter High Sensitivity C-Reactive Protein assay on the Synchron LX-20 analyzer; the inter- and intra-assay precision of this test is <5%. IL-6, VCAM-1 and ICAM-1 were assessed using commercially available ELISA kits from R and D Systems, Minneapolis, MN. Inter- and intra-assay variability for these assays is <10%. WBC count was measured with the Beckman Coulter LH 750 hematology analyzer (Brea, CA). All biochemical assays for each twin pair were processed in the same analytical run.
Other Measurements
A medical history and a physical exam were obtained from all twins. Concentrations of glucose and plasma lipids were measured from overnight fasting blood samples using standardized methods. Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity used in the Atherosclerosis Risk in Communities (ARIC) Study,(24) yielding a global score of physical activity at work, during sports and non-sports activities.
Statistical Analysis
CFR and inflammatory biomarkers were mainly analyzed as continuous variables; inflammatory biomarker levels had a skewed distribution and were log transformed for analysis. All P values were corrected for the correlation between co-twins using generalized estimating equations or mixed-effects models with a random intercept for each pair, depending on variable distribution.
The association between inflammatory markers and microvascular dysfunction was first examined in the whole sample of twins, treating twins as separate observations. In these analyses, all twins were examined regardless of whether their brother was available for analysis, and CFR was categorized according to quartiles. Next, we performed comparisons within twin pairs, where inflammatory marker levels where compared between brothers who differed in CFR. Within-pair differences in CFR were examined in three ways. First, we expressed the within-pair difference in CFR as a continuous variable quantifying the individual twin variation from the twin pair average; this formulation is analogous to fitting the absolute difference between the twins in a pair.(25) Second, we simply classified each twin as having either a higher or a lower CFR than his brother. Finally, we examined discordant twin pairs for a CFR <2.5 which has been suggested as a cut point for microvascular dysfunction, being the lower limit of normal flow reserve in arteries free of obstructive CAD.(26) The within-pair effects are inherently controlled for demographic, shared familial and early environmental influences; in addition, daily activities and other environmental factors during the examination day were controlled since twin pairs were examined together. Because twin pairs were raised together, and monozygotic pairs share 100% of their genetic material, any association between CFR and inflammatory markers within monozygotic pairs cannot be ascribed to genes or early family environment such as parental influences, socioeconomic status, and behaviors learned while growing up. Dizygotic pairs also share familial factors, but on average only share 50% of their genetic material. All analyses used mixed effects regression models and accounted for the twin pair clustering by using a random effect term for each pair. Because the dependent variables were log-transformed, and the association was inverse, we expressed the results as percent difference of the non-transformed values by using the following formula: [1−(expβ)] × 100 (%); β is the regression coefficient and expβ returns the exponential value of the parameter.
Because we were interested in microvascular dysfunction, we adjusted for presence of perfusion abnormalities. Analyses were further adjusted for factors that were significantly different according to CFR status, or that were a priori considered potentially important confounders, including body mass index, previous history of CAD, study year, and the Framingham risk score, which incorporates age, low-density-lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, blood pressure, diabetes mellitus, and smoking.(27) Sensitivity analyses were conducted by adjusting for habitual physical activity (Baecke score), cardiac medications (statins, aspirin, beta-blockers and angiotensin-converting enzyme [ACE]-inhibitors), rate-pressure product during the adenosine infusion, history of major depression (which was a criterion for inclusion in a subsample), and by adjusting for individual CAD risk factors rather than including them in the Framingham risk score. We also repeated the analyses after excluding a few individuals who developed CAD between 1990 and the examination date. All analyses were conducted using SAS software version 9.1 (SAS Institute). Significance level was set at 0.05, two-sided.
Results
Study Sample
Of the 360 twins in THS, 57 were excluded because of incomplete quantitative MBF data, and 35 were excluded because of a previous history of CAD, leaving 268 twins in the analysis: 122 complete pairs (74 monozygotic and 48 dizygotic) and 24 unpaired twins.
The mean CFR in the entire sample was 2.68, with a standard deviation of 0.83. Within the 122 pairs, when each twin was classified as having a higher or a lower CFR than his brother, both twins had similar levels of risk factors, medical history and medication use, with the possible exception of smoking (Table 1). The Framingham risk score was also similar within pairs. The mean CFR was 3.11 in the twins with higher CFR and 2.32 in their matched brothers with lower CFR.
Table 1.
Within-pair comparison of subject characteristics according to CFR status.
| Twin With Lower CFR N=122 | Twin With Higher CFR N=122 | P | |
|---|---|---|---|
| Age, y | 54.0 (53.5–54.6) | 54.0 (53.5–54.6) | __ |
| Systolic blood pressure, mm Hg | 130 (126–132) | 130 (127–132) | 0.74 |
| Diastolic blood pressure, mm Hg | 81.2 (79.2–83.2) | 80.6 (78.7–82.6) | 0.59 |
| LDL-cholesterol, mg/dL | 124 (118–130) | 127 (121–133) | 0.44 |
| HDL-cholesterol, mg/dL | 38.7 (37.1–40.3) | 39.6 (37.8–41.4) | 0.32 |
| Diabetes, % | 8.20 | 8.26 | 0.99 |
| Body mass index | 28.8 (28.0–29.6) | 29.2 (28.4–30.0) | 0.34 |
| Current smoker, % | 22.9 | 15.6 | 0.10 |
| Framingham Risk Score | 5.76 (5.39–6.14) | 5.70 (5.31–6.09) | 0.76 |
| Physical activity (Baecke) score | 7.36 (7.02–7.70) | 7.45 (7.15–7.74) | 0.67 |
| Number of alcoholic beverages in typical week | 4.79 (3.27–6.32) | 5.49 (3.51–7.48) | 0.58 |
| Taking ACE-inhibitors, % | 10.7 | 9.84 | 0.83 |
| Taking statins, % | 22.1 | 17.2 | 0.24 |
| Taking beta-blocker medications, % | 4.92 | 4.10 | 0.71 |
| Taking aspirin, % | 16.4 | 19.7 | 0.51 |
| Taking antidepressants, % | 16.4 | 12.3 | 0.35 |
| CFR | 2.32 (2.21–2.42) | 3.11 (2.95–3.26) | <0.001 |
All data are reported either as means (95% confidence interval) or percentages of subjects. P values are obtained from mixed models for continuous variables or generalized estimating equations (GEE) for categorical variables. P values for inflammatory biomarkers are based on log-transformed data.
HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); ACE, angiotensin-converting enzyme; CFR, coronary flow reserve.
Imaging and Hemodynamics
Less than 30% of the sample had a summed stress score ≥4, which would indicate a significant perfusion abnormality; this proportion was the same comparing twins with higher and lower CFR within pair (Table 2). The overall perfusion defect score, either during adenosine stress or at rest, was also similar, as were hemodynamic responses during adenosine infusion, including maximum systolic blood pressure, maximum heart rate, average and maximum rate-pressure product (Table 2).
Table 2.
Within-pair comparison of myocardial perfusion imaging data according to CFR status.*
| Twin With Lower CFR N=122 | Twin With Higher CFR N=122 | p | |
|---|---|---|---|
| Abnormal perfusion (summed stress score ≥4), No. (%) | 33 (27.3) | 33 (27.3) | 1.00 |
| Summed stress score | 2.75 (0.55) | 2.50 (0.44) | 0.66 |
| Summed rest score | 0.82 (0.28) | 0.73 (0.21) | 0.76 |
| Summed difference score | 1.93 (0.45) | 1.77 (0.37) | 0.77 |
| Average rate-pressure product during adenosine | 92.5 (2.4) | 88.4 (2.1) | 0.12 |
| Maximal rate-pressure product during adenosine | 115 (3) | 110 (3) | 0.16 |
P values are obtained from mixed models for continuous variables or generalized estimating equations for categorical variables, including a with random effect for pair.
Unless otherwise indicated, all data are reported as mean (SE) values.
Inflammatory Biomarkers and CFR
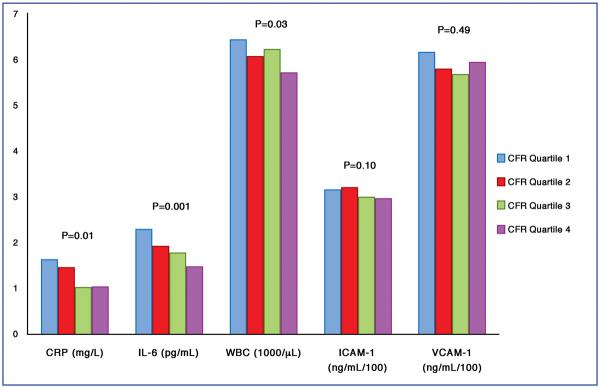
In the overall sample, inflammatory and endothelial activation biomarkers showed a decreasing trend from the lowest to the highest quartile of CFR, which was more pronounced for CRP, IL-6 and WBC than for soluble cell adhesion molecules (Figure 1). Biomarker levels were expressed as geometric means calculated from log-transformed data. Arithmetic means show a larger spread of data; for example, for CRP the arithmetic means going from the lowest to the highest quartile of CFR were 6.10, 2.94, 2.95 and 1.58 mg/L.
Figure 1. Geometric Means of Inflammatory Biomarkers According to Quartiles of CFR.
Quartile ranges: quartile 1: CFR 3.04–6.84; quartile 2: CFR 2.59–3.03; quartile 3: CFR 2.10–2.58; quartile 4: CFR 1.06–2.09. P values refer to tests for trend.
The association was similar when CFR was analyzed as a continuous variable, in the entire myocardium and in specific vascular territories (Table 3). For each unit of increasing CFR in the entire myocardium, CRP decreased 17.0% (p=0.03), IL-6 decreased 14.5% (p=0.01), WBC decreased 4.5% (p=0.02), ICAM decreased 3.5% (p=0.08) and VCAM decreased 3.1% (p=0.22). All inflammatory biomarkers, except VCAM-1, were inversely associated with the CFR in all vascular territories. While absolute maximal flow was not significantly related to inflammation, maximal flow increase from baseline showed similar relationships: p=0.02 for CRP, p=0.003 for IL-6, p=0.005 for WBC, p=0.10 for ICAM, p=0.30 for VCAM. In contrast, none of the biomarkers, except IL-6, were correlated with detectable myocardial perfusion abnormalities as measured by the summed stress score (data not shown).
Table 3.
Percent decrease in inflammatory biomarkers per one unit increase in CFR, in the entire myocardium and in regional coronary artery territories.
| Entire Myocardium |
Left Anterior Descending |
Circumflex |
Right Coronary Artery |
|||||
|---|---|---|---|---|---|---|---|---|
| % | P | % | P | % | P | % | P | |
| CRP (mg/L) | 17.0 | 0.03 | 16.6 | 0.03 | 18.5 | 0.009 | 15.4 | 0.05 |
| IL-6 (pg/dL) | 14.5 | 0.01 | 13.6 | 0.008 | 16.1 | <0.001 | 13.3 | 0.01 |
| WBC (1000/μL) | 4.5 | 0.02 | 4.7 | 0.01 | 4.3 | 0.01 | 4.5 | 0.02 |
| ICAM-1 (ng/mL) | 3.5 | 0.08 | 2.9 | 0.12 | 2.5 | 0.17 | 2.7 | 0.16 |
| VCAM-1 (ng/mL) | 3.1 | 0.22 | 2.5 | 0.27 | 1.5 | 0.48 | 2.4 | 0.31 |
CRP: C-reactive protein; IL-6: interleukin-6; WBC: white blood cell count; VCAM-1: vascular cell adhesion molecule-1; ICAM-1: intercellular adhesion molecule-1.
Values are derived from mixed models with log-transformed biomarker data. Percentages are calculated from regression coefficients.
Within pairs, all inflammatory biomarkers were higher in the twins with lower CFR than in their siblings with higher CFR (Table 4). Of 122 complete twin pairs, 50 pairs were discordant for a CFR of <2.5. Values of all biomarkers except VCAM-1 were significantly higher in twins with a CFR <2.5 than their brothers with a CFR of 2.5 or higher. For example, CRP was 65% higher and IL-6 was 54% higher in the twin with CFR<2.5 than in his brother with CFR ≥2.5. These associations remained statistically significant and fundamentally unchanged after adjusting for the Framingham risk score, body mass index, and summed stress score (Table 5). Adjustment for education, marital status, habitual physical activity, rate-pressure product during the adenosine infusion, previous history of CAD, medications (statins, beta-blockers, aspirin), and history of major depression did not materially change the results. Adjusting for individual risk factors, rather than including them in the Framingham risk score, also did not substantially change the results. Results were similar after stratification by zygosity, indicating that genetic factors do not play an important role in this association.
Table 4.
Unadjusted within-pair differences in plasma inflammatory biomarker levels based on CFR discordance status.
| Geometric Means of Inflammatory Markers (SE) (122 Twin Pairs) |
Geometric Means of Inflammatory Markers (SE) (50 twin pairs) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Twin with Lower CFR | Twin with Higher CFR | Within-Pair Differenc e (%) | P | Twin with CFR <2.5 | Twin with CFR ≥2.5 | Within-Pair Differenc e (%) | P | |
| CRP (mg/L) | 1.40 (1.11) | 1.10 (1.12) | 21.0 | 0.04 | 1.67 (1.18) | 1.01 (1.18) | 65.4 | 0.006 |
| IL-6 (pg/dL) | 2.04 (1.07) | 1.65 (1.07) | 18.7 | 0.02 | 2.65 (1.11) | 1.72 (1.11) | 54.2 | 0.002 |
| WBC (1000/μL) | 6.35 (1.02) | 5.87 (1.02) | 7.5 | 0.005 | 6.44 (1.04) | 5.89 (1.04) | 9.3 | 0.04 |
| ICAM-1 (ng/mL) | 323 (1.02) | 302 (1.02) | 6.6 | 0.02 | 336 (1.04) | 303 (1.04) | 11.0 | 0.02 |
| VCAM-1 (ng/mL) | 614 (1.03) | 573 (1.03) | 6.7 | 0.04 | 603 (1.05) | 580 (1.05) | 4.0 | 0.46 |
CFR: coronary flow reserve; CI: confidence interval; SE: standard error; CRP: C-reactive protein; IL-6: interleukin-6; WBC: white blood cell count; VCAM-1: vascular cell adhesion molecule-1; ICAM-1: intercellular adhesion molecule-1.
CFR is defined as the ratio of myocardial blood flow during adenosine to flow at baseline. All values in table are derived from mixed models with random intercept for pair.
Table 5.
Adjusted within-pair differences in plasma inflammatory biomarker levels based on CFR discordance status.
| Geometric Means of Inflammatory Markers (SE) (122 Twin Pairs) |
Geometric Means of Inflammatory Markers (SE) (50 twin pairs) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Twin with Lower CFR | Twin with Higher CFR | Within-Pair Differenc e (%) | P | Twin with CFR <2.5 | Twin with CFR ≥2.5 | Within-Pair Differenc e (%) | P | |
| CRP (mg/L) | 1.42 (1.10) | 1.11 (1.10) | 22.2 | 0.03 | 1.77 (1.17) | 1.09 (1.17) | 62.9 | 0.006 |
| IL-6 (pg/dL) | 2.05 (1.07) | 1.65 (1.07) | 19.4 | 0.02 | 2.63 (1.11) | 1.74 (1.11) | 51.2 | 0.003 |
| WBC (1000/μL) | 6.38 (1.02) | 5.89 (1.02) | 7.6 | 0.003 | 6.42 (1.04) | 5.94 (1.04) | 8.2 | 0.06 |
| ICAM-1 (ng/mL) | 322 (1.02) | 301 (1.02) | 6.5 | 0.02 | 332 (1.04) | 302 (1.04) | 9.7 | 0.04 |
| VCAM-1 (ng/mL) | 610 (1.03) | 570 (1.03) | 6.6 | 0.049 | 605 (1.05) | 580 (1.05) | 4.3 | 0.44 |
CFR: coronary flow reserve; CI: confidence interval; SE: standard error; CRP: C-reactive protein; IL-6: interleukin-6; WBC: white blood cell count; VCAM-1: vascular cell adhesion molecule-1; ICAM-1: intercellular adhesion molecule-1.
CFR is defined as the ratio of myocardial blood flow during adenosine to flow at baseline. All values in table are derived from mixed models with random intercept for pair.
All estimates adjusted for perfusion defect score, Framingham risk score (including age, current smoking, systolic blood pressure, LDL-cholesterol, HDL-cholesterol and diabetes), body mass index, and study year. Adjusting for additional covariables, including education, marital status, habitual physical activity, rate-pressure product during the adenosine infusion, previous history of coronary heart disease, and medications (statins, beta-blockers, aspirin), did not materially change the results.
Results were also similar when subjects with significant perfusion abnormalities (a summed stress score ≥4) were excluded. These results indicate that global myocardial perfusion, rather than perfusion in specific coronary territories, was reduced in twins with higher inflammation.
Discussion
Using PET myocardial perfusion imaging in a sample of twins without symptomatic CAD, we found a robust association linking biomarkers of systemic inflammation and endothelial cell activation with lower CFR. We also found that this association is independent of CAD risk factors, as well as of familial and genetic factors shared by the twins. In addition, the relationship was independent of perfusion defects, suggesting that the microvascular circulation plays a role. Although no direct causality can be inferred from our data, our results are suggestive of a mechanistic relationship between inflammation and microvascular function and highlight the importance of inflammatory processes in the early phases of CAD.
The role of inflammation in the pathogenesis of atherosclerosis has been widely recognized for many years.(28,29) In addition to extensive experimental research, clinical studies have found a link between peripheral vascular function and inflammation.(5,6) However, data from the peripheral circulation cannot necessarily be extrapolated to the coronary circulation,(30) for which much less is known. Two studies found an attenuated CFR in patients with chronic inflammatory conditions, including rheumatoid arthritis and systemic lupus erythematosus, but without significant coronary stenosis, compared with controls.(31,32) However, two additional studies of patients with chest pain and no obstructive CAD were not able to demonstrate a relationship between invasive angiographic assessment of CFR and levels of inflammatory biomarkers;(7,8) although, in one of these, endothelial function measured with administration of intracoronary acetylcholine was correlated with CRP.(7) These contrasting findings may be due to selected study samples that included symptomatic patients referred for medical evaluation, and/or inadequacies in the selection of control groups. No previous investigation explored the relationship between inflammation and CFR in asymptomatic individuals from the community.
A link between inflammation and coronary microvascular function may result from early atherosclerotic changes, microvascular endothelial or smooth muscle dysfunction, and/or inflammation of the coronary microvasculature. Adenosine-induced hyperemic response incorporates both endothelium- dependent and endothelium-independent pathways.(33) Endothelium-derived nitric oxide is a key modulator of vascular tone and plays an important role in the regulation of the coronary microcirculation.(34) Inhibition of the endothelial nitric oxide synthase by intravenous infusion of L-NG-monomethyl arginine reduces the vasodilatory response to adenosine by about 25% as measured by PET, suggesting a role of the endothelium.(35,36) Although the influence of inflammatory processes on coronary microvascular physiology and pathology has been less studied, our study suggests that similar processes are involved. Increased inflammation in the artery wall may result from oxidative stress due to reduced endothelium-derived nitric oxide. For example, oxidative stress is critical for the activation of nuclear factor kappa B (NF-κB),(37) a transcription factor that increases the expression of pro-inflammatory cytokines, chemokines, and cell adhesion molecules through angiotensin-II stimulation. Increased inflammation may also reflect injury of the arterial wall in the early phases of the atherosclerotic process.(33)
Although our study subjects were asymptomatic and without a prior history of CAD, 27% of them showed perfusion defects by PET imaging. This relatively high prevalence of perfusion abnormalities is not surprising given that CAD risk factors, such as smoking, obesity and hypertension, were common in this sample. The prevalence of myocardial perfusion abnormalities in asymptomatic subjects at risk (either because of family history or because of risk factors) has been reported to be between 20 and 50%.(38–40) Therefore, our data are consistent with previous literature. These perfusion abnormalities may be due to preclinical, mild, or diffuse coronary artery narrowing, or to endothelial dysfunction preceding luminal narrowing. Indeed, adenosine-induced hyperemia is a mixture of both endothelial dependent and independent pathways.(33)
In contrast to their robust relationship with CFR, all inflammatory biomarkers, except IL-6, were not associated with perfusion defects. These results clearly demonstrate that the link between inflammation and CFR in our study was driven by microvascular dysfunction rather than epicardial coronary stenoses. The association, however, could be an epiphenomenon of risk factors such as hypercholesterolemia, hypertension, diabetes and smoking, which have been linked to perfusion abnormalities even in the absence of coronary obstruction.(10–15) We were able to demonstrate that CAD risk factors did not explain the link between microvascular function and inflammation. As a whole, our data highlight the importance of inflammation in the early phases of the atherosclerotic process in asymptomatic individuals, because microvascular dysfunction appears to precede the development of frank CAD.(41) These results may also have implications for prevention, since microvascular dysfunction is potentially reversible and is associated with future cardiovascular events.(16,17)
Our study is cross-sectional, thus limited in the ability to discern the temporal order between inflammation and CFR. Furthermore, as in any study, sample variability in biomarker assays may increase random error. However, all assays used in this study have acceptable levels of precision. Nonetheless, it is possible that the lack of significant findings for ICAM-1 and VCAM-1 is in part due to random error. Because our twins were all middle-aged male military veterans, caution should be used in generalizing our results to women or to younger or older individuals. On the other hand, using a co-twin design, we were able to control for many unmeasured risk factors including maternal factors, early familial environment, and genetic influences, in addition to a comprehensive set of measured CAD risk factors. Finally, CFR measurement based on nitrogen 13 [13N] ammonia PET perfusion imaging is currently limited to institutions with on site cyclotrons, which may limit clinical utility. It is likely that new methods will allow the measurement of myocardial blood flow using agents that do not require a cyclotron in the near future. Until these techniques become available, PET with 13N remains the gold standard method for the non-invasive assessment of CFR.
In conclusion, we found that in asymptomatic middle-aged subjects, a decrease in coronary microvascular function is associated with increased systemic inflammatory response. Given the demonstrated importance of inflammation in the pathogenesis of CAD, our results, using a controlled design of matched twin pairs, support the pathogenic role of inflammation in human microvascular dysfunction.
Acknowledgments
Funding Sources This study was supported by K24HL077506, R01 HL68630 and R01 AG026255 from the National Institutes of Health; by the Emory University General Clinical Research Center MO1-RR00039 and by grant 0245115N from the American Heart Association. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution this research would not have been possible.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures Dr. Tracy Faber is a consultant and shareholder, and receives royalties from Syntermed Inc, which licenses the Emory Cardiac Toolbox, used for some analyses in this study. None of the other authors have disclosures.
References
- 1.Camici PG, Crea F. Coronary microvascular dysfunction. New England Journal of Medicine. 2007;356:830–840. doi: 10.1056/NEJMra061889. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann PA, Camici PG. Myocardial blood flow measurement by PET: technical aspects and clinical applications. J Nucl Med. 2005;46:75–88. [PubMed] [Google Scholar]
- 3.de Silva R, Camici PG. Role of positron emission tomography in the investigation of human coronary circulatory function. Cardiovascular Research. 1994;28:1595–1612. doi: 10.1093/cvr/28.11.1595. [DOI] [PubMed] [Google Scholar]
- 4.Beltrame JF, Crea F, Camici P. Advances in coronary microvascular dysfunction. Heart, Lung and Circulation. 2009;18:19–27. doi: 10.1016/j.hlc.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Fichtlscherer S, Rosenberger G, Walter DH, Breuer S, Dimmeler S, Zeiher AM. Elevated C-reactive protein Levels and impaired endothelial vasoreactivity in patients With coronary artery disease. Circulation. 2000;102:1000–1006. doi: 10.1161/01.cir.102.9.1000. [DOI] [PubMed] [Google Scholar]
- 6.Sinisalo J, Paronen J, Mattila KJ, et al. Relation of inflammation to vascular function in patients with coronary heart disease. Atherosclerosis. 2000;149:403–11. doi: 10.1016/s0021-9150(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 7.Teragawa H, Fukuda Y, Matsuda K, et al. Relation between C reactive protein concentrations and coronary microvascular endothelial function. Heart. 2004;90:750–4. doi: 10.1136/hrt.2003.022269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marroquin OC, Kip KE, Mulukutla SR, et al. Inflammation, endothelial cell activation, and coronary microvascular dysfunction in women with chest pain and no obstructive coronary artery disease. Am Heart J. 2005;150:109–15. doi: 10.1016/j.ahj.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Danesh J, Muir J, Wong YK, Ward M, Gallimore JR, Pepys MB. Risk factors for coronary heart disease and acute-phase proteins. A population-based study. Eur Heart J. 1999;20:954–9. doi: 10.1053/euhj.1998.1309. [DOI] [PubMed] [Google Scholar]
- 10.Dayanikli F, Grambow D, Muzik O, Mosca L, Rubenfire M, Schwaiger M. Early detection of abnormal coronary flow reserve in asymptomatic men at high risk for coronary artery disease using positron emission tomography. Circulation. 1994;90:808–817. doi: 10.1161/01.cir.90.2.808. [DOI] [PubMed] [Google Scholar]
- 11.Yokoyama I, Momomura S, Ohtake T, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. Journal of the American College of Cardiology. 1997;30:1472–1477. doi: 10.1016/s0735-1097(97)00327-6. [DOI] [PubMed] [Google Scholar]
- 12.Laine H, Raitakari OT, Niinikoski H, et al. Early impairment of coronary flow reserve in young men with borderline hypertension. Journal of the American College of Cardiology. 1998;32:147–153. doi: 10.1016/s0735-1097(98)00222-8. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann PA, Gnecchi-Ruscone T, di Terlizzi M, Schafers KP, Luscher TF, Camici PG. Coronary heart disease in smokers: Vitamin C restores coronary microcirculatory function. Circulation. 2000;102:1233–1238. doi: 10.1161/01.cir.102.11.1233. [DOI] [PubMed] [Google Scholar]
- 14.Seiler C, Hess O, Buechi M, Suter T, Krayenbuehl H. Influence of serum cholesterol and other coronary risk factors on vasomotion of angiographically normal coronary arteries. Circulation. 1993;88:2139–2148. doi: 10.1161/01.cir.88.5.2139. [DOI] [PubMed] [Google Scholar]
- 15.Zeiher AM, Schachinger V, Minners J. Long-term cigarette smoking impairs endothelium-dependent coronary arterial vasodilator function. Circulation. 1995;92:1094–1100. doi: 10.1161/01.cir.92.5.1094. [DOI] [PubMed] [Google Scholar]
- 16.Britten MB, Zeiher AM, Schachinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coronary Artery Disease. 2004;15:259–64. doi: 10.1097/01.mca.0000134590.99841.81. [DOI] [PubMed] [Google Scholar]
- 17.Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. Journal of the American College of Cardiology. 2009;54:150–6. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 18.Vaccarino V, Brennan ML, Miller AH, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: A Twin Study. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2008.04.023. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai J, Miller AH, Bremner JD, et al. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men. A twin study. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam Era Twin Registry. Twin Research. 2002;5:476–81. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 21.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–42. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 22.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative dynamic cardiac 82Rb PET using generalized factor and compartment analyses. J Nucl Med. 2005;46:1264–71. [PubMed] [Google Scholar]
- 23.Hachamovitch R, Berman DS, Kiat H, et al. Exercise myocardial perfusion SPECT in patients without known coronary artery disease: incremental prognostic value and use in risk stratification. Circulation. 1996;93:905–914. doi: 10.1161/01.cir.93.5.905. [DOI] [PubMed] [Google Scholar]
- 24.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr., Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 25.Carlin JB, Gurrin LC, Sterne JA, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–99. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 26.Reis SE, Holubkov R, Smith AJC, et al. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: Results from the NHLBI WISE study. American Heart Journal. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–47. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 28.Ross R. Atherosclerosis - an inflammatory disease. New England Journal of Medicine. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 29.Libby P, Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. doi: 10.1161/CIRCULATIONAHA.105.537878. [DOI] [PubMed] [Google Scholar]
- 30.Bottcher M, Madsen MM, Refsgaard J, et al. Peripheral flow response to transient arterial forearm occlusion does not reflect myocardial perfusion reserve. Circulation. 2001;103:1109–14. doi: 10.1161/01.cir.103.8.1109. [DOI] [PubMed] [Google Scholar]
- 31.Recio-Mayoral A, Mason JC, Kaski JC, Rubens MB, Harari OA, Camici PG. Chronic inflammation and coronary microvascular dysfunction in patients without risk factors for coronary artery disease. European Heart Journal. 2009:ehp205. doi: 10.1093/eurheartj/ehp205. [DOI] [PubMed] [Google Scholar]
- 32.Turiel M, Atzeni F, Tomasoni L, et al. Non-invasive assessment of coronary flow reserve and ADMA levels: a case-control study of early rheumatoid arthritis patients. Rheumatology (Oxford) 2009;48:834–9. doi: 10.1093/rheumatology/kep082. [DOI] [PubMed] [Google Scholar]
- 33.Schindler TH, Zhang XL, Vincenti G, Mhiri L, Lerch R, Schelbert HR. Role of PET in the evaluation and understanding of coronary physiology. Journal of Nuclear Cardiology. 2007;14:589–603. doi: 10.1016/j.nuclcard.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimokawa H, Yasuda S. Myocardial ischemia: current concepts and future perspectives. Journal of Cardiology. 2008;52:67–78. doi: 10.1016/j.jjcc.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 35.Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135–2141. doi: 10.1161/01.cir.92.8.2135. [DOI] [PubMed] [Google Scholar]
- 36.Buus NH, Bottcher M, Hermansen F, Sander M, Nielsen TT, Mulvany MJ. Influence of nitric oxide synthase and adrenergic inhibition on adenosine-induced myocardial hyperemia. Circulation. 2001;104:2305–2310. doi: 10.1161/hc4401.098293. [DOI] [PubMed] [Google Scholar]
- 37.Mogensen TH, Melchjorsen J, Hollsberg P, Paludan SR. Activation of NF-kappa B in virus-infected macrophages is dependent on mitochondrial oxidative stress and intracellular calcium: downstream involvement of the kinases TGF-beta-activated kinase 1, mitogen-activated kinase/extracellular signal-regulated kinase kinase 1, and I kappa B kinase. Journal of Immunology. 2003;170:6224–33. doi: 10.4049/jimmunol.170.12.6224. [DOI] [PubMed] [Google Scholar]
- 38.Blumenthal RS, Becker DM, Yanek LR, et al. Detecting occult coronary disease in a high-risk asymptomatic population. Circulation. 2003;107:702–707. doi: 10.1161/01.cir.0000048127.93169.88. [DOI] [PubMed] [Google Scholar]
- 39.Wackers FJT, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes Care. 2004;27:1954–1961. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 40.Sdringola S, Patel D, Gould KL. High prevalence of myocardial perfusion abnormalities on positron emission tomography in asymptomatic persons with a parent or sibling with coronary artery disease. Circulation. 2001;103:496–501. doi: 10.1161/01.cir.103.4.496. [DOI] [PubMed] [Google Scholar]
- 41.Kaul S, Ito H. Microvasculature in acute myocardial ischemia: Part I: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation. 2004;109:146–149. doi: 10.1161/01.CIR.0000111582.02736.CD. [DOI] [PubMed] [Google Scholar]