Abstract
Anti-tumor alkylphospholipids initiate apoptosis in transformed HL-60 and Jurkat cells while sparing their progenitors. Edelfosine like other short-chained phospholipids—inflammatory Platelet-activating Factor (PAF) and apoptotic oxidatively-truncated phospholipids—are proposed to have intracellular sites of action, yet a conduit for these choline phospholipids into mammalian cells is undefined. Edelfosine is also accumulated by Saccharomyces cerevisiae in process requiring the membrane protein Lem3p, and the human genome contains a Lem3p homolog TMEM30a. We show import of choline phospholipids into S. cerevisiae ⊗Lem3 is partially reconstituted by human TMEM30a and by Lem3p-TMEM30a chimeras, showing the proteins are orthologous. TMEM30a-GFP chimeras expressed in mammalian cells localized in plasma membranes, as well as internal organelles, and ectopic TMEM30a expression promoted uptake of exogenous choline and ethanolamine phospholipids. shRNA knockdown of TMEM30a reduced fluorescent choline phospholipid and [3H]PAF import. This knockdown also reduced mitochondrial depolarization from exogenous Edelfosine or the mitotoxic oxidatively truncated phospholipid azelaoyl phosphatidylcholine, and the knockdown reduced apoptosis in response to these two phospholipids. These results show extracellular choline phospholipids with short sn-2 residues can have intracellular roles and sites of metabolism because they are transport substrates for a TMEM30a phospholipid import system. Variation in this mechanism could limit sensitivity to short-chain choline phospholipids such as Edelfosine, PAF, and pro-apoptotic phospholipids.
Introduction
Edelfosine (1-O-alkyl-2-carboxymethyl-sn-glycero-3-phosphocholine) is a pro-apoptotic agent with selectivity towards tumor cells (1) including promyelocytic leukemic HL60 cells (2) and immortalized lymphocytic Jurkat cells (3). Its mode of action is yet to be firmly established, but all of its effects from peroxide-dependent mitochondrial depolarization and apoptosis (2–5) to inhibition of CTP:choline phosphate cytidylyltransferase (6) activity to disruption of lipid raft domains (7–10) all require its internalization. However in contrast to well documented phospholipid export proteins (11–14), evidence for phospholipid import or the proteins that might accomplish this task in mammalian cells is meager. Indeed, the best characterized example ATP8B1, homologous to yeast phospholipid import ATPases, has now been found to neither import phosphatidylserine nor affect its asymmetric distribution across membrane bilayers (15).
The uncertainty of the molecular mechanism of phospholipid import in mammalian cells stands in contrast to our understanding of phospholipid uptake by yeast. Saccharomyces cerevisiae accumulate phospholipids from their external environment, and two independent approaches, one of which was selection for resistance to Edelfosine toxicity, identified the same essential component, Lem3p (16) or Ros3p (17), for this internalization. Lem3p aids import of Edelfosine, fluorescent phosphatidylcholine (16), fluorescent phosphatidylethanolamine (17), and fluorescent lysophosphatidylethanolamine (18), but not fluorescently labeled phosphatidylserine. There may be additional transport conduits since varying the fluorescent group on phosphatidylcholines determines whether import of the lipid is aided by Lem3p (19). Humans express two RNAs, TMEM30a and TMEM3b, with open reading frames that encode proteins of unknown function whose sequences are highly homologous to that of yeast Lem3p (12, 20).
Platelet-activating Factor (1-O-alkyl-2-acetyl-sn-glycero-3-phosphocholine) is an intercellular agonist that activates all cells of the innate immune system, and a number of other cells, through a single G protein coupled PAF receptor expressed on the plasma membrane of responding cells (21–23). However, the PAF receptor is also enriched in an endosomal compartment (24) and at the nuclear membrane (25), and nuclear PAF receptors have unassailably been shown to initiate nuclear Ca++ flux and gene transcription (26, 27)—illuminating a potential intracellular role for PAF. The PAF receptor has a critical role in the metastatic distribution of melanoma cells and is a potential therapeutic target (28). Whether the nuclear PAF receptor responds to PAF produced by distal cells or solely responds to intracellular PAF production is unknown because PAF is not known to readily transit mammalian plasma membrane (29). PAF is, however readily internalized by S. cerevisiae when given the opportunity (30).
Oxidative attack on polyunsaturated phospholipids generates a host of products, some of which are short chain phospholipids created by oxidative scission of the sn-2 polyunsaturated fatty acyl residue. Oxidatively truncated phospholipids, like the abundant oxidation product azelaoyl phosphatidylcholine, induce apoptosis through a direct effect on mitochondria that initiates the intrinsic caspase cascade (31, 32). The rapid incorporation of exogenous azelaoyl phosphatidylcholine into intracellular mitochondrial membranes (32) suggests the presence of a cellular uptake mechanism.
We find (Liu, submitted) that intravascular PAF or oxidatively truncated phospholipids are rapidly (t1/2 ~ 30 seconds) cleared from the circulation, and that the mechanism is not primarily intravascular hydrolysis by plasma PAF acetylhydrolase (33) as expected. Instead, we find that these short chain phospholipids are rapidly internalized as intact phospholipids by an undefined, saturable mechanism. This suggests import of short chained phospholipids has a critical role in mammalian cells in vivo.
Here, we show that TMEM30a is a functional ortholog of S. cerevisiae Lem3p that aids internalization of the anti-tumor phospholipid Edelfosine, the short chain inflammatory lipid PAF, and the mitotoxic phospholipid azelaoyl phosphatidylcholine in yeast and in mammalian cells. Thus, short chain choline phospholipids found in the extracellular environment during atherogensis (34), chronic ethanol ingestion (35), uv irradiation (36), or acute cigarette smoke exposure (37) can be become intracellular effectors.
Material and Methods
Materials
CHO K1 cells and S. cerevisiae stains 201388 and 4001121 were from ATCC. Oligonucleotide primers, MitoTracker Orange CMTMRRos, Organelle Lights Golgi-OFP, Organelle lights ER-OFP, pYES2/CT vector, anti-V5 antibody, Fetal Bovine Serum and S. cerevisiae EasyComp Transformation kit were products of Invitrogen Life Technologies. Human TMEM30a cDNA was purchased from Origene Technologies (Rockville, MD), and shRNA plasmids against human TMEM30a were from SuperArray Bioscience (Frederick, MD). RNeasy Mini kits were from QIAGEN (Valencia, CA). Azelaoyl-PC, NBD-PC, NBD-PE, Edelfosine and PAF were obtained from Avanti Polar lipid, Inc (Alabaster AL). [2-[3H]acetyl]PAF was a product of PerkinElmer Life and Analytical Sciences (Boston, MA). Anti-GFP was from Santa Cruz Biotechnology, and mounting medium was a product of Vector Laboratories (Burlingame, CA). Anti-Na/K ATPase was from Abcam (Cambridge, MA). DMEM, Ham’s F-12, RPMI 1640 media, and penicillin/streptomycin, were obtained from the Cleveland Clinic media core, while yeast nitrogen base with amino acids, synthetic drop-out media supplement without uracil, CelLytic Cell Lysis reagent, and other reagents were obtained from Sigma.
Cell Culture
CHO, HepG2, and Jurkat cells were cultured in DMEM F-12 and RPMI 1640, respectively, with 10% FBS at 37°C in an atmosphere containing 5% CO2. S. cerevisiae strains 4001121 or 201388 were culture in YPD medium with rotation at 270 rpm at 28°C. Transformants were cultured in uracil drop out media with the stated carbon source. Cell number was assessed either by serial two-fold dilutions followed by plating on appropriate agar media, or in liquid culture by scattering at OD600.
Expression constructs
The sequences used to amplify human TMEM30a or S. cerevisiae Lem3 to generate expression constructs or yeast/human chimeras are: Lem3; 5′ GTGCTGGAATTCCATCAGTACAGACCAGAAAA, 3′GGCTCTAGATTTCATATTCCATGACAAAC; hTMEM30a 5′GAATATTAAGCTTACCATGGTAAATAACTATAACGCGAAGGATGAAG, 3′GGCTCTAGAAATGGTAATGTCAGCTGTATTAC; Lem3/hTMEM30a chimera (LT) 5′TAATACGACTCACTATAGGG, 3′CTATCATCTCGAGATAGTACATATCTTCTGTG; TMEM30a/Lem3 chimera (TL) 5′CTGGGGATCCATTTGTGCTGCCATGGCATTG, 3′GCGTGAATGTAAGCGTGAC; TMEM30a/Lem3/TMEM30a chimera (TLT) 5′GGGCTCGAGATTTTAGCGAAGATCAGATA, 3′GGAGATGGATCCACAGCCTCCAATCAGGT. Lem3 sequence was generated by RT-PCR using 201388 yeast strain RNA with SuperScrip III Reverse Transcriptase and Vent polymerase with the stated Lem3 primers. This PCR product was inserted into pYES2/CT by EcoR1 and Xbal digestion, ligated, and transformed into DH5a. The hTMEM30a PCR product was digested with HindIII and Xbal and similarly introduced into pYES2/CT. The Lem3 and TMEM30a chimeric constructs were made starting with the TMEM30a pYES2/CT plasmid, and the primers listed above to produce appropriate Lem3 fragments. The Lem3 fragments and hTMEM30a-Yes2/CT were digested by the stated restriction enzymes and the resulting fragments ligated and transformed according to the manufacturer’s directions. All constructs were confirmed by sequencing and western blotting when appropriate. The TMEM30a-GFP construct PCR product of human TMEM30a with 3′ primer GGCGACCGGTGGAATGGTAATGTCAGCTGTATTAC and 5′ primer GGGGGAATTCCTGTCAGGGTCAGCCGGC was digested by EcoRI and AGeI and inserted to pd2EGFP-N1 vector.
TMTM30a Silencing
shRNA against human TMEM30a with insert sequences GGATGATCTTGAGCACTATTT, CCAGTTTACATTGCTGGATTCT, TGTGAACCTTATCGAAGAAT, CAGTCCCTGTAATAAATGTTT, or control sequence CAGTCCTGTAATAAATGTTT were separately transfected into Jurkat cells by nucleofection before selection in 500 nM neomycin.
TMTM30a expression
Overnight cultures of S. cerevisiae were diluted and grown to confluence in 2% raffinose, recovered by centrifugation, and then grown in uracil drop-out media with glucose or 0.4% galactose containing 2% raffinose such that they grew for ~4 h to log phase (OD600 0.4 to 0.6). The cells were collected by centrifugation (3,000 × g, 5 min, room temperature) and washed twice with sterile Millipore water before being resuspended in CelLytic Cell Lysis reagent. Glass beads (0.5 mm) were added (10% w/v) and cells lysed by shaking twice at 4,800 rpm for 30 seconds in mini-beadbeater. The resulting lysates were centrifuged (12,000 × g, 10 min, 4°C) before the proteins resolved by electrophoresis in a 10% SDS polyacrylamide gel. Anti-V5 (1:2500) and HRP-conjugated goat anti-mouse antibody (1:5000) were used to detect the expression of V5-tagged Lem3, hTMEM30a or chimeric proteins in S. cerevisiae. Human HepG2 cells were lysed by RIPA buffer and centrifuged (12,000×g, 25 min, 4°C) before these supernatants were resolved by 10% SDS-PAGE. Expressed proteins were detected using murine anti-GFP (1:1000) and plasma membrane human (since Chinese hamster is not commercially available) anti-NaK ATPase horseradish peroxidase conjugated goat anti-mouse (1:5000).
Lipid accumulation
Jurkat cells, transfected with TMEM30a shRNA or not, were washed with HBSS twice and resuspended (2 × 106 cells/ml) in 1 μM NDB-PC or NDB-PE and kept at room temperature for 10 min. Competition for NBD-PC (1μM) uptake by TMTM30a-Jurkat cells employed 5 μM unlabeled phospholipid and the same conditions. The labeled cells were washed twice with HBSS containing 1% (w/v) BSA before analysis by flow cytometry. To assess [3H]PAF uptake, transfected CHO cells were seeded in 12 well plates (6 × 105 cells/well) one day before labeling. These were then washed with PBS thrice before incubation with 75,000 dpm [2-[3H]acetyl ]PAF in 500 μl HBSS containing 0.005 % BSA with or without 1μM unlabeled PAF for 10 min either at room temperature or on ice. The cells were then washed twice with cold 1% BSA in PBS, and then once with PBS. The cells were detached with trypsin/EDTA and transferred to Ecolite (CPM Biomedicals, Solon, OH) scintillation fluid for quantitation.
Lipid uptake by S. cerevisiae was determined using sn-1 NBD-phosphatidylcholine, NBD-phosphatidylethanolamine or NBD-phosphatidylserine. S. cerevisiae was grown overnight in uracil dropout media with raffinose as a carbon source. The following morning the cells were diluted to 0.2 OD600 in medium containing glucose or galactose. The cells were cultured for 6 h, washed with PBS thrice before incubation with 1μM NBD-labeled phospholipid in PBS for 10 min at room temperature. The labeled cells were washed with PBS containing 1% BSA thrice and suspended in the same buffer for flow cytometry.
Colony formation on phospholipid agar plates was tested by growing S. cerevisiae in glucose dropout media overnight before dilution to 0.2 OD600 in raffinose dropout media. After growth in log phase to 0.4 to 0.6 OD600, the cells were diluted to 0.01 OD in raffinose dropout media. This was serially diluted two-fold and 3 μl of each dilution was spotted on an agar plate containing the stated reagent before growth in a 30°C humid atmosphere.
Organelle labeling
CHO and HepG2 cells were stably transfect with a human TMEM30a-GFP chimeric expression plasmid and grown in 500 μg/ml G418 before single clones were selected by sorting for GFP. Highly expressing clones were verified by western blot. The cells were seeded on cover slips in 6-well plates one day prior to staining. For organelle labeling, organelle lights Golgi-OFP and organelle lights ER-OFP were used to labeled Golgi body and endoplasmic reticulum, respectively, following the manufacture’s procedure. The culture medium was aspirated and 550 μl organelle lights transduction solution was added to the well before incubation for 4 h at room temperature. After the solution was removed, serum free DMEM F-12 medium containing enhancer was added to the well and incubated for 2h at 37° C in a 5% CO2 atmosphere. The cells were washed in HBSS before fixation in 3.75% formaldehyde for 10 min followed by washing thrice with PBS. The coverslip containing the cells was mounted with DAPI containing media prior to fluorescence imaging. For plasma membrane staining, the cells were washed with serum free medium once and medium containing 2.5 μg/ml CellMask orange plasma membrane stain solution was added for a 5 min incubation at 37°C before the cells were processed as above. Mitochondria were labeled by washing the cells thrice with HBSS before 25 nM MitoTracker orange CMTMRRos in HBSS was added to each well. The cells were incubated at 37°C for 15 min, washed thrice with PBS, mounted, and immediately imaged. All confocal fluorescent micrographs are an image of a single xy plane.
Annexin V labeling
TMEM30a-GFP transfected CHO cells or control cells were detached non-enzymatically with Cellstripper™ (Mediatech, Herndon, VA), washed with complete medium once, and then with annexin V binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) before being suspended (3 × 106 cells/ 100 μl) in this buffer with or without 5 μl annexin V-Alexa647 conjugate (Invitrogen) and kept at room temperature for 15 min. The cells were then diluted with 1 ml binding buffer for flow cytometry.
Cell fractionation
TMEM30a-GFP stable transfectants (2 × 108) were washed thrice with PBS and resuspended in 5 ml extraction buffer (0.25 M sucrose, 10 mM HEPES pH7.4, 1 mM EDTA, protease inhibitor cocktail (Roche) before being equilibrated at 500 psi N2 for 15 min in a nitrogen cavitation bomb (Parr Instrument; Moline, IL). After explosive depressurization, the resulting lysate was centrifuged (500 × g, 5 min, 4°C) to remove cell debris before being layered over a 0.6 M to 2 M continuous sucrose gradient for density separation (28,000 rpm, SW41 rotor) for 90 minutes at 4°C without braking. Aliquots of the fractions collected from the bottom of the gradient were mixed with sample buffer for protein resolution by SDS PAGE followed by western blotting for GFP and plasma membrane markers.
Results
Lem3p enhances short chain phospholipid import in S. cerevisiae
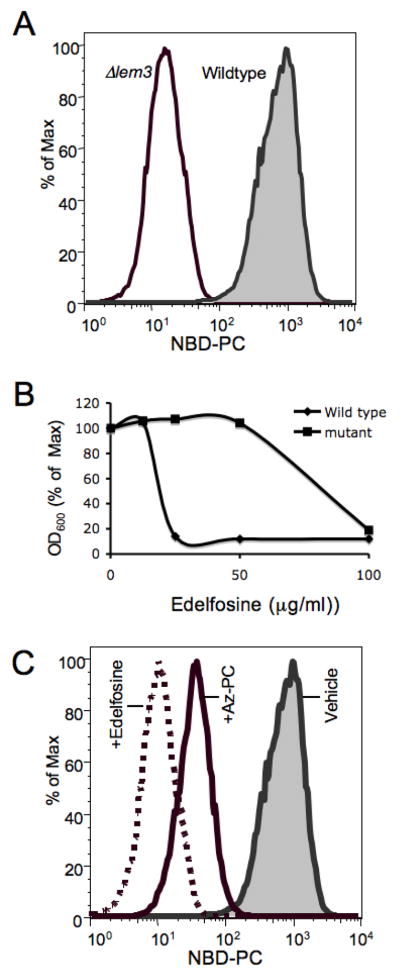
Wild-type S. cerevisiae accumulate NBD-labeled phospholipids, which have relatively high critical micellar concentrations and are soluble, from their environment (Fig. 1A). This uptake was dependent on Lem3p because loss of this membrane protein greatly reduced this influx. The phospholipid Edelfosine, with its very short methyl ether sn-2 residue, also is imported by S. cerevisiae where—as with transformed mammalian cells—it induced cell death (Fig. 1B). Cells in which Lem3 was genetically ablated were resistant to this cytotoxic phospholipid. Short-chained phospholipids, including the truncated phospholipid oxidation product azelaoyl phosphatidylcholine, induce cell death through a direct physical interaction with mitochondria (31, 32)—a process that necessarily includes internalization of the exogenous lipid. We tested whether this phospholipid with a 9-carbon long sn-2 residue was also a Lem3p transport system substrate by examining its ability to compete for uptake of the fluorescent phospholipid NBD-PC. We found that excess Edelfosine effectively reduced uptake of the fluorescently labeled phospholipid (Fig. 1C), and that azelaoyl phosphatidylcholine similarly reduced, although less effectively, uptake of the labeled transport substrate.
Figure 1. Lem3 promotes uptake of short chained phosphatidylcholines by S. cerevisiae.
(A) Flow cytometric analysis of NBD-phosphatidylcholine uptake by wild-type or ⊗Lem3 S. cerevisiae. (B) Edelfosine concentration dependent suppression of wild-type or ΔLem3 growth. (C) Competition for NBD-phosphatidylcholine uptake by wild-type S. cerevisiae by an excess of the cytotoxic choline phospholipid Edelfosine or the oxidatively truncated phosphatidylcholine azelaoyl-PC.
Human TMEM30a partially reconstitutes phospholipid import in ⊗Lem3 S. cerevisiae
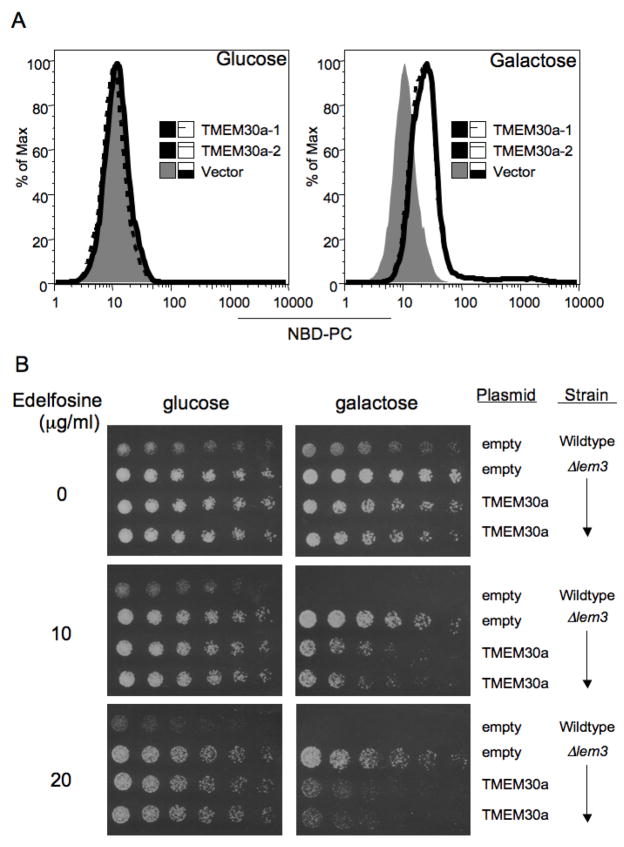
The human genome contains two sequences, transmembrane 30 kDa a and b (TMEM30a, TMEM30b). These sequences would encode proteins that are 39% identical with 54% similar residues to S. cerevisiae Lem3p (Table 1). The human sequence also retains the highly conserved sequence QNHRRYVKS found in nearly all of this family’s homologs. We expressed human TMEM30a in the ⊗Lem3 mutant under the control of a Gal-1 promoter. This transgene had no effect on NBD-PC accumulation in S. cerevisiae grown on glucose where the protein was not expressed, but did promote the uptake of the fluorescent phospholipid in either of two isolates when grown on galactose to induce the protein (Fig. 2A).
Table 1.
 |
Figure 2. Human TMEM30a partially reconstitutes phosphatidylcholine uptake by in S. cerevisiae lacking Lem3p.
(A) ΔLem3 S. cerevisiae transformed with empty vector or two isolates transformed with human TMEM30a were grown on glucose or galactose to induce TMEM30a expression. NBD-phosphatidylcholine uptake was determined by flow cytometry. (B) Concentration dependent effect of Edelfosine on colony growth of serially diluted wild-type S. cerevisiae or ΔLem3 transformed with empty vector or two ΔLem3 isolates transformed with human TMEM30a.
S. cerevisiae is sensitive to the cytotoxic drug Edelfosine, so we determined whether human TMEM30a would increase the sensitivity of the resistant ⊗Lem3 mutant to its toxic effect. Serial dilution of wild-type S. cerevisiae transformed with empty vector followed by culture on galactose plates showed 10 μg/ml Edelfosine reduced viability and that loss of cell viability was more pronounced when the cells were plated on 20 μg/ml Edelfosine (Fig. 2B). The ⊗Lem3 mutant transformed either with empty vector or the TMEM30a vector and grown on repressive glucose were resistant to the cytotoxic effect of even 20 μg/ml Edelfosine. The outcomes differed when the cells were grown on galactose to induce TMEM30a. In this case, ⊗Lem3 transformed with empty vector remained resistant to Edelfosine, while expression of either of two TMEM30a clones sensitized these cells to the effect of 10 and 20 μg/ml Edelfosine.
TMEM30a is orthologous to S. cerevisiae Lem3p
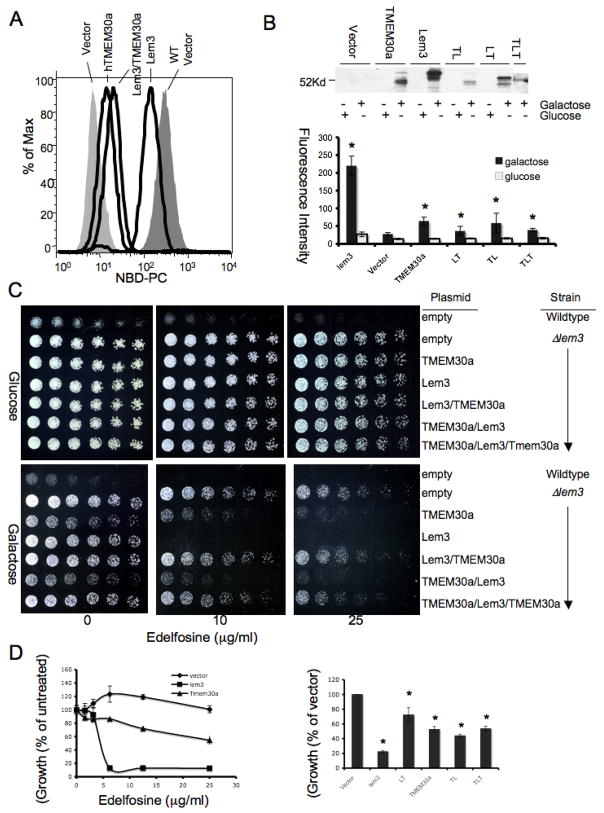
We sought to determine whether human TMEM30a is truly orthologous to Lem3p by forming chimeras of the two proteins by substituting regions defined by the putative transmembrane domains (Table I) to determine whether human encoded regions functionally substitute for the yeast sequences they replaced. Yeast expressed human TMEM30a and all of the chimeras formed with Lem3p, although expression of the chimeras or full length TMEM30a was always significantly less than when Lem3p was reintroduced in ⊗Lem3 (Fig. 3B). We found ⊗Lem3 transformed with Lem3p effectively imported NBD-PC, that human TMEM30a modestly enhanced lipid uptake in the ⊗Lem3 mutant, and that an N-terminal Lem3p/ C-terminal TMEM30a chimera (LT; Table 1) was more effective than the human sequence in promoting fluorescent phospholipid accumulation (Fig. 3A, B). The mirror image chimera (TL) with an N-terminal TMEM30a and C-terminal Lem3p was equally effective in promoting uptake of NBD-PC (Fig. 3B). A chimera (TLT) where only the central portion of the human-yeast-human protein was encoded by Lem3p was less effective than either the yeast-human or human-yeast protein, although its low level of expression compromised its effectiveness. For all of these chimeras, the uptake of NBC-PC was greater when the strains were grown on galactose than on glucose where plasmid proteins are not expressed.
Figure 3. Human TMEM30a-S. cerevisiae Lem3p chimeras partially reconstitute fluorescent phosphatidylcholine uptake by in S. cerevisiae lacking Lem3p.
(A) NBD-phosphatidylcholine uptake determined by flow cytometry for wild-type S. cerevisiae transformed with empty vector or ΔLem3 transformed with Lem3, TMEM30a or a chimera (Table 1) of Lem3 and TMEM30a. (B) Quantitation (n=3) of NBD-phosphatidylcholine uptake by ΔLem3 transformed with Lem3-TMEM30a (LT; see Table 1 for sequence), TMEM30a-Lem3 (TL), or TMEM30a-Lem3-TMEM30a (TLT) chimeras. Western blot (top) for V5 antigen contained in sequences encoding TMEM30a and its chimeras isolated from protein extracts of S. cerevisiae grown in galactose to induce insert expression or non-inducing glucose. (C) Concentration dependent effect of Edelfosine on colony formation on glucose or galactose plates for wild-type S. cerevisiae or ΔLem3 transformed with galactose induced human, yeast or chimeric constructs. (D) Effect of Edelfosine on ΔLem3 viability after introduction of human TMEM30a, yeast Lem3p, or chimeras formed from them. Cell number (OD600) in liquid culture of wildtype or ΔLem3 transformed with the stated vectors at defined concentrations (left) or 12.5 μg/ml (right).
We determined whether human/yeast chimeras increased the sensitivity of ⊗Lem3 to Edelfosine cytotoxicity. As before, the wild-type parent was sensitive to Edelfosine and the ⊗Lem3 mutant insensitive when grown on either glucose or galactose (Fig. 3C). The ⊗Lem3 mutant containing TMEM30a under the control of the Gal-1 promoter behaved just as its empty vector in the ⊗Lem3 control on glucose plates, but became sensitive to Edelfosine when grown on galactose plates when the TMEM30a was expressed. Although not as fully effective as Gal-1 induced Lem3p in reconstituting ⊗Lem3’s sensitivity to Edelfosine, the TMTM30a/Lem3p and Lem3p/TMEM30a/Lem3p chimeras, and especially the TMEM30a/Lem3p chimera, sharply increased the sensitivity of ⊗Lem3 cells to exogenous Edelfosine. The interchangeability of Lem3p and TMEM30a show the proteins are orthologous.
An identical outcome obtained when the experiment was performed in liquid culture where cell numbers could be quantitated. Reintroduction of Lem3p in the ⊗Lem3 strain restored sensitivity to Edelfosine, as did expression of human TMEM30a, although the human sequence was significantly less effective (Fig. 3D). As before, each chimera sensitized the ⊗Lem3 strain to Edelfosine, although in this assay all the chimeras were similarly effective.
TMEM30a-GFP distributes among CHO plasma membrane and internal organelles
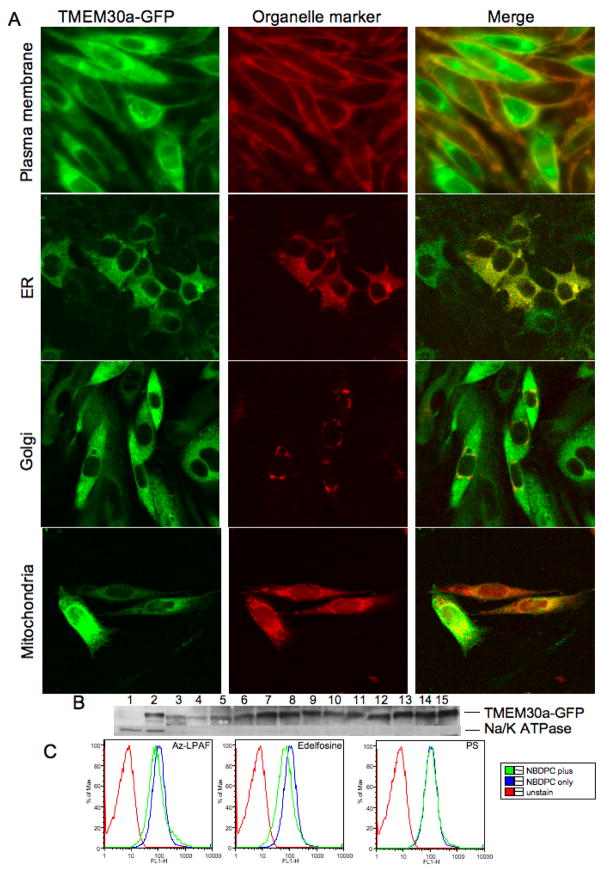
We determined whether human TMEM30a is properly positioned to import phospholipids into mammalian cells using CHO cells stably transfected with TMEM30a-GFP and maintained under G418 selection. We observed by confocal microscopy that one population of cells abundantly expressed the ectopic protein, with staining throughout the cytoplasm and a delimiting rim of green fluorescence (Fig. 4A). Other cells displayed less intense internal expression of the fluorescent chimera, and these cells expressed the TMEM30a-GFP just as a rim of green fluorescence around the cell. We confirmed this rim pattern of fluorescence represented plasma membrane localization of TMEM30a-GFP by staining these cells with the plasma membrane dye CellMask Orange™. The rim pattern of staining of both cells with high expression and those with less abundant TMEM30a-GFP expression produced a rim of orange fluorescence that indicates co-localization at the plasma membrane.
Figure 4. TMEM30a-GFP localizes in the plasma membrane and internal organelles.
(A) CHO cells stably transfected with TMEM30a-GFP and then stained with CellMask™ Orange Plasma Membrane to mark the plasma membrane (top) then imaged by confocal microscopy. Co-expression of the appropriate orange fluorescent protein Organelle Light defined endoplasmic reticulum (row 2), or Golgi (row 3). TMEM30a-GFP expressing CHO cells were labeled with MitoTracker Red to identify polarized mitochondria (bottom). (B) Western blot for GFP or plasma membrane Na/K ATPase in density gradient fractions from HepG2 cells stably expressing TMEM30a-GFP. (C) Fluorescent intensity of TMEM30a-Jurkat cells during flow cytometry after 10 min incubation in the presence of NBD-phosphatidylcholine (1 μM) alone or additionally with 5 μM Az-LPAF or Edelfosine.
Lem3p-GFP is found in discrete patches in the plasma membrane of S. cerevisiae, but also in intracellular structures including the endoplasmic reticulum (17). TMEM30a also was present in CHO cell intracellular structures, and co-expression with endoplasmic reticulum and Golgi Organelle Lights™ show (Fig. 4A) that the mammalian protein co-localized with these organelles in cells that strongly expressed TMEM30a-GFP. TMEM30a also localized with MitoTracker Red that marks polarized mitochondria. The apparent diffuse TMEM30a-GFP staining, then, reflects the wide intracellular distribution of the protein.
We confirmed that TMEM30a-GFP associated with internal organelles by separating membranes of HepG2 cells stably transfected with TMEM30a-GFP by sucrose density centrifugation and immunoblotting for TMEM30a-GFP and the plasma membrane marker Na/K ATPase (Fig. 4B). The TMEM30a chimera was prominent in the plasma membrane fraction, but also was widely distributed among other membrane components.
TMEM30a aided choline phospholipid uptake by CHO cells through a saturable process because excess azelaoyl phosphatidylcholine or Edelfosine decreased fluorescent phosphatidylcholine accumulation by TMEM30a expressing cells (Fig. 4C). Phosphatidylserine, in contrast, did not compete for fluorescent phosphatidylcholine uptake.
TMEM30a enhances phospholipid import into CHO cells
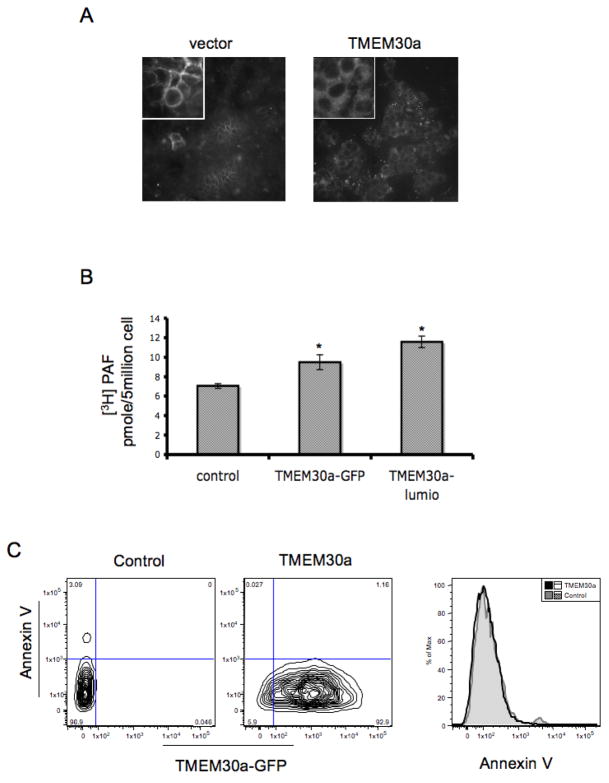
We tested the postulate that TMEM30a participates in phospholipid import in mammalian cells by examining NBD-PC uptake into CHO cells stably expressing TMEM30a. CHO cells transfected with empty vector showed that the NBD-PC associated with these cells was prominently associated with the cell’s plasma membrane (Fig. 5A, inset). In contrast, cells expressing TMEM30a accumulated more NBD-PC and a significant portion of this was intracellular and associated with structures that were clearly excluded from the nucleus.
Figure 5. TMEM30a enhances import of short chained phosphatidylcholines, and not phosphatidylserine, into CHO cells.
(A) NBD-phosphatidylcholine uptake by CHO cells transfected with empty vector or a TMEM30a vector assessed by confocal microscopy (40X). Inset, 60X. (B) Uptake of [3H]PAF by CHO cells expressing TMEM30a containing a GFP or Lumio tag (n=3). (C) Phosphatidylserine surface expression is not reduced in TMEM30a transfected CHO cells. Surface phosphatidylserine was detected (n=3) by flow cytometry with annexin V conjugated with Alexa647 as described in “Methods.”
We confirmed that the TMEM30a-GFP chimera that we used to define the intracellular distribution of TMEM30a retained its function, and that TMEM30a participates in the accumulation of the inflammatory mediator PAF by quantifying the amount of [3H]PAF accumulated by transfected CHO cells. We observed that the expression of TMEM30a-GFP, or a TMME30a modified by a Lumio™ tag that introduces a short sequence that selectively binds a proprietary fluorescent dye, enhanced [3H]PAF uptake (Fig. 5B). Thus, the NBD fluorescent group is not required for phospholipid transport, and exogenous PAF is a transport substrate of TMEM30a—and so has access to intracellular PAF receptors (24–27).
TMTM30a expressed by itself does not increase phosphatidylserine uptake (38), nor does phosphatidylserine compete for the uptake of choline phospholipids (Fig 4C). However, when TMEM30a is co-expressed with the P4-type ATPase ATP8B1 phosphatidylserine uptake is increased and basal surface expression of phosphatidylserine was reduced (38), although this may not be a direct ATP81-mediated function (15). We determined whether expression of TMEM30a in the absence of co-expressed APT8B1 reduced the display of phosphatidylserine on the cell surface. We found that even when TMEM30a-GFP was highly abundant that there was no diminution of annexin V staining of surface phosphatidylserine (Fig. 5C). This indicates that TMEM30a, either through association with another ATPase or alone, has a role in choline phospholipid import that is distinct from its role in enabling ATP8B1 uptake of phosphatidylserine.
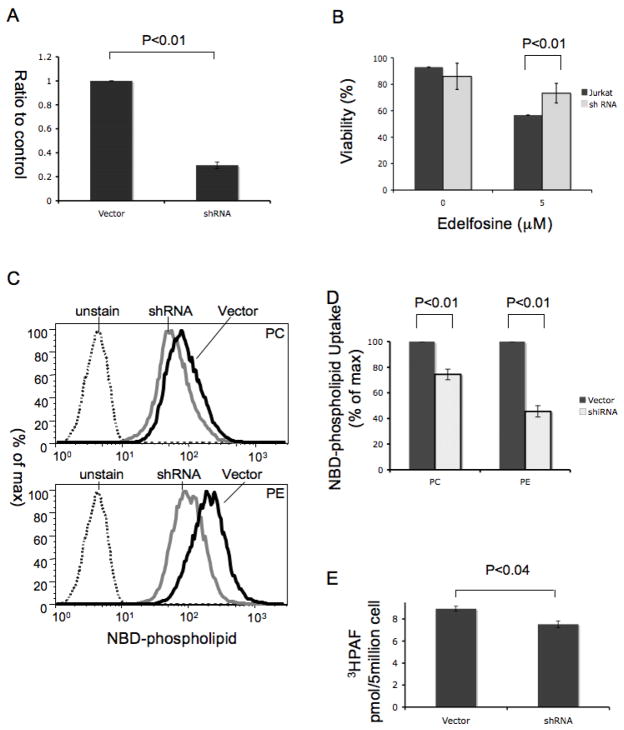
TMEM30a knockdown reduces phospholipid import by Jurkat cells
Edelfosine is selectively cytotoxic for lymphoblastoid Jurkat cells compared to naïve lymphocytes (4, 9, 39–41), suggesting Jurkat cells internalize this short chain phospholipid. We found that Jurkat cells express mRNA for TMEM30a, and that a shRNA directed to it suppressed its expression by 70% (Fig. 6A). Jurkat cells expressing TMEM30a shRNA were less sensitive to the cytotoxic effect of Edelfosine than control cells (Fig. 6B). Jurkat cells accumulated both NBD-PC and NBD-PE as determined by flow cytometry (Fig. 6C), and TMEM30a shRNA reduced the amount of both these phospholipids associated with the cells. The reduction of phospholipid uptake by the TMEM30a knockdown was greater for NBD-PE than NBD-PC (Fig. 6D). We also found that shRNA knockdown of TMEM30a reduced the uptake of radiolabeled PAF (Fig. 6E), so exogenous PAF is internalized with the aid of TMEM30a.
Figure 6. shRNA knockdown of Jurkat cell TMEM30a reduces mitotoxicity of exogenous short chained phosphatidylcholines.
(A) Quantitative PCR for TMEM30a mRNA after transfection by empty vector or one containing TMEM30a shRNA (n=3). (B) Jurkat viability to Edelfosine exposure after transfection with an empty vector or TMEM30a shRNA (n=3). (C) Jurkat cell uptake of fluorescent NBD-phosphatidylcholine (upper) or NBD-phosphatidylethanolamine (lower) by cells expressing TMEM30a shRNA or its vector (n=3). (D) Quantitation of NBD-phosphatidylcholine accumulation by Jurkat cells expressing TMEM30a shRNA or empty vector (n=3). (E) Uptake of [3H]PAF by Jurkat cells is reduced by TMEM30a shRNA knockdown (n=4). All quantitative measures used triplicate determinations in each experiment.
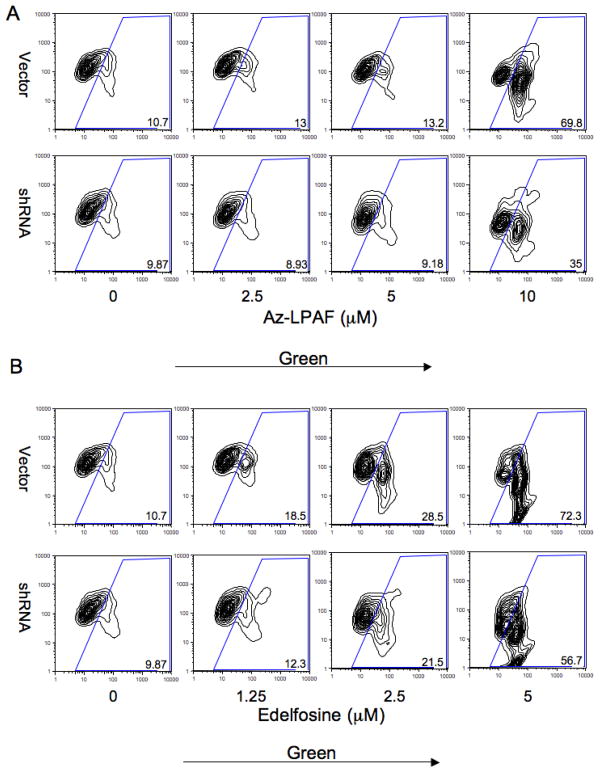
TMEM30a knockdown reduces mitochondrial depolarization by exogenous short-chained phospholipids
Oxidatively truncated phospholipids such as azelaoyl phosphatidylcholine are rapidly internalized, migrate to mitochondria (32) and then initiate the mitochondrial dependent apoptotic cascade (31, 32). We tested the potential role of TMEM30a in internalizing this mitotoxic lipid using shRNA and the potentiometric mitochondrial dye JC-1. This cationic dye is concentrated and precipitated in respiring mitochondria by the electrochemical gradient and so fluoresces red/orange, while monomeric dye in the cytoplasm not incorporated into mitochondria fluoresces in the green channel. We found (Fig. 7A) that suppression of TMEM30a by shRNA expression reduced mitochondrial depolarization caused by azelaoyl phosphatidylcholine. Thus, just 57% of the shRNA transfected cells lost their mitochondrial transmembrane potential after exposure to 10 μM azelaoyl phosphatidylcholine compared to the 72% of vector transfected cells that became depolarized by this oxidatively truncated phospholipid. Intracellular mitochondria are therefore vulnerable to short chained phospholipids in their environment because these phospholipids are internalized in part through a TMEM30a-dependent process.
Figure 7. shRNA knockdown of Jurkat cell TMEM30a reduces azelaoyl choline phospholipid and Edelfosine mitotoxicity.
(A) Flow cytometric analysis of JC-1 green fluorescence (FL1, x axis) and orange/red fluorescence (FL2, y axis) in the presence of the stated azelaoyl lysoPAF concentration in vector and TMEM30a shRNA transfected Jurkat cells. The cationic dye JC1 in functional, polarized mitochondria is aggregated and fluoresces red/orange, while monomeric dye free in the cytoplasm fluoresces green. (B) Flow cytometric analysis of JC-1 fluorescence in the stated concentration of Edelfosine.
Exogenous Edelfosine also affects mitochondrial function and induces mitochondria dependent apoptosis (2, 5). We again found just 10% of vector transfected cells were in the lower rightmost bin with low red/orange mitochondrial fluorescence and high green cytoplasmic fluorescence in the absence of Edelfosine (Fig. 7B). This number increased to 70% when the concentration of exogenous Edelfosine was increased to 10 μg/ml, showing Edelfosine effectively depolarized mitochondria. The number of Jurkat cells transfected with TMEM30a shRNA in the rightmost bin also was 10% of the population in the absence of Edelfosine, but at 10 μg/ml Edelfosine this number increased to just 35% of the population. We conclude exogenous Edelfosine and azelaoyl phosphatidylcholine both access intracellular mitochondria in part through TMEM30a mediated uptake.
Discussion
The primary findings in this work are that mammalian cells readily internalize exogenous short-chained phospholipids, and that they do so using a transport or flipping system orthologous to the phospholipid import system identified in S. cerevisiae. Indeed, we find the human protein TMEM30a functions in S. cerevisiae to increase uptake of exogenous phospholipids in cells whose phospholipid import system had been crippled by ablating the Lem3p component of their transport system. The ability of chimeras of the human and yeast protein to also enhance phospholipid import in this genetic background shows that the function of this protein has been maintained in organisms that diverged ~100 million years ago.
mRNA for TMEM30a is widely expressed, although the presence of the encoded protein has yet to be reported. We used GFP chimeras to determine where TMEM30a distributed within mammalian cells because an anti-peptide antibody to the conserved element we prepared was of too low affinity to be useful in localizing the native protein. We found a portion of the TMEM30a-GFP chimera was associated with the plasma membrane, and this location was the predominant one among cells with lower levels of protein expression. We, and recently others (42), also found that in cells expressing higher amounts of TMEM30a that a large portion remained in the intracellular compartment in organelles identified as endoplasmic reticulum, Golgi, and mitochondria. This intracellular accumulation may have resulted from over-expression, but the S. cerevisiae Lem3p-GFP ortholog is also associated both with the plasma membrane and the endoplasmic reticulum (17) where it participates in endocytic and exocytic processes, maintains amino phospholipid transmembrane asymmetry, and defines cell polarity (43–46).
Edelfosine is a premier member of a small group of anti-cancer, and anti-leishmenaisis, alkyl phospholipids that selectively reduce the viability of metabolically active, proliferating cells (1). Thus leukemic Jurkat cells, in contrast to resting peripheral blood lymphocytes, are sensitive to growth inhibition by alkyl choline phospholipids (9, 39). These choline lipids have several targets, ranging from suppression of CTP:phosphocholine cytidylyltransfersase and hence phosphatidylcholine synthesis (6), to modulation of several kinase signaling pathways (1) to mitochondrial depolarization and apoptotic caspase activation (2). These targets are intracellular, and the sensitivity of various cells to Edelfosine is correlated with the extent to which they can accumulate the lipid (4, 10, 39, 41, 47, 48). Leishmania parasites also express homologs of Lem3p, and manipulation of this import system affects both alkyl phospholipid uptake and their sensitivity to the drug (49, 50).
As with the pro-apoptotic anti-tumor phospholipids, the pro-apoptotic truncated phospholipids have an intracellular site of action. These products of free radical phospholipid oxidation are apoptotic components of oxidized LDL (32) that occur in the circulation of models of oxidative stress (51, 52), after acute exposure to cigarette smoke (53), in developed atherosclerotic lesions (34), and in animals chronically ingesting ethanol (35). Immunosuppressive oxidatively truncated phospholipids are also formed by uv B irradiation of skin (54). The truncated azelaoyl choline phospholipids (Az-PC) are derived from oxidative fragmentation of linoleoyl residues—the most common polyunsaturated sn-2 fatty acyl residue—and are the most potent products that typify oxidatively truncated phospholipids.
One complication that obfuscates our understanding of the substrate selectivity of the lipid import systems is that the nature of the fluorescent moiety affects phospholipid import (19). We found, however, that TMEM30a over-expression increased [3H]PAF uptake, and that internalization of unlabeled Edelfosine or unlabeled azelaoyl phosphatidylcholine was reduced when TMEM30a was suppressed by shRNA. We can therefore be certain that transport substrate selection for these substrates was free from the interference of bulky fluorescent tags.
AzPC, or its alkyl homolog Az-LPAF, rapidly enters cells and associates with respiring mitochondria (32). Thus, these lipid oxidation products, even when exogenously presented, rapidly depolarize intracellular mitochondria in a way enhanced by Bid, a pro-apoptotic member of the Bcl-2 family, and resisted by the anti-apoptotic member Bcl-XL (31, 32). Evidence of rapid internalization of extracellular truncated phospholipids is confirmed by the protection against apoptosis afforded by over-expression of intracellular PAF acetylhydrolase (32), a phospholipase A2 that specifically hydrolyzes short chain phospholipids including Platelet-activating Factor and oxidatively truncated phospholipids (55, 56). Extracellular phospholipids therefore have ready access to intracellular organelles and receptors.
Extension of this observation to the whole animal will have several consequences. First, one route to insensitivity to anti-tumor alkyl phospholipids in mammalian cells, like resistance of Leishmania to these lipids, would derive from reduced TMEM30a lipid transport. Second, plasmalemmal PAF receptors respond to the autacoid PAF, but PAF receptors (and lysophosphatidic acid and prostaglandin E receptors) are also located on nuclear (25) and endosomal (24) membranes. Nuclear PAF receptors are functional and induce intranuclear Ca++ spikes and stimulate gene induction (25, 26), so extracellular PAF—or exogenous oxidatively generated PAF-like lipids (57)—can also directly stimulate nuclear PAF receptors. Third, these results raise the possibility that intravascular hydrolysis of PAF by the circulating plasma PAF acetylhydrolase, also known in the cardiovascular disease literature as lipoprotein-associated phospholipase A2, is not the sole location for the inactivation of PAF and oxidized phospholipids since a portion of this hydrolysis may occur within vascular cells. This downplays the functional role of circulating lipoprotein-associated phospholipase A2, making it primarily a biomarker, although a robust one, for cardiovascular disease (58). This is significant as a large (~15,000 participants) phase III trial, the STABILITY trial, to define the effect of inhibiting circulating PAF acetylhydrolase on cardiovascular disease is underway.
We conclude phosphatidylcholines and phosphatidylethanolamines with short sn-2 residues (or fluorescently tagged phospholipids with increased hydrophilicity) are readily accumulated by mammalian cells by a saturable, energy dependent process that fully resembles that of S. cerevisiae. The protein TMEM30a is homologous, and indeed orthologous, to the lipid import system of this yeast, and prior work showing that mammalian TMEM30a interacts with the ATP8B1 P-type ATPase (38) suggesting it functions as a heterodimeric complex. Since uptake of choline—and not serine phospholipids—is enhanced by TMEM30a in the absence of ATP8B1 over-expression, TMEM30a may interact with members of the P4-ATPase family. This import system is relevant because it provides access for extracellular lipids to alter intracellular lipid metabolism and for oxidatively truncated phospholipids to alter mitochondrial function and cell viability.
Acknowledgments
We appreciate the support from the Lerner Research Institute Imaging and DNA cores. We thank Dr. G. K. Marathe for many helpful discussions over the course of this work.
Footnotes
This work was funded by grants P01 HL087018, HL092747, AA017748, and P50 HL081011 from the National Institutes of Health.
References
- 1.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14:2061–2074. doi: 10.2174/138161208785294636. [DOI] [PubMed] [Google Scholar]
- 2.Gajate C, Santos-Beneit AM, Macho A, Lazaro M, Hernandez-De Rojas A, Modolell M, Munoz E, Mollinedo F. Involvement of mitochondria and caspase-3 in ET-18-OCH(3)-induced apoptosis of human leukemic cells. Int J Cancer. 2000;86:208–218. doi: 10.1002/(sici)1097-0215(20000415)86:2<208::aid-ijc10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 3.Cuvillier O, Mayhew E, Janoff AS, Spiegel S. Liposomal ET-18-OCH(3) induces cytochrome c-mediated apoptosis independently of CD95 (APO-1/Fas) signaling. Blood. 1999;94:3583–3592. [PubMed] [Google Scholar]
- 4.Mollinedo F, Fernandez-Luna JL, Gajate C, Martin-Martin B, Benito A, Martinez-Dalmau R, Modolell M. Selective induction of apoptosis in cancer cells by the ether lipid ET-18-OCH3 (Edelfosine): molecular structure requirements, cellular uptake, and protection by Bcl-2 and Bcl-X(L) Cancer Res. 1997;57:1320–1328. [PubMed] [Google Scholar]
- 5.Vrablic AS, Albright CD, Craciunescu CN, Salganik RI, Zeisel SH. Altered mitochondrial function and overgeneration of reactive oxygen species precede the induction of apoptosis by 1-O-octadecyl-2-methyl-rac-glycero-3-phosphocholine in p53-defective hepatocytes. FASEB J. 2001;15:1739–1744. doi: 10.1096/fj.00-0300com. [DOI] [PubMed] [Google Scholar]
- 6.Boggs KP, Rock CO, Jackowski S. Lysophosphatidylcholine and 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphocholine inhibit the CDP-choline pathway of phosphatidylcholine synthesis at the CTP:phosphocholine cytidylyltransferase step. J Biol Chem. 1995;270:7757–7764. doi: 10.1074/jbc.270.13.7757. [DOI] [PubMed] [Google Scholar]
- 7.Zaremberg V, Gajate C, Cacharro LM, Mollinedo F, McMaster CR. Cytotoxicity of an anti-cancer lysophospholipid through selective modification of lipid raft composition. J Biol Chem. 2005;280:38047–38058. doi: 10.1074/jbc.M502849200. [DOI] [PubMed] [Google Scholar]
- 8.van der Luit AH, Budde M, Ruurs P, Verheij M, van Blitterswijk WJ. Alkyl-lysophospholipid accumulates in lipid rafts and induces apoptosis via raft-dependent endocytosis and inhibition of phosphatidylcholine synthesis. J Biol Chem. 2002;277:39541–39547. doi: 10.1074/jbc.M203176200. [DOI] [PubMed] [Google Scholar]
- 9.Gajate C, Del Canto-Janez E, Acuna AU, Amat-Guerri F, Geijo E, Santos-Beneit AM, Veldman RJ, Mollinedo F. Intracellular triggering of Fas aggregation and recruitment of apoptotic molecules into Fas-enriched rafts in selective tumor cell apoptosis. J Exp Med. 2004;200:353–365. doi: 10.1084/jem.20040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajate C, Gonzalez-Camacho F, Mollinedo F. Involvement of raft aggregates enriched in Fas/CD95 death-inducing signaling complex in the antileukemic action of edelfosine in Jurkat cells. PLoS ONE. 2009;4:e5044. doi: 10.1371/journal.pone.0005044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pomorski T, Holthuis JC, Herrmann A, van Meer G. Tracking down lipid flippases and their biological functions. J Cell Sci. 2004;117:805–813. doi: 10.1242/jcs.01055. [DOI] [PubMed] [Google Scholar]
- 12.Holthuis JC, Levine TP. Lipid traffic: floppy drives and a superhighway. Nat Rev Mol Cell Biol. 2005;6:209–220. doi: 10.1038/nrm1591. [DOI] [PubMed] [Google Scholar]
- 13.Devaux PF, Lopez-Montero I, Bryde S. Proteins involved in lipid translocation in eukaryotic cells. Chem Phys Lipids. 2006;141:119–132. doi: 10.1016/j.chemphyslip.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Daleke DL. Phospholipid flippases. J Biol Chem. 2007;282:821–825. doi: 10.1074/jbc.R600035200. [DOI] [PubMed] [Google Scholar]
- 15.Verhulst PM, van der Velden LM, Oorschot V, van Faassen EE, Klumperman J, Houwen RH, Pomorski TG, Holthuis JC, Klomp LW. A flippase-independent function of ATP8B1, the protein affected in familial intrahepatic cholestasis type 1, is required for apical protein expression and microvillus formation in polarized epithelial cells. Hepatology. 2010;51:2049–2060. doi: 10.1002/hep.23586. [DOI] [PubMed] [Google Scholar]
- 16.Hanson PK, Malone L, Birchmore JL, Nichols JW. Lem3p is essential for the uptake and potency of alkylphosphocholine drugs, edelfosine and miltefosine. J Biol Chem. 2003;278:36041–36050. doi: 10.1074/jbc.M305263200. [DOI] [PubMed] [Google Scholar]
- 17.Kato U, Emoto K, Fredriksson C, Nakamura H, Ohta A, Kobayashi T, Murakami-Murofushi K, Umeda M. A novel membrane protein, Ros3p, is required for phospholipid translocation across the plasma membrane in Saccharomyces cerevisiae. J Biol Chem. 2002;277:37855–37862. doi: 10.1074/jbc.M205564200. [DOI] [PubMed] [Google Scholar]
- 18.Riekhof WR, Voelker DR. Uptake and utilization of lyso-phosphatidylethanolamine by Saccharomyces cerevisiae. J Biol Chem. 2006;281:36588–36596. doi: 10.1074/jbc.M608851200. [DOI] [PubMed] [Google Scholar]
- 19.Elvington SM, Bu F, Nichols JW. Fluorescent, acyl chain-labeled phosphatidylcholine analogs reveal novel transport pathways across the plasma membrane of yeast. J Biol Chem. 2005;280:40957–40964. doi: 10.1074/jbc.M507926200. [DOI] [PubMed] [Google Scholar]
- 20.Katoh Y, Katoh M. Identification and characterization of CDC50A, CDC50B and CDC50C genes in silico. Oncol Rep. 2004;12:939–943. [PubMed] [Google Scholar]
- 21.Braquet P, Touqui L, Shen TY, Vargaftig BB. Perspectives in platelet-activating factor research. Pharmacol Rev. 1987;39:97–145. [PubMed] [Google Scholar]
- 22.Ishii S, Nagase T, Shimizu T. Platelet-activating factor receptor. Prostaglandin other lipid mediat. 2002;68–69:599–609. doi: 10.1016/s0090-6980(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 23.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 24.Ihida K, Predescu D, Czekay RP, Palade GE. Platelet activating factor receptor (PAF-R) is found in a large endosomal compartment in human umbilical vein endothelial cells. J Cell Sci. 1999;112( Pt 3):285–295. doi: 10.1242/jcs.112.3.285. [DOI] [PubMed] [Google Scholar]
- 25.Zhu T, Gobeil F, Vazquez-Tello A, Leduc M, Rihakova L, Bossolasco M, Bkaily G, Peri K, Varma DR, Orvoine R, Chemtob S. Intracrine signaling through lipid mediators and their cognate nuclear G-protein-coupled receptors: a paradigm based on PGE2, PAF, and LPA1 receptors. Can J Physiol Pharmacol. 2006;84:377–391. doi: 10.1139/y05-147. [DOI] [PubMed] [Google Scholar]
- 26.Bazan NG, Squinto SP, Braquet P, Panetta T, Marcheselli VL. Platelet-activating factor and polyunsaturated fatty acids in cerebral ischemia or convulsions: intracellular PAF-binding sites and activation of a fos/jun/AP-1 transcriptional signaling system. Lipids. 1991;26:1236–1242. doi: 10.1007/BF02536539. [DOI] [PubMed] [Google Scholar]
- 27.Marrache AM, Gobeil F, Jr, Bernier SG, Stankova J, Rola-Pleszczynski M, Choufani S, Bkaily G, Bourdeau A, Sirois MG, Vazquez-Tello A, Fan L, Joyal JS, Filep JG, Varma DR, Ribeiro-Da-Silva A, Chemtob S. Proinflammatory gene induction by platelet-activating factor mediated via its cognate nuclear receptor. J Immunol. 2002;169:6474–6481. doi: 10.4049/jimmunol.169.11.6474. [DOI] [PubMed] [Google Scholar]
- 28.Melnikova VO, Balasubramanian K, Villares GJ, Dobroff AS, Zigler M, Wang H, Petersson F, Price JE, Schroit A, Prieto VG, Hung MC, Bar-Eli M. Crosstalk between protease-activated receptor 1 and platelet-activating factor receptor regulates melanoma cell adhesion molecule (MCAM/MUC18) expression and melanoma metastasis. J Biol Chem. 2009;284:28845–28855. doi: 10.1074/jbc.M109.042150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratton DL, Kailey JM, Clay KL, Henson PM. A model for the extracellular release of PAF: the influence of plasma membrane phospholipid asymmetry. Biochim Biophys acta. 1991;1062:24–34. doi: 10.1016/0005-2736(91)90330-b. [DOI] [PubMed] [Google Scholar]
- 30.Foulks JM, Weyrich AS, Zimmerman GA, McIntyre TM. A yeast PAF acetylhydrolase ortholog suppresses oxidative death. Free Radic Biol Med. 2008;45:434–442. doi: 10.1016/j.freeradbiomed.2008.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen R, Feldstein AE, McIntyre TM. Suppression of Mitochondrial Function by Oxidatively-Truncated Phospholipids is Reversible, Aided by Bid, and Suppressed by Bcl-XL. J Biol Chem. 2009;284:26297–26308. doi: 10.1074/jbc.M109.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen R, Yang L, McIntyre TM. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. J Biol Chem. 2007;282:24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2) Cardiovasc Drugs Ther. 2009;23:73–83. doi: 10.1007/s10557-008-6133-8. [DOI] [PubMed] [Google Scholar]
- 34.Podrez EA, Poliakov E, Shen Z, Zhang R, Deng Y, Sun M, Finton PJ, Shan L, Febbraio M, Hajjar DP, Silverstein RL, Hoff HF, Salomon RG, Hazen SL. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, Nagy LE, Feldstein AE, McIntyre TM. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J Biol Chem. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marathe GK, Johnson C, Billings SD, Southall MD, Pei Y, Spandau D, Murphy RC, Zimmerman GA, McIntyre TM, Travers JB. Ultraviolet B radiation generates platelet-activating factor-like phospholipids underlying cutaneous damage. J Biol Chem. 2005;280:35448–35457. doi: 10.1074/jbc.M503811200. [DOI] [PubMed] [Google Scholar]
- 37.Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J Clin Invest. 1997;99:2358–2364. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, Oude Elferink RP. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 39.Quesada E, Delgado J, Gajate C, Mollinedo F, Acuna AU, Amat-Guerri F. Fluorescent phenylpolyene analogues of the ether phospholipid edelfosine for the selective labeling of cancer cells. J Med Chem. 2004;47:5333–5335. doi: 10.1021/jm049808a. [DOI] [PubMed] [Google Scholar]
- 40.Cabaner C, Gajate C, Macho A, Munoz E, Modolell M, Mollinedo F. Induction of apoptosis in human mitogen-activated peripheral blood T-lymphocytes by the ether phospholipid ET-18-OCH3: involvement of the Fas receptor/ligand system. Br J Pharmacol. 1999;127:813–825. doi: 10.1038/sj.bjp.0702606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gajate C, Fonteriz RI, Cabaner C, Alvarez-Noves G, Alvarez-Rodriguez Y, Modolell M, Mollinedo F. Intracellular triggering of Fas, independently of FasL, as a new mechanism of antitumor ether lipid-induced apoptosis. Int J Cancer. 2000;85:674–682. doi: 10.1002/(sici)1097-0215(20000301)85:5<674::aid-ijc13>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 42.van der Velden LM, Wichers CG, van Breevoort AE, Coleman JA, Molday RS, Berger R, Klomp LW, van de Graaf SF. Heteromeric interactions required for abundance and subcellular localization of human CDC50 proteins and class 1 P4 ATPases. J Biol Chem. 2010 doi: 10.1074/jbc.M110.139006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saito K, Fujimura-Kamada K, Furuta N, Kato U, Umeda M, Tanaka K. Cdc50p, a protein required for polarized growth, associates with the Drs2p P-type ATPase implicated in phospholipid translocation in Saccharomyces cerevisiae. Mol Biol Cell. 2004;15:3418–3432. doi: 10.1091/mbc.E03-11-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iwamoto K, Kobayashi S, Fukuda R, Umeda M, Kobayashi T, Ohta A. Local exposure of phosphatidylethanolamine on the yeast plasma membrane is implicated in cell polarity. Genes Cells. 2004;9:891–903. doi: 10.1111/j.1365-2443.2004.00782.x. [DOI] [PubMed] [Google Scholar]
- 45.Furuta N, Fujimura-Kamada K, Saito K, Yamamoto T, Tanaka K. Endocytic recycling in yeast is regulated by putative phospholipid translocases and the Ypt31p/32p-Rcy1p pathway. Mol Biol Cell. 2007;18:295–312. doi: 10.1091/mbc.E06-05-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito K, Fujimura-Kamada K, Hanamatsu H, Kato U, Umeda M, Kozminski KG, Tanaka K. Transbilayer phospholipid flipping regulates Cdc42p signaling during polarized cell growth via Rga GTPase-activating proteins. Dev Cell. 2007;13:743–751. doi: 10.1016/j.devcel.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Zerp SF, Vink SR, Ruiter GA, Koolwijk P, Peters E, van der Luit AH, de Jong D, Budde M, Bartelink H, van Blitterswijk WJ, Verheij M. Alkylphospholipids inhibit capillary-like endothelial tube formation in vitro: antiangiogenic properties of a new class of antitumor agents. Anticancer Drugs. 2008;19:65–75. doi: 10.1097/CAD.0b013e3282f16d36. [DOI] [PubMed] [Google Scholar]
- 48.Alonso MT, Gajate C, Mollinedo F, Modolell M, Alvarez J, Garcia-Sancho J. Dissociation of the effects of the antitumour ether lipid ET-18-OCH3 on cytosolic calcium and on apoptosis. Br J Pharmacol. 1997;121:1364–1368. doi: 10.1038/sj.bjp.0701271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez-Victoria FJ, Sanchez-Canete MP, Castanys S, Gamarro F. Phospholipid translocation and miltefosine potency require both L. donovani miltefosine transporter and the new protein LdRos3 in Leishmania parasites. J Biol Chem. 2006;281:23766–23775. doi: 10.1074/jbc.M605214200. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Canete MP, Carvalho L, Perez-Victoria FJ, Gamarro F, Castanys S. Low plasma membrane expression of the miltefosine transport complex renders Leishmania braziliensis refractory to the drug. Antimicrob Agents Chemother. 2009;53:1305–1313. doi: 10.1128/AAC.01694-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frey B, Haupt R, Alms S, Holzmann G, Konig T, Kern H, Kox W, Rustow B, Schlame M. Increase in fragmented phosphatidylcholine in blood plasma by oxidative stress. J Lipid Res. 2000;41:1145–1153. [PubMed] [Google Scholar]
- 52.Frey B, Johnen W, Haupt R, Kern H, Rustow B, Kox WJ, Schlame M. Bioactive oxidized lipids in the plasma of cardiac surgical intensive care patients. Shock. 2002;18:14–17. doi: 10.1097/00024382-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Lehr HA, Weyrich AS, Saetzler RK, Jurek A, Arfors KE, Zimmerman GA, Prescott SM, McIntyre TM. Vitamin C blocks inflammatory PAF mimetics created by cigarette smoking. J Clin Invest. 1997;99:2358–2364. doi: 10.1172/JCI119417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q, Yao Y, Konger RL, Sinn AL, Cai S, Pollok KE, Travers JB. UVB radiation-mediated inhibition of contact hypersensitivity reactions is dependent on the platelet-activating factor system. J Invest Dermatol. 2008;128:1780–1787. doi: 10.1038/sj.jid.5701251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stremler KE, Stafforini DM, Prescott SM, McIntyre TM. Human plasma platelet-activating factor acetylhydrolase. Oxidatively fragmented phospholipids as substrates. J Biol Chem. 1991;266:11095–11103. [PubMed] [Google Scholar]
- 56.McIntyre TM, Prescott SM, Stafforini D. The emerging roles of PAF acetylhydrolase. J Lipid Res. 2009:S255–S259. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM. Activation of vascular cells by PAF-like lipids in oxidized LDL. Vascul Pharmacol. 2002;38:193–200. doi: 10.1016/s1537-1891(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 58.Munzel T, Gori T. Lipoprotein-associated phospholipase A(2), a marker of vascular inflammation and systemic vulnerability. Eur Heart J. 2009;30:2829–2831. doi: 10.1093/eurheartj/ehp311. [DOI] [PubMed] [Google Scholar]