Abstract
BACKGROUND
Methadone, a full mu-opioid agonist, is the recommended treatment for opioid dependence during pregnancy. However, prenatal exposure to methadone is associated with a neonatal abstinence syndrome (NAS) characterized by central nervous system hyperirritability and autonomic nervous system dysfunction, which often requires medication and extended hospitalization. Buprenorphine, a partial mu-opioid agonist, is an alternative treatment for opioid dependence but has not been extensively studied in pregnancy.
METHODS
We conducted a double-blind, double-dummy, flexible-dosing, randomized, controlled study in which buprenorphine and methadone were compared for use in the comprehensive care of 175 pregnant women with opioid dependency at eight international sites. Primary outcomes were the number of neonates requiring treatment for NAS, the peak NAS score, the total amount of morphine needed to treat NAS, the length of the hospital stay for neonates, and neonatal head circumference.
RESULTS
Treatment was discontinued by 16 of the 89 women in the methadone group (18%) and 28 of the 86 women in the buprenorphine group (33%). A comparison of the 131 neonates whose mothers were followed to the end of pregnancy according to treatment group (with 58 exposed to buprenorphine and 73 exposed to methadone) showed that the former group required significantly less morphine (mean dose, 1.1 mg vs. 10.4 mg; P<0.0091), had a significantly shorter hospital stay (10.0 days vs. 17.5 days, P<0.0091), and had a significantly shorter duration of treatment for the neonatal abstinence syndrome (4.1 days vs. 9.9 days, P<0.003125) (P values calculated in accordance with prespecified thresholds for significance). There were no significant differences between groups in other primary or secondary outcomes or in the rates of maternal or neonatal adverse events.
CONCLUSIONS
These results are consistent with the use of buprenorphine as an acceptable treatment for opioid dependence in pregnant women. (Funded by the National Institute on Drug Abuse; ClinicalTrials.gov number, NCT00271219.)
Opioid dependence during pregnancy is compounded by multiple risk factors contributing to adverse maternal, neonatal, and long-term developmental consequences.1–6 Improved treatment options should reduce the public health and medical costs associated with the treatment of neonates exposed to opioids, which in 2009 was estimated at $70.6 million to $112.6 million in the United States alone.7 Just as the use of methadone in nonpregnant patients with opioid dependence improves patient outcomes,8 its use as part of a comprehensive approach to the care of pregnant women improves maternal and neonatal outcomes, as compared with no treatment and with medication-assisted withdrawal.4,9,10 However, exposure to methadone in utero can result in a neonatal abstinence syndrome (NAS) characterized by hyperirritability of the central nervous system and dysfunction in the autonomic nervous system, gastrointestinal tract, and respiratory system.11 When left untreated, NAS can result in serious illness (e.g., diarrhea, feeding difficulties, weight loss, and seizures) and death.11 Methadone-associated NAS often requires prolonged hospitalization, pharmacologic intervention, and monitoring.
Buprenorphine, a partial mu-opioid agonist and kappa-opioid antagonist, effectively treats opioid dependence.12 Its low intrinsic receptor efficacy results in a less-than-maximal opioid effect13 and a diminished risk of overdose, as compared with methadone. In nonpregnant adults, the effects of abrupt withdrawal of buprenorphine are minimal relative to the effects of withdrawal of full mu-opioid agonists.14,15 Buprenorphine’s pharmacologic advantages led to prospective open-label and controlled studies of its use in prenatal treatment,16–19 and the results of some of these studies suggested that neonates exposed to buprenorphine might be less likely to require treatment for NAS than those exposed to methadone.20 Recent studies of methadone and buprenorphine have had inconsistent results with respect to NAS outcomes.21–26 Given the calls to increase representation of pregnant women in medication research,27 we conducted the Maternal Opioid Treatment: Human Experimental Research (MOTHER) project, a multicenter, randomized, controlled trial comparing buprenorphine with methadone for the treatment of opioid-dependent pregnant patients.28
METHODS
STUDY SITES AND PARTICIPANTS
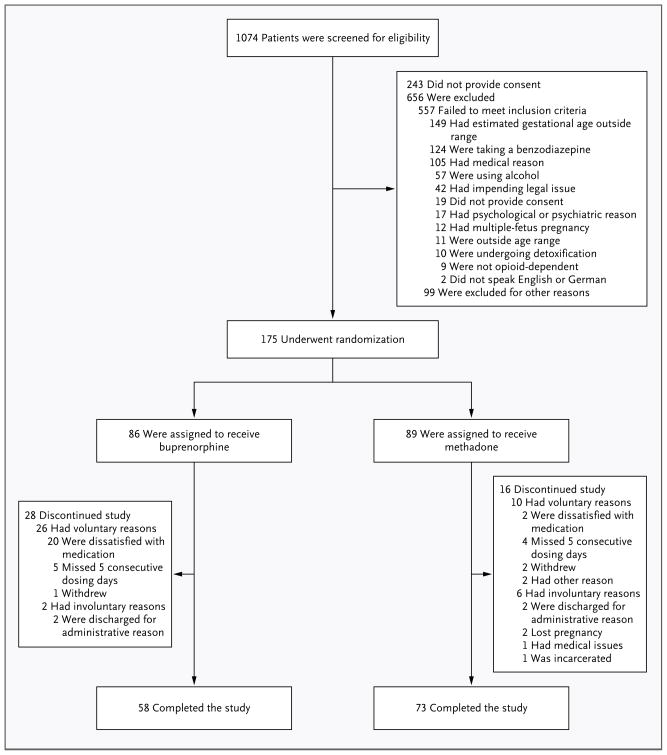
Between May 4, 2005, and October 31, 2008, opioid-dependent women between the ages of 18 and 41 years with a singleton pregnancy between 6 and 30 weeks of gestation (calculated on the basis of the last menstrual period and confirmed by ultrasonographic results) were screened and recruited at eight international sites — six in the United States and one each in Austria and Canada. Seven sites contributed randomized data; one site screened participants but did not complete randomization.
Women were eligible for participation in the study if they had no medical or other conditions contraindicating participation, were not subject to pending legal action that might prevent their participation, had no disorders related to the use of benzodiazepines or alcohol, and did not plan to give birth outside the hospital at the study site (Fig. 1). Study referral sources included community providers, self-referral, and the site’s treatment program.28–30
Figure 1.
Screening, Randomization, and Rate of Treatment Completion, According to Study Group.
Screening for eligibility consisted of a comprehensive battery of tests (see Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The screening tests were performed either at the time of treatment initiation (in the case of patients who were new to treatment) or after a patient’s request for a change in her established treatment (e.g., in the case of patients who were already being treated with a mu-opioid agonist and who agreed to randomization). Patients who were not eligible for participation in the study were so informed and transferred to standard care available at the site’s clinic or at a local community clinic.
Each site’s local institutional review board approved the study. All participants provided written informed consent at the time of screening. Buprenorphine tablets and the associated placebo were supplied by Reckitt Benckiser Health-care, Hull, United Kingdom. These tablets were distributed to U.S. study investigators by the National Institute on Drug Abuse. Schering-Plough distributed buprenorphine tablets and placebo to Austrian investigators. Neither Reckitt Benckiser Healthcare nor Schering-Plough had involvement in the study design; data collection, analysis, or interpretation; or manuscript preparation.
STUDY MEDICATIONS AND PATIENT CARE
Before randomization, all participants received rapid-release morphine sulfate as inpatients to achieve medical stabilization and to ease the transition to the double-blind medication.26,29,31 Qualifying participants underwent randomization and started the assigned study medication as inpatients.
A blinded, individualized dosing schedule was used for the study medications, and a double-blind method was used to implement dose-unit increases or decreases (with dose adjustments of 2 mg for buprenorphine and 5 or 10 mg for methadone). Dose adjustments entailed clinical decisions based on medication adherence, the participant’s request, urine toxicologic results, and self-reported symptoms of withdrawal or craving.26 Tablets of buprenorphine (Subutex, Reckitt Benckiser) were used to avoid prenatal exposure to naloxone. (Neither buprenorphine nor naloxone has been approved by the Food and Drug Administration or the European Medicines Agency for use during pregnancy.) A flexible dose range of 2 to 32 mg of buprenorphine in sublingual tablets was estimated to be equivalent to 20 to 140 mg of methadone on the basis of previously published data from clinical trials.32–34
Participants were required to receive daily medications under observation in the study clinic. They always received seven tablets (three in the size of an 8-mg tablet and four in the size of a 2-mg tablet) to place under the tongue for 5 minutes, or until the tablets dissolved. Each tablet contained buprenorphine or placebo. After receiving these tablets, participants received liquid containing methadone or placebo. Oral methadone and flavor-masking concentrates were diluted to provide the dose in a fixed volume (e.g., 40 ml at U.S. sites and 50 ml in Vienna). Methadone placebo was given in the same fixed volume and included the same flavor-masking concentrates as the active drug concentrate. All medications were dispensed through regulated hospital pharmacies or methadone clinics.
The study sites provided participants with comprehensive care. To promote drug abstinence, patients were given monetary vouchers in exchange for providing urine samples that were negative for opioids (other than buprenorphine and methadone), other illicit drugs, and misuse of prescription medications.26 On completion of the study, participants could receive locally available treatment.
EVALUATION FOR NAS
NAS assessment was performed for a minimum period of 10 days after birth. Hospitalized neonates were examined every 4 hours by trained staff. Neonates discharged from the hospital before postnatal day 10 were expected to reside with the mother in a residential setting, where the evaluation was continued. NAS scores were obtained twice daily, at least 8 hours apart, with the use of a modified Finnegan scale (called the MOTHER NAS scale), which includes 28 items11; 19 items were used for scoring and medication decisions. Scores on the modified scale range from 0 to 42, with higher scores indicating more severe withdrawal. Original NAS-item definitions,35 as well as the morphine medication protocol,26,36 were refined before data collection (Fig. 2 in the Supplementary Appendix); the study was conducted in accordance with the protocol.
An expert rater trained a highly experienced rater at each site; by the end of training, the site raters were required to obtain scores that were within 2 points of the expert rater’s scores. To maintain consistency in the reliability of the ratings at each site, every 6 months the expert rater provided a video of an infant undergoing NAS assessment. An intraclass correlation coefficient (ICC[2,2]) for the degree of agreement37 between the expert rater and the site rater was estimated; the lowest coefficient exceeded 0.94, indicating excellent agreement between the raters.
STUDY OUTCOMES AND ADVERSE EVENTS
The five primary neonatal outcome measures were the number of neonates requiring treatment for NAS, peak NAS score, total amount of morphine needed for treatment of NAS, length of hospital stay, and head circumference. The seven secondary neonatal outcomes were the number of days during which medication was given for NAS, weight and length at birth, preterm birth (defined as birth at <37 weeks of gestation), gestational age at delivery, and 1-minute and 5-minute Apgar scores. The nine secondary maternal outcomes were cesarean section, weight gain, abnormal fetal presentation during delivery, anesthesia during delivery, the results of drug screening at delivery, medical complications at delivery, study discontinuation, amount of voucher money earned for drug-negative tests, and number of prenatal obstetrical visits. Adverse events for all participants were categorized on the basis of the Medical Dictionary for Regulatory Activities (version 10.0) system of organ classes and predefined categories of events.
STATISTICAL ANALYSIS
Bonferroni’s principle was used to set the family-wise alpha level at 0.01 (nominal alpha level, 0.05 ÷ 5) for each of the five primary outcome measures at the time of the initial study design; an interim analysis requested by the data safety and monitoring board resulted in a recalculation of the alpha level on the basis of the O’Brien–Fleming spending function, such that the end-of-trial alpha level was 0.0091 for each primary outcome measure. Bonferroni’s principle was also used to set the family-wise alpha level at 0.003125 (nominal alpha level, 0.05 ÷ 16) for the secondary outcome measures.
There were two fixed-effect factors in all analyses: medication (buprenorphine vs. methadone) and site (U.S. urban [Baltimore; Philadelphia; Detroit; Providence, RI] vs. U.S. rural [Burlington, VT; Nashville] vs. European [Vienna]). Pooling the sites minimized the possibility that site heterogeneity would adversely effect the analyses.30 Poisson regression analyses were conducted for the total amount of morphine needed to treat NAS, neonatal length of stay in the hospital, number of days of treatment for NAS, estimated gestational age at delivery, amount of money earned for drug-negative tests, number of prenatal obstetrical visits, and Apgar scores at 1 minute and 5 minutes. Ordinary least-squares regression analyses were conducted for the peak score on the NAS scale during the assessment period, infant head circumference, and infant weight and length at birth. Logistic-regression analyses were conducted for the remaining dichotomous variables.
For medication effects, model-derived least-squares means are reported for normally distributed outcome variables, model-derived exponentiated estimated means for Poisson-distributed outcome variables, and odds ratios for the logistic regressions. To minimize the possibility that the effects attributed to the assigned medication might be due to differences in participant characteristics, the analyses were repeated with the inclusion of covariates selected on the basis of their potential associations with the outcome variables. (For details on covariates, see Table 1 in the Supplementary Appendix.)
RESULTS
CHARACTERISTICS OF THE STUDY PARTICIPANTS
A total of 16 of the 89 women in the methadone group (18%) and 28 of the 86 women in the buprenorphine group (33%) discontinued treatment before delivery (P = 0.02 with an alpha level of 0.003125 for other secondary maternal outcome measures). The baseline characteristics of participants in the two medication groups, including those who did not complete the study, are shown in Table 1. There were no significant between-group differences in these characteristics, including measures of substance use. Among the women who did not complete treatment, the mean (±SD) number of days in the study was 35.1±35.2 (range, 4 to 155) for those in the methadone group and 8.6±17.2 (range, 0 to 80) for those in the buprenorphine group; 8 participants in the buprenorphine group left the study on the first day. “Dissatisfaction” with the study medication was reported as the reason for discontinuation by 71% of participants in the buprenorphine group, as compared with only 13% of those in the methadone group (Fig. 1). The mean doses of methadone and buprenorphine at the time the participants left the study were 87.3±21.8 mg (range, 41.3 to 133.2) and 14.3±5.9 mg (range, 3.0 to 30.0), respectively.
Table 1.
Baseline Characteristics of Women in the Methadone and Buprenorphine Groups, According to Whether They Completed Treatment.*
| Characteristic | Completed Treatment | Did Not Complete Treatment | ||||
|---|---|---|---|---|---|---|
| Methadone (N = 73) | Buprenorphine (N = 58) | P Value | Methadone (N = 16) | Buprenorphine (N = 28) | P Value | |
| Age (yr) | 27.7±0.7 | 25.3±0.7 | 0.014 | 29.7±1.6 | 29.1±1.7 | 0.75 |
| Race (%)† | 0.26 | 0.26 | ||||
| White | 85 | 91 | 69 | 71 | ||
| Black | 14 | 3 | 31 | 29 | ||
| Other | 1 | 5 | ||||
| Estimated gestational age of fetus (wk) | 18.7±0.8 | 18.7±0.7 | 0.94 | 16.4±1.7 | 19.7±1.3 | 0.13 |
| Education (yr) | 11.3±0.3 | 11.3±0.2 | 0.91 | 11.6±0.4 | 11.3±0.3 | 0.47 |
| Employed (%) | 14 | 19 | 0.41 | 6 | 4 | 0.47 |
| Legal status, criminally unencumbered (%) | 80 | 88 | 0.20 | 69 | 71 | 0.26 |
| Married (%) | 15 | 9 | 0.26 | 13 | 18 | 0.31 |
| Treatment during the previous 30 days (%)‡ | ||||||
| Maintenance therapy with methadone or buprenorphine | 47 | 41 | 0.56 | 50 | 35 | 0.16 |
| Detoxification | 4 | 5 | 0.76 | 0 | 4 | 0.64 |
| Neither maintenance therapy nor detoxification | 52 | 54 | 0.80 | 47 | 70 | 0.17 |
| Current cigarette smoker (%) | 99 | 95 | 0.21 | 88 | 89 | 0.36 |
| Composite score on Addiction Severity Index§ | ||||||
| Drugs | 0.30±0.01 | 0.28±0.01 | 0.16 | 0.29±0.03 | 0.34±0.02 | 0.15 |
| Alcohol | 0±0.01 | 0.01±0.01 | 0.40 | 0±0.01 | 0.02±0.01 | 0.11 |
| Substance use | ||||||
| Cumulative lifetime (mo) | ||||||
| Heroin | 45.7±5.4 | 25.4±6.0 | 0.01 | 60.2±13.5 | 50.7±10.4 | 0.58 |
| Cocaine | 34.1±5.9 | 22.5±6.6 | 0.19 | 40.6±13.6 | 39.3±10.5 | 0.94 |
| Any alcohol | 23.5±4.3 | 13.4±4.8 | 0.13 | 26.6±11.5 | 17.6±8.8 | 0.54 |
| Benzodiazepines | 7.2±2.2 | 7.9±2.5 | 0.83 | 10.4±3.4 | 6.6±2.6 | 0.38 |
| Previous 30 days (days) | ||||||
| Heroin | 8.7±1.5 | 8.7±1.7 | 0.99 | 11.6±3.4 | 16.6±2.6 | 0.25 |
| Cocaine | 3.9±1.0 | 3.6±1.1 | 0.85 | 8.1±2.9 | 7.6±2.3 | 0.93 |
| Any alcohol | 0.3±0.1 | 0.2±0.2 | 0.40 | 0.3±0.5 | 1.1±0.4 | 0.23 |
| Benzodiazepines | 0.8±0.3 | 0.9±0.3 | 0.86 | 0.1±0.5 | 0.8±0.4 | 0.23 |
| Score for extent to which patient was troubled or bothered by drug problems in previous 30 days¶ | 2.8±0.2 | 2.5±0.2 | 0.20 | 2.6±0.4 | 2.7±0.3 | 0.74 |
Plus–minus values are means ±SE. Bonferroni’s principle was used to set the family-wise alpha level at 0.00227 for the between-group comparisons (nominal alpha level, 0.05 ÷ 22 for the number of variables for which inferential tests were conducted); there were no significant medication-group differences in either the sample of participants who completed the study or the sample that did not complete the study.
Race was self-reported. The chi-square goodness-of-fit statistic is reported for a dichotomized variable of white race versus nonwhite race.
Percentages for current treatment do not sum to 100 because the first two categories are not mutually exclusive and because of missing data. In the methadone group that completed treatment, the number of patients with missing data was 2 for maintenance therapy with methadone or buprenorphine and for neither maintenance therapy nor detoxification, whereas in the buprenorphine group that completed treatment, the number of patients with missing data was 1 for detoxification and for neither maintenance therapy nor detoxification. In the methadone group that did not complete treatment, the number of patients with missing data was 1 for detoxification and for neither maintenance therapy nor detoxification, whereas in the buprenorphine group that did not complete treatment, the number of patients with missing data was 1 for maintenance therapy with methadone or buprenorphine and detoxification, 2 for neither maintenance therapy nor detoxification, and 1 for both composite scores on the Addiction Severity Index and for all items on substance use. Patients whose current treatment was characterized as maintenance included those who elected induction and stabilization with an opioid agonist when entering treatment (one or more days before study screening) or who were already receiving maintenance treatment at the time of study screening.
Composite scores for the Addiction Severity Index range between 0 and 1, with higher scores indicating a more severe problem.
The question regarding the extent to which a patient was troubled or bothered by drug problems was answered on a 5-point Likert-type scale, with 0 indicating “not at all” and 4 indicating “extremely.”
Among the 131 participants who completed the study (i.e., gave birth while receiving double-blind study medication), there were no significant differences between the buprenorphine and methadone groups with respect to any of the baseline characteristics, including substance-use measures (P>0.01 for all comparisons, with an alpha level of 0.00227) (Table 1). Analyses of neonatal outcomes are based only on this sample of participants.
PRIMARY OUTCOMES
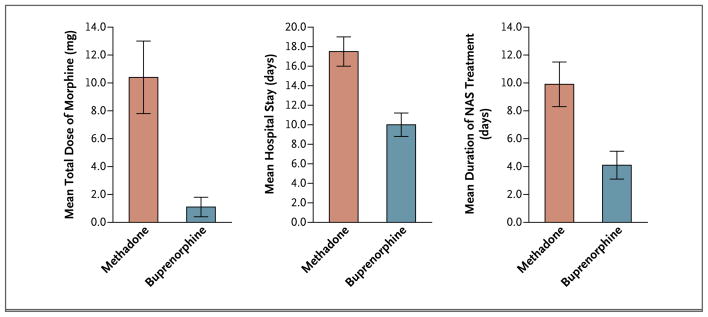
The percentage of neonates requiring NAS treatment did not differ significantly between groups (P = 0.26), nor did the groups differ significantly with respect to the peak NAS score (P = 0.04) or head circumference (P = 0.04). There were significant differences between groups for the other two primary outcome measures: the total amount of morphine needed for the treatment of NAS and the length of the hospital stay for neonates (Table 2 and Fig. 2, and Table 1 in the Supplementary Appendix). On average, neonates exposed to buprenorphine required 89% less morphine than did neonates exposed to methadone (mean total doses of 1.1 mg and 10.4 mg, respectively; P<0.0091 in accordance with prespecified thresholds for significance), and spent, on average, 43% less time in the hospital (10.0 vs. 17.5 days, respectively; P<0.0091). Both these outcome measures also differed significantly between the treatment groups when the analyses were adjusted for selected covariates (Table 1 in the Supplementary Appendix).
Table 2.
Primary and Secondary Outcomes in the Methadone and Buprenorphine Groups.*
| Outcome | Methadone (N = 73) | Buprenorphine (N = 58) | Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Primary outcomes | ||||
| Treated for NAS — no. (%) | 41 (57) | 27 (47) | 0.7 (0.2–1.8) | 0.26 |
| NAS peak score | 12.8±0.6 | 11.0±0.6 | 0.04 | |
| Total amount of morphine for NAS — mg | 10.4±2.6 | 1.1±0.7 | <0.0091† | |
| Duration of infant’s hospital stay — days | 17.5±1.5 | 10.0±1.2 | <0.0091† | |
| Infant’s head circumference — cm | 33.0±0.3 | 33.8±0.3 | 0.03 | |
| Secondary neonatal outcomes | ||||
| Duration of treatment for NAS — days | 9.9±1.6 | 4.1±1.0 | <0.003125† | |
| Weight at birth — g | 2878.5±66.3 | 3093.7±72.6 | 0.03 | |
| Length at birth — cm | 47.8±0.5 | 49.8±0.5 | 0.005 | |
| Preterm, <37 wk — no. (%) | 14 (19) | 4 (7) | 0.3 (0.1–2.0) | 0.07 |
| Gestational age at delivery — wk | 37.9±0.3 | 39.1±0.3 | 0.007 | |
| Apgar score | ||||
| 1 min | 8.0±0.2 | 8.1±0.2 | 0.87 | |
| 5 min | 9.0±0.1 | 9.0±0.1 | 0.69 | |
| Secondary maternal outcomes | ||||
| Cesarean section — no. (%) | 27 (37) | 17 (29) | 0.6 (0.2–2.0) | 0.23 |
| Maternal weight gain — kg | 8.6±1.0 | 8.3±0.9 | 0.80 | |
| Abnormal fetal presentation during delivery — no. (%) | 10 (14) | 3 (5) | 0.3 (0.0–2.4) | 0.09 |
| Analgesia during delivery — no. (%) | 60 (82) | 49 (85) | 1.1 (0.3–4.8) | 0.85 |
| Positive drug screen at delivery — no. (%) | 11 (15) | 5 (9) | 0.5 (0.1–2.7) | 0.27 |
| Medical complications at delivery — no. (%) | 37 (51) | 18 (31) | 0.5 (0.2–0.9) | 0.03 |
| Did not complete study — no. (%) | 16 (18) | 28 (33) | 2.6 (1.3–5.6) | 0.02 |
| Amount of voucher money earned for drug- negative tests — U.S. $ | 1,570.00±121.72 | 1,391.39±123.59 | 0.31 | |
| No. of prenatal obstetrical visits | 8.8±0.5 | 8.7±0.4 | 0.86 | |
Plus–minus values are means ±SE. In accordance with the alpha level chosen for the tests of significance, 99.09% confidence intervals (CIs) were used for the primary outcome measures, and 99.6825% CIs were used for the neonatal and maternal secondary outcome measures. The number of patients who underwent randomization was 175, the number who did not complete the study was 44, and the number who did complete the study was 131. A small percentage of data was missing. For four of the five primary outcomes, the number of patients with missing data was 1 in each medication group except for the outcome on length of hospital stay for neonates, for which no data were missing. For two of the seven secondary neonatal outcomes, the number of patients with missing data was 1 in each medication group for days treated for NAS and 1 in the methadone group for infant length at birth. For four of the nine secondary maternal outcomes, the number of patients with missing data in the methadone group was 2 for maternal weight gain, 2 for abnormal fetal presentation during delivery, 1 for positive drug screen at delivery, and 3 for amount of voucher money earned; the number of patients with missing data in the buprenorphine group was 4 for maternal weight gain, 1 for positive drug screen at delivery, and 1 for voucher money earned.
These P values were calculated in accordance with prespecified thresholds for significance.
Figure 2.
Mean Neonatal Morphine Dose, Length of Neonatal Hospital Stay, and Duration of Treatment for Neonatal Abstinence Syndrome.
SECONDARY OUTCOMES
One of the seven neonatal secondary outcome measures differed significantly between groups: neonates exposed to buprenorphine spent, on average, 58% less time in the hospital receiving medication for NAS than did those exposed to methadone (4.1 days vs. 9.9 days, P<0.003125 in accordance with prespecified thresholds for significance). This difference remained significant in analyses adjusted for selected covariates (Table 1 in the Supplementary Appendix). There were no significant between-group differences in any of the nine maternal secondary outcomes (Table 2, and Table 1 in the Supplementary Appendix).
SUBGROUP ANALYSES
To address the possibility that differences in neonatal outcomes between the two groups might be explained by higher average levels of opioid dependence in women who completed methadone treatment than in those who completed buprenorphine treatment, we performed post hoc analyses that excluded the 25 participants whose methadone dose at delivery exceeded 100 mg. The between-group differences in the amount of morphine required for the treatment of NAS and the length of the hospital stay remained significant (P<0.001 and P = 0.003, respectively). The difference in the secondary outcome of duration of hospitalization while infants were receiving medication was no longer significant (P = 0.01).
ADVERSE EVENTS
Assuming an alpha level of 0.05 (to maximize the detection of differences between medications with respect to adverse events), the methadone group had higher rates of nonserious maternal events overall (P = 0.003) and of nonserious maternal cardiovascular events in particular (P = 0.01). The two medication groups did not differ significantly with respect to any serious maternal or neonatal adverse events or any nonserious neonatal adverse events (Table 3).
Table 3.
Serious and Nonserious Adverse Events Occurring during the Study.*
| Adverse Event | Maternal | Neonatal | ||
|---|---|---|---|---|
| Methadone (N = 89) | Buprenorphine (N = 86) | Methadone (N = 73) | Buprenorphine (N = 58) | |
| number (percent) | ||||
|
Serious events | ||||
| Abnormal fetal health | 3 (3) | 0 | ||
| Abnormal laboratory values | 0 | 0 | 0 | 0 |
| Cardiovascular symptoms | 1 (1) | 0 | 2 (3) | 1 (2) |
| Gastrointestinal symptoms | 1 (1) | 1 (1) | 0 | 1 (2) |
| Genitourinary symptoms | 0 | 1 (1) | 0 | 1 (2) |
| Illicit drug use | 1 (1) | 1 (1) | ||
| Musculoskeletal symptoms | 0 | 0 | 0 | 1 (2) |
| Neurologic symptoms | 0 | 0 | 0 | 1 (2) |
| Obstetrical symptoms | 6 (7) | 2 (2) | 1 (1) | 1 (2) |
| Postsurgical problems | 0 | 0 | 0 | 1 (2) |
| Psychological problems | 1 (1) | 0 | ||
| Psychosocial problems | 1 (1) | 0 | ||
| Respiratory symptoms | 1 (1) | 0 | 2 (3) | 0 |
| Sexually transmitted diseases | 1 (1) | 0 | 0 | 0 |
| Skin conditions | 0 | 1 (1) | 0 | 0 |
| Sleep disturbances | 0 | 1 (1) | ||
| Other | 0 | 0 | 1 (1) | 1 (2) |
| Any serious adverse event | 14 (16) | 8 (9) | 6 (8) | 1 (2) |
|
Nonserious events | ||||
| Abnormal appetite | 2 (2) | 0 | 4 (6) | 1 (2) |
| Abnormal fetal health | 6 (7) | 4 (5) | ||
| Abnormal laboratory values | 10 (11) | 8 (9) | 0 | 0 |
| Blood-borne disorders | 5 (6) | 1 (1) | 0 | 1 (2) |
| Cardiovascular symptoms | 29 (33) | 14 (16) | 8 (11) | 4 (7) |
| Endocrinologic symptoms | 5 (6) | 3 (4) | 1 (1) | 1 (2) |
| Eye, ear, nose, or throat problems | 12 (14) | 15 (17) | 1 (1) | 1 (2) |
| Fever | 3 (3) | 2 (2) | 0 | 0 |
| Gastrointestinal symptoms | 60 (67) | 47 (55) | 5 (7) | 4 (7) |
| Genitourinary symptoms | 23 (26) | 16 (19) | 1 (1) | 0 |
| Hematopoietic or lymphatic symptoms | 14 (16) | 15 (17) | 17 (23) | 14 (24) |
| Illicit drug use | 10 (11) | 8 (9) | 3 (4) | 5 (9) |
| Dental problems | 22 (25) | 15 (17) | 1 (1) | 2 (4) |
| Musculoskeletal symptoms | 38 (43) | 28 (33) | 3 (4) | 1 (2) |
| Neuromuscular symptoms | 33 (37) | 29 (34) | 0 | 0 |
| Neurologic symptoms | 16 (18) | 12 (14) | 0 | 0 |
| Obstetrical problems | 29 (33) | 23 (27) | 3 (4) | 4 (7) |
| Postsurgical problems | 16 (18) | 8 (9) | 3 (4) | 0 |
| Psychological problems | 24 (27) | 21 (24) | ||
| Psychosocial problems | 4 (5) | 5 (6) | ||
| Respiratory symptoms | 29 (33) | 31 (36) | 14 (19) | 12 (21) |
| Sexually transmitted diseases | 8 (9) | 8 (9) | 1 (1) | 1 (2) |
| Skin conditions | 16 (18) | 12 (14) | 7 (10) | 2 (4) |
| Sleep disturbances | 24 (27) | 20 (23) | ||
| Somatic symptoms | 19 (21) | 9 (11) | ||
| Other | 4 (5) | 3 (4) | 2 (3) | 3 (5) |
| Any nonserious adverse event | 83 (93) | 66 (77) | 34 (47) | 29 (50) |
An alpha level of 0.05 was selected for each test of significance. Adverse events related to neonatal appetite included weight loss, need for nutritional support, and feeding intolerance. Cardiovascular events included rapid or slow heart rate and high or low blood pressure. Neonatal obstetrical events included asynclitic presentation and acrocyanosis. Psychosocial events included any stressful life event (e.g., stress surrounding moving, eviction, or death of a family member). A serious adverse event was defined as death or substantial risk of death of the mother or the infant or any medical event that a study investigator or the data and safety monitoring board judged to be serious because it might jeopardize the participant or might require intervention (e.g., hospitalization or extension of hospitalization). Two women in the methadone group had multiple serious adverse events (1 had a positive serologic test for syphilis, overnight hospitalization, and suspected premature rupture of fetal membrane; the other had lack of housing and depression), and 12 women in this group had a single serious adverse event (2 cases each of fetal-heart-rate deceleration, premature labor, and miscarriage and 1 case each of decreased blood flow to the fetus, pathological cardiotocographic deceleration, heroin and cocaine overdose, gastroenteritis requiring hospitalization, amniorrhexis, and pneumonia). Two women in the buprenorphine group had multiple serious adverse events (1 had multicystic kidney and positive drug-screening urinalysis leading to hospitalization; the other had vaginal bleeding and preterm labor), and 6 women in this group had a single serious adverse event (2 cases of vaginal bleeding and 1 case each of methicillin-resistant Staphylococcus aureus, gastric hemorrhage, hospitalization for removal of vaginal condyloma, and false labor). One neonate in the methadone group had multiple serious adverse events (2 surgeries for dextrocardia), and 4 neonates in this group had a single serious adverse event (1 case each of premature delivery [after which the neonate died], suspected apnea, respiratory distress, and cyanosis). One neonate in the buprenorphine group had all 8 serious adverse events listed in the table (e.g., multiple surgeries, renal failure, and hypoxic ischemic encephalopathy) and subsequently died.
DISCUSSION
In this randomized, double-blind trial, infants who had prenatal exposure to buprenorphine required significantly less morphine for the treatment of NAS, a significantly shorter period of NAS treatment, and a significantly shorter hospital stay than did infants with prenatal exposure to methadone. The superiority of buprenorphine over methadone did not extend to differences in the number of neonates requiring NAS treatment, peak NAS score, head circumference, any other neonatal outcome, or any maternal outcome.
Although buprenorphine was superior for two of the five primary outcomes among women who completed treatment, women who were taking buprenorphine were more likely to discontinue treatment. If patients with more severe opioid dependence were more likely to leave the buprenorphine group than the methadone group, this factor could have accounted for better outcomes in the buprenorphine group. However, the absence of significant between-group differences in baseline characteristics and in previous and current substance-use characteristics, both for women who completed treatment and for those who did not, suggests that differences in the rates of treatment completion are unlikely to explain the results. In addition, the significant differences between groups in the amount of morphine required for the treatment of NAS and the duration of the hospital stay remained significant in post hoc analyses that excluded participants receiving 100 mg or more of methadone daily.
Methadone has been the recommended standard of care for opioid-dependent pregnant women, and our double-blind study provides critical data on the outcomes of methadone treatment. Our findings support the safety and usefulness of methadone treatment for opioid dependence during pregnancy, and they also show that the treatment of opioid-dependent pregnant women with buprenorphine results in a clinically meaningful reduction in the severity of NAS in their neonates, as compared with methadone. The mechanisms responsible for this effect remain elusive; variability in the MDR1 genotype may influence the transport of methadone or buprenorphine to the fetus and thus the combination of NAS symptoms exhibited.38,39
Our finding that there was no significant difference between the treatment groups in rates of opioid use during treatment is consistent with observations in previous randomized trials involving nonpregnant patients that methadone and buprenorphine cause similar reductions in illicit opioid use.32 Moreover, the low levels of concomitant use of alcohol and illicit drugs, in combination with the nonsignificant differences in other maternal outcomes between the methadone and buprenorphine groups, suggest that these two medications, in the context of comprehensive care, do not differ markedly in terms of their effect on maternal treatment outcomes at delivery. Thus, the less severe NAS in neonates exposed to buprenorphine as compared with those exposed to methadone cannot be attributed to different effects of these agents on the outcomes of maternal opioid treatment.
These results must be considered in light of the markedly different rates of attrition, which were largely due to greater patient dissatisfaction with buprenorphine than with methadone. Although this finding is similar to the results of trials in nonpregnant patients receiving doses within similar acceptable therapeutic ranges,12 the reasons for the difference in attrition rates are unknown. It is possible that withdrawal was inadequate before the first dose of buprenorphine was administered or that buprenorphine induction was too slow.40,41 In both cases, administering the initial induction dose in smaller increments throughout the day might reduce the dropout rate.42 It is also possible that there is individual variation in the absorption of sublingual buprenorphine tablets. Another possible explanation is that an abrupt cessation of treatment may be more comfortable for patients taking buprenorphine than for those taking methadone because of the milder effects of withdrawal with buprenorphine.43 Buprenorphine may have less potent agonistic effects than methadone in mitigating craving and other symptoms of withdrawal, especially in patients who are highly dependent on opioids. Whatever the reasons, the fact that two primary outcomes remained significant in post hoc analyses omitting participants whose methadone dose at delivery exceeded 100 mg lends support to our general conclusions, particularly given the lost power associated with removing 19% of our sample (25 of 131 participants).
The greater rate of satisfaction with methadone affirms the important role it plays in treating pregnant women who are dependent on opioids. Moreover, given the partial agonistic activity of buprenorphine and its ceiling effect at maximal doses, it will not be the optimal treatment for all pregnant patients with a dependency on opioids. Further research is needed to assess the effectiveness of methods intended to reduce buprenorphine-specific attrition and to examine factors that may predict maternal and neonatal responses to each medication (e.g., pharmacogenomics44), making it feasible to identify subpopulations of pregnant patients who are more likely to have a response to one medication than to the other.
In summary, our findings are consistent with the use of buprenorphine as an alternative to methadone for the treatment of opioid dependency during pregnancy. Although there were no significant differences in overall rates of NAS among infants exposed to buprenorphine and those exposed to methadone, the benefits of buprenorphine in reducing the severity of NAS among neonates with this complication suggest that it should be considered a first-line treatment option in pregnancy. In selecting a course of treatment, however, clinicians should take into account the possibility of reduced adherence and the ceiling effect of this medication as compared with methadone.
Supplementary Material
Acknowledgments
Supported by grants from the National Institute on Drug Abuse (R01 DA015778, to Brown University; R01 DA015764, to Johns Hopkins University; R01 DA018417, to the Medical University of Vienna; R01DA015738, to Thomas Jefferson University; R01 DA015741, to the University of Toronto; R01 DA 018410 and M01 RR109, to the University of Vermont; R01 DA 017513 and M01 RR00095, to Vanderbilt University; and R01DA15832, to Wayne State University).
We thank the patients for their participation in this study; the coinvestigators, clinical and research staff, and members of the Data and Safety Monitoring Board for their effort and dedication to this study; and Reckitt Benckiser Healthcare, Hull, United Kingdom, for providing the buprenorphine and placebo product through the NIDA.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Hulse GK, Milne E, English DR, Holman CD. The relationship between maternal use of heroin and methadone and infant birth weight. Addiction. 1997;92:1571–9. [PubMed] [Google Scholar]
- 2.Kandall SR, Albin S, Gartner LM, Lee KS, Edelman A, Lowinson J. The narcotic-dependent mother: fetal and neonatal consequences. Early Hum Dev. 1977;1:159–69. doi: 10.1016/0378-3782(77)90017-2. [DOI] [PubMed] [Google Scholar]
- 3.Messinger DS, Bauer CR, Das A, et al. The Maternal Lifestyle Study: cognitive, motor, and behavioral outcomes of cocaine-exposed and opiate-exposed infants through three years of age. Pediatrics. 2004;113:1677–85. doi: 10.1542/peds.113.6.1677. [DOI] [PubMed] [Google Scholar]
- 4.Winklbaur B, Kopf N, Ebner N, Jung E, Thau K, Fischer G. Treating pregnant women dependent on opioids is not the same as treating pregnancy and opioid dependence: a knowledge synthesis for better treatment for women and neonates. Addiction. 2008;103:1429–40. doi: 10.1111/j.1360-0443.2008.02283.x. [DOI] [PubMed] [Google Scholar]
- 5.Lester BM, Andreozzi L, Appiah L. Substance use during pregnancy: time for policy to catch up with research. Harm Reduct J. 2004;1:5–49. doi: 10.1186/1477-7517-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lifschitz MH, Wilson GS, Smith EO, Desmond MM. Factors affecting head growth and intellectual function in children of drug addicts. Pediatrics. 1985;75:269–74. [PubMed] [Google Scholar]
- 7.Kandall SR. Improving treatment for drug-exposed infants. Rockville, MD: Department of Health and Human Services; 1993. [Google Scholar]
- 8.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3:CD002209. doi: 10.1002/14651858.CD002209.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones HE, O’Grady KE, Malfi D, Tuten M. Methadone maintenance vs. methadone taper during pregnancy: maternal and neonatal outcomes. Am J Addict. 2008;17:372–86. doi: 10.1080/10550490802266276. [DOI] [PubMed] [Google Scholar]
- 10.Kaltenbach K, Berghella V, Finnegan L. Opioid dependence during pregnancy: effects and management. Obstet Gynecol Clin North Am. 1998;25:139–51. doi: 10.1016/s0889-8545(05)70362-4. [DOI] [PubMed] [Google Scholar]
- 11.Finnegan LP, Kaltenbach K. Neonatal abstinence syndrome. In: Hoekelman RA, Friedman SB, Nelson NM, et al., editors. Primary pediatric care. 2. St. Louis: Mosby; 1992. pp. 1367–78. [Google Scholar]
- 12.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;2:CD002207. doi: 10.1002/14651858.CD002207.pub3. [DOI] [PubMed] [Google Scholar]
- 13.Walsh SL, Preston KL, Bigelow GE, Stitzer ML. Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–72. [PubMed] [Google Scholar]
- 14.Jasinski DR, Pevnick JS, Griffith JD. Human pharmacology and abuse potential of the analgesic buprenorphine: a potential agent for treating narcotic addiction. Arch Gen Psychiatry. 1978;35:501–16. doi: 10.1001/archpsyc.1978.01770280111012. [DOI] [PubMed] [Google Scholar]
- 15.Mello NK, Mendelson JH. Buprenorphine suppresses heroin use by heroin addicts. Science. 1980;207:657–9. doi: 10.1126/science.7352279. [DOI] [PubMed] [Google Scholar]
- 16.Fischer G, Johnson RE, Eder H, et al. Treatment of opioid-dependent pregnant women with buprenorphine. Addiction. 2000;95:239–44. doi: 10.1046/j.1360-0443.2000.95223910.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson RE, Jones HE, Jasinski DR, et al. Buprenorphine treatment of pregnant opioid-dependent women: maternal and neonatal outcomes. Drug Alcohol Depend. 2001;63:97–103. doi: 10.1016/s0376-8716(00)00194-0. [DOI] [PubMed] [Google Scholar]
- 18.Ebner N, Rohrmeister K, Winklbaur B, et al. Management of neonatal abstinence syndrome in neonates born to opioid maintained women. Drug Alcohol Depend. 2007;87:131–8. doi: 10.1016/j.drugalcdep.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 19.Schindler SD, Eder H, Ortner R, Rohrmeister K, Langer M, Fischer G. Neonatal outcome following buprenorphine maintenance during conception and throughout pregnancy. Addiction. 2003;98:103–10. doi: 10.1046/j.1360-0443.2003.00245.x. [DOI] [PubMed] [Google Scholar]
- 20.Hytinantti T, Kahila H, Renlund M, et al. Neonatal outcome of 58 infants exposed to maternal buprenorphine in utero. Acta Paediatr. 2008;97:1040–4. doi: 10.1111/j.1651-2227.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RE, Jones HE, Fischer G. Use of buprenorphine in pregnancy: patient management and effects on the neonate. Drug Alcohol Depend. 2003;70:S87–S101. doi: 10.1016/s0376-8716(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 22.Lejeune C, Simmat-Durand L, Gourarier L, Aubisson S. Prospective multi-center observational study of 260 infants born to 259 opiate-dependent mothers on methadone or high-dose buprenophine substitution. Drug Alcohol Depend. 2006;82:250–7. doi: 10.1016/j.drugalcdep.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Bakstad B, Sarfi M, Welle-Strand GK, Ravndal E. Opioid maintenance treatment during pregnancy: occurrence and severity of neonatal abstinence syndrome: a national prospective study. Eur Addict Res. 2009;15:128–34. doi: 10.1159/000210042. [DOI] [PubMed] [Google Scholar]
- 24.Kakko J, Heilig M, Sarman I. Buprenorphine and methadone treatment of opiate dependence during pregnancy: comparison of fetal growth and neonatal outcomes in two consecutive case series. Drug Alcohol Depend. 2008;96:69–78. doi: 10.1016/j.drugalcdep.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Fischer G, Ortner R, Rohrmeister K, et al. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101:275–81. doi: 10.1111/j.1360-0443.2006.01321.x. [DOI] [PubMed] [Google Scholar]
- 26.Jones HE, Johnson RE, Jasinski DR, et al. Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome. Drug Alcohol Depend. 2005;79:1–10. doi: 10.1016/j.drugalcdep.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Goldkind SF, Sahin L, Gallauresi B. Enrolling pregnant women in research — lessons from the H1N1 influenza pandemic. N Engl J Med. 2010;362:2241–3. doi: 10.1056/NEJMp1003462. [DOI] [PubMed] [Google Scholar]
- 28.Jones HE, Martin PR, Heil SH, et al. Treatment of opioid-dependent pregnant women: clinical and research issues. J Subst Abuse Treat. 2008;35:245–59. doi: 10.1016/j.jsat.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones HE, Johnson RE, Jasinski DR, Milio L. Randomized controlled study transitioning opioid-dependent pregnant women from short-acting morphine to buprenorphine or methadone. Drug Alcohol Depend. 2005;78:33–8. doi: 10.1016/j.drugalcdep.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 30.Unger AS, Martin PR, Kaltenbach K, et al. Clinical characteristics of Central European and North American samples of pregnant women screened for opioid agonist treatment. Eur Addict Res. 2010;16:99–107. doi: 10.1159/000284683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stine SM, Heil SH, Kaltenbach K, et al. Characteristics of opioid-using pregnant women who accept or refuse participation in a clinical trial: final screening results from the MOTHER study. Am J Drug Alcohol Abuse. 2009;35:429–33. doi: 10.3109/00952990903374080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson RE, Chutuape MA, Strain EC, Walsh SL, Stitzer ML, Bigelow GE. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–7. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 33.Ling W, Wesson DR, Charuvastra C, Klett J. A controlled trial comparing buprenorphine and methadone maintenance in opioid dependence. Arch Gen Psychiatry. 1996;53:401–7. doi: 10.1001/archpsyc.1996.01830050035005. [DOI] [PubMed] [Google Scholar]
- 34.Ling W, Wesson DR. Clinical efficacy of buprenorphine: comparisons to methadone and placebo. Drug Alcohol Depend. 2003;70(Suppl):S49–S57. doi: 10.1016/s0376-8716(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 35.D’Apolito K. A scoring system for assessing neonatal abstinence syndrome. Seattle: University of Washington, School of Nursing; 1994. [Google Scholar]
- 36.Jansson LM, Velez M, Harrow C. The opioid-exposed newborn: assessment and pharmacologic management. J Opioid Manag. 2009;5:47–55. [PMC free article] [PubMed] [Google Scholar]
- 37.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psychol Bull. 1979;86:420–8. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 38.Levran O, O’Hara K, Peles E, et al. ABCB1 (MDR1) genetic variants are associated with methadone doses required for effective treatment of heroin dependence. Hum Mol Genet. 2008;17:2219–27. doi: 10.1093/hmg/ddn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hemauer SJ, Patrikeeva SL, Nanovskaya TN, Hankins GD, Ahmed MS. Opiates inhibit paclitaxel uptake by P-glycoprotein in preparations of human placental inside-out vesicles. Biochem Pharmacol. 2009;78:1272–8. doi: 10.1016/j.bcp.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104:1193–200. doi: 10.1111/j.1360-0443.2009.02627.x. [DOI] [PubMed] [Google Scholar]
- 41.Fischer G, Gombas W, Eder H, et al. Buprenorphine versus methadone maintenance for the treatment of opioid dependence. Addiction. 1999;94:1337–47. doi: 10.1046/j.1360-0443.1999.94913376.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosado J, Walsh SL, Bigelow GE, Strain EC. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend. 2007;90:261–9. doi: 10.1016/j.drugalcdep.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mattick RP, Ali R, White JM, O’Brien S, Wolk S, Danz C. Buprenorphine versus methadone maintenance therapy: a randomized double-blind trial with 405 opioid-dependent patients. Addiction. 2003;98:441–52. doi: 10.1046/j.1360-0443.2003.00335.x. [DOI] [PubMed] [Google Scholar]
- 44.Yuferov V, Levran O, Proudnikov D, Nielsen DA, Kreek MJ. Search for genetic markers and functional variants involved in the development of opiate and cocaine addiction and treatment. Ann N Y Acad Sci. 2010;1187:184–207. doi: 10.1111/j.1749-6632.2009.05275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.