Scientific Abstract
Elevated whole blood serotonin (5HT), or hyperserotonemia, is a common biomarker in autism spectrum disorder (ASD). The integrin β3 receptor subunit gene (ITGB3) is a quantitative trait locus for whole blood 5-HT levels. Recent work shows that integrin β3 interacts with the serotonin transporter (SERT) in both platelets and in the midbrain. Furthermore, multiple studies have now reported gene-gene interaction between the integrin β3 and SERT genes in association with ASD. Given the lack of previous data on the impact of integrin β3 on brain or behavioral phenotypes, we sought to compare mice with decreased or absent expression of the integrin β3 receptor subunit (Itgb3 +/- and -/-) with wildtype littermate controls in behavioral tasks relevant to ASD. These mice did not show deficits in activity level in the Open Field or anxiety-like behavior on the Elevated Plus Maze, two potential confounds in the evaluation of mouse social behavior. In the three-chamber social test, mice lacking integrin β3 were shown to have normal sociability but did not show a preference for social novelty. Importantly, the absence of integrin β3 did not impair olfaction or the ability to recall familiar social odors. Additionally, mice lacking integrin β3 showed increased grooming behavior in novel environments. These preliminary studies reveal altered social and repetitive behavior in these mice, which suggests that the integrin β3 subunit may be involved in brain systems relevant to ASD. Further work is needed to fully characterize these behavioral changes and the underlying brain mechanisms.
Keywords: Autism, Genetic, Integrin, Cell Adhesion, Serotonin, Social Memory, Grooming, Obsessive Compulsive Disorder
Introduction
Autism spectrum disorder (ASD) is characterized by deficits in communication and social behaviors coupled with the presence of restricted and repetitive behaviors (American Psychiatric Association, 2000). As a behaviorally defined syndrome without clear boundaries, ASD presents a diagnostic challenge to both clinicians and researchers (Constantino & Todd, 2003; Risi, et al., 2006). Nevertheless, twin studies suggest a strong genetic component to autism susceptibility (Bailey, et al., 1995; Folstein & Rutter, 1977). Linkage and association studies have provided some evidence of common susceptibility alleles in ASD, pointing primarily to genes encoding synaptic proteins, but this evidence has been somewhat inconsistent across studies (Buxbaum, 2009; Geschwind, 2009; O'Roak & State, 2008). This lack of consistency is not surprising given the striking heterogeneity of cognitive and behavioral profiles in autism (Munson, et al., 2008; Richler, et al.). Multiple uncommon copy number and de novo variants have also been identified in ASD (Berkel, et al., 2010; Glessner, et al., 2009; Jamain, et al., 2003; Kim, et al., 2008; Laumonnier, et al., 2004; Marshall, et al., 2008; Pinto, et al., 2010; Weiss, et al., 2008), including some that disrupt synaptic adhesion molecules and scaffolding proteins in individuals.
In the presence of abundant clinical heterogeneity, heritable biomarkers may allow the identification of susceptibility genes specific to a subgroup of individuals with ASD. One such biomarker is elevated whole blood serotonin (5-HT), or hyperserotonemia, which occurs in approximately 30% of individuals with ASD (Cook & Leventhal, 1996; Mulder, et al., 2004; Schain & Freedman, 1961). In the blood, 5-HT is contained almost exclusively in platelets, which take up 5-HT as they pass through the enteric circulation (Anderson, et al., 1987). Elevated whole blood 5-HT levels are also seen in first-degree relatives of probands with ASD (Abramson, et al., 1989; Cross, et al., 2008; Cuccaro, et al., 1993; Leboyer, et al., 1999; Leventhal, et al., 1990). In the Hutterites, a large founder population, whole blood 5-HT levels approach a heritability of 1.0 (Abney, et al., 2001). The integrin β3 gene (ITGB3) and the vitamin D receptor gene (VDR) in an initial genome-wide association study to map whole blood 5-HT quantitative trait loci in the Hutterites (Weiss, et al., 2004). Given that males are much more likely to be affected with ASD (Chakrabarti & Fombonne, 2001), a follow-up study searched for sex-specific loci, finding a stronger association with ITGB3 in males and also finding male-specific association with SLC6A4, which encodes the serotonin transporter (SERT) (Weiss, et al., 2005).
Subsequent studies have considered how integrin β3 may influence whole blood 5-HT levels and autism susceptibility. Two studies confirmed association between polymorphisms in ITGB3 and whole blood 5-HT levels (Coutinho, et al., 2007; Weiss, Kosova, et al., 2006). Carneiro and colleagues in the Blakely lab demonstrated an interaction between integrin β3 and SERT in the platelet. Their proteomic screen for proteins that interact with the C terminus of SERT identified a physical and functional interaction with integrin β3 that may explain the earlier genetic findings (Carneiro, et al., 2008). We have now also identified a similar protein-protein interaction in the midbrain (JV, MC, RB, AC, paper in preparation). Two groups have reported nominally significant association at different ITGB3 single nucleotide polymorphisms (SNPs) in ASD (Ma, et al.; Weiss, Kosova, et al., 2006). Reflecting the interaction between integrin β3 and SERT, however, the most relevant analysis is likely to consider gene-gene interactions. Four independent populations have now reported interaction between ITGB3 and SLC6A4 in association with autism, albeit with different combinations of polymorphisms (Coutinho, et al., 2007; Ma, et al.; Mei, et al., 2007; Weiss, Ober, et al., 2006).
In the context of multiple lines of data pointing to ITGB3 in association with an autism biomarker and perhaps with autism susceptibility itself, we endeavored to understand the role of the corresponding protein in the mouse, focusing on the social and repetitive behaviors that may parallel autistic traits. A knockout mouse that completely lacks expression of a given gene is the classic tool for dissecting the biological role of the corresponding protein. This presents a complication for understanding the role of integrin β3 in social and repetitive behavior, however, because the corresponding protein is a component of the fibrinogen receptor, integrin αIIbβ3. Complete disruption of the integrin β3 gene in the mouse causes impaired platelet aggregation, prolonged bleeding times, and hemorrhages in skin, gastrointestinal tract, and placenta (Hodivala-Dilke, et al., 1999). Hemorrhage leads to a significant loss of homozygous animals in the first 3 weeks of life and throughout the lifespan (Hodivala-Dilke, et al., 1999). In addition to αIIbβ3, the integrin β3 knockout mouse also cannot form the αvβ3 receptor, important in placental implantation, bone resorption, and angiogenesis. Due to osteoclast dysfunction, homozygous knockout mice develop osteosclerosis (McHugh, et al., 2000). None of the reported phenotypes from the integrin β3 null (-/-) mouse are reported in the hemizygous (+/-) mouse that maintains 50% expression.
A variety of behavioral tasks have been proposed as relevant to autism (Blundell, et al.; Etherton, et al., 2009; Lijam, et al., 1997; Moretti, et al., 2005; Moy, Nadler, Poe, et al., 2008; Moy, et al., 2007; Nakatani, et al., 2009; Roullet, et al.; Silverman, et al.; Young, et al.; Zhao, et al.). Given the bleeding phenotype in the Itgb3 -/- animals, we avoided tests of direct social interaction that could include aggression. The 3-chamber sociability and preference for social novelty test (Moy, et al., 2004) allows the evaluation of a test mouse's preferences for proximity to a stimulus mouse while restraining the stimulus mouse within a wire cage. Activity level, anxiety-like behavior, and olfactory memory are potential confounds to this test, which we evaluated in the Open Field, Elevated Plus Maze, and Olfactory Habituation / Dishabituation test, respectively. Over the course of this behavioral testing, we observed that the Itgb3 -/- animals appeared to exhibit increased grooming behavior, which was evaluated using both a standard 10-minute observation of grooming behavior and an extended automated monitoring of a subset of animals in their home cage environment. These initial behavioral analyses are intended as a preliminary assessment of ASD-relevant behavior in mice lacking integrin β3 and motivate further study of this protein using conditional gene knockout strategies to remove the potential confounds of peripheral phenotypes in these mice.
Methods
Mice
All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All procedures were approved by the Vanderbilt University Institutional Animal Care and Use Committee. All mice used in experiments were male progeny of pairings of Itgb3 +/- mice on a C57BL/6J background (backcrossed at least 7 generations from 129S2/SvPas according to the Jackson Laboratory, Bar Harbor, ME) that were housed with 3-5 mixed genotype littermate animals per cage. Mice used within each experiment were greater than 8 weeks of age and were matched by birth date to test animals varying by no more than 4 weeks.
Order of Behavioral Experiments
The first cohort was composed of all littermates born sequentially to Itgb3 +/- breeding pairs and underwent behavioral testing in the following sequence: elevated plus maze, open field testing, tail suspension test (TST, results to be reported elsewhere), 3-chamber sociability and preference for social novelty test. No Itgb3 -/- mice were run on the TST due to concerns about possible bleeding. Mice with signs of active or recent bleeding were excluded from experiments. Mice with open or infected wounds, hunched posture, or difficulty ambulating were euthanized, as well as any mice that would have been singly housed after loss of their cagemates. Of the 8 Itgb3 -/- mice that began in this cohort, 6 had died spontaneously, were euthanized, or were excluded due to signs of bleeding at the time of the 3-chamber sociability and preference for social novelty test. Due to this loss of sample size, 4 additional -/- animals that were the product of the same +/- breeding pairs were also evaluated on the 3-chamber sociability and preference for social novelty test. A second cohort of animals underwent olfactory habituation / dishabituation testing. When grooming was noted to be increased over the course of this testing, a subset of these animals underwent formal testing of grooming behavior along with additional matched littermate animals from the same +/- breeding pairs to increase the sample size.
Elevated Plus Maze (EPM)
The plus maze consists of four arms, 10 × 10 × 30 cm, connected in a plus configuration and elevated approximately 50 cm. Two of the arms have walls, 20 cm high, and two arms have no walls. This test was performed under full light conditions in the last 6 hours of the light period. Mice were placed in the center of the maze at the beginning of the 5-minute test session. The position of the mouse was tracked three times per second, and data were analyzed in real time on a Macintosh computer using Image EP (Miyakawa, et al., 2001) (O'Hara & Co., Tokyo, Japan), a modification of the public domain NIH ImageJ software (Abramoff, et al., 2004). The ratio of time spent in the open arms divided by total time spent in either open or closed arms was analyzed by one-way ANOVA as the primary measure of anxiety-like behavior. The number of entries was analyzed by one-way ANOVA as a secondary measure of activity.
Open Field Behavior
Exploratory locomotor activity was evaluated using activity monitors measuring 27.9 × 27.9 cm (MED Associates, Georgia, VT). Each apparatus contains 16 photocells in each horizontal direction, as well as 16 photocells elevated 4.0 cm to measure rearing. This test was performed under full light conditions in the last 6 hours of the light period. Mice were not previously habituated to the locomotor activity chamber. Mice were placed in the monitors for 15 minutes and allowed to explore freely. Horizontal beam breaks were automatically recorded and represented as distance travelled. Total distance travelled was analyzed by Kruskal-Wallis test as the primary measure of activity. Time spent ambulating, time spent at rest, and time spent vertical rearing were measured as secondary dependent variables and compared by one-way ANOVA.
Three-chamber sociability and preference for social novelty test (Moy, et al., 2004)
Social behavior was evaluated in a 3-chamber polycarbonate apparatus with 4″ sliding gates separating the 7″ × 9″ chambers. The test mouse was initially allowed to explore all 3 chambers for 10 minutes to acclimate to the apparatus. The test mouse was then returned to the center chamber with the gates closed. For the sociability portion of the test, a stimulus mouse (novel mouse) was introduced inside a wire pencil cup (Spectrum Diversified Designs, Inc., Streetsboro, OH) in one side chamber with an empty pencil cup (novel object) introduced in the opposite side chamber. The gates were then opened, and the test mouse was allowed to explore all 3 chambers for 10 minutes. The test mouse was then returned to the center chamber with the gates closed. For the preference for social novelty portion of the test, the empty pencil cup was replaced by a second stimulus mouse (novel mouse), with the original stimulus mouse now becoming a familiar social stimulus (familiar mouse). The gates were again opened, and the test mouse was allowed to explore all 3 chambers for 10 minutes. Sessions were coded for the time that the test mouse spent within 1 cm of each stimulus, which was analyzed by two-way repeated measures ANOVA with Bonferroni post-tests for each portion of the test.
Olfactory Habituation / Dishabituation Test (Yang & Crawley, 2009)
Water, almond, and banana extract were used as non-social odors, with each extract diluted 1:100 in water. Two dirty cages housing unfamiliar novel male mice of the same strain were used to generate two novel social odors. Each test mouse habituated to a new cage for 60 minutes in the testing area. After habituation, each non-social odor was introduced to the cage by dipping the end of a cotton swab in the odor, and then suspending the cotton swab about 4 – 5 cm from the bottom of the cage. Social odors were acquired by swiping the bottom of one of the two dirty cages identified earlier. Each odor was administered in three trials of two minutes per trial with one minute between trials. The odors were presented in the following order: water, almond, banana, social scent 1, and social scent 2. After completion, the mouse was returned to its home cage. An observer with a stopwatch recorded the seconds each mouse spent within 2 cm of each odor stimulus, which was analyzed by two-way repeated measures ANOVA with Bonferroni post-tests for each portion of the test.
Self-grooming in a novel environment (McFarlane, et al., 2008)
Self-grooming behavior was scored by placing each mouse in an empty cage, without bedding. Each mouse was allowed to habituate to this new environment for 10 minutes. An observer with a stopwatch recorded time spent grooming over the next 10 minutes. Total grooming time over 10 minutes was analyzed by Kruskal-Wallis test with Dunn's posttest.
Home Cage Grooming (Steele, et al., 2007)
Individual mice of each genotype were video recorded in their home cage for 24 hours while maintaining their 12:12 light-dark schedule. Automated video analysis was conducted using HomeCageScan (Clever Sys, Inc., Reston, VA) to measure grooming behavior. Time spent grooming in 24 hours was analyzed by one-way ANOVA.
Results
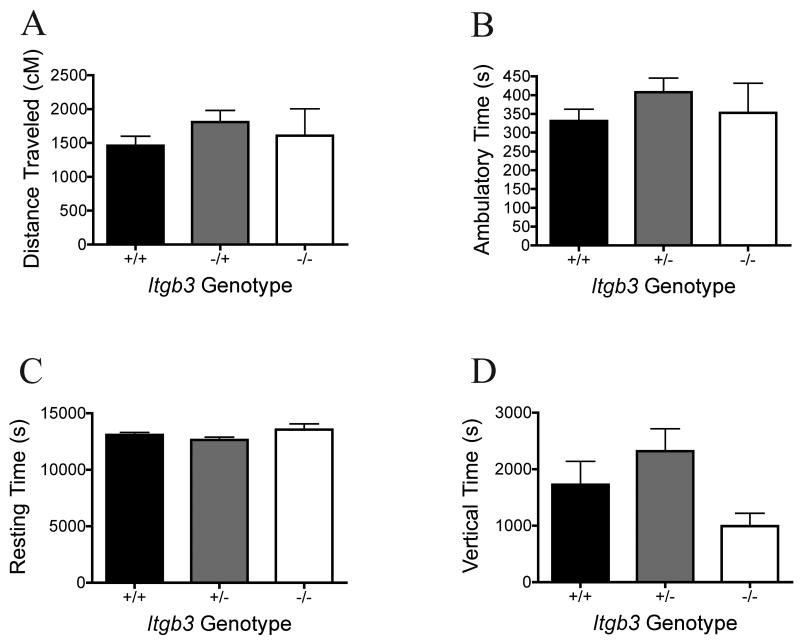
The Itgb3 knockout animals showed no difference in distance traveled, the primary dependent variable, during the 15 minutes spent in the Open Field (OF) (Fig 1A, Kruskal-Wallis test statistic 1.96, P = 0.38; non-parametric analysis chosen due to trend for unequal variances on Bartlett's test, P = 0.06, n = 13 +/+, 23 +/-, 8 -/-). Similarly, no difference was seen on the secondary variables in the OF, including ambulatory time (Fig 1B, one-way ANOVA, F = 0.91, P = 0.41), resting time (Fig 1C, one-way ANOVA, F = 2.10, P = 0.14), and vertical time (Fig 1E, Kruskal-Wallis 3.47, P = 0.18); although significantly decreased variability was seen in Itgb3 -/- animals on vertical time (Bartlett's test for unequal variances P = 0.02).
Figure 1. Open Field Activity.
Mean and standard error of the mean are shown by genotype (13 +/+, 23 +/-, 8 -/-) for cumulative data over 15 minutes spent in the activity chamber for each genotype. A: Distance traveled in cm. B: Time spent in ambulatory movement. C: Time spent not moving. D: Time spent in the vertical position. No significant genotype effects were detected by one-way ANOVA on any of the measures of activity.
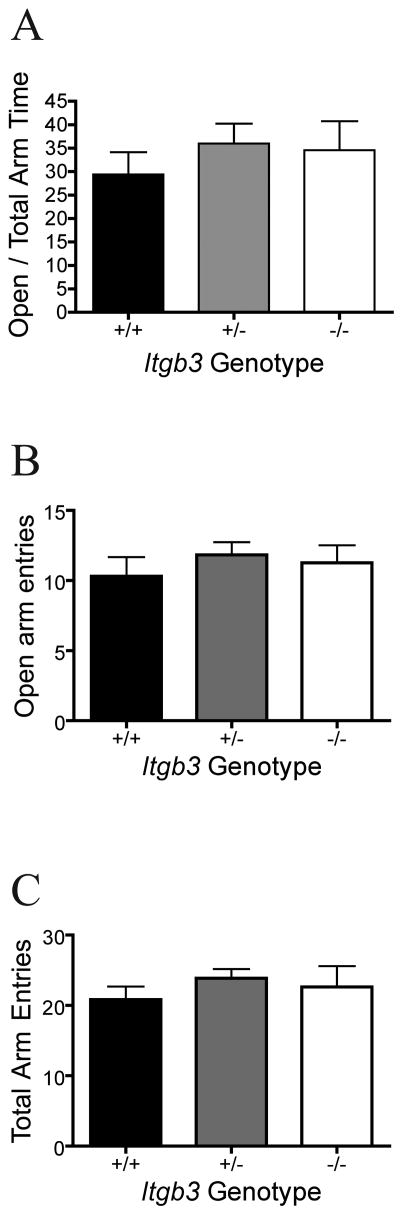
In the Elevated Plus Maze (EPM), the Itgb3 knockout animals showed no difference on the primary dependent variable, the ratio of time spent in the open arms of in contrast with the total time spent in either the open or closed arms (Fig 2A, one-way ANOVA, F = 0.51, P = 0.60, n = 13 +/+, 23 +/-, 8 -/-). Likewise, there was no difference in the number of entries to the open arms (Fig 2B, one-way ANOVA, F = 0.49, P = 0.61). Consistent with the activity data from the OF, there was no difference in total entries on the EPM (Fig 2C, one-way ANOVA, F = 0.84, P = 0.44).
Figure 2. Elevated Plus Maze.
Mean and standard error of the mean are shown by genotype (13 +/+, 23 +/-, 8 -/-) for cumulative data over 5 minutes spent in the elevated plus maze. A: Ratio of time spent in the open arms divided by the time spent in either the open or closed arms. B: Total entries into the open arms. C: Total entries into either open or closed arms. No significant genotype effects were detected by one-way ANOVA on any measure.
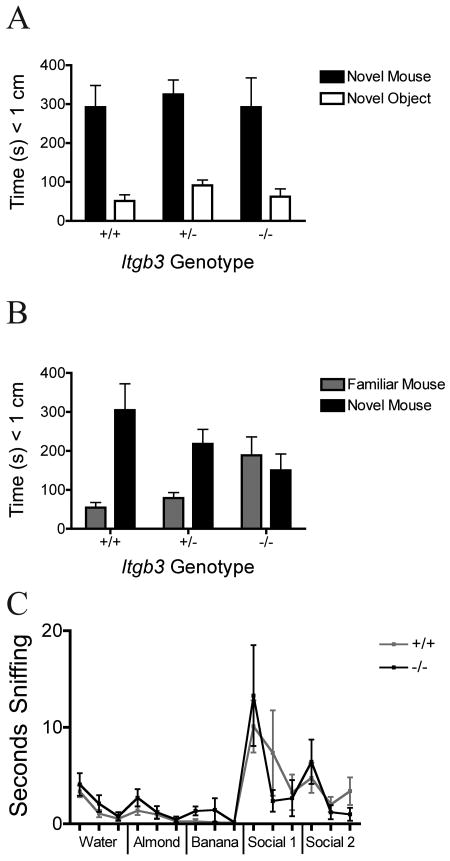
On the 3-chamber test of sociability, all three genotypes showed significant sociability, or preference for the novel mouse over the novel object, with no effect of genotype (Fig 3A, two-way repeated measures ANOVA, genotype by stimulus interaction F = 0.01, P = 0.99; stimulus main effect F = 42.64, P < 0.0001; genotype main effect F = 0.73, P = 0.49; Bonferroni posttests of stimulus effect in all three genotypes, P < 0.05, n = 10 +/+, 20 +/-, 6 -/-). On the second portion of the test, however, there was a significant difference between the genotypes on preference for social novelty (Fig 3B, two-way repeated measures ANOVA, genotype by stimulus interaction F = 4.56, P = 0.018), with Bonferroni posttests showing a preference for social novelty in the wildtype (+/+) animals (t = 4.13, P < 0.001) and the heterozygous (+/-) animals (t = 3.43, P < 0.01) but no such preference in the null (-/-) animals (t = 0.52, P > 0.05).
Figure 3. Social and Olfactory Behavior.
A and B: Three-chamber social test. Mean and standard error of the mean are shown by genotype (10 +/+, 20 +/-, 6 -/-) for the cumulative data over each 10-minute test session. A: Time spent within 1 cm (‘sniffing’) of the wire cage containing the novel mouse or the novel object (empty wire cage). B: Time spent within 1 cm of the wire cage containing the familiar mouse or the novel mouse. C: Olfactory Habituation-Dishabituation. Mean and standard error of the mean are shown by genotype (11 +/+, 8 -/-) for the time spent within 2 cm of the cotton swab containing the odor stimulus over each 2-minute odor presentation.
Despite showing an absence of preference for social novelty on the 3-chamber test, the Itgb3 -/- animals showed intact olfaction and recognition of novel non-social and social odors on the olfactory habituation / dishabituation test (Figure 3C, two-way repeated measures ANOVA, stimulus main effect F = 7.60, P < 0.0001; genotype main effect F = 0.02, P = 0.89; genotype by stimulus interaction F = 0.77, P = 0.70, n = 11 +/+, 8 -/-). Bonferroni posttests revealed no individual odor stimulus presentation that showed a significant difference between wildtype and Itgb3 -/- animals.
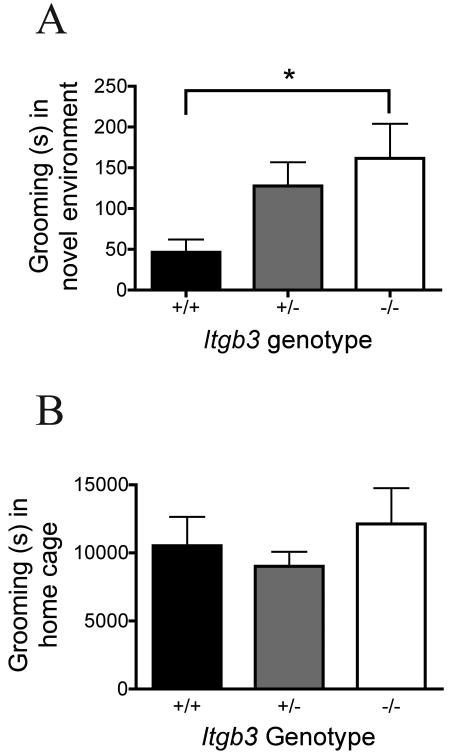
Time spent grooming in a novel environment was significantly different across Itgb3 genotypes (Figure 4A, Kruskal-Wallis test statistic 6.181, P = 0.046; non-parametric analysis chosen due to trend for unequal variances on Bartlett's test, P = 0.08, n = 6 +/+, 6 +/-, 7 -/-). A significant difference in time spent grooming in the novel environment was observed between the wildtype and the Itgb3 -/- animals (Dunn's posttest for multiple comparisons, difference in rank sum -7.38, P < 0.05). In a subset of these animals, time spent grooming over 24 hours in the home cage was not different across Itgb3 genotypes (Figure 4B, one-way ANOVA, F = 0.38, P = 0.69, n = 6 +/+, 3 +/-, 4 -/-).
Figure 4. Grooming behavior.
A: Grooming behavior in the novel environment. Mean and standard error of the mean are shown by genotype (6 +/+, 6 +/-, 7 -/-) for seconds spent grooming in a novel cage scored by stopwatch over a 10 minute observation period. B: Grooming behavior in the home cage. Mean and standard error of the mean are shown by genotype (6 +/+, 3 +/-, 4 -/-) for seconds spent grooming in the home cage as scored by the automated HomeCageScan software (Clever Sys, Inc., Reston, VA).
Discussion
The pattern of diminished preference for social novelty and increased repetitive behavior in a novel environment in the Itgb3 knockout mouse has face validity for some symptoms of autism – that is, it mirrors how we may imagine some human symptoms would be paralleled in a mouse. These preliminary findings raise a number of interesting issues, including the evaluation of a behavioral phenotype in the context of known peripheral phenotypes, the appropriate interpretation of these behavioral phenotypes, the use of a knockout mouse to evaluate the importance of this gene, and the possible biological mechanisms underlying the observed phenotypes.
The known peripheral phenotype in the Itgb3 animals is of obvious concern in interpreting these data. The known anemia, bleeding time, or other peripheral phenotypes (Hodivala-Dilke, et al., 1999; McHugh, et al., 2000) in the Itgb3 -/- mice complicated the behavioral testing, including requiring additional Itgb3 -/- mice from the same breeding pairs to be included to achieve adequate sample size for the 3-chamber social test. The absence of littermate controls for these additional -/- animals is a potential confound given the possibility of parenting or litter effects on this social phenotype. On the other hand, the peripheral phenotypes are difficult to connect directly to the absence of preference for social novelty or the increase in grooming behavior in the novel environment. First, neither behavioral phenotype yields to a straightforward peripheral explanation. For example, if mice with a history of bleeding or other peripheral phenotype would hesitate to interact with a novel animal over a familiar animal due to past experience of aggression, we would also expect to see less sociability on the 3-chamber sociability test. Likewise, if mice with a history of bleeding or other peripheral abnormality were grooming more as a result of itching or wound-healing, we would expect them to show increased grooming at baseline, not just in a novel environment. Second, at least in the case of grooming, the Itgb3 +/- animals appeared to show an intermediate phenotype, supporting both a gene-dosage relationship and a lack of dependence upon bleeding or other peripheral phenotypes, which have not been reported in the hemizygous animals. Third, it is possible that a brain and behavior phenotype could be mediated directly by increased bleeding in the absence of the fibrinogen receptor, but we did not observe any evidence of previous stroke or brain hemorrhage in these mice in multiple fresh dissections of brain tissue or on Nissl stain (JV, MC, AC, paper in preparation). It remains possible that the peripheral phenotypes in the Itgb3 null mouse do impact the observed behavioral changes, and further testing in a neuron-specific Itgb3 null mouse would be necessary to fully rule-out this possibility. Alternatively, parallel testing could be conducted in mice with other causes of bleeding defects, anemia, or osteosclerosis could evaluate the contribution of these peripheral phenotypes to the observed behaviors.
Lack of preference for social novelty is a less straightforward social phenotype than lack of sociability, or the preference for a novel mouse over a novel object, which has been the focus of most behavioral studies of mouse models related to autism (Kwon, et al., 2006; Moy, Nadler, Young, et al., 2008; Silverman, et al., 2010; Tabuchi, et al., 2007). Indeed, absence of preference for people over objects is described in ASD dating back to Leo Kanner's original description of the disorder in 1943 (Kanner, 1943); although lack of sociability is not required for diagnosis of Autistic Disorder (American Psychiatric Association, 2000) and may pertain only to a portion of individuals with ASD (Waterhouse, et al., 1996). Instead, qualitative impairment in social interaction is described as the central deficit in ASD, which may be much more difficult to model in a mouse than absence of sociability itself (American Psychiatric Association, 2000). Lack of preference for exploring novel individuals over familiar individuals could fit as one possible component of this qualitative impairment. Indeed, Kanner included an absence of differentiation between individuals in his first description of autism: “The relation to the members of the household or to other children did not differ from that to the people at the office” (p. 247)(Kanner, 1943). Importantly, however, while this absence of differentiation between individuals may be true of some individuals with ASD, it is again not necessary for diagnosis and is not emphasized in the diagnostic assessment (Lord, et al., 1994).
A number of other mouse mutants have been described with an absence of preference for social novelty. Interestingly, the Dp(11)17/+ mouse model of Potocki-Lupski syndrome, which prominently includes symptoms of autism, lacks a preference for social novelty on the 3-chamber test (Molina, et al., 2008). Mice lacking the oxytocin receptor gene, which has been associated with autism in multiple studies (Gregory, et al., 2009; Jacob, et al., 2007; Lerer, et al., 2008; Liu, et al., 2010; Park, et al., 2010; Wermter, et al., 2010; Wu, et al., 2005), also show an absence of preference for social novelty in one of two studies (Crawley, et al., 2007; Takayanagi, et al., 2005). Using a paradigm of habituation to repeated exposure to a stimulus mouse, other disruptions of the oxytocin system, including knockout of the oxytocin gene itself (Ferguson, et al., 2000) and knockout of the oxytocin-regulating protein CD38 (Jin, et al., 2007), also result in apparent inability to differentiate a familiar from a novel animal. Finally, two mutant mouse lines with no clear genetic relationship to ASD show abnormalities on the preference for social novelty test portion of the 3-chamber test: mice lacking complexin 1 (Cplx1), which is important in synaptic vesicle docking and release (Drew, et al., 2007), and mice hemizygous for the schizophrenia-associated neuregulin-1 (Nrg1) (O'Tuathaigh, et al., 2007).
An increase in baseline grooming behavior has also been reported in a number of mutant mouse models (Chen, et al., 2010; Shmelkov, et al., 2010; Welch, et al., 2007), as well as in the BTBR inbred strain of mice, which show multiple behaviors with face validity for ASD (McFarlane, et al., 2008). In each of these models, mice lose hair or develop skin wounds because of excessive grooming. In the Itgb3 -/- mice, however, we observed increased grooming in novel environments but not in the home cage, at least in a subset of animals. Grooming in novel environments has been described as an indication of anxiety-like behavior in previous studies (Hart, et al., 2010; McNaughton, et al., 2008). The Itgb3 -/- mice do not show any alteration in anxiety-like behavior in the Elevated Plus Maze, however, which would suggest that their increase in grooming behavior in novel environments could reflect an abnormal response to an anxiety-provoking environment, rather than an increase in anxiety-like behavior per se. Of note, increased repetitive behavior is seen in children with ASD in anxiety-provoking settings (Joosten, et al., 2009); although children with ASD might also be expected to have an increased baseline level of repetitive behavior (American Psychiatric Association, 2000).
The genetic link between the integrin β3 gene, whole blood 5-HT levels, and autism susceptibility, led us to evaluate the behavior of the Itgb3 knockout mouse. However, if the hypothesis is correct that increased integrin β3 activity leads to increased SERT transport of 5-HT and therefore hyperserotonemia (Carneiro, et al., 2008), then mice with increased β3 activity would be a better model of the alterations that we might expect to see in ASD. Although such a model of increased integrin β3 function would be extremely interesting, the current data support a role for integrin β3 in modulating social and repetitive behavior. Repeatedly, the ASD genetic literature has identified increases and decreases in expression or function of the same gene or chromosomal region as leading to autism susceptibility, including disruption or duplication of the Rett syndrome gene MECP2 (Ramocki, et al., 2009), maternal duplication or deletion of chromosome 15q (Cook, et al., 1997), and duplication or deletion of chromosome 16p (Kumar, et al., 2008; Weiss, et al., 2008). Importantly, opposite alleles of the promoter polymorphism of SERT, which interacts with integrin β3, are associated with different behavioral phenotypes within ASD (Brune, et al., 2006). As a whole, this literature suggests a narrow range within which activity of autism-associated genes is constrained, with risk alleles leading to expression that is either too high or too low.
The mechanisms underlying the behavioral phenotypes observed in the Itgb3 -/- mice are not obvious. Unlike the neuroligin 3 knockout mouse, which shows absence of preference for social novelty in the context of an olfactory deficit (Radyushkin, et al., 2009), the Itgb3 -/- mice have intact olfaction, as well as intact habituation to olfactory stimuli. Of note, the social scents used in the Olfactory Habituation / Dishabituation test were derived from cages holding multiple animals; therefore, the ability to distinguish the scents of individual animals cannot be directly inferred. The preference for spending time with a novel animal over a familiar animal over 10 minutes is also considerably more complex than the simple investigation of a single novel or familiar scent presented repeatedly. Further testing of social behavior could help clarify the cause of the absence of preference for social novelty, including a longer duration of co-habitation with the familiar stimulus mouse, multiple exposures to the familiar mouse, or tracking of interaction with each stimulus mouse over a longer duration of time. Follow-up testing could also include coding of grooming behavior during the three-chamber social task to evaluate the relationship between these two behavioral domains, which was not possible in this study. Additionally, further testing of cognition in these animals would clarify whether they have a more generalized deficit in preference for novelty, in social memory, in spatial memory, in reversal learning, or in object recognition that may underlie their absence of preference for social novelty.
Previous work on the role of integrin αvβ3 as a cell adhesion molecule in the brain has been limited to neuronal culture and slice preparations of the hippocampus, where it is important in activity-dependent dendritic spine remodeling and scaling of excitatory synapses (Chavis & Westbrook, 2001; Cingolani & Goda, 2008; Shi & Ethell, 2006). Neuronal activity-dependent genes have previously been implicated in ASD (Morrow, et al., 2008), as have synaptic cell adhesion molecules (Bourgeron, 2009; Buxbaum, 2009). It is tempting to interpret the lack of preference for social novelty as a hippocampal-associated memory deficit, but previous work has suggested that social memory may be more dependent upon the amygdala and the olfactory bulb (Adolphs, 2009; Ferguson, et al., 2001; Tobin, et al.). Increased grooming behavior is unlikely to implicate the hippocampus directly, as a corticostriatal circuit has been implicated in previous mouse mutants with increased grooming (Shmelkov, et al., 2010; Welch, et al., 2007).
In summary, these preliminary studies suggest social and repetitive behavior phenotypes in the Itgb3 knockout mice that require further study. Targeted knockout of Itgb3 in the brain is needed to demonstrate independence from the peripheral phenotypes previously described in these mice. Targeted knockout in individual brain regions may clarify the neuronal circuits underlying the particular behavioral abnormalities that we observed. Further behavioral characterization of these targeted knockout animals may be focused on potential memory deficits corresponding to hippocampal abnormalities.
Acknowledgments
We are especially grateful to the families who participated in the original genetic studies. We also thank Randy Blakely for helpful advice and generous mentorship. This work was supported, in part, by NIH grants MH081066 (JV), T32-MH065215 (JV), and HD15052 (Vanderbilt Kennedy Center).
References
- Abney M, McPeek MS, Ober C. Broad and narrow heritabilities of quantitative traits in a founder population. Am J Hum Genet. 2001;68:1302–1307. doi: 10.1086/320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- Abramson RK, Wright HH, Carpenter R, Brennan W, Lumpuy O, Cole E, et al. Elevated blood serotonin in autistic probands and their first-degree relatives. Journal of Autism and Developmental Disorders. 1989;19:397–407. doi: 10.1007/BF02212938. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The social brain: neural basis of social knowledge. Annu Rev Psychol. 2009;60:693–716. doi: 10.1146/annurev.psych.60.110707.163514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders-Text Revision (DSM-IV-TR) 4th. Washington, D.C.: American Psychiatric Association Press Inc.; 2000. [Google Scholar]
- Anderson GM, Feibel FC, Cohen DJ. Determination of serotonin in whole blood, platelet-rich plasma, platelet-poor plasma and plasma ultrafiltrate. Life Sci. 1987;40:1063–1070. doi: 10.1016/0024-3205(87)90568-6. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychological Medicine. 1995;25:63–78. doi: 10.1017/s0033291700028099. [DOI] [PubMed] [Google Scholar]
- Berkel S, Marshall CR, Weiss B, Howe J, Roeth R, Moog U, et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- Blundell J, Blaiss CA, Etherton MR, Espinosa F, Tabuchi K, Walz C, et al. Neuroligin-1 deletion results in impaired spatial memory and increased repetitive behavior. J Neurosci. 2010;30:2115–2129. doi: 10.1523/JNEUROSCI.4517-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeron T. A synaptic trek to autism. Curr Opin Neurobiol. 2009;19:231–234. doi: 10.1016/j.conb.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Brune CW, Kim SJ, Salt J, Leventhal BL, Lord C, Cook EH., Jr 5-HTTLPR Genotype-Specific Phenotype in Children and Adolescents With Autism. Am J Psychiatry. 2006;163:2148–2156. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD. Multiple rare variants in the etiology of autism spectrum disorders. Dialogues Clin Neurosci. 2009;11:35–43. doi: 10.31887/DCNS.2009.11.1/jdbuxbaum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro AM, Cook EH, Murphy DL, Blakely RD. Interactions between integrin alphaIIbbeta3 and the serotonin transporter regulate serotonin transport and platelet aggregation in mice and humans. J Clin Invest. 2008;118:1544–1552. doi: 10.1172/JCI33374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children. Journal of the American Medical Association. 2001;285:3093–3099. doi: 10.1001/jama.285.24.3093. [DOI] [PubMed] [Google Scholar]
- Chavis P, Westbrook G. Integrins mediate functional pre- and postsynaptic maturation at a hippocampal synapse. Nature. 2001;411:317–321. doi: 10.1038/35077101. [DOI] [PubMed] [Google Scholar]
- Chen SK, Tvrdik P, Peden E, Cho S, Wu S, Spangrude G, et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell. 2010;141:775–785. doi: 10.1016/j.cell.2010.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Goda Y. Differential involvement of beta3 integrin in pre- and postsynaptic forms of adaptation to chronic activity deprivation. Neuron Glia Biol. 2008;4:179–187. doi: 10.1017/S1740925X0999024X. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 2003;60:524–530. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- Cook E, Leventhal B. The serotonin system in autism. Current Opinion in Pediatrics. 1996;8:348–354. doi: 10.1097/00008480-199608000-00008. [DOI] [PubMed] [Google Scholar]
- Cook E, Lindgren V, Leventhal B, Courchesne R, Lincoln A, Shulman C, et al. Autism or atypical autism in maternally but not paternally derived proximal 15q duplication. American Journal of Human Genetics. 1997;60:928–934. [PMC free article] [PubMed] [Google Scholar]
- Coutinho AM, Sousa I, Martins M, Correia C, Morgadinho T, Bento C, et al. Evidence for epistasis between SLC6A4 and ITGB3 in autism etiology and in the determination of platelet serotonin levels. Hum Genet. 2007;121:243–256. doi: 10.1007/s00439-006-0301-3. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Chen T, Puri A, Washburn R, Sullivan TL, Hill JM, et al. Social approach behaviors in oxytocin knockout mice: comparison of two independent lines tested in different laboratory environments. Neuropeptides. 2007;41:145–163. doi: 10.1016/j.npep.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Cross S, Kim SJ, Weiss LA, Delahanty RJ, Sutcliffe JS, Leventhal BL, et al. Molecular genetics of the platelet serotonin system in first-degree relatives of patients with autism. Neuropsychopharmacology. 2008;33:353–360. doi: 10.1038/sj.npp.1301406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuccaro ML, Wright HH, Abramson RK, Marsteller FA, Valentine J. Whole-blood serotonin and cognitive functioning in autistic individuals and their first-degree relatives. Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:94–101. doi: 10.1176/jnp.5.1.94. [DOI] [PubMed] [Google Scholar]
- Drew CJ, Kyd RJ, Morton AJ. Complexin 1 knockout mice exhibit marked deficits in social behaviours but appear to be cognitively normal. Hum Mol Genet. 2007;16:2288–2305. doi: 10.1093/hmg/ddm181. [DOI] [PubMed] [Google Scholar]
- Etherton MR, Blaiss CA, Powell CM, Sudhof TC. Mouse neurexin-1alpha deletion causes correlated electrophysiological and behavioral changes consistent with cognitive impairments. Proc Natl Acad Sci U S A. 2009;106:17998–18003. doi: 10.1073/pnas.0910297106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Folstein S, Rutter M. Infantile autism: A genetic study of 21 twin pairs. Journal of Child Psychology, Psychiatry and Allied Disciplines. 1977;18:297–321. doi: 10.1111/j.1469-7610.1977.tb00443.x. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Advances in autism. Annu Rev Med. 2009;60:367–380. doi: 10.1146/annurev.med.60.053107.121225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glessner JT, Wang K, Cai G, Korvatska O, Kim CE, Wood S, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory SG, Connelly JJ, Towers AJ, Johnson J, Biscocho D, Markunas CA, et al. Genomic and epigenetic evidence for oxytocin receptor deficiency in autism. BMC Med. 2009;7:62. doi: 10.1186/1741-7015-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart PC, Bergner CL, Smolinsky AN, Dufour BD, Egan RJ, Laporte JL, et al. Experimental models of anxiety for drug discovery and brain research. Methods Mol Biol. 2010;602:299–321. doi: 10.1007/978-1-60761-058-8_18. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, et al. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob S, Brune CW, Carter CS, Leventhal BL, Lord C, Cook EH., Jr Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417:6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamain S, Quach H, Betancur C, Rastam M, Colineaux C, Gillberg IC, et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat Genet. 2003;34:27–29. doi: 10.1038/ng1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, et al. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Joosten AV, Bundy AC, Einfeld SL. Intrinsic and extrinsic motivation for stereotypic and repetitive behavior. J Autism Dev Disord. 2009;39:521–531. doi: 10.1007/s10803-008-0654-7. [DOI] [PubMed] [Google Scholar]
- Kanner L. Autistic disturbances of affective contact. Nerv Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kim HG, Kishikawa S, Higgins AW, Seong IS, Donovan DJ, Shen Y, et al. Disruption of neurexin 1 associated with autism spectrum disorder. Am J Hum Genet. 2008;82:199–207. doi: 10.1016/j.ajhg.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar RA, KaraMohamed S, Sudi J, Conrad DF, Brune C, Badner JA, et al. Recurrent 16p11.2 microdeletions in autism. Hum Mol Genet. 2008;17:628–638. doi: 10.1093/hmg/ddm376. [DOI] [PubMed] [Google Scholar]
- Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–388. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Bonnet-Brilhault F, Gomot M, Blanc R, David A, Moizard MP, et al. X-Linked mental retardation and autism are associated with a mutation in the NLGN4 Gene, a member of the neuroligin family. Am J Hum Genet. 2004;74:552–557. doi: 10.1086/382137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, et al. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first-degree relatives. Biological Psychiatry. 1999;45:158–163. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- Lerer E, Levi S, Salomon S, Darvasi A, Yirmiya N, Ebstein RP. Association between the oxytocin receptor (OXTR) gene and autism: relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13:980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- Leventhal BL, Cook EH, Jr, Morford M, Ravitz A, Freedman DX. Relationships of whole blood serotonin and plasma norepinephrine within families. J Autism Dev Disord. 1990;20:499–511. doi: 10.1007/BF02216055. [DOI] [PubMed] [Google Scholar]
- Lijam N, Paylor R, McDonald M, Crawley J, Deng CX, Herrup K, et al. Social interaction and sensorimotor gating abnormalities in mice lacking Dvl1. Cell. 1997;90:895–905. doi: 10.1016/s0092-8674(00)80354-2. [DOI] [PubMed] [Google Scholar]
- Liu X, Kawamura Y, Shimada T, Otowa T, Koishi S, Sugiyama T, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55:137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview - Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Ma DQ, Rabionet R, Konidari I, Jaworski J, Cukier HN, Wright HH, et al. Association and gene-gene interaction of SLC6A4 and ITGB3 in autism. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:477–483. doi: 10.1002/ajmg.b.31003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CR, Noor A, Vincent JB, Lionel AC, Feuk L, Skaug J, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–163. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- McHugh KP, Hodivala-Dilke K, Zheng MH, Namba N, Lam J, Novack D, et al. Mice lacking beta3 integrins are osteosclerotic because of dysfunctional osteoclasts. J Clin Invest. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton CH, Moon J, Strawderman MS, Maclean KN, Evans J, Strupp BJ. Evidence for social anxiety and impaired social cognition in a mouse model of fragile X syndrome. Behav Neurosci. 2008;122:293–300. doi: 10.1037/0735-7044.122.2.293. [DOI] [PubMed] [Google Scholar]
- Mei H, Cuccaro ML, Martin ER. Multifactor dimensionality reduction-phenomics: a novel method to capture genetic heterogeneity with use of phenotypic variables. Am J Hum Genet. 2007;81:1251–1261. doi: 10.1086/522307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci. 2001;21:5239–5250. doi: 10.1523/JNEUROSCI.21-14-05239.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J, Carmona-Mora P, Chrast J, Krall PM, Canales CP, Lupski JR, et al. Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome. Hum Mol Genet. 2008;17:2486–2495. doi: 10.1093/hmg/ddn148. [DOI] [PubMed] [Google Scholar]
- Moretti P, Bouwknecht JA, Teague R, Paylor R, Zoghbi HY. Abnormalities of social interactions and home-cage behavior in a mouse model of Rett syndrome. Hum Mol Genet. 2005;14:205–220. doi: 10.1093/hmg/ddi016. [DOI] [PubMed] [Google Scholar]
- Morrow EM, Yoo SY, Flavell SW, Kim TK, Lin Y, Hill RS, et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, et al. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Poe MD, Nonneman RJ, Young NB, Koller BH, et al. Development of a mouse test for repetitive, restricted behaviors: relevance to autism. Behav Brain Res. 2008;188:178–194. doi: 10.1016/j.bbr.2007.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Grossman AW, Murphy DL, et al. Social Approach in Genetically-Engineered Mouse Lines Relevant to Autism. Genes Brain Behav. 2008 doi: 10.1111/j.1601-183X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder EJ, Anderson GM, Kema IP, de Bildt A, van Lang ND, den Boer JA, et al. Platelet serotonin levels in pervasive developmental disorders and mental retardation: diagnostic group differences, within-group distribution, and behavioral correlates. J Am Acad Child Adolesc Psychiatry. 2004;43:491–499. doi: 10.1097/00004583-200404000-00016. [DOI] [PubMed] [Google Scholar]
- Munson J, Dawson G, Sterling L, Beauchaine T, Zhou A, Elizabeth K, et al. Evidence for latent classes of IQ in young children with autism spectrum disorder. Am J Ment Retard. 2008;113:439–452. doi: 10.1352/2008.113:439-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani J, Tamada K, Hatanaka F, Ise S, Ohta H, Inoue K, et al. Abnormal behavior in a chromosome-engineered mouse model for human 15q11-13 duplication seen in autism. Cell. 2009;137:1235–1246. doi: 10.1016/j.cell.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Roak BJ, State MW. Autism genetics: strategies, challenges, and opportunities. Autism Res. 2008;1:4–17. doi: 10.1002/aur.3. [DOI] [PubMed] [Google Scholar]
- O'Tuathaigh CM, Babovic D, O'Sullivan GJ, Clifford JJ, Tighe O, Croke DT, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27. doi: 10.1016/j.neuroscience.2007.03.051. [DOI] [PubMed] [Google Scholar]
- Park J, Willmott M, Vetuz G, Toye C, Kirley A, Hawi Z, et al. Evidence that genetic variation in the oxytocin receptor (OXTR) gene influences social cognition in ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:697–702. doi: 10.1016/j.pnpbp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010 doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radyushkin K, Hammerschmidt K, Boretius S, Varoqueaux F, El-Kordi A, Ronnenberg A, et al. Neuroligin-3-deficient mice: model of a monogenic heritable form of autism with an olfactory deficit. Genes Brain Behav. 2009;8:416–425. doi: 10.1111/j.1601-183X.2009.00487.x. [DOI] [PubMed] [Google Scholar]
- Ramocki MB, Peters SU, Tavyev YJ, Zhang F, Carvalho CM, Schaaf CP, et al. Autism and other neuropsychiatric symptoms are prevalent in individuals with MeCP2 duplication syndrome. Ann Neurol. 2009;66:771–782. doi: 10.1002/ana.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richler J, Huerta M, Bishop SL, Lord C. Developmental trajectories of restricted and repetitive behaviors and interests in children with autism spectrum disorders. Dev Psychopathol. 2010;22:55–69. doi: 10.1017/S0954579409990265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Roullet FI, Wohr M, Crawley JN. Female urine-induced male mice ultrasonic vocalizations, but not scent-marking, is modulated by social experience. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schain RJ, Freedman DX. Studies on 5-hydroxyindole metabolism in autistic and other mentally retarded children. J Pediatrics. 1961;58:315–320. doi: 10.1016/s0022-3476(61)80261-8. [DOI] [PubMed] [Google Scholar]
- Shi Y, Ethell IM. Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci. 2006;26:1813–1822. doi: 10.1523/JNEUROSCI.4091-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmelkov SV, Hormigo A, Jing D, Proenca CC, Bath KG, Milde T, et al. Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice. Nat Med. 2010;16:598–602. doi: 10.1038/nm.2125. 591p following 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele AD, Jackson WS, King OD, Lindquist S. The power of automated high-resolution behavior analysis revealed by its application to mouse models of Huntington's and prion diseases. Proc Natl Acad Sci U S A. 2007;104:1983–1988. doi: 10.1073/pnas.0610779104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science. 2007;318:71–76. doi: 10.1126/science.1146221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci U S A. 2005;102:16096–16101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hashimoto H, Wacker DW, Takayanagi Y, Langnaese K, Caquineau C, et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature. 2010;464:413–417. doi: 10.1038/nature08826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse L, Morris R, Allen D, Dunn M, Fein D, Feinstein C, et al. Diagnosis and classification in autism. J Autism Dev Disord. 1996;26:59–86. doi: 10.1007/BF02276235. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Abney M, Cook EH, Jr, Ober C. Sex-specific genetic architecture of whole blood serotonin levels. Am J Hum Genet. 2005;76:33–41. doi: 10.1086/426697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, Kosova G, Delahanty RJ, Jiang L, Cook EH, Ober C, et al. Variation in ITGB3 is associated with whole-blood serotonin level and autism susceptibility. Eur J Hum Genet. 2006;14:923–931. doi: 10.1038/sj.ejhg.5201644. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Ober C, Cook EH., Jr ITGB3 shows genetic and expression interaction with SLC6A4. Hum Genet. 2006;120:93–100. doi: 10.1007/s00439-006-0196-z. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Shen Y, Korn JM, Arking DE, Miller DT, Fossdal R, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- Weiss LA, Veenstra-Vanderweele J, Newman DL, Kim SJ, Dytch H, McPeek MS, et al. Genome-wide association study identifies ITGB3 as a QTL for whole blood serotonin. Eur J Hum Genet. 2004;12:949–954. doi: 10.1038/sj.ejhg.5201239. [DOI] [PubMed] [Google Scholar]
- Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, et al. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wermter AK, Kamp-Becker I, Hesse P, Schulte-Korne G, Strauch K, Remschmidt H. Evidence for the involvement of genetic variation in the oxytocin receptor gene (OXTR) in the etiology of autistic disorders on high-functioning level. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:629–639. doi: 10.1002/ajmg.b.31032. [DOI] [PubMed] [Google Scholar]
- Wu S, Jia M, Ruan Y, Liu J, Guo Y, Shuang M, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58:74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Yang M, Crawley JN. Simple behavioral assessment of mouse olfaction. Curr Protoc Neurosci. 2009;Chapter 8(Unit 8 24) doi: 10.1002/0471142301.ns0824s48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young DM, Schenk AK, Yang SB, Jan YN, Jan LY. Altered ultrasonic vocalizations in a tuberous sclerosis mouse model of autism. Proc Natl Acad Sci U S A. 2010;107:11074–11079. doi: 10.1073/pnas.1005620107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Fung C, Shin D, Shin BC, Thamotharan S, Sankar R, et al. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol Psychiatry. 2010;15:286–299. doi: 10.1038/mp.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]