Abstract
Parkinson's disease (PD) is a degenerative brain disorder accompanied by the loss of dopaminergic neurons and the presence of motor and non-motor symptoms. We performed a cross-sectional, questionnaire-based analysis of impulsive behavior in our PD clinic population to assess prevalence and associated characteristics. We found a higher prevalence of impulsive behavior (29.7%) than previously reported, and found multiple, concurrent impulsive behaviors in 26% of subjects reporting impulsive behavior. Our findings contribute to the growing awareness of impulsive behavior in PD, and support the need for longitudinal studies to assess changes in impulsive behaviors in Parkinson's patients.
Keywords: impulsive behavior, impulsivity, Parkinson's disease, prevalence
Introduction
Impulse control disorders are a group of disorders including pathological gambling, hypersexuality, and compulsive shopping, that are characterized by compulsive engagement in a behavior despite adverse consequences, diminished control over the problematic behavior, and an urge prior to, and a hedonic sense during, performance of the behavior1. Impulse control disorders have been recently described in patients with Parkinson's disease (PD) 2,3,4. It remains unclear, however, whether these impulse control disorders or impulsive behaviors are a consequence of the disease itself, due to underlying personality traits5, secondary to the pharmacological management of the disease3, or a complex interplay of all these factors6,2.
Impulsive behaviors appear to be related in PD to the phenomenon of dopamine dysregulation syndrome (DDS)2. DDS refers to the behavioral syndrome in PD characterized by dopamine medication abuse, which can be accompanied by a mood disorder and/or impulsive behavior 7.The reported prevalence of impulsive behaviors varies widely, with the variation attributed to particulars of the screening methods and definitions of the abnormal behavior 2. The prevalence of pathological gambling in PD patients, for instance, has been reported variously at 0.05 - 7%3,8. Most studies of PD have focused only on the more common impulse control disorders, such as pathological gambling and compulsive sexual behavior, but have not performed comprehensive analyses of all such disorders.
Other investigators have suggested that the use of dopamine replacement therapies, in particular dopamine agonists, is a risk factor for the development of impulsive behaviors 9,4,3. Other potential risk factors that have been cited include male gender9, younger age of PD onset10,9, pre-existing impulse control or substance abuse disorders, depression, impulsivity11,9, and novelty-seeking personality trait12,4.
Anecdotal evidence in our PD clinic population suggested a higher prevalence of impulsive behaviors than previously reported, with some of the observed behaviors meeting criteria for impulse control disorders. We defined impulsive behavior (IB) as the presence of pathological gambling, trichotillomania, kleptomania, pyromania, compulsive exercise, compulsive buying, compulsive sexual behavior, or intermittent explosive disorder. These disorders were all included as there has been speculation that all may be behavioral manifestations of the same underlying pathophysiology of reward dysregulation 13. Information on binge eating was not collected in our survey. Dopamine abuse and punding were grouped together as Dopamine Dysregulation syndrome.
The aim of this work, therefore, was (1) to determine the prevalence of impulsive behaviors in our PD clinic population assessing for a wider range of impulse control disorders than previously examined, and (2) to identify clinical variables associated with these behaviors. We hypothesized: first, that when behavior is viewed categorically, we would see a higher prevalence of impulsive behavior in our PD population than previously reported; and second, that the presence of impulsive behavior would be associated with male gender, younger age of PD onset, and higher daily dose of dopamine replacement therapy.
Method
Subjects
We performed a cross-sectional study to determine the prevalence of impulsive behaviors in our PD clinic population. Participants included 128 adults aged ≥18 years meeting criteria for PD, were recruited through the University of Minnesota PD database, and were screened for study inclusion and exclusion criteria (see below) based on medical records. Potential subjects were notified by mail of the study and its aims, and then contacted by telephone to determine their willingness to participate. The study was approved by the University of Minnesota Institutional Review Board. Participants provided voluntary, written informed consent, and were recruited over a 2-year period (2007-2009).
Study Inclusion Criteria
Inclusion criteria included a diagnosis of idiopathic PD (U.K. Brain Bank Criteria) and ability to provide informed consent. Subjects included those newly diagnosed, on anti-Parkinson's medications, and those who had undergone deep brain stimulation surgery for PD.
Study Exclusion Criteria
Exclusion criteria included: 1) atypical Parkinsonism, defined as a poor and less-sustained response to traditional anti-Parkinson medications (e.g., L-dopa and the dopamine agonists), more rapid disease progression, and generally a poorer prognosis; 2) abnormal screening brain MRI at 3.0T (performed for routine clinical evaluation of PD); 3) presence of dementia (Mini Mental Status Exam score < 23), or neurological disease other than PD; and 4) inability to understand or complete the surveys.
Assessments
After informed consent, relevant clinical data were collected from medical records. Additionally, participants were mailed (or given during a routine clinic visit) a self-report questionnaire described below. Questionnaire compliance was sought by telephone calls to those subjects who had not returned completed forms within two weeks.
Clinical Characteristics
Data regarding demographics, medical history, medication type and dosage, and history of impulsive behaviors, were collected using the self-report questionnaire.
Levodopa-equivalent daily dose in milligrams (LEDD), including the dopamine agonists, was calculated using the formula [Levodopa/Carbidopa] + [Levodopa/Carbidopa-CR] × 0.75 + [Levodopa/Carbidopa + (Levodopa/Carbidopa-CR × 0.75)] × 0.25 if on Tolcapone + (Pramipexole × 67.75) + (Pergolide × 100) + (Ropinirole × 16.67) + (Bromocriptine × 10) 14.
Disease State Characterization
PD disease severity assessments using the Unified Parkinson's Disease Rating Scale (UPDRS) were obtained from medical records, and had been performed within 48 months of survey completion.
Psychological Assessment
Subjects were asked to record current and past neuropsychiatric diagnoses, including anxiety, depression, and bipolar disorder, and responses were confirmed by chart review for medications, formal neuropsychiatric assessment, and PD Clinic notes.
Behavioral Assessment
A self-report questionnaire was distributed to all participants, and included the following items: The Minnesota Impulse Disorders Interview (MIDI)15 is a semi-structured clinical interview that screens for various impulse control disorders including pathological gambling, trichotillomania, kleptomania, pyromania, intermittent explosive disorder, compulsive buying, compulsive sexual behavior, and compulsive exercise. A diagnosis of an impulsive behavior according to the MIDI was defined by affirmative answers as stated in previous studies 11,4.
Dopamine medication misuse was determined via structured questionnaire (DDS Survey 16). Punding, defined as repetitive, pointless behaviors which have been associated with DDS17, was determined by structured questionnaire which helped subjects recognize patterns of disruptive behaviors (interfering with sleep, social interactions, time spent) that were associated with relief of anxiety.
The Yale-Brown Obsessive Compulsive Symptoms Rating Scale (Y-BOCS; 18) was used to determine the severity of obsessive-compulsive symptoms.
Statistical Analysis
Participant characteristics were summarized with means and standard deviations, or counts and percents, as appropriate. The distributions of LEDD in milligrams (mg) and PD duration in years were highly skewed and were therefore transformed by the natural log to approximate a normal distribution for analysis.
Univariate analyses using logistic regression and chi-square statistics were used to compare characteristics such as age, age at PD onset, PD duration, LEDD, dopamine therapy abuse, gender, DBS surgery, Y-BOCS score and dopamine agonist usage between PD subjects with and without self-reported impulsive behavior. Multiple logistic regression analyses were used to compare the two groups after adjusting for age in years, for those characteristics expected to change with age. All statistical tests were two-sided, and the level of significance was set a priori at alpha (α) = 0.05. Statistical analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
All patients who met eligibility criteria were contacted for study participation by mail, and 128/263 patients (48.7%) agreed to participate. Study participant characteristics are summarized in Table 1. Of non-responders, 63% were male, median age was 66.0 years, and median PD duration was 9.8 years.
Prevalence of impulsive behavior
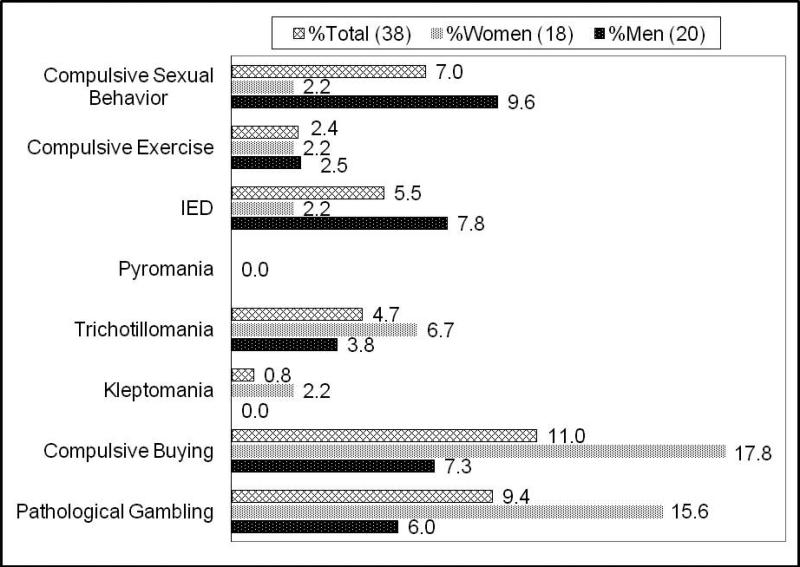
Thirty-eight subjects out of the 128 had at least one current impulsive behavior (prevalence 29.7%). The prevalence of pathological gambling in our study population was 9.4%; compulsive buying was 11.0%; kleptomania was 0.8%; trichotillomania was 4.7% (Figure 1). No subjects reported symptoms of pyromania. Ten of 38 subjects (26.3%) reported two or more impulsive behaviors.
Figure 1.
Percentage of study participants reporting impulsive behavior (IB) (Total N = 38; N for each category listed in parentheses) by category of behavior, based on response to Minnesota Impulsive Disorders Interview (MIDI; IED = Intermittent Explosive Disorder).
The prevalence of impulsive behaviors was higher among women (40%; 18/45) than men (24.1%; 20/83). Compulsive buying, kleptomania and trichotillomania were more common in women, whereas intermittent explosive behavior, compulsive exercise and hypersexuality were more common in men. Men were more likely to report multiple impulsive behaviors (IBs): Five men reported two concurrent IBs, one man reported three, and one man reported four IBs; whereas, two women reported two IBs, and one woman reported three IBs.
Clinical Factors Associated with impulsive behavior
Clinical characteristics, including mean subject age, age at PD onset, PD duration in years, and LEDD in mg, were compared between subjects reporting and those reporting no impulsive behavior (Table 2). Subjects reporting impulsive behavior were significantly younger at time of study and of younger age at PD-onset than non-impulsive subjects. Subjects reporting impulsive behavior had a higher mean value for PD disease duration and LEDD than subjects reporting no impulsive behavior, although these findings did not attain statistical significance.
The proportion of subjects who had undergone deep brain stimulator (DBS) implantation surgery for the treatment of PD was higher, but not significantly so, in subjects reporting impulsive behavior (Table 2).
Y-BOCS testing stratified all subjects into the following severity categories: 71.9% were in the subclinical range (Y-BOCS score 0-7), 18.8% were mild (score 8-15), 7.8% were moderate (score 16-23), and 1.5% were severe (score 24-31), with no patients meeting criteria for the extreme category (Y-BOCS 32-40). A statistically significant difference (p=0.0025) was noted between subjects reporting and those reporting no impulsive behavior for Y-BOCS as a categorical variable based on severity, with 44.7% (17/38) of impulsive behavior subjects reporting Y-BOCS scores ≥ 8, in comparison to 18.9% (17/90) of the non-impulsive behavior subjects.
The prevalence of subjects taking dopamine agonists was higher in subjects reporting impulsive behavior versus those reporting no impulsive behavior, but the difference was not statistically significant. Subjects taking Pramipexole reported slightly more impulsive behavior than those not taking any dopamine agonists (p = 0.06).
The prevalence of dopamine therapy abuse, a symptom of DDS19 was 11.2% (14/125). Of these 14 subjects, 57% (8) were taking dopamine agonists. Abuse of dopamine medication was not more common in the subjects reporting impulsive behavior.
The DBS and non-DBS patients differed significantly in mean age, age of PD onset, duration of PD, and LEDD (Table 3). The distributions of gender and dopamine agonistic medication use were almost the same between the two groups (chi-square of p=0.9 and p=0.8, respectively). When comparing overall impulsive behaviors, the DBS and non-DBS patients were not significantly different from each other after adjusting for age, PD duration, and LEDD (Prevalence odds=1; p=0.9; Table 4). The prevalence of impulsive behavior increased between the fourth and fifth decades, but was similar for the fifth, sixth, and seventh decades of life.
Discussion
This cross-sectional, questionnaire-based study provides evidence of a higher prevalence of impulsive behavior (29.7%) in PD than previously reported. In addition, because 26% of impulsive subjects reported multiple impulsive behaviors, PD patients must be screened for a variety of impulsive behaviors. In contrast to previous reports, we noted a higher prevalence of IBs in women (40.0%) than in men (24.1). The wide variability in reported prevalence of IBs in this population supports the need for standardization of assessment tools for diagnosing, and definitions of, impulsive behavior.
Prevalence of impulsive behavior in PD
An expanding body of literature addresses abnormalities of impulse control in PD 6, although the prevalence of these disorders has been somewhat difficult to assess. Explanations for the conflicting information about prevalence include the previous conception of PD as an exclusively motor disease, the social opprobrium placed on disorders of impulse control—which could be reflected in patient unwillingness to volunteer information and physician discomfort in probing into these disorders—and the confounding effects of different therapies, screening methods20, and definitions of the various impulsive behaviors 2. The potentially devastating psychosocial and financial consequences of these behaviors obligate a careful investigation of their prevalence and associated clinical factors.
The prevalence of all impulsive behaviors surveyed in our PD clinic population was markedly higher than in previous reports. For example, Weintraub and colleagues assessed compulsive gambling, shopping, and sexual behavior in 272 patients and found a combined prevalence of impulse control disorders of 6.6% (18/272)4. Our comprehensive survey tool assessed a wide variety of impulsive behaviors (e.g., kleptomania, compulsive exercise), in contrast to prior studies limited to those specific impulsive behaviors previously highlighted in the literature—e.g., compulsive gambling.
Our methodology—both how we surveyed for impulsive behavior and the definition we used for impulsive behaviors —was different from the majority of those reported in the literature, in that we relied on the self-report-MIDI to screen for impulsive behavior 11 and did not follow this questionnaire with a structured clinical interview, given constraints of our study design. Self-report questionnaires can sometimes overestimate the prevalence of pathological gambling due to retrospective reporting bias 21.Other potential explanations for the high prevalence of impulsive behavior in our population include factors unique to our study population—for instance, the racial and ethnic composition of our study population was almost exclusively Caucasian (99.2%), and of Scandinavian or Western European descent, and the availability of gambling in our region might be higher than in others studied22.
Our finding of a 9.4% prevalence of pathological gambling is consistent with a recent study by Voon and colleagues, who reported a 7.2% lifetime prevalence of pathologic gambling in their PD population12. We also observed a prevalence of 7.0% of compulsive sexual behavior, consistent with other reported rates (range: 2.4% - 10%) 23,11. Our prevalence of compulsive buying was 11.0%--higher than rates reported elsewhere (range 0.4 -10%)23,11. The estimated prevalence of trichotillomania in the general population is 1- 3%13. A 4.7% prevalence of trichotillomania in our study population was interesting as there is only one previous case report of trichotillomania in a patient with PD 24, suggesting that this behavior is either under-recognized, or under-reported, or that our patient population is different from other PD populations described.
The rates of specific impulsive behaviors found in our study are also higher than rates reported in non-PD adults: For instance, lifetime prevalence rates of pathological gambling among adults range from 0.4%-1.6%25; compulsive sexual behavior prevalence in general psychiatric patients is 4.4%26, and in college students is 3.7%27. The higher rates of impulsive behavior in our PD subjects as compared to the general population may result from factors such as altered dopamine regulation, executive dysfunction, having to contend with progressive disability, therapies such as dopaminergic medications and deep brain stimulation, or a complex interplay of many factors.
Clinical factors Associated with impulsive behavior in PD
Subjects reporting impulsive behavior in our study demonstrated a younger age of PD onset than subjects not reporting such behavior, in keeping with previous reports10,9. It remains unclear why younger age of PD onset is associated with impulsive behavior, but there is some evidence that younger PD patients represent a distinct biochemical entity28. Our subject age range was not substantially different from others reported 29,4. In contrast to other studies, however, we did not find male sex to have a statistically significant association with impulsive behavior.
A growing number of reports emphasize an association between impulsive behavior —especially, gambling—and the use of dopamine agonists to treat PD30,31,29,32,12,4, with pramipexole most frequently implicated9, although this remains controversial6,32,9,3,20. However, we have not found a significantly higher prevalence of impulsive behavior in subjects taking dopamine agonists, and we found no association between pramipexole use and impulsive behavior. Thus, other factors should be considered as well, such as the greater availability of gambling opportunities for the population we studied or a more susceptible patient population22.
In our study population, dopamine therapy abuse was reported at a prevalence of 10.9%, in contrast to 3.4 % by Pezzella and colleagues19, and 4% by Giovannoni, et al.16 and punding was observed at a prevalence of 7.8%. The reported prevalence of punding varies (1.4 – 14%), and is suspected to be underreported because of lack of awareness on the part of physicians, and patient reluctance to divulge details of abnormal behavior33.
When comparing overall impulsive behaviors, DBS and non-DBS patients were not significantly different from each other after adjusting for age, PD duration and LEDD. Previous reports have implicated DBS in the onset of pathological gambling34 and in increasing impulsiveness during decision making tasks35, but other investigators have reported a decrease in pathologic gambling 36,8, DDS37, and OCD severity38 following STN DBS surgery. Whether these improvements were a consequence of the reduction in dopaminergic medication doses postoperatively, or a direct effect from stimulation, is unknown. Our results highlight the need for additional studies to determine whether or not DBS causes or exacerbates impulsive behavior.
Study Limitations
Several limitations of this study must be acknowledged. First, not all impulsive behaviors were surveyed (see Bonvin et al., 20078, for instance), due, in part, to the length of time required for subjects to complete our survey instrument. Second, certain potentially important variables were not assessed: For instance, others have demonstrated that a correlation exists between impulse control disorders and positive family history for these disorders20. Unfortunately, the cross-sectional, questionnaire-based nature of our study precluded obtaining detailed information about family history of impulse control disorders and other psychiatric diseases, and detailed subject psychiatric history, as would be obtained in Structured Clinical Interviews. Therefore, we could not definitively rule-out the possibility that some percentage of impulsive behaviors were secondary to bipolar disorder or substance abuse, although all patient medical records were reviewed for such diagnoses. Lastly, 51.3% of clinic patients who were eligible did not consent to participate, and might represent a different sub-population from those who did participate, with respect to impulsive behavior. Although this fact raises the possibility of a non-responder bias, our study design precluded addressing this issue.
Conclusions
Our study provides evidence of a higher prevalence of impulsive behavior (29.7%) in PD than previously reported. In addition, because 26% of impulsive subjects reported multiple impulsive behaviors, PD patients need to be screened for a variety of impulsive behaviors. In contrast to previous reports, we noted that impulsive behaviors were more prevalent in women (40.0%) than men (24.1%). These results support the need for a longitudinal study to assess changes in impulsivity and impulsive behaviors in Parkinson's patients.
Acknowledgement
This research was supported by a Career Development Award from the National Center for Research Resources to Dr. Abosch (5K12-RR03358-03), and from the National Institute of Mental Health (K23 MH069754-01A1) to Dr. Grant. Dr. Grant has received research grants from Forest Pharmaceuticals, GlaxoSmithKline, and Somaxon Pharmaceuticals, and has been a consultant to Pfizer Pharmaceuticals and Somaxon Pharmaceuticals and a consultant for law offices as an expert in pathological gambling. The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Financial Disclosures:
This research was supported by a Career Development Award from the National Center for Research Resources to Dr. Abosch (5K12-RR03358-03), and from the National Institute of Mental Health (K23 MH069754-01A1) to Dr. Grant. Dr. Grant has received research grants from Forest Pharmaceuticals, GlaxoSmithKline, and Somaxon Pharmaceuticals, and has been a consultant to Pfizer Pharmaceuticals and Somaxon Pharmaceuticals and a consultant for law offices as an expert in pathological gambling.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grant JE. Impulse Control Disorders: A Clinician's Guide to Understanding and Treating Behavioral Addictions. 2008.
- 2.Voon V, Potenza MN, Thomsen T. Medication-related impulse control and repetitive behaviors in Parkinson's disease. Curr.Opin.Neurol. 2007;20:484–492. doi: 10.1097/WCO.0b013e32826fbc8f. [DOI] [PubMed] [Google Scholar]
- 3.Driver-Dunckley E, Samanta J, Stacy M. Pathological gambling associated with dopamine agonist therapy in Parkinson's disease. Neurology. 2003;61:422–423. doi: 10.1212/01.wnl.0000076478.45005.ec. [DOI] [PubMed] [Google Scholar]
- 4.Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch.Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbishettar V, Pal PK, Janardhan Reddy YC, et al. Is there a relationship between Parkinson's disease and obsessive-compulsive disorder?Parkinsonism. Relat Disord. 2005;11:85–88. doi: 10.1016/j.parkreldis.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Wolters EC, van der Werf YD, van den Heuvel OA. Parkinson's disease-related disorders in the impulsive-compulsive spectrum. J.Neurol. 2008;255(Suppl 5):48–56. doi: 10.1007/s00415-008-5010-5. [DOI] [PubMed] [Google Scholar]
- 7.Merims D, Giladi N. Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease. Parkinsonism.Relat Disord. 2008;14:273–280. doi: 10.1016/j.parkreldis.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Bonvin C, Horvath J, Christe B, et al. Compulsive singing: another aspect of punding in Parkinson's disease. Ann.Neurol. 2007;62:525–528. doi: 10.1002/ana.21202. [DOI] [PubMed] [Google Scholar]
- 9.Voon V, Thomsen T, Miyasaki JM, et al. Factors associated with dopaminergic drug-related pathological gambling in Parkinson disease. Arch.Neurol. 2007;64:212–216. doi: 10.1001/archneur.64.2.212. [DOI] [PubMed] [Google Scholar]
- 10.Evans AH, Lawrence AD, Potts J, et al. Factors influencing susceptibility to compulsive dopaminergic drug use in Parkinson disease. Neurology. 2005;65:1570–1574. doi: 10.1212/01.wnl.0000184487.72289.f0. [DOI] [PubMed] [Google Scholar]
- 11.Isaias IU, Siri C, Cilia R, et al. The relationship between impulsivity and impulse control disorders in Parkinson's disease. Mov Disord. 2008;23:411–415. doi: 10.1002/mds.21872. [DOI] [PubMed] [Google Scholar]
- 12.Voon V, Hassan K, Zurowski M, et al. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. 2006;66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- 13.Grant JE, Potenza MN. Compulsive aspects of impulse-control disorders. Psychiatr.Clin.North Am. 2006;29:539–51. x. doi: 10.1016/j.psc.2006.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- 15.Christenson GA, Faber RJ, de Zwaan M., et al. Compulsive buying: descriptive characteristics and psychiatric comorbidity. J.Clin.Psychiatry. 1994;55:5–11. [PubMed] [Google Scholar]
- 16.Giovannoni G, O'Sullivan JD, Turner K, et al. Hedonistic homeostatic dysregulation in patients with Parkinson's disease on dopamine replacement therapies. J.Neurol.Neurosurg.Psychiatry. 2000;68:423–428. doi: 10.1136/jnnp.68.4.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson's disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19:397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 18.Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch.Gen.Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- 19.Pezzella FR, Colosimo C, Vanacore N, et al. Prevalence and clinical features of hedonistic homeostatic dysregulation in Parkinson's disease. Mov Disord. 2005;20:77–81. doi: 10.1002/mds.20288. [DOI] [PubMed] [Google Scholar]
- 20.Weintraub D. Dopamine and Impulse Control Disorders in Parkinson's Disease. Ann.Neurol. 2008;64:S93–S100. doi: 10.1002/ana.21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ladouceur R, Jacques C, Chevalier S, Sévigny S, Hamel D. Prevalence of pathological gambling in Quebec in 2002. Can J Psychiatry. 2005;8:451–456. doi: 10.1177/070674370505000804. [DOI] [PubMed] [Google Scholar]
- 22.Pasternak AV, Fleming MF. Prevalence of gambling disorders in a primary care setting. Arch.Fam.Med. 1999;8:515–520. doi: 10.1001/archfami.8.6.515. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub D, Potenza MN. Impulse control disorders in Parkinson's disease. Curr.Neurol.Neurosci.Rep. 2006;6:302–306. doi: 10.1007/s11910-006-0022-y. [DOI] [PubMed] [Google Scholar]
- 24.Machado AG, Hiremath GK, Salazar F, et al. Fracture of subthalamic nucleus deep brain stimulation hardware as a result of compulsive manipulation: case report. Neurosurgery. 2005;57:E1318. doi: 10.1227/01.neu.0000187566.01731.51. [DOI] [PubMed] [Google Scholar]
- 25.Shaffer HJ, Hall MN, Vander Bilt J. Estimating the prevalence of disordered gambling behavior in the United States and Canada: a research synthesis. Am.J.Public Health. 1999;89:1369–1376. doi: 10.2105/ajph.89.9.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grant JE, Levine L, Kim D, et al. Impulse control disorders in adult psychiatric inpatients. Am.J.Psychiatry. 2005;162:2184–2188. doi: 10.1176/appi.ajp.162.11.2184. [DOI] [PubMed] [Google Scholar]
- 27.Odlaug BL, Grant JE. Prevalence of impulse control disorders in a college sample. 2008. [DOI] [PMC free article] [PubMed]
- 28.Gomez Arevalo G., Jorge R, Garcia S, et al. Clinical and pharmacological differences in early- versus late-onset Parkinson's disease. Mov Disord. 1997;12:277–284. doi: 10.1002/mds.870120303. [DOI] [PubMed] [Google Scholar]
- 29.Avanzi M, Uber E, Bonfa F. Pathological gambling in two patients on dopamine replacement therapy for Parkinson's disease. Neurol.Sci. 2004;25:98–101. doi: 10.1007/s10072-004-0238-z. [DOI] [PubMed] [Google Scholar]
- 30.Seedat S, Kesler S, Niehaus DJ, et al. Pathological gambling behaviour: emergence secondary to treatment of Parkinson's disease with dopaminergic agents. Depress.Anxiety. 2000;11:185–186. doi: 10.1002/1520-6394(2000)11:4<185::AID-DA8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 31.Gschwandtner U, Aston J, Renaud S, et al. Pathologic gambling in patients with Parkinson's disease. Clin.Neuropharmacol. 2001;24:170–172. doi: 10.1097/00002826-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Dodd ML, Klos KJ, Bower JH, et al. Pathological gambling caused by drugs used to treat Parkinson disease. Arch.Neurol. 2005;62:1377–1381. doi: 10.1001/archneur.62.9.noc50009. [DOI] [PubMed] [Google Scholar]
- 33.O'Sullivan SS, Evans AH, Lees AJ. Punding in Parkinson's disease. Pract.Neurol. 2007;7:397–399. doi: 10.1136/jnnp.2007.129015. [DOI] [PubMed] [Google Scholar]
- 34.Smeding HM, Goudriaan AE, Foncke EM, et al. Pathological gambling after bilateral subthalamic nucleus stimulation in Parkinson disease. J.Neurol.Neurosurg.Psychiatry. 2007;78:517–519. doi: 10.1136/jnnp.2006.102061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frank MJ, Samanta J, Moustafa AA, et al. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318:1309–1312. doi: 10.1126/science.1146157. [DOI] [PubMed] [Google Scholar]
- 36.Bandini F, Primavera A, Pizzorno M, et al. Using STN DBS and medication reduction as a strategy to treat pathological gambling in Parkinson's disease. Parkinsonism.Relat Disord. 2007;13:369–371. doi: 10.1016/j.parkreldis.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 37.Witjas T, Baunez C, Henry JM, et al. Addiction in Parkinson's disease: impact of subthalamic nucleus deep brain stimulation. Mov Disord. 2005;20:1052–1055. doi: 10.1002/mds.20501. [DOI] [PubMed] [Google Scholar]
- 38.Mallet L, Mesnage V, Houeto JL, et al. Compulsions, Parkinson's disease, and stimulation. Lancet. 2002;360:1302–1304. doi: 10.1016/S0140-6736(02)11339-0. [DOI] [PubMed] [Google Scholar]