Abstract
Neural development requires both synapse elaboration and elimination, yet relatively little is known about how these opposing activities are coordinated. Here we provide evidence Hts/Adducin can serve this function. We show that Drosophila Hts/Adducin is enriched both pre- and postsynaptically at the NMJ. We then demonstrate that presynaptic Hts/Adducin is necessary and sufficient to control two opposing processes associated with synapse remodeling: 1) synapse stabilization as determined by light level, ultrastructural and electrophysiological assays and 2) the elaboration of actin-based, filopodia-like protrusions that drive synaptogenesis and growth. Synapse remodeling is sensitive to Hts/Adducin levels and we provide evidence that the synaptic localization of Hts/Adducin is controlled via phosphorylation. Mechanistically, Drosophila Hts/Adducin protein has actin-capping activity. We propose that phosphorylation-dependent regulation of Hts/Adducin controls the level, localization and activity of Hts/Adducin, influencing actin-based synapse elaboration and spectrin-based synapse stabilization. Hts/Adducin may define a mechanism to switch between synapse stability and dynamics.
INTRODUCTION
It is well established that the developing nervous system requires the combined activities of synapse formation and elimination (Goda and Davis, 2003; Luo and O’Leary, 2005) and there is increasing evidence that this is also true for the maintenance of mature neural circuitry (Holtmaat and Svoboda, 2009; Xu et al., 2009). The molecular mechanisms that control synapse formation have been studied extensively and include modulation of the neuronal cytoskeleton, target recognition, synapse assembly and stabilization (Luo, 2002; Goda and Davis, 2003; Datwani et al., 2009). The opposing mechanisms that disassemble synaptic connections are beginning to emerge and include modulation of growth factor signaling, the submembranous spectrin/ankyrin skeleton, cell adhesion and cellular mechanisms that dismantle the neuronal membrane (Luo and O’Leary, 2005; Nikolaev et al., 2009; Koch et al., 2008; Pielage et al., 2008; Pielage et al., 2005; Watts et al., 2003; Massaro et al., 2009). In general these different molecular mechanisms are studied in isolation. Yet it is also clear that the phenomena of synapse formation and retraction can co-exist within the terminals of single neurons (Walsh and Lichtman, 2003). The mechanisms that serve to balance synapse stabilization and elimination within a neuron to achieve and maintain precise patterns of neural connectivity remain unknown.
To date, relatively few molecular mechanisms have been uncovered that participate in both synapse formation and elimination. Any such signaling system might reasonably be a point of control to balance synapse growth and elimination. Growth factor signaling is a type of global regulation that coordinates synapse formation and elimination with neuronal size (Huang and Reichardt, 2001). However, much less is known about how a balance between synapse stability and growth might be organized and executed locally within a nerve terminal. Potential candidates include adaptive immune signaling (Datwani et al., 2009) and control of cell adhesion. Remarkably, local regulators of the actin and microtubule cytoskeletons capable of balancing growth and elimination have yet to be clearly defined. Here we provide evidence that the actin-capping, spectrin-binding protein Adducin participates in both actin dependent synaptic growth and synapse stabilization. As such, Adducin may serve to coordinate these opposing activities that normally specify the shape, extent and stability of the presynaptic terminal.
The vertebrate genome encodes the three closely related adducin genes α-adducin, b-adducin and γ-adducin that form tetramers composed of either α/β- or α/γ-heterodimers (Matsuoka et al., 2000). Adducin is a key protein involved in the assembly of the sub-membranous Spectrin-actin network (Bennett and Baines, 2001). Adducins contain an N-terminal head domain, a neck domain and a C-terminal tail domain that includes a conserved 22 amino acid MARCKS-related domain (high homology to Myristoylated Alanine-Rich C Kinase Substrate protein) (Matsuoka et al., 2000). Studies using in vitro biochemistry have shown that Adducin tetramers can cap the fast growing ends of actin filaments (Kuhlman et al., 1996) and recruit Spectrin to the ends of these actin filaments (Bennett et al., 1988). The actin binding activity of Adducin has been mapped to the MARCKS domain (Li et al., 1998). In some systems, the phosphorylation of conserved serine residues within the MARCKS domain by protein kinase C abolishes the actin capping and Spectrin recruiting activities of Adducin (Chen et al., 2007; Kuhlman et al., 1996; Matsuoka et al., 2000). Thus, Adducin represents a regulated link between dynamic actin filaments and the stabilizing activity of the spectrin skeleton.
Adducin is highly expressed in the vertebrate nervous system (Bennett et al., 1988; Seidel et al., 1995). It is present in axonal growth cones and is concentrated within both presynaptic nerve terminals and postsynaptic dendritic spines (Matsuoka et al., 2000; Seidel et al., 1995). High levels of phosphorylated Adducin have been observed in hippocampal dendritic spines suggesting that the actin-binding properties of Adducin could be regulated during morphological spine plasticity (Matsuoka et al., 2000). Consistent with this possibility, b-adducin knockout mice have impaired LTP, LTD and learning deficits (Porro et al., 2009; Rabenstein et al., 2005). In addition, increased phosphorylation of γ-Adducin was observed during long-term synaptic facilitation in Aplysia (Gruenbaum et al., 2003).
The Drosophila genome encodes a single adducin homolog, encoded by the hu-li tai shao (hts) gene (Robinson et al., 1994; Yue and Spradling, 1992). In Drosophila, Hts/Adducin was first identified as an essential component of fusomes and ring canals that are required for normal oogenesis (Robinson et al., 1994; Yue and Spradling, 1992). In these tissues Hts/Adducin co-localizes with Spectrin and actin. Importantly, Drosophila hts/adducin encodes an isoform (Hts-M) that includes the highly conserved MARCKS domain required for actin binding (Petrella et al., 2007).
RESULTS
Drosophila hts/adducin encodes 4 potential isoforms that have been previously characterized during Drosophila oogenesis (Petrella et al., 2007). Importantly, two isoforms contain a conserved MARCKS domain at the C-terminus that is essential for the association with spectrin and actin-filaments in vertebrates. We observe only the shorter, 718-amino acid long, isoform in larval brain (Figure 1B and data not shown) and have termed this isoform Hts-M (Add 1 in Petrella et al., 2007). This isoform shares 38% overall identity with vertebrate α– Adducin and 64% identity within the MARCKS domain (www.flybase.org; Blast NCBI). Importantly, the MARCKS domain in Drosophila Hts-M includes a highly conserved central serine residue that can be phosphorylated by PKC/PKA to modulate the actin-recruiting and spectrin-binding properties of Adducin in vertebrates (Matsuoka et al., 2000) (Figure 1A).
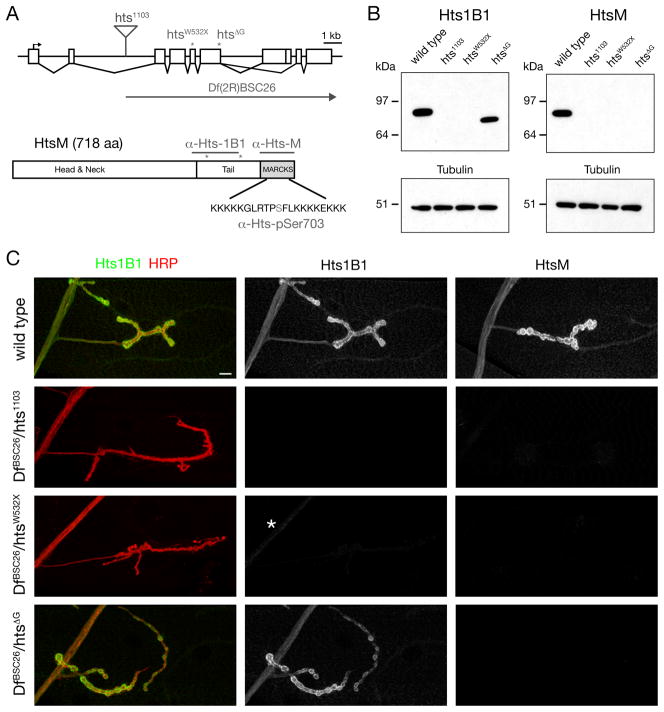
Figure 1. Hts-M is present pre- and postsynaptically at the Drosophila NMJ.
A) Schematic of the Drosophila hts locus. The position of the transposon and EMS-induced mutations are indicated (asterisks). The Hts-M isoform present at the Drosophila NMJ encodes a 718 aa long protein that contains a C-terminal MARCKS domain. Epitopes of the different Hts-M antibodies are indicated.
B) Western Blots of third instar larval brain extracts. Anti-Hts1B1 and anti-Hts-M recognize single protein bands at approximately 80 kDa. The htsΔG mutations results in a truncated protein that can be detected using anti-Hts1B1 but not with anti-Hts-M.
C) Analysis of Hts protein distribution at the Drosophila NMJ using the antibodies Hts-M and Hts1B1. In DfBSC26/hts1103 mutant animals no Hts1B1 or Hts-M staining can be detected. In DfBSC26/htsW532X mutant animals small remnants of protein can be detected by the Hts1B1 antibody in the motoneuron axon (asterisk). In DfBSC26/hts1103mutant animals significant amounts of Hts-M protein can be detected by Hts1B1, however no protein can be detected with the Hts-M antibody.
The organization of the hts locus is shown in a schematic that includes the position of three molecularly defined mutations and a deficiency that we use in this study (Figure 1A; Petrella et al. 2007). In addition, two antibodies, Hts1B1 and Hts-M are available that recognize unique epitopes within Drosophila Hts (Petrella et al., 2007; Zaccai and Lipshitz, 1996) (Figure 1A). These antibodies were used to confirm and define the molecular nature of our mutant hts alleles, and to analyze the presence of the Hts-M isoform at the neuromuscular junction (NMJ). Both antibodies clearly label the Drosophila neuromuscular junction. Staining is present in the presynaptic motor axons, throughout the postsynaptic muscle and is also concentrated within the postsynaptic muscle membrane folds, termed the subsynaptic reticulum (SSR), that surround the NMJ (Figure 1C).
The P-element insertion hts1103 completely eliminates immunostaining assayed in situ and on Western blots of larval brains, demonstrating the specificity of these antibodies (Figure 1B, C). The htsW532X mutation results in a premature stop codon and only a small amount of residual staining is detectable in the motor nerve when using the Hts1B1 antibody (Figure 1C), however no protein can be detected on the Western Blot (Figure 1B). The htsΔG mutation results in a truncated but stable protein lacking the MARCKS domain since staining with the Hts-M antibody is eliminated, while staining with the Hts1B1 antibody is retained. These results are in accordance with prior analysis of the hts mutations in oocytes (Petrella et al., 2007).
Loss of Hts/Adducin causes synapse retraction and elimination
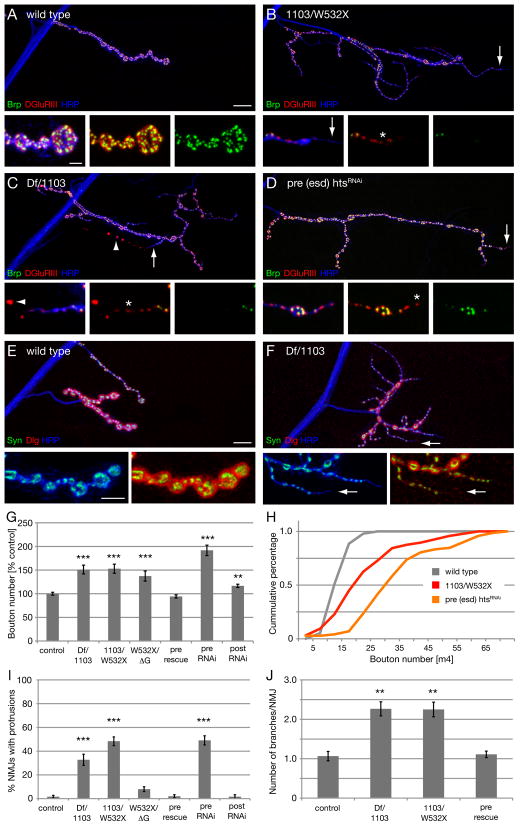
The integrity of the NMJ and of individual synapses within the NMJ can be analyzed by immunolabeling synaptic markers that reside pre- and postsynaptically (Eaton et al., 2002; Koch et al., 2008; Massaro et al., 2009; Pielage et al., 2008; Pielage et al., 2005, 2006). At the wild type NMJ, the active-zone associated protein Bruchpilot resides in precise apposition to clusters of postsynaptic glutamate receptors throughout the NMJ (Figure 2A). In addition, the presynaptic vesicle marker Synapsin and the presynaptic membrane marker anti-HRP are opposed throughout the NMJ by the postsynaptic marker Dlg (Budnik et al., 1996), which labels the SSR (Figure 2A and E). In hts mutant animals, by contrast, we observe large regions of NMJs where postsynaptic antigens are no longer opposed by presynaptic markers. Specifically, within a single NMJ, regions of the presynaptic nerve terminal are devoid of presynaptic Brp but retain postsynaptic glutamate receptor clusters. The regions of the presynaptic nerve terminal that lack Brp have a discontinuous, fragmented presynaptic membrane, whereas regions of the same NMJ that contain Brp have a normal, continuous presynaptic membrane (Figure 2A–D). Consistent with conclusions made in prior publications using an identical assay, the loss of presynaptic antigens and the associated fragmentation of the presynaptic membrane identify sites of synapse disassembly (Eaton et al., 2002; Koch et al., 2008; Massaro et al., 2009; Pielage et al., 2008; Pielage et al., 2005). Staining hts mutant animals with additional pre- and postsynaptic markers further supports this conclusion (Figure S1B).
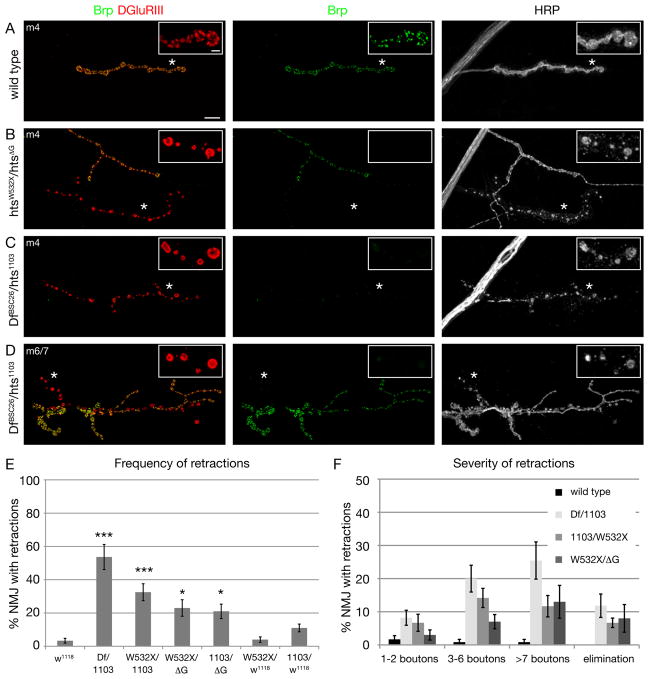
Figure 2. Mutations in hts result in severe synaptic retractions.
A-D) NMJs stained with markers for the presynaptic active zone protein Brp (green), postsynaptic glutamate receptors (DGluRIII, red) and the presynaptic membrane (HRP, white). The insets show individual channels at higher magnifications.
A) Wild type (w1118) muscle 4 NMJ, the presynaptic active zone protein Brp is found in perfect apposition to postsynaptic glutamate receptors.
B) A htsW532X/htsΔG mutant muscle 4 NMJ. Two motoneurons innervate the muscle. One NMJ (arrow) lacks all presynaptic Brp staining. The presynaptic membrane is fragmented and no longer continuous with the main motoneuron nerve. At higher magnification it becomes evident that the glutamate receptor clusters appear fused and are no longer organized into distinct uniform clusters.
C) A DfBSC26/hts1103 mutant muscle 4 NMJ. Only very small amounts of presynaptic Brp can be detected that are no longer organized into distinct puncta (asterisk). In addition, the presynaptic membrane is fragmented and discontinuous (arrow) indicating the retraction of synapses.
D) A DfBSC26/hts1103 mutant muscle 6/7 NMJ. At large parts of the NMJ the presynaptic marker Brp is absent although postsynaptic glutamate receptors are still present. The presynaptic membrane is fragmented at these sites (arrows). Scale bar in A applies to A-D and represents 10 μm; insets 2 μm.
E) Quantification of the frequency of synaptic retractions. All combinations of hts mutants show a significantly higher number of retractions compared to wild type (n > 10 animals and > 100 NMJs each; *** indicates p < 0.001; * indicates p < 0.05, ANOVA). We do not observe significant numbers of retractions in htsW532X/w1118 mutant animals demonstrating that the observed phenotypes in htsW532X/htsΔG are in part due to the lack of the MARCKS domain in the htsΔG mutation.
F) Quantification of the severity of synaptic retractions. The severity was quantified based on the number of postsynaptic bouton profiles that lack the presynaptic marker Brp. Numbers represent percentage of total NMJs scored (n as in E). Elimination indicates the complete absence of distinct Brp signal and the complete fragmentation of the presynaptic membrane from one motoneuron innervation site (examples in B and C). Error bars represent SEM.
The frequency and severity of synapse retractions were quantified in wild type and hts mutant animals. Wild type animals show virtually no evidence of synapse retraction, and when it does occur the retractions only encompass one or two synaptic boutons (Figure 2E, F; wt retraction 3.3%, n = 120). By contrast, hts mutations have a large increase in both the frequency and severity of NMJ retractions (23–53%, n > 100) both on muscle 4 and muscles 6/7 (Figure 2E, F and Figure S8). In addition, the frequency and severity of this phenotype correlates well with the molecular nature of our mutant alleles with hts1103/DfBSC26 representing a strong hypomorph or null combination and showing the most severe phenotype. It is worth noting that the htsΔG mutation, which results in a truncation before the C-terminal MARCKS domain, shows a significant increase in NMJ retractions compared to wild type, suggesting the importance of the Hts/Adducin actin-binding and capping activity for synapse stability (Figure 2E, F). However, the phenotype of the htsΔG mutation is not as severe as the null mutation. One possibility is that the truncated protein retains some actin-capping activity as indicated by in vitro studies (Li et al., 1998). Alternatively, there remains a stabilizing function that is independent of the MARCKS domain in vivo that is not predicted from the in vitro data. From these data we conclude that Hts-M is required for the stabilization of the presynaptic nerve terminal. Adducin interacts with the Spectrin skeleton in Drosophila and other systems (Bennett and Baines, 2001). The demonstration that Hts/Adducin is necessary for synapse stability is consistent with prior studies demonstrating that presynaptic α–/β-Spectrin and the spectrin-interacting adaptor protein Ankyrin2 are required for synapse stability (Koch et al., 2008; Pielage et al., 2008; Pielage et al., 2005). Indeed, the severity of synapse retraction and elimination is comparable in all three mutant genotypes. Consistent with this correlation, we observe a loss of the cell adhesion molecule Fasciclin II, the microtubule associated protein Futsch and Ankyrin2L (Ank2L) within retracting portions of the NMJ (Figure S1 C-H). In addition, we observe that Ank2L staining is perturbed within stable regions of the NMJ (Figure S1 H). Finally, loss of Hts/Adducin potentially has a minor impact on axonal transport. In hts mutant animals we observe increased levels of synaptic antigens in the axon. However, the overall organization of the axonal cytoskeleton is not impaired (Figure S2 A). The potential axonal transport defect is less severe than that observed in mutations in ank2 or after presynaptic knock down of the spectrin cytoskeleton (Figure S2 B; Pielage et al., 2005; Koch et al., 2008). We propose that Hts/Adducin is required to maintain the stability of the spectrin-Ankyrin skeleton, previously shown to be required for NMJ stability (Pielage et al., 2008; Pielage et al., 2005).
Altered Glutamate Receptor Clustering at Sites of Synapse Disassembly
During our analysis of NMJ disassembly, we observed an interesting change in postsynaptic glutamate receptor staining in regions of synapse retraction in hts mutant animals. In wild type, as shown previously, glutamate receptors are organized into discrete clusters within the area of a synaptic bouton (Figure 2A inset). By contrast, at sites of synapse retraction that show fragmentation of the presynaptic membrane, the glutamate receptor clusters appear confluent (Figure 2B–D insets). These receptors are no longer opposed by presynaptic Brp suggesting that they lack a functional presynaptic active zone. We speculate that the altered organization of postsynaptic glutamate receptor clusters could reflect a feature of ongoing synapse disassembly and degeneration, possibly reflecting the loss of trans-synaptic integrity (Eaton et al., 2002).
Ultrastructural Analyses
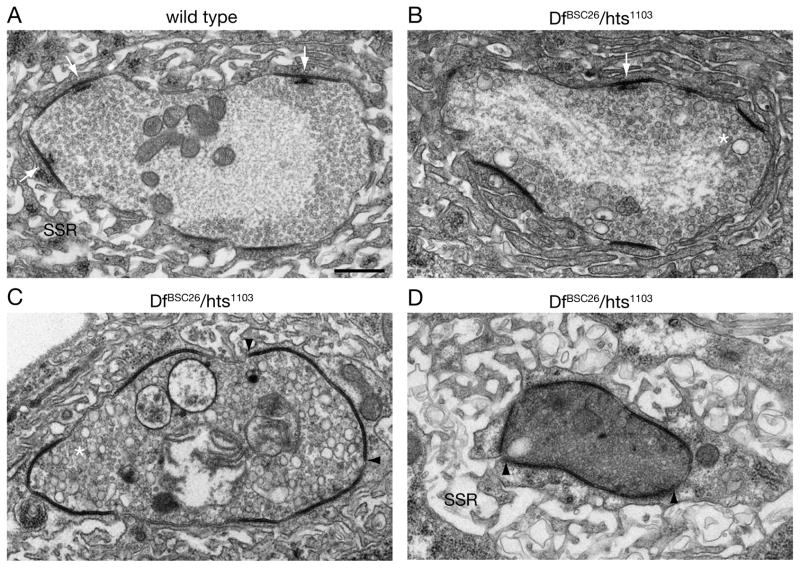
We next examined the NMJ of hts mutant animals at the ultrastructural level. A cross section through a wild type synaptic bouton is shown in Figure 3A. The synaptic bouton contains synaptic vesicles that are concentrated near electron dense active zones that include characteristic, electron dense presynaptic T-bars (white arrows). The bouton is surrounded by complex muscle membrane folds (SSR). We sectioned NMJs of three different DfBSC26/hts1103 mutant animals and present representative images of the retraction phenotype. We observe the appearance of abundant vacuoles within the presynaptic terminal (Figure 3B, C). The presence of vacuoles was previously associated with synapse retraction in dynactin, spectrin-RNAi and ankyrin2 mutant backgrounds (Eaton et al., 2002; Pielage et al., 2008; Pielage et al., 2005). We also observe the expansion of the electron dense membrane domains that are typically associated with active zones. These enlarged electron dense domains do not contain T-bars (Figure 3C, D). This is consistent with our light level analysis using the T-bar associated protein Brp as a marker for presynaptic active zones and glutamate receptor antibodies as a marker for the postsynaptic density (Kittel et al., 2006; Wagh et al., 2006). At retracting nerve terminals, Brp is absent and we observe enlarged confluent domains of glutamate receptor staining (Figure S3 A, B). To further address whether the enlarged electron densities might represent the enlarged glutamate receptor fields, we quantified glutamate receptor fields at the light level, comparing stable synapses (box1 in Figure S3 B) to retracting synapses (box2 in Figure S3 B) on the same muscle. This analysis reveals a significant 6-fold increase in the volume of glutamate receptor clusters and a corresponding 4-fold decrease in the number of separable glutamate receptor clusters per synaptic bouton. These data are consistent with the observation of nearly continuous electron dense regions in our EM analysis (Figure 3C, D). In addition, in sections where there appears to be a severe perturbation of presynaptic ultrastructure, we observe two additional phenotypes: 1) the postsynaptic SSR is less dense, consistent with the disassembly of the postsynaptic SSR as observed previously (Figure 3D) (Eaton et al., 2002; Pielage et al., 2005) and 2) the presynaptic mitochondria are severely perturbed even though muscle mitochondria, in the same image, appear normal. Finally, in all cases, boutons with wild type ultrastructure were also observed within each animal, consistent with our light level observations (data not shown). Thus, our ultrastructural data are consistent with the conclusion that hts is necessary to maintain the stability of the Drosophila NMJ.
Figure 3. Ultrastructural analysis of synapse retraction.
A) A bouton of a wild type muscle 4 NMJ. Electron dense membranes indicate individual release sites (synapses). At multiple synapses presynaptic T-bars are visible (white arrows). The presynaptic vesicles are uniformly distributed within the presynaptic bouton. The postsynaptic SSR is organized into distinct membrane folds.
B-C) Representative examples of three different DfBSC26/hts1103 mutant NMJs are shown.
B) In addition to normal synaptic vesicles larger presynaptic vesicles become apparent (asterisk) at DfBSC26/hts1103 mutant NMJs. Only one T-bar is present at a synapse (white arrow).
C) Larger diameter vesicles are present throughout the presynaptic bouton (asterisk). No T-bars are present and the area of individual electron dense regions is increased (boundaries marked by arrowheads).
D) Presynaptic organelles or vesicles can no longer be identified. Almost the entire membrane of the bouton is electron dense. In addition, the postsynaptic SSR is no longer organized into compact membrane folds.
Scale bar in A applies to A-D and represents 500 nm.
Neuronal Hts is required to maintain NMJ stability
We next sought to define where Hts is required for synapse stability. First, we expressed transgenically encoded RNAi under UAS control (htsRNAi) to knockdown Hts protein in either the presynaptic neuron or postsynaptic muscle. Presynaptic expression of htsRNAi significantly depletes Hts-M protein from the nervous system (Figure 4H). When htsRNAi is expressed presynaptically, with or without co-expression of dicer2 to enhance RNAi efficiency (Dietzl et al., 2007), we observe a significant increase in NMJ retractions compared to control (Gal4-driver lines crossed to w1118) (Figure 4B, G; 36 and 28% of muscle 4 NMJs show retractions compared to 1% in control; n > 98 NMJs for all genotypes). In addition, presynaptic knockdown of Hts shows all hallmarks of synapse retraction observed in hts mutants including loss of presynaptic antigens, persistence of postsynaptic antigens (note the confluence of glutamate receptor staining as documented above, Figure 4B inset) and the fragmentation of the presynaptic nerve membrane (Figure 4B, see below for additional quantification of bouton numbers). By contrast, although muscle-specific expression of htsRNAi was equally efficient at eliminating Hts protein (data not shown), it did not cause an increase in synapse retractions (Figure 4C, G; 2% of NMJs show retractions, n = 60). In addition, the morphology of the NMJ appears grossly normal following postsynaptic knockdown of Hts (Figure 4C, see below for further quantification).
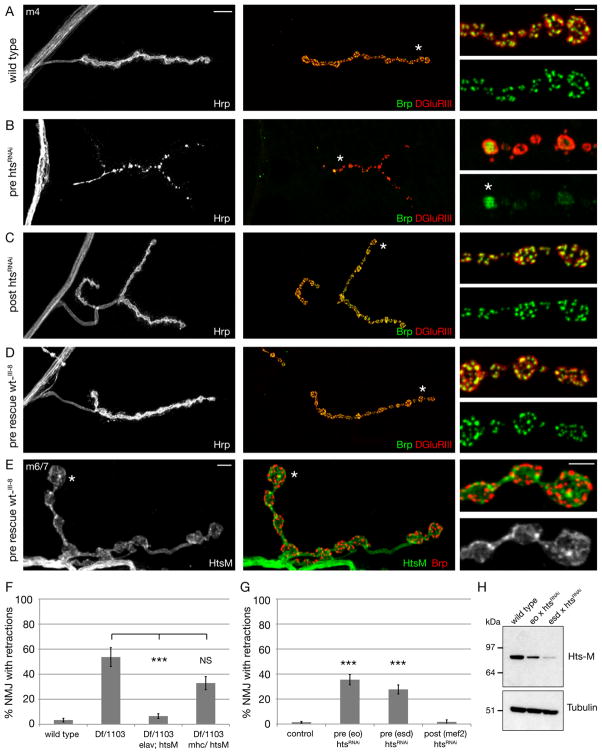
Figure 4. Presynaptic Hts-M is both necessary and sufficient to control synapse stability.
A-E) Muscle 4 NMJs stained for the presynaptic membrane (HRP, white), presynaptic active zones (Brp, green) and postsynaptic glutamate receptors (DGluRIII, red).
A) Wild type NMJ showing perfect apposition between pre- and postsynaptic markers.
B) Presynaptic knock down of hts through the expression of a transgenic UAS-htsRNAi line by a combination of presynaptic Gal4 lines results in severe presynaptic retractions. At some areas remnants of Brp remain, however these are no longer organized into distinct puncta but are uniformly distributed within the altered bouton (asterisk, inset).
C) Postsynaptic knock down of hts in muscle using mef2-Gal4. No defects in synapse stability can be observed.
D) Expression of Hts-M in the presynaptic motoneuron using elav-Gal4 in hts mutant animals (elavGal4; DfBSC26/hts1103; UAS hts-wt_III-8/+) rescues all synapse stability defects associated with the hts mutation. Scale bar in A applies to A-D and represents 10 μm. Scale bar in insets represents 2 μm.
E) In the rescued animals (pre-rescue hts, genotype as in D) we can visualize the localization of presynaptic Hts protein due to the absence of muscle Hts protein. Muscle 6/7 NMJ stained for Hts-M (green), the presynaptic active zone protein Brp (red) and the presynaptic membrane (HRP, white). Scale bar 5 μm, inset 2 μm.
F) Quantification of synapse retractions in hts mutant and rescued animals. Presynaptic expression of Hts-M in hts mutant animals (elavGal4; DfBSC26/hts1103; UAS hts-wt_III-8/+) significantly rescues the synapse retraction phenotype (p>0.001, Anova). Postsynaptic expression of Hts-M in hts mutant animals (DfBSC26/hts1103; UAS hts-wt_III-8/mhcGal4) does not significantly rescue the retraction phenotype. The morphology of the NMJ is severely altered in these animals (see Figure S4). (n = number of NMJs: wt = 120, DfBSC26/hts1103 = 107, DfBSC26/hts1103; elav/Hts-M = 230, DfBSC26/hts1103; mhc/Hts-M = 62).
G) Quantification of synapse retractions after pre- or postsynaptic knock down of Hts. Abbreviations: control = Gal4 x w1118; pre (eo) = elavGal4; ok371Gal4; pre (esd) = elavGal2; scaGal4 UAS-dcr2; post (mef2) = mef2Gal4. (n = number of NMJs: control = 280, pre (eo) htsRNAi = 98, pre (esd) htsRNAi = 110, post (mef2) htsRNAi = 60; *** indicates p>0.001, Anova). Error bars represent SEM.
H) Western blot of Hts protein levels in third instar larval brains of wild type and after presynaptic htsRNAi expression.
To further address the tissue-specific function of Hts, we expressed an hts cDNA in the hts mutant background using the GAL4/UAS expression system. We used a cDNA encoding the 718-aa long isoform of Hts that contains the conserved C-terminal MARCKS domain (Hts-M). The MARCKS domain containing isoform is the major Hts isoform expressed in the larval nervous system as wild type Hts-M and UAS-expressed Hts-M protein run at equal sizes on Western blots of larval brains (see below). We do not detect the OvHts isoform (1156 aa) that is present in nurse cells and oocytes (Petrella et al., 2007; data not shown). We find that presynaptic expression of Hts-M using elav-GAL4 in the hts mutant background (DfBSC26/hts1103) rescues the presynaptic retraction phenotype (Figure 4D, F; retraction frequency 7% compared to 54% in mutant animals, n > 98 NMJ). Importantly, this presynaptic rescue assay allows us to visualize Hts-M protein that is present in the presynaptic nerve terminal because we only re-supply the protein in the motoneuron, not in the muscle. Hts-M protein is present within the presynaptic nerve terminal where it localizes at or near the presynaptic membrane but is not present within active zones marked by Brp (Figure 4E). In contrast, postsynaptic expression of Hts-M causes a slight, though not significant, reduction in the frequency of synapse retraction. However, we find that ectopic expression of Hts-M in muscle severely disrupts muscle and NMJ morphology (Figure S4). Thus, is it not possible to accurately quantify the postsynaptic contribution of Hts-M to NMJ stability when it is expressed via UAS-GAL4. From these data, both RNAi-mediated Hts knockdown and transgenic rescue, we conclude that Hts is required presynaptically to stabilize the NMJ.
Electrophysiological analysis of synaptic transmission
We next analyzed synaptic transmission, comparing wild type with the hts1103/DfBSC26 allelic combination and with animals expressing htsRNAi in presynaptic neurons. We find a significant increase in the average quantal amplitude in the hts1103/DfBSC26 mutant animals and a corresponding decrease in average quantal content (Figure S5 A-C). However, the increased mepsp amplitude was not observed in animals expressing htsRNAi presynaptically. This could be due to a less severe knockdown of Hts protein. Alternatively, the increased average mepsp amplitude could reflect a postsynaptic activity of Hts. This possibility is consistent with the prior demonstration that knockdown of postsynaptic spectrin causes a comparable increase in quantal size (Pielage et al., 2006). We also find that synaptic transmission is considerably more variable in hts loss of function animals (Figure S5 D). We plotted average mepsp versus average quantal content for individual NMJ recordings. There is more variation both in mepsp amplitude and quantal content compared to wild type. This is consistent with prior studies demonstrating highly variable recordings at NMJ undergoing retraction (Massaro et al., 2009; Pielage et al., 2008; Pielage et al., 2005). The observed increase in release variability is less severe than after knockdown of α–/β-spectrin, which might be accounted for by enhanced NMJ growth that is unique to hts mutant animals (see below). Together, these data indicate that Hts/Adducin does not have a strong, direct influence on either spontaneous or evoked vesicle release. The small changes in transmission that we observe are likely to be secondary to changes in NMJ morphology.
Loss of presynaptic Hts/Adducin promotes filopodia-based NMJ extension and synaptogenesis
To this point, the phenotypes caused by loss of hts/adducin strongly resemble the effects observed following loss of presynaptic α-/β-Spectrin (Pielage et al., 2005) or presynaptic Ankyrin2L (Pielage et al., 2008). This is consistent with prior demonstration that Adducin is a component of the submembranous spectrin-Ankyrin lattice (Bennett and Baines, 2001). We now describe a phenotype of NMJ expansion that is completely unique to the loss of hts/adducin.
The loss of Hts causes two striking phenotypes of enhanced synaptic growth. First, the number of type Ib synaptic boutons is increased by approximately 50% in hts mutant animals compared to wild type controls. This increase in bouton number is observed in all of our mutations and is even stronger (192% compared to control) following RNAi-mediated presynaptic knockdown of Hts (Figure 5B–H). Furthermore, this phenotype is completely rescued by presynaptic expression of Hts-M in hts mutant animals (“pre rescue” in Figure 5G). The increase in total bouton number is particularly remarkable given that many of the NMJ that we quantified are also undergoing significant synapse retraction (see above). This aspect is reflected in the large variance of bouton number that we observe in both hts mutant and htsRNAi animals (see histogram, Figure 5H). Thus, the quantification of bouton number most likely underestimates the growth-promoting effect caused by loss of presynaptic Hts/Adducin. Based on these data, we conclude that Hts/Adducin also has a potent activity that restricts the expansion and elaboration of the presynaptic nerve terminal.
Figure 5. Loss of presynaptic hts leads to increased NMJ growth.
A-D) Muscle 4 NMJs stained for presynaptic active zones (Brp, green), postsynaptic glutamate receptors (DGluRIII, red) and the presynaptic membrane (HRP, blue).
A) Wild type NMJ. Insets show the precise apposition between pre- and postsynaptic markers.
B) An hts1103/htsW532X mutant NMJ. Mutant animals show a significant increase in NMJ span and bouton number. Aberrant formation of new synaptic boutons can be observed. Long membrane protrusions form with a smaller diameter than normal type Ib boutons. At the ends of these protrusions (arrow, insets) the appearance of small glutamate receptors can be observed that are not yet opposed by presynaptic Brp (asterisk).
C) A DfBSC26/hts1103 mutant NMJ. On the same muscle a retracting branch and a overgrown NMJ can be observed. The retraction can be clearly identified through the absence of Brp and the simultaneous fragmentation of the presynaptic membrane (arrowhead). In contrast, new synapses form along continuous protrusions of the presynaptic membrane (asterisk).
D) Presynaptic knock down of hts results in an identical phenotype. Scale bar in A applies to A-D and represents 10 μm, scale bar in inset represents 2 μm.
E-F) Muscle 4 NMJs stained for the presynaptic vesicle marker Synapsin (green), the postsynaptic SSR marker Dlg (red) and the presynaptic membrane (HRP, blue). Insets show distal part of the NMJ at higher magnification. Scale bar in E applies to E, F and represents 10 μm, scale bar in inset 5 μm.
G) Quantification of boutons numbers. Numbers are normalized to control genotypes. The NMJ overgrowth can be completely rescued through presynaptic expression of Hts-M in DfBSC26/hts1103 mutant animals (pre rescue). There is no difference in muscle size between genotypes. (n = NMJs per genotype: control = 96, hts1103/DfBSC26 =96, hts1103/htsW532X = 96, htsW532X/htsΔG = 40, pre rescue = 96, pre RNAi = 72, post RNAi = 64; *** indicates p < 0.001, ** indicates p < 0.01 compared to control, ANOVA).
H) Histogram of bouton numbers for selected genotypes from G.
I) Quantification of protrusions. Protrusions were quantified as HRP positive protrusions that show glutamate receptors with little or no opposing Brp staining (n = NMJs per genotype: control = 120, hts1103/DfBSC26 =107, hts1103/htsW532X = 120, htsW532X/htsΔG = 100, pre rescue = 98, pre RNAi = 110, post RNAi = 60; *** indicates p < 0.001 compared to control, ANOVA).
J) Quantification of the number of branches per NMJ (** indicates p>0.01, ANOVA). Numbers as in (I). Error bars represent SEM.
A second remarkable feature of hts mutant NMJs is the appearance of abundant, small caliber membrane protrusions from the NMJ. These membrane protrusions retain presynaptic proteins like Synapsin, and Brp and postsynaptic glutamate receptors, indicating that they may contain functional active zones (Figure 5B, C, D, F). In many cases we observe small glutamate receptor clusters at the distal ends of these protrusions that are not yet opposed by presynaptic Brp. This suggest that these are newly forming synapses as live imaging studies previously demonstrated the appearance of postsynaptic glutamate receptors prior to the appearance of the presynaptic active zone marker Brp (Rasse et al., 2005).
Different motoneurons elaborate terminals of different caliber at the Drosophila NMJ. The type Ib boutons are large diameter boutons. The type Is boutons often co-innervate muscles with type Ib. The type II and type III boutons are much smaller caliber boutons and express peptide neurotransmitters. The small caliber protrusions that we observed originate from existing type Ib boutons, demonstrating that these protrusions represent altered growth of type Ib processes. These small caliber protrusions are longer and smaller in diameter than “satellite boutons” that have been observed previously in mutations that disrupt synaptic vesicle endocytosis or actin regulators (Coyle et al., 2004; Koh et al., 2004; Marie et al., 2004). Thus, these small caliber protrusions represent a distinct modification of normal synaptic growth. To quantify this effect we counted the number of NMJ that contain small caliber protrusions that emerge from existing type Ib terminals and the average number of protrusions per NMJ. The number of synaptic protrusions is significantly increased in all of the hts mutations and after presynaptic knock down of hts and the severity of this phenotype correlates well with the severity of the allelic combination tested (Figure 5I). These protrusions might directly contribute to the altered growth in hts mutant animals because we observe a significant, more than two-fold, increase in the number of branches of type Ib terminals on muscle 4 compared to wild type animals (Figure 5J). Importantly, we can rescue all aspects of altered synapse morphology, protrusions and branching, by presynaptic expression of Hts-M in hts mutant animals (Figure 5I, J).
Synaptic protrusions are actin rich structures
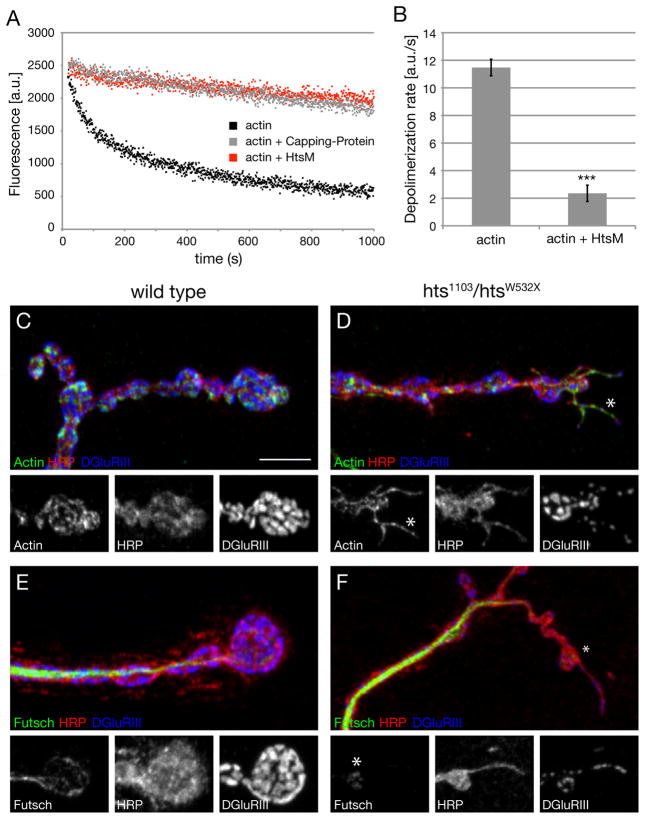
The phenotype of synaptic overgrowth observed in hts mutations is not observed in animals lacking presynaptic α-/β-Spectrin or Ankyrin2L, suggesting that this phenotype may be derived from a unique activity of Hts/Adducin. Prior work in other systems has demonstrated that Adducin is an actin-capping protein that caps the barbed end of actin filaments (Kuhlman et al., 1996; Li et al., 1998). The appearance of small caliber membrane protrusions would correlate well with a loss of actin capping activity within the presynaptic nerve terminal. We tested the potential actin capping activity of Hts-M by monitoring the decay in fluorescence of pyrene labeled actin filaments in the presence of latrunculinB. The addition of purified Hts-M significantly prevents the depolymerization of actin-filaments to a similar extent as Capping Protein. The depolymerization rate drops from 11.7 to 1.2 a.u./s (n = 3) (Figure 6A, B). This demonstrates that Drosophila Hts-M, similar to vertebrate Adducin, has significant actin capping activity. Therefore, we hypothesize that synaptic overgrowth and the appearance of small caliber synaptic protrusions may be related to the loss of actin-capping activity normally provided by presynaptic Hts-M.
Figure 6. Drosophila Hts-M is an actin-capping molecule.
A) Pyrene labeled actin filaments were diluted to 500nM into KMEI plus 1 μM latrunculin B to stimulate depolymerization. The assay was performed alone (black) or in the presence of Hts-M (red) or capping protein (grey). Hts-M protects actin filaments from depolymerization to a similar extent as capping protein.
B) Rate of depolymerization of actin filaments from (A), calculated from the initial 60 seconds of the reactions (3 independent trials per assay). The presence of Hts-M in the assay reduces the depolymerization rate from 11.696 to 1.221 a.u./s., *** indicates p< 0.001, Student’s T-test; Error bars represent SEM).
C) Presynaptic actin filaments were visualized through the expression of the GFP-tagged f-actin binding domain of Drosophila Moesin (UAS-GMA).
D) In the absence of Hts-M, membrane protrusions are evident at many sites of the presynaptic nerve terminal. Actin filaments are present throughout the protrusions (asterisk, insets).
E) In wild type, microtubules are present and form loops in distal synaptic boutons (anti-Futsch).
F) In hts mutant animals Futsch staining is absent in the distal parts of the protrusions. Scale bar in C corresponds to C-F 5 μm.
Recently, actin-capping proteins have been hypothesized to regulate a balance between actin-based filopodial extension and the formation of lamellipodial actin networks by Arp2/3-mediated branching (Akin and Mullins, 2008; Bear et al., 2002; Iwasa and Mullins, 2007; Mejillano et al., 2004; van der Gucht et al., 2005). Monomeric actin has a higher affinity for the barbed end of elongating actin filaments than for Arp2/3, and increasing the concentration of capping protein in vitro inhibits filament elongation to promote lamellipod formation by Arp2/3 (Akin and Mullins, 2008). Thus, loss of an actin capping protein might be expected to bias a cell toward actin filament extension. Consistent with this hypothesis, depletion of capping protein results in a dramatic increase of actin-rich filopodia in tissue culture (Mejillano et al., 2004). Similarly, loss of Eps8, a protein with actin-capping activity, causes the appearance of actin-rich filopodia in cultured neurons (Menna et al., 2009). Based upon these prior studies, we hypothesize that loss of Hts-M/Adducin-mediated actin capping causes actin-based filopodia extensions at the nerve terminal. If so, the small caliber protrusions that we observe at the NMJ should be actin-rich structures.
We examined filamentous actin within the presynaptic nerve terminal of wild type and hts mutant animals by expression of the f-actin binding domain of Drosophila Moesin (UAS-GMA) (Dutta et al., 2002). Consistent with prior studies examining actin at the Drosophila NMJ (Nunes et al., 2006), actin is organized into a network near the plasma membrane including the presence of actin patches that are distributed throughout the NMJ (Figure 6C). In hts mutants, we find that the small caliber nerve terminal protrusions that are opposed by small postsynaptic glutamate receptor clusters are actin rich structures, resembling actin-based filopodia extensions in other systems (Figure 6D, insets).
We next asked whether the small caliber protrusions also contain bundled microtubules. In wild type animals, the microtubule-associated protein Futsch labels a core of bundled microtubules that extend throughout the NMJ including all distal boutons (Figure 6E, Figure S6 C; Roos et al., 2000). Futsch-positive microtubules do not invade the small caliber, actin-based protrusions we observed in the hts mutants (Figure 6F, Figure S6 D, insets). We then analyzed the distribution of the spectrin adaptor protein Ank2L. In wild type NMJ Ank2L is present beneath the plasma membrane and provides a potential link between cell adhesion molecules, the spectrin skeleton and presynaptic microtubules (Figure S6 A, C; Koch et al., 2008; Pielage et al., 2008). Similar to Futsch, Ank2L is not present in the distal parts of the actin-rich protrusions (Figure S6 B, D, inset). Finally, we stained the small caliber protrusions in hts mutants for the cell adhesion molecule Fasciclin II (FasII). FasII is essential for the maintenance of the NMJ as a trans-synaptic homophilic cell adhesion molecule and normally delineates the NMJ (Schuster et al., 1996) (Figure S6 E). We find that FasII is present in the small caliber protrusions indicating that these structures may be stabilized by homophilic cell adhesion (Figure S6 F). However, postsynaptic Dlg levels are low, providing additional evidence that these structures may be newly formed, prior to the elaboration of the postsynaptic SSR (Figure S6 F). Based upon these data, we propose that the small caliber protrusions observed in hts mutants are derived from increased actin-filament formation caused by the lack of Hts-M-dependent actin capping. Note, however, that in the htsΔG mutation lacking the MARCKS domain we do not observe significant protrusions but we do observe increased growth. It is possible that some actin capping activity is retained in this mutant based upon prior in vitro biochemistry on vertebrate Adducin proteins (Li et al., 1998) and this is sufficient to suppress protrusion formation (see also discussion).
Hts/Adducin overexpression blocks extension of small caliber presynaptic terminals
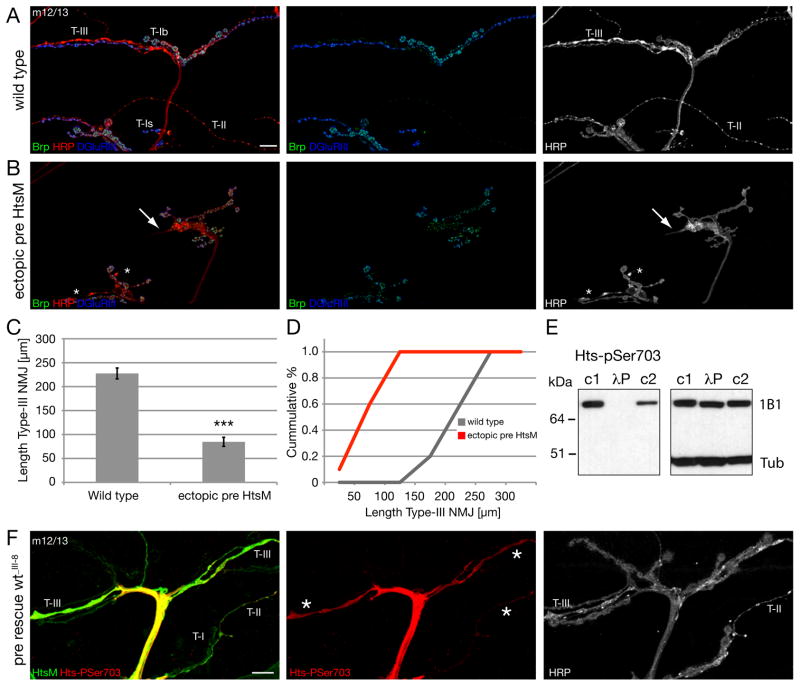
If loss of the actin-capping activity of Hts promotes the formation of actin-based filopodial extensions from an existing nerve terminal, then over-expression of Hts-M should block this process. We overexpressed high levels of Hts-M presynaptically and examined synapse morphology at muscles 12 and 13. These muscles are innervated by motoneurons that form large diameter type Ib boutons as well as small caliber type II and type III nerve terminals (Figure 7A). Overexpression of Hts-M severely impacts the extension and growth of the small caliber type II and type III synaptic bouton arborizations (Figure 7B). The motoneurons navigate to the NMJ, but fail to extend on the muscle surface. In addition, the morphology of the remaining type III terminals is clearly altered (Figure 7B arrows). By contrast, the large caliber type Ib boutons are present and elaborate at the nerve terminal. The quantification of the total length of type III terminals on muscle 12 reveals a significant, 2.7 fold reduction (Figure 7C, D). These data support the hypothesis that the actin-capping activity of Hts/Adducin may control the shape and extent of nerve terminal growth, particularly of the small caliber synaptic aborizations. Interestingly, the small caliber nerve terminals (type II and type III) are the most dynamic structures in the neuromuscular system and are strongly influenced by changes in neural activity (Budnik et al., 1990). This raises the possibility that Hts activity might be regulated to control synaptic growth.
Figure 7. Ectopic expression of Hts-M can restrict synapse formation and growth.
A-C) NMJs on muscles 12/13 stained for the presynaptic active zone protein Brp (green), the presynaptic membrane (HRP, red) and postsynaptic glutamate receptors (DGluRIII).
A) Wild type NMJ. Type II and III synapses cover large areas of muscles 12 and 13.
B) Expression of high levels of Hts-M presynaptically in a wild type background (elavGal4; scaGal4 crossed to 2 copies of UAS-Hts-M). Synapse formation of type II and type III synapses is severely suppressed. The arrows indicate the tip of type III synapses with altered morphology and the asterisks indicate type II synapses.
C, D) Quantification of NMJ length of type III synapses. The ectopic expression of Hts-M reduces the mean total length of type III synapses on muscle 12 from 227 μm to 84 μm (n = 10); p < 0.001 compared to wild type, ANOVA; Error bars represent SEM).
E) The Hts-pSer703 (UPS) antibody recognizes only phosphorylated Hts-M. In untreated larval brain extracts (c1 and c2) anti-Hts-pSer703 detects a protein of identical size as the Hts-1B1 antibody (right blot). The signal is absent in larval brain extracts treated for 5 min with l-phosphatase (lP). c1: identical buffer as in lP treated lane including phosphatase inhibitors; c2: standard Ripa buffer including phosphatase inhibitors. The image on the right is the same Western blot after stripping analyzed with anti-Hts-1B1 and anti-Tubulin antibody.
F) To analyze the distribution and phosphorylation level of presynaptic Hts-M we stained hts mutant animals that expressed Hts-M only presynaptically (elavGal4; DfBSC26/hts1103; UAS hts-wt_III-8/+). In these animals all phenotypes associated with hts mutations are rescued. We analyzed the distribution of the Hts-M and phospho-Hts at muscles 12/13 where we can simultaneously analyze type I, II and III synapses as stated in A. While we detect no or only very low levels of phosphorylated Hts-M in type I boutons we observe high levels of phosphorylated Hts-M in both type II and type III synapses. Scale bar in A corresponds to A, B, F 10 μm.
Evidence for phosphorylation-dependent control of synaptic Hts/Adducin
The spectrin binding and actin recruiting functions of Adducin, as well as its subcellular localization, are controlled by phosphorylation in several tissues in vertebrates. For example, in resting platelets, dephosphorylated Adducin is complexed with the submembranous spectrin skeleton where it may cap actin filaments and inhibit filopodia formation. During platelet activation Adducin becomes phosphorylated, released from the submembranous spectrin skeleton and aggregates in the cell interior. It is believed that the translocation of Adducin removes actin-capping activity from the membrane and enables the observed change in platelet cell shape that include the formation of numerous filopodia (Barkalow et al., 2003). By extension, we might expect to observe phosphorylated Hts/Adducin at synapses undergoing actin-based extension and growth. We tested this possibility using available phospho-specific antibodies. The anti-phospho-Adducin (Ser724) antibody (Upstate/Millipore) was raised against a peptide corresponding to amino acids 720–728 of rat α-Adducin. This peptide FRTP[pS]FLKK is, except of the first amino acid, identical to the core sequence of the MARCKS domain of Drosophila Hts-M (see Figure 1A). We refer to this antibody as Hts-pSer703 based on the position of the phosphorylated Serine in the Drosophila Hts-M sequence.
First, we demonstrate that Hts-pSer703 is, indeed, a phospho-specific Hts antibody that works in situ at the Drosophila NMJ (Figure S7 A, B). Hts-pSer703 staining is observed both in the presynaptic motor nerve and throughout the muscle (Figure S7 A) where it co-localizes with Hts-M. Importantly, all staining is absent in hts mutant animals indicating specificity for Drosophila Hts-M (Figure S7 B). To demonstrate that Hts-pSer703 only recognizes phosphorylated Hts-M we analyzed larval brain extracts in the presence or absence of λ-phosphatase. The λ-phosphatase treatment completely abolishes any signal on the Western Blot (Figure 7E). In addition, a small downshift of the Hts-M protein can be detected when analyzing the extract with a general Hts antibody suggesting several phosphorylations of Hts-M in vivo (Figure 7E, right blot Hts1B1). Therefore, we can conclude that a subset of Hts-M is phosphorylated both in the presynaptic nerve and in postsynaptic muscle.
In order to determine whether phosphorylated Hts-M is present within the presynaptic nerve terminal we used our presynaptic rescue assay that allows the visualization of presynaptic Hts-M protein in absence of postsynaptic Hts-M protein. We, first, examined type II and III terminals on muscle 12/13 that are most sensitive to Hts-M over-expression (see above, Figure 7B–D). We observe Hts-M and Hts-pSer703 staining throughout the terminal of both type II and type III boutons (Figure 7F). Interestingly, while Hts-M is clearly present in type Is and Ib terminals on the same muscles we do not observe significant levels of Hts-pSer703 staining in these boutons. Similarly, if we analyze Hts-M and Hts-pSer703 in type Ib boutons on muscle 4 we find that Hts-pSer703 staining is restricted to the motor nerve and stops just prior to where the motoneuron contacts the muscle cell. There is no, or only very low levels, of phosphorylated Hts-M protein in the presynaptic terminal of type Ib boutons, although there is clearly abundant Hts-M protein within the presynaptic nerve terminal (Figure S7 C). We conclude that Hts-M is de-phosphorylated within type Ib boutons. We hypothesize, therefore, that Hts-M may be regulated by posttranslational phosphorylation within small caliber type II and type III terminals, and possibly maintained in a de-phosphorylated state in type Ib boutons. This differential regulation may account for the enhanced dynamics and plasticity of type II and III nerve terminals compared to the larger caliber type Ib terminals.
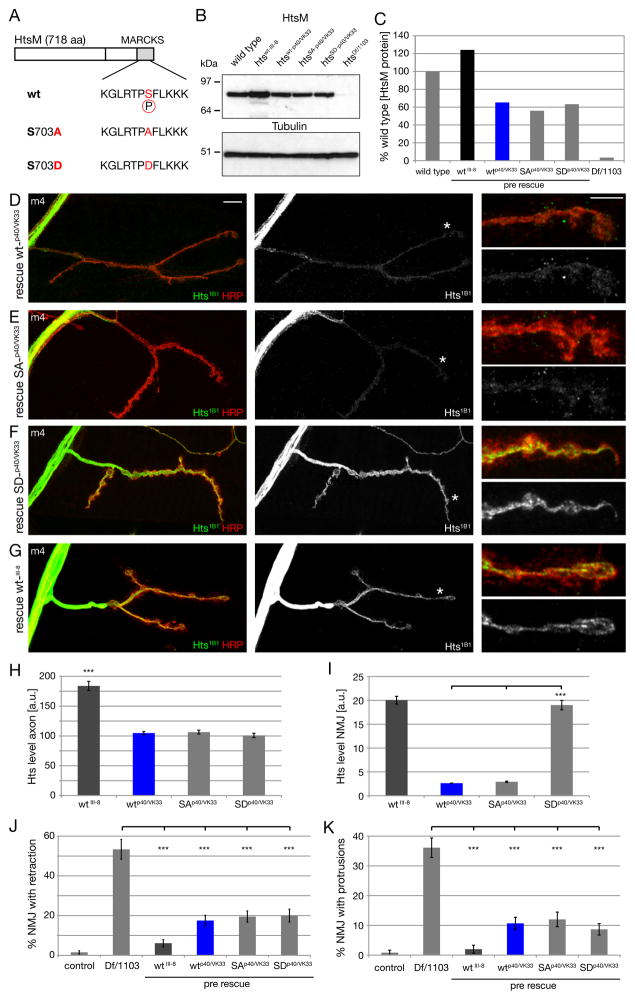
We next tested the hypothesis that the phosphorylation status of Hts-M might control the localization of the Hts protein and the function of Hts during NMJ growth and stabilization. Therefore, we generated htsM-transgenes harboring either phospho-mimic (S703D) or non-phosphorylatable (S703A) mutations within the HtsM MARCKS domain (Figure 8A). It was necessary to precisely control transgene expression levels in order to compare synaptic protein levels between phospho-mimic and non-phosphorylatable transgenes. To address this, we took advantage of the recently development phi-mediated site-specific integration system in Drosophila (Venken and Bellen, 2006). We generated transgenic lines with transgenes inserted at specific genomic integration sites for wild type (wt), phospho-mimic (SD) and non-phosphorylatable (SA) forms of Hts-M. We had to generate stocks that allow presynaptic expression of two UAS-insertions (attP40 and VK00033 insertions of the same transgenes) in the background of the hts mutation to achieve significant expression levels in motoneurons (see methods). First, we assayed expression levels of HtsM protein in the larval brains of these rescued animals (e.g. for wild type: htswt-p40/VK33 = elavGal4; hts1103 UAS-htsM-wtp40/Df(2R)BSC26; UAS-htsM-wtVK33). We find equivalent protein expression levels for each genotype assayed by Western Blot (Figure 8B, C). Each of these site integrated Hts-M variants is expressed at approximately 60% of wild type Hts-M levels (Figure 8B, C). By comparison, the wild type Hts-M transgene (wtIII-8 = random P-element insertion on the third chromosome) that we used in our prior rescue experiments is expressed at approximately 120% of wild type levels (Figure 8B, C). Thus, we have a system that allows us to express wild type and modified Hts proteins in the hts mutant background and make direct comparisons between these genotypes regarding synaptic protein levels and phenotypic rescue.
Figure 8. Control of synaptic Hts-M localization via phosphorylation of S703.
A) Schematic of the wild type, non-phosphorylatable (S703A) and phospho-mimic (S703D) Hts-M sequences.
B) Western Blot of third instar larval brains comparing Hts-M levels in wild type, mutant and the different presynaptic rescue animals.
C) Quantification of the Western Blots. We observe identical expression levels between the site-integrated constructs wt-p40/VK33, SA-p40/VK33 and SD-p40/VK33 in the mutant background. The P-element mediated insertion wt_III-8 expresses approximately 2-fold higher levels of Hts-M compared to the site-integrated constructs and approximately 120% of wild type. The site-integrated insertions express only approximately 60% of wild type Hts-M levels.
D-G) Visualization of axonal and presynaptic HtsM levels in rescued animals expressing different HtsM constructs presynaptically (genotypes as in B, C) on muscle 4. The site-integrated constructs (D-F) express identical levels of wt, SA and SD mutant forms of HtsM. Animals are stained with Hts-1B1 and Hrp to mark the presynaptic membrane.
D) Expression of wild type HtsM results in very low levels of HtsM within the presynaptic nerve terminal.
E) Expression of non-phosphorylatable HtsM (SA) results in similarly low levels of HtsM within the presynaptic nerve terminal.
F) Expression of a phospho-mimic form of HtsM (SD) results in highly abundant HtsM protein within the presynaptic nerve terminal.
G) The P-element insertion wt_III-8 used for all phenotypic rescues (Fig. 4) expresses high levels of HtsM in the presynaptic nerve terminal.
H) Quantification of HtsM levels in the axon. Identical levels can be observed for the three site-integrated constructs while the p-element insertion shows an approximate 2-fold increase, corresponding to the 2-fold higher expression levels observed in B-C.
I) Quantification of HtsM levels within the presynaptic nerve terminal. The presynaptic levels of the Hts-SD variant are approximately 5-fold increased compared to wt and SA variants.
J) Quantification of synapse retractions at m4 for the different phenotypes.
K) Quantification of protrusions on m4. All constructs significantly rescue the protrusion phenotype. The original wild type construct that expresses higher levels of Hts rescues better than the site-integrated versions (n > 150 for all genotypes for J, K; *** indicates p < 0.001, ANOVA; Error bars represent SEM).
The first striking observation is that the phospho-mimic transgene (htsSD-p40/VK33) results in significantly higher levels of synaptic Hts-M protein compared to either the wild type (htswt-p40/VK33) or the non-phosphorylatable transgene (htsSA-p40/VK33) (Figure 8D–F). This difference in synaptic localization is a reproducible and quantifiable difference (Figure 8I; SD is more than 5-times more abundant within the presynaptic nerve terminal compared to wt and SA). By contrast, there is no difference in the levels of axonal protein levels between the three transgenes, consistent with equivalent protein expression levels detected in larval brain extracts (Figure 8H). Furthermore, expression of our original wild type transgene (wt_III-8) shows increased protein levels both in the axon and at the synapse compared to the phi-integrated wild type transgenes (htswt-p40/VK33) (Figure 8G, H, I). From these data we conclude that the phospho-mimic S703D mutation facilitates trafficking of Hts M protein into the presynaptic nerve terminal, which could include mechanisms of protein transport or stabilization.
We next asked whether the mutant transgenes had a differential ability to rescue the phenotypes of synaptic retraction, bouton number and the appearance of growth-related protrusions in the hts mutant. Both the phospho-mimic and non-phosphorylatable transgenes were able to significantly rescue synapse retractions (Figure 8J), protrusions (Figure 8K) and bouton numbers (Supplemental Figure 7). Thus, the primary effect of the phospho-mimic mutation appears to be the control of synaptic translocation of the Hts-M protein. However, if one takes into account the different levels of synaptic protein present in wt, phospho-mimic and non-phosphorylatable genotypes, then some phenotypic differences can be observed. For example, comparing htswt_III-8 to htsSD-p40/VK33, which show equivalent synaptic Hts-M protein levels, reveals that htsSD-p40/VK33 does not rescue as well (Figure 8J, K). This could indicate that the mutant protein is not fully functional or that the phosphorylation-dependent localization of the mutant protein is not optimal. Regardless, the major effect of S703 phosphorylation within the MARCKS domain appears to be to control Hts synaptic protein levels, a parameter that we have shown can strongly influence synapse stability and growth.
DISCUSSION
Here we provide evidence that Hts/Adducin is an important player in the mechanisms that control both the stability and growth of the NMJ. We demonstrate that hts mutations cause a profound destabilization of the presynaptic nerve terminal. These data are consistent with the well-established function of Adducin as a spectrin binding protein that participates in the stabilization of the submembranous spectrin-actin skeleton (Bennett and Baines, 2001; Matsuoka et al., 2000). Remarkably, hts mutations also promote the growth and elaboration of new processes at the NMJ. Indeed, the elaboration of new processes and increased growth overcome the effects of synapse destabilization such that, on average, the NMJ is significantly larger in the hts mutant animals compared to wild type. Process elaboration is accompanied by the extension of small caliber, actin-rich protrusions that contain the necessary machinery for synaptic transmission including essential components of the active zone, postsynaptic glutamate receptors and homophilic cell adhesion molecules. This phenotype has not been observed in animals lacking presynaptic α-/β-Spectrin or Ankyrin2 (Pielage et al., 2008; Pielage et al., 2005), indicating that Hts/Adducin has a specific activity relevant to the formation of these new synaptic processes.
We go on to provide biochemical insight into how Hts/Adducin might control new process formation at the NMJ. We demonstrate that Drosophila Hts-M has actin capping activity similar to its vertebrate homolog. Based on recent work in other systems, loss of actin capping activity at the plasma membrane could reasonably favor the formation of actin-based filopodia that might promote the elaboration of small caliber synaptic protrusions (Bear et al., 2002; Mejillano et al., 2004; Menna et al., 2009). Consistent with such a model, overexpression of Hts/Adducin, thereby increasing the amount of actin capping protein at the NMJ, is sufficient to inhibit the growth and elaboration of small caliber type II and type III nerve terminals. Finally, we demonstrate that synaptic localization of Hts/Adducin is controlled via phosphorylation of a conserved serine residue in the C-terminal MARCKS domain. Based on these and additional data discussed below, we present a model in which Hts/Adducin functions as a molecular keystone, stabilizing the submembranous spectrin skeleton to achieve synapse stability and, simultaneously, capping actin filaments at the plasma membrane to influence the shape and growth potential of the presynaptic nerve terminal. Modulation of Adducin activity, either through changes in protein abundance or phospho-regulation, might then influence the balance of growth versus stability.
Mechanisms of Remodeling and Degeneration
Synapse retraction at the Drosophila NMJ occurs without cell death (Eaton et al., 2002; Massaro et al., 2009) implying a local degenerative process. Mechanistically, withdrawal of target derived BMP signaling causes retraction (Eaton and Davis, 2005) while overexpression of BMPs can suppress retractions (Massaro et al., 2009) suggesting similarities with developmental degeneration. However, we have identified several mutations that cause NMJ retraction that are linked to neuromuscular degeneration in human (Eaton et al., 2002; Pielage et al., 2005). Furthermore, over-expression of a WldS (wallarian degeneration slowed) transgene is able to significantly suppress synapse retraction at the NMJ, implying a degenerative mechanism similar to that observed in mammalian motoneurons (Massaro et al., 2009). Based upon these data, we hypothesize that synapse retraction at the Drosophila NMJ is driven by local, degenerative processes that are similar to those observed during neural development and the early stages of neurodegeneration in other systems.
Our data demonstrating the involvement of Hts/Adducin in both NMJ degeneration and nerve terminal sprouting/growth is quite unique. It may be possible to partition these functions to the spectrin-binding and actin-capping activities of Adducin. It is particularly intriguing that the subcellular distribution of Adducin can be regulated by phosphorylation. Might changes in the local concentration of Adducin be involved in developmental pruning and neuromuscular synapse elimination or degeneration in other systems? If so, our data seem to highlight an increasingly opaque distinction between degenerative and developmental mechanisms.
Enhanced NMJ Growth, Altered NMJ Morphology and Regulation of Synaptic Actin
Two general phenotypes of synaptic overgrowth have been previously documented at the Drosophila NMJ. The first phenotype involves a uniform and dramatic expansion NMJ (DiAntonio et al., 2001; Wan et al., 2000; Sweeney and Davis, 2002). The second phenotype involves the formation of highly ramified clusters of synaptic boutons that have been termed satellite boutons (Marie et al., 2004). This phenotype has been linked to disruption of endocytic proteins such as Dap160/Intersectin, Dynamin and Endophilin (Koh et al., 2004; Marie et al., 2004) as well as mutations that disrupt actin regulatory molecules including NWASP, Arp2/3 and Nervous Wrecked (Coyle et al., 2004).
By contrast, the phenotypes documented in hts/adducin are quite different from any previously reported mutation. The NMJ is transformed into a hybrid structure consisting of normal type 1b boutons that support the extension of long, small diameter synaptic protrusions. This phenotype is robust and highly penetrant. Adducin has two prominent functions. It participates in the stabilization of the spectrin-ankyrin skeleton and it caps actin filaments. By comparison of our data with prior genetic analyses of α-/β-spectrin and ankyrin2L (Koch et al., 2008; Pielage et al., 2008; Pielage et al., 2005), we can partition these two functions of hts/adducin. α-/β-Spectrin and Ankyrin2L are necessary for NMJ stability, but do not influence NMJ growth. Thus, we propose that Adducin is required to stabilize the nerve terminal through a well-established association with the spectrin/ankyrin skeleton. By extension, we propose that the actin-capping activity of Adducin regulates NMJ growth. One possibility is that loss of actin-capping at the nerve terminal membrane promotes filopodia formation and this drives the extension of the observed small-caliber protrusions. However, the loss of actin-capping activity alone may not be sufficient since it occurs in the presence of an impaired spectrin/ankyrin/adducin submembranous skeleton. An alternative possibility is that loss of Adducin causes two simultaneous effects. First, it relieves a constraining influence of the spectrin skeleton (Pielage et al., 2005). Second, in this relaxed context, increased filopodia formation is able to efficiently drive new nerve-terminal extension. Such a model could explain why the htsΔG mutation does not have prominent protrusions despite showing increased growth. If the htsΔG mutation retains some actin binding activity, as suggested by in vitro data (Li et al., 1998), and retains some stabilizing activity (Figure 2), then the combined effect might be sufficient to suppress protrusion formation while allowing enhanced synaptic growth. Interestingly, the association of Adducin with the submembranous spectrin skeleton can be controlled via phosphorylation downstream of growth factor signaling in other systems (Fukata et al., 1999; Pariser et al., 2005).
Experimental Procedures
Fly stocks
Flies were maintained at 25°C on standard fly food. The following strains were used in this study: w1118 (as wild type), hts1103, Df(2R)BSC26, ank2518, ank22001 elav-GAL4, mef2-GAL4 and mhc-GAL4 (all Bloomington stock center), UAS-drc2, htsRNAi (lines 103631 and 29102, Vienna Drosophila RNAi center), htsW532X, and htsΔG (gift of L. Cooley, New Haven, USA).
Generation of hts constructs, transgenes and protein
See supplemental methods.
Immunohistochemistry
Primary antibodies were used at the following dilutions: anti-Bruchpilot (nc82) 1:200; anti-Fasciclin II (1D4) 1:50; anti-Synapsin 1:50; anti-Futsch (22C10) 1:500; anti-Hts-1B1 1:50 (all provided by the Developmental Studies Hybridoma Bank, Iowa); rat anti-Ank2L 1:500 (gift from M. Hortsch); rabbit anti-Dlg 1:5.000 (gift from V. Budnik); rabbit anti-Syt 1:500; mouse anti-phospho-Adducin (Ser274) (= anti p703-Hts-M) (Upstate/Millipore) 1:400; rabbit anti-Hts-M 1:1000 (gift from L. Cooley); anti-DGluRIII was raised by David’s Biotechnology (Regensburg, Germany) against the 22 c-terminal amino acids of DGluRIII as previously reported (Marrus et al., 2004), affinity purified and used at 1: 4000. Alexa488/568/647 conjugated secondary antibodies were obtained from Invitrogen and used at 1:1000. Cy3 and Cy5 conjugated anti-HRP were obtained from Jackson Immunoresearch Laboratories and Molecular Probes and used at 1:1000 dilutions for 1–2 hours at room temperature (RT). Larval preparations were mounted in Prolong. Images were captured at room temperature using a Leica SPE confocal microscope with a HCX PLAPO 63x objective (Aperture 1.4). Imaris software (Bitplane) was used to process and analyze images and to quantify phenotypes.
Western Blot
See supplemental methods.
Electrophysiology
Third instar larvae were selected and dissected according to previously published techniques (Pielage et al., 2008). Whole muscle recordings were performed on muscle 6 in abdominal segment A3 using sharp microelectrodes (12–16MΩ). Recordings were selected for analysis only if resting membrane potentials were more hyperpolarized than −60 mV and if input resistances were greater than 5MΩ. See supplemental methods for additional detail.
Electron microscopy
Methods have been previously published (Pielage et al., 2005; 2006). See also supplemental methods.
Actin capping assay
Actin was purified from Acanthamoeba castellani as described (Gordon et al., 1976), labeled with pyrene iodoacetamide as described (Cooper et al., 1983), and stored on ice. Actin depolymerization assays were performed as described, with minor modification (Zuchero et al., 2009). See supplemental methods for additional detail.
Supplementary Material
Acknowledgments
We would like to thank V. Budnik, M. Hortsch and L. Cooley for generous gifts of antibodies and fly stocks. This work was supported by the Novartis research foundation (JP) and NIH grant (NS047342) (GWD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jan Pielage, Email: jan.pielage@fmi.ch.
Graeme W. Davis, Email: Graeme.davis@ucsf.edu.
References
- Akin O, Mullins RD. Capping protein increases the rate of actin-based motility by promoting filament nucleation by the Arp2/3 complex. Cell. 2008;133:841–851. doi: 10.1016/j.cell.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkalow KL, Italiano JE, Chou DE, Matsuoka Y, Bennett V, Hartwig JH. Alpha-adducin dissociates from F-actin and spectrin during platelet activation. J Cell Biol. 2003;161:557–570. doi: 10.1083/jcb.200211122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- Bennett V, Gardner K, Steiner JP. Brain adducin: a protein kinase C substrate that may mediate site-directed assembly at the spectrin-actin junction. J Biol Chem. 1988;263:5860–5869. [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–640. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budnik V, Zhong Y, Wu CF. Morphological plasticity of motor axons in Drosophila mutants with altered excitability. J Neurosci. 1990;10:3754–3768. doi: 10.1523/JNEUROSCI.10-11-03754.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Hsieh YT, Chen HC. Phosphorylation of adducin by protein kinase Cdelta promotes cell motility. J Cell Sci. 2007;120:1157–1167. doi: 10.1242/jcs.03408. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Walker SB, Pollard TD. Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J Muscle Res Cell Motil. 1983;4:253–262. doi: 10.1007/BF00712034. [DOI] [PubMed] [Google Scholar]
- Coyle IP, Koh YH, Lee WCM, Slind J, Fergestad T, Littleton JT, Ganetzky B. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004;41:521–534. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- Datwani A, McConnell MJ, Kanold PO, Micheva KD, Busse B, Shamloo M, Smith SJ, Shatz CJ. Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009;64:463–470. doi: 10.1016/j.neuron.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiAntonio A, Haghighi AP, Portman SL, Lee JD, Amaranto AM, Goodman CS. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature. 2001;412:449–452. doi: 10.1038/35086595. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dutta D, Bloor JW, Ruiz-Gomez M, VijayRaghavan K, Kiehart DP. Real-time imaging of morphogenetic movements in Drosophila using Gal4-UAS-driven expression of GFP fused to the actin-binding domain of moesin. Genesis. 2002;34:146–151. doi: 10.1002/gene.10113. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Oshiro N, Kinoshita N, Kawano Y, Matsuoka Y, Bennett V, Matsuura Y, Kaibuchi K. Phosphorylation of adducin by Rho-kinase plays a crucial role in cell motility. J Cell Biol. 1999;145:347–361. doi: 10.1083/jcb.145.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda Y, Davis GW. Mechanisms of synapse assembly and disassembly. Neuron. 2003;40:243–264. doi: 10.1016/s0896-6273(03)00608-1. [DOI] [PubMed] [Google Scholar]
- Gordon DJ, Eisenberg E, Korn ED. Characterization of cytoplasmic actin isolated from Acanthamoeba castellanii by a new method. J Biol Chem. 1976;251:4778–4786. [PubMed] [Google Scholar]
- Gruenbaum LM, Gilligan DM, Picciotto MR, Marinesco S, Carew TJ. Identification and characterization of Aplysia adducin, an Aplysia cytoskeletal protein homologous to mammalian adducins: increased phosphorylation at a protein kinase C consensus site during long-term synaptic facilitation. J Neurosci. 2003;23:2675–2685. doi: 10.1523/JNEUROSCI.23-07-02675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa JH, Mullins RD. Spatial and temporal relationships between actin-filament nucleation, capping, and disassembly. Curr Biol. 2007;17:395–406. doi: 10.1016/j.cub.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, et al. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–1054. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- Li X, Matsuoka Y, Bennett V. Adducin preferentially recruits spectrin to the fast growing ends of actin filaments in a complex requiring the MARCKS-related domain and a newly defined oligomerization domain. J Biol Chem. 1998;273:19329–19338. doi: 10.1074/jbc.273.30.19329. [DOI] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Luo L, O’Leary DDM. Axon retraction and degeneration in development and disease. Annu Rev Neurosci. 2005;28:127–156. doi: 10.1146/annurev.neuro.28.061604.135632. [DOI] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massaro CM, Pielage J, Davis GW. Molecular mechanisms that enhance synapse stability despite persistent disruption of the spectrin/ankyrin/microtubule cytoskeleton. J Cell Biol. 2009;187:101–117. doi: 10.1083/jcb.200903166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–373. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Menna E, Disanza A, Cagnoli C, Schenk U, Gelsomino G, Frittoli E, Hertzog M, Offenhauser N, Sawallisch C, Kreienkamp HJ, et al. Eps8 regulates axonal filopodia in hippocampal neurons in response to brain-derived neurotrophic factor (BDNF) PLoS Biol. 2009;7:e1000138. doi: 10.1371/journal.pbio.1000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A, McLaughlin T, O’Leary DDM, Tessier-Lavigne M. APP binds DR6 to trigger axon pruning and neuron death via distinct caspases. Nature. 2009;457:981–989. doi: 10.1038/nature07767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Nunes P, Haines N, Kuppuswamy V, Fleet DJ, Stewart BA. Synaptic vesicle mobility and presynaptic F-actin are disrupted in a N-ethylmaleimide-sensitive factor allele of Drosophila. Mol Biol Cell. 2006;17:4709–4719. doi: 10.1091/mbc.E06-03-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariser H, Herradon G, Ezquerra L, Perez-Pinera P, Deuel TF. Pleiotrophin regulates serine phosphorylation and the cellular distribution of beta-adducin through activation of protein kinase C. Proc Natl Acad Sci USA. 2005;102:12407–12412. doi: 10.1073/pnas.0505901102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella LN, Smith-Leiker T, Cooley L. The Ovhts polyprotein is cleaved to produce fusome and ring canal proteins required for Drosophila oogenesis. Development. 2007;134:703–712. doi: 10.1242/dev.02766. [DOI] [PubMed] [Google Scholar]
- Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15:918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. A postsynaptic spectrin scaffold defines active zone size, spacing, and efficacy at the Drosophila neuromuscular junction. J Cell Biol. 2006;175:491–503. doi: 10.1083/jcb.200607036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. Rate constants for the reactions of ATP- and ADP-actin with the ends of actin filaments. J Cell Biol. 1986;103:2747–2754. doi: 10.1083/jcb.103.6.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro F, Rosato-Siri M, Leone E, Costessi L, Iaconcig A, Tongiorgi E, Muro A. beta-adducin (Add2) KO mice show synaptic plasticity, motor coordination and behavioral deficits accompanied by changes in the expression and phosphorylation levels of the alpha- and gamma-adducin subunits. Genes Brain Behav. 2009;9:8–96. doi: 10.1111/j.1601-183X.2009.00537.x. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Addy NA, Caldarone BJ, Asaka Y, Gruenbaum LM, Peters LL, Gilligan DM, Fitzsimonds RM, Picciotto MR. Impaired synaptic plasticity and learning in mice lacking beta-adducin, an actin-regulating protein. J Neurosci. 2005;25:2138–2145. doi: 10.1523/JNEUROSCI.3530-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasse TM, Fouquet W, Schmid A, Kittel RJ, Mertel S, Sigrist CB, Schmidt M, Guzman A, Merino C, Qin G, et al. Glutamate receptor dynamics organizing synapse formation in vivo. Nat Neurosci. 2005;8:898–905. doi: 10.1038/nn1484. [DOI] [PubMed] [Google Scholar]
- Robinson DN, Cant K, Cooley L. Morphogenesis of Drosophila ovarian ring canals. Development. 1994;120:2015–2025. doi: 10.1242/dev.120.7.2015. [DOI] [PubMed] [Google Scholar]
- Roos J, Hummel T, Ng N, Klämbt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–382. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Seidel B, Zuschratter W, Wex H, Garner CC, Gundelfinger ED. Spatial and sub-cellular localization of the membrane cytoskeleton-associated protein alpha-adducin in the rat brain. Brain Res. 1995;700:13–24. doi: 10.1016/0006-8993(95)00962-p. [DOI] [PubMed] [Google Scholar]
- Sweeney ST, Davis GW. Unrestricted synaptic growth in spinster-a late endosomal protein implicated in TGF-beta-mediated synaptic growth regulation. Neuron. 2002;36:403–416. doi: 10.1016/s0896-6273(02)01014-0. [DOI] [PubMed] [Google Scholar]
- van der Gucht J, Paluch E, Plastino J, Sykes C. Stress release drives symmetry breaking for actin-based movement. Proc Natl Acad Sci U S A. 2005;102:7847–7852. doi: 10.1073/pnas.0502121102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venken KJT, He Y, Hoskins RA, Bellen HJ. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 2006;314:1747–1751. doi: 10.1126/science.1134426. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Dürrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, et al. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–844. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Walsh MK, Lichtman JW. In vivo time-lapse imaging of synaptic takeover associated with naturally occurring synapse elimination. Neuron. 2003;37:67–73. doi: 10.1016/s0896-6273(02)01142-x. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Hoopfer ED, Luo L. Axon pruning during Drosophila metamorphosis: evidence for local degeneration and requirement of the ubiquitin-proteasome system. Neuron. 2003;38:871–885. doi: 10.1016/s0896-6273(03)00295-2. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik A, Tobin W, Zweig J, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009 doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L, Spradling AC. hu–li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992;6:2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]
- Zaccai M, Lipshitz HD. Differential distributions of two adducin-like protein isoforms in the Drosophila ovary and early embryo. Zygote. 1996;4:159–166. doi: 10.1017/s096719940000304x. [DOI] [PubMed] [Google Scholar]
- Zuchero JB, Coutts AS, Quinlan ME, Thangue NBL, Mullins RD. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat Cell Biol. 2009;11:451–459. doi: 10.1038/ncb1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.