Abstract
This study investigated the potential of creating a charged polymeric micelle-based nucleic acid delivery system that could easily be reconstituted by the addition of water. (PLGA36kDa)2-b-bPEI25kDa (PLGA MW 36kDa, bPEI Mw 25kDa, PLGA:bPEI block ratio = 2) was synthesized and used to prepare cationic micelles. The copolymer retained proton-buffering capability from the bPEI block within the endosomal pH range. Micelle/pDNA complexes retained their particle size (100–150 nm) and surface charge (30–40 mV) following reconstitution. It was found that adding a small amount of low molecular weight bPEI (1.8 kDa) completely shielded pDNA in the micelle/pDNA complexes and enhanced transfection efficiency 50–100 fold for both fresh and reconstituted complexes without affecting complex size. Transfection efficiency for “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes was 16-fold higher than its “fresh” counterpart. Although transfection levels achieved using “reconstituted” micelle/pDNA/bPEI1.8kDa complexes were 3.6-fold lower than control “fresh” bPEI25kDa/pDNA (N/P 5) complexes, transfection levels were 39-fold higher than “reconstituted” bPEI25kDa/pDNA (N/P 5) complexes. The micelle/pDNA/bPEI1.8kDa system showed very low cytotoxicity in MCF7 cells even with pDNA doses up to 20 μg, and transfection levels increased linearly with increasing pDNA dose. These results indicate that this PLGA-b-bPEI polymeric micelle-based system is well suited as a reconstitutable gene delivery system, and has high potential for use as a delivery system for gene therapy applications.
1. Introduction
Gene therapy is a technique that uses genes to treat or prevent disease. There are several applications for gene therapy including replacing a mutated gene with a healthy copy of gene [1, 2], introducing a new gene to help fight disease [3, 4], or inactivating a mutated gene that is not functioning correctly [5]. This technique has the potential for broad scale application and impact in treating both acquired and inherited diseases. Although there has been some success using viral vector systems as vehicles for gene transfection in vitro and in vivo, the large number of drawbacks associated with viral-based carrier systems has increased interest in the development of non-viral delivery systems [6]. Concerns about the integration of delivered exogeneous DNA into the host genome [7–9], cytotoxicity [10], tumorigenicity [11], and immunogenicity [12] have increased interest in finding alternative delivery systems [6, 13].
Non-viral based delivery systems are typically classified into two main categories – lipids and polymers. With attractive biological traits such as low immunogenicity, no tumorigenicity, and excellent biocompatibility, polymer-based systems offer the ability to engineer carrier systems with customized features that can be adapted to suit any system [6]. Functionalities include attaching targeting moieties to the non-viral polymer carrier for targeting specific cell types [14–16], adding endosomolytic agents to disrupt the endosome [17, 18], increasing nuclear delivery by targeting the cell nucleus [19, 20], or designing degradable polymers to decrease toxicity [21–23].
Many different combinations of polymeric/oligomeric blocks in single polymer systems have been created and studied in an effort to increase utility. Block copolymer micelle systems in particular have gained interest in recent years as promising drug delivery vehicles because of their ability to prolong circulation in the blood and their ability to modulate the pharmacokinetics of drugs [24]. For gene delivery, micelle copolymers having a hydrophobic block-charged hydrophilic block architecture usually assemble into a core-shell micelle structure, creating the potential to load hydrophobic drugs and gene drugs into the different compartments (core and shell) respectively. Some recent examples of such copolymers include poly(ε-caprolactone)-bPEI1.8kDa [25], poly((N-methyldietheneamine sebacate)-co-[(cholesteryl oxocarbonylamido ethyl) methyl bis(ethylene) ammonium bromide] sebacate) [26], and poly(dimethylaminoethyl methacrylate)-poly(ε-caprolactone)-poly(dimethylaminoethyl methacrylate) [27].
Even so, non-viral systems are still a long way from being readily available as a mainstream therapeutic option. Some important formulation considerations for clinical usage are drug concentration [28], ease of administration [29], and formulation stability during storage [30, 31]. Many formulations made from liposomes or polymer-based systems exist as colloidal liquids, which are generally stable for short periods of time [32]. However, some polymer systems are designed to be gradually degradable by hydrolysis so long-term storage in liquid form is not a viable option for these types of systems [33]. In addition, many formulations can become unstable if kept in liquid form during long-term storage due to a variety of issues including degradation of the non-viral vector and/or drug [34], formation of insoluble aggregates [35], unwanted drug release [36], and loss of bioactivity [37]. One method to overcome the stability limitations of storing liquid formulations is to lyophilize the formulation, allowing storage in a powder formulation. When the formulation is required, it can be reconstituted though the simple addition of water or buffer. While some success has been seen using this method, many groups have additionally added cryoprotective agents [38, 39] or modified the formulation in some way such as through PEGylation [40, 41] or physical cross-linking [42]. Unfortunately these formulations showed some changes in their characteristics following reconstitution such as increases in particle size [38]. The ideal scenario would be to have a formulation that can be lyophilized, be reconstituted easily by simply adding water or buffer, and most importantly retain its original chemical, physical and biological properties.
This study was designed to investigate the possibility of creating a charged polymeric micelle-based gene therapeutic delivery system that maintains efficacy following lyophilization and reconstitution. Branched polyethyleneimine (bPEI) and poly(lactide-co-glycolide) (PLGA) were selected to create our micelle system. The hydrophilic cationic component bPEI25kDa (Mw 25 kDa) has been used as the gold standard for polymeric vectors but its viscosity and sticky characteristics have limited its use in a dried state. The hydrophobic degradable component PLGA has been used in dried nano/microparticles form but its water-insolubility limits its use for creating formulations containing biological therapeutics in aqueous buffers. In order to extract the favorable features from both bPEI25kDa and PLGA36kDa, these two polymers were chemically linked together and the resulting block copolymer was used to create charged micelles which could interact with biological agents in aqueous solution and could also be stored as a dried formulation. Physicochemical and biological comparisons between freshly-prepared micelle-based gene complexes and reconstituted micelle-based gene complexes were evaluated in terms of particle size, surface charge, pDNA condensation ability, and cell transfection efficiency.
2. Materials and methods
2.1. Materials
Poly(lactide-co-glycolide) (PLGA36kDa; Resomer®503H; lactide:glycolide = 1:1 (mole/mole); approximate MW 36 kDa) having a carboxylic group at one end was purchased from Boehringer Ingelheim Pharm GmbH & Co. KG (Germany). Two bPEIs having Mr 1.8 kDa (bPEI1.8kDa) and Mw 25 kDa (bPEI25kDa; Mn 10 kDa) were purchased from Polysciences, Inc. (Warrington, PA) and Sigma-Aldrich Co. (St. Louis, MO) respectively. Triethylamine (TEA), N,N′-dicyclohexylcarbodiimide (DCC), N-hydroxysuccinimide (NHS), RPMI1640 cell culture medium powder, sodium bicarbonate, D-glucose, 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid sodium salt (HEPES), human recombinant insulin, Ca2+-free and Mg2+-free Dulbecco’s phosphate buffered saline (DPBS), 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), and agarose were purchased from Sigma-Aldrich Co. (St. Louis, MO). Fetal bovine serum (FBS), penicillin/streptomycin, and 0.25% trypsin/EDTA were purchased from Gibco BRL (Grand Island, NY). YOYO-1 dye was purchased from Invitrogen, Inc (Carslbard, CA). Ethidium bromide (EtBr) and the Biocinchoninic Acid Protein Assay Kit (BCA) were obtained from Thermo Fisher Scientific, Inc. (Waltham, MA). Luciferase assay kit was obtained from Promega Co. (Madison, WI). Spectrapor dialysis membrane MWCO 15 kDa was purchased from Spectrum Laboratories, Inc. (Rancho Dominguez, CA). Plasmid DNA (pDNA) encoded with the firefly luciferase reporter gene (gWiz-Luc) was purchased from Aldevron, Inc. (Fargo, ND).
2.2. Cell culture
In this study, MCF7 cells (human breast adenocarcinoma cell line) were used for determining the cytotoxicity of micelles and polyplexes and for polyplex transfection. All experiments used MCF7 cells cultured in RPMI1640 cell culture medium supplemented with insulin (4 mg/L), glucose (2 g/L), 10% heat-inactivated fetal bovine serum (FBS) and 1% penicillin/streptomycin antibiotics at 37 °C in humidified air containing 5% CO2.
2.3. Synthesis and characterization of amphiphilic block copolymers
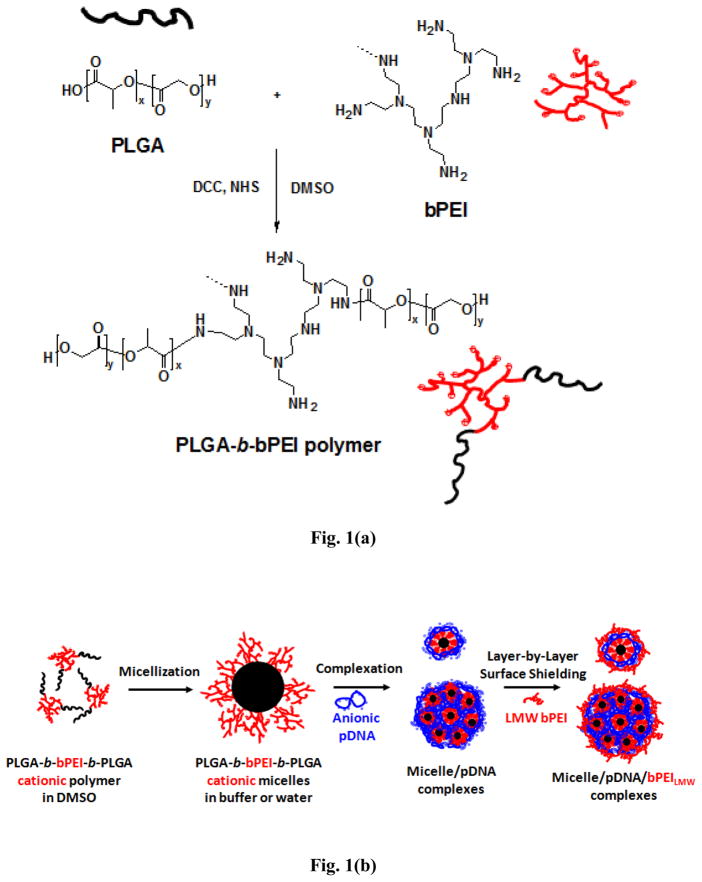
A block copolymer composed of PLGA36kDa and bPEI25kDa ((PLGA36kDa)2-b-bPEI25kDa) was synthesized by conventional condensation between the carboxylic acid group from PLGA36kDa and the amine groups from bPEI25kDa as shown in Fig. 1(a). In detail, PLGA36kDa (100 μmol) and bPEI25kDa (50 μmol) were dissolved separately in DMSO (100 mL) and stirred independently for 4 hours. Then the two polymer solutions were mixed together along with DCC (1 mmol), NHS (1 mmol), and TEA (0.1 mL) and polymerization was carried out at room temperature (RT) for 48 hours under constant stirring. About 100 mL of deionized (DI) water was added to the reaction mixture and stirred for 2 hours; then the polymer solution was transferred to a dialysis tube (MWCO 15 kDa) and dialyzed against DI water for two days to remove any unreacted bPEI25kDa. Unreacted PLGA36kDa was removed by filtration at a later stage following micelle formation. The dialysis solution was lyophilized to obtain the resulting block copolymer. The reaction yield was over 95%. The chemical structure was confirmed by 1H-NMR spectroscopy.
Fig. 1.
(a) Reaction schematic for (PLGA36kDa)2-b-bPEI25kDa copolymer and (b) schematic representation of the structures of (PLGA36kDa)2-b-bPEI25kDa micelles, micelle/pDNA complexes, and micelle/pDNA/bPEILMW complexes.
2.4. Preparation and characterization of micelles
Micelles were prepared using standard dialysis techniques. As shown in Fig. 1(b), (PLGA36kDa)2-b-bPEI25kDa was dissolved in DMSO at a concentration of 10 mg/mL and stirred for 4 hours at RT. HEPES buffer (20 mM, pH 7.4) was added to the reaction mixture at an equivalent volume to DMSO and stirred for an additional 2 hours. The reaction mixture was transferred to a dialysis membrane (MWCO 15 kDa) and dialyzed against DI water for 24 hours. The resulting micelle solution was filtered using a 0.22 μm filter to remove any unreacted PLGA36kDa remaining from the synthesis (which precipitates in water) and the remaining solution was lyophilized and stored at −20°C until needed.
Particle size and surface charge of freshly-prepared micelles and reconstituted micelles were measured at RT using a Zetasizer 3000HSA (Malvern Instruments Inc., Worcestershire, UK) with a fixed wavelength of 677 nm and a constant angle of 90°.
To evaluate whether the (PLGA36kDa)2-b-bPEI25kDa micelles maintained any proton buffering capacity from the bPEI25kDa block, micelles were titrated using traditional acid-base titration methods [18, 23]. (PLGA36kDa)2-b-bPEI25kDa micelles were dispersed in 150 mM NaCl and titrated from pH 7.4 to pH 3 using 0.1 N HCl. The proton buffering ability of the (PLGA36kDa)2-b-bPEI25kDa micelles was compared to bPEI25kDa alone within the pH range 7.4-5.1 because this pH range correlates with typical endolysosomal pHs. Buffering capacity (%) of the polymers was calculated using the following equation [43]:
where ΔVHCl is the volume of the HCl solution (0.1 M) which decreased the pH value of the polymer solution from pH 7.4 to pH 5.1, CHCl is the concentration of the HCl solution (0.1 M), and N is the total moles of protonable amine groups contained in the polymer.
Cytotoxicity of (PLGA36kDa)2-b-bPEI25kDa micelles was assessed using standard protocols for MTT-based cell viability assay [18, 23]. MCF7 breast cancer cells were seeded in a 96-well plate at a density of 5000 cells/well in culture medium (0.1 mL). Sample solutions (10 μL) at various concentrations were added to each well and incubated for 24 hours. MTT solution (10 μL, 5 mg/mL) was added to each well and the plates were incubated for 4 hours at 37°C in a cell culture incubator. Media was completely removed, 100 μL of DMSO was added to each well to dissolve the formazan metabolites, and the plate was incubated for 10 minutes at 37°C. Absorbance was measured at 570 nm using a microplate reader (SpectraMax® M2, Molecular Devices, Sunnyvale, CA) and used to calculate cell viability.
2.5. Preparation and characterization of polyplexes
Micelle solutions prepared by standard dialysis techniques and used immediately are referred to as “fresh” micelles. “Fresh” micelles that were lyophilized and then reconstituted by adding DI water are referred to as “reconstituted” micelles. Micelle solutions (fresh or reconstituted) were mixed with pDNA in HEPES buffer (20 mM, pH 7.4) containing 5% glucose (HBG) and incubated at RT for 30 minutes to form polyplexes. Some polyplexes were additionally mixed with a small amount of bPEI1.8kDa following complexation at a weight ratio of 2.5μg bPEI1.8kDa per 1μg pDNA. This method was used to create polyplexes for particle size and zeta potential measurements, gel retardation studies, gene transfection and cell viability tests.
Complexation between micelles and pDNA was evaluated by gel retardation and dye quenching methods [18, 44]. For the gel retardation studies, polyplex solution (0.5 μg pDNA in 10 μL) was loaded onto a 0.8 % agarose gel containing Ethidium Bromide (EtBr) (100 ng/mL). Electrophoresis was run using 0.5X Tris-buffer containing boric acid and EDTA (TBE) at 100 V for 60 minutes. The gel was imaged using an Alpha Innotech FluorChem FC2® instrument with Alpha View Software (Cell Biosciences, Santa Clara CA).
For the dye quenching assays, pDNA was mixed with EtBr at a mole ratio of five nucleotides per one EtBr and pre-incubated in the dark at RT for 30 minutes. Using EtBr-intercalated pDNA, polyplexes were prepared following the same method described above. Polyplexes were excited at 515 nm and the emitted fluorescence was measured at 595 nm using a Spectramax® M2 spectraphotometer (Molecular Devices, Sunnyvale CA).
Particle size and surface charge of fresh polyplexes and reconstituted polyplexes were measured at RT using a Zetasizer 3000HSA (Malvern Instruments Inc., Worcestershire, UK) with a fixed wavelength of 677 nm and a constant angle of 90°.
In vitro transfection of fresh polyplexes and reconstituted polyplexes was evaluated in MCF7 cells using previously reported methods [45]. The cells were seeded at a density of 5 × 105 cells/well in 6-well plates and cultured for 24 hours in culture medium (2 mL). One hour prior to transfection, the cell culture medium was removed and replaced with serum-free insulin-free culture medium (2 mL). Polyplexes were added to the wells (20 μL volume, pDNA content was fixed at 1 μg/well) and cells were incubated for 4 hours at 37°C. The medium was replaced with the complete culture medium (supplemented with serum and insulin) and incubated for another 44 hours. Following incubation, culture medium was removed and cells were rinsed once with DPBS and lysed using a reporter lysis buffer. Luciferase gene expression (relative luminescence units (RLU)) was quantified by following the manufacturer’s protocol for the luciferase assay. Protein content in the cells was evaluated by the BCA™ protein assay. Gene transfection efficiency is reported as RLU/mg protein.
In vitro cytotoxicity of fresh polyplexes and reconstituted polyplexes with or without bPEI1.8kDa was monitored by MTT-based cell viability assay. The experimental procedure was the same as previously described for in vitro transfection except the cell number used (2.5×105 cells/well; 12-well plates) and the polyplex dose (10 μL; 0.5 μg pDNA/well). After completing the 48 hour transfection procedure, MTT solution (0.1 mL; 5 mg/mL) was added to the cells (in 1 mL of culture medium). After an additional 4-hour incubation, the MTT-containing medium was removed. The resulting formazan crystals produced by living cells were dissolved in 1 mL of DMSO and absorbance was measured at 570 nm using a microplate reader.
For the pDNA dose-dependent transfection and cytotoxicity studies, the final glucose concentrations of transfection media and culture media with polyplex solutions were adjusted to 2 g/L to avoid any potential glucose-induced effects on cell viability/toxicity.
Cellular uptake of the polyplexes was monitored using flow cytometry following methods described elsewhere [23]. Briefly, polyplexes were prepared using pDNA stained with YOYO-1 dye and added to cells pre-seeded in 6-well culture plates. After incubation for 4 hours, cells were detached and fixed with 4% paraformaldehyde solution. Flow cytometry (FACScan Analyzer, Becton-Dickinson, Franklin Lakes, NJ) was used to monitor cells containing fluorescence and a primary argon laser (488nm) and fluorescence detector (530 ± 15nm) were used to detect the presence of YOYO-1 dye.
The statistical significance of the data was evaluated by conducting unpaired Student’s t-test with a confidence level of p<0.05, one-variable Analysis of Variance (ANOVA) and two-variable Analysis of Variance (ANOVA).
3. Results and Discussion
3.1. Synthesis and characterization of (PLGA36kDa)2-b-bPEI25kDa block polymers
Cationic amphiphilic block copolymers composed of PLGA36kDa and bPEI25kDa were synthesized by a conjugation reaction between monocarboxylated PLGA36kDa and the primary amines of bPEI25kDa (Fig. 1(a)). Copolymer synthesis and block ratio (PLGA36kDa to bPEI25kDa) were confirmed by 1H-NMR. In d6-DMSO, (PLGA36kDa)2-b-bPEI25kDa polymer was confirmed by observing peaks centered around δ=5.20 for C(=O)-CH2-O of glycolide, δ=1.45 for CH3- of lactide, δ=4.85 for C(=O)-CH(CH3)-O of lactide, and δ= 3.35, 3.80, and 4.10 for the -CH2-groups neighboring the primary, secondary, tertiary amines of bPEI25kDa (Fig. S1). However, the -CH2- groups of bPEI25kDa showed broad absorption due to the overlapping absorption of the amine groups of bPEI25kDa [46] as seen in Fig. S1. In addition, the ratio of lactide and glycolide in (PLGA36kDa)2-b-bPEI25kDa from the spectra taken in DMSO did not match the original 1:1 mole ratio contained in the PLGA36kDa. This could be due to the weakly soluble character of bPEI25kDa in DMSO; some fraction of the lactide and glycolide could have been hidden from detection by the bPEI25kDa domain. Thus, to obtain accurate block ratios for (PLGA36kDa)2-b-bPEI25kDa, the PLGA36kDa block was completely degraded into its glycolic acid and lactic acid units by base-catalyzed hydrolysis in D2O containing 1 N NaOH. The degradation products of (PLGA36kDa)2-b-bPEI25kDa were very soluble in the aqueous solution. Based on the integration ratio of glycolic acid (or lactic acid) and bPEI25kDa (Fig. S2), the block ratio of PLGA36kDa per bPEI25kDa was 1.93 and the estimated molecular weight (based on 1H-NMR data) was approximately 79.5 kDa based on Mn (~94.5 kDa based on Mw). Therefore, as designed, the block copolymer is very close to a triblock copolymer of PLGA36kDa-b-bPEI25kDa-b-PLGA36kDa ((PLGA36kDa)2-b-bPEI25kDa).
3.2. Preparation and characteristics of (PLGA36kDa)2-b-bPEI25kDa micelles
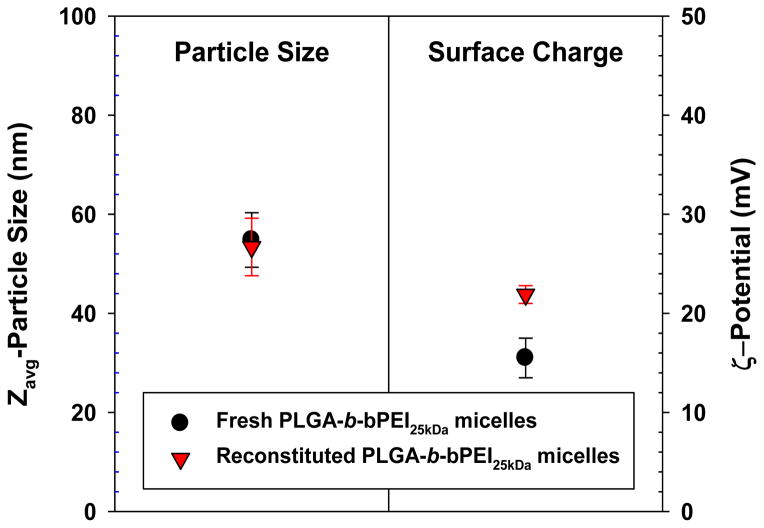
Utilizing the amphiphilic character of the block copolymer, positively-charged polymeric micelles were formed using conventional dialysis methods (Fig. 1(b)). The micelles formed a core-shell structure where PLGA36kDa formed the core and bPEI25kDa formed the corona (or shell). Cationic micelles made from (PLGA36kDa)2-b-bPEI25kDa had an average diameter of 50–60 nm (Zavg-particle size relevant to hydrodynamic size) and surface charge of 15–25 mV (Fig. 2). Cationic micelles in aqueous solution maintained their size and surface charge because the strong positively-charged corona prevented the formation of aggregated micelles due to repulsion. The micelle core made from relatively high molecular weight PLGA (MW 36 kDa) can be characterized by strong hydrophobicity [47–49] and slow degradation rate [47, 50, 51].
Fig. 2.
Particle size and surface charge of (PLGA36kDa)2-b-bPEI25kDa micelles before and after reconstitution. (n=5; mean ± SD)
Although the aqueous colloidal stability of (PLGA36kDa)2-b-bPEI25kDa micelles can be assured for up to 4 days when stored at 4°C, long-term exposure to various storage conditions (humidity, temperature, aqueous solution, etc.) may alter the physicochemical characteristics (particle size and surface charge) of the micelles and could damage the biological function of any biotherapeutic agents loaded into the micelles. Storage in a powder form could alleviate the potential pitfalls associated with storage in solution form. Thus, particle size and surface charge of the micelles were monitored to evaluate whether the colloidal stability of the micelles was preserved after lyophilization. As shown in Fig. 2, particle size and surface charge of reconstituted micelles were similar to those of fresh micelles.
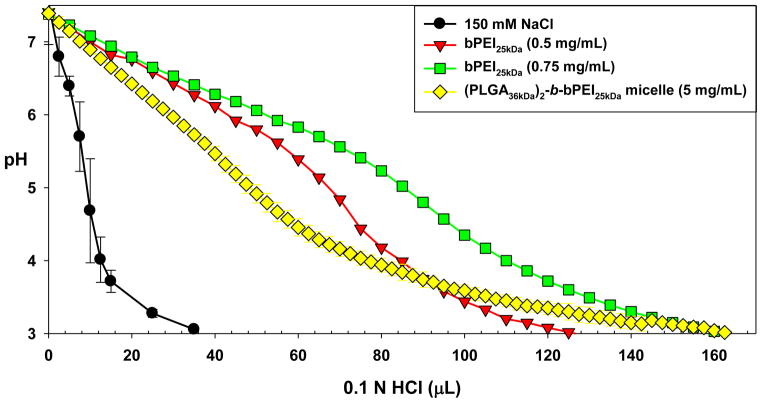
It is well known that bPEI25kDa has very strong proton-buffering capabilities. To determine how much proton-buffering capacity was preserved in the (PLGA36kDa)2-b-bPEI25kDa micelles, (PLGA36kDa)2-b-bPEI25kDa (5 mg/mL; 43.88 μmol of amines in 3 mL) was dissolved in 150 mM NaCl and titrated from pH 7.4 to pH 3.0 by adding 0.1 N HCl. The buffering capacity of the copolymer was compared to bPEI25kDa at 0.5 mg/mL (34.88 μmol of amines in 3 mL) and 0.75 mg/mL (52.33 μmol of amines in 3 mL) because the weight percent of bPEI25kDa in the copolymer was approximately 12.6% (based on Mn). As shown in Fig. 3, the (PLGA36kDa)2-b-bPEI25kDa copolymer retained broad proton buffering ability within the endosomal pH range (specifically pH 5.1 to pH 7.4) similar to bPEI25kDa, although the buffering capacity (10.6%) was lower after copolymerization as compared to free bPEI25kDa (average 17.2% from two different concentrations). The reason for this decrease is most likely because there is decreased accessibility of protons to protonate secondary or tertiary amines located near the linkage to the strong hydrophobic PLGA block which prevents them from becoming ionized as effectively following polymerization, thus reducing the overall buffering capability.
Fig. 3.
Proton buffering capacity of (PLGA36kDa)2-b-bPEI25kDa micelles and bPEI25kDa. (n=3; mean ± SD)
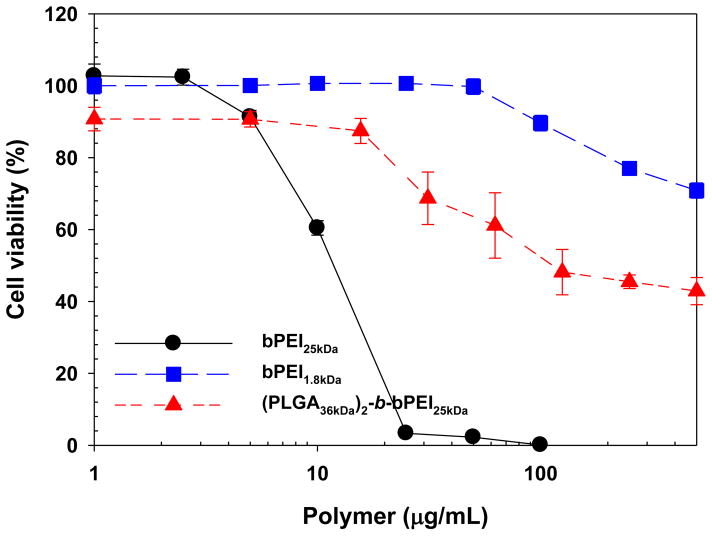
The toxicity of the polymeric micelles was compared to the toxicity of control polymers (i.e., bPEI25kDa and bPEI1.8kDa) as shown in Fig. 4. As expected, the cytotoxicity of the micelles was quite low compared to bPEI25kDa alone. The IC50 of (PLGA36kDa)2-b-bPEI25kDa was approximately 110 μg/mL, much higher than bPEI25kDa (~ 12 μg/mL). However, if the weight fraction of bPEI25kDa contained in the copolymer is considered, then the IC50 of PEI25kDa in (PLGA36kDa)2-b-bPEI25kDa (13.9 μg/mL) is not significantly different from bPEI25kDa alone. In addition, the cytotoxicity contribution from bPEI1.8kDa was negligible within the concentration range used in the transfection studies (2.5 μg per 1 μg pDNA; 1.25–25 μg/mL bPEI1.8kDa for 1–20 μg pDNA), with cell viabilities higher than 90% for the concentrations of bPEI1.8kDa used.
Fig. 4.
Cytotoxicity of (PLGA36kDa)2-b-bPEI25kDa micelles, bPEI25kDa, and bPEI1.8kDa in MCF7 cells. (n=6; mean ± SD)
3.3. Preparation and characteristics of (PLGA36kDa)2-b-bPEI25kDa micelle-based polyplexes
Reconstituted (PLGA36kDa)2-b-bPEI25kDa micelles were complexed with pDNA and characterized to further address whether (PLGA36kDa)2-b-bPEI25kDa micelle/pDNA complexes (abbreviated as micelle/pDNA complexes) could be used to produce exogenous proteins in cells via transfection of exogenous therapeutic pDNA. To evaluate this potential, the first step was to form electrostatic complexes between cationic micelles and anionic pDNA. Although most pDNA appeared to complex with the micelles at low weight ratios (WR) of micelle to pDNA (e.g., WR <3), some pDNA remained uncomplexed (Fig. 5). With higher WRs (e.g., WR 25), all of the pDNA complexed with the micelles but some pDNA still appeared to be exposed or only weakly complexed (Fig. S3). Nevertheless, micelle/pDNA complexes having WR ≥ 15 were approximately 50–120 nm in diameter and had a strong positive surface charge (20–25 mV) (Fig. S4). When micelle/pDNA complexes with WR 15–30 were used to transfect MCF7 cells, the micelle/pDNA complexes showed approximately 103–104 lower transfection efficiency than bPEI25kDa/pDNA complexes (N/P 5) (Fig. S5). This result may be due to either weak complexation between pDNA and the micelles or the presence of partially exposed pDNA on the surface of the micelle/pDNA complexes.
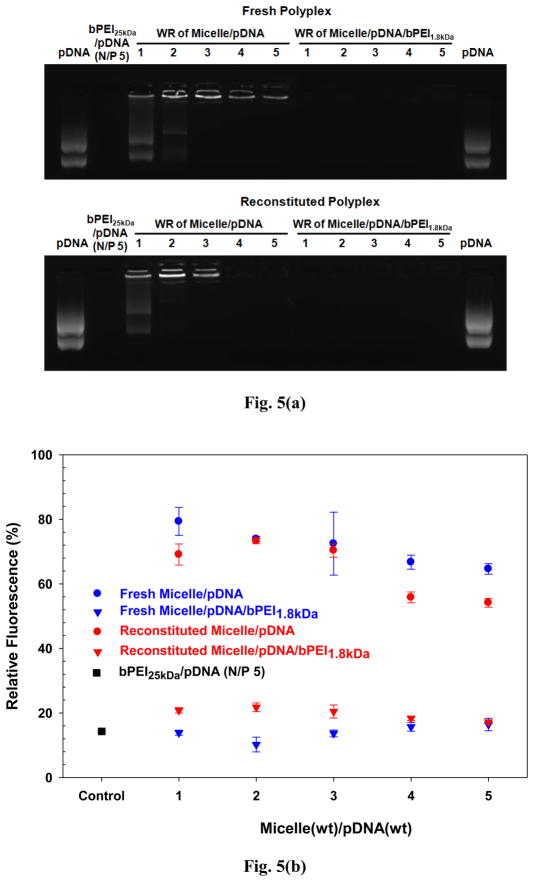
Fig. 5.
Surface shielding effect of bPEI1.8kDa on gene condensation of micelle/pDNA complexes: (a) agarose gel retardation and (b) dye quenching assay (n=3; mean ± SD).
To solve the aforementioned possible issues with micelle/pDNA complexes, low molecular weight (LMW) bPEIs were introduced into the micelle/pDNA complexes. LMW bPEIs could shield any exposed pDNA on the surface of the complexes through electrostatic layer-by-layer (LBL) attraction. In addition, LMW bPEIs could fill up any free volume between the cationic micelles and pDNAs and induce tighter complexation. From among the commercially available bPEIs, the lowest MW bPEI (i.e., bPEI0.8kDa) was selected as the first candidate to prove these hypotheses. However, micelle/pDNA complexes shielded with bPEI0.8kDa (micelle/pDNA/bPEI0.8kDa complexes) still had some exposed pDNA, as illustrated by the detection of EtBr-intercalated pDNA from those complexes in the gel retardation data (Fig. S6). The next smallest available bPEI (bPEI1.8kDa) was selected next to see if it could endow stronger interaction between the LMW bPEIs and pDNA. Introducing bPEI1.8kDa resulted in no detection of EtBr-intercalated pDNA in “fresh” micelle/pDNA/bPEI1.8kDa complexes (Fig. 5(a)). In addition, dye quenching results clearly indicate that bPEI1.8kDa completely prevented pDNA exposure on polyplex surface (Fig. 5(b)). These findings indicate that bPEI1.8kDa completely shielded any exposed pDNA on the surface of “fresh” micelle/pDNA complexes or that bPEI1.8kDa strengthened the complexation between micelles and pDNA.
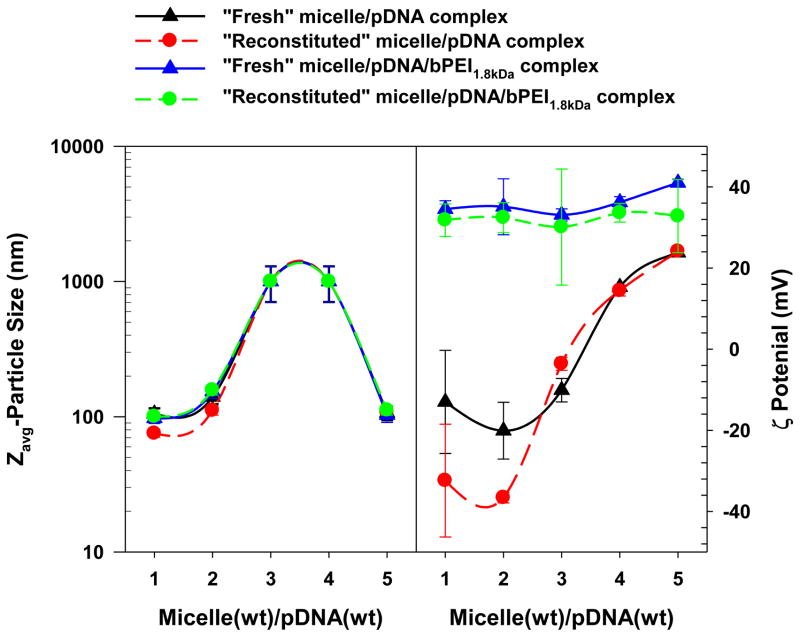
Particle size and surface charge of “fresh” micelle/pDNA complexes and “fresh” micelle/pDNA/bPEI1.8kDa complexes were further investigated to address whether bPEI1.8kDa mainly affected the surface shielding on the micelle/pDNA complexes or induced tighter complexation in the micelle/pDNA complexes. As shown in Fig. 6, the particle sizes of “fresh” micelle/pDNA complexes and “fresh” micelle/pDNA/bPEI1.8kDa complexes were not significantly different. This finding indicates that the major effect of bPEI1.8kDa treatment is probably not the induction of tighter complexation. However, the introduction of bPEI1.8kDa strongly increased the surface charge to more positive values (30–40 mV) compared to micelle/pDNA complexes where the pDNA was fully exposed (approximately −20 mV at WR 1 to 2) or partially pDNA exposed (approximately 0–20 mV at WR 3 to 5). These results may indicate that bPEI1.8kDa bound to and protected any pDNA exposed on the surface of micelle/pDNA complexes.
Fig. 6.
Particle size and surface charge of micelle/pDNA complexes and micelle/pDNA/bPEI1.8kDa complexes before and after reconstitution. (n=5; mean ± SD)
After examining the physicochemical similarities between “fresh” micelles and “reconstituted” micelles, the effects of reconstitution on polyplexes were characterized in terms of complexation, particle size, and surface charge. Although EtBr-intercalated pDNA was detected for “fresh” micelle/pDNA complexes (WRs 4–5), EtBr-intercalated pDNA was not detectable for “reconstituted” micelle/pDNA complexes (WRs 4–5) (Fig. 5). It is possible that the lyophilization process may decrease the distance between phosphate groups in the pDNA and the primary amines of bPEI25kDa corona and thus further increase their electrostatic attraction. These tighter interactions could then prevent EtBr from intercalating with pDNA. However, in the case of bPEI1.8kDa-shielded micelle/pDNA complexes, there was no apparent lyophilization effect because the polyplexes (WRs 1–5) had completely shielded pDNA regardless of whether the polyplexes were “fresh” or “reconstituted” (Fig. 5). Monitoring particle size of the polyplexes confirmed that the lyophilization and reconstitution process did not significantly affect particle size of “reconstituted” polyplexes compared to “fresh” polyplexes regardless of whether they were bPEI1.8kDa-shielded or unshielded micelle/pDNA complexes (Fig. 6). In addition, the surface charge of “reconstituted” polyplexes was not significantly different from “fresh” polyplexes (Fig. 6). These facts support the theory that the lyophilization and reconstitution of polyplexes may not negatively impact the physicochemical characteristics of “fresh” polyplexes.
3.4. Biological Characteristics of (PLGA36kDa)2-b-bPEI25kDa micelle/pDNA/bPEI1.8kDa complexes
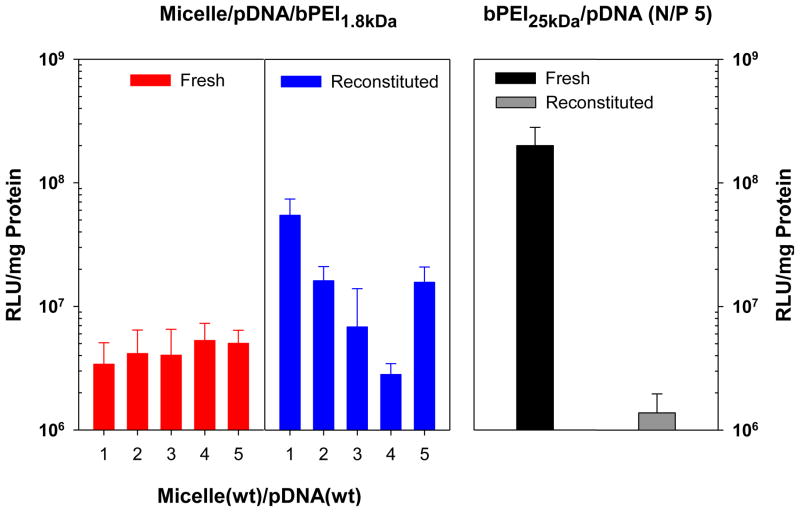
Transfection experiments using micelle/pDNA/bPEI1.8kDa complexes were performed in MCF7 breast cancer cells, using bPEI25kDa/pDNA (N/P 5) complexes as a control. As shown in Fig. 7, transfection efficiencies for “fresh” micelle/pDNA/bPEI1.8kDa complexes (WRs 1–5) were much higher (50–100 fold) than for micelle/pDNA complexes (WRs 15–30) (Fig. S5). This indicates that the surface shielding conferred by the addition of bPEI1.8kDa may prevent bioactivity loss of pDNA via protection from nuclease-mediated degradation. Nevertheless, “fresh” micelle/pDNA/bPEI1.8kDa complexes (WRs 1–5) still had 20–40-fold lower transgene expression levels than “fresh” bPEI25kDa/pDNA (N/P 5) complexes (Fig. 7).
Fig. 7.
Transfection efficiency of fresh and reconstituted micelle/pDNA/bPEI1.8kDa complexes (1 μg of pDNA) in MCF7 cells (5×105 cells at seeding). (n=8; mean ± SD)
Interestingly however, “reconstituted” micelle/pDNA/bPEI1.8kDa complexes (WRs 1–5) had enhanced transfection efficiencies up to 16 times higher than the transfection values of their “fresh” micelle/pDNA/bPEI1.8kDa complex counterparts (WRs 1–5) (Fig. 7). This was especially true for micelle/pDNA/bPEI1.8kDa complexes that were less than 200 nm in diameter (i.e., WRs 1, 2, and 5), where the process of lyophilization and reconstitution significantly enhanced their transfection efficiencies compared to their “fresh” polyplex counterparts (p<0.001). The transfection efficiency of “reconstituted” micelle/pDNA/bPEI1.8kDa complexes (WR 1) was much closer (3.6 times lower) to transfection levels using “fresh” bPEI25kDa/pDNA (N/P 5) complexes compared to “fresh” micelle/pDNA/bPEI1.8kDa complexes (WR 1; 59-fold lower) (Fig. 7). Rather interestingly “reconstituted” micelle/pDNA/bPEI1.8kDa complexes (WR 1) had approximately 39-fold higher transfection efficiency than “reconstituted” bPEI25kDa/pDNA (N/P 5) complexes prepared from sticky and lyophilized bPEI25kDa/pDNA (N/P 5). Given the effect of reconstitution on bPEI25kDa/pDNA complexes, the benefit of transfection enhancement for “reconstituted” micelle/pDNA/bPEI1.8kDa complexes (WR 1) may increase its appeal as a potential alternative to bPEI25kDa as a transfection reagent because the micelle system was also considerably less cytotoxic than bPEI25kDa.
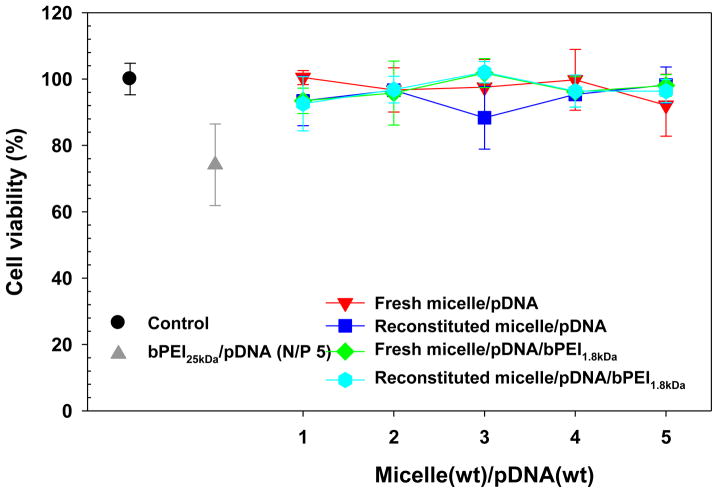
Although the micelle possesses more biocompatibility than bPEI25kDa, polyplex cytotoxicity can also significantly affect transfection results. Thus, the cytotoxicity of “fresh” and “reconstituted” polyplexes (WRs 1–5) was evaluated in MCF7 cells and compared to the cytotoxicity of “fresh” bPEI25kDa/pDNA complexes (N/P 5). As shown in Fig. 8, the cell viability of “fresh” micelle/pDNA complexes was over 90% compared to the untransfected control cells and was not statistically different from “reconstituted” micelle/pDNA complexes. Although bPEI1.8kDa was introduced into the micelle/pDNA complexes, the polyplexes still showed cell viability of approximately 90% regardless of whether they were subjected to lyophilization and reconstitution. Overall, the cytotoxicity of micelle-based polyplexes was significantly lower than “fresh” bPEI25kDa/pDNA complexes (>90% versus <75%, p<0.01).
Fig. 8.
Cytotoxicity of fresh and reconstituted micelle/pDNA/bPEI1.8kDa complexes (0.5 μg of pDNA) in MCF7 cells (2.5×105 cells at seeding). (n=4; mean ± SD)
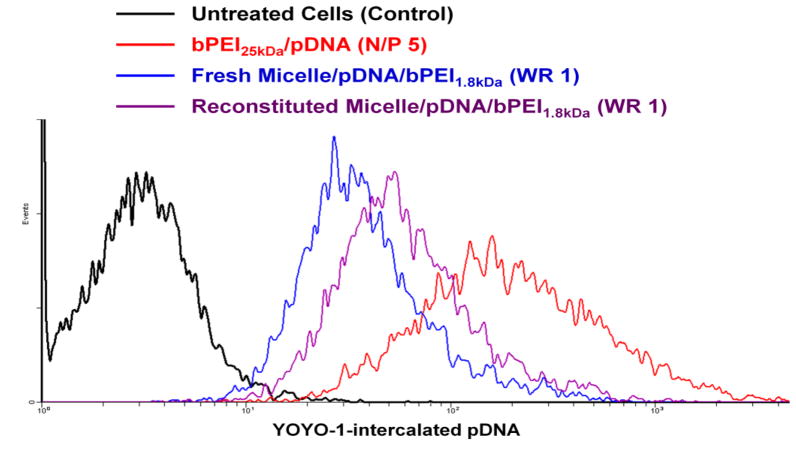
To investigate why “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes showed higher transfection efficiency than “fresh” micelle/pDNA/bPEI1.8kDa (WR 1) complexes but lower transfection efficiency than “fresh” bPEI25kDa/pDNA (N/P 5) complexes, cellular uptake of complexed pDNA was analyzed in MCF7 cells. As shown in Fig. 9, from among the different polyplexes tested, “fresh” bPEI25kDa/pDNA (N/P 5) showed the highest cellular uptake. “Reconstituted” micelle/pDNA/bPEI1.8kDa complexes had higher up-take compared to their “fresh” counterparts. These differences in the amount of intracellular pDNA support the benefit of reconstitution on increasing the transfection efficiency of micelle/pDNA/bPEI1.8kDa (WR 1).
Fig. 9.
Cellular uptake of “fresh” and “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) and “fresh” bPEI25kDa/pDNA (N/P 5) complexes in MCF7 cells.
3.5. Scalability of (PLGA36kDa)2-b-bPEI25kDa micelle/pDNA/bPEI1.8kDa complexes
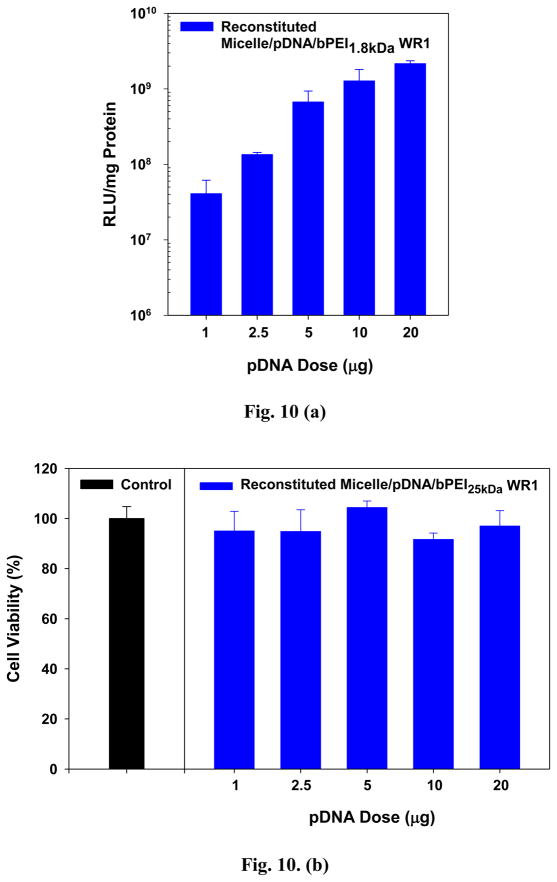
While the transfection results using “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes appear very promising in vitro, in vivo and clinical applications of polymeric gene vectors would require large scale polyplex preparation with limited polyplex volume. In addition, one concern in gene therapy is whether transfection and cytotoxicity results obtained using small scale polyplex doses can be scaled up proportionately and translated to reflect the transfection and cytotoxicity associated with large scale polyplex doses. Thus, pDNA dose-dependent transfection and cytotoxicity studies of “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes were conducted in MCF7 cells to evaluate the effects of increasing pDNA doses.
MCF7 cells were transfected with “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes at varying final concentrations of pDNA ranging from 0.5–10 μg/mL (1–20 μg pDNA delivered to 500,000 cells at initial seeding). As shown in Fig. 10(a), transfection levels increased linearly with increasing pDNA dose; after accounting for cell viability, transfection increased nearly 50-fold with a pDNA dose of 20 μg (10 μg/mL) versus 1 μg (0.5 μg/mL). The cytotoxicity of micelle/pDNA/bPEI1.8kDa complexes was fairly low, with cell viabilities of 80% or higher at every pDNA concentration tested (Fig. 10(b)). Thus, this (PLGA36kDa)2-b-bPEI25kDa polymeric micelle-based system could be used to delivery larger quantities of pDNA without causing undue toxicity.
Fig. 10.
pDNA-dose dependent transfection characteristics of “reconstituted” micelle/pDNA/bPEI1.8kDa (WR 1) complexes: (a) transfection efficiency in MCF7 cells (5×105 cells at seeding) and (b) cytotoxicity in MCF7 cells (2.5×105 cells at seeding). (n=4; mean ± SD)
Based on our initial findings, this micelle/pDNA/bPEI1.8kDa polyplex system has many favorable characteristics that make it an attractive gene delivery system. In addition, the structure of the particular copolymer used allows the possibility to bestow additional functionalities and further optimize the carrier. For instance, cationic polyplexes tend to have decreased colloidal stability due to non-specific interactions with serum proteins and non-target cells. This shortcoming could be overcome by conjugating poly(ethylene glycol) (PEG), which would reduce protein interactions and increase polyplex stability. Another option is to modify the carrier by conjugating targeting moieties to the polymeric micelle-surface which would enhance cellular internalization in the cell population of interest. Finally, because of the core-shell structure of our micelle system, there is the potential to utilize the core by loading it with hydrophobic therapeutic agents such as doxorubicin, dexamethasone, or taxol among others, thus creating a dual-agent delivery system that can be lyophilized for storage and easily reconstituted when needed.
4. Conclusions
The goal of this study was to create a charged polymeric micelle-based system for gene therapeutics delivery that maintained efficacy following lyophilization and reconstitution. Using our (PLGA36kDa)2-b-bPEI25kDa block copolymer, we were able to create a micelle system that remained stable following reconstitution. After complexation with pDNA, the micelle/pDNA system retained its particle size and surface charge characteristics following lyophilization and reconstitution. Finally, the reconstituted system was able to successfully transfect breast cancer cells using a reporter gene with minimal toxicity to the cells. Although extrapolating the use of non-viral systems to treat human disease is still somewhat premature, we are encouraged by evidence from our lab showing the potential of this particular system as a reconstitutable charged polymeric micelle gene delivery system.
Supplementary Material
Acknowledgments
This work was partially supported by NIH GM82866. Utah-Inha DDS Center supported DM for one year.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Louis A, Bartke A, Masternak MM. Effects of growth hormone and thyroxine replacement therapy on insulin signaling in Ames Dwarf mice. J Gerontol A Biol Sci Med Sci. 2010;65:344–352. doi: 10.1093/gerona/glq018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pawlyk BS, Bulgakov OV, Liu X, Xu X, Adamian M, Sun X, et al. Replacement gene therapy with a human RPGRIP1 sequence slows photoreceptor degeneration in a murine model of leber congenital amaurosis. Hum Gene Ther. 2010;21:993–1004. doi: 10.1089/hum.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang G, Qiu J, Wang R, Krause A, Boyer JL, Hackett NR, et al. Persistent expression of biologically active anti-HER2 antibody by AAVrh.10-mediated gene transfer. Cancer Gene Ther. 2010;17:559–570. doi: 10.1038/cgt.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou S, Mody D, DeRavin SS, Hauer J, Lu T, Ma Z, et al. A self-inactivating lentiviral vector for SCID-X1 gene therapy that does not activate LMO2 expression in human T cells. Blood. 2010;116:900–908. doi: 10.1182/blood-2009-10-250209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.López-Fraga M, Wright N, Jiménez A. RNA interference-based therapeutics: new strategies to fight infectious disease. Infect Disord Drug Targets. 2008;8:262–273. doi: 10.2174/187152608786734223. [DOI] [PubMed] [Google Scholar]
- 6.Kang HC, Lee M, Bae YH. Polymeric gene carriers. Crit Rev Eukaryot Gene Expr. 2005;15:317–342. doi: 10.1615/critreveukargeneexpr.v15.i4.30. [DOI] [PubMed] [Google Scholar]
- 7.Deyle D, Russell D. Adeno-associated virus vector integration. Curr Opin Mol Ther. 2009;11:442–447. [PMC free article] [PubMed] [Google Scholar]
- 8.Bosticardo M, Ghosh A, Du Y, Jenkins NA, Copeland NG, Candotti F. Self-inactivating retroviral vector-mediated gene transfer induces oncogene activation and immortalization of primary murine bone marrow cells. Mol Ther. 2009;17:1910–1918. doi: 10.1038/mt.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano G, Marino I, Pentimalli F, Adamo V, Giordano A. Insertional mutagenesis and development of malignancies induced by integrating gene delivery systems: implications for the design of safer gene-based interventions in patients. Drug News Perspect. 2009;22:185–196. doi: 10.1358/dnp.2009.22.4.1367704. [DOI] [PubMed] [Google Scholar]
- 10.Beer S, Bellovin DI, Lee JS, Komatsubara K, Wang LS, Koh H, et al. Low-level shRNA cytotoxicity can contribute to MYC-induced hepatocellular carcinoma in adult mice. Mol Ther. 2009;18:161–170. doi: 10.1038/mt.2009.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kay MA. AAV vectors and tumorigenicity. Nat Biotechnol. 2007;25:1111–1113. doi: 10.1038/nbt1007-1111. [DOI] [PubMed] [Google Scholar]
- 12.Wu TL, Ertl HC. Immune barriers to successful gene therapy. Trends Mol Med. 2009;15:32–39. doi: 10.1016/j.molmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Li SD, Huang L. Non-viral is superior to viral gene delivery. J Control Release. 2007;123:181–183. doi: 10.1016/j.jconrel.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Ogris M, Wagner E. Tumor-targeted gene transfer with DNA polyplexes. Somat Cell Mol Genet. 2002;27:85–95. doi: 10.1023/a:1022988008131. [DOI] [PubMed] [Google Scholar]
- 15.Schatzlein AG. Targeting of synthetic gene delivery systems. J Biomed Biotechnol. 2003;2003:149–158. doi: 10.1155/S1110724303209116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HC, Kim S, Lee M, Bae YH. Polymeric gene carrier for insulin secreting cells: Poly(l-lysine)-g-sulfonylurea for receptor mediated transfection. J Control Release. 2005;105:164–176. doi: 10.1016/j.jconrel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Ferrer-Miralles N, Vazquez E, Villaverde A. Membrane-active peptides for non-viral gene therapy: making the safest easier. Trends Biotechnol. 2008;26:267–275. doi: 10.1016/j.tibtech.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Kang HC, Bae YH. pH-Tunable endosomolytic oligomers for enhanced nucleic acid delivery. Adv Funct Mater. 2007;17:1263–1272. [Google Scholar]
- 19.Chan CK, Jans DA. Using nuclear targeting signals to enhance non-viral gene transfer. Immunol Cell Biol. 2002;80:119–130. doi: 10.1046/j.1440-1711.2002.01061.x. [DOI] [PubMed] [Google Scholar]
- 20.Pouton CW, Wagstaff KM, Roth DM, Moseley GW, Jans DA. Targeted delivery to the nucleus. Adv Drug Deliv Rev. 2007;59:698–717. doi: 10.1016/j.addr.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30:2180–2198. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Kim YH, Park JH, Lee M, Kim YH, Park TG, Kim SW. Polyethylenimine with acid-labile linkages as a biodegradable gene carrier. J Control Release. 2005;103:209–219. doi: 10.1016/j.jconrel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 23.Kang HC, Kang H-J, Bae YH. A reducible polycationic gene vector derived from thiolated low molecular weight branched polyethyleneimine linked by 2-iminothiolane. Biomaterials. 2011;32:1193–1203. doi: 10.1016/j.biomaterials.2010.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47:113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 25.Qiu L, Bae YH. Self-assembled polyethylenimine-graft-poly(ε-caprolactone) micelles as potential dual carriers of genes and anticancer drugs. Biomaterials. 2007;28:4132–4142. doi: 10.1016/j.biomaterials.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Ke C-Y, Weijie Beh C, Liu S-Q, Goh S-H, Yang Y-Y. The self-assembly of biodegradable cationic polymer micelles as vectors for gene transfection. Biomaterials. 2007;28:5358–5368. doi: 10.1016/j.biomaterials.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Jung S, Luo S, Meng F, Zhu X, Park TG, et al. Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA-PCL-PDMAEMA triblock copolymers. Biomaterials. 2010;31:2408–2416. doi: 10.1016/j.biomaterials.2009.11.077. [DOI] [PubMed] [Google Scholar]
- 28.Mishra V, Gupta U, Jain NK. Biowaiver: an alternative to in vivo pharmacokinetic bioequivalence studies. Pharmazie. 2010;65:155–161. [PubMed] [Google Scholar]
- 29.Fuchs GS, Mikkelsen S, Knudsen TK, Kappelgaard A-M. Ease of use and acceptability of a new pen device for the administration of growth hormone therapy in pediatric patients: An open-label, uncontrolled usability test. Clin Ther. 2009;31:2906–2914. doi: 10.1016/j.clinthera.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 30.Collier J, Shah R, Gupta A, Sayeed V, Habib M, Khan M. Influence of Formulation and Processing Factors on Stability of Levothyroxine Sodium Pentahydrate. AAPS PharmSciTech. 2010;11:818–825. doi: 10.1208/s12249-010-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCluskey SV, Graner KK, Kemp J, Aloumanis V, Ben M, Kupiec T, et al. Stability of fentanyl 5 μg/mL diluted with 0.9% sodium chloride injection and stored in polypropylene syringes. Am J Health Syst Pharm. 2009;66:860–863. doi: 10.2146/ajhp080255. [DOI] [PubMed] [Google Scholar]
- 32.Craig DQ. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharm. 2002;231:131–144. doi: 10.1016/s0378-5173(01)00891-2. [DOI] [PubMed] [Google Scholar]
- 33.Bulmus V, Chan Y, Nguyen Q, Tran HL. Synthesis and characterization of degradable p(HEMA) microgels: use of acid-labile crosslinkers. Macromol Biosci. 2007;7:446–455. doi: 10.1002/mabi.200600258. [DOI] [PubMed] [Google Scholar]
- 34.Molina MdC, Allison SD, Anchordoquy TJ. Maintenance of nonviral vector particle size during the freezing step of the lyophilization process is insufficient for preservation of activity: Insight from other structural indicators. J Pharm Sci. 2001;90:1445–1455. doi: 10.1002/jps.1096. [DOI] [PubMed] [Google Scholar]
- 35.Flores-Fernández GM, Solá RJ, Griebenow K. The relation between moisture-induced aggregation and structural changes in lyophilized insulin. J Pharm Pharmacol. 2009;61:1555–1561. doi: 10.1211/jpp/61.11.0016. [DOI] [PubMed] [Google Scholar]
- 36.Thompson C, Hansford D, Higgins S, Rostron C, Hutcheon G, Munday D. Preparation and evaluation of microspheres prepared from novel polyester-ibuprofen conjugates blended with non-conjugated ibuprofen. J Microencapsul. 2009;26:676–683. doi: 10.3109/02652040802656333. [DOI] [PubMed] [Google Scholar]
- 37.Miene C, Klenow S, Veeriah S, Richling E, Glei M. Impact of apple polyphenols on GSTT2 gene expression, subsequent protection of DNA and modulation of proliferation using LT97 human colon adenoma cells. Mol Nutr Food Res. 2009;53:1254–1262. doi: 10.1002/mnfr.200800444. [DOI] [PubMed] [Google Scholar]
- 38.Holzer M, Vogel V, Mäntele W, Schwartz D, Haase W, Langer K. Physico-chemical characterisation of PLGA nanoparticles after freeze-drying and storage. Eur J Pharm Biopharm. 2009;72:428–437. doi: 10.1016/j.ejpb.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Anhorn MG, Mahler H-C, Langer K. Freeze drying of human serum albumin (HSA) nanoparticles with different excipients. Int J Pharm. 2008;363:162–169. doi: 10.1016/j.ijpharm.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Stark B, Pabst G, Prassl R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effects of cryoprotectants on structure. Eur J Pharm Sci. 2010;41:546–555. doi: 10.1016/j.ejps.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 41.Kuntsche J, Freisleben I, Steiniger F, Fahr A. Temoporfin-loaded liposomes: Physicochemical characterization. Eur J Pharm Sci. 2010;40:305–315. doi: 10.1016/j.ejps.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 42.Miyata K, Kakizawa Y, Nishiyama N, Yamasaki Y, Watanabe T, Kohara M, et al. Freeze-dried formulations for in vivo gene delivery of PEGylated polyplex micelles with disulfide crosslinked cores to the liver. J Control Release. 2005;109:15–23. doi: 10.1016/j.jconrel.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 43.Ou M, Xu R, Kim SH, Bull DA, Kim SW. A family of bioreducible poly(disulfide amine)s for gene delivery. Biomaterials. 2009;30:5804–5814. doi: 10.1016/j.biomaterials.2009.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang HC, Kim S, Lee M, Bae YH. Polymeric gene carrier for insulin secreting cells: Poly(l-lysine)-g-sulfonylurea for receptor mediated transfection. J Control Release. 2005;105:164–176. doi: 10.1016/j.jconrel.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Kang HC, Samsonova O, Bae YH. Trafficking microenvironmental pHs of polycationic gene vectors in drug-sensitive and multidrug-resistant MCF7 breast cancer cells. Biomaterials. 2010;31:3071–3078. doi: 10.1016/j.biomaterials.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silverstein RM, Bassler GC, Morrill TC. Spectrometric identification of organic compounds. 5. John Wiley & Sons; 1991. [Google Scholar]
- 47.Mittal G, Sahana DK, Bhardwaj V, Ravi Kumar MNV. Estradiol loaded PLGA nanoparticles for oral administration: Effect of polymer molecular weight and copolymer composition on release behavior in vitro and in vivo. J Control Release. 2007;119:77–85. doi: 10.1016/j.jconrel.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Lee W-k, Park J-y, Yang EH, Suh H, Kim SH, Chung DS, et al. Investigation of the factors influencing the release rates of cyclosporin A-loaded micro- and nanoparticles prepared by high-pressure homogenizer. J Control Release. 2002;84:115–123. doi: 10.1016/s0168-3659(02)00239-0. [DOI] [PubMed] [Google Scholar]
- 49.Park TG. Degradation of poly(lactic-co-glycolic acid) microspheres: effect of copolymer composition. Biomaterials. 1995;16:1123–1130. doi: 10.1016/0142-9612(95)93575-x. [DOI] [PubMed] [Google Scholar]
- 50.Braunecker J, Baba M, Milroy GE, Cameron RE. The effects of molecular weight and porosity on the degradation and drug release from polyglycolide. Int J Pharm. 2004;282:19–34. doi: 10.1016/j.ijpharm.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Jalil R, Nixon JR. Biodegradable poly(lactic acid) and poly(lactide-co-glycolide) microcapsules: problems associated with preparative techniques and release properties. J Microencapsul. 1990;7:297–325. doi: 10.3109/02652049009021842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.