Summary
The worldwide prevalence of childhood obesity has increased greatly over the past 3 decades. The increasing occurrence in children of disorders, such as type 2 diabetes, is believed to be a consequence of this obesity epidemic. Much progress has been made in understanding the genetics and physiology of appetite control and from this, the elucidation of the causes of some rare obesity syndromes. However, these rare disorders have so far taught us only limited lessons on how to prevent or reverse obesity in most children. Calorie intake and activity recommendations need to be re-assessed and better quantified, on a population level, given the more sedentary life of children today. For individual treatment, the currently recommended calorie prescriptions may be too conservative given the evolving insight on the “energy gap.” Whilst quality of research in both prevention and treatment has improved, there is still a need for high-quality multi-centre trials with long-term follow-up. Meanwhile, prevention and treatment approaches that aim to increase energy expenditure and decrease intake need to continue. Most recently, the spiralling increase in obesity prevalence may be abating for children. Thus, even greater efforts need to be made on all fronts to continue this potentially exciting trend.
Introduction
It has been 8 years since the last Lancet Seminar on childhood obesity.1 The goal of this Seminar is to review new information relevant to childhood obesity and outline some of the challenges that remain.
Epidemiology
A 2006 review of secular trends in childhood overweight/obesity concluded that its prevalence had increased over the last two to three decades in most industrialized countries except for Russia and Poland and in several lower income countries, particularly in urban areas.2 The prevalence doubled or trebled between the early 1970’s and late 1990’s in Australia, Brazil, Canada, Chile, Finland, France, Germany, Greece, Japan, the UK, and the US.2 It was predicted that by 2010, over 40% of children in the North American and Eastern Mediterranean WHO regions, 38% in the European region, 27% in the Western Pacific region, and 22% in South-East Asian region would be overweight/obese. However, this review predates most recent data, which although it is too soon to be certain, suggests that the increase in childhood obesity in the US, UK and Sweden may be abating.3–5
Definition of childhood obesity
There are internationally agreed thresholds of body-mass index (BMI) for defining under-weight, normal weight, overweight, and obesity in adults, but in children, the marked effects of age, gender, pubertal status and race/ethnicity on growth make classification difficult. There are two challenges in defining (i) a standard age-related growth chart; (ii) cut-points for overweight and obesity that are clinically meaningful. The International Obesity Taskforce (IOTF) have developed an international standard growth chart, which enables comparison of prevalence globally.6 However, many countries continue to use their own country-specific charts, including US, whose standards are based on a national survey from early 1960’s, before the current epidemic.7
Commonly used cut points for childhood overweight and obesity include: 110% or 120% of ideal weight for height; weight-for-height Z-scores of >1 and >2, and BMI at the 85th, 90th, 95th, and 97th percentiles (based on international or country-specific reference populations).2 The IOTF recommend using their international growth charts and age and gender specific cut points that, on average, correspond to the adult thresholds. The IOTF classification has been shown to have high specificity but low sensitivity.8
Determinants and risk factors for childhood obesity
It is a historical convergence of forces, biological and technological, that has produced the obesity epidemic seen today. Over millennia of frequent food scarcities, natural selection likely favoured those with more parsimonious energy metabolism, known as the “thrifty gene” hypothesis.9 Although the advent of agriculture about 14,000 years ago ensured stable food supply, activities of daily living still required considerable energy expenditure until about 50 years ago, when radical changes occurred in food availability and required energy expenditures. The current obesity epidemic is then likely the result of our evolutionary legacy interacting with today’s technologically-advanced, consumerist society. Population groups in North America who have preserved traditional lifestyles with significant embedded physical activity show considerably lower prevalence of obesity.10 Likewise, in low and middle income countries the obesity epidemic is largely occurring in urbanized areas with easy access to energy dense cheap foods and less energy requirements in daily life.2
More specifically, obesity is a complex condition which is influenced by a wide-range of genetic and non-genetic factors, with interactions between many of these. We focus primarily on current knowledge related to prevention and treatment in this Seminar. Table 1 provides a summary of determinants or risk factors that have been associated with childhood obesity and/or variation in adiposity, with Figure 1 showing a simplified model of the leptin signalling pathway, the key biological pathway that controls energy balance. For several risk factors, evidence is currently weak and, whilst there have been important advances, it still remains unclear how to incorporate the available information effectively and cost-effectively in prevention programs for children across the world.
Table 1.
Determinants or Risk Factors for Development of Childhood Obesity or Greater Adiposity
| Risk factor | Nature of association | Currently potentially modifiable | Type of evidence* |
|---|---|---|---|
| Genetic variation | Rare single gene defects in which obesity is the specific abnormality, e.g. those related to the leptin signaling pathway (Figure 1). | No, except for leptin replacement in the handful of leptin-deficient individuals | Basic science studies, case series, family linkage, and genetic-association studies86 |
| Obesity is a manifestation of several genetic syndromes (Figure 2). Prader-Willi syndrome is associated with hyperghrelinemia, but the mechanism of hyperphagia remains unclear.87,88 Animal models of ciliopathies (Bardet-Biedl and Alstrom syndromes) have defects in leptin pathway signaling.89,90 Haploinsufficiency of BDNF, a downstream mediator of leptin action, is associated with hyperphagia and obesity in children with WAGR syndrome.91 | No | Genetic-association studies86 | |
| Genome wide association studies have identified several common genetic variants associated with greater adiposity and obesity; each with weak/modest effects. | No | Genome wide association studies92 | |
| Epigenetics | The mechanism whereby in utero factors (see below) can produce heritable changes in adiposity has been suggested to be due to DNA methylation or histone modification of DNA in gene regulatory regions. However, evidence from humans is currently lacking. | Possibly, in animals, maternal consumption of folate, methionine, and vitamin B12 during pregnancy can affect DNA methylation in offspring | Non-systematic review of evidence (largely from basic science and animal studies)93 |
| Endocrine disease | Classically, hypothyroidism, growth hormone deficiency or resistance, and cortisol excess. Polycystic ovarian syndrome (PCOS) is a consequence of but also possible contributor to obesity. The obesity associated with pseudohypoparathyroidism (caused by Gαs inactivating mutation) may be due to defective signaling at G-protein coupled receptors, including the melanocortin receptor of the leptin pathway.94 | Some, e.g. thyroxine and growth hormone replacement, surgical treatment of Cushing syndrome; for PCOS, oral contraceptives, anti-androgens, and insulin-sensitizers have been used, but long-term large RCTs lacking in adolescents95 | Non-systematic review of evidence (basic science, epidemiology, clinical)96 |
| Central nervous system pathology | Congenital or acquired hypothalamic abnormalities have been associated with a severe form of obesity in children and adolescents. | Possibly, but still under investigation; hyperinsulinemia due to increased vagal tone has been postulated as a contributing factor prompting studies using octreotide, which prevented further weight gain in a small RCT, but long-term larger RCTs are needed.97 | Non-systematic review of evidence (basic science, epidemiology, clinical)98 |
| Intrauterine exposure to gestational diabetes | In populations at high risk of obesity and diabetes (e.g. Pima Indians) exposure to gestational diabetes is associated with increased risk of childhood and early adult obesity in offspring. Evidence for similar associations in other populations is currently lacking. | Yes | Review of observational studies in Pima99 Prospective cohorts in other populations100 (and other studies cited in this reference) |
| Intrauterine exposure to greater maternal adiposity | A study comparing obesity in children whose mothers had received bariatric surgery for extreme morbid obesity found siblings born before surgery (when mother was extremely obese) were more obese than their siblings born after weight loss in response to surgery. Evidence that less extreme variation in maternal adiposity affects offspring obesity is lacking. | Yes | Within sibling comparisons,101 prospective cohort studies, 99,102 and Mendelian randomization study102 |
| Birth weight | Higher birth weight is associated with increased offspring fat and lean mass. Small-for-gestational age babies who exhibit catch-up growth may be at risk for childhood obesity, but this may simply reflect greater growth resulting in larger size. | Safe means of modifying birth weight to improve health are unknown | Prospective cohort studies50,103 |
| BMI rebound | Early age at BMI rebound is associated with greater risk of obesity, but this may be statistical artifact. | No, since can only be determined retrospectively in individuals | Non-systematic review of largely prospective cohort studies104 |
| Diet | Breast feeding is unlikely to be causally protective of childhood obesity. | Yes | Systematic review of prospective cohort studies,105 RCT106 |
| High quality prospective evidence is sparse. Available evidence suggests that high energy intake in early infancy and high consumption of sweetened drinks in childhood is prospectively associated with childhood obesity risk. Lack of evidence for other dietary characteristics may reflect poor study design and difficulties of accurately assessing diet in children. | Yes | Non-systematic review of observational studies107 | |
| Energy expenditure | Low levels of physical activity are associated with childhood obesity risk. | Yes | Systematic review of observational studies108 |
| Television viewing | Greater hours spent screening viewing associated with childhood obesity risk. | Yes | Systematic review of observational and experimental studies109 |
| Sleep | Shorter sleep duration in infancy and childhood associated with childhood obesity risk. | Possibly | Prospective cohort study110 |
| Microbial infection | Potential role of microbial infection (e.g. adenovirus Ad-36) and composition of gut flora (e.g. ratio of Firmicutes to Bacteroidetes species) in the pathogenesis of obesity. However, epidemiological evidence in the non-selected general population is lacking. | Yes | Cross-sectional studies 111,112 |
| Iatrogenic | Cranial irradiation or surgery causing hypothalamic damage (see above). Psychotropic medication (e.g., olanzapine and risperidone), chemotherapeutics (e.g. treatment acute lymphocytic leukemia even without cranial irradiation), and hormonal contraception (e.g., depot medroxyprogesterone acetate) have been associated with greater weight gain in children and adolescents. | Depends upon disease/treatment and risk-benefit considerations | Non-systematic review of evidence (basic science, epidemiology, clinical)98 and prospective cohort studies113–115 |
| Ethnic origin | Some ethnic groups, e.g. Hispanic and South Asian, appear to be more likely to become obese. At a given BMI children and infants of South Asian origin have higher adiposity. | No | Cross-sectional studies116,117 |
| Country of birth | Children from low and middle income countries tend to be stunted and underweight but with sufficient nutrition gain healthy weight and with overnutrition are prone to obesity. | No | Cross-sectional and ecological studies118 |
| Urban versus rural area of residence | Children in urban areas are more likely to be obese than those in rural areas in many countries including high and low-middle income countries. | Unlikely to be able to change where families live but may be able to modify underlying reasons for association | Cross-sectional studies2 |
| Socioeconomic position | In high income countries generations born prior to 1950’s–1960’s did not show socioeconomic differentials in adiposity/obesity in childhood (though do as adults). Some evidence that contemporary populations of children do show higher rates of obesity in those from lowest socioeconomic groups in high income countries. | Yes with major political and cultural changes. May be able to modify the underlying reasons for association | Prospective two-generational cohort study119 |
The most recent systematic review of highest level of evidence is cited for most risk factors rather than providing a comprehensive list of all papers for each risk factor which would be beyond the scope of this seminar.
Abbreviation: RCT, randomized controlled trial. This table has been modified and updated from a similar table published previously.4
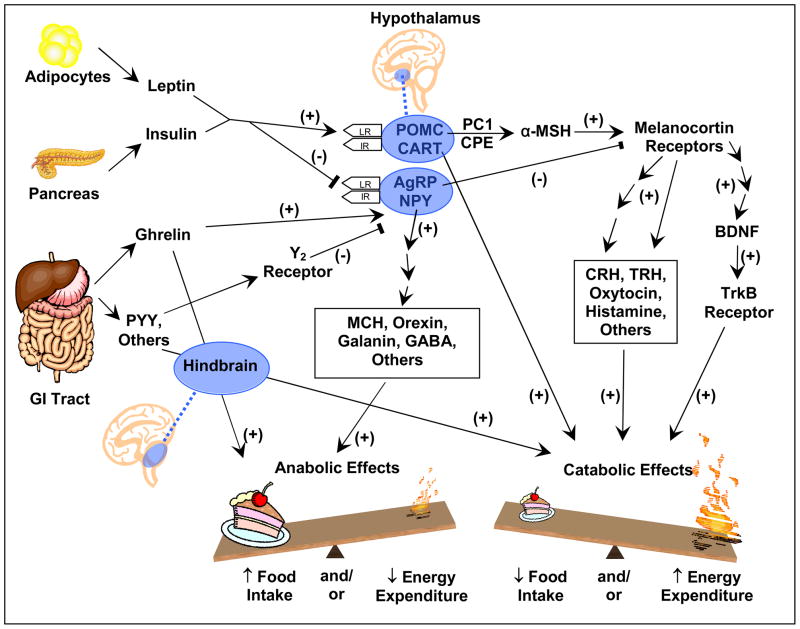
Figure 1. A simplified model of the leptin signalling pathway.
Since the discovery of leptin, understanding of the mechanisms that control energy balance has rapidly advanced. Unfortunately, except for leptin replacement therapy in a handful of leptin deficient individuals, there is yet to emerge any interventions that effectively prevent or treat obesity in the general population. Both insulin and leptin are secreted in proportion to body fatness and serve as adiposity signals, acting on the same neurons of the hypothalamic arcuate nucleus to regulate energy homeostasis. Ghrelin, which is secreted by the stomach and duodenum, serves as a hunger signal at the hypothalamus and brainstem, while other peptides secreted by the GI tract, including PYY, act as satiation signals. The ligands leptin,120 POMC, 121,122 CART,123 and BDNF,91,124 the receptors for leptin, 125,126 melanocortins,127–130 and BDNF,131 and the enzyme PC1132,133 have been found to have function-altering mutations associated with obesity in children. Mutations in the ligands and receptors for NPY,134 AGRP,135 CPE,136,137 and MCH138 have been found to alter energy balance in rodents, but have not been as convincingly shown to be associated with human obesity. Lines with arrowheads indicate stimulatory action. Lines ending with a perpendicular end-block indicate inhibitory action. Abbreviations: GI, gastrointestinal; PYY, peptide YY, IR, insulin receptor; LR, leptin receptor, NPY, neuropeptide Y; AGRP, agouti-related protein; POMC, pro-opiomelanocortin; CART, cocaine-amphetamine related transcript; PC1, prohormone convertase 1; CPE, carboxypeptidase E; MSH, melanocyte stimulating hormone; TRH, thyrotropin-releasing hormone; MCH, melanin concentrating hormone; GABA, gamma amino butyric acid; BDNF, brain-derived neurotrophic factor; TrkB, tyrosine kinase receptor B.
Differential diagnosis of childhood obesity
In evaluation of the paediatric patient with obesity, the possibility of endocrine diseases, congenital and acquired hypothalamic defects, genetic syndromes, and usage of medications affecting appetite should be considered (Figure 2). The vast majority of patients, however, will not have any of these identifiable conditions. Regardless of aetiology, all patients should be considered for modifiable lifestyle risk factors and screened for the complications of obesity.
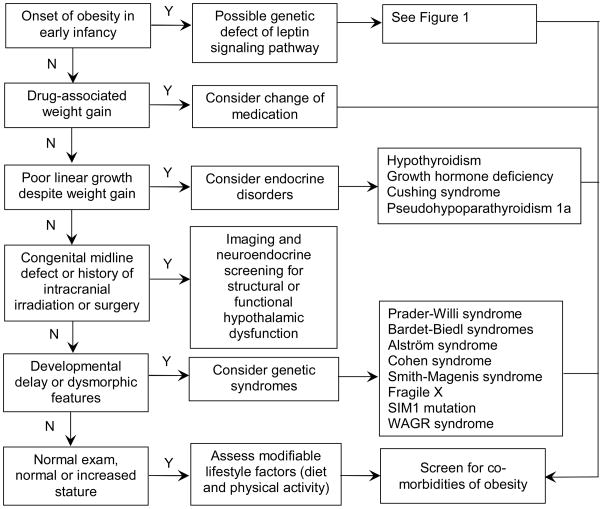
Figure 2. Recommended evaluation of childhood-onset obesity.
The clinical history and examination of the patient should guide the care provider in forming a differential diagnosis. Onset of marked obesity during early infancy raises the suspicion for function-altering genetic mutations affecting the leptin signalling pathway, but these are exceedingly rare conditions, with the most common, melanocortin-4 receptor defects, comprising <5% of children with early-onset obesity.130 When evaluating new onset of excessive weight gain, potential side effect from a recently initiated medication should be taken into consideration, as weight gain may be associated with administration of insulin or insulin secretagogues, glucocorticoids, hormonal contraceptives (e.g., depot medroxyprogesterone acetate), psychotropic medications (including atypical antipsychotics (e.g., clozapine, olanzapine, risperidone), mood stabilizers (e.g. lithium), tricyclic antidepressants (e.g., amitriptyline, imipramine, and nortriptyline), and anticonvulsants (e.g., valproic acid, gabapentin, and carbamazepine)), antihypertensives (e.g., propranolol and clonidine), and antihistamines.139 In patients with decreased growth velocity despite continued weight gain, an endocrinopathy should be considered; measurement of TSH and free T4, and referral to a paediatric endocrinologist are recommended. All patients, regardless of the aetiology of obesity, should be assessed for modifiable lifestyle factors, including physical activity and diet, and screened for complications of obesity, including measurement of fasting lipids, fasting glucose, and alanine amino transferase. If fasting glucose is 100 – 126 mg/dL, an oral glucose tolerance test is recommended. Screening for vitamin D and iron deficiency should also be considered.
Complications associated with childhood obesity
Childhood obesity can adversely affect nearly every organ system (Figure 3) and often cause serious consequences, including hypertension, dyslipidemia, insulin resistance/diabetes, fatty liver disease, and psychosocial complications.11 One study showed that being overweight or obese between age 14 to 19 was associated with increased adult mortality (from age 30) from a wide variety of systemic diseases.12 Atherosclerotic process13 appears to be accelerated in the obese child and almost half of children with BMI ≥ 97th percentile have one or more of the conditions which comprise the metabolic syndrome.14 Childhood and adolescent BMI is associated with increased risk of cardiovascular disease in adulthood.15 Pulmonary disorders, including obstructive sleep apnoea and reactive airway disease16 are seen more frequently among obese children. Asthma severity, however, does not seem to be altered by obesity,17 leaving open the possibility that weight-related but non-asthmatic airflow limitations are being misdiagnosed as asthma in some obese children.18
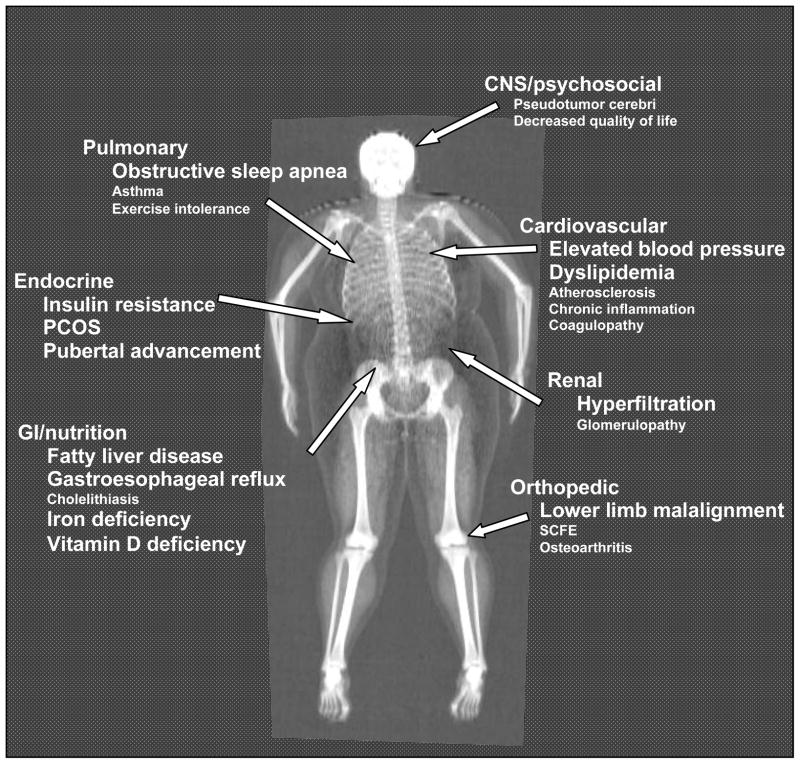
Figure 3. Complications associated with childhood obesity.
Image obtained by dual energy X-ray absorptiometry from a teenage girl with BMI 38 kg/m2. Conditions that are of high prevalence and are well-established in their association with childhood obesity are shown in larger font size.
Certain nutritional deficiencies often accompany childhood obesity. Higher BMI and greater adiposity have been associated with lower vitamin D levels in children.19 The mechanism underlying hypovitaminosis D in obesity is unclear, but has been proposed to be greater storage of vitamin D in adipose tissue.20 Overweight/obese children are also at least twofold more likely to be iron-deficient than normal weight children.21 Obesity leads to increased production of pro-inflammatory cytokines that in turn promote the release of hepcidin, a peptide hormone produced by the liver and adipocytes, which decreases iron absorption from the gut.22
Complications of childhood obesity include acceleration in the timing of thelarche and menarche in girls,23,24 pubertal advancement in boys25 and adverse effects on maturation26 and alignment27 of developing bones in both. Advanced skeletal maturation has been attributed to increased adipose tissue aromatization of weak androgens into more potent estrogens. Obesity may also impact pubertal timing through nutritionally related signals (e.g., insulin and leptin) on the reproductive axis.28 Orthopaedic complaints, including fractures, musculoskeletal discomfort, impaired mobility, and lower limb malalignment may be more common in obese compared to non-overweight children.27 Serious orthopaedic complications of childhood obesity include tibia vara (Blount’s disease or adolescent bowing of the legs)29 and slipped capital femoral epiphyses.30 By contrast, however, obesity may provide some beneficial effect with regards to bone mineral density. A recent study, using variation in the FTO gene as an instrumental variable, suggests that greater fat mass in children is causally associated with greater total, spinal, and limb bone mineral content.31
Prevention
Prevention, especially in the young, is universally viewed as the best approach for reversing the rising global prevalence of obesity. Despite the seeming allure of prevention, to date there is limited evidence regarding the most effective means of preventing obesity development in children. This may in part be related to relatively small sample sizes for the expected effect size and insufficient length of follow-up in many prevention trials. These trials have also been criticized for frequently not being based on sound theory of behaviour change and for having inadequate feasibility and pilot work.32
Past trials of prevention interventions may also have failed to show notable effects in part because they failed to adequately address the “energy gap”33 that separates those children who remain lean from those who gain weight throughout childhood.34 Butte and Ellis calculated that an energy deficit of >250 kcal/day is required to prevent further weight gain in 90% of overweight children35 – equivalent to a child walking an additional 1–2 hours/day at 1.9 km/hour – or consuming roughly one-fifth fewer calories than usual per day.35
Prevention is multi-level and measures can be instituted at individual, household, institutional, community, and health care levels:
Individual level prevention
Caregivers would need to be targeted rather than the children themselves at young age, and there may be reasons for focusing on mothers. First, it has been suggested that developmental or foetal over nutrition as a result of gestational diabetes or maternal obesity may have fuelled the current obesity epidemic (see Table 1).1 To date, there have been no intervention studies that have examined the long-term effect of reducing gestational diabetes or maternal adiposity on future offspring obesity risk. Second, it has been suggested that breast-feeding might prevent childhood obesity. However, systematic reviews suggest that observational associations might be explained largely by residual confounding and/or publication bias, and a large randomized trial of a breast feeding promotion intervention suggested no causal effect of breast feeding on childhood body weight or obesity risk.36 Third, mother’s might influence offspring diet more so than fathers.37 However, there are no intervention trials of maternal only interventions to prevent childhood obesity.
Household (family) level prevention
Encouraging parents to offer appropriate food portions, foster physical activity, maximize activities of daily living, and minimize sedentary behaviours are viewed as basic measures of prevention.38 Most government guidelines have traditionally focused on ensuring adequate nutritional intake.39 However, these may be less useful for assuring energy intakes that are appropriate for contemporary sedentary lifestyles. We are unaware of any randomised controlled trials to date that focus solely on household/family based interventions to prevent childhood obesity.
Institutional (nursery or school) level prevention
Most randomized prevention trials have been in the school setting since it is viewed as a universal “encatchment” for children. Most prevention programs involve altering the caloric content of school meals and increasing physical activity. One area hotly debated in the US involves the removal of vending machine from schools to curb the availability of energy dense snack foods. However, a national survey in the US revealed that snack foods from vending machines contributed only 1.3% of total daily calories from snacks, while home snacks contributed 69.1 %.40 There have been at least 9 systematic reviews of randomised controlled trials of school-based childhood obesity prevention programmes32,41 (see also citations of other systematic reviews within these). Earlier reviews noted a lack of evidence of effectiveness and the poor quality of studies; whereas more recent reviews suggest that school based interventions may be effective. A recent review identified 19 high-quality trials of school based interventions and found reduced odds of being overweight or obese in intervention compared to control groups (pooled odds ratio 0.74 (95%CI: 0.60, 0.92).41 The majority of studies were conducted in the US. There is a need to determine what the key effective characteristics are of such programmes and whether they are effective outside of the US. Although initiatives have also been aimed at children in kindergarten/nurseries,42 the relatively few controlled trials in this setting have yet to be systematically reviewed. One area yet to be addressed is the built environment of schools/nurseries. Architectural designs of school buildings and their environment can be re-examined for opportunities to impose increased energy expenditure. A multistory building with purposefully designed class schedules could lead to significant stair (or ramp) climbing during the school day.
Community-level prevention
Prevention at the community level includes public policies and mass media campaigns.43,44 For the past decade, there has been increasing pressure for labelling caloric contents on restaurant menus, especially at fast food restaurants. However, there are limited data regarding the effectiveness of such labelling for preventing childhood obesity.45 In 2002, the Centers for Disease Control launched a 2-year marketing campaign in the US via media advertisements to promote physical activity in children aged 9–13 years.46 Children’s physical activity (assessed by self-report) increased,43,44 but effects on BMI were not assessed. Currently, there is a strong movement toward government involvement in several countries for addressing the “toxic environment” by levying taxes on sugared beverages and fast foods, though the effectiveness of such measurements is currently unknown.47
The popular media in several countries have given much attention to the topic of obesity, but there is no objective information on the effect of these messages on the public.
Public health surveillance and screening for childhood obesity has been implemented in some communities. In the US, Arkansas was the first state to pass legislation in 2003 for mandatory BMI assessments of children in public schools with annual reporting to parents. This approach has subsequently been followed in thirteen other states.48,49 In 2005, an annual National Child Measurement Programme was introduced in the UK for surveillance of two school year groups. In 2007, the British government introduced legislation to give parents the results of their child’s measurements. Evidence to date is unclear as to whether surveillance or screening of childhood obesity will be valuable in its prevention.
Health care settings for prevention
Infants and young children are seen frequently in medical settings for well child and acute care. These visits present a potentially unique opportunity to detect upward deviations in a child’s growth rate, thus, placing the primary care provider at the strategic first line of defence before BMI exceeds recommended levels. However, data for the efficacy of such counselling for obesity prevention are lacking.
Critical periods for prevention
Certain periods during childhood present both challenges and windows of opportunity for obesity prevention as they have been observed to be associated with notable changes in adiposity growth velocity or obesity related behaviour. They include the first year of life,50 the period of “adiposity rebound” (between ages 3–7), and menarche.51 The transition years from childhood to adolescence are a time of marked behavioural changes, which include a precipitous decline in physical activity.52 Although it is unclear whether preventive measures instituted during these times will prevent excessive growth, these should be investigated further.
Discussion on prevention
Common sense supports a key role for decreased energy intake and increased energy expenditure in humans who have adapted through evolutionary processes to a parsimonious energy metabolism. Thus, prevention programs should aim to decrease energy intake, increase activity, and decrease sedentary behaviour. To balance the need for more definitive research on which interventions best achieve changes in these behaviours with the “pressure to act now” to halt and reverse the obesity epidemic, we need to continue with both prevention activities and research to better understand the means of inducing behavioural changes and their impact on childhood obesity.
In closing, for prevention, one might recall the words of Rudolph Virchow, a 19th century German pathologist, who wrote that “epidemics appear, and often disappear without traces, when a new culture period has started” and that mass diseases are “due to … disturbances of human culture.”53 Geoffrey Rose promulgated the notion further that whole populations can be sick (such as the case of obesity), and that political action may be needed to improve population health.54 Thus, we must continue to seek opportunities for prevention at all levels of society including having responsible public policies to modifying our manner of living since there remain many untapped resources and untried venues.
Treatment of childhood obesity
We recommend that children with BMI ≥ 95th percentile or BMI ≥ 85th percentile accompanied by co-morbidities of overweight, such as hypertension, hyperlipidaemia, or impaired glucose tolerance be considered for treatment.
Non-pharmacologic treatment
Non-pharmacologic treatment should be the foundation of all obesity treatments, especially in children and should always be considered as Step 1 therapy. A recent systematic review of randomised controlled trials of treatments for childhood obesity identified 64 trials, 54 of which assessed non-pharmacological lifestyle intervention.55 The 54 lifestyle intervention trials were generally of small sample size (16 to 218 participants), with 70% including fewer than 30 participants. The majority of trials had significant methodological limitations and were of relatively short-term follow-up. Despite these limitations the authors concluded that “… this review shows that family-based, lifestyle interventions with a behavioural program aimed at changing diet and physical activity and thinking patterns provide significant and clinically meaningful decreases in overweight in both children and adolescents … in the short- and the long-term” These findings are encouraging and provide a useful guidance for treatment of currently obese children, but they also highlight the need for additional large randomised controlled trials with long term follow-up. The following is a summary of approaches that form the basis of most non-pharmacologic intervention strategies.
Energetics (thermodynamics) of weight reduction
Inducing weight loss requires a catabolic state of stored energy. The latest guidelines from the American Academy of Pediatrics recommend that weight reducing diets contain “less energy than that required to maintain weight but not less than 1200 kilocalories a day.”56 Equivalent UK guidance emphasises energy balance between intake and expenditure but does not specify amounts of intake.57 Another recommended approach is to construct a diet that is 300–400 kcal/d below weight–maintenance requirements as assessed by dietary history or as calculated on the basis of a formula relating anthropometry to energy expenditure, such as the Harris-Benedict equation. Given the magnitude of the “energy gap,” a sizeable energy deficit would be needed to induce appreciable weight reduction in an obese child, and many of the currently used weight loss diets may be energy neutral in a young child or even lead to weight gain in a sedentary female adolescent.33,35
Some guidelines (e.g. UK) and commentators emphasize behavioural strategies that do not specify actual caloric intake amount. A well-conducted randomized trial of behavioural treatment without specified calorie limits showed no effect on BMI.58,59 The protein-sparing modified fast has a very low calorie regimen (600–800 kcal/day) and appears to be promising, but this concept has not progressed since it was first reported.60 Transient growth deceleration was observed but growth was normalized by 14 months.61 However, this was not a randomized trial and had limited follow-up data.
Fostering increased energy expenditure for weight reduction has not received the same attention as dietary prescriptions. We found only one randomized controlled trial of 6–11 year old obese children that compared hypocaloric diet, 90 minutes of moderate exercise 3 days/week, or both. Weight loss was greater in the diet or diet plus exercise group (being similar in these two) than the exercise-only group, but there was no “control” group and follow-up was for just 9-months.62 Interventions to decrease sedentary activity, such as limiting television viewing, has been examined and shown to be promising.63
Macronutrient composition of weight reducing diets
The macronutrient composition of diets has been examined for differential weight loss benefits. Several popular diets such as the Atkin’s diet have emphasized increasing protein but not changing the energy content of the diet. Although some studies show greater weight loss with this diet in adults64–66 long-term studies generally show no difference in weight loss between diets of varying macronutrient contents that do not change total energy intake.67,68 Demol et al, found no differences in BMI decrease in obese adolescents on different macronutrient diets.67
The glycaemic index of diets has also been implicated in weight reduction.69 Two small, short-term studies of obese adolescents reported greater weight loss on a reduced glycaemic load diet, but the numbers were small and long-term effects are unknown.70,71
Behavioural strategies
Strategies to alter dietary habits to a more calorie-reduced intake are based on behavioural principles with Bandura’s social cognitive model as the most widely used.72 It is based on the notion that lifestyle changes succeed through cognitively driven, intentional behaviours such as self-monitoring, goal setting, and rewarding successful change. A widely adopted approach in children uses the “traffic light” system developed by Epstein et al.73
Motivational interviewing has been recently advocated as an especially useful technique for those who may not feel ready for change.74 It is an empathetic “way of being”, including reflective listening, shared decision making, and agenda setting.75 A recent guideline from the American Heart Association recommends motivational interviewing for paediatric weight management.76 However, the efficacy of this approach versus other behavioural approaches is not known.
Venues for weight reduction programs
Most weight reduction programs are provided by outpatient clinics. One study examined an inpatient intervention and found no evidence of its efficacy.77,78 Although the school setting has not been regarded as a site for treatment of childhood obesity (as opposed to its prevention), the promising results from a randomized trial of classroom-based weight reduction in obese Mexican-American children suggest that this venue needs further examination.79 Residential summer camp for obese adolescents has been examined and shown to have short-term efficacy,80 but long-tem effects after camp remain unknown. Internet intervention for obese adolescents has been examined without promising results to date.81
Discussion on non-pharmacologic treatment
In summary, there is urgent need to improve research on non-pharmacologic treatment, especially on the extent of caloric restriction and the effectiveness of increasing energy expenditure. Consensus guidelines for age-appropriate safety monitoring of weight reducing regimens are also needed to ensure appropriate height growth and biologic and social development. Since randomized clinical trials are costly, multi-centre collaborative research with common protocols might be cost-effective and more generalizable. Given the aging populations worldwide and increasing use of technology-intensive medical treatments, allocation of increasingly scarce medical resources will demand more evidence-based information for treatment of childhood obesity. Questions such as how often an obese child should go for dietary counselling will not find ready answers unless better evidence is made available.
Pharmacologic treatment
The recent Cochrane review identified 10 randomised controlled trials of pharmacological treatments for obese children.55 These trials were largely of small sample size (range 24 to 539 participants, with 60% including fewer than 30 participants), but most were high quality. With one exception, all of the pharmacological treatment trials were in older children/adolescents (minimum age 12 years); the one exception included individuals aged 9–18 years. Trials meeting criteria for pooled meta-analysis included only two drugs: orlistat (a lipase inhibitor that prevents absorption of dietary fat from the gut) and sibutramine (an inhibitor of serotonin, norepinephrine, and dopamine reuptake). The additional effect of orlistat over placebo when given in combination with a lifestyle intervention was a difference in BMI of −0.76 kg/m2 (95% CI: −1.07 to −0.44) at 6 months follow-up. The additional effect of sibutramine over placebo when given in combination with a lifestyle intervention was difference in BMI of −1.66 kg/m2 (95% CI: −1.89 to −1.43) at 6 months follow-up. For long-term outcomes, there has been only one randomized trial of orlistat, which reported a change in BMI of −0.55 kg/m2 with orlistat versus +0.31 kg/m2 with placebo at 12 months (p=0.001),82 and only one randomized trial of sibutramine, which reported a change in BMI of −2.9 kg/m2 with sibutramine versus −0.3 kg/m2 with placebo at 12 months (p<0.001).83 Side effects (reported as prevalence in excess of that reported for placebo) of orlistat included oily stool (42%), abdominal pain (11%), faecal incontinence (9%), and new cholelithiasis (2%).82 Side effects of sibutramine included tachycardia (6%), dry mouth (5%), constipation (4%), dizziness (4%), insomnia (3%), and hypertension (2%).83 Thus, whilst there is evidence for modest effectiveness of orlistat and sibutramine when combined with lifestyle intervention, treatment with these medications is associated with more adverse effects than lifestyle intervention alone.
Surgical treatment
To date there are no randomised controlled trials of bariatric surgery in children or adolescents.55 A recent systematic review of observational studies reporting outcome data in patients ≤ 21y (range 9–21y, mean 16.8y) with a minimum of 12 months follow-up identified 4 studies of Roux-en-Y gastric bypass (RYGB, a restrictive and malabsorptive procedure, Figure 4a) and 6 studies of laparoscopic adjustable gastric banding (LAGB, a purely restrictive procedure, Figure 4b) that met inclusion criteria for meta-analysis. 84 For RYGB, the 95% CI for change in BMI compared to baseline was −17.8 to −22.3 kg/m2 at 1–6.3y follow-up. 84 For LAGB, the 95% CI for change in BMI compared to baseline was −13.7 to −10.6 kg/m2 at 1–3y follow-up.84 For RYGB, complications included pulmonary embolism, shock, intestinal obstruction, postoperative bleeding, staple line leak, and severe malnutrition.84 For LAGB, complications included band slippage/erosion, micronutrient deficiency, port/tube dysfunction, hiatal hernia, wound infection, and pouch dilation.84 Long-term prospective studies are needed to establish the safety and efficacy of restrictive and malabsorptive procedures and to determine whether reductions in morbidity and mortality outweigh the risks of serious surgical complications and life-long nutritional deficiencies.
Figure 4. Operations performed for weight loss.
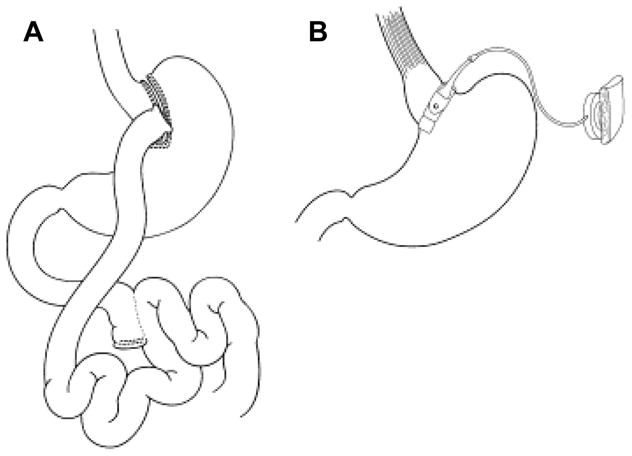
(A) Roux-en-Y gastric bypass, (B) Adjustable gastric band. (Reprinted from Inge TH, Zeller MH, Lawson ML, Daniels SR. A critical appraisal of evidence supporting a bariatric surgical approach to weight management for adolescents. J Pediatr 2005;147(1):10-9 with permission from Elsevier Limited.)140
Discussion on pharmacologic and surgical treatment
There is a need for large trials that are sufficiently powered to examine long-term effects and that allow direct comparisons of non-pharmacologic, pharmacologic, and surgical treatments. In light of the paucity of data and limited efficacy and unknown risks for long-term medication use, we suggest following the conservative approach, namely to employ pharmacotherapy only in those with BMI ≥ 95th percentile who have significant medical complications of obesity and after a reasonable period of behavioural intervention. The risks of bariatric surgery are considerable, and its long-term safety and efficacy in children remain largely unknown. Therefore, surgery should be reserved for only the most severely obese (BMI ≥ 50 kg/m2 or BMI ≥ 40 kg/m2 with significant co-morbidities), and even then, considered with extreme caution.
Conclusion: progress, challenges, and future directions
There has been much progress made in understanding the genetics and physiology of appetite control and from this, the elucidation of the causes of some exceedingly rare obesity syndromes. Much work still remains to be done since these rare disorders have so far taught us only limited lessons about how to prevent or reverse obesity in most children. There is currently no evidence-based, clinically meaningful definition of childhood obesity. Calorie intake and activity recommendations need to be re-assessed and better quantified, at a population level, given the more sedentary life of children today. For individual treatment, the currently recommended calorie prescriptions may be too conservative given evolving insight on the “energy gap.” The quality of scientific reports needs to improve to permit comparisons between interventions and pooling of studies. Because obesity is a chronic condition in need of ongoing management, long-term clinical trials are needed to demonstrate safety and efficacy of treatments not only for a few months but over the critical period of active growth and maturation. In children, the safety of treatments needs to be examined as a co-equal outcome to their efficacy.
Despite the challenges that remain, there are glimmers of hope. Recent statistics suggest the prevalence of childhood obesity may be stabilizing in Western countries. All past efforts made in the prevention and treatment of obesity, though not of evident individual notable effect in trials, may still have contributed collectively to this trend.54 The increased attention directed to obesity by the media may have helped increase the public’s awareness regarding energy balance. The private sector’s expanding food product availability and increasing food labelling may have also helped the consumer to make better informed choices. One cannot wait to delineate the complex “causal web” of the obesity epidemic. Unravelling even one thread of the causal web may allow for an important degree of prevention.85 Thus, prevention efforts should continue at all levels with the goal that the outcome should be greater than the sum of its parts. These efforts should be in tandem with an increased commitment to more robust research.
We anticipate that the next 10 years will be a time of new discoveries and collective societal actions that will help eliminate this scourge of the new millennium.
Acknowledgments
Dr. Han receives research support from the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The funding source had no direct role in writing this Seminar. Prof. Lawlor receives funding from the US NIH (R01 DK077659), UK MRC (G0600705 & G0801456) and NIHR (RP-PG-0407-10044) for her work in the area of childhood obesity and determining causality from observational research. The views in this review are those of the authors and not necessarily any funding body.
Footnotes
Disclaimer: J Han is a commissioned officer in the U.S. Public Health Service, DHHS, but the opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the U.S. Public Health Service.
Search strategy: We identified original research, reviews, and commentaries by searching computer databases—e.g., Medline, PsychINFO, Agricola, Lexis-Nexis—and by reviewing issues of journals that publish obesity research. We directed special attention towards publications since 2002. Research developments and published work were also identified by discussions with specialists in the areas of paediatric obesity, nutrition, and public health.
Contributors
All three authors participated in deciding content, reviewing evidence and the writing of this manuscript.
Conflict of interest statement
Dr. Kimm serves as a member of the Medical Advisory Board of the Aspartame Resource Center, from which she received no support for her research or her effort in this manuscript.
References
- 1.Ebbeling CB, Pawlak DB, Ludwig DS. Childhood obesity: public-health crisis, common sense cure. Lancet. 2002;360(9331):473–82. doi: 10.1016/S0140-6736(02)09678-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. Jama. 2008;299(20):2401–5. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 4.Kipping RR, Jago R, Lawlor DA. Obesity in children. Part 1: Epidemiology, measurement, risk factors, and screening. Bmj. 2008;337:a1824. doi: 10.1136/bmj.a1824. [DOI] [PubMed] [Google Scholar]
- 5.Sundblom E, Petzold M, Rasmussen F, Callmer E, Lissner L. Childhood overweight and obesity prevalences levelling off in Stockholm but socioeconomic differences persist. Int J Obes (Lond) 2008;32(10):1525–30. doi: 10.1038/ijo.2008.104. [DOI] [PubMed] [Google Scholar]
- 6.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. Bmj. 2000;320(7244):1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troiano RP, Flegal KM, Kuczmarski RJ, Campbell SM, Johnson CL. Overweight prevalence and trends for children and adolescents. The National Health and Nutrition Examination Surveys, 1963 to 1991. Arch Pediatr Adolesc Med. 1995;149(10):1085–91. doi: 10.1001/archpedi.1995.02170230039005. [DOI] [PubMed] [Google Scholar]
- 8.Neovius MG, Linne YM, Barkeling BS, Rossner SO. Sensitivity and specificity of classification systems for fatness in adolescents. Am J Clin Nutr. 2004;80(3):597–603. doi: 10.1093/ajcn/80.3.597. [DOI] [PubMed] [Google Scholar]
- 9.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–62. [PMC free article] [PubMed] [Google Scholar]
- 10.Bassett DR. Physical activity of Canadian and American children: a focus on youth in Amish, Mennonite, and modern cultures. Appl Physiol Nutr Metab. 2008;33(4):831–5. doi: 10.1139/H08-044. [DOI] [PubMed] [Google Scholar]
- 11.Daniels SR. Complications of obesity in children and adolescents. Int J Obes (Lond) 2009;33 (Suppl 1):S60–5. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- 12.Bjorge T, Engeland A, Tverdal A, Smith GD. Body mass index in adolescence in relation to cause-specific mortality: a follow-up of 230,000 Norwegian adolescents. Am J Epidemiol. 2008;168(1):30–7. doi: 10.1093/aje/kwn096. [DOI] [PubMed] [Google Scholar]
- 13.Freedman DS, Patel DA, Srinivasan SR, et al. The contribution of childhood obesity to adult carotid intima-media thickness: the Bogalusa Heart Study. Int J Obes (Lond) 2008;32(5):749–56. doi: 10.1038/sj.ijo.0803798. [DOI] [PubMed] [Google Scholar]
- 14.Calcaterra V, Klersy C, Muratori T, et al. Prevalence of metabolic syndrome (MS) in children and adolescents with varying degrees of obesity. Clin Endocrinol (Oxf) 2008;68(6):868–72. doi: 10.1111/j.1365-2265.2007.03115.x. [DOI] [PubMed] [Google Scholar]
- 15.Owen CG, Whincup PH, Orfei L, et al. Is body mass index before middle age related to coronary heart disease risk in later life? Evidence from observational studies. Int J Obes (Lond) 2009;33(8):866–77. doi: 10.1038/ijo.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilliland FD, Berhane K, Islam T, et al. Obesity and the risk of newly diagnosed asthma in school-age children. Am J Epidemiol. 2003;158(5):406–15. doi: 10.1093/aje/kwg175. [DOI] [PubMed] [Google Scholar]
- 17.Santamaria F, Montella S, De Stefano S, et al. Asthma, atopy, and airway inflammation in obese children. J Allergy Clin Immunol. 2007;120(4):965–7. doi: 10.1016/j.jaci.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Sutherland ER. Obesity and asthma. Immunol Allergy Clin North Am. 2008;28(3):589–602. ix. doi: 10.1016/j.iac.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alemzadeh R, Kichler J, Babar G, Calhoun M. Hypovitaminosis D in obese children and adolescents: relationship with adiposity, insulin sensitivity, ethnicity, and season. Metabolism. 2008;57(2):183–91. doi: 10.1016/j.metabol.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Yanoff LB, Parikh SJ, Spitalnik A, et al. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf) 2006;64(5):523–9. doi: 10.1111/j.1365-2265.2006.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nead KG, Halterman JS, Kaczorowski JM, Auinger P, Weitzman M. Overweight children and adolescents: a risk group for iron deficiency. Pediatrics. 2004;114(1):104–8. doi: 10.1542/peds.114.1.104. [DOI] [PubMed] [Google Scholar]
- 22.McClung JP, Karl JP. Iron deficiency and obesity: the contribution of inflammation and diminished iron absorption. Nutr Rev. 2009;67(2):100–4. doi: 10.1111/j.1753-4887.2008.00145.x. [DOI] [PubMed] [Google Scholar]
- 23.Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84–8. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- 24.Bau AM, Ernert A, Schenk L, et al. Is there a further acceleration in the age at onset of menarche? A cross-sectional study in 1840 school children focusing on age and bodyweight at the onset of menarche. Eur J Endocrinol. 2009;160(1):107–13. doi: 10.1530/EJE-08-0594. [DOI] [PubMed] [Google Scholar]
- 25.Mamun AA, Hayatbakhsh MR, O’Callaghan M, Williams G, Najman J. Early overweight and pubertal maturation--pathways of association with young adults’ overweight: a longitudinal study. Int J Obes (Lond) 2009;33(1):14–20. doi: 10.1038/ijo.2008.220. [DOI] [PubMed] [Google Scholar]
- 26.Denzer C, Weibel A, Muche R, Karges B, Sorgo W, Wabitsch M. Pubertal development in obese children and adolescents. Int J Obes (Lond) 2007;31(10):1509–19. doi: 10.1038/sj.ijo.0803691. [DOI] [PubMed] [Google Scholar]
- 27.Taylor ED, Theim KR, Mirch MC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117(6):2167–74. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiVall SA, Radovick S. Endocrinology of female puberty. Curr Opin Endocrinol Diabetes Obes. 2009;16(1):1–4. doi: 10.1097/med.0b013e3283207937. [DOI] [PubMed] [Google Scholar]
- 29.Gordon JE, Hughes MS, Shepherd K, et al. Obstructive sleep apnoea syndrome in morbidly obese children with tibia vara. J Bone Joint Surg Br. 2006;88(1):100–3. doi: 10.1302/0301-620X.88B1.16918. [DOI] [PubMed] [Google Scholar]
- 30.Murray AW, Wilson NI. Changing incidence of slipped capital femoral epiphysis: a relationship with obesity? J Bone Joint Surg Br. 2008;90(1):92–4. doi: 10.1302/0301-620X.90B1.19502. [DOI] [PubMed] [Google Scholar]
- 31.Timpson NJ, Sayers A, Davey-Smith G, Tobias JH. How does body fat influence bone mass in childhood? A Mendelian randomization approach. J Bone Miner Res. 2009;24(3):522–33. doi: 10.1359/jbmr.081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Summerbell CD, Waters E, Edmunds LD, Kelly S, Brown T, Campbell KJ. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2005;(3):CD001871. doi: 10.1002/14651858.CD001871.pub2. [DOI] [PubMed] [Google Scholar]
- 33.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299(5608):853–5. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- 34.Swinburn BA, Sacks G, Lo SK, et al. Estimating the changes in energy flux that characterize the rise in obesity prevalence. Am J Clin Nutr. 2009;89(6):1723–8. doi: 10.3945/ajcn.2008.27061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butte NF, Ellis KJ. Comment on Obesity and the environment: where do we go from here? Science. 2003;301(5633):598. doi: 10.1126/science.1085985. author reply 598. [DOI] [PubMed] [Google Scholar]
- 36.Kramer MS, Matush L, Vanilovich I, et al. A randomized breast-feeding promotion intervention did not reduce child obesity in Belarus. J Nutr. 2009;139(2):417S–21S. doi: 10.3945/jn.108.097675. [DOI] [PubMed] [Google Scholar]
- 37.Brion M-J, Ness A, Rogers I, et al. Maternal macronutrient and energy intake in pregnancy and offspring intake at 10 years: exploring parental comparisons and prenatal effects. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.28623. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins SS, Cole TJ, Law C. An ecological systems approach to examining risk factors for early childhood overweight: findings from the UK Millennium Cohort Study. J Epidemiol Community Health. 2009;63(2):147–55. doi: 10.1136/jech.2008.077917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Welsh SO, Davis C, Shaw A. USDA’s Food Guide: Background and Development. Hyattsville, MD: United States Department of Agriculture, Human Nutrition Information Service; 1993. [Google Scholar]
- 40.Nielsen SJ, Siega-Riz AM, Popkin BM. Trends in energy intake in U.S. between 1977 and 1996: similar shifts seen across age groups. Obes Res. 2002;10(5):370–8. doi: 10.1038/oby.2002.51. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Suarez C, Worley A, Grimmer-Somers K, Dones V. School-based interventions on childhood obesity: a meta-analysis. Am J Prev Med. 2009;37(5):418–27. doi: 10.1016/j.amepre.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 42.Jouret B, Ahluwalia N, Dupuy M, et al. Prevention of overweight in preschool children: results of kindergarten-based interventions. Int J Obes (Lond) 2009;33(10):1075–83. doi: 10.1038/ijo.2009.166. [DOI] [PubMed] [Google Scholar]
- 43.Berkowitz JM, Huhman M, Nolin MJ. Did augmenting the VERB campaign advertising in select communities have an effect on awareness, attitudes, and physical activity? Am J Prev Med. 2008;34(6 Suppl):S257–66. doi: 10.1016/j.amepre.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Huhman ME, Potter LD, Duke JC, Judkins DR, Heitzler CD, Wong FL. Evaluation of a national physical activity intervention for children: VERB campaign, 2002–2004. Am J Prev Med. 2007;32(1):38–43. doi: 10.1016/j.amepre.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto JA, Yamamoto JB, Yamamoto BE, Yamamoto LG. Adolescent fast food and restaurant ordering behavior with and without calorie and fat content menu information. J Adolesc Health. 2005;37(5):397–402. doi: 10.1016/j.jadohealth.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Wong F, Huhman M, Heitzler C, et al. VERB - a social marketing campaign to increase physical activity among youth. Prev Chronic Dis. 2004;1(3):A10. [PMC free article] [PubMed] [Google Scholar]
- 47.Brownell KD, Frieden TR. Ounces of prevention--the public policy case for taxes on sugared beverages. N Engl J Med. 2009;360(18):1805–8. doi: 10.1056/NEJMp0902392. [DOI] [PubMed] [Google Scholar]
- 48.Justus MB, Ryan KW, Rockenbach J, Katterapalli C, Card-Higginson P. Lessons learned while implementing a legislated school policy: body mass index assessments among Arkansas’s public school students. J Sch Health. 2007;77(10):706–13. doi: 10.1111/j.1746-1561.2007.00255.x. [DOI] [PubMed] [Google Scholar]
- 49.Nihiser A, Lee S, Wechsler H, et al. BMI Measurement in School. Pediatrics. 2009;124:S89–97. doi: 10.1542/peds.2008-3586L. [DOI] [PubMed] [Google Scholar]
- 50.Ong KK, Loos RJ. Rapid infancy weight gain and subsequent obesity: systematic reviews and hopeful suggestions. Acta Paediatr. 2006;95(8):904–8. doi: 10.1080/08035250600719754. [DOI] [PubMed] [Google Scholar]
- 51.Cole TJ. Children grow and horses race: is the adiposity rebound a critical period for later obesity? BMC Pediatr. 2004;4(1):6. doi: 10.1186/1471-2431-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kimm SY, Glynn NW, Kriska AM, et al. Decline in physical activity in black girls and white girls during adolescence. N Engl J Med. 2002;347(10):709–15. doi: 10.1056/NEJMoa003277. [DOI] [PubMed] [Google Scholar]
- 53.Stamler J. Population-wide adverse dietary patterns: a pivotal cause of epidemic coronary heart disease/cardiovascular disease. J Am Diet Assoc. 2008;108(2):228–32. doi: 10.1016/j.jada.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 54.Rose G, Khaw K-T, Marmot M. Rose’s Strategy of Preventive Medicine. Oxford: Oxford University Press; 2008. [Google Scholar]
- 55.Oude Luttikhuis H, Baur L, Jansen H, et al. Interventions for treating obesity in children. Cochrane Database Syst Rev. 2009;(1):CD001872. doi: 10.1002/14651858.CD001872.pub2. [DOI] [PubMed] [Google Scholar]
- 56.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120 (Suppl 4):S254–88. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 57.National Institute for Health and Clinical Excellence; 2006. Obesity: the prevention, identification, assessment and management of overweight and obesity in adults and children. http://www.nice.org.uk/guidance/index.jsp?action=byID&o=11000. [PubMed] [Google Scholar]
- 58.Stewart L, Houghton J, Hughes AR, Pearson D, Reilly JJ. Dietetic management of pediatric overweight: development and description of a practical and evidence-based behavioral approach. J Am Diet Assoc. 2005;105(11):1810–5. doi: 10.1016/j.jada.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 59.Hughes AR, Stewart L, Chapple J, et al. Randomized, controlled trial of a best-practice individualized behavioral program for treatment of childhood overweight: Scottish Childhood Overweight Treatment Trial (SCOTT) Pediatrics. 2008;121(3):e539–46. doi: 10.1542/peds.2007-1786. [DOI] [PubMed] [Google Scholar]
- 60.Figueroa-Colon R, von Almen TK, Franklin FA, Schuftan C, Suskind RM. Comparison of two hypocaloric diets in obese children. Am J Dis Child. 1993;147(2):160–6. doi: 10.1001/archpedi.1993.02160260050021. [DOI] [PubMed] [Google Scholar]
- 61.Sothern, Udall JN, Jr, Suskind RM, Vargas A, Blecker U. Weight loss and growth velocity in obese children after very low calorie diet, exercise, and behavior modification. Acta Paediatr. 2000;89(9):1036–43. doi: 10.1080/713794562. [DOI] [PubMed] [Google Scholar]
- 62.Shalitin S, Ashkenazi-Hoffnung L, Yackobovitch-Gavan M, et al. Effects of a twelve-week randomized intervention of exercise and/or diet on weight loss and weight maintenance, and other metabolic parameters in obese preadolescent children. Horm Res. 2009;72(5):287–301. doi: 10.1159/000245931. [DOI] [PubMed] [Google Scholar]
- 63.Robinson TN. Reducing children’s television viewing to prevent obesity: a randomized controlled trial. Jama. 1999;282(16):1561–7. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- 64.Foster GD, Wyatt HR, Hill JO, et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med. 2003;348(21):2082–90. doi: 10.1056/NEJMoa022207. [DOI] [PubMed] [Google Scholar]
- 65.Gardner CD, Kiazand A, Alhassan S, et al. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. Jama. 2007;297(9):969–77. doi: 10.1001/jama.297.9.969. [DOI] [PubMed] [Google Scholar]
- 66.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 67.Demol S, Yackobovitch-Gavan M, Shalitin S, Nagelberg N, Gillon-Keren M, Phillip M. Low-carbohydrate (low & high-fat) versus high-carbohydrate low-fat diets in the treatment of obesity in adolescents. Acta Paediatr. 2009;98(2):346–51. doi: 10.1111/j.1651-2227.2008.01051.x. [DOI] [PubMed] [Google Scholar]
- 68.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Jama. 2002;287(18):2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 70.Sondike SB, Copperman N, Jacobson MS. Effects of a low-carbohydrate diet on weight loss and cardiovascular risk factor in overweight adolescents. J Pediatr. 2003;142(3):253–8. doi: 10.1067/mpd.2003.4. [DOI] [PubMed] [Google Scholar]
- 71.Ebbeling CB, Leidig MM, Sinclair KB, Hangen JP, Ludwig DS. A reduced-glycemic load diet in the treatment of adolescent obesity. Arch Pediatr Adolesc Med. 2003;157(8):773–9. doi: 10.1001/archpedi.157.8.773. [DOI] [PubMed] [Google Scholar]
- 72.Bandura A. Social Foundations of Thought and Action. Englewood Cliffs, NJ: Prentice Prentice-Hall; 1986. [Google Scholar]
- 73.Epstein LH, Valoski A, Wing RR, McCurley J. Ten-year follow-up of behavioral, family-based treatment for obese children. Jama. 1990;264(19):2519–23. [PubMed] [Google Scholar]
- 74.Miller W, Rollnick S. Motivational Interviewing: Preparing People for Change. New York: Guilford Press; 2002. [Google Scholar]
- 75.Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: Conceptual issues and evidence review. J Am Diet Assoc. 2006;106(12):2024–33. doi: 10.1016/j.jada.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 76.Daniels SR, Jacobson MS, McCrindle BW, Eckel RH, Sanner BM. American Heart Association Childhood Obesity Research Summit Report. Circulation. 2009;119(15):e489–517. doi: 10.1161/CIRCULATIONAHA.109.192216. [DOI] [PubMed] [Google Scholar]
- 77.Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity (Silver Spring) 2006;14(1):148–55. doi: 10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- 78.Braet C, Tanghe A, Bode PD, Franckx H, Winckel MV. Inpatient treatment of obese children: a multicomponent programme without stringent calorie restriction. Eur J Pediatr. 2003;162(6):391–6. doi: 10.1007/s00431-003-1155-5. [DOI] [PubMed] [Google Scholar]
- 79.Johnston CA, Tyler C, McFarlin BK, et al. Weight loss in overweight Mexican American children: a randomized, controlled trial. Pediatrics. 2007;120(6):e1450–7. doi: 10.1542/peds.2006-3321. [DOI] [PubMed] [Google Scholar]
- 80.Gately PJ, Cooke CB, Barth JH, Bewick BM, Radley D, Hill AJ. Children’s residential weight-loss programs can work: a prospective cohort study of short-term outcomes for overweight and obese children. Pediatrics. 2005;116(1):73–7. doi: 10.1542/peds.2004-0397. [DOI] [PubMed] [Google Scholar]
- 81.Doyle AC, Goldschmidt A, Huang C, Winzelberg AJ, Taylor CB, Wilfley DE. Reduction of overweight and eating disorder symptoms via the Internet in adolescents: a randomized controlled trial. J Adolesc Health. 2008;43(2):172–9. doi: 10.1016/j.jadohealth.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chanoine JP, Hampl S, Jensen C, Boldrin M, Hauptman J. Effect of orlistat on weight and body composition in obese adolescents: a randomized controlled trial. Jama. 2005;293(23):2873–83. doi: 10.1001/jama.293.23.2873. [DOI] [PubMed] [Google Scholar]
- 83.Berkowitz RI, Fujioka K, Daniels SR, et al. Effects of sibutramine treatment in obese adolescents: a randomized trial. Ann Intern Med. 2006;145(2):81–90. doi: 10.7326/0003-4819-145-2-200607180-00005. [DOI] [PubMed] [Google Scholar]
- 84.Treadwell JR, Sun F, Schoelles K. Systematic review and meta-analysis of bariatric surgery for pediatric obesity. Ann Surg. 2008;248(5):763–76. doi: 10.1097/SLA.0b013e31818702f4. [DOI] [PubMed] [Google Scholar]
- 85.MacMahon B, Pugh TF. Chapter 2: Concepts of Cause1970 Epidemiology Principles and Methods. Boston: Little, Brown and Company; 1970. [Google Scholar]
- 86.Farooqi S, O’Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27(7):710–18. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 87.Feigerlova E, Diene G, Conte-Auriol F, et al. Hyperghrelinemia precedes obesity in Prader-Willi syndrome. J Clin Endocrinol Metab. 2008;93(7):2800–5. doi: 10.1210/jc.2007-2138. [DOI] [PubMed] [Google Scholar]
- 88.Cummings DE, Clement K, Purnell JQ, et al. Elevated plasma ghrelin levels in Prader Willi syndrome. Nat Med. 2002;8(7):643–4. doi: 10.1038/nm0702-643. [DOI] [PubMed] [Google Scholar]
- 89.Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Hum Mol Genet. 2009;18(7):1323–31. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Davenport JR, Watts AJ, Roper VC, et al. Disruption of intraflagellar transport in adult mice leads to obesity and slow-onset cystic kidney disease. Curr Biol. 2007;17(18):1586–94. doi: 10.1016/j.cub.2007.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han JC, Liu QR, Jones M, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N Engl J Med. 2008;359(9):918–27. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Willer CJ, Speliotes EK, Loos RJ, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41(1):25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Campion J, Milagro FI, Martinez JA. Individuality and epigenetics in obesity. Obes Rev. 2009;10(4):383–92. doi: 10.1111/j.1467-789X.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 94.Long DN, McGuire S, Levine MA, Weinstein LS, Germain-Lee EL. Body mass index differences in pseudohypoparathyroidism type 1a versus pseudopseudohypoparathyroidism may implicate paternal imprinting of Galpha(s) in the development of human obesity. J Clin Endocrinol Metab. 2007;92(3):1073–9. doi: 10.1210/jc.2006-1497. [DOI] [PubMed] [Google Scholar]
- 95.Pfeifer SM, Kives S. Polycystic ovary syndrome in the adolescent. Obstet Gynecol Clin North Am. 2009;36(1):129–52. doi: 10.1016/j.ogc.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Weaver JU. Classical endocrine diseases causing obesity. Front Horm Res. 2008;36:212–28. doi: 10.1159/000115367. [DOI] [PubMed] [Google Scholar]
- 97.Lustig RH, Hinds PS, Ringwald-Smith K, et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J Clin Endocrinol Metab. 2003;88(6):2586–92. doi: 10.1210/jc.2002-030003. [DOI] [PubMed] [Google Scholar]
- 98.Lee M, Korner J. Review of physiology, clinical manifestations, and management of hypothalamic obesity in humans. Pituitary. 2009;12(2):87–95. doi: 10.1007/s11102-008-0096-4. [DOI] [PubMed] [Google Scholar]
- 99.Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30 (Suppl 2):S169–74. doi: 10.2337/dc07-s211. [DOI] [PubMed] [Google Scholar]
- 100.Lawlor DA, Fraser A, Lindsay RS, et al. Association of existing diabetes, gestational diabetes and glycosuria in pregnancy with macrosomia and offspring body mass index, waist and fat mass in later childhood: findings from a prospective pregnancy cohort. Diabetologia. 2009 doi: 10.1007/s00125-009-1560-z. [DOI] [PubMed] [Google Scholar]
- 101.Kral JG, Biron S, Simard S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics. 2006;118(6):e1644–9. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- 102.Lawlor DA, Timpson NJ, Harbord RM, et al. Exploring the developmental overnutrition hypothesis using parental-offspring associations and FTO as an instrumental variable. PLoS Med. 2008;5(3):e33. doi: 10.1371/journal.pmed.0050033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rogers IS, Ness AR, Steer CD, et al. Associations of size at birth and dual-energy X-ray absorptiometry measures of lean and fat mass at 9 to 10 y of age. Am J Clin Nutr. 2006;84(4):739–47. doi: 10.1093/ajcn/84.4.739. [DOI] [PubMed] [Google Scholar]
- 104.Rockell JE, Green TJ, Skeaff CM, et al. Season and ethnicity are determinants of serum 25-hydroxyvitamin D concentrations in New Zealand children aged 5–14 y. J Nutr. 2005;135(11):2602–8. doi: 10.1093/jn/135.11.2602. [DOI] [PubMed] [Google Scholar]
- 105.Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115(5):1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- 106.Kramer MS, Matush L, Vanilovich I, et al. Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86(6):1717–21. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- 107.Moreno LA, Rodriguez G. Dietary risk factors for development of childhood obesity. Curr Opin Clin Nutr Metab Care. 2007;10(3):336–41. doi: 10.1097/MCO.0b013e3280a94f59. [DOI] [PubMed] [Google Scholar]
- 108.Jimenez-Pavon D, Kelly J, Reilly JJ. Associations between objectively measured habitual physical activity and adiposity in children and adolescents: Systematic review. Int J Pediatr Obes. 2009:1–16. doi: 10.3109/17477160903067601. [DOI] [PubMed] [Google Scholar]
- 109.Marshall SJ, Biddle SJ, Gorely T, Cameron N, Murdey I. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. Int J Obes Relat Metab Disord. 2004;28(10):1238–46. doi: 10.1038/sj.ijo.0802706. [DOI] [PubMed] [Google Scholar]
- 110.Al Mamun A, Lawlor DA, Cramb S, O’Callaghan M, Williams G, Najman J. Do childhood sleeping problems predict obesity in young adulthood? Evidence from a prospective birth cohort study. Am J Epidemiol. 2007;166(12):1368–73. doi: 10.1093/aje/kwm224. [DOI] [PubMed] [Google Scholar]
- 111.Atkinson RL, Dhurandhar NV, Allison DB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. Int J Obes (Lond) 2005;29(3):281–6. doi: 10.1038/sj.ijo.0802830. [DOI] [PubMed] [Google Scholar]
- 112.Turnbaugh PJ, Hamady M, Yatsunenko T, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. Jama. 2009;302(16):1765–73. doi: 10.1001/jama.2009.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mayer EI, Reuter M, Dopfer RE, Ranke MB. Energy expenditure, energy intake and prevalence of obesity after therapy for acute lymphoblastic leukemia during childhood. Horm Res. 2000;53(4):193–9. doi: 10.1159/000023566. [DOI] [PubMed] [Google Scholar]
- 115.Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med. 2006;160(1):40–5. doi: 10.1001/archpedi.160.1.40. [DOI] [PubMed] [Google Scholar]
- 116.Freedman DS, Khan LK, Serdula MK, Ogden CL, Dietz WH. Racial and ethnic differences in secular trends for childhood BMI, weight, and height. Obesity (Silver Spring) 2006;14(2):301–8. doi: 10.1038/oby.2006.39. [DOI] [PubMed] [Google Scholar]
- 117.Yajnik CS, Lubree HG, Rege SS, et al. Adiposity and hyperinsulinemia in Indians are present at birth. J Clin Endocrinol Metab. 2002;87(12):5575–80. doi: 10.1210/jc.2002-020434. [DOI] [PubMed] [Google Scholar]
- 118.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicentre Growth Reference Study: planning, study design, and methodology. Food Nutr Bull. 2004;25(1 Suppl):S15–26. doi: 10.1177/15648265040251S103. [DOI] [PubMed] [Google Scholar]
- 119.Li L, Moira APd, Power C. Changing influences on childhood obesity: a study of two generations of the 1958 British birth cohort. J Epidemiol Community Health. 2009;63(Suppl 2):27. doi: 10.1136/jech.2009.096719a. [DOI] [Google Scholar]
- 120.Montague CT, Farooqi IS, Whitehead JP, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–8. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 121.Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nat Genet. 1998;19(2):155–7. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- 122.Creemers JW, Lee YS, Oliver RL, et al. Mutations in the amino-terminal region of proopiomelanocortin (POMC) in patients with early-onset obesity impair POMC sorting to the regulated secretory pathway. J Clin Endocrinol Metab. 2008;93(11):4494–9. doi: 10.1210/jc.2008-0954. [DOI] [PubMed] [Google Scholar]
- 123.Challis BG, Pritchard LE, Creemers JW, et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Hum Mol Genet. 2002;11(17):1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- 124.Gray J, Yeo GS, Cox JJ, et al. Hyperphagia, severe obesity, impaired cognitive function and hyperactivity associated with functional loss of one copy of the BDNF gene. Diabetes. 2006 doi: 10.2337/db06-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Farooqi IS, Wangensteen T, Collins S, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N Engl J Med. 2007;356(3):237–47. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Clement K, Vaisse C, Lahlou N, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392(6674):398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- 127.Yeo GS, Farooqi IS, Aminian S, Halsall DJ, Stanhope RG, O’Rahilly S. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nat Genet. 1998;20(2):111–2. doi: 10.1038/2404. [DOI] [PubMed] [Google Scholar]
- 128.Lee YS, Poh LK, Loke KY. A novel melanocortin 3 receptor gene (MC3R) mutation associated with severe obesity. J Clin Endocrinol Metab. 2002;87(3):1423–6. doi: 10.1210/jcem.87.3.8461. [DOI] [PubMed] [Google Scholar]
- 129.Feng N, Young SF, Aguilera G, et al. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes. 2005;54(9):2663–7. doi: 10.2337/diabetes.54.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Farooqi IS, Yeo GS, Keogh JM, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. J Clin Invest. 2000;106(2):271–9. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yeo GS, Connie Hung CC, Rochford J, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7(11):1187–9. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 132.Jackson RS, Creemers JW, Ohagi S, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nat Genet. 1997;16(3):303–6. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- 133.Farooqi IS, Volders K, Stanhope R, et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. J Clin Endocrinol Metab. 2007;92(9):3369–73. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- 134.Lin S, Boey D, Herzog H. NPY and Y receptors: lessons from transgenic and knockout models. Neuropeptides. 2004;38(4):189–200. doi: 10.1016/j.npep.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 135.Moussa NM, Claycombe KJ. The yellow mouse obesity syndrome and mechanisms of agouti-induced obesity. Obes Res. 1999;7(5):506–14. doi: 10.1002/j.1550-8528.1999.tb00440.x. [DOI] [PubMed] [Google Scholar]
- 136.Naggert JK, Fricker LD, Varlamov O, et al. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995;10(2):135–42. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- 137.Berman Y, Mzhavia N, Polonskaia A, Devi LA. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J Biol Chem. 2001;276(2):1466–73. doi: 10.1074/jbc.M008499200. [DOI] [PubMed] [Google Scholar]
- 138.Chen AS, Marsh DJ, Trumbauer ME, et al. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat Genet. 2000;26(1):97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- 139.Malone M. Medications associated with weight gain. Ann Pharmacother. 2005;39(12):2046–55. doi: 10.1345/aph.1G333. [DOI] [PubMed] [Google Scholar]
- 140.Inge TH, Zeller MH, Lawson ML, Daniels SR. A critical appraisal of evidence supporting a bariatric surgical approach to weight management for adolescents. J Pediatr. 2005;147(1):10–9. doi: 10.1016/j.jpeds.2005.03.021. [DOI] [PubMed] [Google Scholar]