Abstract
Background/Aims
Chronic hepatitis C virus infection can cause chronic liver disease, cirrhosis and liver cancer. The HALT-C Trial was a prospective, randomized controlled study of long-term, low-dose peginterferon therapy in patients with advanced chronic hepatitis C who had failed to respond to a previous course of optimal antiviral therapy. The aim of this follow-up analysis was to describe the frequency and causes of death among this cohort of patients.
Methods
Deaths occurring during and after the HALT-C Trial were reviewed by a committee of investigators to determine the cause of death and to categorize each death as liver- or non-liver-related and as related or not to complications of peginterferon. Rates of liver transplantation were also assessed.
Results
Over a median of 5.7 years, 122 deaths occurred among 1,050 randomized patients (12%) of which 76 were considered liver-related (62%) and 46 non-liver-related (38%); 74 patients (7%) underwent liver transplantation. At 7 years, the cumulative mortality rate was higher in the treatment compared to the control group (20% versus 15%, p=0.049); the primary difference in mortality was in patients in the fibrosis compared to the cirrhosis stratum (14% versus 7%, p=0.01); comparable differences were observed when liver transplantation was included. Excess mortality, emerging after 3 years of treatment, was related largely to non-liver-related death; liver-related mortality was similar in the treatment and control groups. No specific cause of death accounted for the excess mortality, and only one death was suspected to be a direct complication of peginterferon.
Conclusions
Long-term maintenance peginterferon in patients with advanced chronic hepatitis C is associated with an excess overall mortality, which was primarily due to non-liver-related causes among patients with bridging fibrosis.
Keywords: hepatitis C, natural history, peginterferon, mortality rates, end-stage liver disease, liver transplantation, hepatocellular carcinoma
Introduction
Hepatitis C virus (HCV) infection is the most important cause of chronic hepatitis in the United States and is a major cause of morbidity and mortality resulting from cirrhosis and hepatocellular carcinoma (HCC).1–3 While successful antiviral therapy with clearance of HCV appears to decrease the rate of progression of disease and death from chronic hepatitis C, no known beneficial therapy is available currently for patients who fail to respond to standard treatment.4 The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial was a large, randomized controlled trial to evaluate the effects of a three-and-a-half year course of low-dose, maintenance therapy with peginterferon compared to no therapy in retarding the progression of liver disease and in preventing end-stage liver disease, HCC, and death in patients with advanced chronic hepatitis C who had failed to achieve a sustained response to a previous course of optimal antiviral therapy.5 The HALT-C Trial was initiated in 2000 and the randomized treatment phase completed in 2007. The results of the randomized phase showed the lack of a beneficial effect of long-term peginterferon on clinical outcomes or death.6 Moreover, excess mortality occurred in the treatment group among patients with advanced fibrosis but without cirrhosis. To investigate whether this difference in mortality persisted with longer follow-up and to evaluate its possible explanations, the HALT-C Trial cohort was followed for an additional 3 years and analyzed for the causes of deaths.
Patients and Methods
Study Design
The design of the HALT-C Trial has been described.5,6 Briefly, between August, 2000 and August, 2004, patients meeting the following criteria were enrolled at 10 clinical centers in the United States: (1) failure to achieve a sustained virological response with previous interferon-based therapy; (2) presence of advanced hepatic fibrosis on liver biopsy categorized as either fibrosis without cirrhosis (Ishak Stage 3 or 4, “fibrosis stratum”) or cirrhosis (Ishak Stage 5 or 6, “cirrhosis stratum”)7; (3) a history of compensated liver disease (i.e., absence of a history of hepatic decompensation or HCC); and (4) absence of exclusion criteria (e.g., liver disease other than hepatitis C, uncontrolled medical or psychiatric conditions, or interferon contraindications).
Participants in the HALT-C Trial were treated initially with peginterferon alfa-2a (180 μg subcutaneously weekly) and ribavirin (1,000–1,200 mg daily) in a 24-week lead-in phase, and those who failed to clear HCV RNA by week 20 were categorized as nonresponders and were eligible for randomization to treatment with half-dose, maintenance peginterferon alfa-2a (90 μg weekly) or to an untreated control group for 3.5 years. Patients in whom HCV RNA was undetectable at week-20 were categorized as responders and continued full-dose combination therapy for up to 48 weeks. Initial responders were eligible for randomization into the Trial if virologic breakthrough occurred during extended therapy or relapse followed 48 weeks of therapy. In addition, patients who were treated with peginterferon and ribavirin outside the lead-in phase of the HALT-C Trial were also eligible for randomization (“express” group) if they met criteria for nonresponse, breakthrough, or relapse. This approach to enrollment ensured that all patients had received optimal therapy with peginterferon and ribavirin8,9 before they were enrolled into this long-term Trial, during which they might not be treated.5
After randomization, patients in both groups were seen at 3-month intervals for 3.5 years, at which point peginterferon was discontinued in the treatment group. Nine patients assigned to the control group were treated “off-protocol” by non-study physicians, but they were included as controls in our intention-to-treat analysis. Thereafter, all patients remained untreated and were seen at 6-month intervals. At each visit, the occurrence of clinical outcomes (which had been established prospectively) was noted, including clinical events and laboratory markers of hepatic decompensation, HCC, or death. Although not a primary clinical outcome in the HALT-C Trial, liver transplantation was included in this mortality analysis, because these patients were likely to have died in the absence of liver transplantation. Most deaths were identified by study coordinators interacting with family members via telephone. In addition, periodic on-line searches were performed of the U.S. Social Security Death Index (SSDI) (http://ssdi.rootsweb.com/), which is generated from the U.S. Social Security Administration's Death Master File. The SSDI was queried for any participant with whom the study site had no contact for at least six months. The last search of the SSDI was conducted in October, 2009. To account for the potential lag between date of death and report to the SSDI, we included in our analysis deaths occurring on or before December 31, 2008.
All deaths were reviewed by a seven-person, central review committee consisting of HALT-C Trial investigators blinded to the identity of the subject, study site, fibrosis versus cirrhosis stratum, but not randomization allocation (treatment or control), this information being required to assess treatment relatedness. The committee classified the primary cause of death into one of 15 categories (Supplementary Table 1). Furthermore, the likelihood that hepatitis C-related liver disease (“liver-related”) or that peginterferon were contributing causes of death (“treatment-related”) was assessed as “unlikely” (<25% likelihood), “possible” (25 to 49% likelihood), “probable” (50 to 75% likelihood) or “highly likely” (>75% likelihood). Differences of opinion were resolved by discussion among the group to achieve consensus or by majority vote. For purposes of this analysis, when the role of hepatitis C or treatment was judged to be unlikely or only possible (i.e., <50% likelihood), the death was categorized as nonrelated (to hepatitis C and/or treatment), whereas the role of hepatitis C or treatment in any death considered probable or highly likely (≥50% likelihood) was classified as related.
Data analysis and statistics
Statistical analyses were performed at the Data Coordinating Center with SAS release 9.1 (SAS Institute, Cary, NC). Time-to-event analytic methods were used to compare survival distributions in the groups defined by randomization group and cirrhosis stratum at baseline. Significance was tested with the Log-Rank test of equality of survival distributions. Time-to-event was defined as the time between randomization and date of death if before December 31, 2008 or the date the participant was last known to be alive. Participants not known to have died were censored at the date of last study contact or December 31, 2008, whichever occurred first. Last study contact included the latest of the following: last study visit, last telephone contact, last biopsy, liver transplantation, study outcome (excluding death), or date of randomization. Participants who died after December 31, 2008 were censored at that date.
We report the p-value for the test of the overall hypothesis of equality of survival distributions and the 7-year cumulative death rates as a measure of the size of the difference at the end of the observation period.
Results
Patient characteristics
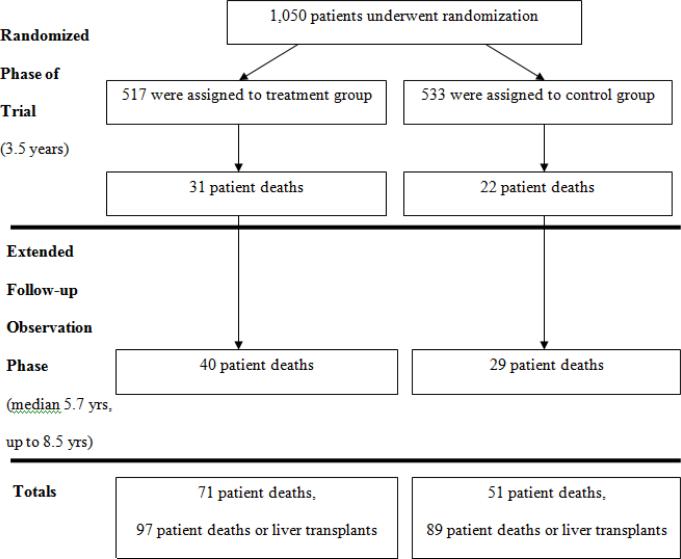
Extensive details on the composition of the HALT-C Trial cohort have been provided in previous publications.5,6 The 1,050 randomized patients all had chronic hepatitis C, active viremia, and a liver biopsy showing advanced fibrosis (n = 622) or cirrhosis (n = 428). Participants were predominantly male (n = 745, 71%), and half were older than 49 years (range 19 to 80, median 49 years). Most patients were non-Hispanic white (n = 812, 77%), 108 (10%) were non-Hispanic black, 107 (10%) Hispanic, and 23 (2%) were of other or mixed ethnicity. The sample included 306 (29%) people who reported being current smokers and 221 (21%) who were diabetic. The overall design, numbers of patients, and flow of patients in the treatment and control arms at the different time points are shown in Figure 1.
Figure 1.
Overview of the HALT-C Trial, showing flow of the patient cohort from time of randomization, through the 3.5 years of the randomized trial phase, and during the extended follow-up phase showing numbers of deaths.
Mortality rates, overall and by cirrhosis status
A total of 122 deaths occurred among 1,050 randomized patients (12%) over a median period of 5.7 years (range 0 to 8 years). In addition, 74 patients (7%) underwent liver transplantation, 10 of whom subsequently died and were included in the total number of deaths (Table 1). 53 deaths (43%) occurred during the randomized phase of the trial, defined as 3.8 years (3.5 years plus a 3-month window around the final study milestone) after randomization when patients were being treated actively with peginterferon or followed on no therapy. The remaining 69 deaths (57%) occurred after the conclusion of the randomized phase when all patients were being followed but no study treatment was offered.
Table 1.
Death according to baseline stratum and liver-relatedness.
| Stratum | Outcome | Death* |
|---|---|---|
| Cirrhosis (n = 428) | Liver-related | |
| End-stage liver disease | 3 (4) | |
| HCC | 12 (3) | |
| Other | 6 | |
| Non-liver-related | 28 (1) | |
|
| ||
| Total | 80 (8) | |
|
| ||
| Fibrosis (n = 622) | Liver-related | |
| End-stage liver disease | 13 (1) | |
| HCC | 9 | |
| Other | 2 (1) | |
| Non-liver-related | 18 | |
|
| ||
| Total | 42 (2) | |
Numbers in parenthesis represent the patients who died after liver transplantation.
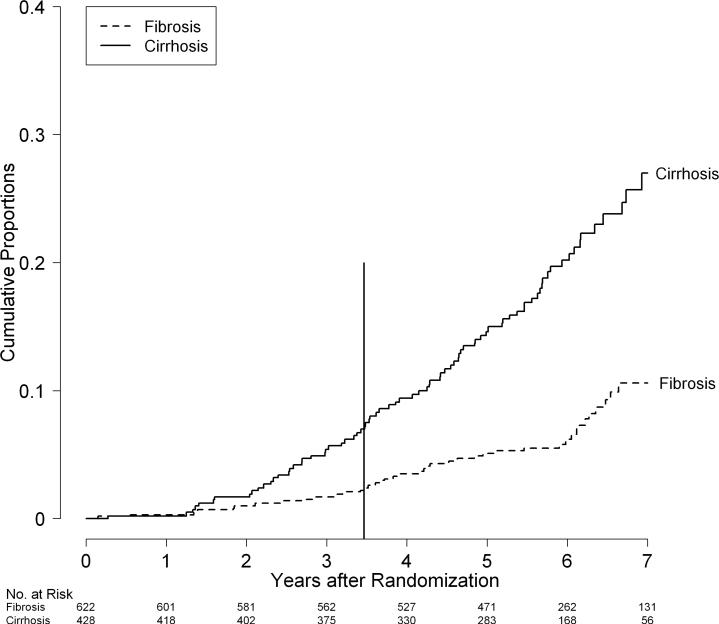
More deaths occurred in patients in the cirrhosis stratum (n = 80) than the fibrosis stratum (n = 42), and the survival distributions differed significantly (p<0.0001, Figure 2). Seven-year cumulative mortality rates were more than two times higher in patients in the cirrhosis stratum than the fibrosis stratum (27% versus 11%) which is equivalent to average annual death rates of 3.9% in the cirrhosis and 1.5% in the fibrosis stratum. Similarly, the distributions of the combined outcome of death or liver transplantation differed significantly in the two strata (p < 0.0001) resulting in a 7-year cumulative rate of 36% (n = 120) in the cirrhosis stratum compared to 16% (n = 66) in the fibrosis stratum.
Figure 2.
Cumulative rates of death by fibrosis/cirrhosis stratum in the HALT-C Trial cohort: Kaplan-Meier analysis of 622 patients in the fibrosis stratum (Fibrosis: dotted line) versus 428 patients in the cirrhosis stratum (Cirrhosis: solid line). The vertical line at 3.5 years marks the end of the randomized trial phase.
Of the 122 deaths, 76 were categorized as liver-related (62%) and 46 as non-liver-related (38%) (Table 1). The majority of liver-related deaths were attributable directly to complications of end-stage chronic hepatitis C or HCC; however, eight deaths (11%) were attributed to liver disease even though other potentially fatal medical conditions were present (e.g., cancer other than HCC, septicemia, influenza and pneumonia, or accident). The proportion of liver-related deaths was slightly higher among patients in the cirrhosis stratum compared to those with in the fibrosis stratum, but this difference was not statistically significant (65% versus 57%, p = 0.39).
Mortality rates by treatment group and fibrosis stratum
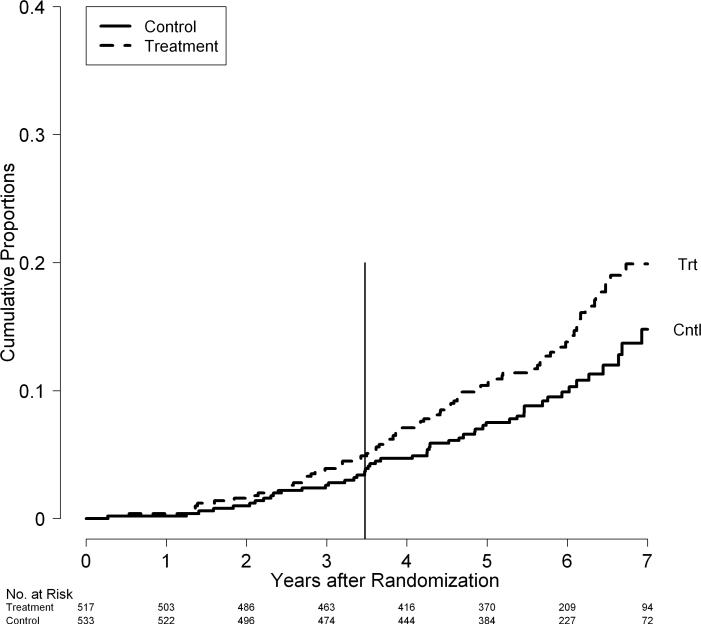
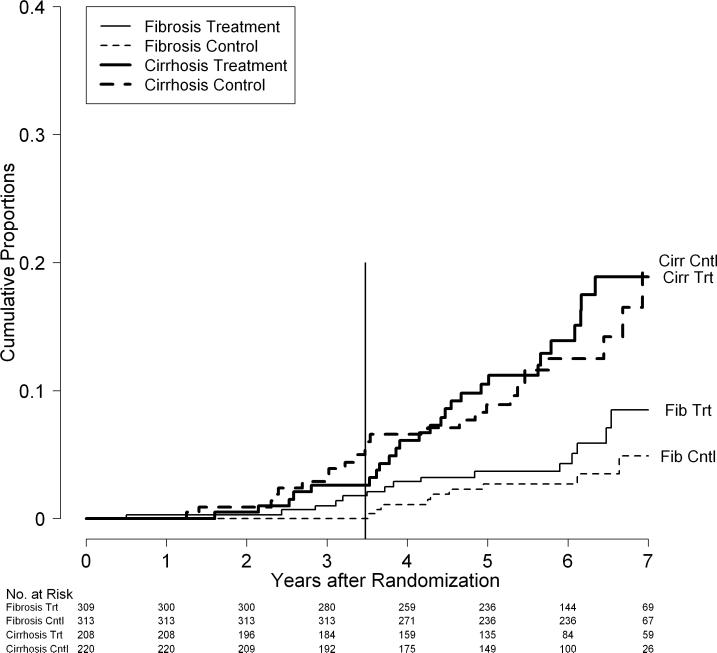
Overall, as well as within each stratum, the death rate was higher in patients in the treatment group compared to patients in the control group (p = 0.049, Figure 3). The cumulative 7-year death rate was 20% in treated and 15% in control patients. The mortality rates began to separate after 3 years of therapy and continued to separate during the 2 to 3 years of follow-up observation after treatment. The difference in mortality rates between patients in the treatment and control groups was statistically significant in the fibrosis stratum (p = 0.01) but was not significant in the cirrhosis stratum (p = 0.49) (Figure 4A). In the fibrosis stratum, at the end of the randomized phase (3.8 years), the cumulative mortality rate was 5.0% in patients in the treatment group compared to 1.9% in patients in the control group (p = 0.04).6 By 7 years, these rates increased to 14% and 7%, respectively. In the cirrhosis stratum, the mortality rates in patients in the treatment and control groups were 9.1% and 8.4% at the end of the randomized phase6 and, during follow-up observation, increased to 28% and 26%. In the fibrosis stratum, as in the group overall, the major separation of mortality rates occurred after three years of treatment.
Figure 3.
Cumulative rates of death by randomization group in the HALT-C Trial cohort: Kaplan-Meier analysis of deaths in 517 patients randomized to the treatment group (Trt: dotted line) versus 533 patients randomized to the controls group (Cntl: solid line). The vertical line at 3.5 years marks the end of the randomized trial phase
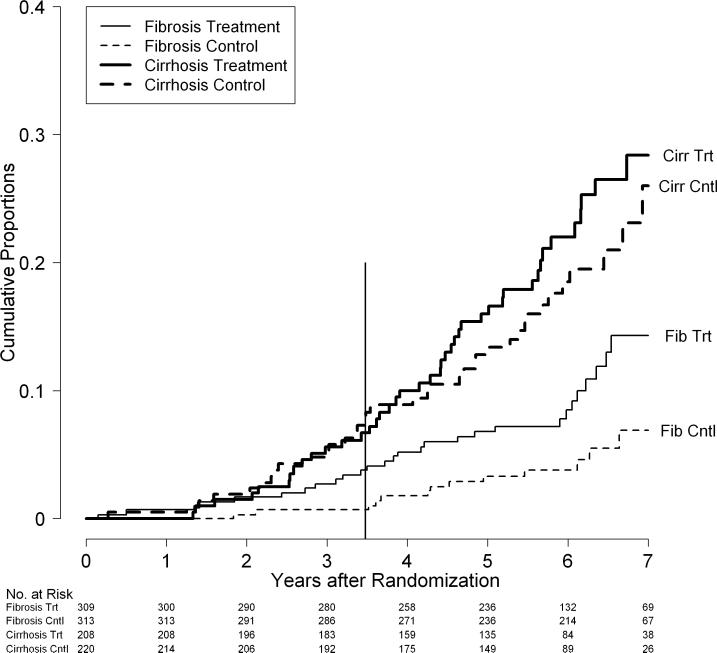
Figure 4.
Cumulative rates of death by fibrosis/cirrhosis stratum and randomization group in the HALT-C Trial cohort: All deaths (A), Liver-related deaths only (B), and Non-liver-related deaths only (C). Kaplan-Meier analysis of deaths in 309 patients in the fibrosis stratum and treatment group (Fib Trt), 313 patients in the fibrosis stratum and control group (Fib Cntl),208 patients in the cirrhosis stratum and treatment group (Cirr Trt), and220 patients in the cirrhosis stratum and control group (Cirr Cntl). The vertical line at 3.5 years marks the end of the randomized trial phase.
Death and liver transplantation as an endpoint
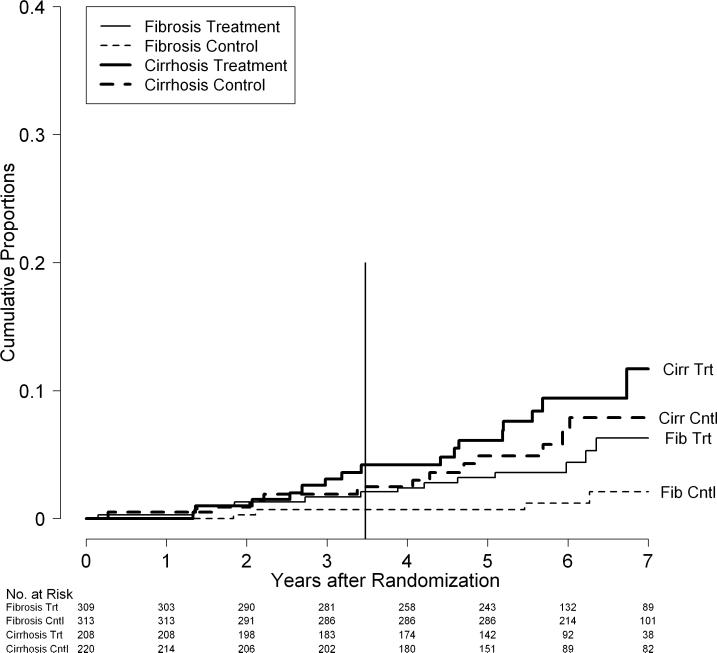
The results were similar when liver transplantation and death were combined as outcomes. Among the 1,050 patients enrolled, 186 patients (18%) either died or underwent liver transplantation. The rates of death or transplantation were minimally higher in the treated than control patients, and the difference was not statistically significant (p = 0.45) with 7-year cumulative rates of 25% and 24%, respectively. When separated by fibrosis stratum, however, the differences were statistically significant (Supplementary Figure 1). In the fibrosis stratum, the rates of death or transplantation were significantly higher in treated compared to control patients (p = 0.02) with 7-year cumulative rates of 19% and 12%, respectively, whereas in the cirrhosis stratum, rates of death or transplantation were similar in the two groups (p = 0.46) with 7-year cumulative rates of 34% and 39%, respectively.
Specific causes of death and liver relatedness
When causes of death were categorized by liver-relatedness, the excess mortality in the treatment group fibrosis stratum was primarily from non-liver causes. Thus, rates of liver-related deaths were similar in the treatment and the control groups (p = 0.42) with 7-year cumulative liver-related death rates of 12% and 11%, respectively. The rates of liver-related deaths in treatment and control groups were similar in both the fibrosis stratum (p = 0.21) and the cirrhosis stratum (p = 0.85), although the 7-year death rates did begin to show some separation in the fibrosis stratum (8% versus 5%) but not in the cirrhosis stratum (19% versus 20%, Figure 4B). On the other hand, non-liver related deaths were significantly more frequent among patients in the treatment group compared to the control group (p = 0.03) with 7-year cumulative mortality rates of 8% and 4%, respectively. These differences were more marked in the fibrosis stratum (p = 0.03) than in the cirrhosis stratum (p = 0.36, Figure 4C). The cumulative 7-year mortality rates were 6% and 2% in the treatment and control groups, respectively, in the fibrosis stratum and 12% and 8%, respectively, in the cirrhosis stratum.
Causes of non-liver related deaths
Examination of the specific causes of non-liver-related deaths failed to identify an excess frequency of any single diagnosis or category of diseases as a cause of death. The non-liver-related deaths reflected a spectrum of expected conditions, including non-HCC cancer as well as cardiac and cerebrovascular disease (Table 2). The distribution of these categories of illness appeared to be similar between those in the fibrosis and cirrhosis strata and in treated versus untreated patients. Cases of death resulting from malignant neoplasms other than HCC were assessed further in an attempt to identify a pattern (Table 3). The distribution of cancers appeared to mirror their relative frequencies in the general population—among the 11 patients whose deaths were attributed to cancer, four died of lung and two of colon cancer. Among the five deaths resulting from septicemia (two liver-related and three not liver-related: Table 2), three occurred in association with underlying cardiac or pulmonary disease, one in association with acute pancreatitis, and one approximately 2 years after liver transplantation. Deaths in nine patients were categorized as “other” and included trauma (3), intoxication or overdose (2), pneumonia and respiratory failure (2), ischemic colitis (1) and status epilepticus (1).
Table 2.
Causes of death by fibrosis stratum, treatment group, and liver-relatedness.
| Cause of Death | Fibrosis | Cirrhosis | |||
|---|---|---|---|---|---|
| Treatment | Control | Treatment | Control | Totals | |
| n = 309 | n = 313 | n = 208 | n = 220 | n = 1050 | |
| Liver-related deaths | |||||
| Chronic liver disease | 8 | 5 | 20 | 14 | 47 |
| HCC | 6 | 3 | 3 | 9 | 21 |
| Malignant neoplasm (not HCC)* | 0 | 1 | 0 | 1 | 2 |
| Septicemia* | 1 | 0 | 1 | 0 | 2 |
| Accidental* | 0 | 0 | 0 | 1 | 1 |
| Influenza and pneumonia* | 0 | 0 | 0 | 1 | 1 |
| Unknown* | 0 | 0 | 2 | 0 | 2 |
|
| |||||
| Totals | 15 | 9 | 26 | 26 | 76 |
|
| |||||
| Non-liver-related deaths | |||||
| Malignant neoplasm (not HCC) | 5 | 0 | 2 | 2 | 9 |
| Other | 1 | 0 | 5 | 3 | 9 |
| Accidental | 2 | 1 | 1 | 2 | 6 |
| Heart disease | 2 | 0 | 2 | 2 | 6 |
| Septicemia | 3 | 0 | 0 | 0 | 3 |
| Cerebrovascular disease | 0 | 0 | 2 | 0 | 2 |
| Influenza and pneumonia | 0 | 1 | 0 | 0 | 1 |
| Unknown | 1 | 2 | 4 | 3 | 10 |
|
| |||||
| Totals | 14 | 4 | 16 | 12 | 46 |
These eight deaths were attributed to liver disease even though other potentially fatal medical conditions were present.
Table 3.
Malignant neoplasms other than HCC causing death in 11 patients
| Stratum | Randomization group | Diagnosis |
|---|---|---|
| Liver-related * | ||
| Fibrosis | Control | Colon cancer with liver metastases |
| Cirrhosis | Control | Lymphoma |
| Non-liver-related | ||
| Fibrosis | Treatment | Non small cell lung cancer |
| Fibrosis | Treatment | Non small cell lung cancer |
| Fibrosis | Treatment | Squamous cell lung cancer |
| Fibrosis | Treatment | Prostate cancer |
| Fibrosis | Treatment | Colon cancer with liver metastases |
| Cirrhosis | Treatment | Gallbladder cancer |
| Cirrhosis | Treatment | Pancreatic cancer with lung metastases |
| Cirrhosis | Control | Lung cancer |
| Cirrhosis | Control | Gastric adenocarcinoma |
Death was considered to be liver-related in these patients, because, even though they had otherwise potentially fatal non-liver diseases, the circumstances of their deaths were most consistent with advanced liver disease, which may have been exacerbated by treatment of the malignancies.
Role of peginterferon side effects in excess mortality
Special attention was given to whether peginterferon therapy was a direct contributing factor or cause of death in the treatment group. Most deaths in the treated group occurred well after peginterferon was stopped, with only eight patients dying within 2 months of receiving peginterferon (11% of deaths in the treatment group). Independent assessment identified only one death as probably peginterferon-related. A 52 year old man with chronic hepatitis C and advanced fibrosis had an episode of severe S. aureus septicemia followed by multiorgan failure and death within a week of a last injection of peginterferon and after almost 2 years of maintenance therapy.
Discussion
The overall death rate in this cohort of patients with advanced chronic hepatitis C was remarkably high. Of the 1,050 patients, 18% died or underwent liver transplantation during a median followup time of 5.7 years, and approximately two thirds of deaths (62%) could be attributed to end-stage liver disease or HCC. Among patients with cirrhosis at baseline, the rate of death or liver transplantation was particularly high (7-year cumulative rate 36%, annualized rate 5.2%). Among those with fibrosis without cirrhosis at baseline, rates of all outcomes were less frequent, and the overall rate of death or liver transplantation was lower (7-year cumulative rate 16%, annualized rate 2.2%).
Several prospective studies have shown that chronic HCV infection is associated with an increased mortality rate,10–17 but the degree of this increase has been difficult to ascertain.18 The mortality rates observed in the HALT-C Trial cohort were similar to those reported in similar cohorts from other areas of the world. For example, in a recent systematic analysis of natural history studies, the annual rate of death or transplantation among patients with compensated cirrhosis associated with hepatitis C averaged 4.6%.19 In comparison, the annual mortality rate among HALT-C Trial patients in the compensated cirrhosis stratum was 3.9%, and the annual rate of death or transplantation in this stratum was 5.2%. Although the HALT-C Trial did not include uninfected control patients for comparison, the high mortality rates observed, particularly in the cirrhosis stratum, confirm the poor outcomes among patients with chronic hepatitis C and advanced hepatic fibrosis.
The unique finding of higher mortality among patients in the peginterferon-treatment group noted in the initial report of the randomized phase of the HALT-C Trial6 persisted when analyzed with a longer period of follow-up, the focus of the current analysis. An important feature of the current analysis was that all deaths were reviewed by a central committee blinded to the patient's identify. A cause of death was determined by committee consensus based upon pre-selected criteria. Because reviewers were also asked to assess possible causality due to interferon treatment, they could not be blinded to group assignment. During subsequent follow-up, the difference in death rates between patients in the treatment and control groups remained statistically significant and actually increased further. This was particularly true in the noncirrhotic fibrosis stratum, the trial subset in which increased mortality was found to be associated with maintenance therapy during the randomized phase.6 The difference in mortality in the cirrhosis stratum between patients in the treatment and control groups also increased during extended observation but did not reach statistical significance.
Of note, the excess mortality in the treatment group cohort did not begin to arise until 3 years into treatment and continued for several years after peginterferon was stopped. Nevertheless, a review of each case failed to identify an immediate or direct relationship between the increased death rate and peginterferon therapy. Interferon therapy has been associated in rare instances with fatal severe adverse events, including suicide, acute myocardial infarction, cerebrovascular accident, precipitation of severe autoimmune disease, and septicemia.20 These complications, however, did not account for the increased rate of death associated with treatment in the HALT-C Trial cohort. Indeed, of the 71 deaths that occurred in patients in the treatment group, only eight occurred within 2 months of a peginterferon injection, and in only one instance was peginterferon thought to have probably played a contributing role (an episode of septicemia in close temporal proximity to a peginterferon injection).
Importantly, the excess mortality in the treatment group resulted largely from non-liver-related causes. Indeed, rates of death attributable to end-stage liver disease and HCC were similar in the treatment and control groups. Careful review of the non-liver-related deaths, however, failed to reveal a specific disease category associated with this excess mortality, although the combination of death by non-HCC malignancy and systemic infection might suggest a potential effect on host immunity. Nevertheless, the excess mortality arising after 3 years of peginterferon treatment remains unexplained and did not result from a specific adverse effect of treatment or single type of fatal condition (such as heart disease, lung disease, or cancer); the link between treatment and mortality was both non-cause-specific and delayed. Risk factors for non-liver-related death such as obesity and smoking were included in the analysis but did not obviously account for the excess deaths (data not shown).
Theoretical explanations might be offered to explain excess mortality associated with long-term peginterferon treatment. Interferon-alpha is not a direct acting antiviral but rather acts on cell-surface receptors to trigger signaling pathways that activate “interferon-stimulated genes,” which render the cell resistant to viral infection and less capable of supporting viral replication.21,22 The basis of the antiviral activity of alpha interferon is complex and involves multiple, often redundant cellular pathways, such as those involved in regeneration; cell turnover; apoptosis; and protein, lipid, and carbohydrate metabolism. Possibly, the continuous stimulation of interferon-induced genes by long-term maintenance therapy is detrimental, particularly to cells and tissues without active viral replication. These effects may be diverse and, therefore, not manifested as a single adverse reaction.
An alternative explanation for the difference in mortality between the treatment and control groups in the HALT-C Trial is the presence of an undefined confounding factor, such as baseline difference in the randomization groups, or difference in subsequent management. However, given the size of the trial, the success of randomization,6 and the uniformity of management in the two groups, these differences are unlikely to have accounted for a statistical difference in mortality rates. Currently, hypotheses to explain excess mortality linked to interferon are not supported by clinical or experimental observations but warrant further study.
Thus, the HALT-C Trial was not able to show a benefit of long-term peginterferon maintenance on rates of clinical progression, histologic progression to cirrhosis, hepatic decompensation, HCC, or death.6 In this extended follow-up analysis, as in the analysis of the randomized trial, the mortality rate appeared to be higher among patients in the peginterferon treatment group. In other post-hoc analyses of the HALT-C Trial cohort, long-term peginterferon therapy appeared to be associated with a lower rate of late HCC, diverging from the control group after four years of observation, but only in patients with cirrhosis at baseline.23 As shown in the current analysis, the lower rate of late HCC was not accompanied by a lower rate of death or liver transplantation.
In summary, long-term observation of a large cohort of patients with chronic hepatitis C and advanced hepatic fibrosis revealed a high rate of death, particularly among those with cirrhosis at baseline. Approximately two-thirds of deaths were attributable to liver disease. An increase in mortality occurred in patients in the long-term peginterferon treatment group, but this increase in mortality was attributed to non-liver-related deaths and occurred largely among patients with pre-cirrhotic advanced fibrosis at baseline. No pattern to this excess mortality was discernible; deaths were unrelated to direct effects of peginterferon treatment. These findings suggest that the long-term use of interferon should be evaluated cautiously and that attention to unrelated complications is warranted.
Supplementary Material
Cumulative rates of death or liver transplantation (combined) by fibrosis/cirrhosis stratum and randomization group in the HALT-C Trial cohort: Kaplan-Meier analysis of deaths in 309 patients in the fibrosis stratum and treatment group (Fib Trt), 313 patients in the fibrosis stratum and control group (Fib Cntl), 208 patients in the cirrhosis stratum and treatment group (Cirr Trt), and 220 patients in the cirrhosis stratum and control group (Cirr Cntl). The vertical line at 3.5 years marks the end of the randomized trial phase.
ACKNOWLEDGMENTS
The authors thank Jay H. Hoofnagle for his suggestions on the design of this analysis and help with writing. This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID); the National Cancer Institute; the National Center for Minority Health and Health Disparities; by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (NIH grant numbers are listed below); and by the Intramural Research Program of the NIH, NIDDK (M.G.Ghany). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., (now Genentech) through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, David P. Lundmark
University of Colorado Denver, School of Medicine, Aurora, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) Gregory T. Everson, MD, Thomas Trouillot, MD, Marcelo Kugelmas, MD, S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Karen L. Lindsay, MD, MMM, Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Richard K. Sterling, MD, MSc, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, MPH, Patricia R. Robuck, PhD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Teresa M. Curto, MSW, MPH, Margaret C. Bell, MS, MPH
Inova Fairfax Hospital, Falls Church, VA: Zachary D. Goodman, MD, PhD, Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Conflicts of Interest: Financial relationships of the authors with Hoffmann-La Roche, Inc. (now Genentech), are as follows: A.M. Di Bisceglie is a consultant and receives research support; M.L. Shiffman is a consultant, on the speaker's bureau and receives research support; W.M. Lee receives research support; R.J. Fontana is on the speaker's bureau; and T.R. Morgan receives research support.
Abbreviations
- HCV
hepatitis C virus
- HCC
hepatocellular carcinoma
- HALT-C
Hepatitis C Antiviral Long-term Treatment against Cirrhosis
- SSDI
Social Security Death Index
Footnotes
Authors with no financial relationships related to this project are: A.M. Stoddard, J.L. Dienstag, L.B. Seeff, H.L. Bonkovsky, C. Morishima, E.C. Wright, K.K. Snow, and M.G. Ghany.
This is publication #65 of the HALT-C Trial.
References
- 1.Alter MJ. Epidemiology of hepatitis C virus infection. World J Gastroenterol. 2007;13:2436–41. doi: 10.3748/wjg.v13.i17.2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29(Suppl 1):74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Chang H-G, McNutt L-A, Smith PF. Estimating the mortality rate of hepatitis C using multiple data sources. Epidemiol Infect. 2005;133:121–125. doi: 10.1017/s0950268804003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghany MG, Strader DB, Thomas DL, Seeff LB, American Association for the Study of Liver Diseases Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335–74. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG, Morishima C, Wright EC, Everhart JE, HALT-C Trial Group Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–92. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie AM, Shiffman ML, Everson GT, Lindsay KL, Everhart JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL, The HALT-C Trial Group Prolonged therapy of advanced chronic hepatitis C with low dose peginterferon. N Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishak KG. Chronic hepatitis: morphology and nomenclature. Modern Pathology. 1994;7:690–713. [PubMed] [Google Scholar]
- 8.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–65. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 9.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–82. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 10.Tong MJ, El-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 11.Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT, Thomas H, Njapoum C, Casarin C, Bonetti P, Fuschi P, Basho J, Tocco A, Bhalla A, Galassini R, Noventa F, Schalm SW, Realdi G. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–72. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 12.Kenny-Walsh E, The Irish Hepatology Research Group Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. N Engl J Med. 1999;340:1228–1233. doi: 10.1056/NEJM199904223401602. [DOI] [PubMed] [Google Scholar]
- 13.Seeff LB, Hollinger FB, Alter HJ, Wright EC, Cain CM, Buskell ZJ, Ishak KG, Iber FL, Toro D, Samanta A, Koretz RL, Perrillo RP, Goodman ZD, Knodell RG, Gitnick G, Morgan TR, Schiff ER, Lasky S, Stevens C, Vlahcevic RZ, Weinshel E, Tanwandee T, Lin HJ, Barbosa L. Long-term mortality and morbidity of transfusion-associated non-A, non-B, and type C hepatitis: A National Heart, Lung, and Blood Institute collaborative study. Hepatology. 2001;33:455–63. doi: 10.1053/jhep.2001.21905. [DOI] [PubMed] [Google Scholar]
- 14.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744–749. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iacobellis A, Siciliano M, Perri F, Annicchiarico BE, Leandro G, Caruso N, Accadia L, Bombardieri G, Andriulli A. Peginterferon alfa-2b and ribavirin in patients with hepatitis C virus and decompensated cirrhosis: a controlled study. J Hepatol. 2007;46:206–12. doi: 10.1016/j.jhep.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 16.Neal KR, The Trent Hepatitis C Study group Excess mortality rates in a cohort of patients infected with the hepatitis C virus: a prospective study. Gut. 2007;56:1098–1104. doi: 10.1136/gut.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toshikuni N, Izumi A, Nishino K, Inada N, Sajanoue R, Yamato R, Suehiro M, Kawanaka M, Yamada G. Comparison of outcomes between patients with alcoholic cirrhosis and those with hepatitis C virus-related cirrhosis. J Gastroenterol Hepatol. 2009;24:1276–1283. doi: 10.1111/j.1440-1746.2009.05851.x. [DOI] [PubMed] [Google Scholar]
- 18.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36(5 Suppl 1):S35–46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 19.Alazawi W, Cunningham M, Dearden J, Foster GR. Systematic review: outcome of compensated cirrhosis due to chronic hepatitis C infection. Aliment Pharmacol Ther. 2010;32:344–55. doi: 10.1111/j.1365-2036.2010.04370.x. [DOI] [PubMed] [Google Scholar]
- 20.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(Suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 21.Samuel CE. Antiviral actions of interferons. Clin Microbiol Rev. 2001;14:778–809. doi: 10.1128/CMR.14.4.778-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436:967–72. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 23.Lok AS, Everhart JE, Wright EC, Di Bisceglie AM, Kim H-Y, Sterling RK, Everson GT, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in advanced hepatitis C. Gastroenterology. 2011 doi: 10.1053/j.gastro.2010.11.050. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cumulative rates of death or liver transplantation (combined) by fibrosis/cirrhosis stratum and randomization group in the HALT-C Trial cohort: Kaplan-Meier analysis of deaths in 309 patients in the fibrosis stratum and treatment group (Fib Trt), 313 patients in the fibrosis stratum and control group (Fib Cntl), 208 patients in the cirrhosis stratum and treatment group (Cirr Trt), and 220 patients in the cirrhosis stratum and control group (Cirr Cntl). The vertical line at 3.5 years marks the end of the randomized trial phase.