Abstract
Acquiring the behavioral significance of a sound has repeatedly been shown to correlate with long term changes in response properties of neurons in the adult primary auditory cortex. However, the molecular and cellular basis for such changes is still poorly understood. To address this, we have begun examining the auditory cortical expression of an activity-dependent effector immediate early gene (IEG) with documented roles in synaptic plasticity and memory consolidation in the hippocampus: Arc/Arg3.1. For initial characterization, we applied a repeated 10 minute (24 hour separation) sound exposure paradigm to determine the strength and consistency of sound-evoked Arc/Arg3.1 mRNA expression in the absence of explicit behavioral contingencies for the sound. We used 3D surface reconstruction methods in conjunction with fluorescent in-situ hybridization (FISH) to assess the layer-specific sub-cellular compartmental expression of Arc/Arg3.1 mRNA. We unexpectedly found that both the intranuclear and cytoplasmic patterns of expression depended on the prior history of sound stimulation. Specifically, the percentage of neurons with expression only in the cytoplasm increased for repeated versus singular sound exposure, while intranuclear expression decreased. In contrast, the total cellular expression did not differ, consistent with prior IEG studies of primary auditory cortex. Our results were specific for cortical layers 3–6, as there was virtually no sound driven Arc/Arg3.1 mRNA in layers 1–2 immediately after stimulation. Our results are consistent with the kinetics and/or detectability of cortical sub-cellular Arc/Arg3.1 mRNA expression being altered by the initial exposure to the sound, suggesting exposure-induced modifications in the cytoplasmic Arc/Arg3.1 mRNA pool.
Keywords: immediate early gene, mouse, catFISH, novelty, familiarity, synaptic plasticity
Response properties of sensory cortical neurons change as a stimulus gains behavioral relevance (Weinberger, 2004). On short time scales, such changes may represent attentional effects (Fritz et al., 2007), but on longer time scales, they provide a basis for the distributed storage of sensory memories (Sutherland and McNaughton, 2000; Kilgard et al., 2002; Sacco and Sacchetti, 2010). The molecular mechanisms underlying such long term cortical plasticity and how each sensory experience engages these mechanisms are poorly understood. Sensory stimuli likely induce plasticity-related genomic responses (Mello et al., 1992; Velho et al., 2005; Pinaud et al., 2008; Dong et al., 2009), but these responses have not been well investigated in mammalian sensory cortex compared to electrophysiological measures of plasticity.
In considering such molecular mechanisms, Arc/Arg3.1 (hereafter referred to as Arc) is of particular interest since it acts as a key regulator of translation-dependent synaptic plasticity in the hippocampus (reviewed by (Bramham et al., 2010)). Arc mRNA can be rapidly induced by synaptic activity (Link et al., 1995; Lyford et al., 1995) and transported into dendrites (Dynes and Steward, 2007), accumulating specifically near activated synapses (Moga et al., 2004). Arc protein then becomes enriched at the site of local synaptic activity (Steward et al., 1998; Yin et al., 2002; Moga et al., 2004; Rodríuez et al., 2005), where it regulates glutamate receptor trafficking, postsynaptic density remodeling and spine morphology (Chowdhury et al., 2006; Rial Verde et al., 2006; Messaoudi et al., 2007; Peebles et al., 2010). A single, brief exploratory experience can drive an initial and a temporally delayed (8–24 hour (h)) wave of hippocampal Arc protein expression, possibly representing a reactivation of a subnet of neurons encoding that experience (Wilson and McNaughton, 1994; Ramirez-Amaya et al., 2005).
Arc’s role in hippocampal-based memory consolidation (Guzowski et al., 2000; Plath et al., 2006) has therefore made it a promising target for investigating molecular mechanisms of long term cortical plasticity (Mahlke and Wallhäusser-Franke, 2004; Sun et al., 2005; Wang et al., 2006; Tan et al., 2007; Carpenter-Hyland et al., 2010; Gao et al., 2010; Gusev and Gubin, 2010). However, basic knowledge about Arc’s pattern of expression across layer-dependent cortical networks is incomplete. Our objective was to characterize both the baseline and stimulus-induced layer-specific Arc expression in a simple stimulus exposure paradigm to lay the groundwork for future studies using a more explicit associative learning context. Our data revealed a significant relation between the history of prior sound exposure and the compartmentalization of the evoked Arc mRNA expressed in the thalamorecipient and infragranular layers, demonstrating that a single exposure to a stimulus can leave a sub-cellular molecular trace in primary sensory cortical neurons.
EXPERIMENTAL PROCEDURES
All procedures were approved by the Emory Institutional Animal Care and Use Committee. Experiments were performed on CBA/CaJ mice (15–18 weeks old). Mice were kept under a reversed light cycle and housed individually at least one day prior to the start of experiments, performed during the dark cycle. On a given experiment day, a mouse in its home cage was placed into a silent anechoic chamber (44” × 27” × 24”, W × D × H inner dimensions, Acoustic Systems, Austin, TX) for 4 h, followed by a 10 minute (min) test period of either additional silence or continuous sound stimulation. The latter consisted of a dynamic sequence of 32 kHz tones at 40 dBSPL, with random durations (60 ± 24 ms, mean ± standard deviation) and inter-tone intervals (206 ± 49 ms). Sounds were generated by an RX6 digital signal processor and attenuated by a PA5 programmable attenuator (Tucker Davis Technologies, Alachua, FL). Further details are described in the Results.
Tissue preparation
Mice were sacrificed by CO2 inhalation. In most cases, their brains were then removed rapidly, covered with OCT media (VWR International, West Chester, PA), and frozen immediately in liquid nitrogen. Serial 20 um coronal sections cut by cryostat (Leica, Richmond, IL) were mounted onto slides and stored at −80° C. In some cases where a tangential slice through the auditory cortex was desired, mice were instead perfused with phosphate buffer saline (pH 7.4) for 2–3 min after CO2 inhalation. The brain was removed and divided into left and right hemispheres. The left hemisphere was flattened between two glass slides, and together with a block from the right hemisphere, postfixed in 4% paraformaldehyde for 1 h followed by soaking in 30% sucrose overnight. Both hemispheres were then covered with OCT media, frozen in liquid nitrogen, and stored at −80°C. Tangential (left hemisphere) or coronal (right hemisphere) sections 40 um in thickness were cut by serial cryostat, mounted onto slides and stored at −80° C.
FISH for Arc mRNA
FISH was performed on slide-mounted brain sections following protocols previously described in detail elsewhere (Guzowski et al., 1999; Muddashetty et al., 2007). Briefly, frozen sections were fixed in 4% paraformaldehade for 5 min.; slides were rinsed in cold 2× SSC (sodium citrate chloride), and treated with 0.5% acetic anhydride in 0.1M triethanolamine-HCl buffer (pH 8.0) and dehydrated in acetone:methanol (1:1) at room temperature. After washing again in 2× SSC, slides were incubated with prehybridization buffer (2× SSC, 25% formamide, 1% Denhardt’s reagent, 50% dextran sulfate, 25mg/ml yeast tRNA, and 10 mg/ml of denatured salmon sperm DNA) in wet chamber at room temperature for 1 h. Digoxigenin-labeled Arc antisense and sense riboprobes (NCBI accession number NM_018790.2, nt 273–1369) were prepared using a commercial kit (Roche Molecular Biochemicals, Nutley, NJ). After riboprobe hybridization (16 h, 56°C), slides were washed with 2× SSC and treated with RNaseA (10 mg/ml) at 37°C for 30 min. After a graduated series of washes in SSC, slides were incubated in 3% hydrogen peroxide for 15 min, followed by incubation in block buffer TNB (0.1% Tris HCl, pH 7.5; 0.15M NaCl; 5% Blocking-Reagent (Roche)) for 30 min, and then TNB containing anti-digoxigenin-POD, Fab fragments (Roche Diagnostics, Indianapolis, IN), for 2 h at room temperature. Arc probes were detected with TSA-Direct Cyanine-3 fluorescence amplification kit (TSA Amp Kit, PerkinElmer, Boston, MA). Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI, Electron Microscopy Sciences, Hatfield, PA).
Confocal microscopy and cell counting
Stained slides were analyzed using a Zeiss LSM 510 confocal microscope. For tangential sections through layer 4, auditory cortex was identified by relative alignment with the barrel cortex (Caviness and Frost, 1980). Coronal sections were matched to a standard mouse atlas (Paxinos and Franklin, 2001) to identify the anatomically labeled primary auditory cortex (Au1); sections fell between −2.30 mm and −2.80 mm relative to Bregma. The shape of the hippocampus was an excellent guide for localization, as validated in additional experiments in adult female mice where auditory cortex was located electrophysiologically. Details about electrophysiological methods can be found in our previous publications (Galindo-Leon et al., 2009; Lin and Liu, 2010). Positions of cortical layers in the coronal slices were determined by aligning the DAPI staining of nuclei to layers previously delineated by Nissl staining (Anderson et al., 2009). Our analysis focused only on 3 groups of cortical layers corresponding to the supragranular (layers 1–2), thalamorecipient (layers 3–4 in auditory cortex (Cruikshank et al., 2002; Winer et al., 2005)) and infragranular (layers 5–6) layers of the auditory cortex, due to the labor-intensive nature of the 3D quantification (see Results). For consistency, images were targeted to the middle of each of these 3 groups rather than across their entire widths.
In coronal sections, the threshold level for Arc positive fluorescence could be set by referencing to the positive-labeled cells in the hippocampus. For each experimental group and layer, typically 2 non-overlapping z-stacks at a given cortical depth were imaged per slide, for a median of 6 (range 5–9) z-stacks per group per layer. Each z-stack, imaged at 63X, consisted of single planes spaced 0.5 µm apart. The median image thickness was 16.5 um (34 planes per z-stack). Each z-stack was then viewed in the Imaris 3D software (Bitplane Scientific Software, Zurich, Switzerland), which generated artificial surfaces for DAPI-stained nuclei and fluorescently labeled Arc mRNA. The threshold for the DAPI surfaces was set so that the surface would just fully encompass the stained volume. The threshold for the Arc surfaces was determined by building the Arc surface in a random sample of the images to determine an average value that created a good visual match between the Arc surface and the raw Arc signal; this was then applied consistently across images.
Since glial cells are not known to express Arc (Cirelli and Tononi, 2000), we excluded glial-like nuclei based on the intensity, texture and homogeneity of the DAPI stain, following previously published criteria (Chawla et al., 2004); nuclei that were not spherical or oval-shaped were also suspected to be glial cells and were often removed from consideration. In quantifying images, nuclei that were substantially cut off at any of the 6 edges of the image volume were also removed. Remaining cells were classified as either being positive or negative for Arc mRNA. Positive neurons were sub-classified as having Arc mRNA expressed in “intranuclear foci only,” in the perinuclear “cytoplasm only,” or simultaneously in “both” compartments (see Results). To suppress unspecific background fluorescence in our quantification, we required Arc mRNA fluorescence surfaces to be within (i.e. intranuclear) or outside but in contact with (i.e. perinuclear cytoplasmic) the DAPI surface. Percentages of expressing cells were computed for each layer’s images based on these compartmental pattern-specific values. Percentages were also computed for nuclear-positive (sum of each image’s “intranuclear foci only” and “both” percentages), cytoplasm-positive (“cytoplasm only” and “both” percentages) and total cellular expression (sum of all three compartmental pattern percentages). The total cellular expression plus the negative staining percentage totaled 100%. Percentages were analyzed by ANOVA, with post-hoc multiple comparisons carried out by the Tukey-Kramer Honestly Significant Difference (HSD) test. Differences at the p≤0.05 level were considered significant.
RESULTS
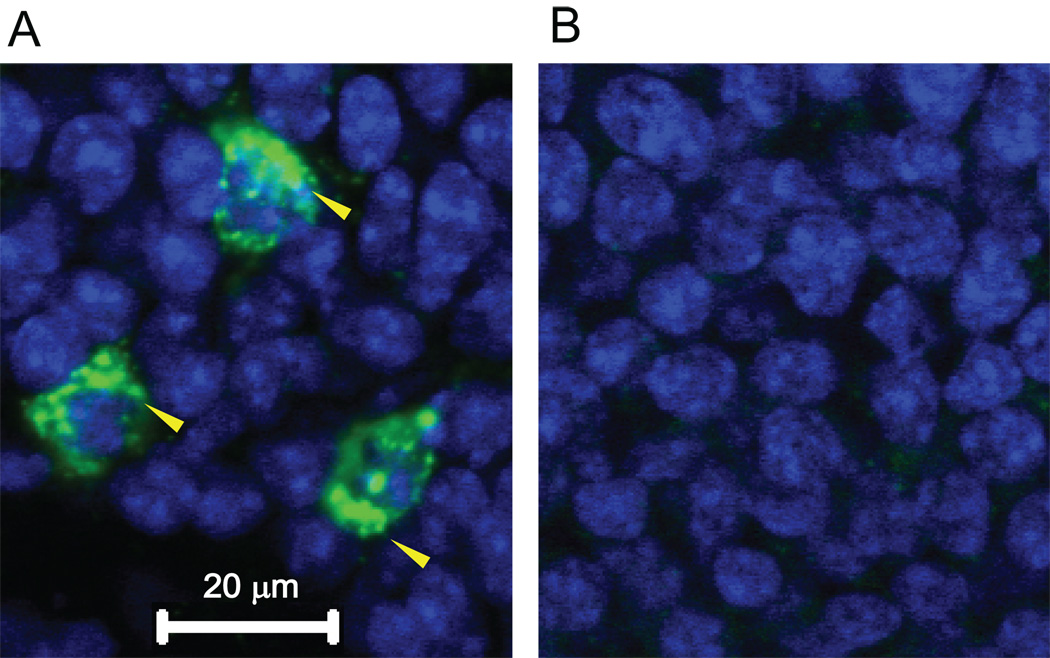
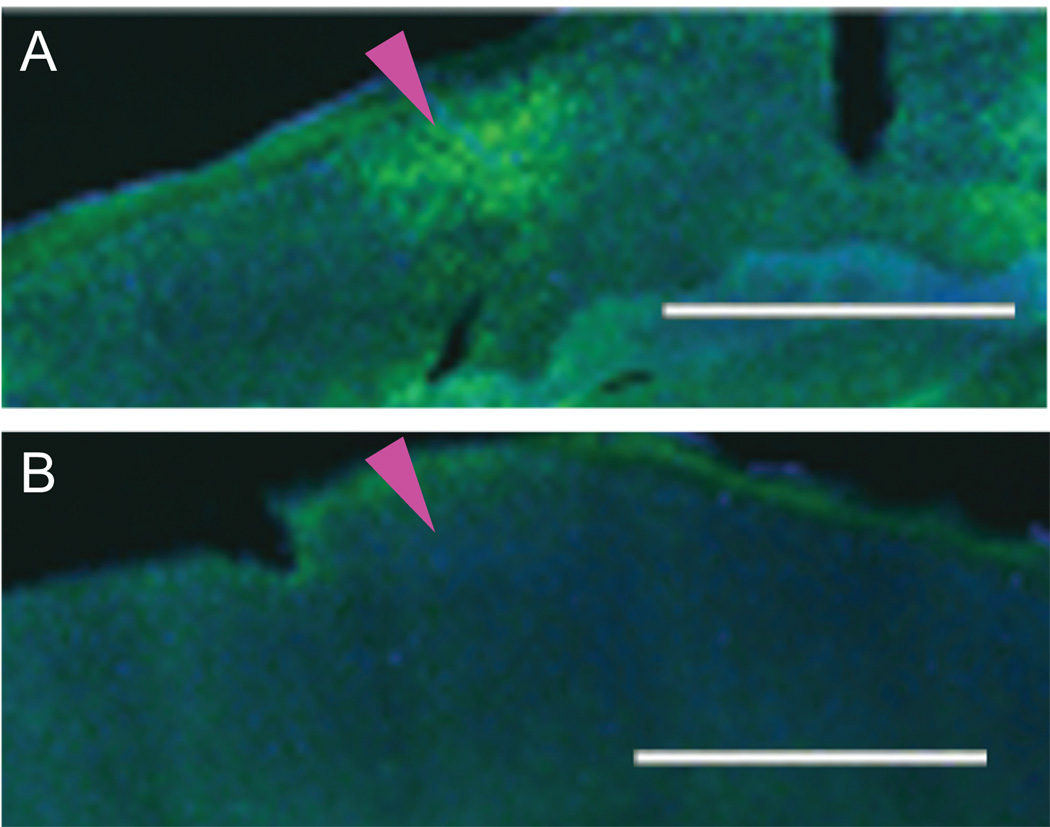
We first tested whether our sound stimulus (dynamic 32kHz, 40dBSPL tone) could evoke measurable Arc mRNA expression in the auditory cortex. Mice (3 animals) received 10 min of sound stimulation in an anechoic chamber after a 4 h silent habituation period and were kept for an additional 30 min in silence before decapitation to ensure robust expression (Guzowski et al., 1999; Velho et al., 2005). Successful FISH for Arc mRNA was confirmed by observing cellular cytoplasmic expression within the granule cell layer of the dentate gyrus (Fig. 1A) (Guzowski et al., 1999). Such expression was Arc mRNA-specific since tissue processed with Arc sense riboprobes showed no staining (Fig. 1B). Extensive Arc mRNA expression after sound stimulation was seen across a flattened tangential section through layer 4 of primary auditory cortex (Fig. 2A, magenta arrowhead, Brodmann area 41). In contrast, spontaneous Arc mRNA expression (Fig. 2B) from animals kept in the anechoic chamber without sound stimulus was negligible.
Figure 1.
Arc mRNA expression in the dentate gyrus after sound stimulation. (A) Image (63X) of a coronal section through the dentate gyrus processed for FISH using a digoxigeninlabeled riboprobe (antisense) shows Arc mRNA expression (yellow arrowheads) around granule cell soma. (B) Image after hybridization using the Arc sense probe remained unstained.
Figure 2.
Single plane images of Arc mRNA expression in a tangential section (40 µm) through cortical layer 4 of auditory cortex (magenta arrowhead) after (A) sound stimulation and (B) silence. Blue represents DAPI staining of nuclei. Green represents Arc mRNA expression. Scale bar is 1000 µm.
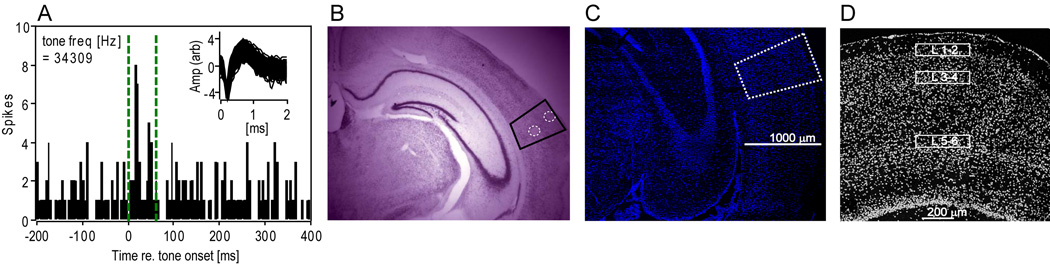
Sound-evoked expression in the tangential slice was sufficiently distributed to be expected to be observed in several serial coronal slices near the center of primary auditory cortex. This center was targeted in coronal slices by comparing the shape of the hippocampus and thalamic nuclei to a standard mouse atlas (Paxinos and Franklin, 2001). Separate in vivo electrophysiological experiments validated the presence of primary-like tone responses (Fig. 3A) at such a location, which was histologically marked with electrolytic lesions after recordings (Fig. 3B). Note that this anatomically-defined Au1 presumably includes both the anterior auditory field and the primary auditory field (Stiebler et al., 1997). In slices subjected to FISH, those with a nuclear DAPI stain (Fig. 3C) matching the target region (see Experimental Procedures) were then imaged at high resolution in the middle of the supragranular (1–2), thalamorecipient (3–4) and infragranular (5–6) cortical layers (Fig. 3D).
Figure 3.
Location within primary auditory cortex (Au1) of confocal image analyses. (A) Peristimulus time histogram of responses of a neuronal unit (all action potential waveforms depicted in the Inset) to a 34 kHz pure tone (duration of tone marked by dashed vertical green lines). (B) Cresyl violet stain (2X magnification) of lesioned sites (dashed circle) within areas showing primary-like auditory responses (A), presumably corresponding to primary auditory cortex (black box). This site corresponds to −2.54 mm re. Bregma according to the Paxinos and Franklin (2001) mouse brain atlas. (C) DAPI nuclear staining viewed under the confocal microscope at 10X magnification through an anatomically similar slice through primary auditory cortex (white dashed box). (D) Expanded grayscale view of the boxed area in (C) showing nuclear density variations similar to that seen in the cresyl violet stain (B). This enabled targeting of specific, identifiable regions in the middle of the supragranular (layers 1–2), thalamorecipient (layers 3–4) and infragranular (layers 5–6) layers for confocal image analysis (rectangular zones).
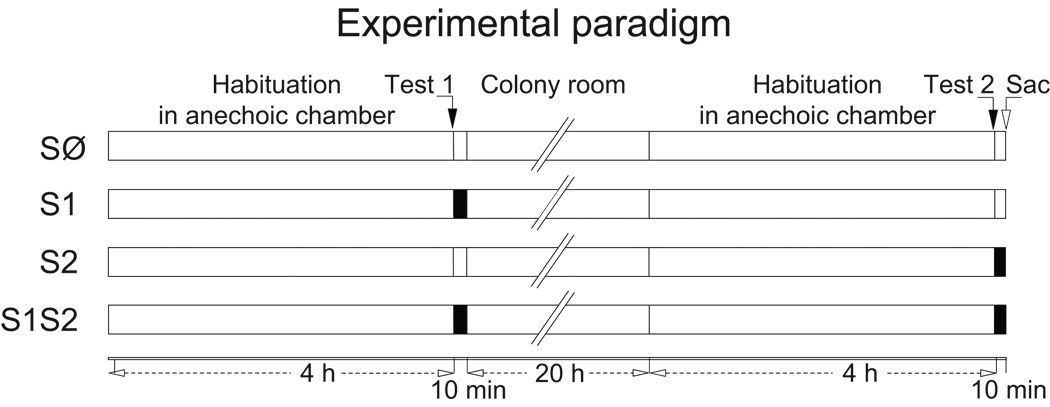
Using these coronal slices, we examined whether sound-induced auditory cortical Arc mRNA expression in adult mice shows changes after simply repeating sound exposure. We compared the layer-specific and compartmental pattern of Arc mRNA expression across four groups of animals (3–4 mice per group). All mice received two test epochs, separated by 24 h (with 4 h silent habituation periods), before being sacrificed immediately after Test 2 (Fig. 4). A familiar sound group received stimuli during both Tests 1 and 2 (S1S2) for 10 min each, which was sufficient to induce both nuclear and cytoplasmic expression. A novel sound group received stimulation with sound only during Test 2 (S2). As a control, one group received sound during Test 1 but not Test 2 (S1), to determine whether initial sound stimulation affects Arc mRNA expression 24 h later in the absence of further stimulation (Ramirez-Amaya et al., 2005). Finally, a no sound (SØ) control group experienced the anechoic environment without any sound stimulation, thus providing a baseline for auditory cortical expression in silence.
Figure 4.
Experimental paradigm. Segments indicate amount of time in each behavioral state. Filled and unfilled Test segments indicate presence or absence, respectively, of a 32 kHz dynamic tone stimulus.
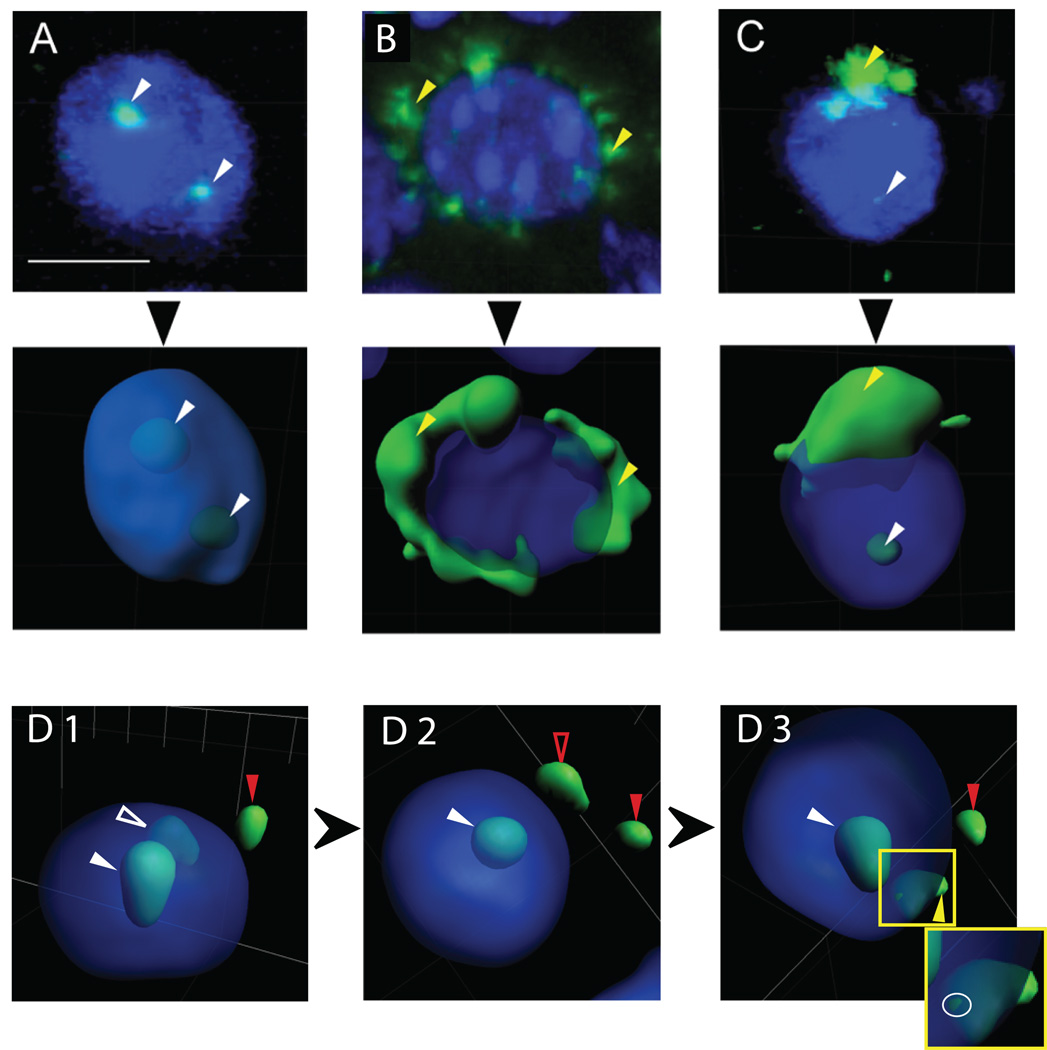
For each confocal z-stack, a 3D reconstruction of fluorescence surfaces was performed, and the sub-cellular compartmental location of the Arc mRNA expression was determined (see Experimental Procedures). Figure 5A–B illustrates examples of cells labeled as having Arc mRNA fluorescence in “intranuclear foci” (Fig. 5A, white arrowheads), and in the perinuclear “cytoplasm only” (Fig. 5B, yellow arrowheads). The top row shows the projection of the full z-stacks into 2D images, while the middle row plots the reconstructed 3D surfaces. Figure 5C shows a cell with “both” intranuclear (white arrowhead) and cytoplasmic (yellow arrowhead) expression. Figure 5D demonstrates the utility of the 3D reconstruction, which allows the rotation of an image to conclusively separate intranuclear from extranuclear (i.e. cytoplasmic or background) Arc fluorescence signals.
Figure 5.
Classification of Arc mRNA expression. Examples of Arc mRNA expressed in (A) intranuclear foci only, (B) perinuclear cytoplasm only, or (C) both intranuclear foci and perinuclear cytoplasm. Top panels are each confocal z-stacks (projection onto 2D) from a tissue section subjected to FISH. Middle panels represent their respective 3D surface reconstructions. These high-magnification views (63X) illustrate individual nuclear foci (white arrowheads) and diffuse perinuclear cytoplasmic signals (yellow arrowheads). Blue represents DAPI staining of nuclei. Note the detection of two Arc mRNA foci within the nucleus in (A), suggesting active transcription at each allele. (D1–3) The classification advantage of 3D reconstruction is evident from successively rotated views of the same nucleus. The solid red arrowhead in each panel points to a fluorescence signal labeled as background and left unclassified, since it cannot be associated with a specific cell. The solid white arrowhead in each panel indicates a fluorescence signal classified as a focal point of intranuclear Arc expression, since it remains wholly within the nucleus in all views. The open white arrowhead in D1 indicates a potential site of intranuclear expression based on the transparent view, but upon rotation (D2), this fluorescence appears to fall outside the nucleus, potentially representing background expression (open red arrowhead). Further rotation (D3) reveals that the fluorescence surface intersects the DAPI stained nuclear surface (highlighted by white ring around intersection in the Inset, which expands the image within the yellow box in D3), resulting in its classification as a point of perinuclear cytoplasmic expression (yellow arrowhead). This cell is therefore classified as having “both” intranuclear and cytoplasmic expression. Common scale bar shown in (A) is 10 µm.
Upon quantifying the expression in this way, our experiments revealed that the sub-cellular pattern of Arc mRNA expression immediately after sound stimulation depends on both the specific layer within the auditory cortex and the sound history. A 3-way ANOVA on the percentage of cells with different compartmental patterns of Arc mRNA expression showed significant main effects of group (F(3,204)=14.35, p«0.001), compartment (F(2,204)=24.29, p«0.001) and layer (F(2,204)=37.96, p«0.001). Importantly, we also found interactions between group × compartment (F(6,204)=14.39, p«0.001), compartment × layer (F(4,204)=5.24, p≤0.0005), and group × compartment × layer (F(12,204)=2.65, p≤0.005), indicating that factors were not simply independent. In particular, the nature of Arc mRNA expression in layers 1–2 differed fundamentally from that of 3–4 and 5–6. Unlike the latter, expression in the former was never significantly different from silent controls for any group in any compartment (HSD, p>0.05). This was also true of the total cellular expression (2-way ANOVA for group and layer; group effect F(3,68)=14.59, p«0.001; layer effect F(2,68)=38.59, p«0.001; pairwise HSD for layers 1–2, p>0.05). Our sound stimulation thus induced layer-specific Arc mRNA expression, with groups that received sound just prior to sacrifice (S2 and S1S2) showing significantly higher expression in layers 3–4 and 5–6 versus 1–2 (Fig. 6A, HSD, p<0.05), in contrast to a lack of layer differences in the silent control (SØ, HSD, p>0.05). Intriguingly, even S1 had significantly elevated layers 5–6 expression compared to layers 1–2, the first indication that prior experience with sound in the anechoic chamber left a long-lasting molecular signature on primary auditory cortex.
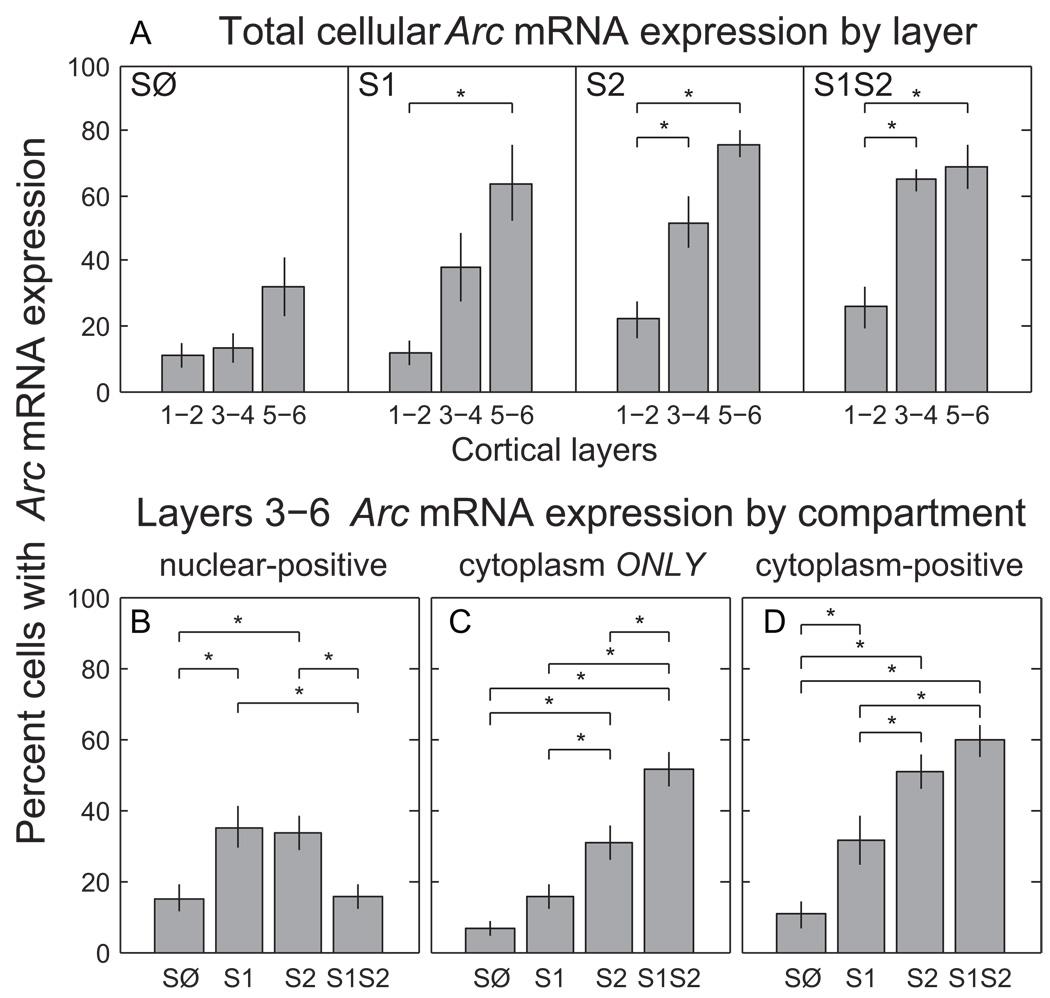
Figure 6.
Arc mRNA expression across behavioral groups, cortical layer and sub-cellular compartments. (A) Average percentage of cells positive for Arc mRNA (in nucleus and/or cytoplasm) in images from cortical layers 1–2, 3–4, and 5–6 for each behavioral group. The difference in layer-dependent expression between SØ and S1 mice, both of which only had silence just before sacrifice, indicates an effect of prior sound exposure. (B–D) Average percentage of Arc mRNA expression in each compartment, was pooled across layers 3–6, according to group. Nuclear-positive (cytoplasm-positive) cells have expression in the nucleus (cytoplasm), but may also have cytoplasmic (nuclear) expression. Cytoplasm ONLY cells express Arc mRNA only in the cytoplasm. Differences between S2 and S1S2 in panels B and C, and SØ and S1 in B and D indicate an effect of prior sound exposure. Asterisks in each graph represent significance by posthoc HSD test (p<0.05) for indicated pairs. Error bars represent standard error.
To investigate this in more detail, we dropped layers 1–2 and pooled layers 3–4 and 5–6 by group for compartmental analysis. This was justified because post-hoc comparisons between them for any matching combination of group and compartment were not different (HSD, p>0.05). Note that the absolute level of expression was on average slightly higher for layers 5–6 compared to 3–4 (noticeable for each group in Fig. 6A, albeit not significant), although the effects of group and compartment appeared to be similar for the two (0.90 correlation coefficient, p«0.001). A 2-way ANOVA on the pooled layers 3–6 data remained significant for group (F(3,147)=12.16, p«0.001), compartment (F(2,147)=22.56, p«0.001) and their interaction (F(6,147)=13.96, p«0.001).
The interaction implies that the pattern of Arc mRNA expression across the animal groups depended on the sub-cellular compartment. The percentage of neurons with Arc mRNA appearing as “intranuclear foci only” was not significantly different between any group (HSD, p>0.05,data not shown) The percentage of neurons labeled as having expression in “both” the nucleus and cytoplasm was also not different between groups, except between S2 and SØ (HSD, p<0.05). Analyzing the overall percentage of nuclear-positive neurons (those with expression in “intranuclear foci only” and “both” compartments) showed a significantly higher level of expression for S2 compared to SØ (Fig. 6B, 1-way ANOVA F(3,49)=5.53, p≤0.005; HSD, p<0.05). In this case, S2 was also greater than S1S2 (Fig. 7, bottom row, cyan asterisks), which was not different from SØ, demonstrating that even though initial (i.e. novel) stimulation increased total intranuclear expression, this evoked response was suppressed after a repeat stimulation 24 h later. Finally, the nuclear-positive percentage for S1 was as large as S2 (HSD, p>0.05), and significantly greater than S1S2 and SØ (HSD, p<0.05), again indicating initial sound exposure affects subsequent sub-cellular processes within primary auditory cortical neurons.
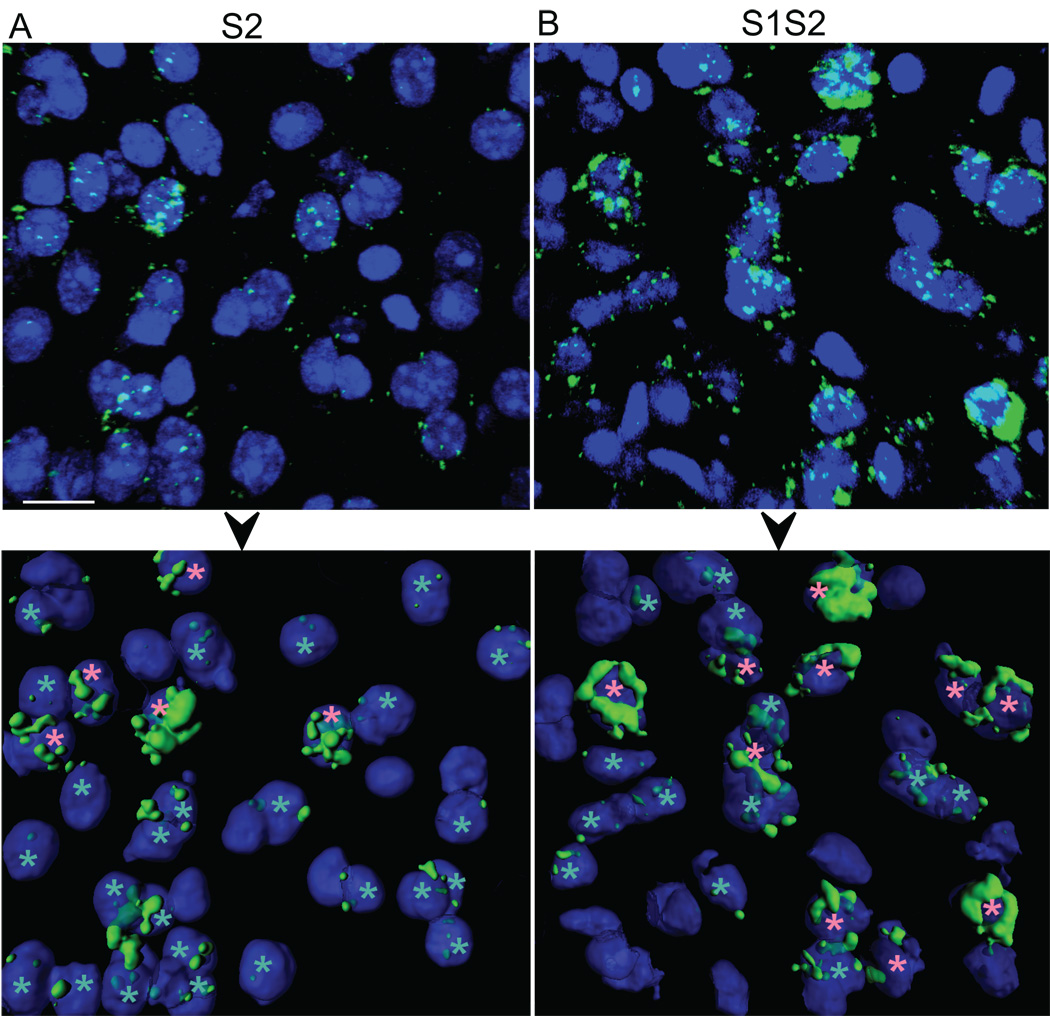
Figure 7.
Examples of sub-cellular compartmental Arc mRNA expression in layers 5–6 for sound-stimulated groups (A) S2 and (B) S1S2. Top panels show raw confocal z-stacks (63X) from tissue sections subjected to FISH. Bottom panels represent the respective 3D surface reconstruction, retaining only probably neuronal (and not glial) cell bodies that are predominantly within the edges of the image. Pink asterisks mark cells with Arc mRNA expression in the perinuclear “cytoplasm only”; cyan asterisks mark those with nuclear-positive Arc mRNA expression (either “intranuclear foci only,” or “both”). Blue represents DAPI staining of nuclei. Scale bar is 15 µm.
In contrast to results for the intranuclear compartment, the percentage of neurons with Arc mRNA expressed in the “cytoplasm only” was highly differentiated across groups (Fig. 6C). In this case, recent sound exposure was needed to evoke sound-induced expression, since S1 was not different from SØ (HSD, p>0.05), but both were significantly less than either S2 or S1S2 (HSD, p<0.05). More importantly, the “cytoplasm only” pattern of compartmental expression was observed significantly more often for S1S2 than S2 (Fig. 6C, HSD, p<0.05; Fig. 7, bottom row, pink asterisks), even though neither the overall cytoplasm-positive percentage (those with expression in “cytoplasm only” or “both” compartments) (Fig. 6D, 1-way ANOVA F(3,49)=17.14, p«0.001; HSD, p>0.05) nor the total cellular mRNA expression (Fig. 6A, HSD, p>0.05) was different between the two. Since the difference between “cytoplasm only” and cytoplasm-positive expression was the proportion of neurons with Arc mRNA expressed in both the nuclear and cytoplasmic compartments, our data suggests the earlier exposure to the sound may change the kinetics and/or detectability of cytoplasmic Arc mRNA expression in primary auditory cortex.
DISCUSSION
We found the history of sound stimulation affects the sub-cellular distribution of an mRNA encoding an important synaptic plasticity effector IEG, Arc, across the layers of the adult mammalian primary auditory cortex. This novel finding demonstrates that compartmental analysis of Arc mRNA, which has been used for neuronal activity mapping in Cellular compartmental Analysis of Temporal activity by FISH (catFISH) paradigms (Guzowski et al., 1999; Ramirez-Amaya et al., 2005; Barot et al., 2008; Marrone et al., 2008), can be a sensitive tool for differentiating the transcription-dependent response of cortical neurons even when using just a single recent stimulus experience (Test 2). Our main discovery was the percentage of layers 3–6 neurons expressing Arc mRNA only in the cytoplasmic compartment depended significantly on whether this recent sound exposure was familiar or novel. This result was unanticipated since sound exposure alone without behavioral contingency does not induce long term receptive field plasticity within the adult primary auditory cortex (Recanzone et al., 1993; Polley et al., 2006), and thus was also not expected to modulate Arc mRNA expression. In fact, an earlier study in rats that assayed only total cellular expression of the transcription factor IEG, c-Fos, found no effect of familiarity in primary auditory cortex (Wan et al., 2001). Consistent with this, we observed that the total cellular Arc mRNA expression was not different between S1S2 and S2 (Fig. 3B). Hence, whereas total cellular expression is independent of the sound stimulation history, a previous sound experience increases a neuron’s capability to accumulate Arc mRNA in the cytoplasm in response to the same sound 24 h later. As discussed below, this Arc mRNA sensitization may prime the molecular machinery underlying synaptic plasticity and facilitate learning if a sound is re-experienced with behavioral contingencies.
Our S1S2 vs. S2 differences might be explained by a combination of two non-exclusive hypotheses. First, prior experience with the sound may have predisposed neurons to have a sound-evoked genomic response (consisting of transcriptional and posttranscriptional events underlying gene induction) early in Test 2, so that after 10 min, much of the Arc mRNA transcribed in the nucleus was already targeted into the cytoplasm. In support of this, nuclear-positive Arc expression for S1 was indeed significantly higher than SØ (Fig. 6B), suggesting that baseline activation of primary auditory cortical neurons during silence was elevated for animals placed back in an environment in which a sound was originally experienced. This presumably occurred throughout both the silent habituation and Test 2 period. The mRNA transcribed earlier during silence may then have been targeted into the cytoplasm, giving rise to a significantly higher cytoplasm-positive (Fig. 6D) and total cellular (not shown for pooled layer 3–6 data, but can be inferred from Fig. 6A) expression for S1 than SØ, even though neither was stimulated with sound just before sacrifice. Repeat stimulation (S1S2) further enhanced the cytoplasmic expression (Figs. 6C–D), yet significantly reduced the nuclear-positive expression (Fig. 6B) compared to S1, which might be attributed to the enhanced processing and/or export of Arc mRNA into the cytoplasm.
However, rather than affect the kinetics of the genomic response in the nucleus, sound familiarity might have instead changed the detectability of Arc mRNA within the cytoplasm, possibly by affecting the distribution of mRNA in the cytoplasm. Arc, like other dendritically-targeted mRNAs, can have their sub-cellular distribution altered by activity (Bramham and Wells, 2007). In response to high frequency stimulation, Arc mRNA can be transported into distal laminae and accumulate at activated synaptic sites (Steward et al., 1998); yet in the presence of NMDA receptor antagonists, Arc mRNA is only diffusely distributed within dendrites and fails to accumulate at discrete laminae (Steward and Worley, 2001). The sorting of Arc mRNA within the somatodendritic compartment in vivo is influenced by numerous signaling pathways, such as Rho kinase, MAP kinase and extracellular signal-regulated kinase, ERK, as well as the actin cytoskeleton (Huang et al., 2007). The precise cellular mechanisms involved in activity-dependent regulation of Arc mRNA localization in the cytoplasm are unclear, but seem to involve the assembly of Arc mRNA molecules into transport granules which traffic from the soma into dendrites of cultured neurons (Dynes and Steward, 2007). Arc RNA granules are heterogeneous in size, suggesting varying amounts of Arc mRNA within each packet. Previous studies on other types of RNA granules in vitro have suggested that they assemble in the soma, and that their size and distribution within the somatodendritic compartment can be regulated by neuronal activity and signaling pathways (Tiruchinapalli et al., 2003; Kiebler and Bassell, 2006). Hence, one possibility is that initial exposure to the sound left low and/or diffuse levels of Arc mRNA in the cytoplasm, rendering it undetectable by our FISH methods until the second stimulation. Existing Arc mRNA could then have redistributed or clustered in the cytoplasm to produce a stronger FISH signal, possibly by stimulating the recruitment of mRNAs into granules and their trafficking throughout the soma and into dendrites (Tiruchinapalli et al., 2003). The assembly and trafficking of mRNAs in granules appears, at least in vitro, to be an essential prerequisite for activity dependent local protein synthesis at synapses. It will therefore be interesting in the future to relate such studies to the present data suggesting differential levels of Arc mRNA redistribution in the cytoplasm in response to novel and familiar experiences.
Arc was previously shown to be necessary for several types of synaptic plasticity and learning and memory (e.g. (Plath et al., 2006; Waung et al., 2008). In particular, recent studies suggest an important role of Arc for synaptic homeostasis and network stability by regulating GluR1 internalization (Chowdhury et al., 2006; Shepherd et al., 2006; Gao et al., 2010; Peebles et al., 2010), for example during visual experience. Here we report that acoustic stimulation induces Arc mRNA expression in an experience- and cell compartment-dependent manner. In the future it will be interesting to assess the importance of Arc expression for auditory plasticity by testing whether auditory learning is impaired in Arc knockout mice (Plath et al., 2006). The necessity of auditory cortical Arc for forming behaviorally-relevant auditory memories could also be tested by blocking Arc transcription (Guzowski et al., 2000) in an operant learning paradigm (Carpenter-Hyland et al., 2010) or in the natural context of acquiring a communication sound’s significance (Liu et al., 2006; Liu and Schreiner, 2007; Galindo-Leon et al., 2009). Furthermore, Arc’s role in network homeostasis suggests that Arc expression must be tightly regulated during synaptic plasticity. Apart from its induction following synaptic activity, the stability and/or translation of the mRNA might also be regulated, for example by specific mRNA-binding proteins. Arc mRNA was shown to associate with FMRP (Zalfa et al., 2005) and to be prone to nonsense-mediated decay (Giorgi et al., 2007). To assess their possible role for sound-induced Arc regulation and auditory experience, it will be interesting to analyze the sound-induced localization of FMRP and other mRNA-binding proteins known to be present in dendritic granules (e.g. Staufen) (Martin and Ephrussi, 2009).
Although finding a sub-cellular Arc mRNA dependence on sound exposure history in primary auditory cortex was unexpected, the modulation of plasticity-related IEGs by stimulus familiarity has been observed in higher-order auditory areas. In rats (Wan et al., 2001) and songbirds (Jarvis et al., 1995; Mello et al., 1995), repeated sound stimulation decreased overall cellular expression of transcriptional regulators c-Fos or zenk (zif268/egr1/ngfi-a/krox-24) in auditory association areas hypothesized to be responsible for recognition memory. In the case of the rat, this area, TE3, receives direct input from TE1 (LeDoux et al., 1991), which encompasses the primary auditory cortical area probed here. Hence, future studies should investigate whether higher order auditory areas might show even larger changes in compartmental expression of Arc. In the case of songbirds, debate continues as to the precise mammalian analog of the relevant area (caudomedial neopallium, NCM) (Jarvis et al., 2005), but it is clearly beyond the main target of auditory thalamic projections (Field L2a). Interestingly, Field L2a does not express zenk or Arc in response to hearing song (Mello et al., 1992; Velho et al., 2005), a striking difference from the Arc mRNA expression in thalamorecipient layers 3–4 of primary auditory cortex that we observed in mice (Fig. 3B). This species difference might be reconciled though if the actual thalmorecipient neurons within layers 3–4 in the mouse are not the ones expressing Arc mRNA.
The cortical layer dependence of Arc expression has previously been studied (Burke et al., 2005; Gusev and Gubin, 2010), but not at the sub-cellular compartmental level. In the auditory cortex, thalamic input from the ventral division of the medial geniculate body mainly forms synapses in layers 3 and 4 (Cruikshank et al., 2002; Winer et al., 2005), thus motivating their combination into a thalamorecipient layer group. This was justified over grouping layer 3 with layer 2, as is often done, because evidence in mice suggests that pyramidal cells in the former are distinct from those in the latter (Oviedo et al., 2010). Instead, to keep our labor-intensive image 3D analysis manageable, we grouped layer 2 with layer 1 as a supragranular layer group, and layer 5 and 6 were combined into an infragranular layer group. The absence of Arc mRNA expression immediately after stimulation in our supragranular layers is consistent with a visual cortex study using Arc-GFP mice that showed a substantially delayed Arc protein response there after visual input, while the thalmorecipient and infragranular layers exhibited a protein response within 30 min, as expected (Wang et al., 2006). The similarity of the Arc response in these two groups despite the former’s role as input and the latter’s role as subcortical output of a cortical column is consistent with the strong interlaminar connection from layers 3 and 4 to layer 5 in the mouse auditory cortex (Llano and Sherman, 2009).
In summary, although analysis of compartmental and temporal Arc mRNA expression has been studied extensively during spatial learning paradigms, our study demonstrates for the first time that a differential compartmental analysis of activity-regulated transcripts can be useful to study the details of neural changes that may underlie auditory cortical synaptic plasticity. Our findings showing a correlation between the layer-specific sub-cellular Arc mRNA distribution and previous sound stimulation will motivate further experiments to analyze the molecular mechanisms underlying synaptic plasticity in the auditory cortex. Finally, we speculate that the subtle, sensory-induced sensitization of Arc mRNA may provide a substrate to bias the participation of specific neurons (Ramirez-Amaya et al., 2005) within the cortical network to store the long-term, distributed memory trace of a sensory event (Sutherland and McNaughton, 2000).
ACKNOWLEDGEMENTS
We thank Edgar Galindo-Leon for experimental assistance, and David Nicholson for comments on a prior version of the paper. This work was supported by NSF CBN IBN-9876754 (RCL) and NIH DC008343 (RCL) and MH085617 (GJB), and by the Neuronal Imaging Core of the Emory Neuroscience NINDS core facility (P30NS055077).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anderson LA, Christianson GB, Linden JF. Mouse auditory cortex differs from visual and somatosensory cortices in the laminar distribution of cytochrome oxidase and acetylcholinesterase. Brain Res. 2009;1252:130–142. doi: 10.1016/j.brainres.2008.11.037. [DOI] [PubMed] [Google Scholar]
- Barot SK, Kyono Y, Clark EW, Bernstein IL. Visualizing stimulus convergence in amygdala neurons during associative learning. Proc Natl Acad Sci U S A. 2008;105:20959–20963. doi: 10.1073/pnas.0808996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Chawla MK, Penner MR, Crowell BE, Worley PF, Barnes CA, McNaughton BL. Differential encoding of behavior and spatial context in deep and superficial layers of the neocortex. Neuron. 2005;45:667–674. doi: 10.1016/j.neuron.2005.01.042. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Plummer TK, Vazdarjanova A, Blake DT. Arc expression and neuroplasticity in primary auditory cortex during initial learning are inversely related to neural activity. Proc Natl Acad Sci U S A. 2010;107:14828–14832. doi: 10.1073/pnas.1008604107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness VS, Jr, Frost DO. Tangential organization of thalamic projections to the neocortex in the mouse. J Comp Neurol. 1980;194:335–367. doi: 10.1002/cne.901940205. [DOI] [PubMed] [Google Scholar]
- Chawla MK, Lin G, Olson K, Vazdarjanova A, Burke SN, McNaughton BL, Worley PF, Guzowski JF, Roysam B, Barnes CA. 3D-catFISH: a system for automated quantitative three-dimensional compartmental analysis of temporal gene transcription activity imaged by fluorescence in situ hybridization. J Neurosci Methods. 2004;139:13–24. doi: 10.1016/j.jneumeth.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruikshank SJ, Rose HJ, Metherate R. Auditory thalamocortical synaptic transmission in vitro. J Neurophysiol. 2002;87:361–384. doi: 10.1152/jn.00549.2001. [DOI] [PubMed] [Google Scholar]
- Dong S, Replogle KL, Hasadsri L, Imai BS, Yau PM, Rodriguez-Zas S, Southey BR, Sweedler JV, Clayton DF. Discrete molecular states in the brain accompany changing responses to a vocal signal. Proc Natl Acad Sci U S A. 2009;106:11364–11369. doi: 10.1073/pnas.0812998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynes JL, Steward O. Dynamics of bidirectional transport of Arc mRNA in neuronal dendrites. J Comp Neurol. 2007;500:433–447. doi: 10.1002/cne.21189. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Does attention play a role in dynamic receptive field adaptation to changing acoustic salience in A1? Hear Res. 2007;229:186–203. doi: 10.1016/j.heares.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galindo-Leon EE, Lin FG, Liu RC. Inhibitory Plasticity in a Lateral Band Improves Cortical Detection of Natural Vocalizations. Neuron. 2009;62:705–716. doi: 10.1016/j.neuron.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK. A specific requirement of Arc/Arg3.1 for visual experience-induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci. 2010;30:7168–7178. doi: 10.1523/JNEUROSCI.1067-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgi C, Yeo GW, Stone ME, Katz DB, Burge C, Turrigiano G, Moore MJ. The EJC factor eIF4AIII modulates synaptic strength and neuronal protein expression. Cell. 2007;130:179–191. doi: 10.1016/j.cell.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Gusev PA, Gubin AN. Arc/Arg3.1 mRNA global expression patterns elicited by memory recall in cerebral cortex differ for remote versus recent spatial memories. Front Integr Neurosci. 2010;4:15. doi: 10.3389/fnint.2010.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Mello CV, Nottebohm F. Associative learning and stimulus novelty influence the song-induced expression of an immediate early gene in the canary forebrain. Learn Mem. 1995;2:62–80. doi: 10.1101/lm.2.2.62. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, et al. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebler MA, Bassell GJ. Neuronal RNA granules: movers and makers. Neuron. 2006;51:685–690. doi: 10.1016/j.neuron.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Pandya PK, Engineer ND, Moucha R. Cortical network reorganization guided by sensory input features. Biol Cybern. 2002;87:333–343. doi: 10.1007/s00422-002-0352-z. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Farb CR, Romanski LM. Overlapping projections to the amygdala and striatum from auditory processing areas of the thalamus and cortex. Neuroscience Letters. 1991;134:139–144. doi: 10.1016/0304-3940(91)90526-y. [DOI] [PubMed] [Google Scholar]
- Lin FG, Liu RC. Subset of thin spike cortical neurons preserve the peripheral encoding of stimulus onsets. J Neurophysiol. 2010 doi: 10.1152/jn.00295.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci U S A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Schreiner CE. Auditory Cortical Detection and Discrimination Correlates with Communicative Significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RC, Linden JF, Schreiner CE. Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci. 2006;23:3087–3097. doi: 10.1111/j.1460-9568.2006.04840.x. [DOI] [PubMed] [Google Scholar]
- Llano DA, Sherman SM. Differences in intrinsic properties and local network connectivity of identified layer 5 and layer 6 adult mouse auditory corticothalamic neurons support a dual corticothalamic projection hypothesis. Cereb Cortex. 2009;19:2810–2826. doi: 10.1093/cercor/bhp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Mahlke C, Wallhäusser-Franke E. Evidence for tinnitus-related plasticity in the auditory and limbic system, demonstrated by arg3.1 and c-fos immunocytochemistry. Hearing Research. 2004;195:17–34. doi: 10.1016/j.heares.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Schaner MJ, McNaughton BL, Worley PF, Barnes CA. Immediate-Early Gene Expression at Rest Recapitulates Recent Experience. J Neurosci. 2008;28:1030–1033. doi: 10.1523/JNEUROSCI.4235-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Ephrussi A. mRNA Localization: Gene Expression in the Spatial Dimension. Cell. 2009;136:719–730. doi: 10.1016/j.cell.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated exposure to one song leads to a rapid and persistent decline in an immediate early gene's response to that song in zebra finch telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Natl Acad Sci U S A. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga DE, Calhoun ME, Chowdhury A, Worley P, Morrison JH, Shapiro ML. Activity-regulated cytoskeletal-associated protein is localized to recently activated excitatory synapses. Neuroscience. 2004;125:7–11. doi: 10.1016/j.neuroscience.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile × syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo HV, Bureau I, Svoboda K, Zador AM. The functional asymmetry of auditory cortex is reflected in the organization of local cortical circuits. Nat Neurosci. 2010;13:1413–1420. doi: 10.1038/nn.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates, Deluxe 2nd Edition. Boston: Elsevier Academic Press; 2001. [Google Scholar]
- Peebles CL, Yoo J, Thwin MT, Palop JJ, Noebels JL, Finkbeiner S. Arc regulates spine morphology and maintains network stability in vivo. Proc Natl Acad Sci U S A. 2010;107:18173–18178. doi: 10.1073/pnas.1006546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinaud R, Osorio C, Alzate O, Jarvis ED. Profiling of experience-regulated proteins in the songbird auditory forebrain using quantitative proteomics. Eur J Neurosci. 2008;27:1409–1422. doi: 10.1111/j.1460-9568.2008.06102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial Exploration-Induced Arc mRNA and Protein Expression: Evidence for Selective, Network-Specific Reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez JJ, Davies HA, Silva AT, De Souza IEJ, Peddie CJ, Colyer FM, Lancashire CL, Fine A, Errington ML, Bliss TVP, Stewart MG. Long-term potentiation in the rat dentate gyrus is associated with enhanced Arc/Arg3.1 protein expression in spines, dendrites and glia. European Journal of Neuroscience. 2005;21:2384–2396. doi: 10.1111/j.1460-9568.2005.04068.x. [DOI] [PubMed] [Google Scholar]
- Sacco T, Sacchetti B. Role of secondary sensory cortices in emotional memory storage and retrieval in rats. Science. 2010;329:649–656. doi: 10.1126/science.1183165. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Worley PF. A cellular mechanism for targeting newly synthesized mRNAs to synaptic sites on dendrites. Proc Natl Acad Sci U S A. 2001;98:7062–7068. doi: 10.1073/pnas.131146398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Stiebler I, Neulist R, Fichtel I, Ehret G. The auditory cortex of the house mouse: left-right differences, tonotopic organization and quantitative analysis of frequency representation. J Comp Physiol [A] 1997;181:559–571. doi: 10.1007/s003590050140. [DOI] [PubMed] [Google Scholar]
- Sun W, Mercado E, 3rd, Wang P, Shan X, Lee TC, Salvi RJ. Changes in NMDA receptor expression in auditory cortex after learning. Neurosci Lett. 2005;374:63–68. doi: 10.1016/j.neulet.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton B. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Current Opinion in Neurobiology. 2000;10:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Tan J, Rüttiger L, Panford-Walsh R, Singer W, Schulze H, Kilian SB, Hadjab S, Zimmermann U, Köpschall I, Rohbock K, Knipper M. Tinnitus behavior and hearing function correlate with the reciprocal expression patterns of BDNF and Arg3.1/arc in auditory neurons following acoustic trauma. Neuroscience. 2007;145:715–726. doi: 10.1016/j.neuroscience.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Tiruchinapalli DM, Oleynikov Y, Kelic S, Shenoy SM, Hartley A, Stanton PK, Singer RH, Bassell GJ. Activity-dependent trafficking and dynamic localization of zipcode binding protein 1 and beta-actin mRNA in dendrites and spines of hippocampal neurons. J Neurosci. 2003;23:3251–3261. doi: 10.1523/JNEUROSCI.23-08-03251.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velho TAF, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. European Journal of Neuroscience. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- Wan H, Warburton EC, Ku015Bmierek P, Aggleton JP, Kowalska DM, Brown MW. Fos imaging reveals differential neuronal activation of areas of rat temporal cortex by novel and familiar sounds. European Journal of Neuroscience. 2001;14:118–124. doi: 10.1046/j.0953-816x.2001.01625.x. [DOI] [PubMed] [Google Scholar]
- Wang KH, Majewska A, Schummers J, Farley B, Hu C, Sur M, Tonegawa S. In vivo two-photon imaging reveals a role of arc in enhancing orientation specificity in visual cortex. Cell. 2006;126:389–402. doi: 10.1016/j.cell.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [see comments] [DOI] [PubMed] [Google Scholar]
- Winer JA, Miller LM, Lee CC, Schreiner CE. Auditory thalamocortical transformation: structure and function. Trends Neurosci. 2005;28:255–263. doi: 10.1016/j.tins.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Adinolfi S, Napoli I, Kuhn-Holsken E, Urlaub H, Achsel T, Pastore A, Bagni C. Fragile × mental retardation protein (FMRP) binds specifically to the brain cytoplasmic RNAs BC1/BC200 via a novel RNA-binding motif. J Biol Chem. 2005;280:33403–33410. doi: 10.1074/jbc.M504286200. [DOI] [PubMed] [Google Scholar]