Abstract
The effects of the polyunsaturated omega-3 (n-3) and omega-6 (n-6) fatty acids (FA) on hematopoiesis are complex in that both FA forms are processed into leukotrienes, eicosanoids, and prostaglandins, which can have independent effects. These FA have antagonistic effects in that n-6 FA prostaglandins tend to be pro-proliferative and pro-inflammatory, while the effects of n-3 FA prostaglandins are the opposite. We have previously shown that diets high in n-3 FA reduce the size of the middle to later stage myeloid progenitor compartment in FVB X sv129 F1hybrid mice. To assay the effects of high n-3 FA diets on earlier stages of myelopoiesis, we fed C57BL/6J mice diets high in n-3 FA or levels of n-3/n-6 FA similar to western diets and assayed the effects on myelopoiesis with flow cytometry and colony forming cell assays. Results indicate an expansion of the common myeloid progenitor cell compartment in high n-3 FA diets, which does not persist into later stages where the number of progenitor cells is actually lower in high n-3 FA fed animals. Investigations in vitro with the hematopoietic stem cell line EML-clone 1 indicate that cells cultured with eicosapentaenoic acid (n-3 FA) or arachidonic acid (n-6 FA) have no differences in cell viability but that arachidonic acid more rapidly produces progenitors with low levels of the macrophage developmental marker, F4/80.
Keywords: Omega fatty acids, EPA, DHA, Immunology, Stem cells, Progenitor cells, Bone marrow, Nutrition
Introduction
The examination of omega-3 (n-3) and omega-6 (n-6) fatty acids (FA) effects on hematopoiesis is important to patient care. The inhibitory effect of n-3 FA on inflammation has led to the use of fish oils that are high in these FA in the management of several inflammatory and autoimmune diseases [1]. n-3 FA affect hematopoietic differentiation by influencing myeloid progenitor cells [2]. n-3 FA are known to affect immune system function by reducing several aspects of neutrophil, monocyte, and lymphocyte function [1]. Suppression of n-6 derived eicosanoids has been proposed as a strategy for chemoprevention and as an adjunct for treatment of cancer [3-6].
n-3 and n-6 FA are incorporated into cell membranes either directly or after elongation and then desaturation by Δ6 and Δ5 desaturases. Dietary linoleic acid (LNA, 18 carbons, n-6 FA) is generally considered to be the major source of tissue arachidonic acid (ARA, 20 carbons, n-6 FA) although meat fat can be a direct source of ARA [7]. n-3 FA have greater affinity for the Δ5 and Δ6 desaturases than n-6 FA. Consequently, increasing dietary intake of n-3 FA reduces the desaturation of LNA and reduces the production of ARA [8]. All three major n-3 FA—a linolenic acid (ALA, 18:3, n-3)—eicosapentaenoic acid (EPA, 20:5, n-3), and docosahexaenoic acid (DHA, 22:6, n-3), directly inhibit the production of ARA from LNA [8].
Both ARA and EPA can be cleaved from the cell membrane phospholipids stores by phospholipase A2 and acted on by cyclooxygenases (either the constitutive COX1 or the inducible COX2) to produce prostaglandin precursors which are isomerized by prostaglandin synthases to produce prostaglandins. COX activity on ARA forms the two-series prostaglandins that tend to be pro-proliferative and pro-inflammatory in most tissues [9]. Micromolar concentrations of prostaglandin E2 increase human myeloid progenitor cell proliferation [2]. However, COX activity on EPA forms the three-series prostaglandins that tend to have anti-proliferative and anti-inflammatory properties [9]. In addition to prostaglandins, leukotrienes and eicosanoids are formed from FA through activity of various lipoxygenases. These have been shown to have varying and sometimes controversial effects on either hematopoietic stem cell or myeloid progenitor cell differentiation [2, 10]. A model system approach is needed to effectively dissect the net effect of dietary fatty acids on hematopoiesis in vivo.
In this study, we examined the effects of n-3 and n-6 fatty acids in vivo in the mouse, with an analysis at the level of stem and progenitor subtypes. Our results indicate that, compared to diets rich in n-6 FA, diets rich in n-3 FA induce lower levels of later stage myeloid progenitor cells in mice, but that there is a higher frequency of the earliest stage myeloid progenitor cells in these mice. Our in vitro results indicate that aspects of the in vivo effects of n-3 and n-6 FA can be modeled using the EML cell culture system to ascertain the mechanisms involved.
Materials and Methods
Animals
Mice were housed in the AAALAC accredited animal facilities of the Marshall Universitiy School of Medicine. All animal use and care was approved by the Marshall University Institutional Animal and Use Committee. The mice were housed 3–4 in a cage and individually numbered for identification. Mice were fed either a fish oil diet (n = 8) or a corn oil diet (n = 8) from 6 weeks of age until 20 weeks of age (100 days on diet).
Diet
The base diet was an AIN-76A diet modified by substitution of 5% sucrose for 5% more oils to contain a total of 10% w/w oil (Tables 1, 2). The fish oil diet contained 3.65% n-3 FA and 1.3% n-6 FA. The corn oil diet contained 0.1% n-3 FA and 6.1% n-6 FA. Diets were prepared in the Marshall University School of Medicine animal diet prep room. Diet composition is shown in Table 1 and was formulated to be isocaloric, isonutrient, and relevant to human consumption. The AIN-76A diet is adequate for the nutritional support of the mice [11]. The dry ingredients of the diet were obtained in bulk from MP Biomedicals (Solon, OH, USA), sugar, corn, and canola oil were purchased locally (100% canola oil, 100% corn oil, no additives or preservatives). The n-3 supplement (OmegaRx Liquid) was purchased from Zone Labs, Danvers, MA. Batches of diet were prepared as needed, about every 2 weeks. The diet mixture was pressed into trays. Food (25–30 g) was stored in sealed containers at −20 °C to prevent oxidation of the fat and bacterial growth in the food. Mice had free access to food and water and were fed fresh food 5 days per week. Food removed from cages was discarded.
Table 1.
Modified AIN-76A diet composition
| Ingredient | Diet composition |
|
|---|---|---|
| wt% | Amount/100 g | |
| Casein (protein) | 20 | 20 |
| Sucrose | 45 | 45 |
| Corn starch (carbs) | 15 | 15 |
| Alphacel (fiber) | 5 | 5 |
| Choline bitartrate | 0.2 | 0.2 |
| DL-Methionine | 0.3 | 0.3 |
| Mineral mix | 3.5 | 3.5 |
| Vitamin mix | 1.0 | 1 |
| Fat | 10 | 10 |
| Total | 100 | 100 |
| Total fat | 10 | |
| Total protein | 20 | |
| Total carbohydrate | 60 | |
The base diet is an AIN-76A diet modified by substitution of 5% sucrose for 5% more oils to contain a total of 10% w/w oil. The mouse food recipe contains 10% fat in each diet supplied through these oils
Table 2.
Compositions of dietary fats (approximate %)
| Saturated FA | Linoleic acid (omega 6) | Total omega 3 | Monounsaturated FA | |
|---|---|---|---|---|
| Corn oila | 13 | 61 | 1 | 26 |
| Canola oila | 6 | 20 | 10 | 62 |
| n-3 suppb | 9 | 6 | 63 | 21 |
The corn oil diet is the low n-3:n-6 FA diet containing 10% w/w corn oil as the source of all fat. The fish oil diet is our high n-3:n-6 FA diet containing 5% w/w canola oil and 5% w/w n-3 FA supplement. The corn oil diet contains 0.1% n-3 FA and 6.1% n-6 FA, which is a relative ratio of n-6 to n-3 of 61:1. The fish oil diet contains 3.65% n-3 FA and 1.3% n-6 FA, which is a relative ratio of 1:2.8
From: manufacturer's certified analyses
Colony Forming Cell (CFC) Assays
CFC assays were performed upon bone marrow isolated from the mice as previously discussed [12, 13]. Briefly, bone marrow was harvested by flushing from the femurs with Iscove's modified Dulbecco's medium and cells were counted and seeded in 4-well plates at a density determined empirically by a pilot study conducted with a broad and consistent range of seeding densities. After linear response of colony production to seeding density was insured by the pilot study, seeding densities were chosen to produce 10–30 colonies for each well (4-well plates). Bone marrow was cultured in 1% semi-solid Methocult M3434 (StemCell Technologies), supplemented with 0.4% autochthonous sera. Cells were incubated for 6–7 days to allow colony formation. Colonies with a minimum cell number of 20 were scored as positive using an inverted microscope at 40× magnification. Colonies were counted based on morphological features that are associated with each progenitor type.
Cell Cycle Analysis
EML cells were seeded at 2 × 105 cells/mL and treated with vehicle, 60 μM ARA (Sigma–Aldrich, cat # A3555), and 60 μM EPA (Sigma–Aldrich, cat # E2011). Cell counts were taken at 24 and 48 h. After 48 h, cells were collected, centrifuged (500g) and washed once with PBS. The cells were then resuspended in PBS (without Ca2+ or Mg2+) and incubated with 70% EtOH for >2 h, at which time they were again centrifuged (500g) and washed once with PBS. The cells were then resuspended in PBS (without Ca2+ or Mg2+) containing 50 μg/mL of propidium iodide and 250 lg/mL of RNase A (both purchased from Sigma, St. Louis, MO, USA) and incubated at 37 °C for 30 min. Cells were then analyzed for fluorescence by flow cytometry on a BD FACSAria.
Flow Cytometry
The preparation of bone marrow for flow cytometry was performed as previously described [12]. EML cells were prepared by washing twice with FACS buffer (PBS supplemented with 0.5% bovine serum albumin and 2 mM EDTA) and collecting by centrifugation. Thereafter, samples of both type were incubated with 2% autochthonous sera obtained from cardiac puncture just before marrow harvest to prevent nonspecific binding by blocking the Fc receptors for half an hour at 4 °C. The cells were washed again and labeled with antibodies for 30 min on ice. The following antibodies were used in the bone marrow studies with Streptavidin-Pacific Blue to detect biotinylated antibodies: PE-Cy7 conjugated Sca-1 (clone D7, eBiosciences #25-5981-81), APC-eFluor 750 conjugated CD117 (clone 2B8, eBiosciences #47-1171-80), biotinylated lineage panel (BD Biosciences #559971), biotinylated IL-7Rα (clone B12-1, BD Biosciences #555288), APC conjugated FCRγ (clone 93, eBiosciences #17-0161-81), and PE conjugated CD34 (clone RAM34, BD Biosciences 551387). The following antibodies were used in labeling the EML-clone1 cells in labeling pairs with Streptavidin-APC (BD Biosciences #554067) to detect biotinylated antibodies: biotinylated Ly6G/C (clone RB6-8CS, BD Biosciences #553125) + PE conjugated CD117 (clone 2B8, BD Biosciences #553355), biotinylated CD11b (clone M1/70, BD Biosciences #553309) + PE conjugated Sca-1 (clone D7, BD Biosciences #553108), and biotinylated CD45 (clone RA3-6B2, BD Biosciences #553086) + PE conjugated F4/80 (clone BM8, Caltag #MF48004). Data acquisition was performed using BD FACS Aria I sorter and data analysis/compensation was performed using FlowJo v. 7.6 software (Treestar, Ashland, OR, USA) with super-enhanced Dmax subtraction analysis for determination of differences in histograms.
In Vitro Culture and Differentiation
EML C1 cells were the kind gift of Dr. Schickwann Tsai and were maintained in Iscove's modified Dulbecco medium (IMDM,) supplemented with 20% horse serum (American Type culture collection, ATCC, Manassas, VA, USA) and 10% BHK/MKL-conditioned medium [14]. For differentiation studies, EML cells were induced to differentiate into myeloid cells with 10 μM all-trans retinoic acid (ATRA; sigma, St. Louis, MO, USA), 10% BHK conditioned medium (source of stem cell factor) and 15% WEHI conditioned medium (source of interleukin-3a) for 3 days. EML-clone1 cells were seeded at 2 × 105 cells/mL and cultured for 24 h in 60 μM FA (same formulations as stated previously) in standard growth medium (20% horse serum, 70% Dulbecco's modified eagle medium, and 10% BHK conditioned medium). After 24 h cells were placed in differentiation medium with 60 μM FA at 2.0 × 105 cells/mL. Cell counts were performed using trypan blue at 24, 48, 72, and 96 h. After 96 h cells were processed for flow cytometry analysis.
FA Metabolism Studies
Cells were seeded at 2 × 105 cells/mL and treated with vehicle or 60 μM FA (same formulations as stated previously). Cell counts were performed at 24, 48, 72, and 96 h using trypan blue. At each time point, half the total volume of the culture was taken as a sample and replaced with untreated growth media. Each sample was centrifuged (500g), the supernatant removed and the pellet store at −20 °C until analyzed by gas chromatography.
At the time of gas chromatography, cells were homogenized in 0.1% butylated hydroxytoluene in 70% methanol/distilled water to prevent FA oxidation. Lipids were extracted with chloroform/methanol and methylated. Methylated lipids were separated and identified using gas chromatography as previously published [15]. FA methyl ester standards (Nu-Chek-Prep, Elysian, MN, USA) were used for peak identification. The FA methyl esters were reported as the percent of the total methylated FA (area under the curve).
Statistical Analysis
Statistical analyses were run using SAS software release 9.2 (SAS Institute Inc. Cary, NC, USA). Student's t tests were used to detect differences between the experimental groups (corn versus fish diet) of colony forming cells (n = 8 for each group) and between the experimental groups (corn vs. fish diet) in flow cytometry assays (n = 8 for each group). To determine statistical significance in gas chromatography experiments and differentiation studies the levels of fatty acids of a particular type were analyzed using either one-way analysis of variance (ANOVA) or by one-way Kruskal–Wallis analysis of variance on ranks. Dunnett multiple comparison tests were used for testing if any treatments are significantly different from a single control for all main effects means in the MEANS statement.
Results
Fish Oil Diets Induce Changes in the Frequency of Various Myeloid Progenitor Cell Types in the Bone Marrow
In our previous investigations of the effect of fish oil diets on murine hematopoiesis, we found a down-regulation in the frequency of myeloid progenitor cells in bone marrow in mice fed fish oil diets compared to those on corn oil diets [16]. Other investigators have reported that bone marrow of rodents readily changes composition based upon dietary FA sources [17]. However, our investigations using colony forming cell assays limited our ability to assay the frequency of more immature cell types, such as the common myeloid progenitor (CMP) and hematopoietic stem cell (HSC). In order to assay the effects of high n-3 FA diets on these cell types, we verified that the effects seen in hybrid F1 animals were also seen in C57BL/6 mice where flow cytometry markers for examining these rare cell types have been verified [18]. In the experiments presented here, mice were fed diets rich in n-3 FA (fish oil diet) or rich in n-6 FA (corn oil diet) from 6 weeks of age until 20 weeks of age (Tables 1, 2, 100 days on diet). Bone marrow was then harvested and analyzed by colony forming cell assay and flow cytometry. There was no difference in density of cells in the bone marrow (Fig. 1a). Our results (Fig. 1b) indicated an overall lower frequency of mid to late stage progenitors when mice were fed n-3 FA rich diets compared to those that were fed diets rich in n-6 FA rich diets. There was a significantly lower (26%, p < 0.01) overall myeloid progenitor cell frequency, with significant reductions (p < 0.05) in colony forming unit granulocyte-macrophage (CFU-GM) and colony forming unit macrophage (CFU-M).
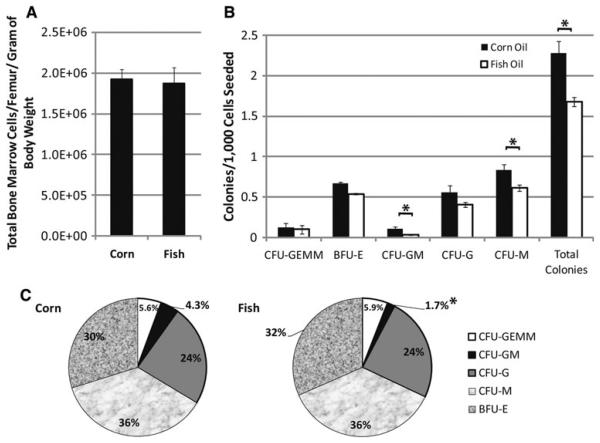
Fig. 1.
Reduction in middle to latter stage myeloid progenitor cell types by high n-3 diet. Mice were fed either corn oil or fish oil diets for x days followed by harvesting of bone marrow from femurs. The number of cells harvested from the mice on the different diets were similar (a). Colony forming cell assays were performed to enumerate the frequency of middle to latter stage progenitor types. The frequency of each progenitor cell type (b) and the relative distribution of progenitor types (c) in the total progenitors assayed are shown. Significant differences as measured by Student's t tests (p<0.05) are indicated by asterisk. Error bars represent SEM (n = 8). SEMs in c for the corn oil diet data are BFU-E, 2.4%, CFU-GEMM, 0.8%, CFU-GM, 1.1%, CFU-G, 2.7%, CFU-M, 1.8% and for the fish oil diet are BFU-E, 2.4%, CFU-GEMM, 0.6%, CFU-GM, 0.5%, CFU-G, 2.1%, CFU-M, 2.1%. CFU colony forming unit, BFU blast forming unit, GEMM granulocyte erythrocyte monocyte macrophage, E erythrocyte, GM granulocyte monocyte, G granulocyte, and M macrophage
When we examined the relative contributions of each progenitor cell type to the overall myeloid progenitor cell pool in the bone marrow (Fig. 1c), we found that there is a significant reduction in CFU-GM proportion in mice on the fish oil diet (p<0.05). The comparison of proportions of subtypes of myeloid progenitors is different than what we observed in FVB X sv129 F1 hybrid mice [16], where we observed an overall shift to more later stage progenitor types [16]. Thus, though there was a reduction in myeloid progenitor cell frequencies in the marrow and a twofold reduction in proportion of CFU-GM, there was little shift in proportions of the other progenitor subtypes. This may be due to particular characteristics of the C57BL/6 inbred strain that are not seen in F1 hybrid animals, due to the lessening of homozygous recessive mutations in hybrid animals. We have shown that mice vary significantly in myeloid progenitor cell frequencies in a previous publication [12]. These data indicate that C57BL/6 mice responded to high n-3 FA diets with a reduction in overall myeloid progenitors cell frequencies, and significantly altering the proportions of the CFU-GM present.
The Frequency of the Common Myeloid Progenitor Fraction is Increased in Mice Fed Fish Oil Diets
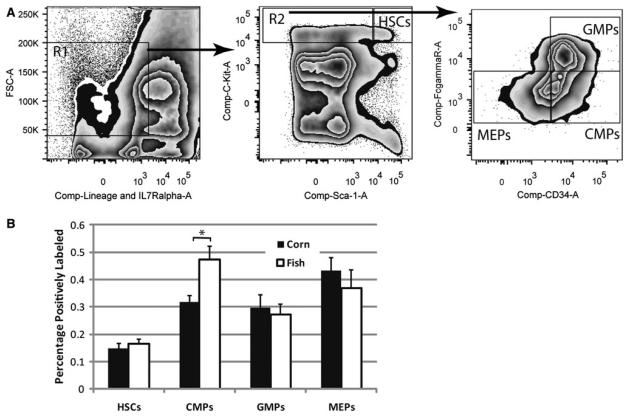
In order to assay the frequencies of earlier stage stem and progenitors cells in mice fed corn and fish oil diets, flow cytometry studies were conducted using established gating parameters for the assay of HSC, CMP, granulocyte–macrophage progenitors (GMP), and megakaryocyte–erythrocyte progenitors (HSC) in C57BL/6 mice[18] (Fig. 2a). Bone marrow was first gated by excluding high and low forward scatter cells along with those labeled positively for one of the differentiation antigens in the lineage panel or IL-7Rα, which marks lymphoid cells. These FSCmidIL-7Rα−Lin− were further separated into stem (FSCmidIL-7Rα−Lin−Sca-1+c-Kit+) and progenitor (FSCmidIL-7Rα−Lin−Sca-1−c-Kit+) cell fractions by expression of c-Kit and Sca-1 as shown. The progenitor pool was also separated based upon the expression of CD34 and FcγR to delineate CMP (FSCmidIL-7Rα−Lin−Sca-1+ c-Kit+FcγRloCD34+), GMP (FSCmidIL-7Rα−Lin−Sca-1+ c-Kit+FcγRhiCD34+), and HSC (FSCmidIL-7Rα−Lin-Sca-1+c-Kit+FcγRloCD34−). These data show a 50% increase (p = 0.01) in the frequency of the CMP fraction in mice fed fish oil diets (Fig. 2b). There were no significant differences in frequency of HSC, GMP, or MEP fractions indicated in these studies. These data suggest that the frequency of the CMP has been increased by administration of a diet containing high levels of n-3 FA.
Fig. 2.
Increase in early myeloid progenitors by high n-3 diets. Mice were fed either corn oil or fish oil diets for x days followed by harvesting of bone marrow from femurs. Bone marrow was analyzed by flow cytometry first by FSC X lineage panel + IL7Ra, then by C-kit and Sca-1 expression, followed by FcγR X CD34 expression to differentiate between various stem or progenitor cell types (a). The percentage of the total marrow cells labeling positive for each fraction are shown (b). Significant differences as measured by Student's t tests (p<0.05) are indicated by asterisk. Error bars represent SEM (n = 8). HSC hematopoietic stem cells, CMP common myeloid progenitors, GMP granulocyte macrophage progenitors, and HSC megakaryocyte erythrocyte progenitors
FA Applied to EML Cell Culture Medium are Incorporated and Processed
In order to study the observed effects of n-3 versus n-6 FA on early myeloid progenitor cells in more detail, we examined the effects of the polyunsaturated fatty acids EPA and ARA on EML cells in culture. We chose to compare these two FA because EPA (20:5, n-3) is the fatty acid most molecularly similar to ARA (20 carbons, n-6 FA) available. DHAn-3 (22:6, n-3) has two more carbons in the chain. The EML cell line is a stem cell factor dependent multipotent cell line with erythroid (E), myeloid (M), and lymphoid (L) potential. It was established from DBA/2 mouse bone marrow infected with a retroviral vector (LRARα403SN) harboring a dominant negative retinoic acid receptor [14]. It is a suspension cell line consisting of mostly blast like cells with 20–30% hand mirror shaped cells. EML cells serve as an excellent model to study hematopoietic differentiation. It can be induced to differentiate towards granulocyte/monocyte progenitors by high concentration of all-trans retinoic acid (ATRA) in the presence of interleukin-3 (IL-3).
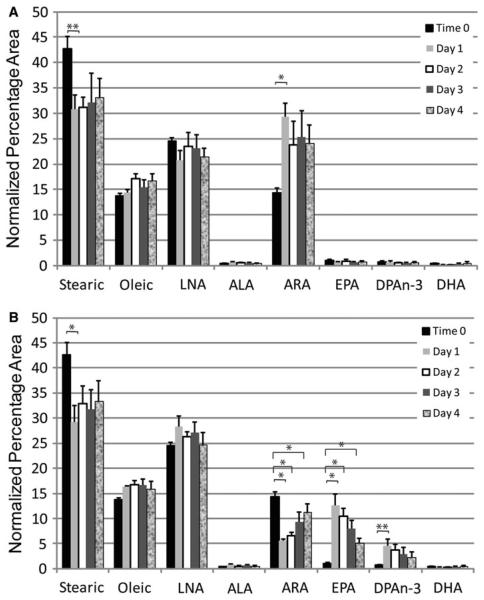
To verify the incorporation and processing by EML cells of FA placed in the culture medium, we spiked the culture medium with 60 μM ARA (Fig. 3a) or 60 μM EPA (Fig. 3b) and analyzed the levels of various FA present in the cells over the course of 4 days. Cells were analyzed with gas chromatography daily to determine the uptake and processing of FA. The major FA in untreated EML cell membranes are steric acid, oleic acid, LNA, and ARA (time 0). There are only faint traces of n-3 FA such as ALA, EPA, docosapentaenoic acid (DPA), and DHA in EML cell membranes under normal culturing conditions (time 0). In both cultures, the percentage of the major constituent of cell membranes (steric acid) is reduced as this is replaced by either ARA or EPA (Fig. 3a, b, 24 h). Thus, FA supplied in culture medium were being incorporated into cellular membranes and this occurs within 24 h. Supplementation of ARA in the medium caused an increase in the already substantial levels of ARA in cellular membranes (Fig. 3a). There was a significant (p < 0.05) two-fold increase in ARA at 24 h that drops to a 66% increase at 48 h and was maintained for the remainder of the 4 days. The addition of EPA to the culture medium caused a significant (p<0.001) increase in the n-3 FA EPA and elevated levels (p value significant before correction for multiple comparisons) of the EPA elongation metabolite DPA, with a corresponding significant (p<0.001) decrease in ARA by 24 h (Fig. 3b). Interestingly, there was no increase in DHA, which is the Δ4 desaturase metabolite of DPA, indicating EML cells do not have this enzymatic activity. Thus, addition of FA to the cell medium resulted in incorporation of these FA into cellular membranes and processing of these FA by 24 h.
Fig. 3.
EML cells take up and metabolize FA. EML cells were placed in growth medium containing 60 lM arachidonic (a) or eicosapentaenoic (b) acid. The uptake and processing of these FA was assayed over the course of 4 days. Samples were taken just before addition of the FA (t = 0), 24, 48, 72, and 96 h for analysis by gas chromatography. The gas chromatography data for each FA is shown above for the five data points in temporal order. Data displayed are the results of three independent experiments with the mean and SEM displayed. LNA linoleic acid, ALA alpha-linolenic acid, ARA arachidonic acid, EPA eicosapentaenoic acid, DPA docosapentaenoic acid, and DHAn-3 docosahexaenoic acid. * indicate statistically significant (p<0.05) differences based on one-way ANOVA test or Kruskal–Wallis test with Dunnett multiple comparisons adjustment. ** indicate statistically significant (p<0.05) differences based on Student's t test or Mann–Whitney rank sum test without multiple comparison adjustment
Incubation of EML Cells with FA has Effects on Immunophenotype and Cell Viability
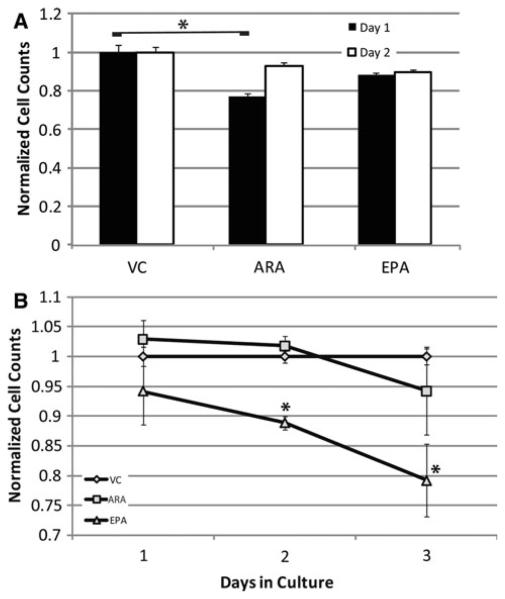
To determine if the effects of n-3 versus n-6 FA seen in our in vivo experiment are due to viability/proliferation differences induced by the FA, we analyzed the effect of n-3 and n-6 FA on EML cells in culture (Fig. 4a) for 2 days, since we have shown the greatest levels of incorporation of fatty acids supplied through the culture medium occur at 24 h according to the gas chromatography. EML cells in their native state are a model for hematopoietic stem cells. We found that cultures treated with 60 μM ARA had significantly reduced (p<0.05) cell counts after 1 day compared to the vehicle control. EPA treated cultures had slightly lower cell counts at day one, but the difference was not statistically significant. Neither of the treated cultures had statistically significant differences from the control at day 2, indicating that the effects of treatment with ARA were transitory. These results are consistent with the in vivo results, where we saw no difference in the stem cell compartment of fish oil (high n-3) versus corn oil (high n-6) fed mice.
Fig. 4.
In vitro culture of EML cells shows a slight increased viability in n-3 versus n-6 augmented cultures. EML cells in standard culture were augmented with either vehicle control, arachidonic acid (n-6), or eicosapentaenoic acid (n-3) for 48 h with cell counts performed at 24 and 48 h to determine viability (a). EML cells were cultured in either vehicle control, arachidonic acid (n-6), or eicosapentaenoic acid (n-3) for 24 h then induced to differentiate for another 72 h down myeloid lineages (b). Data displayed are the results of two (a) or three (b) independent experiments with the mean and SEM displayed of culture counts normalized to the vehicle control. * indicate statistically significant (p<0.05) difference based on one-way ANOVA test with Dunnett multiple comparisons adjustment
To observe cell autonomous effects of n-3 versus n-6 FA on cell viability and proliferation during the GMP myeloid progenitor cell differentiation stage we primed EML cells for 24 h with 60 μM EPA or ARA, then induced differentiation into macrophage/granulocytic lineages with ATRA, stem cell factor (SCF), and IL-3. After 72 h in the differentiation medium, cell counts were performed on these EML cell cultures. Viability/proliferation was measured by trypan blue exclusion (Fig. 4b). The viability of the cell cultures was very similar with the various treatments at each stage with a fairly consistent viability as the cultures differentiated (measured by non-stained cells/total cells, data not presented). All cultures were proliferating robustly with a doubling time starting at 18 h and shortening as differentiation proceeded (data not presented). The major finding of this study was a significant reduction (p<0.05) in cell counts in the EPA treatment group at days 2 (11% reduction) and 3 (21% reduction) with a suggestive reduction at day 1 (Fig. 4b). There was no reduction in viability of the cultures at these time points, suggesting the difference was due to cell proliferation. These results are consistent with our in vivo results that show a higher frequency of middle-later stage myeloid progenitors with high n-6 diets.
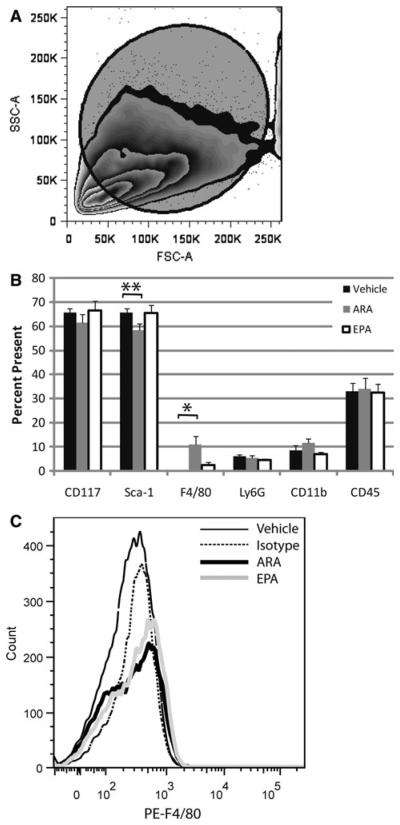
To observe cell autonomous effects of n-3 versus n-6 FA on myeloid progenitor cell differentiation we primed EML cells for 24 h with 60 μM EPA or ARA, then induced differentiation into macrophage/granulocytic lineages with ATRA, stem cell factor (SCF), and IL-3. After 72 h in the differentiation medium, EML cells were immunophenotyped for the early markers Sca-1 and CD117, as well as the differentiation markers Ly6G, CD11b, CD45, and F4/80 (Fig. 5b). There were no significant decreases in Sca-1 levels (the first sign of differentiation in this model) with treatment, though the decrease with ARA treatment is suggestive (p value significant before correction for multiple comparisons, but not after the appropriate Dunnett multiple comparison correction) when comparing treatments to vehicle control. There was significant (p<0.05) difference in the percentage of cells labeling with the F4/80 antigen in the ARA treatment group compared to the vehicle control. The sizeable increase in F4/80 positive cells coupled with the suggestive decrease for the Sca-1 antigen in the treatments with ARA compared to vehicle (Fig. 5b) indicates the production of a granulocyte/macrophage progenitor population with ARA treatment. These two results are consistent in that there is a lower frequency of the marker for stem cells and an increase in frequency of the differentiation marker F4/80. This result is also consistent with the in vivo data that found an increase in production of granulocyte and macrophage progenitor subtypes at later stages. The expression of F4/80 appears to be at a low abundance on a per cell basis in both ARA and EPA treated samples, but much more prevalent in ARA treated samples (Fig. 5c). Thus, our model suggests that the differences seen in the CMP compartment seen in our in vivo experiment are not due to changes in rate of differentiation in the bone marrow and that n-6 FA supplementation produces a shift to macrophage/granulocytic lineages as seen in vivo.
Fig. 5.
No significant differences in myeloid progenitor cell maturation rate between n-3 and n-6 FA. EML cells were cultured in either vehicle control, arachidonic acid (n-6), or eicosapentaenoic acid (n-3) for 24 h then induced to differentiate for another 72 h down myeloid lineages. Flow cytometry was performed analyzing the larger FSC population of non-apoptotic cells (a). The percentage of cells displaying various markers of differentiation are shown for each culture type (b). Representative histograms are displayed for the isotype control (black, narrow line), vehicle control (dotted line), EPA treated cultures (gray line), and ARA treated cultures (black, thick line)(c). Both fatty acid treated culture display a shift over to low level expression (a small increase in fluorescence) of the antigen, but the ARA treated cultures show a greater shift (more cells present in the higher fluorescence channels). Data represents the results from five independent experiments with the mean and SEM displayed. * indicates statistically significant (p<0.05) difference based on one-way Kruskal–Wallis analysis of variance on ranks followed by Dunnett test. ** indicates statistically significant (p<0.05) difference based on Student's t test without multiple comparison adjustment
Discussion
Our primary objective in this study was to determine if the reduction of later myeloid progenitor subtypes by diets high in n-3 FA seen previously [12] was the continuation of differences induced at earlier stages of myeloid progenitor development. The data from these experiments indicate that this is not the case. The effect of lowering the frequency of myeloid progenitors of the later stages in mice fed n-3 FA rich diet was recapitulated in the present study, but we did not see a lowering of the stem/early progenitor cell fractions in the bone marrow. Conversely, we observed an increase in the common myeloid progenitor cell fraction with flow cytometry. Thus, the effects of n-3 FA on stem/progenitor cell biology in bone marrow are not a simple lowering of the overall frequency of these cell types. Determination of the exact mechanism of n-3 FA effects will entail studies at multiple levels of maturation in bone marrow. Possible mechanisms include effects through metabolites such as leukotrienes, resolvins, incorporation into cell signaling molecules such as hedgehog, or production of prostaglandins. Our data suggest that these mechanisms are eventually triggering a slowed differentiation between the early and middle myeloid progenitor phases resulting in an increase in early progenitors and a decrease in later stage progenitors in high n-3 diets. Understanding the mechanisms involved will enable targeted therapies to hematopoietic disorders involving problematic differentiation.
Our in vivo studies indicate that diets rich in n-3 FA when compared to n-6 FA have an effect of increasing the frequency of CMP, but no effect on HSC (Fig. 2). The changes seen in early stem/progenitor cell biology are then reversed, in that diets rich in n-6 FA produce more later stage progenitor cells of the granulocyte and macrophage lineage (Fig. 1). The results of our stem cell culture model found no lasting effects on stem cell viability or proliferation when cultured in n-3 versus n-6 fatty acids (Fig. 4a), similar to that seen in vivo. It is interesting that we did not see an increase in HSC with the corn oil diet containing high n-6 FA levels considering the recent results indicating one of the products of n-6 FA, prostaglandin E2, can increase HSC production in vivo [19-22]. This may be due to the complex metabolism of FA and use of them in biomolecules or the genetics of the C57BL/6 model. We found a low level expression of F4/80 antigen upon treatment with ARA treatment, characteristic of macrophage progenitor development [23-26], consistent with these progenitors being greater in vivo in corn oil fed mice compared to fish oil fed mice (Fig. 1). No other significant changes were seen in differentiation state upon treatment with ARA or EPA in comparison to vehicle controls. We observed a lower proliferation rate during differentiation of EML cells while cultured in EPA compared to vehicle controls that was not seen with ARA treatment. This is consistent with an overall lowering of middle-later stage myeloid progenitor cell numbers in mice fed fish oil diets relative to corn oil diets (Fig. 1). An important consideration in extrapolating these in vitro experiments to our in vivo results is that the EML cell model only takes into account cell autonomous factors. The stem cell niche is an important part of stem cell biology and this is not recapitulated in this model.
These data indicate that the effect of n-3 FA in the diet of mice on reducing later stage myeloid progenitors is not a simple reduction of HSC in the bone marrow relative to n-6 FA fed mice. n-3 FA appear to have a diverse effect depending upon the stage of progenitor cell development. Understanding the mechanisms involved will allow a more targeted approach to using n-3 FA clinically in differentiation therapies. We are currently investigating whether the use of n-3 fatty acids as a mechanism of induced differentiation is applicable to reduction of the progression of chronic myelogenous leukemia (CML) to the lethal blast crisis phase in a murine model as well as clinical trials with B-cell malignancies ([27] and unpublished results).
Acknowledgments
This work was supported by NIH grants 1R03 CA129790 to VES, 1R01 CA114018 to WEH, COBRE (5P20RR020180) to Richard M. Niles, and the WV-INBRE Program (5P20RR016477) to Gary O. Rankin, as well as with support from the NASA WV Space Grant Consortium issued by NASA Goddard Space Flight Center, Grant Number NNG05GF80H. We would like to acknowledge John Wilkinson IV for assistance in discussion of results.
Abbreviations
- n-3
Omega-3
- n-6
Omega-6
- ARA
Arachidonic acid
- ALA
α Linolenic acid
- AIN-76A diet
American institute of nutrition 76A diet
- APC
Allophycocyanin
- ATRA
all-trans Retinoic acid
- BHK
Baby hamster kidney
- CFC
Colony forming cell(s)
- CFU-GM
Colony forming unit granulocyte–macrophage
- CFU-M
Colony forming unit macrophage
- CMP
Common myeloid progenitor(s)
- COX
Cyclooxygenase
- DHAn-3
Docosahexaenoic acid
- DPA
Docosapentaenoic acid
- EDTA
Ethylenediaminetetraacetic acid
- EML
Erythroid myeloid lymphoid
- EPA
Eicosapentaenoic acid
- FA
Fatty acid(s)
- FCRγ
Fragment crystallizable receptor γ
- FSC
Forward scatter
- GMP
Granulocyte-macrophage progenitor(s)
- HSC
Hematopoietic stem cell(s)
- IL-3
Interleukin-3
- IL-7Rα
Interleukin-7 receptor α
- LNA
Linoleic acid
- MEP
Megakaryocyte–erythrocyte progenitor
- PE
Phycoerythrin
- PBS
Phosphate-buffered saline
- SCF
Stem cell factor
- SSC
Side scatter
References
- 1.Kelley DS. Modulation of human immune and inflammatory responses by dietary fatty acids. Nutrition. 2001;17(7–8):669–673. doi: 10.1016/s0899-9007(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 2.Dupuis F, et al. Effects of lipidic mediators on the growth of human myeloid and erythroid marrow progenitors. J Lipid Mediat Cell Signal. 1997;16(3):117–125. doi: 10.1016/s0929-7855(97)00007-2. [DOI] [PubMed] [Google Scholar]
- 3.Germain E, et al. Dietary n-3 polyunsaturated fatty acids and oxidants increase rat mammary tumor sensitivity to epirubicin without change in cardiac toxicity. Lipids. 1999;34(Suppl):S203. doi: 10.1007/BF02562290. [DOI] [PubMed] [Google Scholar]
- 4.Hardman WE, et al. Effects of iron supplementation and ET-18-OCH3 on MDA-MB 231 breast carcinomas in nude mice consuming a fish oil diet. Br J Cancer. 1997;76(3):347–354. doi: 10.1038/bjc.1997.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karmali RA. Historical perspective and potential use of n-3 fatty acids in therapy of cancer cachexia. Nutrition. 1996;12(1 Suppl):S2–S4. doi: 10.1016/0899-9007(96)90008-8. [DOI] [PubMed] [Google Scholar]
- 6.Oberley LW, Spitz DR. Assay of superoxide dismutase activity in tumor tissue. Methods Enzymol. 1984;105:457–464. doi: 10.1016/s0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- 7.Li D, et al. Contribution of meat fat to dietary arachidonic acid. Lipids. 1998;33(4):437–440. doi: 10.1007/s11745-998-0225-7. [DOI] [PubMed] [Google Scholar]
- 8.Hagve TA, Christophersen BO. Effect of dietary fats on arachidonic acid and eicosapentaenoic acid biosynthesis and conversion to C22 fatty acids in isolated rat liver cells. Biochim Biophys Acta. 1984;796(2):205–217. doi: 10.1016/0005-2760(84)90349-7. [DOI] [PubMed] [Google Scholar]
- 9.Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83(3):217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- 10.Rizzo MT. The role of arachidonic acid in normal and malignant hematopoiesis. Prostaglandins Leukot Essent Fat Acids. 2002;66(1):57–69. doi: 10.1054/plef.2001.0331. [DOI] [PubMed] [Google Scholar]
- 11.Report of the American Institute of Nutrition ad hoc committee on standards for nutritional studies. J Nutr. 1977;107(7):1340–1348. doi: 10.1093/jn/107.7.1340. [DOI] [PubMed] [Google Scholar]
- 12.Sollars VE, et al. Analysis of expansion of myeloid progenitors in mice to identify leukemic susceptibility genes. Mamm Genome. 2006;17(8):808–821. doi: 10.1007/s00335-006-0017-7. [DOI] [PubMed] [Google Scholar]
- 13.Miller CL, Lai B. In: Human and mouse hematopoietic colony-forming cell assays, in basic cell culture protocols. Helgason CD, Miller, editors. Humana Press Inc; Totowa, NJ: 2005. pp. 71–90. [DOI] [PubMed] [Google Scholar]
- 14.Tsai S, et al. Lymphohematopoietic progenitors immortalized by a retroviral vector harboring a dominant-negative retinoic acid receptor can recapitulate lymphoid, myeloid, and erythroid development. Genes Dev. 1994;8(23):2831–2841. doi: 10.1101/gad.8.23.2831. [DOI] [PubMed] [Google Scholar]
- 15.Hardman WE, Ion G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr Cancer. 2008;60(5):666–674. doi: 10.1080/01635580802065302. [DOI] [PubMed] [Google Scholar]
- 16.Varney ME, Hardman WE, Sollars VE. Omega 3 fatty acids reduce myeloid progenitor cell frequency in the bone marrow of mice and promote progenitor cell differentiation. Lipids Health Dis. 2009;8:9. doi: 10.1186/1476-511X-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson TG, Barker HJ, Meckling-Gill KA. Incorporation of long-chain n-3 fatty acids in tissues and enhanced bone marrow cellularity with docosahexaenoic acid feeding in post-weanling Fischer 344 rats. Lipids. 1997;32(3):293–302. doi: 10.1007/s11745-997-0036-x. [DOI] [PubMed] [Google Scholar]
- 18.Akashi K, et al. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404(6774):193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 19.Frisch BJ, et al. In vivo prostaglandin E2 treatment alters the bone marrow microenvironment and preferentially expands short-term hematopoietic stem cells. Blood. 2009;114(19):4054–4063. doi: 10.1182/blood-2009-03-205823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoggatt J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113(22):5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goessling W, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell. 2009;136(6):1136–1147. doi: 10.1016/j.cell.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lord AM, North TE, Zon LI. Prostaglandin E2: making more of your marrow. Cell Cycle. 2007;6(24):3054–3057. doi: 10.4161/cc.6.24.5129. [DOI] [PubMed] [Google Scholar]
- 23.Gordon S, et al. Localization and function of tissue macrophages. Ciba Found Symp. 1986;118:54–67. doi: 10.1002/9780470720998.ch5. [DOI] [PubMed] [Google Scholar]
- 24.McKnight AJ, et al. Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J Biol Chem. 1996;271(1):486–489. doi: 10.1074/jbc.271.1.486. [DOI] [PubMed] [Google Scholar]
- 25.Torroella-Kouri M, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69(11):4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, et al. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28(3):620–632. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Witte TR, et al. RBC and WBC fatty acid composition following consumption of an omega 3 supplement: lessons for future clinical trials. Lipids Health Dis. 2010;9:31. doi: 10.1186/1476-511X-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]