Abstract
We tested hypotheses that disproportionately large placental size and vascular lesions were associated with high systolic blood pressure (SBP); and these associations might be more evident with age. The sample included 13,273 out of 40,666 full-term singletons in the Collaborative Perinatal Project. Placentas were examined by pathologists blinded of pregnancy courses and outcomes. The 4-month and 7-year SBP were measured with palpation and auscultation methods, respectively. We found that placental weight (adjusted mean difference corresponding to an increase by 1 standard deviation, 0.50 [95% confidence interval, 0.33 to 0.68]) and placenta-fetus weight ratio (0.37 [95% CI, 0.19 to 0.54]) was positively associated with 7-year SBP, but not associated with 4-month SBP. Placental largest and smallest diameters, and area were negatively associated with 4-month SBP, but positively with 7-year SBP. Placental thickness was negatively associated with 4-month SBP only. Placental volume was negatively associated with 4-month SBP (−0.60 [95% CI, −0.85 to −0.35]), but positively associated with 7-year SBP (0.48 [95% CI, 0.30 to 0.67]). Thrombi in cord vessels (adjusted mean difference vs absence, 2.73 [95% CI, −0.03 to 5.50]) and decidual vessels (2.58 [95% CI, 0.24 to 4.91]), villous microinfarcts (1.63 [95% CI, 0.71 to 2.55]), necrosis at the decidual margin (1.57 [95% CI, 0.54 to 2.59]) and basalis (3.44 [95% CI, 1.55 to 5.32]) were associated with higher 4-month SBP only. We conclude that placental inefficiency, reflected by disproportionately large weight and size, predicts long-term blood pressure, while vascular resistance and lesions may only influence short-term blood pressure.
Keywords: placenta, placental insufficiency, placental circulation, fetal development, blood pressure
BACKGROUND
As the main organ supplying nutrients, oxygen, and hormones to the fetus, the placenta can be a key to understand fetal programming of blood pressure.1–3 Placental efficiency refers to the ability of the placenta to extract and transfer nutrients and oxygen from the mother to the fetus. It is commonly defined as the grams of fetus that can be supported by each gram of placenta,4 and simply calculated as the ratio between fetus and placenta weight.5, 6 However, previous studies examining the associations between placenta-fetus weight ratio and offspring blood pressure have not reached consistency: some found positive,6–8 but others found no associations.9, 10 One possible reason is that intrauterine environmental insults may either constrain or stimulate placental growth11, and both placental restricted growth and overgrowth can initiate hypertension in offspring.12
Other placental size measures, such as placental area and thickness, provide more valuable information for placental efficiency and growth. First, they mark two different dimensions of placental growth: area reflects lateral spreading/expansion of the anchoring villi, while thickness indicates vertical arborization of the villous tree.5, 12 Second, they can proximately reflect different timing of intrauterine environment sufficiency. Placental area growth is almost completed by early third trimester whereas placental thickness growth mainly occurs in late third trimester.5 Third, they may be directly linked to the burden of the fetal cardiovascular system including cardiac workload and haemodynamic burden.13 However, little is known about the association between placental area or thickness and offspring blood pressure. Only two studies12, 14 have tried to examine these associations and reported inconsistent results for placental area: positive association with blood pressure in one study12 but null in the other.14 The first study12 also found that placental thickness (derived from weight/area) was not associated with adult hypertension.
Placental vascular pathological lesions, such as thrombus, infarct, necrosis, and hemorrhage, may be indicators of low uterus-placenta or fetus-placenta blood flow15 and early vascular impairments. These vascular pathological lesions themselves can lead to further reduction in placental blood flow, and also induce high vascular resistance. Animal experiments suggest that insufficient placental blood flow leads to increased fibrosis in the heart and kidneys, increased aortic wall thickening, and reduced number of kidney glomeruli in adolescent offspring.16, 17 On the other hand, the high vascular resistance can increase fetal cardiac workload and haemodynamic burden,13 and thus induce temporary and possibly permanent changes in cardiovascular physiology and function. But no human studies have examined the associations between placental vascular pathological lesions and offspring blood pressure.
Repeatedly measuring offspring blood pressure since birth can help to better understand the role of the placenta on programming blood pressure, especially to distinguish placental disorders with short- and long-term effects on offspring blood pressure. For example, infancy blood pressure can be a good marker for some short-term effects of intrauterine environments (e.g. the placenta),18 while blood pressure in later life may reflect the latent or long-term effects of the placenta.
Therefore, we had two aims in this analysis: 1) to examine the associations between detailed placental morphology measures (size and vascular lesions) and childhood systolic blood pressure (SBP); 2) to examine whether the observed associations differed in infancy and middle childhood. We hypothesized that, disproportionately large placental size (relative to birth weight) and vascular lesions were associated with high SBP; and these associations should be more evident with age.
METHODS
Study sample
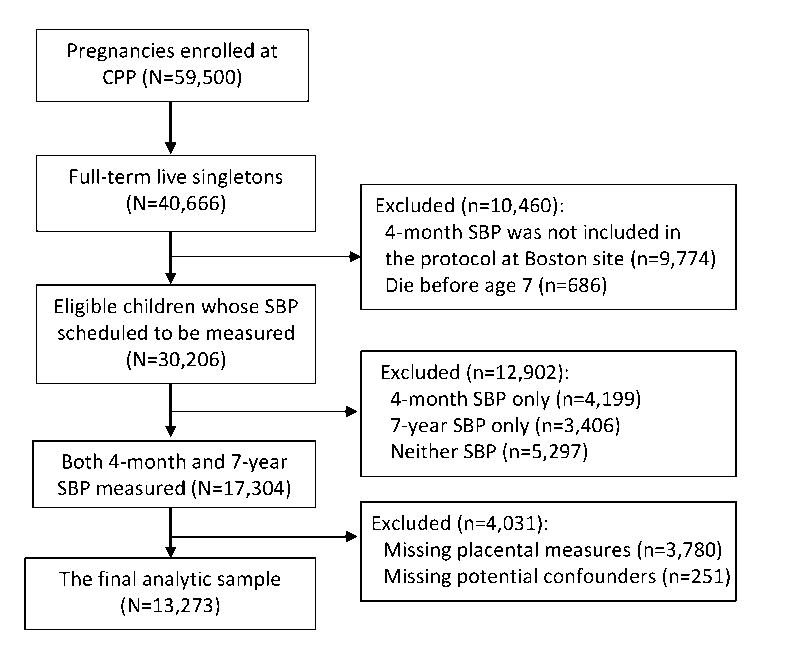
We used the data from the Collaborative Perinatal Project (CPP), a cohort study conducted in 12 cities throughout the United States.19, 20 About 59,500 pregnancies were enrolled at prenatal care (mostly in 2nd trimester) between 1959 and 1965. Approximately 58,000 live born infants were followed up until 8 years of age and assessed for health status periodically. For the purpose of this analysis, we only included 13,273 full-term singletons who had complete data on placental measures of interest, childhood systolic blood pressure, and potential confounders. Figure 1 shows the flow chart of the analytic sample.
Figure 1. Flow chart of the analytic sample.
SBP, systolic blood pressure; CPP, the Collaborative Perinatal Project.
Outcome measures
In CPP, data on systolic blood pressure (SBP) of the child was available at 4-month and 7-year follow-ups. SBP was measured once by physicians or nurses with the palpation method at 4 months of age and the auscultation method (manual sphygmomanometer) at the 7 years of age.21, 22 Blood pressure was obtained from the right arm with the child at rest for 10 to 15 minutes in a recumbent position. Approximately 2/3 of the upper arm was covered with the blood pressure cuff with appropriate size (at least a 4-inch cuff for 7-year SBP). All infants were awake when blood pressure was measured. Blood pressure was not measured if the child could not be put in a resting state (e.g. crying, excited, or apprehensive).
Exposure measures
Placentas were collected, prepared, and examined according to a standard protocol “Examination of the placenta”.23 Pathologists who examined placentas were blinded of pregnancy courses and outcomes. In this analysis, we focused on the following placental size measures and vascular pathological lesions.
Placental size
Placental size measures were obtained from gross examination completed in the delivery room. Briefly, the placenta without trimming of the cord, membranes, and large clots was weighted and recorded in grams. The largest and smallest diameters were measured and recorded in centimeters. The thickness was measured at the center of the placental tissue by piercing it with a knitting needle or similar object calibrated in centimeters. Based on these original measures, several other measures were derived with well-established mathematic formulas:12, 24, 25 placenta-fetus weight ratio=placental weight (g)/birth weight (g); placental surface area= π × the smallest diameter (cm) × the largest diameter (cm)/4; placental volume=surface area (cm2) × thickness (cm); and placental density=placental weight (g)/volume (cm3).
Vascular pathological lesions
Selected measures (e.g. number, location, and size) of placental vascular pathological lesions were obtained from both gross and microscopic examination. Thrombi were classified according to affected locations: the thrombi in vessels (umbilical cord, fetal, and decidual vessels; Yes/No) and intervillous thrombi (the number: 0, 1, and ≥2). Infarct measures included the infarcts at cutsurface (the number: 0, 1, 2, and ≥3) and villous microinfarcts (Yes/No). Necrosis measures included necrosis at decidual basalis and margin (Yes/No). Measures for retroplacental hemorrhage included the binary classification (Yes/No) and the shortest distance (<1cm and ≥1cm) from hemorrhage edge to placental margin.
Covariate measures
Birth weight was measured immediately after delivery and recorded in grams. Gestational age was defined as the interval between the last menstrual period and delivery date. Full-term was defined as the gestational age between 37 and 42 complete weeks. Potential confounders included family socio-economic status (SES) percentile; maternal age at pregnancy, race (white, black, and others), marital status (married vs unmarried), parity (primiparity vs multiparity), chronic hypertension, and preeclampsia-eclampsia; the child’s sex and gestational age; and the study site. Family SES percentile was based on a composite index adapted from the United States Bureau of the Census that averaged percentiles of family income as well as the household head’s education and occupation.26 We obtained the diagnoses of chronic hypertension, preeclampsia, and eclampsia from obstetric forms of CPP. Obstetricians made these diagnoses according to the American Committee on Maternal Welfare classification of toxemia published in 1952.27
Statistical analysis
Pearson correlation coefficients were used to assess the correlations among different placental size measures. Adjusted mean differences in SBP and their 95% confidence intervals were estimated from regression models. Given that each child was measured for SBP at two different ages (4 months and 7 years), we fit multivariable linear regression models with generalized estimate equations (GEEs).
Based on Q-Q plot and Kolmogorov-Smirnov test, blood pressure and most placental size measures were normally distributed, except the placental thickness which was often conventionally recorded as rounded numbers (1.5, 2, 2.5, or 3.0 cm) although its distribution was still symmetric. We derived the percentiles for each placental size measure within the analytic sample, correcting for sex,28 gestational age,28, 29 and delivery method (vaginal vs cesarean).29 Quintiles of placental size measures were used to examine their potential non-linear associations (e.g. U-shape3) with childhood SBP. Trend tests were conducted by including quintiles as continuous variables in regression models. If the trend test was significant, the linear association was then assessed by using z-score of the placental size measure which was calculated as (individual value – group mean)/standard deviation. Given the multicollinearity between placental size measures, each regression model included one of them and potential confounders. To assess placental efficiency (placental size relative to birth weight),5, 6 we fit two sets of models for placental size measures with or without adjusting birth weight, and then compared the estimated associations in the two sets of models. For placental pathological lesion measures, we included them simultaneously and potential confounders in the regression models. All of the analysis was completed in SAS version 9.1 (SAS Institute Inc. Cary, NC).
RESULTS
Sample characteristics
Table 1 shows maternal, offspring, and placental characteristics in the final analytic sample (N=13,273) and full eligible sample of full-term singletons (N=30,206, see the sample definition in Figure 1). Overall, there were no substantial differences in most characteristics between these two samples. Among children in the final analytic sample, 50.8% were boys, mean gestational age was 39.7 weeks, mean birth weight was 3,217 g, and mean placental weight was 437 g. Mean SBP was 85.6 mmHg (standard deviation, 15.5) and 101.2 mmHg (standard deviation, 9.7) at 4-month and 7-year follow-up, respectively. Pearson correlation coefficient between 4-month and 7-year SBP was 0.04.
Table 1.
Characteristics of mothers, offspring, and placentas
| Characteristic | Full eligible sample* (N=30,206) | Final analytic sample (N=13,273) |
|---|---|---|
| Mother | ||
| Pregnancy age (years), mean (SD) | 23.9 (6.0) | 24.3 (6.1) |
| Race, % | ||
| White | 11,008 (36.4) | 4,854 (36.6) |
| Black | 16,547 (54.8) | 7,650 (57.6) |
| Others | 2,651 (8.8) | 769 (5.8) |
| Marital status, % | ||
| Unmarried | 7,931 (26.3) | 3,266 (24.6) |
| Married | 22,275 (73.7) | 10,007 (75.4) |
| Family SES percentile, mean (SD) | 43.7 (21.0) | 45.6 (21.9) |
| Parity, % | ||
| Primiparity | 8,256 (27.5) | 3,624 (27.3) |
| Multiparity | 21,795 (72.5) | 9,649 (72.7) |
| Chronic hypertension, % | 1,102 (3.7) | 541 (4.1) |
| Preeclampsia-eclampsia, % | 5,396 (17.9) | 2,594 (19.5) |
| Offspring | ||
| Sex, % | ||
| Male | 15,164 (50.3) | 6,739 (50.8) |
| Female | 14,990 (49.7) | 6,534 (49.2) |
| Gestational age (weeks), mean (SD) | 39.7 (1.4) | 39.7 (1.4) |
| Birth weight (g), mean (SD) | 3,202 (474) | 3,217 (472) |
| SBP (mmHg), mean (SD) | ||
| At 4 months of age | 86.1 (16.3) | 85.6 (15.5) |
| At 7 years of age | 101.4 (10.2) | 101.2 (9.7) |
| Placenta, mean (SD) | ||
| Placental weight (g) | 439 (93) | 437 (93) |
| Placental largest diameter (cm) | 18.9 (2.2) | 18.9 (2.2) |
| Placental smallest diameter (cm) | 16.5 (2.0) | 16.5 (1.9) |
| Placental area (cm2) | 248 (51) | 247 (51) |
| Placental thickness (cm) | 2.1 (0.5) | 2.1 (0.5) |
| Placental volume (cm3) | 529 (164) | 529 (164) |
| Placental density (g/cm3) | 0.9 (0.4) | 0.9 (0.4) |
SD, standard deviation; SBP, systolic blood pressure; SES, socio-economic status.
See Figure 1 for the sample definition. The sum of categories might be not equal to the total because of missing data.
Correlation between placental size measures
Table 2 shows the correlation matrix for placental size measures. Most pairwise correlations were in strong (|r| ≥0.5) or moderate (0.3 ≤|r| <0.5) range. However, there was no substantial correlation (|r| <0.1) between placental thickness and largest diameter (r=−0.04), smallest diameter (r=0.02), or area (r=−0.01); and between placental density and placental weight (r=0.05) or placenta-fetus weight ratio (r=0.09).
Table 2.
Pearson correlation matrix for placental size measures*
| Placental size measure | Placental weight | Placenta-fetus weight ratio | Largest diameter | Smallest diameter | Area | Thickness | Volume | Density |
|---|---|---|---|---|---|---|---|---|
| Placental weight | 1 | |||||||
| Placenta-fetus weight ratio | 0.70 | 1 | ||||||
| Largest diameter | 0.50 | 0.28 | 1 | |||||
| Smallest diameter | 0.50 | 0.26 | 0.55 | 1 | ||||
| Area | 0.56 | 0.30 | 0.87 | 0.88 | 1 | |||
| Thickness | 0.32 | 0.22 | −0.04 | 0.02 | −0.01 | 1 | ||
| Volume | 0.62 | 0.37 | 0.55 | 0.61 | 0.66 | 0.73 | 1 | |
| Density | 0.05 | 0.09 | −0.11 | −0.23 | −0.19 | −0.46 | −0.45 | 1 |
Strong (|r| ≥ 0.5) or moderate (0.3 ≤ |r| <0.5) correlations are shown in bold.
P-value<0.05 for all pairwise correlation coefficients.
Associations between placental size measures and SBP
Table 3 shows adjusted mean difference in SBP across quintiles and z-scores of placental size measures. Placental weight was not associated with 4-month SBP, but positively associated with the mean of 7-year SBP (adjusted mean difference corresponding to an increase by 1 standard deviation, 0.50 [95% CI, 0.33, 0.68]). Placenta-fetus weight ratio was also positively associated with 7-year SBP only (0.37 [95% CI, 0.19 to 0.54]). Both placental largest and smallest diameters were negatively associated with 4-month SBP, but positively with 7-year SBP. Similarly, placenta area was negatively associated with 4-month SBP (−0.45 [95% CI, −0.70 to −0.20]), but positively with 7-year SBP (0.59 [95% CI, 0.41 to 0.78]). Placental thickness was negatively associated with 4-month SBP only (−0.40 [−0.65 to −0.15]). Placental volume was negatively associated with 4-month SBP (−0.60 [95% CI, −0.85 to −0.35]) but positively associated with 7-year SBP (0.48 [95% CI, 0.30 to 0.67]). Placental density was positively associated with 4-month SBP only. Overall, adjustment for birth weight did not change directions of the above associations; rather, it augmented the magnitude of the associations of placental size measures with 4-month SBP, whereas attenuated their associations with 7-year SBP.
Table 3.
Associations between placental size measures and childhood systolic blood pressure
| At 4 months of age |
At 7 years of age |
||||
|---|---|---|---|---|---|
| Placental size measure | n | Adjusted mean difference (95% CI)* | Adjusted mean difference (95% CI)† | Adjusted mean difference (95% CI)* | Adjusted mean difference (95% CI)† |
| Placental weight | |||||
| 1 to 20th percentile | 2,747 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 2,721 | 1.45 (0.69,2.21) | 1.33 (0.57,2.10) | 0.44 (−0.09,0.97) | 0.33 (−0.21,0.86) |
| 41 to 60th percentile | 2,570 | 0.18 (−0.59,0.95) | 0.00 (−0.78,0.78) | 0.78 (0.24,1.32) | 0.60 (0.04,1.16) |
| 61 to 80th percentile | 2,682 | 0.78 (0.03,1.53) | 0.54 (−0.24,1.32) | 0.97 (0.43,1.51) | 0.73 (0.16,1.30) |
| 81 to 100th percentile | 2,553 | 0.24 (−0.53,1.02) | −0.12 (−0.95,0.71) | 1.40 (0.85,1.95) | 1.03 (0.41,1.66) |
| Increase by 1 SD | --‡ | --‡ | 0.50 (0.33,0.68) | 0.37 (0.16,0.58) | |
| Placenta-fetus weight ratio | |||||
| 1 to 20th percentile | 2,667 | Reference | Reference | ||
| 21 to 40th percentile | 2,660 | 0.51 (−0.27,1.28) | -- | 0.12 (−0.42,0.66) | -- |
| 41 to 60th percentile | 2,648 | 0.12 (−0.66,0.90) | -- | 0.50 (−0.04,1.04) | -- |
| 61 to 80th percentile | 2,656 | −0.45 (−1.22,0.33) | -- | 1.07 (0.52,1.61) | -- |
| 81 to 100th percentile | 2,642 | −0.13 (−0.90,0.64) | -- | 0.88 (0.33,1.42) | -- |
| Increase by 1 SD | --‡ | -- | 0.37 (0.19,0.54) | -- | |
| Largest diameter | |||||
| 1 to 20th percentile | 3,844 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 2,759 | 0.48 (−0.22,1.18) | 0.36 (−0.35,1.06) | −0.18 (−0.67,0.30) | −0.31 (−0.79,0.18) |
| 41 to 60th percentile | 2,491 | 0.05 (−0.69,0.78) | −0.13 (−0.87,0.61) | 0.52 (0.01,1.02) | 0.34 (−0.18,0.86) |
| 61 to 80th percentile | 2,184 | −0.24 (−1.00,0.53) | −0.48 (−1.26,0.29) | 0.84 (0.30,1.38) | 0.60 (0.04,1.15) |
| 81 to 100th percentile | 1,995 | −1.12 (−1.90, −0.34) | −1.43 (−2.22, −0.64) | 1.52 (0.95,2.09) | 1.21 (0.62,1.81) |
| Increase by 1 SD | −0.37 (−0.61, −0.12) | −0.48 (−0.73, −0.23) | 0.52 (0.35,0.70) | 0.41 (0.22,0.60) | |
| Smallest diameter | |||||
| 1 to 20th percentile | 3,979 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 2,791 | 0.32 (−0.37,1.01) | 0.20 (−0.49,0.89) | 0.03 (−0.44,0.51) | −0.09 (−0.57,0.39) |
| 41 to 60th percentile | 2,646 | 0.56 (−0.15,1.27) | 0.38 (−0.34,1.10) | 0.44 (−0.05,0.94) | 0.26 (−0.25,0.77) |
| 61 to 80th percentile | 2,051 | 0.00 (−0.78,0.78) | −0.25 (−1.04,0.53) | 0.84 (0.29,1.38) | 0.58 (0.02,1.15) |
| 81 to 100th percentile | 1,806 | −1.25 (−2.05, −0.44) | −1.62 (−2.44, −0.79) | 1.21 (0.61,1.81) | 0.84 (0.21,1.47) |
| Increase by 1 SD | −0.37 (−0.61, −0.12) | −0.49 (−0.75, −0.24) | 0.49 (0.31,0.67) | 0.36 (0.17,0.56) | |
| Area | |||||
| 1 to 20th percentile | 2,852 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 2,823 | 0.27 (−0.47,1.01) | 0.14 (−0.61,0.88) | 0.04 (−0.47,0.56) | −0.09 (−0.61,0.43) |
| 41 to 60th percentile | 2,562 | 0.06 (−0.70,0.82) | −0.15 (−0.92,0.62) | 0.33 (−0.20,0.86) | 0.13 (−0.41,0.67) |
| 61 to 80th percentile | 2,561 | 0.22 (−0.55,0.98) | −0.07 (−0.85,0.71) | 0.98 (0.44,1.52) | 0.69 (0.13,1.25) |
| 81 to 100th percentile | 2,475 | −1.20 (−1.98, −0.42) | −1.60 (−2.40, −0.79) | 1.36 (0.79,1.92) | 0.96 (0.36,1.57) |
| Increase by 1 SD | −0.45 (−0.70, −0.20) | −0.59 (−0.85, −0.33) | 0.59 (0.41,0.78) | 0.45 (0.25,0.65) | |
| Thickness | |||||
| 1 to 20th percentile | 3,012 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 4,483 | −1.29 (−1.97, −0.61) | −1.34 (−2.03, −0.66) | −0.05 (−0.54,0.43) | −0.11 (−0.59,0.38) |
| 41 to 60th percentile | 765 | −2.27 (−3.43, −1.11) | −2.36 (−3.53, −1.20) | 1.52 (0.68,2.36) | 1.42 (0.59,2.26) |
| 61 to 80th percentile | 3,016 | −1.40 (−2.16, −0.65) | −1.52 (−2.28, −0.76) | 0.46 (−0.10,1.02) | 0.34 (−0.22,0.90) |
| 81 to 100th percentile | 1,997 | −1.69 (−2.51, −0.88) | −1.87 (−2.69, −1.05) | 0.60 (−0.01,1.21) | 0.42 (−0.19,1.04) |
| Increase by 1 SD¶ | −0.40 (−0.65, −0.15) | −0.46 (−0.72, −0.21) | 0.13 (−0.06,0.31) | 0.07 (−0.12,0.26) | |
| Volume | |||||
| 1 to 20th percentile | 2,691 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 2,745 | −0.70 (−1.46,0.07) | −0.83 (−1.59, −0.06) | 0.04 (−0.50,0.57) | −0.09 (−0.63,0.45) |
| 41 to 60th percentile | 2,622 | −1.18 (−1.96, −0.40) | −1.40 (−2.19, −0.61) | 0.53 (0.00,1.07) | 0.31 (−0.23,0.86) |
| 61 to 80th percentile | 2,612 | −1.27 (−2.06, −0.49) | −1.59 (−2.38, −0.79) | 1.26 (0.70,1.82) | 0.94 (0.37,1.52) |
| 81 to 100th percentile | 2,603 | −1.73 (−2.51, −0.95) | −2.18 (−2.98, −1.38) | 1.31 (0.74,1.89) | 0.86 (0.25,1.47) |
| Increase by 1 SD | −0.60 (−0.85, −0.35) | −0.77 (−1.03, −0.51) | 0.48 (0.30,0.67) | 0.32 (0.12,0.52) | |
| Density | |||||
| 1 to 20th percentile | 2,665 | Reference | Reference | Reference | Reference |
| 21 to 40th percentile | 2,660 | 0.74 (−0.02,1.51) | 0.75 (−0.02,1.51) | 0.21 (−0.34,0.76) | 0.21 (−0.34,0.76) |
| 41 to 60th percentile | 2,652 | 1.39 (0.61,2.17) | 1.39 (0.60,2.17) | −0.29 (−0.85,0.28) | −0.29 (−0.86,0.27) |
| 61 to 80th percentile | 2,661 | 2.12 (1.34,2.90) | 2.12 (1.33,2.90) | −1.11 (−1.68, −0.54) | −1.11 (−1.68, −0.54) |
| 81 to 100th percentile | 2,635 | 2.40 (1.62,3.18) | 2.41 (1.63,3.19) | −0.34 (−0.92,0.24) | −0.33 (−0.91,0.25) |
| Increase by 1 SD | 0.54 (0.27,0.81) | 0.54 (0.27,0.81) | 0.08 (−0.07,0.22) | 0.08 (−0.07,0.22) | |
SD, standard deviation.
Adjusted for family socio-economic percentile, maternal characteristics (age at pregnancy, race, marital status, parity, chronic hypertension, and preeclampsia-eclampsia), the child's sex and gestational age, and the study site.
Additionally adjusted for birth weight.
P-value for the trend test >0.05.
This should be interpreted with caution because the recorded placental thickness was not normally distributed.
Associations between placental vascular pathological lesions and SBP
Table 4 shows adjusted mean differences in childhood SBP by placental vascular pathological lesions. Thrombi in cord vessels (adjusted mean difference vs absence, 2.73 [95% confidence interval, −0.03 to 5.50]) and decidual vessels (2.58 [95% CI, 0.24 to 4.91]) were associated with higher 4-month SBP. Villous microinfarcts (1.63 [95% CI, 0.71 to 2.55]), necrosis at the decidual margin (1.57 [95% CI, 0.54 to 2.59]) and basalis (3.44 [95% CI, 1.55 to 5.32]) were also associated with higher 4-month SBP. Thrombi in fetal vessels (−2.11 [95% CI, −3.68 to −0.53]) were associated with lower 7-year SBP. Intervillous thrombi, cutsurface infarcts, and retroplacental hemorrhage were not associated with either 4-month or 7-year SBP.
Table 4.
Associations between placental vascular pathological lesions and childhood systolic blood pressure
| At 4 months of age |
At 7 years of age |
||
|---|---|---|---|
| Placental vascular pathological lesion | n | Adjusted mean difference (95% CI)* | Adjusted mean difference (95% CI)* |
| Thrombus | |||
| Thrombi in cord vessels | |||
| No | 13,164 | Reference | Reference |
| Yes | 109 | 2.73 (−0.03,5.50) | −1.76 (−3.79,0.26) |
| Thrombi in fetal vessels | |||
| No | 13,113 | Reference | Reference |
| Yes | 160 | 0.25 (−1.88,2.39) | −2.11 (−3.68, −0.53) |
| Number of ntervillous thrombi | |||
| 0 | 11,958 | Reference | Reference |
| 1 | 802 | −0.27 (−1.31,0.77) | 0.50 (−0.17,1.17) |
| ≥2 | 513 | 0.57 (−0.64,1.78) | 0.28 (−0.57,1.14) |
| Thrombi in decidual vessels | |||
| No | 13,106 | Reference | Reference |
| Yes | 167 | 2.58 (0.24,4.91) | −0.14 (−1.87,1.59) |
| Infarct | |||
| Number of cutsurface infarcts | |||
| 0 | 10,875 | Reference | Reference |
| 1 | 1,479 | −0.12 (−0.90,0.66) | 0.53 (−0.01,1.07) |
| 2 | 484 | −0.72 (−1.93,0.48) | 0.66 (−0.23,1.55) |
| ≥3 | 435 | 0.85 (−0.51,2.21) | 0.01 (−0.93,0.94) |
| Villous microinfarcts | |||
| No | 11,910 | Reference | Reference |
| Yes | 1,363 | 1.63 (0.71,2.55) | −0.22 (−0.90,0.45) |
| Necrosis | |||
| Decidual necrosis, margin | |||
| No | 12,225 | Reference | Reference |
| Yes | 1,048 | 1.57 (0.54,2.59) | −0.15 (−0.85,0.55) |
| Decidual necrosis, basalis | |||
| No | 13,003 | Reference | Reference |
| Yes | 270 | 3.44 (1.55,5.32) | 0.48 (−0.86,1.81) |
| Hemorrhage | |||
| Retroplacental hemorrhage | |||
| No | 12,933 | Reference | Reference |
| Yes, distance to margin ≥ <1cm | 232 | 0.04 (−1.94,2.02) | −0.69 (−2.07,0.69) |
| Yes, distance to margin 1cm | 108 | 1.45 (−1.50,4.39) | 0.56 (−1.24,2.36) |
Adjusted for family socio-economic percentile, maternal characteristics (age at pregnancy, race, marital status, parity, chronic hypertension, and preeclampsia-eclampsia), the child's sex and gestational age, and the study site.
DISCUSSION
Summary of results
In a national prospective cohort, we examined the associations of placental morphology with infancy (4-month) and middle childhood (7-year) SBP. We found that placental weight and placenta-fetus weight ratio were only associated with middle childhood SBP; large placental size (i.e. diameters, area, and volume) was associated with lower infancy SBP but higher middle childhood SBP; placental vascular pathological lesions were only associated with high infancy SBP. These associations could be explained by placental inefficiency and vascular resistance.
Placental weight, size and efficiency
Placental inefficiency has been hypothesized to predict high blood pressure in offspring.30 This was supported by our finding that high placental weight and placenta-fetus weight ratio predicted higher middle childhood SBP. But we did not find their associations with infancy SBP, which suggests that placental weight may have some latent link to offspring SBP that is undetectable at infancy. But whether this is a causal or non-causal link remains unclear, because some genetics, maternal factors (e.g. nutrition, stress, smoking, hypoxemia, and anemia), and fetal exposures (e.g. excessive glucocorticoid and hypoxia) may influence both placental weight and long-term SBP.3, 31, 32 Alternatively, placental insufficiency may also be the mediator for these predisposed factors. In addition, intrauterine insults can either constrain or stimulate placental growth, depending on their timing and severity as well as maternal nutritional status.12, 33 Thus, using non-invasive methods (e.g. ultrasound) to monitor placental development throughout pregnancy can contribute to better understanding how placental weight is related to long-term SBP.
Existing evidence regarding the association between placental area and offspring blood pressure is inconsistent. One previous study12 found that the smallest diameter of the placenta was negatively associated with the risk of hypertension among adult offspring (mean age 62 years) of shorter mother (height ≤ 160cm), whereas the largest diameter was independent of adult hypertension. Another study did not find any association between placental area and blood pressure in adults aged 50 years.14 Our own findings supported the link between placental area and childhood SBP. The largest and smallest diameters were similarly associated with childhood SBP. Interestingly, these two diameters and area were negatively associated with infancy SBP but positively with middle childhood SBP. Our explanation for this paradox is that a greater placental diameter or area presents lower placental vascular resistance5 which is associated with lower short-term SBP, but a greater diameter or area relative to fetal size also marks placental inefficiency which predicts higher long-term SBP.30
We found that a too thin placenta was associated with high infancy SBP. Too thin placentas usually have inadequate branching or arborization of the villous tree, and thus insufficient exchange surface for oxygen and nutrients.5 However, in line with a previous study,12 we did not find any association between placental thickness and middle childhood SBP. So, placental thickness does not seem to play an important role in programming long-term SBP. Alternatively, this null association may be due to that the errors (conventional rounding) of our thickness measure outweigh the modest but meaningful difference.
As a summary measure of area and thickness, placental volume had dose-response associations with offspring SBP in our sample. Unexpectedly, the association direction was negative for infancy SBP but positive for middle childhood SBP. This paradox may be due to that large placental volume is a marker of both low vascular resistance (low density) and placental inefficiency. More specifically, the former association (4-month SBP) might be explained by low vascular resistance, 5 while the latter one (7-year SBP) could be explained placental inefficiency.30 One previous study found that placental volume measured with ultrasound at 20 weeks of gestation was negatively associated with blood pressure in children aged 1 to 3.5 years.34 Taken together, the association between placental volume and offspring blood pressure may change from a negative to positive direction between early and middle childhood.
Placental vascular pathological lesions
Some of our findings support the hypothesis that placental vascular pathological lesions may impact the development of organs and neuroendocrine functions related to blood pressure control.16, 17 For example, thrombi in cord and decidual vessels, villous microinfarcts, necrosis at decidual magin and basalis were associated with higher infancy SBP. These vascular lesions may narrow vascular diameter, reduce the fetus-placenta blood flow, and increase the vascular resistance upon the fetus’s heart. As an adaptive response, the fetus’ heart has to work harder to pump the blood flow against the increased vascular resistance.13 The combination of narrow vascular diameter and forceful heart pump can substantially elevate systolic blood pressure. The elevated SBP in offspring may last for a period after birth and is thus detected in infancy. However, effects of these vascular lesions seem to diminish or disappear with age because they were not associated with high SBP in middle childhood in our sample.
Strengths
This was the first comprehensive analysis to examine the associations between placental morphology and offspring blood pressure in human population. Besides conventional measures of placental weight and placenta-fetus weight ratio, we also extensively examined placental diameters, area, thickness, volume, density, and vascular pathological lesions. The large and national sample in CPP provided good generalizability of our findings as well as sufficient statistical power (especially for some uncommon vascular pathological lesions). With measured 4-month and 7-year SBP, we were able to explore the age trend in these associations. The blindness of pathologists to pregnancy courses and outcomes could reduce information bias. We also controlled for many important potential confounders.
Limitations
First, the considerable amount of missing data on placental measures and childhood SBP might introduce selection bias. However, we did not find any substantial differences in most characteristics of mothers, offspring, and placentas between the final analytic sample and the full eligible sample. Second, compared to oscillometry and Doppler ultrasound, the palpation method was insensitive for measuring infancy blood pressure especially in those infants with small stroke volume.35 This might contribute to part of high variation for 4-month SBP. In addition, blood pressure was measured only once at each follow-up. However, these measurement errors were very likely to be independent of placental measures and thus should not lead to substantial bias.36 Third, placental area, volume, and density were derived with mathematical formulas and thus were not very accurate. Finally, the CPP data was collected several decades ago. However, biological effects of the placenta should not change with time substantively, and therefore new findings from this historical project are still very informative to current practice.
Perspectives
We have found that high placental weight and placenta-fetus weight ratio are associated with higher middle childhood SBP; large placental size is associated with lower infancy SBP but higher middle childhood SBP, while placental vascular pathological lesions only predict high infancy SBP. Despite uncertain causality, these novel findings can advance current limited knowledge in this field. Placental inefficiency predicts long-term blood pressure, whereas vascular resistance and lesions may only influence short-term blood pressure. Using state-of-the-art technology to monitor placental growth, blood flow, vascular resistance, hormones (e.g. growth factor and 11β-hydroxysteroid dehydrogenase type 2), and epigenetic markers, can assure whether, and reveal how, this important “black box” (the placenta) influences offspring’s long-term cardiovascular health.
Acknowledgments
The authors appreciate the assistance of Dr. Michelle L. Rogers on the data preparation.
SOURCES OF FUNDING
This analysis was supported by Flight Attendants Medical Research Institute and by grant R40MC03600-01-00 from the Maternal and Child Health Bureau, Department of Health and Human Services; National Institutes of Health (NIH) Transdisciplinary Tobacco Use Research Center (TTURC) Award (P50 CA084719) by the National Cancer Institute; the National Institute on Drug Abuse; and the Robert Wood Johnson Foundation.
Footnotes
DISCLOSURES
None
Contributor Information
Xiaozhong Wen, Epidemiology Section, Department of Community Health, Brown University, Providence, RI, Center for Population Health and Clinical Epidemiology, Department of Community Health, Brown University, Providence, RI
Elizabeth W. Triche, Epidemiology Section, Department of Community Health, Brown University, Providence, RI, Center for Population Health and Clinical Epidemiology, Department of Community Health, Brown University, Providence, RI
Joseph W. Hogan, Center for Statistical Sciences, Department of Community Health, Brown University, Providence, RI
Edmond D. Shenassa, Maternal and Child Health Program, Department of Family Science, University of Maryland, College Park, MD; Epidemiology Section, Department of Community Health, Brown University, Providence, RI
Stephen L. Buka, Epidemiology Section, Department of Community Health, Brown University, Providence, RI, Center for Population Health and Clinical Epidemiology, Department of Community Health, Brown University, Providence, RI
References
- 1.Harding JE. The nutritional basis of the fetal origins of adult disease. Int J Epidemiol. 2001;30:15–23. doi: 10.1093/ije/30.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Fowden AL, Forhead AJ, Coan PM, Burton GJ. The placenta and intrauterine programming. J Neuroendocrinol. 2008;20:439–450. doi: 10.1111/j.1365-2826.2008.01663.x. [DOI] [PubMed] [Google Scholar]
- 3.Godfrey KM. The role of the placenta in fetal programming-a review. Placenta. 2002;23 (Suppl A):S20–27. doi: 10.1053/plac.2002.0773. [DOI] [PubMed] [Google Scholar]
- 4.Wilson ME, Ford SP. Comparative aspects of placental efficiency. Reprod Suppl. 2001;58:223–232. [PubMed] [Google Scholar]
- 5.Salafia CM, Charles AK, Maas EM. Placenta and fetal growth restriction. Clin Obstet Gynecol. 2006;49:236–256. doi: 10.1097/00003081-200606000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hemachandra AH, Klebanoff MA, Duggan AK, Hardy JB, Furth SL. The association between intrauterine growth restriction in the full-term infant and high blood pressure at age 7 years: Results from the collaborative perinatal project. Int J Epidemiol. 2006;35:871–877. doi: 10.1093/ije/dyl080. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ, Godfrey KM, Osmond C, Bull A. The relation of fetal length, ponderal index and head circumference to blood pressure and the risk of hypertension in adult life. Paediatr Perinat Epidemiol. 1992;6:35–44. doi: 10.1111/j.1365-3016.1992.tb00741.x. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson J, Forsen T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. doi: 10.1161/01.hyp.36.5.790. [DOI] [PubMed] [Google Scholar]
- 9.Forsen T, Nissinen A, Tuomilehto J, Notkola IL, Eriksson J, Vinni S. Growth in childhood and blood pressure in finnish children. J Hum Hypertens. 1998;12:397–402. doi: 10.1038/sj.jhh.1000643. [DOI] [PubMed] [Google Scholar]
- 10.Whincup P, Cook D, Papacosta O, Walker M. Birth weight and blood pressure: Cross sectional and longitudinal relations in childhood. BMJ. 1995;311:773–776. doi: 10.1136/bmj.311.7008.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumey LH. Compensatory placental growth after restricted maternal nutrition in early pregnancy. Placenta. 1998;19:105–111. doi: 10.1016/s0143-4004(98)90105-9. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ, Thornburg KL, Osmond C, Kajantie E, Eriksson JG. The surface area of the placenta and hypertension in the offspring in later life. Int J Dev Biol. 2010;54:525–530. doi: 10.1387/ijdb.082760db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salafia CM, Maas E. The twin placenta: Framework for gross analysis in fetal origins of adult disease initiatives. Paediatr Perinat Epidemiol. 2005;19 (Suppl 1):23–31. doi: 10.1111/j.1365-3016.2005.00576.x. [DOI] [PubMed] [Google Scholar]
- 14.Roseboom TJ, van der Meulen JH, Ravelli AC, van Montfrans GA, Osmond C, Barker DJ, Bleker OP. Blood pressure in adults after prenatal exposure to famine. J Hypertens. 1999;17:325–330. doi: 10.1097/00004872-199917030-00004. [DOI] [PubMed] [Google Scholar]
- 15.Naeye RL. Disorders of the placenta, fetus, and neonate: Diagnosis and clinical significance. 1. St. Louis, MO: Mosby Year Book Inc; 1992. [Google Scholar]
- 16.Briscoe TA, Rehn AE, Dieni S, Duncan JR, Wlodek ME, Owens JA, Rees SM. Cardiovascular and renal disease in the adolescent guinea pig after chronic placental insufficiency. Am J Obstet Gynecol. 2004;191:847–855. doi: 10.1016/j.ajog.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 17.Loeliger M, Briscoe T, Lambert G, Caddy J, Rehn A, Dieni S, Rees S. Chronic placental insufficiency affects retinal development in the guinea pig. Invest Ophthalmol Vis Sci. 2004;45:2361–2367. doi: 10.1167/iovs.03-1349. [DOI] [PubMed] [Google Scholar]
- 18.Gillman MW, Rich-Edwards JW, Rifas-Shiman SL, Lieberman ES, Kleinman KP, Lipshultz SE. Maternal age and other predictors of newborn blood pressure. J Pediatr. 2004;144:240–245. doi: 10.1016/j.jpeds.2003.10.064. [DOI] [PubMed] [Google Scholar]
- 19.Hardy JB. The collaborative perinatal project: Lessons and legacy. Ann Epidemiol. 2003;13:303–311. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 20.Niswander KR, Gordon M. The women and their pregnancies: The collaborative perinatal study of the national institute of neurological diseases and stroke. Washington, DC: US Goverment Print Office; 1972. [Google Scholar]
- 21.Bordley J, 3rd, Connor CA, Hamilton WF, Kerr WJ, Wiggers CJ. Recommendations for human blood pressure determinations by sphygmomanometers. Circulation. 1951;4:503–509. doi: 10.1161/01.cir.4.4.503. [DOI] [PubMed] [Google Scholar]
- 22.Kirkendall WM, Burton AC, Epstein FH, Freis ED. Recommendations for human blood pressure determination by sphygmomanometers. Circulation. 1967;36:980–988. doi: 10.1161/01.cir.36.6.980. [DOI] [PubMed] [Google Scholar]
- 23.Benirschke K. Examination of the placenta. Obstetrics and gynecology. 1961;18:309–333. [Google Scholar]
- 24.Salafia CM, Zhang J, Charles AK, Bresnahan M, Shrout P, Sun W, Maas EM. Placental characteristics and birthweight. Paediatr Perinat Epidemiol. 2008;22:229–239. doi: 10.1111/j.1365-3016.2008.00935.x. [DOI] [PubMed] [Google Scholar]
- 25.Salafia CM, Zhang J, Miller RK, Charles AK, Shrout P, Sun W. Placental growth patterns affect birth weight for given placental weight. Birth Defects Res A Clin Mol Teratol. 2007;79:281–288. doi: 10.1002/bdra.20345. [DOI] [PubMed] [Google Scholar]
- 26.Myrianthopoulos NC, French KS. An application of the u.S. Bureau of the census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2:283–299. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 27.Eastman NJ, Bell ET, Dieckmann WJ, Kellogg FS, Mussey RD, Chesley LC, Peters JP, Page EW, Ross RA, Johnson HW, Van Wyck HB. Definition and classification of toxemias brought up-to-date. Chicago: American Committee on Maternal Welfare; 1952. [Google Scholar]
- 28.Thompson JM, Irgens LM, Skjaerven R, Rasmussen S. Placenta weight percentile curves for singleton deliveries. BJOG. 2007;114:715–720. doi: 10.1111/j.1471-0528.2007.01327.x. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt T, Schaffer L, Schneider C, Zimmermann R, Kurmanavicius J. Reference values for the weight of freshly delivered term placentas and for placental weight-birth weight ratios. Eur J Obstet Gynecol Reprod Biol. 2006;128:248–252. doi: 10.1016/j.ejogrb.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 30.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Louey S, Cock ML, Harding R. Postnatal development of arterial pressure: Influence of the intrauterine environment. Arch Physiol Biochem. 2003;111:53–60. doi: 10.1076/apab.111.1.53.15137. [DOI] [PubMed] [Google Scholar]
- 32.Seckl JR, Holmes MC. Mechanisms of disease: Glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 33.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 34.Thame M, Osmond C, Wilks RJ, Bennett FI, McFarlane-Anderson N, Forrester TE. Blood pressure is related to placental volume and birth weight. Hypertension. 2000;35:662–667. doi: 10.1161/01.hyp.35.2.662. [DOI] [PubMed] [Google Scholar]
- 35.Park MK, Menard SM. Accuracy of blood pressure measurement by the dinamap monitor in infants and children. Pediatrics. 1987;79:907–914. [PubMed] [Google Scholar]
- 36.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3. Philadelphia, PA: Lippincott Williams & Wilkins; 2008. pp. 142–143. [Google Scholar]