Abstract
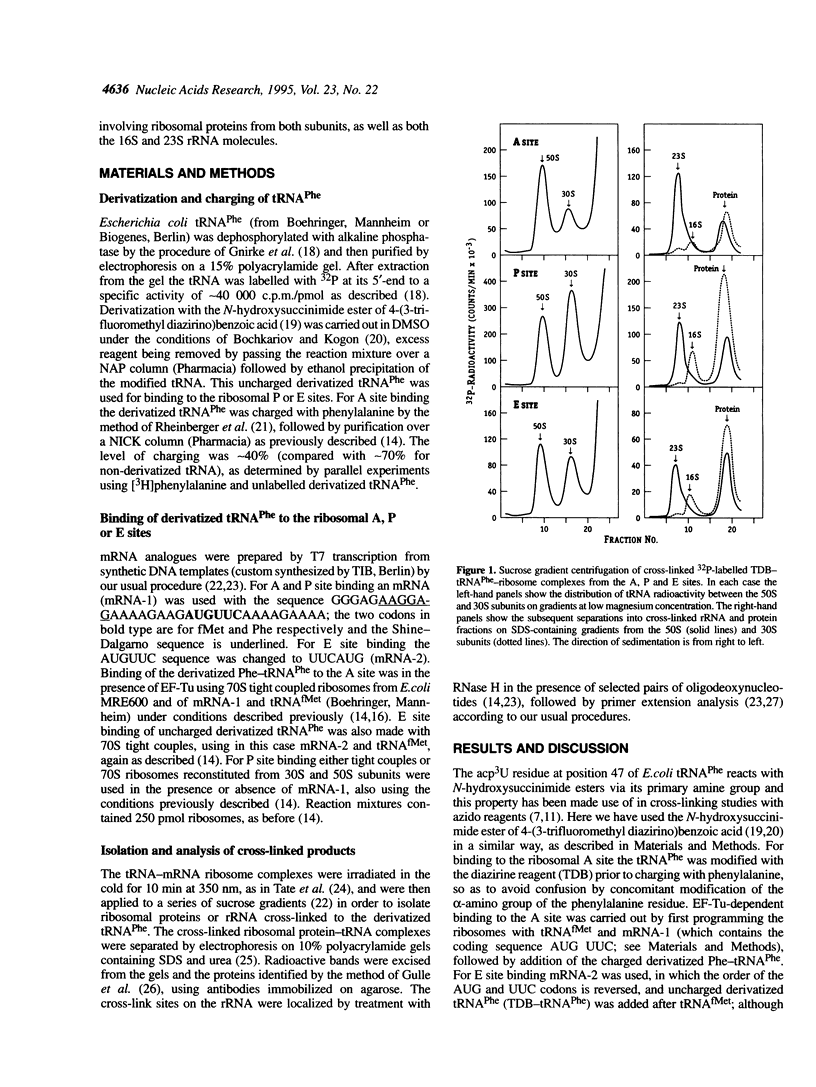
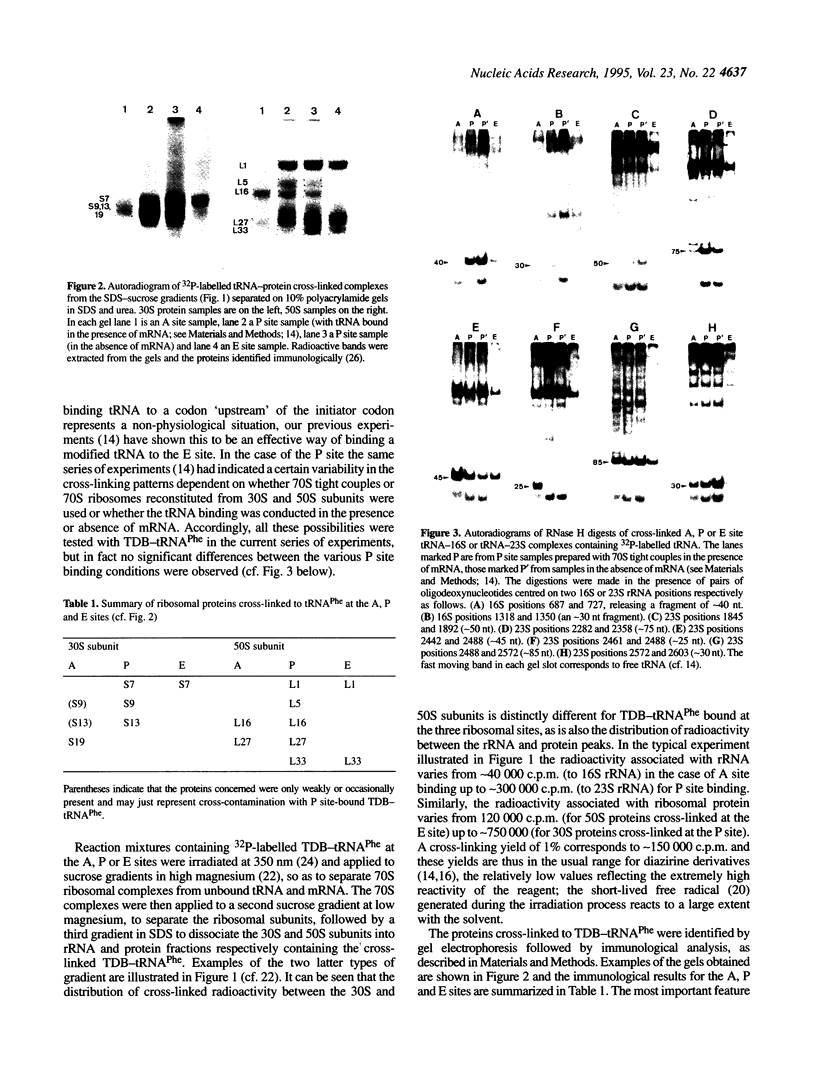
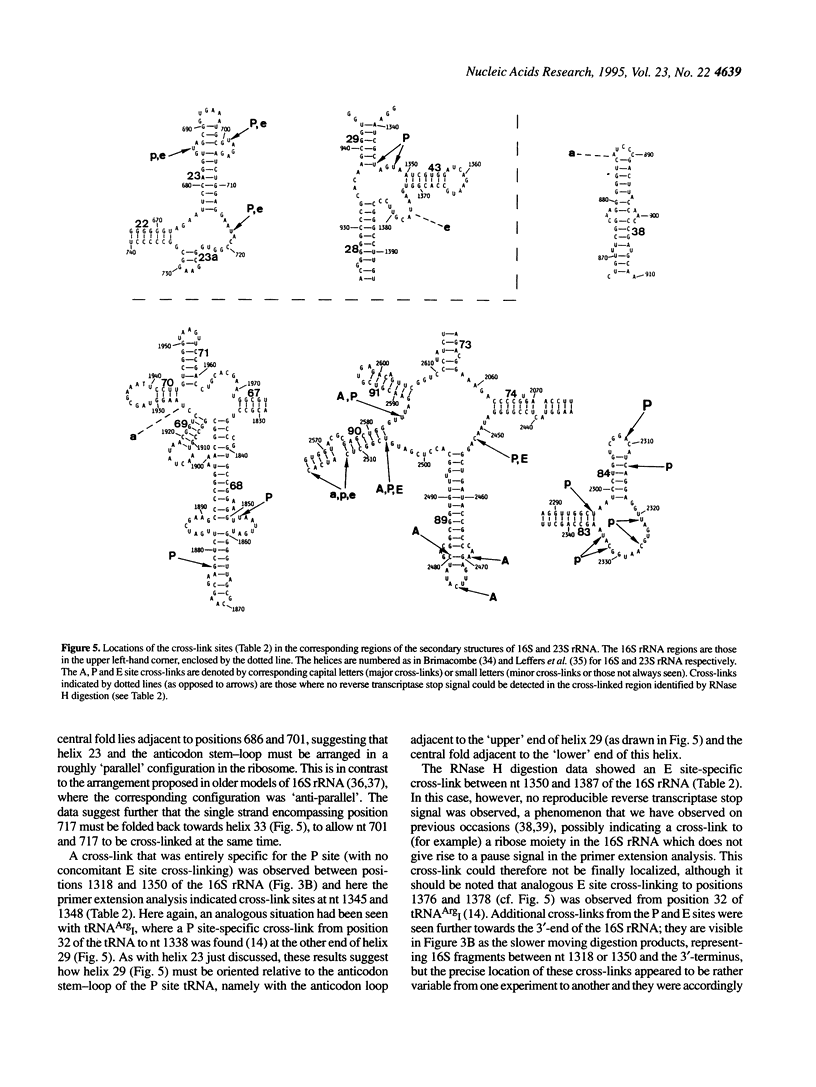
The naturally occurring nucleotide 3-(3-amino-3-carboxy-propyl)uridine (acp3U) at position 47 of tRNA(Phe) from Escherichia coli was modified with a diazirine derivative and bound to ribosomes in the presence of suitable mRNA analogues under conditions specific for the ribosomal A, P or E sites. After photo-activation at 350 nm the cross-links to ribosomal proteins and RNA were identified by our standard procedures. In the 30S subunit protein S19 (and weakly S9 and S13) was the target of cross-linking from tRNA at the A site, S7, S9 and S13 from the P site and S7 from the E site. Similarly, in the 50S subunit L16 and L27 were cross-linked from the A site, L1, L5, L16, L27 and L33 from the P site and L1 and L33 from the E site. Corresponding cross-links to rRNA were localized by RNase H digestion to the following areas: in 16S rRNA between positions 687 and 727 from the P and E sites, positions 1318 and 1350 (P site) and 1350 and 1387 (E site); in the 23S rRNA between positions 865 and 910 from the A site, 1845 and 1892 (P site), 1892 and 1945 (A site), 2282 and 2358 (P site), 2242 and 2461 (P and E sites), 2461 and 2488 (A site), 2488 and 2539 (all three sites) and 2572 and 2603 (A and P sites). In most (but not all) cases, more precise localizations of the cross-link sites could be made by primer extension analysis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Determination of tRNA nucleotide residues directly interacting with proteins in the post- and pretranslocated ribosomal complexes. FEBS Lett. 1990 Sep 3;269(2):398–401. doi: 10.1016/0014-5793(90)81202-y. [DOI] [PubMed] [Google Scholar]
- Abdurashidova G. G., Tsvetkova E. A., Budowsky E. I. Nucleotide residues of tRNA, directly interacting with proteins within the complex of the 30 S subunit of E. coli ribosome with poly(U) and NAcPhe-tRNA(Phe). FEBS Lett. 1989 Jan 30;243(2):299–302. doi: 10.1016/0014-5793(89)80149-8. [DOI] [PubMed] [Google Scholar]
- Bochkariov D. E., Kogon A. A. Application of 3-[3-(3-(trifluoromethyl)diazirin-3-yl)phenyl]-2,3- dihydroxypropionic acid, carbene-generating, cleavable cross-linking reagent for photoaffinity labeling. Anal Biochem. 1992 Jul;204(1):90–95. doi: 10.1016/0003-2697(92)90144-v. [DOI] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brimacombe R. RNA-protein interactions in the Escherichia coli ribosome. Biochimie. 1991 Jul-Aug;73(7-8):927–936. doi: 10.1016/0300-9084(91)90134-m. [DOI] [PubMed] [Google Scholar]
- Brimacombe R. The structure of ribosomal RNA: a three-dimensional jigsaw puzzle. Eur J Biochem. 1995 Jun 1;230(2):365–383. [PubMed] [Google Scholar]
- Brunner J., Senn H., Richards F. M. 3-Trifluoromethyl-3-phenyldiazirine. A new carbene generating group for photolabeling reagents. J Biol Chem. 1980 Apr 25;255(8):3313–3318. [PubMed] [Google Scholar]
- Dohme F., Nierhaus K. H. Role of 5S RNA in assembly and function of the 50S subunit from Escherichia coli. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2221–2225. doi: 10.1073/pnas.73.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontsova O., Dokudovskaya S., Kopylov A., Bogdanov A., Rinke-Appel J., Jünke N., Brimacombe R. Three widely separated positions in the 16S RNA lie in or close to the ribosomal decoding region; a site-directed cross-linking study with mRNA analogues. EMBO J. 1992 Aug;11(8):3105–3116. doi: 10.1002/j.1460-2075.1992.tb05383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dontsova O., Tishkov V., Dokudovskaya S., Bogdanov A., Döring T., Rinke-Appel J., Thamm S., Greuer B., Brimacombe R. Stem-loop IV of 5S rRNA lies close to the peptidyltransferase center. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4125–4129. doi: 10.1073/pnas.91.10.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döring T., Mitchell P., Osswald M., Bochkariov D., Brimacombe R. The decoding region of 16S RNA; a cross-linking study of the ribosomal A, P and E sites using tRNA derivatized at position 32 in the anticodon loop. EMBO J. 1994 Jun 1;13(11):2677–2685. doi: 10.1002/j.1460-2075.1994.tb06558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Penczek P., Grassucci R., Srivastava S. Three-dimensional reconstruction of the 70S Escherichia coli ribosome in ice: the distribution of ribosomal RNA. J Cell Biol. 1991 Nov;115(3):597–605. doi: 10.1083/jcb.115.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J., Zhu J., Penczek P., Li Y., Srivastava S., Verschoor A., Radermacher M., Grassucci R., Lata R. K., Agrawal R. K. A model of protein synthesis based on cryo-electron microscopy of the E. coli ribosome. Nature. 1995 Aug 3;376(6539):441–444. doi: 10.1038/376441a0. [DOI] [PubMed] [Google Scholar]
- Gnirke A., Geigenmüller U., Rheinberger H. J., Nierhaus L. H. The allosteric three-site model for the ribosomal elongation cycle. Analysis with a heteropolymeric mRNA. J Biol Chem. 1989 May 5;264(13):7291–7301. [PubMed] [Google Scholar]
- Gulle H., Hoppe E., Osswald M., Greuer B., Brimacombe R., Stöffler G. RNA-protein cross-linking in Escherichia coli 50S ribosomal subunits; determination of sites on 23S RNA that are cross-linked to proteins L2, L4, L24 and L27 by treatment with 2-iminothiolane. Nucleic Acids Res. 1988 Feb 11;16(3):815–832. doi: 10.1093/nar/16.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffers H., Kjems J., Ostergaard L., Larsen N., Garrett R. A. Evolutionary relationships amongst archaebacteria. A comparative study of 23 S ribosomal RNAs of a sulphur-dependent extreme thermophile, an extreme halophile and a thermophilic methanogen. J Mol Biol. 1987 May 5;195(1):43–61. doi: 10.1016/0022-2836(87)90326-3. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Kahan L., Ofengand J. Crosslinking of phenylalanyl-tRNA to the ribosomal A site via a photoaffinity probe attached to the 4-thiouridine residue is exclusively to ribosomal protein S19. J Mol Biol. 1984 Jan 5;172(1):77–86. doi: 10.1016/0022-2836(84)90415-7. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Osswald M., Brimacombe R. Identification of intermolecular RNA cross-links at the subunit interface of the Escherichia coli ribosome. Biochemistry. 1992 Mar 24;31(11):3004–3011. doi: 10.1021/bi00126a023. [DOI] [PubMed] [Google Scholar]
- Mitchell P., Stade K., Osswald M., Brimacombe R. Site-directed cross-linking studies on the E. coli tRNA-ribosome complex: determination of sites labelled with an aromatic azide attached to the variable loop or aminoacyl group of tRNA. Nucleic Acids Res. 1993 Feb 25;21(4):887–896. doi: 10.1093/nar/21.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Interaction of tRNA with 23S rRNA in the ribosomal A, P, and E sites. Cell. 1989 May 19;57(4):585–597. doi: 10.1016/0092-8674(89)90128-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Podkowinski J., Gornicki P. Neighbourhood of the central fold of the tRNA molecule bound to the E. coli ribosome--affinity labeling studies with modified tRNAs carrying photoreactive probes attached to the dihydrouridine loop. Nucleic Acids Res. 1991 Feb 25;19(4):801–808. doi: 10.1093/nar/19.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podkowiński J., Górnicki P. Ribosomal proteins S7 and L1 are located close to the decoding site of E. coli ribosome--affinity labeling studies with modified tRNAs carrying photoreactive probes attached adjacent to the 3'-end of the anticodon. Nucleic Acids Res. 1989 Nov 11;17(21):8767–8782. doi: 10.1093/nar/17.21.8767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinberger H. J., Geigenmüller U., Wedde M., Nierhaus K. H. Parameters for the preparation of Escherichia coli ribosomes and ribosomal subunits active in tRNA binding. Methods Enzymol. 1988;164:658–670. doi: 10.1016/s0076-6879(88)64076-6. [DOI] [PubMed] [Google Scholar]
- Rinke-Appel J., Jünke N., Brimacombe R., Dukudovskaya S., Dontsova O., Bogdanov A. Site-directed cross-linking of mRNA analogues to 16S ribosomal RNA; a complete scan of cross-links from all positions between '+1' and '+16' on the mRNA, downstream from the decoding site. Nucleic Acids Res. 1993 Jun 25;21(12):2853–2859. doi: 10.1093/nar/21.12.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinke-Appel J., Jünke N., Stade K., Brimacombe R. The path of mRNA through the Escherichia coli ribosome; site-directed cross-linking of mRNA analogues carrying a photo-reactive label at various points 3' to the decoding site. EMBO J. 1991 Aug;10(8):2195–2202. doi: 10.1002/j.1460-2075.1991.tb07755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen K. V., Alexander R. W., Wower J., Zimmermann R. A. Mapping the central fold of tRNA2(fMet) in the P site of the Escherichia coli ribosome. Biochemistry. 1993 Nov 30;32(47):12802–12811. doi: 10.1021/bi00210a032. [DOI] [PubMed] [Google Scholar]
- Stade K., Jünke N., Brimacombe R. Mapping the path of the nascent peptide chain through the 23S RNA in the 50S ribosomal subunit. Nucleic Acids Res. 1995 Jul 11;23(13):2371–2380. doi: 10.1093/nar/23.13.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Riens S., Bochkariov D., Brimacombe R. Contacts between the growing peptide chain and the 23S RNA in the 50S ribosomal subunit. Nucleic Acids Res. 1994 Apr 25;22(8):1394–1399. doi: 10.1093/nar/22.8.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stade K., Rinke-Appel J., Brimacombe R. Site-directed cross-linking of mRNA analogues to the Escherichia coli ribosome; identification of 30S ribosomal components that can be cross-linked to the mRNA at various points 5' with respect to the decoding site. Nucleic Acids Res. 1989 Dec 11;17(23):9889–9908. doi: 10.1093/nar/17.23.9889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H., Mueller F., Orlova E. V., Schatz M., Dube P., Erdemir T., Zemlin F., Brimacombe R., van Heel M. The 70S Escherichia coli ribosome at 23 A resolution: fitting the ribosomal RNA. Structure. 1995 Aug 15;3(8):815–821. doi: 10.1016/s0969-2126(01)00216-7. [DOI] [PubMed] [Google Scholar]
- Stern S., Weiser B., Noller H. F. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol. 1988 Nov 20;204(2):447–481. doi: 10.1016/0022-2836(88)90588-8. [DOI] [PubMed] [Google Scholar]
- Sylvers L. A., Kopylov A. M., Wower J., Hixson S. S., Zimmermann R. A. Photochemical cross-linking of the anticodon loop of yeast tRNA(Phe) to 30S-subunit protein S7 at the ribosomal A and P sites. Biochimie. 1992 Apr;74(4):381–389. doi: 10.1016/0300-9084(92)90116-v. [DOI] [PubMed] [Google Scholar]
- Tate W., Greuer B., Brimacombe R. Codon recognition in polypeptide chain termination: site directed crosslinking of termination codon to Escherichia coli release factor 2. Nucleic Acids Res. 1990 Nov 25;18(22):6537–6544. doi: 10.1093/nar/18.22.6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. The importance of highly conserved nucleotides in the binding region of chloramphenicol at the peptidyl transfer centre of Escherichia coli 23S ribosomal RNA. EMBO J. 1988 Nov;7(11):3577–3587. doi: 10.1002/j.1460-2075.1988.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vester B., Garrett R. A. The importance of highly conserved nucleotides in the binding region of chloramphenicol at the peptidyl transfer centre of Escherichia coli 23S ribosomal RNA. EMBO J. 1988 Nov;7(11):3577–3587. doi: 10.1002/j.1460-2075.1988.tb03235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walleczek J., Schüler D., Stöffler-Meilicke M., Brimacombe R., Stöffler G. A model for the spatial arrangement of the proteins in the large subunit of the Escherichia coli ribosome. EMBO J. 1988 Nov;7(11):3571–3576. doi: 10.1002/j.1460-2075.1988.tb03234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Malloy T. A., 4th, Hixson S. S., Zimmermann R. A. Probing tRNA binding sites on the Escherichia coli 30 S ribosomal subunit with photoreactive analogs of the anticodon arm. Biochim Biophys Acta. 1990 Aug 27;1050(1-3):38–44. doi: 10.1016/0167-4781(90)90138-r. [DOI] [PubMed] [Google Scholar]
- Wower J., Scheffer P., Sylvers L. A., Wintermeyer W., Zimmermann R. A. Topography of the E site on the Escherichia coli ribosome. EMBO J. 1993 Feb;12(2):617–623. doi: 10.1002/j.1460-2075.1993.tb05694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]