Abstract
Phytoestrogens are plant derived compounds found in a wide variety of foods, most notably soy. A litany of health benefits including a lowered risk of osteoporosis, heart disease, breast cancer, and menopausal symptoms, are frequently attributed to phytoestrogens but many are also considered endocrine disruptors, indicating that they have the potential to cause adverse health effects as well. Consequently, the question of whether or not phytoestrogens are beneficial or harmful to human health remains unresolved. The answer is likely complex and may depend on age, health status, and even the presence or absence of specific gut microflora. Clarity on this issue is needed because global consumption is rapidly increasing. Phytoestrogens are present in numerous dietary supplements and widely marketed as a natural alternative to estrogen replacement therapy. Soy infant formula now constitutes up to a third of the US market, and soy protein is now added to many processed foods. As weak estrogen agonists/antagonists with molecular and cellular properties similar to synthetic endocrine disruptors such as Bisphenol A (BPA), the phytoestrogens provide a useful model to comprehensively investigate the biological impact of endocrine disruptors in general. This review weighs the evidence for and against the purported health benefits and adverse effects of phytoestrogens.
Keywords: Soy, Isoflavones, Genistein, Equol, Endocrine disruption, Estrogen, ERα, ERβ, Brain, Hypothalamus
1. Introduction
Asian populations have historically had lower rates of cardiovascular disease, menopausal symptoms, breast cancer (and other hormone dependent cancers), diabetes and obesity than Western populations [4]. Soy is the cornerstone of a traditional Asian diet, an observation which has long fueled the widely held belief that consumption of soy foods reduces the risk of disease. But is there any evidence to support such an association and if so, which of the many compounds contained in soy could be responsible for alleviating these and other health conditions? One group of compounds that has received considerable attention is the phytoestrogens, many of which are now recognized to be endocrine disruptors. Although they behave similarly to man-made endocrine disrupting compounds (EDCs) on numerous molecular and cellular targets, the attitude of the general public and clinicians toward soy phytoestrogens are generally positive, while their synthetic counterparts are increasingly the subject of mounting public and congressional concern. Moreover, while exposure to most synthetic EDCs such as pesticides (e.g. DDT and methoxychlor), industrial lubricants (e.g. PCBs) and plasticizers (phthalates and Bisphenol A) is frequently associated with alarming statistics regarding declining reproductive health and increasing rates of cancer and obesity [50,74], the phytoestrogens remain widely believed to provide an array of beneficial effects, including preventative or therapeutic actions in carcinogenesis, atherosclerosis, and osteoporosis [170,169,127,47,36]. Source, rather than evidence for effects likely contributes to this incongruous attitude. A growing body of work now cautions that the health benefits frequently attributed to soy may be overstated [16,115,234]. Clinical and experimental studies examining the impact of soy or soy phytoestrogen consumption on human health have produced mixed and often conflicting results. Of even greater concern is that emerging evidence suggests that exposure to these compounds may, in fact, pose a risk to some groups, particularly infants and the unborn [231,187]. So are they helpful or harmful? The answer is undoubtedly complex and may ultimately depend on age, health status, level of consumption, and even the composition of an individual’s intestinal microflora. This review examines the pros and cons of phytoestrogen consumption on human health, focusing primarily on the neuroendocrine system.
2. Consumption levels, metabolism and absorption of common phytoestrogens
Phytoestrogens are naturally-occurring plant compounds that are structurally and/or functionally similar to mammalian estrogens and their active metabolites (Table 1) [294]. One major class is the lignans, which are components of plant cell walls and found in many fiber-rich foods such as berries, seeds (particularly flaxseeds), grains, nuts and fruits. Most phytoestrogens, however, are phenolic compounds of which the isoflavones and coumestans are the most widely researched groups. Isoflavones are present in berries, wine, grains and nuts, but are most abundant in soybeans and other legumes [136]. The isoflavone content of an array of foods is now published in numerous on-line databases (reviewed in Schwartz et al. [242]) the most accessible of which for consumers is maintained by the USDA (Table 2) United States Department of Agriculture [281].
Table 1.
Classification, dietary sources and structure of common phytoestrogens. The structure of 17β-estradiol is provided as a reference for comparison.
| Group | Subgroup | Examples | Dietary sources | Basic structure |
|---|---|---|---|---|
| 17β-Estradiol | Endogenous Estrogen | N/A | N/A | 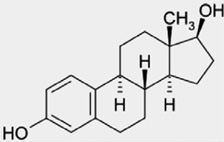 |
| Polyphenols | Resveratrol | Grape skin, red wine | 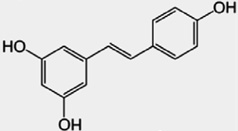 |
|
| Flavonoids | Flavanones | Eriodictyol, Hesperetin, Homoeriodictyol,Naringenin |
Citrus fruits and juices |  |
| Flavones | Apigenin, Luteolin, Tangeritin | Parsley, celery, capsicum pepper |  |
|
| Flavonols | Fisetin, Kaempferol, Myricetin, Pachypodol, Quercetin, Rhamnazin |
Kale, broccoli, onions tomatoes, lettuce, apples, grapes, red wine |
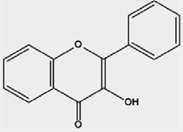 |
|
| Catechins | Proanthocyanides | Chocolate, green tea, beans, apricots, cherries, berries |
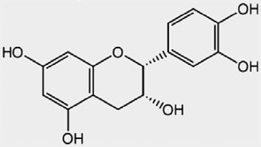 |
|
| Isoflavonoids | Isoflavones | Biochanin A, Clycitein, Daidzein, Formononetin, Genistein |
Soy beans, and other legumes | 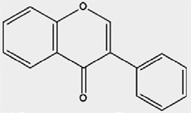 |
| Isoflavans | Equol | Metabolite of daidzein | 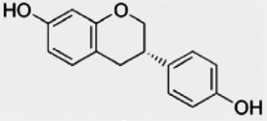 |
|
| Coumestans | Coumestrol | Clover, alfalfa, spinach | 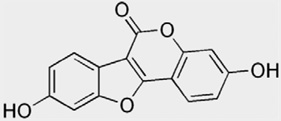 |
Table 2.
Isoflavone content of a representative sample of food products. Values obtained from the USDA Database on the Isoflavone Content of Selected Foods, Release 2.0 (accessed January 19, 2010). When multiple values were listed, they were averaged and the mean is presented in this table. Absolute isoflavone levels can differ considerably by brand, lot number, and season so all values should be used as a general guide for each type of product.
| Food product | Genistein (mg/100g) |
Daidzein (mg/100g) |
Total isoflavones (mg/100 g) |
|---|---|---|---|
| Soy Infant Formula (powder) | 13.5 | 6.32 | 26.3 |
| Edamame (raw green soybeans) |
22.6 | 20.3 | 48.9 |
| Miso | 23.2 | 16.4 | 41.5 |
| Silken tofu | 8.4 | 9.2 | 18.0 |
| Raw tofu, regular | 13 | 9 | 23 |
| Textured soy flour | 89.4 | 67.7 | 172.6 |
| Soy protein isolate | 57 | 31 | 91 |
| Soy-based sliced cheese | 6.5 | 5.1 | 14.5 |
| Soy-based bacon bits | 45.8 | 64.4 | 118.5 |
| Soy-based burgers | 5.0 | 2.4 | 6.4 |
| Red clover | 10 | 11 | 21 |
| Multigrain bread | 0.2 | 0.2 | 0.4 |
| KASHI Go Lean cereal | 7.7 | 8.4 | 17.4 |
| Green tea, Japanese | 0.02 | 0.01 | 0.02 |
| Flaxseeds | 0.04 | 0.02 | 0.07 |
| Raw broccoli | 0.00 | 0.04 | 0.25 |
Daidzein and genistein are the two most well characterized isoflavones and human exposure to these compounds occurs primarily through the consumption of soy-based food and beverage products. Unbeknownst to most consumers, in addition to well recognized soy products such as soy milk, tofu and tempeh, soy is found in upwards of 60% of processed foods [280]. Textured soy protein (50–70% soy protein) is a meat substitute found in hotdogs, hamburgers, sausages and other meat products while soy protein isolate (90% soy protein) is used to enrich energy bars, sports drinks, infant formula, cereals, granola bars, imitation dairy products, ice cream, cheese and even doughnuts. In addition, textured soy protein is used to fortify numerous products in the school breakfast and lunch programs as well as other federal assistance programs [274,243]. Soy is a popular food additive because it is a cholesterol-free, vegetable protein rich in complex carbohydrates and unsaturated fats, high in fiber, and free of lactose. It also contains upwards of 100 or more phytoestrogens.
In 1999, the US Food and Drug Administration (FDA) approved the use of the health claim that daily consumption of soy is effective in reducing the risk of coronary artery disease [75], a move which had a rapid and profound impact on the prevalence of soy products. Sales data illustrate this rapid rise in popularity. For example, between 2000 and 2007, more than 2700 new soy-based food products were introduced in the US market and sales of foods containing soy increased from approximately $300 million in 1992 to over $4 billion in 2008 [265]. According to a 2009 report published by the United Soybean Board [282], 84% of US consumers perceive soy as healthy and 32% purposefully consume soy products at least once a month. Soy isoflavones and other phytoestrogens are also widely available as dietary supplements [250,217] typically at far higher concentrations than found in soy-based foods. Overall, these sales and marketing data indicate that phytoestrogen intake is rapidly increasing, emphasizing the need to critically evaluate their potential health effects, both beneficial and detrimental, across age groups and populations.
In foods, phytoestrogens are present as mixtures, of which the isoflavones may only constitute a small part. Isoflavones are naturally found as biologically inactive glycoside conjugates containing glucose or carbohydrate moieties. The unconjugated from (aglycone) is the bioactive form. The proportion of conjugated to unconjugated forms varies substantially among foods, but fermented soy foods, such as miso or tempeh, often contain higher levels of the aglycone than other soy-based foods. Once consumed, they are rapidly metabolized and absorbed, entering systemic circulation predominantly as conjugates with limited bioavailability. For example, “free” genistein typically represents only 1–3% of total plasma genistein. Conjugated isoflavones then undergo enterohepatic circulation and return to the intestine where they may be further deconjugated by intestinal microbes. Genistein and daidzein can be derived from their glucosides or from the precursors biochanin A and formononetin, respectively, by the action of intestinal glucosidases [13]. Notably, a very specific type of intestinal microbe is need to bioconvert daidzein to its metabolite equol, and it appears that, at best, only 30–50% of individuals are capable of making that conversion with vegetarians and individuals of Asian origin being most likely [252,140]. The alternative metabolite is O-desmethylangiolensin (O-DMA). It has not yet been clearly elucidated what factors influence the capacity to produce equol vs. O-desmethylangolensin, but genetics, gut physiology, and diet purportedly contribute to interindividual differences. How these metabolic phenotypes affect human health outcomes is poorly characterized but considerable attention has recently been devoted to understanding the significance of this metabolic step because equol has a higher estrogenic potency than daidzein [251] and some researchers have hypothesized that having the ability to produce equol may be critical for obtain the health benefits associated with consuming a soy-rich diet [139].
Blood isoflavone levels vary widely, and can be orders of magnitude different between individuals. Part of this variation results from local and/or seasonal differences in food phytoestrogen content. For example, a recent study found that the total isoflavone content of raw soy beans can range from 18 to 562 mg/100 g [175]. Dietary preferences and individual differences in phytoestrogen absorption and metabolism have an even greater impact [108,160,228]. Predictably, people who eat more soy-based foods have higher blood levels of isoflavones. Soy is abundant in traditional Asian diets, resulting in isoflavone consumption as high as 50 mg/kg body weight/day [175]. In the US, estimates range from 1 to 3 mg/day for individuals eating a typical “Western” diet [175,44,45,105]. A vegetarian lifestyle or use of supplements can easily elevate phytoestrogen intake to levels at or above Asian levels [217,175,171]. This level of intake, even among Western Caucasians, is higher than for most synthetic endocrine disruptors, including Bisphenol A (BPA) which was recently estimate to be approximately 35 ng/kg per day [138]. Blood genistein levels are generally in the range of 25 ng/mL for Asian women, slightly less for vegetarian women, and under 2 ng/ml for US women [287]. For their weight, infants exclusively fed soy-based formula have the highest mean daily consumption of total isoflavones (ranging from 6 to 9 mg/kg per day in 4-month-old infants) resulting in plasma levels approaching 1000 ng/mL (Table 3). A more recent study confirmed these levels and found some levels to be even higher with the 25th, 50th, and 75th quartiles as high as 405.3, 890.7 and 1455.1 ng/ml [34]. In contrast, infants fed cow’s milk formula or human breast milk have plasma isoflavone levels of 9.4 and 4.7 ng/ml, respectively [248,249,81]. Soy formula has been estimated to constitute approximately 25% of the infant formula market [14,77,20] and at least one report suggests that for women who would prefer to breast feed, but are unable, soy formula is their substitute of choice [20].
Table 3.
Comparison of estradiol Bisphenol A (BPA) and genistein levels in newborns, infants, and adults. For all age groups and in all fluids listed, genistein levels are higher than BPA levels. Compiled values represent the range of those previously reported and do not take into account methodological differences or sample sizes. Thus, values presented should be considered representative. Circulating estradiol levels were obtained from references [294,73,266] and the UK General Practice Notebook (http://www.gpnotebook.co.uk/simplepage.cfm?ID=570818627&linkID=24801&cook=yes). (See below mentioned reference for further information.)
| E2 (ng/mL) | Genistein (ng/mL) | Bisphenol A (BPA, ng/ml) | Reference(s) | |
|---|---|---|---|---|
| Plasma, Adult Western Woman (follicular phase) | 0.25–1.8 | 1–2 | 0.3–5 | [283,61,96,241,210] |
| Plasma, Adult Western Woman (preovulatory peak) | 1.4–5.4 | 1–2 | 0.3–5 | [283,6] |
| Amniotic Fluid, Western | 0.4–1.7 | [69,78] | ||
| Cord blood, Western | 1–3 | [241] | ||
| Plasma, Adult Japanese Woman | 7.2–83 | 1.4–2 | [275,180,11,112] | |
| Amniotic Fluid, Japan | 15 | 1.1–8.3 | [112,7] | |
| Cord blood, Japan | 19.4–45 | 2.2 | [275,180,112,7] | |
| Breast Milk | 8–13.5 | 1.3 | [303,80] | |
| Plasma, Breast-Fed Infant | <0.04–0.08 | 2–4.7 | [248,82] | |
| Plasma, Infant fed Bovine Formula | <0.04–0.08 | 9.4 | [248] | |
| Plasma, Infant fed Soy Formula | <0.04–0.08 | 684–757 | [34,248] |
3. Multiple modes of action including endocrine disruption
How could the phytoestrogens act within the body to confer all the purported health benefits attributed to them? Some isoflavones, most notably genistein, inhibit pathways important for cell growth and proliferation, an effect which affects multiple organ systems. Genistein inhibits the activity of protein tyrosine kinases (PTKs) in numerous tissues including breast cancer cells [216,31]. PTKs catalyze phosphorylation of their own tyrosine residues and those of other proteins, including growth factors involved in tumor cell proliferation [155]. By inhibiting PTKs, genistein can potentially slow tumorigenesis, an effect that has let many laboratories to explore its therapeutic potential for breast and prostate cancer [8]. PTKs are also highly expressed in several brain regions, including the hippocampus, and phosphoregulation of PTKs is critical for numerous brain responses including synaptic plasticity, neurode-generation and response to neuronal injury [142]. At high doses, genistein suppresses PTK expression in the brain, an effect which is interpreted to be neuroprotective [151]. Inhibition of PTK activity may also play a role in improving cardiovascular function [199] and impeding the vascularization of tumors. In addition to PTKs, genistein can also inhibit other DNA replication enzymes associated with tumorigenesis including DNA topoisomerases I and II [136,191] and matrix metalloprotein (MMP9). It can also down-regulate the expression of vascular endothelial growth factor (VEGF) along with other related growth factor genes [221]. Phytoestrogens are often good antioxidants and anti-inflamatory agents; genistein and resveratrol are particularly powerful in this regard [227,233]. These estrogen receptor (ER)-independent properties of genistein, resveratrol and other isoflavones, indicate that they have the potential to affect a wide array of intracellular signaling mechanisms important for regulating cellular growth and protection [157].
Resveratrol, found naturally in grapes and red wine, has also gained considerable attention of late because high doses have now been shown to significantly extend lifespan in numerous species, including Saccharomyces cerevisiae, Caenorhabditis elegans and Drosophila melanogaster [106,297,94]. This effect appears to be conferred by the upregulation of the Sir2 gene (mammalian homolog is SIRT 1, SIR2L1 or Sir2α), a member of the sirtuin family, long hypothesized to play a role in the lifespan-extending effects of caloric restriction [143]. Sir2 is a highly conserved deacetylating enzyme, and overexpression in mice results in lower cholesterol, blood glucose and insulin levels. One notable study using mice fed a high fat diet found that high dose resveratrol (22 mg/kg) could effectively stave off many of the adverse health effects of obesity, resulting in significantly improved survival rates [23]. Although exciting, most of these studies have been generated from the same research team so adequate replication of these effects has not yet been achieved, and there is still debate as to whether or not resveratrol can influence SIRT 1 activity [194,24].
Perhaps the most well characterized mode of phytoestrogen action is estrogen receptor (ER) binding. There are two major ER subtypes in mammals, ERα and ERβ (also referred to as ESR1 and ESR2, respectively). As such, phytoestrogens, particularly the isoflavones, fit the Environmental Protection Agency’s definition of an endocrine disruptor which characterizes these compounds as those which, “alter the structure or function(s) of the endocrine system and cause adverse effects.” This definition includes disruption of lactation, the timing of puberty, the ability to produce viable, fertile offspring, sex specific behavior, premature reproductive senescence and compromised fertility. In animal models, isoflavones produce all of these effects. Recognition of the endocrine disrupting properties of phytoestrogens dates back to the 1940’s when ewes grazing on clover rich pastures in Australia were observed to have abnormally high rates of infertility, abortion, and reproductive abnormalities in their offspring [26,25]. It was ultimately determined coumestrol was primarily responsible for the observed effects [32,1,2]. Decades later, a singular case of infertility and liver disease in captive cheetahs placed on a soy-based diet was ultimately attributed to isoflavones [246]. These incidents have raised concerns that isoflavone intake, by mimicking or interfering with endogenous estrogens, could pose a risk to human reproductive health.
In vitro assays have found that, although most phytoestrogens, including the isoflavones, bind both ERα and ERβ, and activate ER-dependent gene transcription through both subtypes, they generally have a higher relative binding affinity for ERβ than ERα [133,215,35,132]. Genistein is 7- to 48-fold more selective for ERβ than ERα, depending on the assay used [133,132,17,107]. The relative estrogenic potency of genistein for ERβ is approximately 30-fold higher than for ERα. Potency estimates vary considerably depending on the assay used [117], but as a general principle, most isoflavones bind and activate both ERα and ERβ more readily than synthetic EDCs including BPA [133]. Once bound, isoflavones do not act like typical estrogen agonists, but rather more like selective estrogen receptor modulators (SERMS) such as the breast cancer drug tamoxifen which is an ER agonist in the uterus and bone but an antagonist in the breast [193]. This differential activity by phytoestrogens and SERMS results, in part, from the profile of co-activator and co-repressor proteins present in the cell. It also now apparent that each ER ligand induces unique conformational changes, which then influences the recruitment of co-regulator proteins and interactions with the estrogen response element (ERE) [159]. In the presence of phytoestrogens and other endocrine disruptors it appears that ERβ is more efficient than ERα at recruiting coactivators including TIF2 and SRC-1a [230].
The fact that most phytoestrogens bind ERβ more readily than ERα is likely functionally significant because ERα and ERβ are differentially distributed throughout the body and the brain and appear to upregulate different gene families [257,305,213,128, 64,40]. In breast tumor cells, for example, the suite of genes upregulated by ERβ activation enhance cell cycle progression and generally suppress proliferation while activation of ERα does largely the opposite [40]. In addition to the breast, ERβ is strongly expressed in bone, the cardiovascular system, uterus, bladder, prostate, lung, ovarian granulosa cells and testicular Sertoli and germ cells [132,158,131,284]. This distribution can change over the lifespan and is sexually dimorphic, particularly in the brain, suggesting that the two ER subtypes regulate different aspects of reproduction, behavior and neuroendocrine function and likely have differential roles across the lifespan [224,258]. For example, the paraventricular nucleus of the hypothalamus (PVN), a region important for the coordination of reproductive, social, and stress related behaviors, primarily expresses ERβ and agonism of ERβ is now recognized to be anxiolytic in castrated rodents [154,97]. ERβ is also expressed at higher levels than ERα in the basal forebrain, hippocampus and cerebral cortex in the adult [257,305], all brain regions critical to memory function and vulnerable to Alzheimer’s disease.
Once bound to ERs, phytoestrogens can then initiate transcription either classically through interactions with EREs or by binding early immediate genes, such as Jun and Fos [137]. These non-classical, rapid effects have only recently begun to be elucidated. Steroid hormones, particularly estrogens, are now recognized to initiate rapid, nongenomic actions at the cell surface via a range of mechanisms, including the binding of specialized steroid membrane receptors [72,147,172,173,285]. Ligand binding to these membrane receptors causes rapid (less than 10 min) and transient (a few hours) activation of second messenger pathways, such as increased intracellular calcium or cAMP levels, resulting in the stimulation of signal transduction pathways important for neuronal signaling, differentiation, and other cellular processes. Initially viewed with some skepticism, these alternative mechanisms of steroid action are rapidly becoming more widely appreciated as possible pathways by which endocrine disruptors can produce biological effects. For example, the first accepted transmembrane ER, GPR30, has proven to be capable of binding a wide range of EDCs including genistein [273]. The functional significance of this pathway, or its disruption, has yet to be fully established. A recent study has now revealed that GPR30 may not have intrinsic estrogenic activity, but rather the potential to induce the activity of a truncated, 36-kDa variant of ERα, called ERα36 [291], which is expressed on the plasma membrane [123]. Further studies are needed to better characterize the relationship between GPR30 and ERα36 as well as the potential for phytoestrogens and other endocrine disruptors to influence the signaling pathways associated with these receptors.
Phytoestrogens can also manipulate steroid biosynthesis and transport by, for example, stimulating hormone-binding globulin (SHBG) synthesis in liver cells [5], and competitively displacing either 17β-estradiol or testosterone from plasma SHBG [53]. This subtle deflection of the quantity or availability of SHBG by phytoestrogens changes the free fraction of endogenous hormones in circulation, either systemically or locally. Phytoestrogens can also manipulate endogenous hormone levels by interfering with the enzymes needed for steroid biosynthesis. Coumestrol, for example, attenuates the conversion of [3H]-estrone to [3H]-estradiol in vitro by inhibiting the enzyme 17β-hydroxysteroid oxidoreductase Type 1 in a dose-dependent fashion [79]. Genistein, though weaker, has a similar dose-dependent inhibitory effect. Disruption of aromatase [289,211,42] and 5α-reductase [70] by a number of phytoestrogens has also been demonstrated in vitro.
4. Pros: evidence for health benefits in humans
Numerous epidemiological and clinical studies have now evaluated the relationship between phytoestrogen consumption and human disease outcomes, but the results have not yielded a clear picture as to whether or not these compounds have therapeutic potential [36,261]. Dose, dietary composition, phytoestrogens administered, and duration of use vary considerably across epidemiological studies making them difficult to intercompare. Some feeding trials are limited by markedly small sample sizes, and several widely popularized studies were funded, at least in part, by the manufacturers of soy and soy-based supplements leading to some mistrust. Nonetheless, there is considerable public interest in the legitimacy of the claims being made regarding benefits to heart, bone, breast and menopausal symptoms. Unfortunately, the data supporting these claims are not strong in most cases, thus the degree to which phytoestrogen consumption confers meaningful health benefits remains unresolved (Fig. 1).
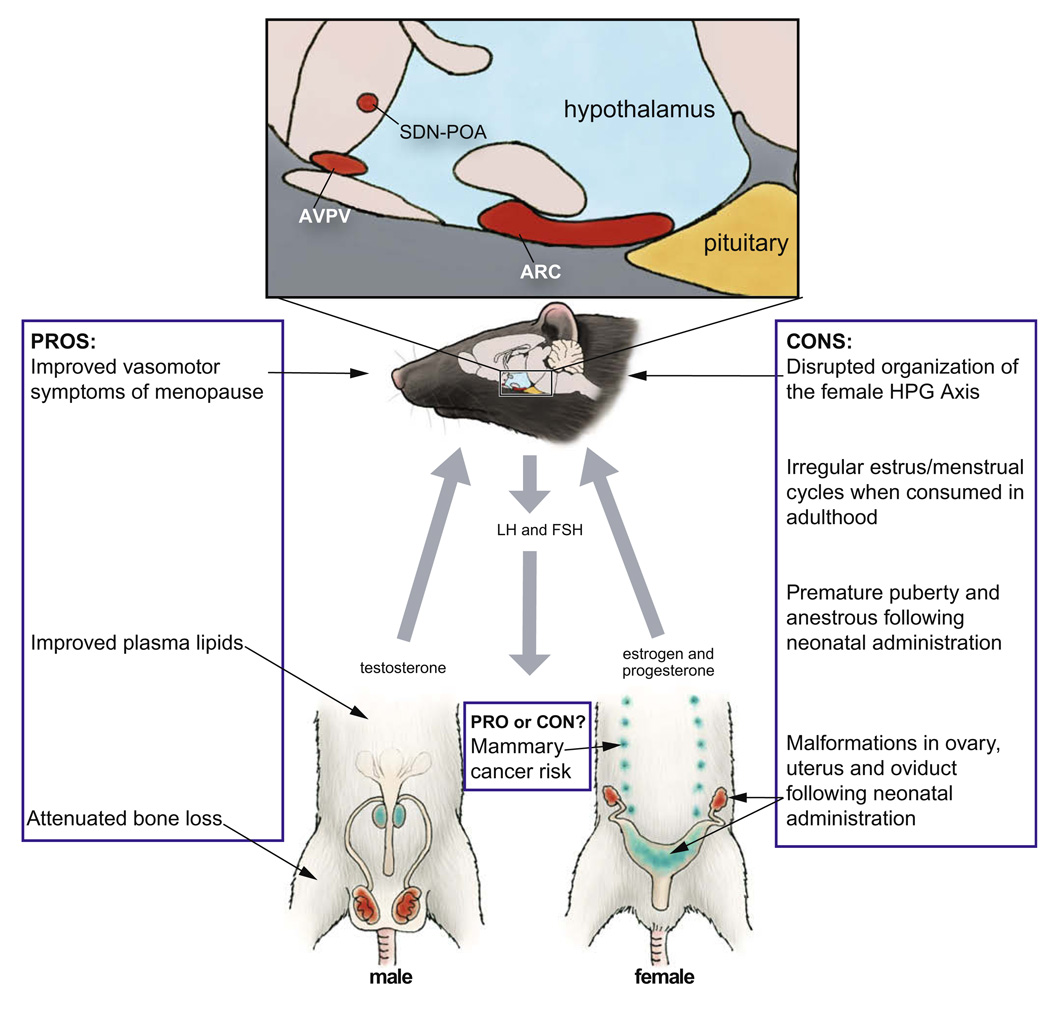
Fig. 1.
Summary of the pros and cons of genistein. The illustration depicts the rat hypothalamic–pituitary–gonadal (HPG) axis, indicating key components (in red) known to be vulnerable to disruption by genistein administration during critical windows of development. Developmental exposure to genistein can also result in abnormal development of the accessory sex glands (blue). Artwork by Barbara Aulicino. Edited and reprinted by permission of American Scientist, magazine of Sigma Xi, The Scientific Research Society.
4.1. Menopausal symptoms
The first widely attributed health benefit of phytoestrogen consumption was relief from vasomotor perimenopausal symptoms, including hot flushes and night sweats. For some women, the severity of these symptoms can markedly diminish their quality of life and interfere with daily activities. Although pharmaceutical hormone replacement therapy is effective in most cases, this option has fallen out of favor because of fears that its use increases the risk of developing breast cancer risk [238,27]. Incidence of vasomotor symptoms is higher in Western countries (70–80% of women) than in Asian countries (10–20%) [135], an observation which has led to the now popularly held belief that soy phytoestrogens may bring relief. Unfortunately, demonstrable evidence for such an association is weak at best, with most clinical trials showing no or minimal relief. One feature that stands out in nearly all studies is a large placebo effect [115,135,254,134]. In 2004 the North American Menopause Society issued a position statement which read, in part, “Among nonprescription remedies, clinical trial results are insufficient to either support or refute efficacy for soy foods and isoflavone supplements (from either soy or red clover), black cohosh, or vitamin E [277].” Despite this uncertainty, dietary supplements continue to be popular, particularly among women seeking a “natural” alternative to hormone replacement therapy [134,266].
4.2. Prevention of osteoporosis
Another consequence of aging is the progressive loss of bone-mineral density, a process that accelerates during perimenopause and increases fracture risk. Estrogens help maintain normal bone density, and it has been hypothesized that phytoestrogens may confer similar benefits. Results from animal studies, although inconsistent and negative in some cases, are nonetheless encouraging. Numerous phytoestrogens including coumestrol, genistein, daidzein and others have been reported to have bone sparing effects in the rat [36,59,63,10,102,149,302,244] but efficacy appears to depend on dose, route and duration of administration, and, to some degree, the animal model employed. In ovariectomized (OVX) post-menopausal monkeys, soy phytoestrogens were ineffective, even after 3 years of intake [222]. Evidence for measurable effects in humans is equally mixed. At least one study has found that post-menopausal women consuming high quantities of soy foods have better femoral and/or lumbar spine density compared to women who consume less soy [301]. A 2009 meta-analysis of randomized clinical trials conducted in humans, however, found only a weak association between increased consumption of soy isoflavones and improved bone-mineral density, leading the authors to conclude that soy isoflavones were unlikely to meaningfully reduce the risk of osteoporosis [152].
Bone sparing benefits may depend on the ability to bioconvert daidzein to equol. Equol production may also at least partially explain why the beneficial effects of isoflavones observed in laboratory rodents have not been easily recapitulated in humans, because while the most laboratory species (rats, mice and monkeys) consistently produce high levels of equol, only approximately a third to half of the human population appears capable of doing so [252,140]. Equol is a chiral molecule with the natural enantiomer, S-equol having a 13-fold higher relative binding affinity for ERβ than ERα [177]. In contrast, the R-enantiomer has a stronger affinity for ERα. S-equol also binds ERβ better than its parent compound, daidzein [177]. It is not yet clear which enantiomer has more potent bone sparing effects. In OVX rats, daily consumption of 200 mg/kg racemic equol for 8 weeks moderately elevated femoral calcium concentrations but also increased uterine weight [144]. Lower doses also appear to be effective in bone without inducing uterine proliferation, yielding hope that it might be beneficial for human bone without unwanted estrogenic side effects [166]. To date, few clinical trials have considered equol production as a potentially important variable, but at least one found that consumption of isoflavones (18 g soy protein powder or 105 mg isoflavone aglycone equivalents) for a year failed to improve bone-mineral density in post-menopausal women, even among equol producers [126]. This finding, however, conflicts with prior studies [244] including one which found a 2.4% increase in lumbar spine bone-mineral density among equol producers following ingestion of an isoflavone-rich soy milk over 2 years [156]. Dose, duration of therapy and subject age may have contributed to the incongruous results emphasizing the need to understand more about the mechanism by which equol and other phytoestrogens act to enhance bone density. Interest in the potential for equol to improve bone and other aspects of human health remains a hot topic of investigation and a potentially fruitful area of research [139]. For many women, adding soy to an already healthful diet may be an appealing choice to help stave off bone loss in mid-life.
4.3. Cardiovascular health and the prevention of heart disease
The purported health benefit that has arguably received the greatest attention and, consequently, stimulated the rapid proliferation and adoption of soy foods in Western countries, is a reduced risk of cardiovascular disease. The 1999 approval by FDA of the health claim that daily consumption of soy is effective in reducing the risk of coronary artery disease [75] has undoubtedly cemented the idea in the minds of many that soy is beneficial for human health. It is impossible to walk among US grocery store aisles without spotting this claim which reads, “Diets low in saturated fat and cholesterol that include 25 grams of soy protein a day may reduce the risk of heart disease. One serving of (this food) provides (this amount) of soy protein.” Similar claims and labeling policies quickly followed in other countries including the United Kingdom, Japan, Korea and South Africa. However, because of mounting evidence that such a claim might be spurious, in December of 2007, the FDA announced its intent to reevaluate the data justifying this claim [76], a process than was still ongoing as this review went to press. Similarly discouraging, the American Heart Association issued a statement in August of 2005 warning that “Earlier research indicating that soy protein as compared with other proteins has clinically important favorable effects on low density lipoprotein (LDL) cholesterol and other cardiovascular disease risk factors has not been confirmed by many studies reported during the past 10 years [234].” A recent review of the animal data also concluded that although marginal benefits have been observed, the impact of soy consumption on LDL levels and other cardiovascular risk factors are smaller than previously hoped [47].
There are numerous risk factors for cardiovascular disease including high blood pressure, obesity, C-reactive protein levels, systemic arterial compliance and the ratio of “good” (high density lipoprotein, HDL) to “bad” (LDL) cholesterol. Of these, only marginally reduced LDL levels are a consistent feature of human and animal studies of soy intervention. The most significant reductions in LDL cholesterol levels are generally small (3% or less) and only seen in individuals with high cholesterol who replace a substantial portion of their animal protein intake with soy, consuming between 40 and 318 mg isoflavones per day [234]. For example, daily administration of soy supplements providing 0.7–1.5 mg/kg isoflavones over 5 or 12 weeks did not alter serum lipids in men or women with average cholesterol levels [87,176,103,182], but lowered LDL levels have been reported in hypercholesterolemic women following soy isoflavone therapy [220].
Intriguingly, removal of the isoflavones from the soy protein does not eliminate the modest impact on LDL levels, suggesting that the soy protein itself, rather than the isoflavones, is producing the modest benefit [56]. An important consequence of this finding is the implication that use of phytoestrogen supplements, rather than substituting soy protein for animal protein as part of a balanced diet, is unlikely to confer meaningful cardiovascular benefits. Whether or not the individual isoflavones or another aspect of soy protein itself is responsible for producing the modest improvement in cardiovascular health must be disentangled. Regardless, people at risk for heart disease may want to consider replacing at least a portion of their meat intake with soy.
5. Breast cancer: pro or con?
Determining if phytoestrogens increase or reduce the risk of developing breast cancer has proven to be one of the most challenging human health impacts to address. It is well established that estrogens promote breast tumorigenesis, and that parameters which increase lifetime estrogen exposure (such as early menarche, short duration breastfeeding, and low parity) are associated with elevated breast cancer risk. Because they bind ERs with relatively high affinity, some researchers and clinicians are concerned that high phytoestrogen intake may increase the risk of carcinogenesis and put breast cancer survivors at risk for reoccurrence. Others have proposed that the opposite is true, citing traditionally low cancer rates in Asia as evidence [141]. Depending on the assay used, levels of endogenous estrogen present, life stage, and tumor type, genistein can act as both a proliferative and an antiproliferative agent [272,30]. For example, in vitro, genistein can inhibit proliferation of ER-positive and ER-negative breast cancer cells at high doses (>10 M), but, paradoxically, promote tumor growth at lower, more physiological doses [171,290]. Tamoxifen and other selective estrogen receptor modulators (SERMs) used for breast cancer therapy can also have mixed effects depending on dose and tissue type [192]. The SERM-like activity of soy phytoestrogens makes dietary guidelines particularly difficult to issue with confidence.
A relatively large number of studies have taken an epidemiological approach to address these concerns, but the results have differed by region and patient population. A Dutch study comparing plasma isoflavone levels in women with and without breast cancer found that high plasma levels of genistein were associated with a 32% reduction in breast cancer risk [286]. Most studies, however, have failed to corroborate such a profoundly beneficial effect of genistein. A meta-analysis, supported in part by the Susan G Koman Breast Cancer Foundation, concluded that, for Asian women, the risk of developing breast cancer drops as soy intake rises. As little as 10 mg of soy per day was sufficient to decrease breast cancer risk by 12% [299]. This association was not found for Caucasian women, but average daily isoflavone intake in this group was considerably lower (under 1 mg per day). Thus, it is unclear, if higher intake levels would have been beneficial for Caucasian as well as Asian women. Paradoxically, a different meta-analysis of 18 studies published between 1978 and 2004 found a protective effect of soy in pre-menopausal Caucasian women, but not women of Asian descent [278].
Dietary intervention studies have generally produced negative results. One of the largest found that consumption of 50–100 mg isoflavones per day for 1–2 years did not reduce mammographic density, a biomarker of increased risk [161,163]. Administration of a dietary supplement containing red clover derived isoflavones also failed to alter mammographic breast density after 1 year [12]. The impact of soy on breast cancer survivors is also unclear and appears to differ by ethnicity. The most recent study on breast cancer survivors examined 5042 Chinese women aged 20–75, and found that soy intake was significantly associated with a decreased risk of death and/or recurrence [255]. These results are consistent with a prior study, also done in Chinese women, which found chemopreventitive effects of soy consumption, particularly among pre-menopausal women [306]. As described previously, equol production has emerged as an important variable for achieving bone sparing effects and it may also prove to be an important predictor of cancer protection. An association between equol production and reduced breast cancer risk has been observed in at least one study of Caucasian women [88]. Additional studies are needed to further explore the relationship between equol production and breast cancer risk.
Phytoestrogens may have the biggest impact on lifetime risk when exposure occurs prior to puberty and possibly before birth. Although not an initial goal of the study, a Hawaiian research group found an association between high soy intake during early life and increased breast density, a risk factor for breast cancer [163]. The study consisted of 220 pre-menopausal women and was designed to determine if consumption of approximately 50 mg of isoflavones over 2 years in adulthood could reduce breast density. This intervention failed but life history data obtained during the process led the authors to conclude that Caucasian women who ate more soy over their lifetime had denser breast tissue than those who did not. This observation is not consistent with an earlier study, which found that, in Chinese women, high intake over a lifetime is directly correlated with reduced risk of cancer [141].
Results from perinatal exposure in animals have also been mixed. For example, one early study of this hypothesis found that rat pups born to mothers that consumed genistein (25 or 250 mg/kg diet) during gestation and lactation developed fewer breast tumors [84]. A more recent study, however, found that neonatal, subcutaneous administration of 5 or 50 mg/kg genistein stunted mammary gland development and the animals, particularly those given the higher dose, exhibited abnormal ductal morphology including reduced lobular alveolar development, and focal areas of “beaded” ducts lined with hyperplastic ductal epithelium [195]. Subcutaneous administration of a lower dose (0.5 mg/kg genistein), produced the opposite effect. In these animals mammary gland development was advanced and no significant ductal malformations were observed in adulthood suggesting that accelerated differentiation might reduce cancer risk. This biphasic effect of genistein on breast tissue development and differentiation indicates that dose may be an important factor when considering risk. The hypothesis that exposure to soy phytoestrogens early in life can alter the timing and character of breast development is supported by a 2008 cross-sectional study of 694 girls in Israel, which found increased prevalence of breast buds in 2-year old girls fed soy formula as infants [308]. It is unclear how this may impact their lifetime risk of developing breast cancer but argues for a more thorough investigation of the possible relationship between early life phytoestrogen exposure, premature thelarche, and breast cancer risk.
Overall, although research in this area has been intense over the past two decades, results from both in vivo and in virto studies have been frustratingly incongruous [68]. Recent, comprehensive reviews of the human studies suggest a modest inverse association between risk and high soy intake [278,300] but this trend is generally not supported by data from the animal literature [292]. To date, no clear consensus has been reached on whether or not phytoestrogens are helpful or harmful, or when they might be contraindicated for some groups. Unfortunately, despite the need for guidance, in many published reviews of the topic too many authors shy away from making definitive recommendations and instead suggest that women “discuss the issue with their health care provider.” This directive is unhelpful because it abdicates responsibility to clinicians, who are no more capable of giving informed opinions on the subject than research scientists. Although a myriad of factors such as patient age, hormone receptor status of breast tumors, ethnicity, alcohol consumption, and other dietary habits likely all interact and complicate the potential impact of soy consumption on breast tumor proliferation, movement towards a clear consensus-based set of guidelines is badly needed [276,99]. Given the evidence that adding soy foods to an already healthy diet may have modest but measurable benefits on bone and cardiovascular health, women without serious risk factors for breast cancer or a family history of breast cancer could likely incorporate soy into their diet without significant concern.
6. Cons: the endocrine disrupting properties of phytoestrogens in the adult brain and reproductive tract
In a 2008 clinical case report, physicians at SUNY Downstate Medical Center treated three women (aged 35–56) for a similar suite of symptoms including abnormal uterine bleeding, endometrial pathology and dysmenorrhea. In all three cases, symptoms ameliorated after soy intake was reduced or eliminated, demonstrating that consumption of particularly high isoflavone levels can compromise female reproductive health [38]. The youngest of the three had been on a soy-rich diet since age 14 and was experiencing secondary infertility, a condition that resolved and resulted in a pregnancy once she reduced her soy consumption. Isoflavone intake was not quantified, but estimated to exceed 40 g per day in the oldest of the three patients. It remains to be determined if these cases are atypical or sentinels of a legitimate public health concern. Because soy consumption is increasing so rapidly, and so many products now contain soy, along with its isoflavones and other phytoestrogens, this possibility clearly warrants greater attention.
6.1. Disruption of endogenous hormone levels and the ovulatory cycle
Animal and human studies evaluating phytoestrogen effects on the adult hypothalamic–pituitary–gonadal (HPG) axis following adult exposures have been fairly consistent and reveal the potential for suppression. Multiple studies have documented the estrogenic activity of phytoestrogens in ovariectomized rodents [54,235,239] and, in humans, it is generally accepted that consumption of isoflavones-rich soy foods suppresses circulating estrogen and progesterone levels and can attenuate the preovulatory surge of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [298,179,65]. Notably, however, a handful of studies have found no impact of isoflavones on female hormone levels at all. One of the most recent of these followed 34 women consuming 100 mg of isoflavones per day for a year and found no changes in luteal phase levels of estradiol, estrone, progesterone, SHBG, FSH or LH at months 1, 3, 6 or 12 [162]. Another also failed to find altered circulating gonadal hormone levels in 14 women given soy cookies containing 52 mg of isoflavones or isoflavone-free cookies for 5 days. Interestingly, at least one study found suppressed luteal estradiol levels following increased soy intake, but only in women of Asian descent [298], indicating ethnicity could be an underappreciated factor when considering the potential human health effects of soy isoflavones. With such small samples sizes in all of these studies, however, they may have been too underpowered to detect effects. A 2009 meta-analysis concluded that, in pre-menopausal women, isoflavone intake increases cycle length and suppresses LH and FSH levels [104]. This conclusion is consistent with the clinical case report from SUNY Downstate Medical Center [38] and indicates that use of soy foods should be approached with caution in women attempting to become pregnant or experiencing menstrual cycle irregularities.
6.2. Behavior
Our research has revealed that isoflavone intake can suppress female sex behavior in rats. Administration of a commercially prepared phytoestrogen supplement to adult female rats, at a dose that results in serum levels between those seen in Western and Asian (human) adults, attenuated lordosis to the same degree as the SERM tamoxifen [207,204]. The supplement treated group displayed significantly fewer proceptive behaviors than the tamoxifen treated group, demonstrating the potential for soy isoflavones to suppress sexual motivation. Intriguingly, administration of genistein alone did not recapitulate these effects on sexual behavior [205] suggesting that a compound, or mixture of compounds, other than genistein was responsible for the suppressed sexual behavior. Studies using ERαKO mice have shown that ERα is required for the normal expression of both male and female sexual behavior [189,225,190] indicating that activity through ERα may be mediating this behavioral change.
Other behaviors may be affected as well including social, aggressive, and anxiety-related behaviors. Increased aggression and circulating testosterone levels have been reported in male Syrian hamsters maintained for 5 weeks on the soy-rich Purina 5001 diet compared to control animals fed a soy-free diet [174]. Animals on the phytoestrogen- rich diet also had lower vasopressin receptor (V1a) expression in the lateral septum but higher V1a expression in lateral hypothalamus indicating that the altered behavior might result from changes within vasopressin signaling pathways. Similarly, male rats maintained on a diet containing 150 µg/g genistein and daidzein displayed increased anxiety and elevated stress-induced plasma vasopressin and corticosterone levels [98]. Elevated hypothalamic vasopressin has also been reported in rats fed a diet containing 1250 ppm genistein across the lifespan [236]. Anxiolytic effects of phytoestrogen-rich diets have also been reported in gonadally intact male and female rats exposed over their entire lifetimes but not when administered briefly in adulthood [145]. Phytoestrogen intake can also affect behavior in non-human primates. Male cynomolgus monkeys fed soy protein isolate containing 1.88 mg isoflavones/g protein over 18 months demonstrated higher frequencies of intense aggressive (67% higher) and submissive (203% higher) behaviors as well as a decreased proportion of time (68% reduction) spent in physical contact with other monkeys [260].
6.3. ER-dependent gene expression in the brain
Phytoestrogens have widespread effects in the adult brain which have previously been reviewed in detail elsewhere [145, 201,146,202]. Both the PVN and the ventromedial nucleus (VMN) are critical nuclei for the initiation and regulation of sex behavior [214] and each contains primarily one ER subtype. Only ERβ is expressed in the PVN while the VMN contains primarily ERα [256]. Coumestrol and genistein stimulate ERβ mRNA expression in the PVN, an effect opposite to that of 17β-estradiol [205,203]. It is not yet clear what the functional significance of this might be but the PVN is the primary site of oxytocin (OT) production, a peptide hormone important for social behavior and the facilitation of sexual behavior [296,33,229]. Estrogen stimulates OT production, a process that is regulated through ERβ [206]. Oxytocin then binds to its receptor (OTR) in the VMN, a nucleus critical for mediating the lordosis response in females [214]. ERα is required for the upregulation of OTR [206,304]. Consumption of the commercially prepared phytoestrogen supplement described previously, attenuated the estrogen-dependent up-regulation of OTR in the rat VMN [204], an effect which was accompanied by reduced lordosis.
7. Cons: evidence for endocrine disruption during development
A possibility of increasing concern is that phytoestrogens may interfere with the organizational role of estrogen in the developing brain and reproductive system (Fig. 1). Regardless of animal model used, manipulation of estrogen during specific critical windows of development throughout gestation and early infancy leads to a myriad of adverse health outcomes including malformations in the ovary, uterus, mammary gland and prostate, early puberty, reduced fertility, disrupted brain organization, and reproductive tract cancers [50,258,183,89,90,150]. These effects mirror some very disturbing public health trends in Western nations. For example, in the United States and Europe median age at menarche, first breast development, and sexual precocity has steadily advanced, especially among minority populations [91,98]. Similar trends have been documented among children adopted from developing countries by Western parents [9]. There are also indications that female fecundity is declining, even among young women, although the rate and degree to which this is occurring has been difficult to quantify. Among men, sperm counts in the United States and Europe appear to have declined by roughly half over the past 50 years [270]. In Demark, it is now estimated that more than 10% of men have sperm counts in the infertile range and up to 30% are in the subfertile range [121]. Rates of testicular cancer also appear to be increasing. Another study showed that infant boys born to vegetarian mothers had increased incidence of hypospadias (malformation of the male external genitalia) [186] suggesting that dietary components (perhaps phytoestrogens) cross the placenta and cause adverse effects on the developing fetus. The causes of these reproductive health trends are likely complex and multi-faceted, but rapidity of the increase in reproductive and behavioral disorders suggests an environmental, endocrine disrupting component [164]. Whether or not isoflavone phytoestrogens could be one such component is now the subject of rigorous debate and has caught the attention of public health officials in the US and abroad.
7.1. Soy-based infant formulas: prevalence and phytoestrogen content
Isoflavones can pass from mother to fetus through the placenta, and have been found in human umbilical cord blood and amniotic fluid at levels comparable to concentrations seen in maternal plasma, indicating that fetal exposure is possible (Table 3) [7]. These levels are considerably lower, however, than blood levels in infants exclusively fed soy formula. Initially developed as an alternative to bovine milk formulas for babies with a milk allergy, use of soy infant formula in the US has steadily risen in popularity. An estimated 25% of US infants, approximately one million each year, are now raised on soy formula, largely because of perceived health benefits or to maintain a vegetarian lifestyle, rather than concerns about cow milk allergies, colic, or other health concerns [14,77,20]. The widespread prevalence of popular media articles touting the beneficial effects of soy have undoubtedly contributed to its selection by mothers trying to make the most healthful choice for their babies.
Total isoflavone content in soy infant formula varies widely due to environmental and genetic differences between batches and sources, but is consistently higher than in most other food sources [248,81,245,122]. Infants on soy formula consume approximately 6–9 mg isoflavones per kg body weight per day, an amount, when adjusted for body weight, that is up to seven times higher than for adults meeting the FDA soy consumption guideline, or Asians consuming a traditional soy-based diet (0.3–1.2 mg/kg per day) [248,81,18]. It was initially thought that, because the gastrointestinal tract of infants is undeveloped compared to adults, they would not be able to completely absorb the isoflavones. At least one study, however, reports that infants as young as 4 weeks can digest, absorb and excrete isoflavones as effectively as adults [113]. Moreover, plasma isoflavone levels are an order of magnitude higher in infants than adults, even when levels of intake are similar [249]. Infants fed soy formula have circulating phytoestrogen concentrations of approximately 1000 ng/ml, 13,000–22,000 times higher than their own endogenous estrogen levels, 50–100 times higher than estradiol levels in pregnant women and 3000 times higher than estradiol levels at ovulation [249,14,295]. These blood levels are high enough to produce many of the physiological effects observed in research animals and human adults. In addition, they are at least a level of magnitude higher than those reported for other endocrine disruptors including BPA (Table 3) and the phthalates [50]. A recent prospective study in human infants observed that female infants on soy-based infant formulas exhibit estrogenized vaginal epithelium at times when their breast fed or cow based formula counterparts did not, suggesting estrogenic activity of the soy infant formula [28]. Determining if use of soy infant formulas can have long term reproductive health effects is a public health imperative.
7.2. Diethylstilbestrol (DES): lessons from history
Effects of endocrine disruption in the developing fetus are likely to be subtle and not readily apparent at birth, a lesson learned from the tragic case of diethylstilbestrol (DES), a synthetic estrogen prescribed to upwards of 10 million women between 1938 and 1971 [183,85]. Initially thought to reduce the risk of miscarriage, a claim that ultimately turned out to be incorrect, belief in its healthful benefits was so widespread, that it was eventually recommended as a routine prophylactic for all pregnant women, regardless of miscarriage history [58,262,232,197]. Non-medical use of DES was also common, and therefore a significant source of DES exposure for millions of others, albeit at lower doses. Products containing DES included lotions, shampoo and growth enhancers for chicken and cattle. Unfortunately, many children, both male and female, born to women prescribed DES during pregnancy subsequently developed reproductive health problems as adults. Adverse effects were first detected in 1971, decades after DES use had become commonplace. Two physicians noted that an extremely rare form of vaginal clear cell carcinoma was appearing in DES daughters more frequently than in unexposed women, and at a much younger age [100,101]. It was subsequently established that DES exposure in utero can also result in vaginal dysplasia, vaginal and/or cervical adenosis, malformations of the uterus, cervix and vagina, increased risk of testicular cancer, lower sperm count, undescended testes, infertility, late spontaneous abortion, premature delivery, and other pregnancy complications [183,232,197]. In general, the severity of the health effects correspond with timing and level of exposure, an observation which was the first, clear demonstration of how important it is to consider “critical windows of exposure” when attempting to predict potential consequences of human exposure to endocrine disruptors, such as the phytoestrogens.
So is another reproductive health tragedy silently unfolding with the use of soy infant formula? Most of the reproductive outcomes following fetal exposure to DES were predicted by or replicated in animal models [183,168] emphasizing the importance of animal models for adumbrating potential adverse effects of endocrine disruptors in humans. Evidence for concern is emerging from a steady stream of laboratory animal data. The difficulty for public health agencies, parents and physicians is determining if the observed effects reliably predict what is likely to be happening in humans.
7.3. Animal data: disruption of brain sexual differentiation
In the rat, genistein readily crosses the placenta and in the fetal brain, the bioactive aglycone form is present at levels comparable to circulating levels in the dam [60]. In addition, the transfer of genistein to the brain from systemic circulation appears to be more efficient in prenatal animals than adults [39] indicating that it and other isoflavone phytoestrogens could interfere with the organization of estrogen sensitive neuroendocrine signaling pathways. Hormone mediated architectural and functional changes within the HPG axis occur during a series of well defined critical periods spanning gestation through puberty, resulting in sex specific physiology and behavior in the adult animal [258,90,48]. Interference with the hormone-sensitive formation of these pathways could result in irreversible developmental defects, potentially making development one of the most susceptible periods for phytoestrogen and EDC exposure over the lifespan.
The sexually dimorphic brain region that is most frequently used as a “biomarker” of endocrine disruption in rats is the sexually dimorphic nucleus of the preoptic area (SDN-POA). The volume of the SDN-POA is enhanced by estradiol aromatized from perinatal, testicular androgen [90], and is five to six times larger in males than females [92]. Both ERα and ERβ are expressed in the SDN-POA across the lifespan but ERα appears to play a dominant role in masculinizing SDN-POA morphometrics [213,256,200]. In rats, numerous studies have been undertaken to determine the extent to which phytoestrogens and other EDCs can alter SDN-POA volume in both sexes. Although not always in complete accordance, the data have generally shown the potential for genistein and other phytoestrogens to act as estrogen agonists in the brain. When administered prenatally through adulthood, genistein increases SDN-POA volume in males but not females [237]. No enhancement, however, was observed in males exposed from birth through weaning [148] or in males exposed on only the first few days of life [208] suggesting that exposure must be ongoing to maintain the enlargement. Consistent with this hypothesis, males fed a phytoestrogen-rich diet during development and puberty, then switched to a phytoestrogen-free diet in adulthood had smaller SDN-POA volumes than males fed the phytoestrogen diet across the entire lifespan [153] but these results are difficult to interpret because unexposed, control animals were not included in the analysis. Effects in female rats have been less robust but consistent with an estrogenic effect of genistein, at least at high doses. Masculinizing effects on female SDN-POA volume have been reported following subcutaneous administration on PND 0–10 of 500 or 1000 µg of genistein but not lower amounts [71]. Other studies have not seen genistein-induced changes in female SDN-POA volume, even at doses high enough to be uterotrophic [148,165].
Another sexually dimorphic region sensitive to endocrine disruption is the locus coeruleus (LC), a noradrenergic brain stem nucleus that is an important component of the stress axis and may play a role in depression and Parkinson’s Disease [223,66]. The LC is normally larger in females and contains a greater density of cells than the male LC. Pre- and postnatal exposure to moderate levels of the phytoestrogen resveratrol, abolished the sex difference in LC volume and cell density by demasculinizing the male brain [130]. In this case, no changes in SND-POA volume were found underscoring the complexity and site-specificity of isoflavone action in the brain. Both ERα and ERβ are expressed in the adult rat LC [256] but only ERα is expressed in the postnatal LC [213] suggesting that resveratrol may be acting through an ERα-dependent mechanism.
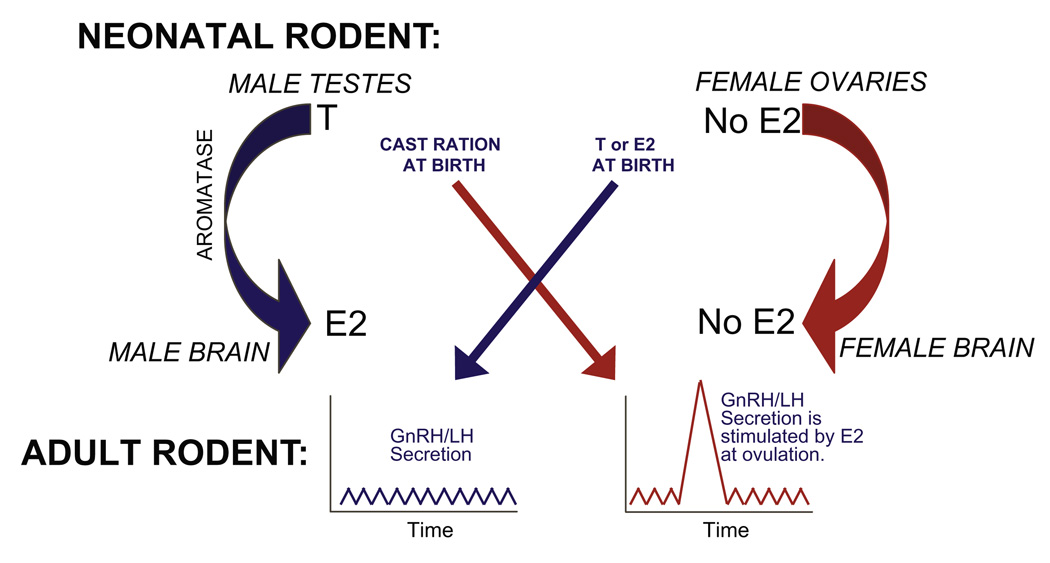
Recent studies in our laboratory have focused on the organization of sexually dimorphic hypothalamic nuclei critical for preovulatory gonadatropin release in female rats. The neural components of the HPG axis that coordinate the secretion of gonadotropin releasing hormone (GnRH), are sexually differentiated by endogenous gonadal hormones during a series of pre- and perinatal critical periods [49]. In female rodents, the population of GnRH neurons lying just rostrally to the anterior ventral periventricular nucleus (AVPV), respond to rising levels of estrogen and generate the surge of luteinizing hormone (LH) that precedes ovulation [91,67]. This population is often referred to as the “GnRH surge center.” In humans, the GnRH surge center appears to be functional by the end of the first trimester [95], while in rodents this system does not sexually differentiate until the first few days of the neonatal period [52]. Within the male rodent brain, testicular testosterone is converted to estrogen by the enzyme aromatase, and it is this estrogen that is primarily responsible for defeminizing/masculinizing the hypothalamic nuclei of the HPG axis during development. At birth, if endogenous estrogen is blocked in males, either by castration, by blocking the action of aromatase, or by blocking hypothalamic estrogen receptors, the HPG axis fails to defeminize and the capacity to elicit a gonadal surge remains (Fig. 2). In contrast, neonatal estrogen can defeminize the female HPG axis and thus eliminate the future emergence of the female estrous cycle. Therefore, interference with estrogen at birth, either by blocking estrogen activity in males or by triggering estrogen signaling in females, can result in the improper differentiation and function of the HPG axis across the lifespan.
Fig. 2.
During the neonatal period, estrogen (E2), aromatized from testicular testosterone (T), defeminizes the male rodent brain such that a GnRH surge cannot be elicited by steroid positive feedback. If E2 action is blocked, by castration, inhibition of aromatase, or disruption by endocrine disruptors, then the female GnRH pattern results. In females, the ovaries are quiescent at birth. Thus, the female-typical GnRH secretion pattern develops in the absence of E2. The administration of E2 during this critical period produces the male pattern, and the loss of the preovulatory GnRH surge. We have found that administration of 10 mg/kg genistein to neonatal females in abrogated GnRH activation by steroid hormones, an effect which is consistent with defeminization.
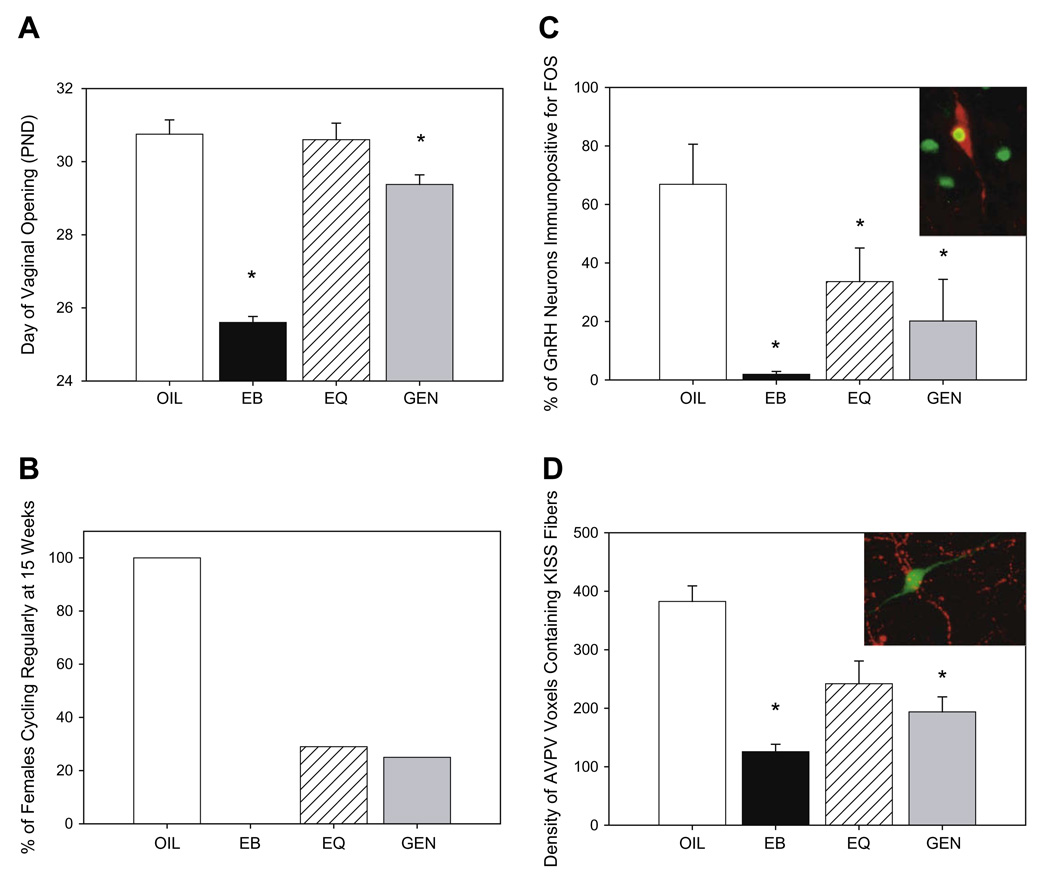
We have found that subcutaneous administration of 10 mg/kg genistein, a dose that is approximately equivalent to the total amount of isoflavones ingested by infants fed soy formula, over the first 4 days of life, advanced vaginal opening and compromised the ability to maintain a regular estrous cycle in female rats [21], effects which have also been observed by other research groups [129]. These physiological changes were accompanied by an impaired ability to stimulate GnRH neuronal activity (as measured by the immunoreactivity of both of GnRH and FOS) following ovariectomy and hormone priming (Fig. 3). This finding indicates that the capacity to generate a GnRH surge is compromised. Because it has long been appreciated that estrogen administration during this neonatal critical period typically induces a similar suite of effects, our data indicate that neonatal genistein exposure has a defeminizing effect on the organization of the female rat HPG axis.
Fig. 3.
Summary of the disruptive effects of neonatal genistein or equol exposure in the female rat HPG axis. (A) Day of vaginal opening (a hallmark of puberty in the rat) is advanced in female rats neonatally exposed to estradiol benzoate (EB) or genistein (GEN) but not equol (EQ) during the first 4 days of life compared to vehicle treated controls (OIL). (B) By 15 weeks of age only 29% of GEN and 25% of EQ exposed animals displayed a regular estrous cycle compared to 100% of the control animals and none of the EB treated animals. (C) Immunolabeling of GnRH (red) and Fos (green) was used as an indicator of GnRH activation following hormone priming. The percentage of GnRH neurons immunopositive for Fos was significantly lower in the animals neonatally exposed to GEN or EQ indicating that both defeminized steroid positive feedback signaling pathways. (D) The density of neuronal fibers immunopositive for kisspeptin (KISS, red) surrounding GnRH neurons (green) was significantly lower in animals neonatally treated with EB or GEN but not EQ. Reduced stimulation of GnRH by kisspeptin neurons may be a mechanism by which GnRH activity is impaired in females exposed to GEN and possibly other isoflavones during development. (Means ± SEM, *p ≤ 0.05.)
It is important to emphasize that in humans androgen, rather than estrogens, is thought to be most important for masculinizing the brain during development [95,288]. This species difference makes organizational neuroendocrine effects in animals difficult to apply to human risk assessment because it is not readily apparent how estrogenic compounds, like the phytoestrogens, might impact the sexual differentiation of the human hypothalamus or other brain regions. Our lab has tried to address this by exploring the impact of perinatal phytoestrogen exposure on a neuroendocrine system that was initially discovered in humans: the kisspeptin system.
It has rapidly become apparent that neurons which express the kiss1 gene are the primary gatekeepers of GnRH release in many species, including rats and humans [188,125,263,264,181]. This gene codes for a family of peptides called kisspeptins (previously called metastins). In rodents, kisspeptin neurons lie predominantly within two sexually dimorphic hypothalamic regions, both of which were already well appreciated for their importance in regulating GnRH secretion: the AVPV and arcuate (ARC) nucleus [256,96,219,218,259]. AVPV kisspeptin neurons are more numerous in females than males and are thought to be essential for steroid positive feedback and the initiation of the preovulatory GnRH surge [125,264,226,93,114,46]. In contrast, kiss1 mRNA expression in the ARC is not thought to be sexually dimorphic and appears to be important for the regulation of steroid negative feedback and, possibly, pubertal onset [125,124]. We recently showed that neonatal exposure to 10 mg/kg genistein significantly decreases the density of neuronal fibers immunoreactive for kisspeptin in the AVPV but not the ARC of female rats (Fig. 3) [209,22]. This was accompanied by early vaginal opening (a hallmark of puberty in the rat), premature anestrous and blunted GnRH activation. Our findings suggest that disrupted organization of kisspeptin signaling pathways by genistein may be a novel yet comprehensive mechanism underlying a suite of reproductive abnormalities in females.
7.4. Animal data: abnormal development of the female reproductive tract
Altered timing of pubertal onset and estrous cyclicity following perinatal phytoestrogen exposure has been shown by us and others in rodents [21,129,118,185]. Mice or rats treated perinatally with lower doses of genistein (0.5–10 mg/kg) advance the timing of vaginal opening while higher doses (50 mg/kg) have no impact or delay it. Estrous cyclicity is also disrupted following developmental exposure to genistein. Mice or rats treated neonatally with genistein spend significantly longer periods of time in the estrous phase of the cycle; this abnormality increases in severity with increasing dose as well as increasing age [21,129,118]. Other investigators have shown similar estrous cycle alterations in experimental animal models including a study by Nikaido et al. which reported developmental exposure to numerous environmental estrogens, including genistein, resveratrol, zearalenone, and Bisphenol A, resulted in extended estrous cycles when the animals became adults [185]. This is similar to mice exposed perinatally to DES [167] further confirming the idea that developmental exposure to estrogens causes disruptions in estrous cyclicity. These abnormalities could result from organizational disruptions anywhere within the HPG axis, including the ovary. Other aspects of reproductive tract development may be vulnerable as well.
In rodents, neonatal exposure to genistein alters ovarian differentiation, reduces fertility and causes uterine cancer later in life. Most of these studies have been done with subcutaneous injections of the aglycone form of the compound, genistein. Mice treated by subcutaneous injection of genistein at a dose of 50 mg/kg/day have peak serum circulating levels of genistein of 3.4–6.2 µM [61]. These levels are similar to infants on soy-based infant formulas (1– 5.4 µM) [34,247]. This level of genistein exhibits estrogenic activity in the neonatal mouse as measured by uterine wet weight gain during the time of treatment [184].
Neonatal, subcutaneous injection of genistein at doses of 0.5, 5 and 50 mg/kg/day produced a dose-dependent increase in the number of mice with multi-oocyte follicles (MOFs) in the ovary prior to puberty with almost all of the mice in the highest treatment group having MOFs [116]. This effect is mediated through ERβ since mice lacking ERβ do not develop MOFs following neonatal treatment with genistein [116]. The formation of MOFs was further characterized by observing ovarian development during the time of neonatal treatment [119]. At birth, mice have large oocyte clusters or nests; these nests dissociate into individual oocytes surrounded by granulosa cells during the first week of life [212]. Treatment with genistein at a dose of 50 mg/kg on days 1–5 inhibits this differentiation process leaving the oocytes together in nests and still attached to each other by intercellular bridges. This study also showed a higher percentage of unassembled oocytes (those not completely surrounded with granulosa cells) further supporting the limited differentiation of the ovary in genistein treated mice. MOFs have also been found in rats treated during development with genistein suggesting that this occurrence is not limited to mice [178]. Further, the presence of MOFs has also been noted in humans, supporting the idea that effects observed in animal models translate to humans, although the cause in humans is still not known. Several other estrogenic compounds have also been found to cause MOFs if exposure occurs during development including 17β-estradiol, DES and Bisphenol A [111,109,110,269] confirming that estrogenic substances alter ovarian differentiation. It has also been shown that oocytes derived from MOFs have reduced quality since in vitro fertilization of these oocytes is markedly reduced compared to single oocyte follicles [111]. However, a recent study in our laboratory showed that eggs from mice treated neonatally with genistein had normal morphology (normal meiotic spindles) and fertilized equally as well as eggs from control mice suggesting the major contributor of infertility in these mice is not egg quality [120]. One caveat is that these studies were done in young mice (2 months of age) and it is not known if the quality of the eggs decreases later in life.
Altered ovarian function has also been observed following developmental exposure to genistein. A recent study from our laboratory showed the complete lack of corpora lutea (CL) and anovulation at 4 months of age following neonatal exposure to genistein at 50 mg/kg on days 1–5 indicating ovarian function was disrupted [3]. Doses of genistein lower than 50 mg/kg showed enhanced ovulation rates as evidenced by increased numbers of oocytes ovulated following exogenous gonadotropins at 26 days of age [116] as well as increased numbers of CLs at 4 months of age [118]. Whether these effects are due to a direct effect on the ovary or an indirect effect on the HPG axis is not fully elucidated, but an indirect mechanism appears most likely, as shown by the following studies. Exogenous gonadotropins restored ovarian function in mice treated with the high dose of genistein as evidenced by similar numbers of oocytes ovulated compared to controls. In addition, higher neonatal doses of genistein exposure were associated with decreased pituitary responsiveness and suppressed LH production in response to GnRH stimulation [71]. The LH surge is necessary for ovulation so lower levels of LH may explain the lack of ovulation seen in the high dose treated mice. Interestingly, in that same study rats treated with lower doses of genistein (0.01 mg/kg) were hyper-responsive to GnRH stimulation leading to enhanced ovulation rates similar to data from our laboratory using younger mice treated neonatally with low doses of genistein and again in older mice with increased CLs at 4 months of age [71,118,116]. These data are consistent with our own demonstrating that neonatal exposure to genistein in rats abrogates GnRH neuronal activation following hormone priming as measured by fos co-immunoreactivity with GnRH in neurons [21]. These data taken together, suggest that the HPG axis is disrupted following developmental exposure to genistein.
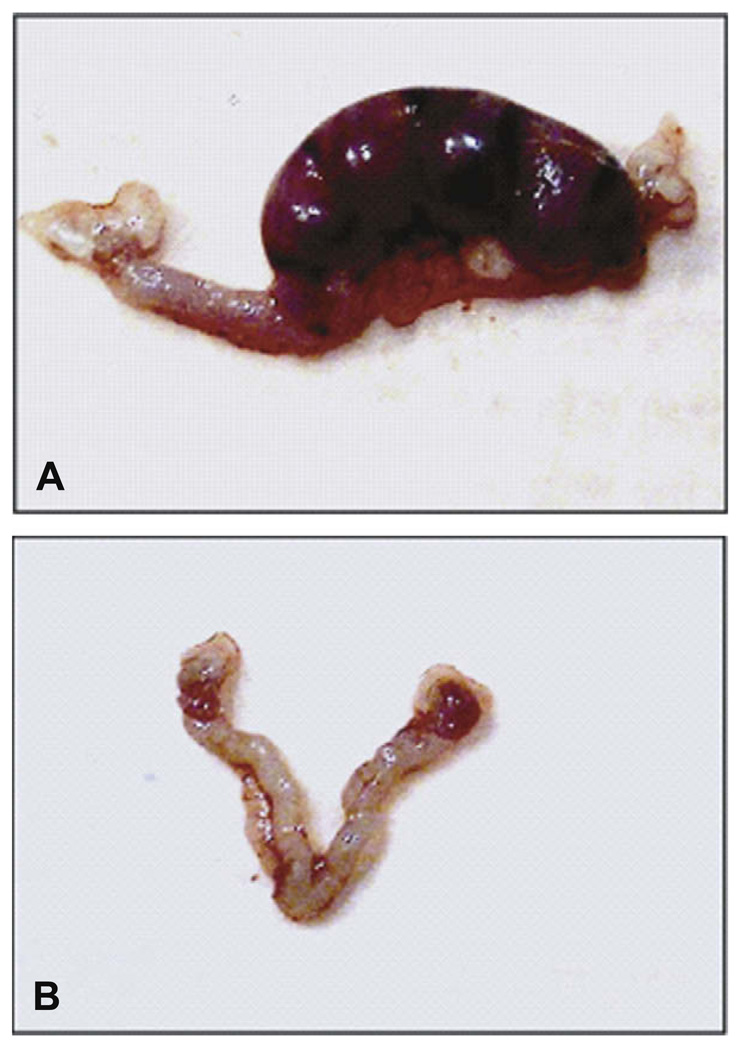
Female fertility is also disrupted in rodents following developmental exposure to genistein. Female mice treated with lower doses of genistein (0.5 and 5 mg/kg) showed no difference in the numbers of mice delivering live pups compared to controls at 2 and 4 months of age. However, by 6 months of age, a reduction in the percentage of mice delivering live pups in both treatment groups compared to controls was seen as well as a reduction in the number of live pups in the mice that delivered; these findings suggest early reproductive senescence [118]. Mice treated neonatally with genistein (25 mg/kg) exhibit reduced fertility at 2 months of age with only 4 out of 8 (50%) plug positive mice delivering live pups. Female mice treated neonatally with genistein (50 mg/kg) did not deliver any live pups at 2 months of age (0/8 mice) suggesting mice exposed developmentally to this dose are infertile. A study from another laboratory supports our findings since rats treated with genistein (100 mg/kg) also showed disruption of fertility [178]. Since mice treated with genistein 50 mg/kg did not deliver live pups, additional studies were conducted to characterize the source of infertility. Less than half of the genistein treated mice showed signs of pregnancy following vaginal plug positive compared to almost all of the controls. In addition to low numbers of pregnancies, the females that were pregnant had smaller and fewer implantation sites compared to controls. There were also visible reabsorption sites in some of the genistein treated mice. One possible explanation for these implantation defects is that the environment of the uterus or the hormonal milieu is not suitable for implantation. However, serum hormones measured during pregnancy did not reveal any deficiencies in hormones needed to maintain pregnancy such as progesterone and estradiol suggesting that these are not a likely cause of the implantation problems [118]. Another possibility for implantation problems and early pregnancy loss is that the oocyte itself is of poor quality. As mentioned previously, this is not the case since eggs retrieved from genistein treated mice have good morphology and are equally fertilizable as control eggs [120]. Further study revealed that the oviductal and uterine environments are not suitable to maintain pregnancy. There is a 50% loss of embryos during transit through the oviduct of genistein treated mice and embryo transfer experiments showed that the uterus of genistein treated mice is not capable of sustaining pregnancy even if the blastocysts are from control mice (Fig. 4) [120]. These data suggest that there is a permanent change in the function of the female reproductive tract that leads to complete infertility in these mice.
Fig. 4.
Implantation sites following transfer of control blastocysts into control and genistein-treated recipient mice. (A) Uterus of a control mouse 8 days after transfer of control blastocysts (4 normal implantation sites). (B) Uterus of a genistein-treated mouse 8 days after transfer of control blastocysts (no implantation sites visible).
One of the main criticisms of these studies and others is the route of exposure, subcutaneous injection of the aglycone form, genistein. To address these concerns a recent study was conducted to compare oral exposure of the glycosylated form, genistin with the previous work. Genistin is the predominant form found in soy based products and human infants are exposed orally. Mice exposed on neonatal days 1–5 with oral genistein at doses of 25 and 37.5 mg/kg/day exhibit estrogenic activity similar to mice exposed by subcutaneous injection with genistein at doses of 20 and 25 mg/kg [120]. It takes about 80% of the subcutaneous dose to cause the same level of uterine weight gain as the oral dose suggesting the compound is readily absorbed and reaches the target organs (e.g. reproductive tract). Circulating levels of total genistein reached peak values higher than following subcutaneous injection (19.2 µM vs. 6.2 µM) but the total area under the curve was very similar, 83% of the subcutaneous dose. The aglycone levels were about 50% of the subcutaneous dose but the biological activity was around 80%. This data suggests that resulting biological activity is complex and the peak and total circulating levels may contribute to the overall biological effect. It is not possible to conduct these type of experiments in human infants so the only levels we have are single point levels at unknown times post feeding so they cannot be directly compared; however, similar levels are attained in both rodents and human (µM range).
Female mice treated orally with genistein at doses of 6.25, 12.5, 25 and 37.5 mg/kg developed multi-oocyte follicles, altered vaginal opening and altered estrous cyclicity in a dose dependent manner similar to previous studies using subcutaneous injections of genistein [120]. These mice also developed subfertility increasing in severity with increasing dose and over time similar to mice exposed by subcutaneous injection of genistein. This study confirms that it is not the route of exposure that is important but the amount of biologically active compound that gets to the target.
7.5. Animal data: abnormal development of the male reproductive tract
There is a surprising paucity of data on the developmental effects of phytoestrogens in males (reviewed in 2009) [37]. To date, no data on the long term consequences of gestation-only exposure are currently available. There was a large multi-generational study conducted in rats exposed to genistein through the diet throughout the lifetime of the animal [55]. The dose range finding portion of this study exposed rats to genistein in the diet starting on gestational day 7 and through lactation until weaning and then in the diet until postnatal day 50 (adulthood). The doses used in that study were approximately 0.3, 1.7, 6.4, 16, 38, and 72 mg/kg to the dam and 0.6, 3, 11, 29, 69, and 166 mg/kg to the pups after weaning. In addition to females developing ductal/alveolar hyperplasia in the mammary gland, males in this study developed mammary gland hypertrophy at doses at or above 11 mg/kg and mammary gland hyperplasia at doses at or above 29 mg/kg. Males in this study had reduced prostate weight following the highest dose. There were very few additional effects on the male reproductive tract in this study. However, studies examining the actual dose of the chemical that entered circulation revealed that there was very minimal exposure to the neonates through lactation so the male rats were exposed primarily prenatally and in adulthood but not during the neonatal period [62]. More studies should be conducted to determine potential adverse effects in males during critical periods of development, particularly during neonatal life. A pair of studies that have had a profound impact on the field, and greatly contributed to health advisories in Europe was conducted in marmosets. Twins were fed either soy formula or milk formula. Males on the soy diet had lower serum testosterone concentrations and higher numbers of Leydig cells at the discontinuation of soy formula use. As adults, the soy fed marmoset had larger testes and lower serum testosterone levels than its twin demonstrating that the impacts were persistent [271,253].
8. Con: evidence for long term health consequences of soy infant formula in humans
The question of whether or not soy formula is safe has been extensively debated across the globe for more than a decade, and a litany of review articles and position papers have been published on the subject [248,29,15,19,86,279,307,41] so an extensive overview will not be recapitulated here. The National Toxicology Program recently completed its most recent safety assessment of soy infant formula (available at http://cerhr.niehs.nih.gov/chemicals/genistein-soy/SoyFormulaUpdt/FinalEPReport_508.pdf) and concluded there is “minimal concern for adverse developmental effects.” To put this classification in context, the NTP uses a 5 level scale and this is level 2 (with 5 being the most serious level of concern). It is the same levels of concern initially expressed for BPA until mounting evidence for wide ranging health effects pressed the FDA to elevate that advisory to “some concern” in January, 2010. The isoflavone phytoestrogens genistein and daidzien, as well as many others, have far greater relative binding affinities for both ERα and ERβ than BPA, raising concern that these compounds pose an underappreciated threat to infant development.
In 2008 the American Academy of Pediatricians (AAP) concluded that in terms of nutritional quality “isolated soy protein-based formula has no advantage over cow milk protein-based formula” and that soy formula has “no proven value in the prevention or management of infantile colic or fussiness” but stopped short of recommending against its use. Internationally, use of soy formula is viewed more cautiously than in the US. For example, the United Kingdom, Australia and New Zealand all caution against indiscriminate use [41]. Other nations offer it only by prescription and, consequently, use of soy formula is customarily lower in these countries. These types of advisories are confusing for parents and have done little to quash the idea that soy formula is a healthful alternative to breastfeeding.
An apparent lack of notable long term effects is one reason why so many consumers, clinicians and public health agencies consider regular use of soy formula to be safe, even beneficial. This near absence of documented effects in soy-fed infants is not entirely reassuring, however, because although soy infant formula has been widely available for more than four decades, surprisingly little work has been done regarding its potential long term effects on reproduction, fertility or behavior. Historically, epidemiological studies have mainly focused on nutritional status, growth parameters, and impacts on the thyroid system because soy has long been recognized to induce hypothyroidism and goiter when not counteracted with elevated iodine intake [43,57]. Very few have explored the possibility that soy formula use can impact reproductive development or function and those which have, are hampered by insufficient sample sizes and the absence of appropriately sensitive measures. The task is not easy because, as with many endocrine disrupting compounds, isoflavone effects, if present, may not manifest for years, even decades, and are likely mild enough to escape clinical detection.
The earliest evidence for reproductive health effects came from two studies, conducted in the mid 1980’s, which associated neonatal phytoestrogen exposure with thelarche before age 2 in a population of Puerto Rican girls. A number of confounding factors, however, including the consumption of chicken that had been fattened with potent estrogens, make the data problematic and difficult to interpret [83,240]. Within the last decade, a retrospective cohort study of 952 women found that young women fed soy-based infant formula (248 women) as part of a controlled, University of Iowa feeding study, reported longer menstrual bleeding and menstrual discomfort than those who were fed a non-soy-based formula (563 women) [268]. At the time the study was conducted, the women were too young to comprehensively examine pregnancy or fertility outcomes, but, now that nearly a decade has past, this area is ripe for reevaluation. Most recently, soy formula consumption has been linked to a greater risk of developing uterine fibroids [51]. The study population included over 19,000 women enrolled in the Sister Study, making it one of the largest and most highly powered epidemiological evaluations of the long term reproductive health impacts of soy formula.
When considering the potential safety of soy formula, one argument that frequently comes up is that Asian populations have been consuming soy for a long time, with no obvious consequences. This argument fails to recognize, however, that intake levels between Asians consuming a traditional soy-rich diet and Caucasians eating a typical “Western” diet differ dramatically over the lifespan. This temporal divergence may explain why there appear to be differences in both the pros and cons of phytoestrogen exposure between the two populations. In Asian populations, soy consumption is high across the entire lifespan, except for a brief 6–8 month neonatal breastfeeding window. In Westerners feeding their babies soy infant formula the pattern is just the opposite, and the highest consumption levels occur in the first year of life then drop to near zero. In Asia, soy is consumed mostly in the form of tofu, tempeh, and other unprocessed foods, not as dietary supplements or products enriched with soy protein isolate. Asian populations also eat considerably higher levels of seafood and low levels of animal fat than Western populations. These variables make the two populations quite distinct in terms of lifestyle, dietary habits, and lifetime phytoestrogen exposure. Thus, phytoestrogen effects may differ between the two groups, a possibility that should be taken into account when interpreting epidemiological data.
9. Conclusions
Phytoestrogens are intriguing because, although they behave similarly to numerous synthetic compounds in laboratory models of endocrine disruption, society embraces these compounds at the same time it rejects, often with vigor, use of synthetic endocrine disruptors in household products. Thus, phytoestrogens both expand our view of environmental endocrine disruptors and propound that the source of the compound in question can influence the direction and interpretation of research and available data. While the potentially beneficial effects of phytoestrogen consumption have been eagerly pursued, and frequently overstated, the potentially adverse effects of these compounds are likely underappreciated. The opposite situation exists for synthetic endocrine disruptors, most of which have lower binding affinities for classical ERs than any of the phytoestrogens but can sometime produce similar biological effects. Animal data reveal that the isoflavones have a wide range of molecular, cellular and behavioral effects at doses and plasma concentrations attainable in humans. In vivo isoflavone responses have been reported for a wider range of tissues and processes than the endpoints generally used to evaluate most synthetic EDCs [293], yet only minimal concern has been raised about their increasing use. Infants fed soy formula have the highest exposure to any nonpharmacological source of estrogen-like compounds, yet we know virtually nothing about how the use of these phytoestrogen-rich formulas might impact their future reproductive health. Although relative few adverse effects have been detected, that may simply be because a surprising paucity of large-scale, comprehensive studies have been undertaken to address this issue, especially in boys. That may change in the near future because the health effects of endocrine disrupting compounds in general are receiving more attention from public health agencies, and the public at large.
As with many other compounds, like alcohol or caffeine, there are many pros and cons associated with moderate soy intake. Consumers should be aware that soy contains endocrine disrupting compounds and make dietary choices accordingly. For a typical consumer, alarm over soy products is likely unnecessary but so is the belief that a soy-rich diet will alleviate all ills. Women who are pregnant, nursing, or attempting to become pregnant should use soy foods with caution and be aware that soy formula may not be the best option for their babies. Older individuals, especially those with high cholesterol, may experience modest benefits including improved bone and cardiovascular health, and perhaps a decreased risk of carcinogenesis. Moderation is likely key and the incorporation of real foods, as opposed to supplements or processed foods to which soy protein is added, is probably essential for maximizing health benefits. Finally, the relative importance of the soy protein itself, compared to the isoflavones, on health outcomes such as lipid levels, reduced risk of carcinogenesis, and fracture risk must be resolved. If something other than the isoflavone phytoestrogens is producing the mild but measurable health benefits of soy foods, this would considerably help shape the development of dietary guidelines for both adults and children.
Acknowledgments
The authors would like to thank Karina Todd for her invaluable comments in preparation of this review and Barbara Aulicino for the artwork depicted in Fig. 1.
References
- 1.Adams NR. Detection of the effects of phytoestrogens on sheep and cattle. J. Anim. Sci. 1995;73:1509–1515. doi: 10.2527/1995.7351509x. [DOI] [PubMed] [Google Scholar]
- 2.Adams NR. Organizational and activational effects of phytoestrogens on the reproductive tract of the ewe. Proc. Soc. Exp. Biol. Med. 1995;208:87–91. doi: 10.3181/00379727-208-43837. [DOI] [PubMed] [Google Scholar]
- 3.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal Bisphenol-A exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin releasing hormone neurons. Biol. Reprod. 2009 doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adlercreutz H, Mazur W. Phyto-oestrogens and western diseases. Ann. Med. 1997;29:95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 5.Adlercreutz H, Mousavi Y, Clark J, Höckersted K, Hämäläinen EK, Wähälä K, Mäkelä T, Hase T. Dietary phytoestrogens and cancer: in vitro and in vivo studies. J. Steroid Biochem. Mol. Biol. 1992;41:331–337. doi: 10.1016/0960-0760(92)90359-q. [DOI] [PubMed] [Google Scholar]
- 6.Adlercreutz H, Fotsis T, Watanabe S, Lampe J, Wahala K, Makela T, Hase T. Determination of lignans and isoflavonoids in plasma by isotope dilution gas chromatography-mass spectrometry. Cancer Detect Prev. 1994;18:259–271. [PubMed] [Google Scholar]
- 7.Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am. J. Obstet. Gynecol. 1999;180:737–743. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R. Cell signaling and regulators of cell cycle as molecular targets for prostate cancer prevention by dietary agents. Biochem. Pharmacol. 2000;60:1051–1059. doi: 10.1016/s0006-2952(00)00385-3. [DOI] [PubMed] [Google Scholar]
- 9.Aksglaede L, Sorensen K, Petersen JH, Skakkebaek NE, Juul A. Recent decline in age at breast development: the copenhagen puberty study. Pediatrics. 2009;123:e932–e939. doi: 10.1542/peds.2008-2491. [DOI] [PubMed] [Google Scholar]
- 10.Anderson JJ, Ambrose WW, Garner SC. Biphasic effects of genistein on bone tissue in the ovariectomized, lactating rat model. Proc. Soc. Exp. Biol. Med. 1998;217:345–350. doi: 10.3181/00379727-217-44243. [DOI] [PubMed] [Google Scholar]
- 11.Arai Y, Uehara M, Sato Y, Kimira M, Eboshida A, Adlercreutz H, Watanabe S. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J. Epidemiol. 2000;10:127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson C, Warren RM, Sala E, Dowsett M, Dunning AM, Healey CS, Runswick S, Day NE, Bingham SA. Red-clover-derived isoflavones and mammographic breast density: a double-blind, randomized, placebo-controlled trial [ISRCTN42940165] Breast Cancer Res. 2004;6:R170–R179. doi: 10.1186/bcr773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Soya - a dietary source of the non-steroidal oestrogen equol in man and animals. J. Endocrinol. 1984;102:49–56. doi: 10.1677/joe.0.1020049. [DOI] [PubMed] [Google Scholar]
- 14.Badger TM, Ronis MJ, Hakkak R, Rowlands JC, Korourian S. The health consequences of early soy consumption. J. Nutr. 2002;132:559S–565S. doi: 10.1093/jn/132.3.559S. [DOI] [PubMed] [Google Scholar]
- 15.Badger TM, Gilchrist JM, Pivik RT, Andres A, Shankar K, Chen JR, Ronis MJ. The health implications of soy infant formula. Am. J. Clin. Nutr. 2009;89:1668S–1672S. doi: 10.3945/ajcn.2009.26736U. [DOI] [PubMed] [Google Scholar]
- 16.Balk E, Chung M, Chew P, Ip S, Raman G, Kupelnick B, Tatsioni A, Sun Y, Devine D, Lau J. Effects of soy on health outcomes. Evid. Rep. Technol. Assess. (Summ) 2005:1–8. doi: 10.1037/e439502005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barkhem T, Carlsson B, Nilsson Y, Enmark E, Gustafsson JA, Nilsson S. Differential response of estrogen receptor α and estrogen receptor β to partial estrogen agonists/antagonists. Mol. Pharmacol. 1998;54:105–112. doi: 10.1124/mol.54.1.105. [DOI] [PubMed] [Google Scholar]
- 18.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J. Nutr. 1995;125:S777–S783. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 19.Barrett JR. Soy and children’s health: a formula for trouble. Environ. Health Perspect. 2002;110:A294–A296. doi: 10.1289/ehp.110-a294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett JR. The science of soy: what do we really know? Environ Health Perspect. 2006;114:A352–A358. doi: 10.1289/ehp.114-a352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–997. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008 doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, Pistell PJ, Poosala S, Becker KG, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein KW, Spencer RG, Lakatta EG, Le Couteur D, Shaw RJ, Navas P, Puigserver P, Ingram DK, de Cabo DK, Sinclair DA. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beher D, Wu J, Cumine S, Kim KW, Lu SC, Atangan L, Wang M. Resveratrol is not a direct activator of SIRT 1 enzyme activity. Chem. Biol. Drug Des. 2009;74:619–624. doi: 10.1111/j.1747-0285.2009.00901.x. [DOI] [PubMed] [Google Scholar]
- 25.Bennetts HHW, Underwood EEJ. The oestrogenic effects of subterranean clover (trifolium subterraneum); uterine maintenance in the ovariectomised ewe on clover grazing. Aust. J. Exp. Biol. Med. Sci. 1951;29:249–253. doi: 10.1038/icb.1951.29. [DOI] [PubMed] [Google Scholar]
- 26.Bennetts HW, Underwood EJ, Shier FL. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust. Vet. J. 1946;22:2. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 27.Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 28.Bernbaum JC, Umbach DM, Ragan NB, Ballard JL, Archer JI, Schmidt-Davis H, Rogan WJ. Pilot studies of estrogen-related physical findings in infants. Environ. Health Perspect. 2008;116:416–420. doi: 10.1289/ehp.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121:1062–1068. doi: 10.1542/peds.2008-0564. [DOI] [PubMed] [Google Scholar]
- 30.Bouker KB, Hilakivi-Clarke L. Genistein: does it prevent or promote breast cancer? Environ Health Perspect. 2000;108:701–708. doi: 10.1289/ehp.00108701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boutin JA. Minireview - Tyrosine protein kinase inhibition and cancer. Int. J. Biochem. Cell Biol. 1994;26:1203–1226. doi: 10.1016/0020-711x(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 32.Braden A, Hart N, Lamberton J. Oestrogenic activity and metabolism of certain isoflavones in sheep. Aust. J. Agric. Res. 1967;18:348–355. [Google Scholar]
- 33.Caldwell JD, Johns JM, Faggin BM, Senger MA, Pedersen CA. Infusion of an oxytocin antagonist into the medial preoptic area prior to progesterone inhibits sexual receptivity and increases rejection in female rats. Horm. Behav. 1994;28:288–302. doi: 10.1006/hbeh.1994.1024. [DOI] [PubMed] [Google Scholar]
- 34.Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J. Expo. Sci. Environ. Epidemiol. 2009;19:223–234. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Casanova M, You L, Gaido K, Archibeque-Engle S, Janszen D, Heck H. Developmental effects of dietary phytoestrogens in Sprague-Dawley rats and interactions of genistein and daidzein with rat estrogen receptors alpha and beta in vitro. Toxicol. Sci. 1999;51:236–244. doi: 10.1093/toxsci/51.2.236. [DOI] [PubMed] [Google Scholar]
- 36.Cassidy A, Albertazzi P, Lise Nielsen I, Hall W, Williamson G, Tetens I, Atkins S, Cross H, Manios Y, Wolk A, Steiner C, Branca F. Critical review of health effects of soyabean phyto-oestrogens in post-menopausal women. Proc. Nutr. Soc. 2006;65:76–92. doi: 10.1079/pns2005476. [DOI] [PubMed] [Google Scholar]
- 37.Cederroth CR, Auger J, Zimmermann C, Eustache F, Nef S. Soy, phytooestrogens and male reproductive function: a review. Int. J. Androl. 2009 doi: 10.1111/j.1365-2605.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 38.Chandrareddy A, Muneyyirci-Delale O, McFarlane SI, Murad OM. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complement Ther. Clin. Pract. 2008;14:132–135. doi: 10.1016/j.ctcp.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Chang HC, Churchwell MI, Delclos KB, Newbold RR, Doerge DR. Mass spectrometric determination of genistein tissue distribution in diet-exposed Sprague-Dawley rats. J. Nutr. 2000;130:1963–1970. doi: 10.1093/jn/130.8.1963. [DOI] [PubMed] [Google Scholar]
- 40.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Impact of estrogen receptor beta on gene networks regulated by estrogen receptor alpha in breast cancer cells. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 41.Chen A, Rogan WJ. Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu. Rev. Nutr. 2004;24:33–54. doi: 10.1146/annurev.nutr.24.101603.064950. [DOI] [PubMed] [Google Scholar]
- 42.Chen S, Kao YC, Laughton CA. Binding characteristics of aromatase inhibitors and phytoestrogens to human aromatase. J. Steroid Biochem. Mol. Biol. 1997;61:107–115. [PubMed] [Google Scholar]
- 43.Chorazy PA, Himelhoch S, Hopwood NJ, Greger NG, Postellon DC. Persistent hypothyroidism in an infant receiving a soy formula: case report and review of the literature. Pediatrics. 1995;96:148–150. [PubMed] [Google Scholar]
- 44.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 45.Chun OK, Chung SJ, Song WO. Urinary isoflavones and their metabolites validate the dietary isoflavone intakes in US adults. J. Am. Diet Assoc. 2009;109:245–254. doi: 10.1016/j.jada.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 46.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE. Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. J. Neurosci. 2008;28:8691–8697. doi: 10.1523/JNEUROSCI.1775-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cooke GM. A review of the animal models used to investigate the health benefits of soy isoflavones. J. AOAC Int. 2006;89:1215–1227. [PubMed] [Google Scholar]
- 48.Cooke B, Hegstrom C, Villeneuve L, Breedlove S. Sexual differeniation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 49.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front. Neuroendocrinol. 1998;19:323–362. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 50.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil. Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Association of intrauterine and early life exposures with diagnosis of uterine leiomyomata by age 35 in the sister study. Environ. Health Perspect. 2009 doi: 10.1289/ehp.0901423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis EC, Shryne JE, Gorski RA. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology. 1996;63:142–148. doi: 10.1159/000126950. [DOI] [PubMed] [Google Scholar]
- 53.Dechaud H, Ravard C, Claustrat F, Brac de la Perriere A. Xenoestrogen interaction with human sex hormone-binding globulin (hSHBG) Steroids. 1999;64:328–334. doi: 10.1016/s0039-128x(98)00114-7. [DOI] [PubMed] [Google Scholar]
- 54.Degen GH, Janning P, Diel P, Bolt HM. Estrogenic isoflavones in rodent diets. Toxicol. Lett. 2002;128:145–157. doi: 10.1016/s0378-4274(02)00009-7. [DOI] [PubMed] [Google Scholar]
- 55.Delclos KB, Newbold R. NTP toxicity report of reproductive dose range-finding study of genistein (CAS No. 446-72-0) administered in feed to Sprague-Dawley rats. Toxicol. Rep. Ser. 2007 1-C2. [PubMed] [Google Scholar]
- 56.Demonty I, Lamarche B, Jones PJ. Role of isoflavones in the hypocholesterolemic effect of soy. Nutr. Rev. 2003;61:189–203. doi: 10.1301/nr.2003.jun.189-203. [DOI] [PubMed] [Google Scholar]
- 57.Divi RL, Chang HC, Doerge DR. Anti-thyroid isoflavones from soybean: isolation, characterization, and mechanisms of action. Biochem. Pharmacol. 1997;54:1087–1096. doi: 10.1016/s0006-2952(97)00301-8. [DOI] [PubMed] [Google Scholar]
- 58.Dodds EC, Goldberg L, Larson W, Robinson R. Estrogenic activity of certain synthetic compounds. Nature. 1938;141:247. [Google Scholar]
- 59.Dodge JA, Glasebrook AL, Magee DE, Phillips DL, Sato M, Short LL, Bryant HU. Environmental estrogens: effects on cholesterol lowering and bone in the ovariectomized rat. J. Steroid Biochem. Mol. Biol. 1996;59:155–161. doi: 10.1016/s0960-0760(96)00104-5. [DOI] [PubMed] [Google Scholar]
- 60.Doerge DR, Churchwell MI, Chang HC, Newbold RR, Delclos KB. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod. Toxicol. 2001;15:105–110. doi: 10.1016/s0890-6238(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 61.Doerge DR, Twaddle NC, Banks EP, Jefferson WN, Newbold RR. Pharmacokinetic analysis in serum of genistein administered subcutaneously to neonatal mice. Cancer Lett. 2002;184:21–27. doi: 10.1016/s0304-3835(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 62.Doerge DR, Twaddle NC, Churchwell MI, Newbold RR, Delclos KB. Lactational transfer of the soy isoflavone, genistein, in Sprague-Dawley rats consuming dietary genistein. Reprod. Toxicol. 2006;21:307–312. doi: 10.1016/j.reprotox.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 63.Draper CR, Edel MJ, Dick IM, Randall AG, Martin GG, Prince RL. Phytoestrogens reduce bone loss and bone resorption in oophorectomized rats. J. Nutr. 1997;127:1795–1799. doi: 10.1093/jn/127.9.1795. [DOI] [PubMed] [Google Scholar]
- 64.Drummond A, Fuller P. The importance of ER{beta} signalling in ovarian function. J. Endocrinol. 2009 [Google Scholar]
- 65.Duncan AM, Merz BE, Xu X, Nagel TC, Phipps WR, Kurzer MS. Soy isoflavones exert modest hormonal effects in premenopausal women. J. Clin. Endocrinol. Metab. 1999;84:192–197. doi: 10.1210/jcem.84.1.5387. [DOI] [PubMed] [Google Scholar]
- 66.Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Ann. NY Acad. Sci. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- 67.Elkind-Hirsch K, King JC, Gerall AA, Arimura AA. The luteinizing hormone-releasing hormone (LHRH) system in normal and estrogenized neonatal rats. Brain Res. Bull. 1981;7:645–654. doi: 10.1016/0361-9230(81)90112-x. [DOI] [PubMed] [Google Scholar]
- 68.Enderlin CA, Coleman EA, Stewart CB, Hakkak R. Dietary soy intake and breast cancer risk. Oncol. Nurs. Forum. 2009;36:531–539. doi: 10.1188/09.ONF.531-539. [DOI] [PubMed] [Google Scholar]
- 69.Engel SM, Levy B, Liu Z, Kaplan D, Wolff MS. Xenobiotic phenols in early pregnancy amniotic fluid. Reprod. Toxicol. 2006;21:110–112. doi: 10.1016/j.reprotox.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 70.Evans BA, Griffiths K, Morton MS. Inhibition of 5 alpha-reductase in genital skin fibroblasts and prostate tissue by dietary lignans and isoflavonoids. J. Endocrinol. 1995;147:295–302. doi: 10.1677/joe.0.1470295. [DOI] [PubMed] [Google Scholar]
- 71.Faber KA, Hughes CL., Jr Dose-response characteristics of neonatal exposure to genistein on pituitary responsiveness to gonadotropin releasing hormone and volume of the sexually dimorphic nucleus of the preoptic area (SDN-POA) in postpubertal castrated female rats. Reprod. Toxicol. 1993;7:35–39. doi: 10.1016/0890-6238(93)90007-t. [DOI] [PubMed] [Google Scholar]
- 72.Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones - a focus on rapid, nongenomic effects. Pharmacol. Rev. 2000;52:513–556. [PubMed] [Google Scholar]
- 73.Farquharson RG, Klopper AI. A study of maternal, retroplacental and umbilical cord estradiol levels in term infants delivered by caesarean section. Eur. J. Obst. Gynecol. Reprod. Biol. 1984;16:315–320. doi: 10.1016/0028-2243(84)90159-x. [DOI] [PubMed] [Google Scholar]
- 74.Fisher JS. Environmental anti-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- 75.Food and Drug Administration. Food labeling: health claims; soy protein and coronary heart disease. Food and Drug Administration, HHS. Final rule. Fed. Regist. 1999;64:57700–57733. [PubMed] [Google Scholar]
- 76.Food and Drug Administration. Health claims and qualified health claims; dietary lipids and cancer, soy protein and coronary heart diease, antioxidant vitamins and certain cancers, and selenium and certain cancers. Reevaluation. 2007 [Google Scholar]
- 77.Forsyth BW, McCarthy PL, Leventhal JM. Problems of early infancy, formula changes, and mothers’ beliefs about their infants. J. Pediatr. 1985;106:1012–1017. doi: 10.1016/s0022-3476(85)80260-2. [DOI] [PubMed] [Google Scholar]
- 78.Foster WG, Chan S, Platt L, Hughes CL., Jr Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol. Lett. 2002;129:199–205. doi: 10.1016/s0378-4274(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 79.Franke AA, Custer LJ. High-performance liquid chromatographic assay of isoflavonoids and coumestrol from human urine. J. Chromatogr. B: Biomed. Appl. 1994;662:47–60. doi: 10.1016/0378-4347(94)00390-4. [DOI] [PubMed] [Google Scholar]
- 80.Franke AA, Custer LJ. Daidzein and genistein concentrations in human milk after soy consumption. Clin. Chem. 1996;42:955–964. [PubMed] [Google Scholar]
- 81.Franke AA, Custer LJ, Tanaka Y. Isoflavones in human breast milk and other biological fluids. Am. J. Clin. Nutr. 1998;68:1466S–1473S. doi: 10.1093/ajcn/68.6.1466S. [DOI] [PubMed] [Google Scholar]
- 82.Franke AA, Halm BM, Custer LJ, Tatsumura Y, Hebshi S. Isoflavones in breastfed infants after mothers consume soy. Am. J. Clin. Nutr. 2006;84:406–413. doi: 10.1093/ajcn/84.1.406. [DOI] [PubMed] [Google Scholar]
- 83.Freni-Titulaer LLW, Cordero JJF, Haddock LL, Lebrón GG, Martínez RR, Mills JJL. Premature thelarche in Puerto Rico. A search for environmental factors. Am. J. Dis. Child. 1986;140:1263–1267. doi: 10.1001/archpedi.1986.02140260065028. [DOI] [PubMed] [Google Scholar]
- 84.Fritz WA, Coward L, Wang J, Lamartiniere CA. Dietary genistein: perinatal mammary cancer prevention, bioavailability and toxicity testing in the rat. Carcinogenesis. 1998;19:2151–2158. doi: 10.1093/carcin/19.12.2151. [DOI] [PubMed] [Google Scholar]
- 85.Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann. Intern. Med. 1995;122:778–788. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- 86.Goldman LR, Newbold R, Swan SH. Exposure to soy-based formula in infancy. JAMA. 2001;286:2402–2403. doi: 10.1001/jama.286.19.2402. [DOI] [PubMed] [Google Scholar]
- 87.Goodman MT, Wilkens LR, Hankin JH, Lyu L-C, Wu AH, Kolonel LN. Association of soy and fiber consumption with the risk of endometrial cancer. Am. J. Epidemiol. 1997;146:294–306. doi: 10.1093/oxfordjournals.aje.a009270. [DOI] [PubMed] [Google Scholar]
- 88.Goodman MT, Shvetsov YB, Wilkens LR, Franke AA, Le Marchand L, Kakazu KK, Nomura AM, Henderson BE, Kolonel LN. Urinary phytoestrogen excretion, postmenopausal breast cancer risk: the multiethnic cohort study. Cancer Prev. Res. (Phila Pa) 2009;2:887–894. doi: 10.1158/1940-6207.CAPR-09-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gorski RA. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am. J. Physiol. 1963;205:842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- 90.Gorski RA. Sexual dimorphisms of the brain. J. Anim. Sci. 1985;61 Suppl. 3:38–61. doi: 10.1093/ansci/61.supplement_3.38. [DOI] [PubMed] [Google Scholar]
- 91.Gorski RA, Mennin SP, Kubo K. The neural and hormonal bases of the reproductive cycle of the rat. Adv. Exp. Med. Biol. 1975;54:115–153. doi: 10.1007/978-1-4684-8715-2_6. [DOI] [PubMed] [Google Scholar]
- 92.Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;143:333–346. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- 93.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- 94.Gruber J, Tang SY, Halliwell B. Evidence for a trade-off between survival and fitness caused by resveratrol treatment of Caenorhabditis elegans. Ann. NY Acad. Sci. 2007;1100:530–542. doi: 10.1196/annals.1395.059. [DOI] [PubMed] [Google Scholar]
- 95.Grumbach MM. The neuroendocrinology of human puberty revisited. Horm. Res. 2002;57 Suppl. 2:2–14. doi: 10.1159/000058094. [DOI] [PubMed] [Google Scholar]
- 96.Gu GB, Simerly RB. Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. J. Comp. Neurol. 1997;384:142–164. [PubMed] [Google Scholar]
- 97.Handa RJ, Weiser MJ, Zuloaga DG. A role for the androgen metabolite, 5alpha-androstane-3beta, 17beta-diol, in modulating oestrogen receptor beta-mediated regulation of hormonal stress reactivity. J. Neuroendocrinol. 2009;21:351–358. doi: 10.1111/j.1365-2826.2009.01840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hartley DE, Edwards JE, Spiller CE, Alom N, Tucci S, Seth P, Forsling ML, File SE. The soya isoflavone content of rat diet can increase anxiety and stress hormone release in the male rat. Psychopharmacology (Berl) 2003;167:46–53. doi: 10.1007/s00213-002-1369-7. [DOI] [PubMed] [Google Scholar]
- 99.Helferich WG, Andrade JE, Hoagland MS. Phytoestrogens and breast cancer: a complex story. Inflammopharmacology. 2008;16:219–226. doi: 10.1007/s10787-008-8020-0. [DOI] [PubMed] [Google Scholar]
- 100.Herbst AL, Green TH, Jr, Ulfelder H. Primary carcinoma of the vagina. An analysis of 68 cases. Am. J. Obstetr. Gynecol. 1970;106:210–218. doi: 10.1016/0002-9378(70)90265-6. [DOI] [PubMed] [Google Scholar]
- 101.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New Engl. J. Med. 1971;284:878–881. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 102.Hertrampf T, Schleipen B, Offermanns C, Velders M, Laudenbach U, Diel P. Comparison of the bone protective effects of an isoflavone-rich diet with dietary and subcutaneous administrations of genistein in ovariectomized rats. Toxicol. Lett. 2009;184:198–203. doi: 10.1016/j.toxlet.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Hodgson JM, Puddey IB, Beilin LJ, Mori TA, Croft KD. Supplementation with isoflavonoid phytoestrogens does not alter serum lipid concentrations: a randomized controlled trial in humans. J. Nutr. 1998;128:728–732. doi: 10.1093/jn/128.4.728. [DOI] [PubMed] [Google Scholar]
- 104.Hooper L, Ryder JJ, Kurzer MS, Lampe JW, Messina MJ, Phipps WR, Cassidy A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: a systematic review and meta-analysis. Human Reprod. Update. 2009;15:423–440. doi: 10.1093/humupd/dmp010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Horn-Ross PL, Barnes S, Lee VS, Collins CN, Reynolds P, Lee MM, Stewart SL, Canchola AJ, Wilson L, Jones K. Reliability and validity of an assessment of usual phytoestrogen consumption (United States) Cancer Causes Control. 2006;17:85–93. doi: 10.1007/s10552-005-0391-6. [DOI] [PubMed] [Google Scholar]
- 106.Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 107.Hsieh RW, Rajan SS, Sharma SK, Guo Y, DeSombre ER, Mrksich M, Greene GL. Identification of ligands with bicyclic scaffolds provides insights into mechanisms of estrogen receptor subtype selectivity. J. Biol. Chem. 2006;281:17909–17919. doi: 10.1074/jbc.M513684200. [DOI] [PubMed] [Google Scholar]
- 108.Hutchins AM, Slavin JL, Lampe JW. Urinary isoflavonoid phytoestrogen and lignan excretion after consumption of fermented and unfermented soy products. J. Am. Diet. Ass. 1995;95:545–551. doi: 10.1016/S0002-8223(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 109.Iguchi T, Takasugi N. Polyovular follicles in the ovary of immature mice exposed prenatally to diethylstilbestrol. Anat. Embryol. (Berl) 1986;175:53–55. doi: 10.1007/BF00315455. [DOI] [PubMed] [Google Scholar]
- 110.Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34:29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- 111.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol. Reprod. 1990;43:478–484. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 112.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of Bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 113.Irvine CH, Shand N, Fitzpatrick MG, Alexander SL. Daily intake and urinary excretion of genistein and daidzein by infants fed soy- or dairy-based infant formulas. Am. J. Clin. Nutr. 1998;68:1462S–1465S. doi: 10.1093/ajcn/68.6.1462S. [DOI] [PubMed] [Google Scholar]
- 114.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 115.Jacobs A, Wegewitz U, Sommerfeld C, Grossklaus R, Lampen A. Efficacy of isoflavones in relieving vasomotor menopausal symptoms - a systematic review. Mol. Nutr. Food Res. 2009;53:1084–1097. doi: 10.1002/mnfr.200800552. [DOI] [PubMed] [Google Scholar]
- 116.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol. Reprod. 2002;67:1285–1296. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 117.Jefferson WN, Padilla-Banks E, Clark G, Newbold RR. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;777:179–189. doi: 10.1016/s1570-0232(02)00493-2. [DOI] [PubMed] [Google Scholar]
- 118.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol. Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 119.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol. Reprod. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 120.Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol. Reprod. 2009;80:425–431. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Joensen UN, Jorgensen N, Rajpert-De Meyts E, Skakkebaek NE. Testicular dysgenesis syndrome and Leydig cell function. Basic Clin. Pharmacol. Toxicol. 2008;102:155–161. doi: 10.1111/j.1742-7843.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- 122.Johns P, Dowlati L, Wargo W. Determination of isoflavones in ready-to-feed soy-based infant formula. J. AOAC Int. 2003;86:72–78. [PubMed] [Google Scholar]
- 123.Kang L, Zhang X, Xie Y, Tu Y, Wang D, Liu Z, Wang ZY. Involvement of estrogen receptor variant ER-{alpha}36, not GPR30, in nongenomic estrogen signaling. Mol. Endocrinol. 2010 doi: 10.1210/me.2009-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kauffman AS. Sexual differentiation and the Kiss1 system: hormonal and developmental considerations. Peptides. 2009;30:83–93. doi: 10.1016/j.peptides.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 126.Kenny AM, Mangano KM, Abourizk RH, Bruno RS, Anamani DE, Kleppinger A, Walsh SJ, Prestwood KM, Kerstetter JE. Soy proteins and isoflavones affect bone mineral density in older women: a randomized controlled trial. Am. J. Clin. Nutr. 2009;90:234–242. doi: 10.3945/ajcn.2009.27600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim J. Protective effects of Asian dietary items on cancers - soy and ginseng. Asian Pac. J. Cancer Prev. 2008;9:543–548. [PubMed] [Google Scholar]
- 128.Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endocr. Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- 129.Kouki T, Kishitake M, Okamoto M, Oosuka I, Takebe M, Yamanouchi K. Effects of neonatal treatment with phytoestrogens, genistein and daidzein, on sex difference in female rat brain function: estrous cycle and lordosis. Horm. Behav. 2003;44:140–145. doi: 10.1016/s0018-506x(03)00122-3. [DOI] [PubMed] [Google Scholar]
- 130.Kubo K, Arai O, Omura M, Watanabe R, Ogata R, Aou S. Low dose effects of Bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 2003;45:345–356. doi: 10.1016/s0168-0102(02)00251-1. [DOI] [PubMed] [Google Scholar]
- 131.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Hilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138:863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 133.Kuiper GGJM, Lemmen JG, Carlsson B, Corton JC, Van Der Safe SH, Van Der Saag PT, Berg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor β. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 134.Kurzer MS. Phytoestrogen supplement use by women. J. Nutr. 2003;133:1983S–1986S. doi: 10.1093/jn/133.6.1983S. [DOI] [PubMed] [Google Scholar]
- 135.Kurzer MS. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology. 2008;16:227–229. doi: 10.1007/s10787-008-8021-z. [DOI] [PubMed] [Google Scholar]
- 136.Kurzer MS, Xu X. Dietary phytoestrogens. Annu. Rev. Nutr. 1997;17:353–381. doi: 10.1146/annurev.nutr.17.1.353. [DOI] [PubMed] [Google Scholar]
- 137.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J. Steroid Biochem. Mol. Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 138.Lakind JS, Naiman DQ. Daily intake of Bisphenol A and potential sources of exposure: 2005–2006 National Health and Nutrition Examination Survey. J. Expo. Sci. Environ. Epidemiol. 2010 doi: 10.1038/jes.2010.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lampe JW. Is equol the key to the efficacy of soy foods? Am J. Clin. Nutr. 2009;89:1664S–1667S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lampe J, Karr S, Hutchins A, Slavin J. Urinary equol excretion with a soy challenge: influence of habitual diet. PSEBM. 1998;217:335–339. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 141.Lee HP, Gourley L, Duffy SW, Esteve J, Lee J, Day NE. Dietary effects on breast-cancer risk in Singapore. Lancet. 1991;337:1197–1200. doi: 10.1016/0140-6736(91)92867-2. [DOI] [PubMed] [Google Scholar]
- 142.Lee YB, Lee HJ, Sohn HS. Soy isoflavones and cognitive function. J. Nutr. Biochem. 2005;16:641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 143.Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc. Natl. Acad. Sci. USA. 2008;105:3374–3379. doi: 10.1073/pnas.0712145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Legette LL, Martin BR, Shahnazari M, Lee WH, Helferich WG, Qian J, Waters DJ, Arabshahi A, Barnes S, Welch J, Bostwick DG, Weaver CM. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J. Nutr. 2009;139:1908–1913. doi: 10.3945/jn.109.108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lephart ED, West T, Weber KS, Rhees RW, Setchell K, Adlercreutz H, Lund T. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol. Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 146.Lephart ED, Setchell KD, Lund TD. Phytoestrogens: hormonal action and brain plasticity. Brain Res. Bull. 2005;65:193–198. doi: 10.1016/j.brainresbull.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 147.Levin ER. Membrane oestrogen receptor alpha signalling to cell functions. J. Physiol. 2009;587:5019–5023. doi: 10.1113/jphysiol.2009.177097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lewis RW, Brooks N, Milburn GM, Soames A, Stone S, Hall M, Ashby J. The effects of the phytoestrogen genistein on the postnatal development of the rat. Toxicol. Sci. 2003;71:74–83. doi: 10.1093/toxsci/71.1.74. [DOI] [PubMed] [Google Scholar]
- 149.Li B, Yu S. Genistein prevents bone resorption diseases by inhibiting bone resorption and stimulating bone formation. Biol. Pharm. Bull. 2003;26:780–786. doi: 10.1248/bpb.26.780. [DOI] [PubMed] [Google Scholar]
- 150.Lindzey J, Korach KS. Developmental and physiological effects of estrogen receptor gene disruption in mice. Trends Endocrinol. Metab. 1997;8:137–145. doi: 10.1016/s1043-2760(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 151.Linford NJ, Dorsa DM. 17beta-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. Steroids. 2002;67:1029–1040. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 152.Liu J, Ho SC, Su YX, Chen WQ, Zhang CX, Chen YM. Effect of long-term intervention of soy isoflavones on bone mineral density in women: a meta-analysis of randomized controlled trials. Bone. 2009;44:948–953. doi: 10.1016/j.bone.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 153.Lund T, Rhees R, Setchell K, Lephart E. Altered sexually dimorphic nucleus of the preoptic area (SDN-POA) volume in adult Long-Evans rats by dietary soy phytoestrogens. Brain Res. 2001;914:92–99. doi: 10.1016/s0006-8993(01)02779-2. [DOI] [PubMed] [Google Scholar]
- 154.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 155.Lurje G, Lenz HJ, FR EG. EGFR signaling and drug discovery. Oncology. 2009;77:400–410. doi: 10.1159/000279388. [DOI] [PubMed] [Google Scholar]
- 156.Lydeking-Olsen E, Jensen JBE, Setchell KDR, Daamhus M, Jensen TH. Isoflavone-rich soymilk prevents bone-loss in the lumbar spine of postmenopasual women. A 2 year study. J. Nutr. 2002;132:581S. (abstract). [Google Scholar]
- 157.Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the nervous system: mechanisms and nonreproductive functions. Annu. Rev. Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- 158.Makela S, Savolainen H, Aavik E, Myllarniemi M, Strauss L, Taskinen E, Gustafsson JA, Hayry P. Differentiation between vasculoprotective and uterotrophic effects of ligands with different binding affinities to estrogen receptors alpha and beta. Proc. Natl. Acad. Sci. USA. 1999;96:7077–7082. doi: 10.1073/pnas.96.12.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Manas ES, Xu ZB, Unwalla RJ, Somers WS. Understanding the selectivity of genistein for human estrogen receptor-beta using X-ray crystallography and computational methods. Structure. 2004;12:2197–2207. doi: 10.1016/j.str.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 160.Markiewicz L, Garey J, Adlercreutz H, Gurpide E. In vitro bioassays of non-steroidal phytoestrogens. J. Steroid Biochem. Mol. Biol. 1993;45:399–405. doi: 10.1016/0960-0760(93)90009-l. [DOI] [PubMed] [Google Scholar]
- 161.Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol. Biomarkers Prev. 2002;11:195–201. [PubMed] [Google Scholar]
- 162.Maskarinec G, Williams AE, Inouye JS, Stanczyk FZ, Franke AA. A randomized isoflavone intervention among premenopausal women. Cancer Epidemiol. Biomarkers Prevent. 2002;11:195–201. [PubMed] [Google Scholar]
- 163.Maskarinec G, Takata Y, Franke AA, Williams AE, Murphy SP. A 2-year soy intervention in premenopausal women does not change mammographic densities. J. Nutr. 2004;134:3089–3094. doi: 10.1093/jn/134.11.3089. [DOI] [PubMed] [Google Scholar]
- 164.Massart F, Parrino R, Seppia P, Federico G, Saggese G. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr. 2006;58:247–254. [PubMed] [Google Scholar]
- 165.Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–170. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 166.Mathey J, Mardon J, Fokialakis N, Puel C, Kati-Coulibaly S, Mitakou S, Bennetau-Pelissero C, Lamothe V, Davicco MJ, Lebecque P, Horcajada MN, Coxam V. Modulation of soy isoflavones bioavailability and subsequent effects on bone health in ovariectomized rats: the case for equol. Osteopor. Int. 2007;18:671–679. doi: 10.1007/s00198-007-0351-y. [DOI] [PubMed] [Google Scholar]
- 167.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–3999. [PubMed] [Google Scholar]
- 168.McLachlan JA, Newbold RR, Shah HC, Hogan MD, Dixon RL. Reduced fertility in female mice exposed transplacentally to diethylstilbestrol (DES) Fertil. Steril. 1982;38:364–371. doi: 10.1016/s0015-0282(16)46520-9. [DOI] [PubMed] [Google Scholar]
- 169.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr. Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 170.Messina M, Gardner C, Barnes S. Gaining insight into the health effects of soy but a long way still to go: commentary on the fourth international symposium on the role of soy in preventing and treating chronic disease. J. Nutr. 2002;132:547S–551S. doi: 10.1093/jn/132.3.547S. [DOI] [PubMed] [Google Scholar]
- 171.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 172.Micevych P, Dominguez R. Membrane estradiol signaling in the brain. Front. Neuroendocrinol. 2009;30:315–327. doi: 10.1016/j.yfrne.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Micevych P, Kuo J, Christensen A. Physiology of membrane oestrogen receptor signalling in reproduction. J. Neuroendocrinol. 2009;21:249–256. doi: 10.1111/j.1365-2826.2009.01833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Moore TO, Karom M, O’Farrell L. The neurobehavioral effects of phytoestrogens in male Syrian hamsters. Brain Res. 2004;1016:102–110. doi: 10.1016/j.brainres.2004.04.073. [DOI] [PubMed] [Google Scholar]
- 175.Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, Cassidy A, Magee P, Millar J, Hall WL, Kramer Birkved F, Sorensen IK, Sontag G. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol. Nutr. Food Res. 2009;53 Suppl. 2:S266–S309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 176.Murkies A, Lombard C, Strauss B, Wilcox G, Burger H, Morton M. Dietary flour supplementation decreases post-menopausal hot flushes: effect of soy and wheat. Maturitas. 1995;21:189–195. doi: 10.1016/0378-5122(95)00899-v. [DOI] [PubMed] [Google Scholar]
- 177.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg. Med. Chem. 2004;12:1559–1567. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 178.Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod. Toxicol. 2001;15:399–411. doi: 10.1016/s0890-6238(01)00141-1. [DOI] [PubMed] [Google Scholar]
- 179.Nagata C, Takatsuka N, Inaba S, Kawakami N, Shimizu H. Effect of soymilk consumption on serum estrogen concentrations in premenopausal Japanese women. J. Natl. Cancer Inst. 1998;90:1830–1835. doi: 10.1093/jnci/90.23.1830. [DOI] [PubMed] [Google Scholar]
- 180.Nagata C, Iwasa S, Shiraki M, Ueno T, Uchiyama S, Urata K, Sahashi Y, Shimizu H. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan) Cancer Causes Control. 2006;17:1107–1113. doi: 10.1007/s10552-006-0044-4. [DOI] [PubMed] [Google Scholar]
- 181.Navarro VM, Fernandez-Fernandez R, Castellano JM, Roa J, Mayen A, Barreiro ML, Gaytan F, Aguilar E, Pinilla L, Dieguez C, Tena-Sempere M. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J. Physiol. 2004;561:379–386. doi: 10.1113/jphysiol.2004.072298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nestel PJ, Pomeroy S, Kay S, Komesaroff P, Behrsing J, Cameron JD, West L. Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J. Clin. Endocrinol. Metab. 1999;84:895–898. doi: 10.1210/jcem.84.3.5561. [DOI] [PubMed] [Google Scholar]
- 183.Newbold RR. Prenatal exposure to diethylstilbestrol (DES) Fertil. Steril. 2008;89:e55–e56. doi: 10.1016/j.fertnstert.2008.01.062. [DOI] [PubMed] [Google Scholar]
- 184.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–4328. [PubMed] [Google Scholar]
- 185.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 2004;18:803–811. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 186.North K, Golding J. A maternal vegetarian diet in pregnancy is associated with hypospadias. The ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. BJU Int. 2000;85:107–113. doi: 10.1046/j.1464-410x.2000.00436.x. [DOI] [PubMed] [Google Scholar]
- 187.NTP Multigenerational Reproductive Study of Genistein (CAS No. 446-72-0) in Sprague–Dawley Rats (Feed Study) Natl. Toxicol. Program Tech. Rep. Ser. 2008. pp. 1–266. [PubMed] [Google Scholar]
- 188.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr. Rev. 2009;30:713–743. doi: 10.1210/er.2009-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ogawa S, Taylor JA, Lubahn DB, Korach KS, Pfaff DW. Reversal of sex roles in genetic female mice by disruption of estrogen receptor gene. Neuroendocrinology. 1996;64:467–470. doi: 10.1159/000127154. [DOI] [PubMed] [Google Scholar]
- 190.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–5081. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 191.Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of [Val 12] Ha-ras-transformed NIH 3T 3 cells. Biochem. Biophys. Res. Commun. 1988;157:183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- 192.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–1665. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med. 2008;74:1656–1665. doi: 10.1055/s-0028-1088304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, Qiu X, Stockman B, Thanabal V, Varghese A, Ward J, Withka J, Ahn K, RT S. SRT 2183, SRT 1460, and resveratrol are not direct activators of SIRT 1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. (1720) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Padilla-Banks E, Jefferson WN, Newbold RR. Neonatal exposure to the phytoestrogen genistein alters mammary gland growth and developmental programming of hormone receptor levels. Endocrinology. 2006;147:4871–4882. doi: 10.1210/en.2006-0389. [DOI] [PubMed] [Google Scholar]
- 196.Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal Bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Palmlund I. Exposure to a xenoestrogen before birth: the diethylstilbestrol experience. J. Psychosomatic Obstetrics Gynaecol. 1996;17:71–84. doi: 10.3109/01674829609025667. [DOI] [PubMed] [Google Scholar]
- 198.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends and changes after migration. Endocr. Rev. 2003;24:668–693. doi: 10.1210/er.2002-0019. [DOI] [PubMed] [Google Scholar]
- 199.Park D, Huang T, Frishman WH. Phytoestrogens as cardioprotective agents. Cardiol. Rev. 2005;13:13–17. doi: 10.1097/01.crd.0000126084.68791.32. [DOI] [PubMed] [Google Scholar]
- 200.Patchev AV, Gotz F, Rohde W. Differential role of estrogen receptor isoforms in sex-specific brain organization. FASEB J. 2004;18:1568–1570. doi: 10.1096/fj.04-1959fje. [DOI] [PubMed] [Google Scholar]
- 201.Patisaul HB. Phytoestrogen action in the adult and developing brain. J. Neuroendocrinol. 2005;17:57–64. doi: 10.1111/j.1365-2826.2005.01268.x. [DOI] [PubMed] [Google Scholar]
- 202.Patisaul HB, Polston EK. Influence of endocrine active compounds on the developing rodent brain. Brain Res. Rev. 2008;57:352–362. doi: 10.1016/j.brainresrev.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 203.Patisaul HB, Whitten PL, Young L. Regulation of estrogen receptor beta mRNA in the brain: opposite effects of 17β-estradiol and the phytoestrogen coumestrol. Brain Res: Mol. Brain Res. 1999;67:165–171. doi: 10.1016/s0169-328x(99)00058-3. [DOI] [PubMed] [Google Scholar]
- 204.Patisaul HB, Dindo M, Whitten PL, Young LJ. Soy isoflavone supplements antagonize reproductive behavior and ERα- and ERβ-dependent gene expression in the brain. Endocrinology. 2001;142:2946–2952. doi: 10.1210/endo.142.7.8241. [DOI] [PubMed] [Google Scholar]
- 205.Patisaul HB, Melby M, Whitten PL, Young LJ. Genistein affects ERβ- but not ERα-dependent gene expression in the hypothalamus. Endocrinology. 2002;143:2189–2197. doi: 10.1210/endo.143.6.8843. [DOI] [PubMed] [Google Scholar]
- 206.Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J. Neuroendocrinol. 2003;15:787–793. doi: 10.1046/j.1365-2826.2003.01061.x. [DOI] [PubMed] [Google Scholar]
- 207.Patisaul HB, Luskin JR, Wilson ME. A soy supplement and tamoxifen inhibit sexual behavior in female rats. Horm. Behav. 2004;45:270–277. doi: 10.1016/j.yhbeh.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 208.Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and Bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 209.Patisaul HB, Todd KL, Mickens JA, Adewale HB. Impact of neonatal exposure to the ERα agonist PPT, Bisphenol-A or phytoestrogens on hypothalamic kisspeptin fiber density in male and female rats. Neurotoxicology. 2009;30(3):350–357. doi: 10.1016/j.neuro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Peeters PH, Slimani N, van der Schouw YT, Grace PB, Navarro C, Tjonneland A, Olsen A, Clavel-Chapelon F, Touillaud M, Boutron-Ruault MC, Jenab M, Kaaks R, Linseisen J, Trichopoulou A, Trichopoulos D, Dilis V, Boeing H, Weikert C, Overvad K, Pala V, Palli D, Panico S, Tumino R, Vineis P, Bueno-de-Mesquita HB, van Gils CH, Skeie G, Jakszyn P, Hallmans G, Berglund G, Key TJ, Travis R, Riboli E, Bingham SA. Variations in plasma phytoestrogen concentrations in European adults. J. Nutr. 2007;137:1294–1300. doi: 10.1093/jn/137.5.1294. [DOI] [PubMed] [Google Scholar]
- 211.Pelissero C, Lenczowski M, Chinzi D, Davail-Cuisset B, Sumpter J, Fostier A. Effects of flavonoids on aromatase activity, and in vitro study. J. Steroid Biochem. Mol. Biol. 1996;57:215–223. doi: 10.1016/0960-0760(95)00261-8. [DOI] [PubMed] [Google Scholar]
- 212.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev. Biol. 2001;234:339–351. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 213.Perez SE, Chen EY, Mufson EJ. Distribution of estrogen receptor alpha and beta immunoreactive profiles in the postnatal rat brain. Brain Res. Dev. Brain Res. 2003;145:117–139. doi: 10.1016/s0165-3806(03)00223-2. [DOI] [PubMed] [Google Scholar]
- 214.Pfaff D. Drive. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- 215.Pfitscher A, Reiter E, Jungbauer A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J. Steroid Biochem. Mol. Biol. 2008;112:87–94. doi: 10.1016/j.jsbmb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 216.Piontek M, Hangels KJ, Porschen R, Strohmeyer G. Anti-proliferative effect of tyrosine kinase inhibitors in epidermal growth factor-stimulated growth of human gastric cancer cells. Anticancer Res. 1993;13:2119–2123. [PubMed] [Google Scholar]
- 217.Piotrowska E, Jakobkiewicz-Banecka J, Wegrzyn G. Different amounts of isoflavones in various commercially available soy extracts in the light of gene expression-targeted isoflavone therapy. Phytother. Res. 2009 doi: 10.1002/ptr.2944. [DOI] [PubMed] [Google Scholar]
- 218.Polston EK, Simerly RB. Ontogeny of the projections from the anteroventral periventricular nucleus of the hypothalamus in the female rat. J. Comp. Neurol. 2006;495:122–132. doi: 10.1002/cne.20874. [DOI] [PubMed] [Google Scholar]
- 219.Polston EK, Gu G, Simerly RB. Neurons in the principal nucleus of the bed nuclei of the stria terminalis provide a sexually dimorphic GABAergic input to the anteroventral periventricular nucleus of the hypothalamus. Neuroscience. 2004;123:793–803. doi: 10.1016/j.neuroscience.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 220.Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW., Jr Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr. 1998;68:1375S–1379S. doi: 10.1093/ajcn/68.6.1375S. [DOI] [PubMed] [Google Scholar]
- 221.Ravindranath MH, Muthugounder S, Presser N, Viswanathan S. Anticancer therapeutic potential of soy isoflavone, genistein. Adv. Exp. Med. Biol. 2004;546:121–165. doi: 10.1007/978-1-4757-4820-8_11. [DOI] [PubMed] [Google Scholar]
- 222.Register TC, Jayo MJ, Anthony MS. Soy phytoestrogens do not prevent bone loss in postmenopausal monkeys. J. Clin. Endocrinol. Metabol. 2003;88:4362–4370. doi: 10.1210/jc.2003-030493. [DOI] [PubMed] [Google Scholar]
- 223.Remy P, Doder M, Lees A, Turjanski N, Brooks D. Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain. 2005;128:1314–1322. doi: 10.1093/brain/awh445. [DOI] [PubMed] [Google Scholar]
- 224.Rissman EF. Roles of oestrogen receptors alpha and beta in behavioural neuroendocrinology: beyond Yin/Yang. J. Neuroendocrinol. 2008;20:873–879. doi: 10.1111/j.1365-2826.2008.01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout by knockout studies: neuroendocrine and behavioral aspects. Horm. Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 226.Roa J, Vigo E, Castellano JM, Navarro VM, Fernandez-Fernandez R, Casanueva FF, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. Hypothalamic expression of KiSS-1 system and gonadotropin-releasing effects of kisspeptin in different reproductive states of the female Rat. Endocrinology. 2006;147:2864–2878. doi: 10.1210/en.2005-1463. [DOI] [PubMed] [Google Scholar]
- 227.Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem. Biophys. Res. Commun. 2008;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- 228.Rolwand I, Wiseman H, Sanders T, Adlercreutz H, Bowey E. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr. Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 229.Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front. Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Routledge EJ, White R, Parker MG, Sumpter JP. Differential effects of xenoestrogens on coactivator recruitment by estrogen receptor (ER) alpha and ERbeta. J. Biol. Chem. 2000;275:35986–35993. doi: 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- 231.Rozman KK, Bhatia J, Calafat AM, Chambers C, Culty M, Etzel RA, Flaws JA, Hansen DK, Hoyer PB, Jeffery EH, Kesner JS, Marty S, Thomas JA, Umbach D. NTP-CERHR expert panel report on the reproductive and developmental toxicity of genistein. Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:485–638. doi: 10.1002/bdrb.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Rubin MM. Antenatal exposure to DES: lessons learned.future concerns. Obstetr. Gynecol. Survey. 2007;62:548–555. doi: 10.1097/01.ogx.0000271138.31234.d7. [DOI] [PubMed] [Google Scholar]
- 233.Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- 234.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 235.Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J. Nutr. 1997;127:263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- 236.Scallet AC, Wofford M, Meredith JC, Allaben WT, Ferguson SA. Dietary exposure to genistein increases vasopressin but does not alter beta-endorphin in the rat hypothalamus. Toxicol. Sci. 2003;72:296–300. doi: 10.1093/toxsci/kfg029. [DOI] [PubMed] [Google Scholar]
- 237.Scallet AC, Divine RL, Newbold RR, Delclos KB. Increased volume of the calbindin D28k–labeled sexually dimorphic hypothalamus in genistein and nonylphenol-treated male rats. 1. Toxicol. Sci. 2004;82:570–576. doi: 10.1093/toxsci/kfh297. [DOI] [PubMed] [Google Scholar]
- 238.Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L, Hoover R. Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. JAMA. 2000;283:485–491. doi: 10.1001/jama.283.4.485. [DOI] [PubMed] [Google Scholar]
- 239.Schmidt S, Degen GH, Seibel J, Hertrampf T, Vollmer G, Diel P. Hormonal activity of combinations of genistein, Bisphenol A and 17beta-estradiol in the female Wistar rat. Arch. Toxicol. 2006;80:839–845. doi: 10.1007/s00204-006-0102-4. [DOI] [PubMed] [Google Scholar]
- 240.Schoental R. Precocious sexual development in Puerto Rico and oestrogenic mycotoxins (zearalenone) Lancet. 1983;1:537. doi: 10.1016/s0140-6736(83)92229-8. [DOI] [PubMed] [Google Scholar]
- 241.Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent Bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Schwartz H, Sontag G, Plumb J. Inventory of phytoestrogen databases. Food Chem. 2009;113:736–747. [Google Scholar]
- 243.Senti F. Soy protein foods in U.S. assistance programs. J. Am. Oil Chem. Soc. 1974;51:138A–140A. [Google Scholar]
- 244.Setchell KD, Lydeking-Olsen E. Dietary phytoestrogens and their effect on bone: evidence from in vitro amd in vivo, human observational, and dietary intervention studies. Am. J. Clin. Nutr. 2003;78:593S–609S. doi: 10.1093/ajcn/78.3.593S. [DOI] [PubMed] [Google Scholar]
- 245.Setchell KDR, Welsh MB. High-performance liquid chromatographic analysis of phytoestrogens in soy protein preparations with ultraviolet electrochemical and thermospray mass spectrometric detection. J. Chromatogr. A. 1987;386:315–323. doi: 10.1016/s0021-9673(01)94608-4. [DOI] [PubMed] [Google Scholar]
- 246.Setchell KKD, Gosselin SSJ, Welsh MMB, Johnston JJO, Balistreri WWF, Kramer LLW, Dresser BBL, Tarr MMJ. Dietary estrogens - a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93:225–233. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- 247.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 248.Setchell KDR, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997;350:23–27. doi: 10.1016/S0140-6736(96)09480-9. [DOI] [PubMed] [Google Scholar]
- 249.Setchell KD. L. Zimmer-Nechemias, J. Cai, J.E. Heubi, Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am. J. Clin. Nutr. 1998;68:1453S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 250.Setchell KD, Brown NM, Desai P, Zimmer-Nechemias L, Wolfe BE, Brashear WT, Kirschner AS, Cassidy A, Heubi JE. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001;131:1362S–1375S. doi: 10.1093/jn/131.4.1362S. [DOI] [PubMed] [Google Scholar]
- 251.Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol - a clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002;132:3577–3584. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 252.Setchell KD, Brown NM, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose- response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J. Nutr. 2003;133:1027–1035. doi: 10.1093/jn/133.4.1027. [DOI] [PubMed] [Google Scholar]
- 253.Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum. Reprod. 2002;17:1692–1703. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- 254.Shen W, Stearns V. Treatment strategies for hot flushes. Expert. Opin. Pharmacother. 2009;10:1133–1144. doi: 10.1517/14656560902868217. [DOI] [PubMed] [Google Scholar]
- 255.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, Lu W. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–2443. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Shughrue PL, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor a and b mRNA in the rat central nervous system. J. Comp. Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 257.Shughrue P, Lane M, Scrimo P, Merchenthaler I. Comparative distribution of estrogen receptor-α (ERα) and β (ERβ) mRNA in the rat pituitary gonad and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 258.Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu. Rev. Neurosci. 2002;25:507–536. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- 259.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 260.Simon NG, Kaplan JR, Hu S, Register TC, Adams MR. Increased aggressive behavior and decreased affiliative behavior in adult male monkeys after long-term consumption of diets rich in soy protein and isoflavones. Horm. Behav. 2004;45:278–284. doi: 10.1016/j.yhbeh.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 261.Sirtori CR, Arnoldi A, Johnson SK. Phytoestrogens: end of a tale? Ann Med. 2005;37:423–438. doi: 10.1080/07853890510044586. [DOI] [PubMed] [Google Scholar]
- 262.Smith OW. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. Am. J. Obst. Gynecol. 1948;56:821–834. [PubMed] [Google Scholar]
- 263.Smith JT, Clifton DK, Steiner RA. Regulation of the neuroendocrine reproductive axis by kisspeptin-GPR54 signaling. Reproduction. 2006;131:623–630. doi: 10.1530/rep.1.00368. [DOI] [PubMed] [Google Scholar]
- 264.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J. Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Soyfoods Associations of North America. Soyfood Sales and Trends. 2009. [Google Scholar]
- 266.Speroff L. Alternative therapies for postmenopausal women. Int. J. Fertil. Womens Med. 2005;50:101–114. [PubMed] [Google Scholar]
- 267.Stricker R, Eberhart R, Chevailler MC, Quinn FA, Bischof P. Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin. Chem. Lab. Med. 2006;44:883–887. doi: 10.1515/CCLM.2006.160. [DOI] [PubMed] [Google Scholar]
- 268.Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA. 2001;286:807–814. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 269.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T. Developmental effects of perinatal exposure to Bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod. Toxicol. 2002;16:107–116. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 270.Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tan KA, Walker M, Morris K, Greig I, Mason JI, Sharpe RM. Infant feeding with soy formula milk: effects on puberty progression, reproductive function and testicular cell numbers in marmoset monkeys in adulthood. Human Reprod. 2006;21:896–904. doi: 10.1093/humrep/dei421. [DOI] [PubMed] [Google Scholar]
- 272.Taylor CK, Levy RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr. Rev. 2009;67:398–415. doi: 10.1111/j.1753-4887.2009.00213.x. [DOI] [PubMed] [Google Scholar]
- 273.Thomas P, Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: a potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 274.Thomas JM, Lutz SF. Soy protein lowers fat and saturated fat in school lunch beef and pork entrees. J. Am. Diet. Assoc. 2001;101:461–463. doi: 10.1016/S0002-8223(01)00118-3. [DOI] [PubMed] [Google Scholar]
- 275.Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, Osada H, Ikezuki Y, Tsutsumi O, Iguchi T, Mori C. Fetal exposure to phytoestrogens - the difference in phytoestrogen status between mother and fetus. Environ. Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 276.Tomar RS, Shiao R. Early life and adult exposure to isoflavones and breast cancer risk. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2008;26:113–173. doi: 10.1080/10590500802074256. [DOI] [PubMed] [Google Scholar]
- 277.Treatment of menopause-associated vasomotor symptoms: position statement of The North American Menopause Society. Menopause. 2004;11:11–33. doi: 10.1097/01.GME.0000108177.85442.71. [DOI] [PubMed] [Google Scholar]
- 278.Trock BJ, Hilakivi-Clarke L, Clarke R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006;98:459–471. doi: 10.1093/jnci/djj102. [DOI] [PubMed] [Google Scholar]
- 279.Tuohy PG. Soy infant formula and phytoestrogens. J. Paediatr. Child Health. 2003;39:401–405. doi: 10.1046/j.1440-1754.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 280.UK-Committee-on-Toxicity, Phytoestrogens and Health, Committee on Toxicity of Chemicals in Food, Consumer Products and the Environment. London: 2003. [Google Scholar]
- 281.United States Department of Agriculture (Agricultural Research Service) Database for the Isoflavone Content of Selected Foods, Release 2.0. 2008. [Google Scholar]
- 282.U.S. Board. 16th Annual Survey: Consumer Attitudes About Nutrition. 2009. [Google Scholar]
- 283.Valentin-Blasini L, Blount BC, Caudill SP, Needham LL. Urinary and serum concentrations of seven phytoestrogens in a human reference population subset. J. Expos. Anal. Environ. Epidemiol. 2003;13:276–282. doi: 10.1038/sj.jea.7500278. [DOI] [PubMed] [Google Scholar]
- 284.van Pelt AM, de Rooij DG, van der Burg B, Saag PT, Gustafsson JA, Kuiper GG. Ontogeny of estrogen receptor-beta expression in rat testis. Endocrinology. 1999;140:478–483. doi: 10.1210/endo.140.1.6438. [DOI] [PubMed] [Google Scholar]
- 285.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front. Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 286.Verheus M, van Gils CH, Keinan-Boker L, Grace PB, Bingham SA, Peeters PH. Plasma phytoestrogens and subsequent breast cancer risk. J. Clin. Oncol. 2007;25:648–655. doi: 10.1200/JCO.2006.06.0244. [DOI] [PubMed] [Google Scholar]
- 287.Verkasalo PK, Appleby PN, Allen NE, Davey G, Adlercreutz H, Key TJ. Soya intake and plasma concentrations of daidzein and genistein: validity of dietary assessment among eighty British women (Oxford arm of the European Prospective Investigation into Cancer and Nutrition) Br. J. Nutr. 2001;86:415–421. doi: 10.1079/bjn2001424. [DOI] [PubMed] [Google Scholar]
- 288.Wallen K. Hormonal influences on sexually differentiated behavior in nonhuman primates. Front. Neuroendocrinol. 2005;26:7–26. doi: 10.1016/j.yfrne.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 289.Wang C, Makela T, Hase TA, Adlercreutz CHT, Kurzer MS. Lignans and isoflavonoids inhibit aromatase enzyme in human preadipocytes. J. Steroid Biochem. Mol. Biol. 1994;50:205–212. doi: 10.1016/0960-0760(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 290.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 291.Wang Z, Zhang X, Shen P, Loggie BW, Chang Y, Deuel TF. Identification, cloning, and expression of human estrogen receptor-alpha36, a novel variant of human estrogen receptor-alpha66. Biochem. Biophys. Res. Commun. 2005;336:1023–1027. doi: 10.1016/j.bbrc.2005.08.226. [DOI] [PubMed] [Google Scholar]
- 292.Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br. J. Cancer. 2008;98:1485–1493. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Whitten PL, Patisaul HB. Cross-species and interassay comparisons of phytoestrogen action. Environ. Health Perspect. 2001;109:5–23. doi: 10.1289/ehp.01109s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Whitten PL, Kudo S, Okubo KK. Isoflavonoids. In: D’Mello JPF, editor. Handbook of Plant and Fungal Toxicants. Boca Raton: CRC Press; 1997. pp. 117–137. [Google Scholar]
- 295.Winter JSD, Hughes IA, Reyes FI, Faiman C. Pituitary-gonadal relations in infancy: patterns of serum gonadal steroid concentrations in man from birth to two years of age. J. Clin. Endocrinol. Metabol. 1976;42:679–686. doi: 10.1210/jcem-42-4-679. [DOI] [PubMed] [Google Scholar]
- 296.Witt DM, Insel TR. A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology. 1991;128:3269–3276. doi: 10.1210/endo-128-6-3269. [DOI] [PubMed] [Google Scholar]
- 297.Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 298.Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, Pike MC. Effects of soy foods on ovarian function in premenopausal women. Br. J. Cancer. 2000;82:1879–1886. doi: 10.1054/bjoc.1999.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Wu AH, Yu MC, Tseng CC, Pike MC. Epidemiology of soy exposures and breast cancer risk. Br. J. Cancer. 2008;98:9–14. doi: 10.1038/sj.bjc.6604145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Yamori Y, Moriguchi EH, Teramoto T, Miura A, Fukui Y, Honda KI, Fukui M, Nara Y, Taira K, Moriguchi Y. Soybean isoflavones reduce postmenopausal bone resorption in female Japanese immigrants in Brazil: a ten-week study. J. Am. Coll. Nutr. 2002;21:560–563. doi: 10.1080/07315724.2002.10719255. [DOI] [PubMed] [Google Scholar]
- 302.Ye SF, Saga I, Ichimura K, Nagai T, Shinoda M, Matsuzaki S. Coumestrol as well as isoflavones in soybean extract prevent bone resorption in ovariectomized rats. Endocr. Regulat. 2003;37:145–152. [PubMed] [Google Scholar]
- 303.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Measuring environmental phenols and chlorinated organic chemicals in breast milk using automated on-line column-switching-high performance liquid chromatography-isotope dilution tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006;831:110–115. doi: 10.1016/j.jchromb.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 304.Young LJ, Wang Z, Donaldson R, Rissman EF. Estrogen receptor α is essential for induction of oxytocin receptor by estrogen. NeuroReport. 1998;9:933–936. doi: 10.1097/00001756-199803300-00031. [DOI] [PubMed] [Google Scholar]
- 305.Zhang JQ, Cai WQ, Zhou de S, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 306.Zhang C, Ho SC, Lin F, Cheng S, Fu J, Chen Y. Soy product and isoflavone intake and breast cancer risk defined by hormone receptor status. Cancer Sci. 2009 doi: 10.1111/j.1349-7006.2009.01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zoppi G, Guandalini S. The story of soy formula feeding in infants: a road paved with good intentions. J. Pediatric Gastroenterol. Nutr. 1999;28:541–543. doi: 10.1097/00005176-199905000-00022. [DOI] [PubMed] [Google Scholar]
- 308.Zung A, Glaser T, Kerem Z, Zadik Z. Breast development in the first 2 years of life: an association with soy-based infant formulas. J. Pediatric Gastroenterol. Nutr. 2008;46:191–195. doi: 10.1097/MPG.0b013e318159e6ae. [DOI] [PubMed] [Google Scholar]