Abstract
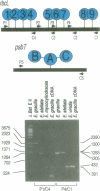
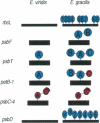
The origin of present day introns is a subject of spirited debate. Any intron evolution theory must account for not only nuclear spliceosomal introns but also their antecedents. The evolution of group II introns is fundamental to this debate, since group II introns are the proposed progenitors of nuclear spliceosomal introns and are found in ancient genes from modern organisms. We have studied the evolution of chloroplast introns and twintrons (introns within introns) in the genus Euglena. Our hypothesis is that Euglena chloroplast introns arose late in the evolution of this lineage and that twintrons were formed by the insertion of one or more introns into existing introns. In the present study we find that 22 out of 26 introns surveyed in six different photosynthesis-related genes from the plastid DNA of Euglena gracilis are not present in one or more basally branching Euglena spp. These results are supportive of a late origin for Euglena chloroplast group II introns. The psbT gene in Euglena viridis, a basally branching Euglena species, contains a single intron in the identical position to a psbT twintron from E.gracilis, a derived species. The E.viridis intron, when compared with 99 other Euglena group II introns, is most similar to the external intron of the E.gracilis psbT twintron. Based on these data, the addition of introns to the ancestral psbT intron in the common ancester of E.viridis and E.gracilis gave rise to the psbT twintron in E.gracilis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cavalier-Smith T. Intron phylogeny: a new hypothesis. Trends Genet. 1991 May;7(5):145–148. [PubMed] [Google Scholar]
- Cech T. R. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell. 1986 Jan 31;44(2):207–210. doi: 10.1016/0092-8674(86)90751-8. [DOI] [PubMed] [Google Scholar]
- Chanfreau G., Jacquier A. Catalytic site components common to both splicing steps of a group II intron. Science. 1994 Nov 25;266(5189):1383–1387. doi: 10.1126/science.7973729. [DOI] [PubMed] [Google Scholar]
- Copertino D. W., Christopher D. A., Hallick R. B. A mixed group II/group III twintron in the Euglena gracilis chloroplast ribosomal protein S3 gene: evidence for intron insertion during gene evolution. Nucleic Acids Res. 1991 Dec 11;19(23):6491–6497. doi: 10.1093/nar/19.23.6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertino D. W., Hall E. T., Van Hook F. W., Jenkins K. P., Hallick R. B. A group III twintron encoding a maturase-like gene excises through lariat intermediates. Nucleic Acids Res. 1994 Mar 25;22(6):1029–1036. doi: 10.1093/nar/22.6.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertino D. W., Hallick R. B. Group II and group III introns of twintrons: potential relationships with nuclear pre-mRNA introns. Trends Biochem Sci. 1993 Dec;18(12):467–471. doi: 10.1016/0968-0004(93)90008-b. [DOI] [PubMed] [Google Scholar]
- Copertino D. W., Hallick R. B. Group II twintron: an intron within an intron in a chloroplast cytochrome b-559 gene. EMBO J. 1991 Feb;10(2):433–442. doi: 10.1002/j.1460-2075.1991.tb07965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copertino D. W., Shigeoka S., Hallick R. B. Chloroplast group III twintron excision utilizing multiple 5'- and 3'-splice sites. EMBO J. 1992 Dec;11(13):5041–5050. doi: 10.1002/j.1460-2075.1992.tb05611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Doolittle W. F. Speculations on the early course of evolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1271–1275. doi: 10.1073/pnas.83.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorit R. L., Gilbert W. The limited universe of exons. Curr Opin Genet Dev. 1991 Dec;1(4):464–469. doi: 10.1016/s0959-437x(05)80193-5. [DOI] [PubMed] [Google Scholar]
- Ferat J. L., Michel F. Group II self-splicing introns in bacteria. Nature. 1993 Jul 22;364(6435):358–361. doi: 10.1038/364358a0. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Marchionni M., McKnight G. On the antiquity of introns. Cell. 1986 Jul 18;46(2):151–153. doi: 10.1016/0092-8674(86)90730-0. [DOI] [PubMed] [Google Scholar]
- Gingrich J. C., Hallick R. B. The Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. I. Complete DNA sequence and analysis of the nine intervening sequences. J Biol Chem. 1985 Dec 25;260(30):16156–16161. [PubMed] [Google Scholar]
- Gockel G., Hachtel W., Baier S., Fliss C., Henke M. Genes for components of the chloroplast translational apparatus are conserved in the reduced 73-kb plastid DNA of the nonphotosynthetic euglenoid flagellate Astasia longa. Curr Genet. 1994 Sep;26(3):256–262. doi: 10.1007/BF00309557. [DOI] [PubMed] [Google Scholar]
- Hallick R. B., Hong L., Drager R. G., Favreau M. R., Monfort A., Orsat B., Spielmann A., Stutz E. Complete sequence of Euglena gracilis chloroplast DNA. Nucleic Acids Res. 1993 Jul 25;21(15):3537–3544. doi: 10.1093/nar/21.15.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton T. A., Graham M. W. A simple and efficient method for direct cloning of PCR products using ddT-tailed vectors. Nucleic Acids Res. 1991 Mar 11;19(5):1156–1156. doi: 10.1093/nar/19.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L., Hallick R. B. A group III intron is formed from domains of two individual group II introns. Genes Dev. 1994 Jul 1;8(13):1589–1599. doi: 10.1101/gad.8.13.1589. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Base-pairing interactions involving the 5' and 3'-terminal nucleotides of group II self-splicing introns. J Mol Biol. 1990 Jun 5;213(3):437–447. doi: 10.1016/S0022-2836(05)80206-2. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoop V., Brennicke A. Evidence for a group II intron in Escherichia coli inserted into a highly conserved reading frame associated with mobile DNA sequences. Nucleic Acids Res. 1994 Apr 11;22(7):1167–1171. doi: 10.1093/nar/22.7.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learn G. H., Jr, Shore J. S., Furnier G. R., Zurawski G., Clegg M. T. Constraints on the evolution of plastid introns: the group II intron in the gene encoding tRNA-Val(UAC). Mol Biol Evol. 1992 Sep;9(5):856–871. doi: 10.1093/oxfordjournals.molbev.a040765. [DOI] [PubMed] [Google Scholar]
- Madhani H. D., Guthrie C. A novel base-pairing interaction between U2 and U6 snRNAs suggests a mechanism for the catalytic activation of the spliceosome. Cell. 1992 Nov 27;71(5):803–817. doi: 10.1016/0092-8674(92)90556-r. [DOI] [PubMed] [Google Scholar]
- Michel F., Dujon B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 1983;2(1):33–38. doi: 10.1002/j.1460-2075.1983.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Ferat J. L. Structure and activities of group II introns. Annu Rev Biochem. 1995;64:435–461. doi: 10.1146/annurev.bi.64.070195.002251. [DOI] [PubMed] [Google Scholar]
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Mohr G., Perlman P. S., Lambowitz A. M. Evolutionary relationships among group II intron-encoded proteins and identification of a conserved domain that may be related to maturase function. Nucleic Acids Res. 1993 Nov 11;21(22):4991–4997. doi: 10.1093/nar/21.22.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod C., Takahashi Y., Goldschmidt-Clermont M., Rochaix J. D. The chloroplast ycf8 open reading frame encodes a photosystem II polypeptide which maintains photosynthetic activity under adverse growth conditions. EMBO J. 1994 Jun 15;13(12):2747–2754. doi: 10.1002/j.1460-2075.1994.tb06568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Podar M., Boulanger S. C., Perlman P. S. The stereochemical course of group II intron self-splicing. Science. 1994 Dec 9;266(5191):1685–1688. doi: 10.1126/science.7527587. [DOI] [PubMed] [Google Scholar]
- Palmer J. D., Logsdon J. M., Jr The recent origins of introns. Curr Opin Genet Dev. 1991 Dec;1(4):470–477. doi: 10.1016/s0959-437x(05)80194-7. [DOI] [PubMed] [Google Scholar]
- Roger A. J., Doolittle W. F. Molecular evolution. Why introns-in-pieces? Nature. 1993 Jul 22;364(6435):289–290. doi: 10.1038/364289a0. [DOI] [PubMed] [Google Scholar]
- Saldanha R., Mohr G., Belfort M., Lambowitz A. M. Group I and group II introns. FASEB J. 1993 Jan;7(1):15–24. doi: 10.1096/fasebj.7.1.8422962. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schopf J. W. Microfossils of the Early Archean Apex chert: new evidence of the antiquity of life. Science. 1993 Apr 30;260:640–646. doi: 10.1126/science.260.5108.640. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Siemeister G., Buchholz C., Hachtel W. Genes for ribosomal proteins are retained on the 73 kb DNA from Astasia longa that resembles Euglena chloroplast DNA. Curr Genet. 1990 Dec;18(5):457–464. doi: 10.1007/BF00309917. [DOI] [PubMed] [Google Scholar]
- Siemeister G., Buchholz C., Hachtel W. Genes for the plastid elongation factor Tu and ribosomal protein S7 and six tRNA genes on the 73 kb DNA from Astasia longa that resembles the chloroplast DNA of Euglena. Mol Gen Genet. 1990 Feb;220(3):425–432. doi: 10.1007/BF00391749. [DOI] [PubMed] [Google Scholar]
- Siemeister G., Hachtel W. Organization and nucleotide sequence of ribosomal RNA genes on a circular 73 kbp DNA from the colourless flagellate Astasia longa. Curr Genet. 1990 May;17(5):433–438. doi: 10.1007/BF00334524. [DOI] [PubMed] [Google Scholar]
- Siemeister G., Hachtel W. Structure and expression of a gene encoding the large subunit of ribulose-1,5-bisphosphate carboxylase (rbcL) in the colourless euglenoid flagellate Astasia longa. Plant Mol Biol. 1990 May;14(5):825–833. doi: 10.1007/BF00016515. [DOI] [PubMed] [Google Scholar]
- Sontheimer E. J., Steitz J. A. The U5 and U6 small nuclear RNAs as active site components of the spliceosome. Science. 1993 Dec 24;262(5142):1989–1996. doi: 10.1126/science.8266094. [DOI] [PubMed] [Google Scholar]
- Wise J. A. Guides to the heart of the spliceosome. Science. 1993 Dec 24;262(5142):1978–1979. doi: 10.1126/science.8266091. [DOI] [PubMed] [Google Scholar]