Abstract
Since the discovery in the early eighties of the Human Immunodeficiency Virus (HIV) that causes acquired immunodeficiency syndrome (AIDS), there have been reports of people who were completely resistant to infection with HIV and others who progressed at slower rates to AIDS. The present article summarises the mechanisms involved in resistance against HIV infection and progression to AIDS. The paper will specifically focus on the role of immunological mechanisms, genetics, ethnicity and cultural practices such as male circumcision in mitigating infection. The current understanding on host natural resistance against HIV infection and progression to AIDS would potentially contribute to better prevention strategies, delayed onset of AIDS in people living with HIV, the identification of more efficient types of therapy for AIDS patients and, possibly, appropriate vaccines against HIV/AIDS. This area of research has important implications for patient care through controlling factors that contribute to AIDS progression.
Keywords: HIV; AIDS; Resistance, Natural; Disease, Progression
Acquired immunodeficiency syndrome (AIDS) was first reported in the United States of America in 1981 and has since become a major worldwide epidemic with over 40 million people infected. AIDS is caused by a retrovirus named the human immunodeficiency virus (HIV).1, 2, 3 By damaging or killing cells of the body’s immune system, HIV progressively destroys the body’s ability to fight infections and certain cancers. People diagnosed with AIDS may get life-threatening diseases and opportunistic infections (OI).4
HIV is spread most commonly by having unprotected sex with an infected partner.5, 6 HIV is also spread through contact with infected blood. The virus is frequently spread among injection drug users by the sharing of needles or syringes contaminated with very small quantities of blood from someone infected with the virus. Women can transmit HIV to their babies during pregnancy or birth. Approximately one-quarter to one-third of all untreated pregnant women infected with HIV will pass the infection to their babies.7, 8 HIV can also be spread to babies through the breast milk of mothers infected with the virus. If HIV-infected pregnant women are treated appropriately by antiretroviral drugs and deliver their babies by cesarean section, the chances of the baby being infected can be reduced to a rate of 1%.
Many people do not experience symptoms when first infected with HIV, however some have a flu-like illness within a month or two after exposure to the virus. Even during the asymptomatic period, the virus is actively multiplying, infecting and killing cells of the immune system. The virus can also hide within infected cells and lie dormant. The most obvious effect of HIV infection is a decline in the number of CD4 positive T (CD4+) cells, the immune system’s key infection fighters. The virus slowly disables or destroys these cells without causing symptoms.9
As the function of the immune system deteriorates, a variety of complications start to take over. The term AIDS applies to the most advanced stages of HIV infection. The Centre for Disease Control (CDC), in Atlanta Georgia (USA) has developed official criteria for the definition of AIDS.10, 11 The CDC’s definition of AIDS includes all HIV-infected people who have fewer than 200 CD4+ T cells per cubic millimeter of blood (healthy adults usually have CD4+ T-cell counts of ≥1,000 per mm3). In addition, the definition includes 26 clinical conditions that affect people with advanced HIV disease. Most of these conditions are opportunistic infections that generally do not affect healthy people. During the course of HIV infection, most people experience a gradual decline in the number of CD4+ T cells, although some may have abrupt and dramatic drop in their CD4+ T-cell counts. A person with CD4+ T cells above 200 may experience some of the early symptoms of HIV disease. Others may have no symptoms even though their CD4+ T-cell count is ≤200.
Recently and as a result of intensive research globally, two important breakthroughs in the AIDS epidemic have come to light. First, AIDS is now considered as a chronic medical condition, which is controllable by long-term therapy. Advances in HIV/AIDS research have been able to change the fatal outlook of HIV infection into a more or less living condition. The second important breakthrough has been the discovery that there are certain individuals who may possess natural mechanisms to resist HIV infection and/or, if they do become infected, are able to control the infection so effectively that they remain asymptomatic for long periods of time.12 A small number of people first infected with HIV ≥ 15 years ago have not developed symptoms of AIDS.13 Although these cases are still comparatively rare, studies of them have provided very important insights into natural mechanisms for resistance, or for overcoming HIV infection, which could be exploited for developing new antiretroviral drugs or HIV vaccines. Scientists are trying to determine what factors may account for their lack of progression to AIDS, such as: whether their immune systems have particular characteristics, whether they were infected with a less aggressive strain of the virus, or whether their genes may protect them from the effects of HIV.
It is known that many factors contribute to resisting infection with HIV; these include biological factors, host factors (e.g. immunologic such as CD8+ cell response), genetic factors (e.g. HLAs, Chemokine receptors etc.), viral factors (e.g. nef gene) and others including life style (see below). Natural resistance to HIV/AIDS can be considered at different levels: 1) resistance to becoming infected with the virus; 2) resistance to progression of the disease after infection e.g. long-term non-progressors; 3) resistance after symptoms appear (CD4+<200) e.g. long-term AIDS survivors. In this review, we will discuss each of these issues in some detail with up to date knowledge on each issue. In addition, we will touch on the role of the immune response in the resistance to HIV infection.
MECHANISMS OF RESISTANCE TO INFECTION BY HIV
There are individuals who are repeatedly exposed to HIV infection but remain sero-negative.14 They are also referred to as highly exposed sero-negative individuals. They include persons who continue to indulge in high-risk activities, such as numerous unprotected sexual contacts with multiple partners, yet still remain antibody negative. In fact, the transmission of HIV is very inefficient in comparison to other human viruses. It is therefore difficult to establish how often exposures to HIV in nature, which fail to establish infection, are due to resistance or are merely part of the intrinsic inefficiency of HIV transmission. There are, however, a number of examples of defined instances of HIV exposure, which provide opportunities for the study of resistance to HIV infection.
Persons in “high risk” populations, who are repeatedly exposed to HIV infection yet remain sero-negative to HIV, include groups of female prostitutes in Nairobi and the Gambia and male homosexuals in Los Angeles who have been subject to intense investigation.15 These individuals continue to engage in high risk, unprotected sexual activities yet remain antibody negative. There are also some long-term sexual-partners of HIV positive individuals who remain sero-negative, for example spouses of persons who have been infected by blood or blood products. Finally, some seronegative infants are born to HIV positive mothers (see later section).
Two types of resistance to HIV infection have been recognized: 1) resistance due to mutations in co-receptors used by HIV to establish infection; 2) when HIV infection does become established, there are some individuals who appear to be able to mount a particularly vigorous and effective immune response which is able to overcome and clear the infection.
MUTATION IN THE HIV CO-RECEPTOR
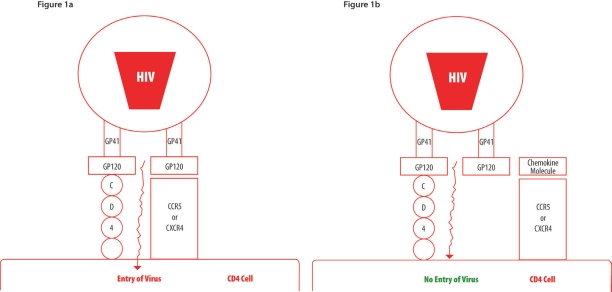
CD4, the main receptor for HIV, is a membrane bound extracellular receptor used by some immune cells, such as T helpers, macrophages, dendritic cells and monocytes, to help detect foreign cells in the body.16 It helps to amplify the action of T cell receptors after stimulation with the major histocompatibility complex. Upon finding a cell with a CD4 receptor, HIV (through its surface glycoprotein GP120) will bind to CD4 and another protein on the surface of the cell.17 This other protein is commonly a receptor which detects immune signals (a chemokine receptor, commonly CCR5 or CXCR4).18 Once HIV has bound to either of these two proteins it will be able to infect the cell [Figure 1a]. Some people have a slightly differently shaped immune signal receptor, commonly due to a mutation, which leaves it slightly truncated. This means that HIV cannot bind to it and as such cannot infect the cell [Figure 1b].
Figures 1a & 1b:
A simplified diagram showing that HIV requires a chemokine co-receptor (CCR5 or CXCR-4) in addition to the CD4, molecule the main receptor, to gain entry into the cell
Figure 1a - Virus entry due to availability of both CD4 receptor and a chemokine co-receptor
Figure 1b - No virus entry due to availability of only CD4 receptor as the chemokine co-receptor (CCR5 or CXCR4) is engaged in the binding to its ligand
The role of the chemokine receptors, which serve as co-receptors for HIV infection, has been established.19 Not surprisingly, therefore, individuals with mutations in the alleles coding for these receptors have been demonstrated to be resistant to HIV infection. Chemokines are well known chemical messengers, which transmit activation signals to recipient cells. Chemokines have been shown to be able effectively to inhibit HIV infection [Figure 1b].17, 20, 21 The receptor for the CC chemokines is called CCR-5 and this has been demonstrated to be a crucial co-receptor for macrophage tropic (M-tropic) strains of HIV-1. The M-tropic strains of HIV-1 are the main strains, which are responsible for establishing infection and they predominate in the early stages of infection.22 These strains target macrophages and CD4+ lymphocytes. T-tropic strains predominate in the latter stages of infection and infect CD4+ lymphocytes, but not macrophages, and utilize another co-receptor called fusin.
Many chemokine co-receptors, including the CCR-5 and fusin, are known to act as secondary receptors for virus entry.17, 20, 21, 23, 24 Based on the identification of its chemokine ligand, fusin was renamed CXCR-4. The discovery that CXCR-4 constitutes a functional co-receptor for T-tropic HIV-1 strains gave added significance to the earlier finding that the C-C chemokines (MIP-1-alpha, MIP-1-beta and RANTES) are able to block infection of CD4+ human T-cells by M-tropic but not T-tropic HIV-1.25
CCR5 appears to be important for non-syncitium inducing (NSI) strains of HIV (the strains most common in early disease), while CXCR4 appears to be more important for syncytium inducing (SI) strains (a more aggressive strain seen in some people with more aggressive disease).26 NSI strains of HIV are the most common sexually transmitted form of the virus. This type of HIV preferentially infects macrophages (often found in the skin and mucous membrane) rather than T-cells. Therefore, it is M-tropic. When HIV is transmitted sexually, it first establishes itself as an M-tropic virus, later developing into T-cell-tropic viruses in some people. These T-tropic strains that prefer to infect T-cells are SI viruses and may become more prevalent during later stages of the disease. It is unclear why the virus converts from an NSI to an SI strain in some people. Approximately 50% of people who die of AIDS still have a predominant NSI strain of virus. The SI strain of HIV is more aggressive and its prevalence correlates with more rapid disease progression.27 Additionally, anti-HIV drugs generally have less activity against SI strains of HIV.26, 28
The CCR-5 receptor is responsible for the penetration and entry of HIV-1 into the cell after the virus has attached to the CD4+ cell surface antigen by its envelope glycoprotein GP120 [Figure 1]. Mutations in the CCR-5 receptor could therefore be expected to result in cells becoming resistant to HIV-1. A small number of individuals who are homozygous for the allele of CCR-5 have been found amongst the highly exposed group of sero-negative individuals. Lymphocytes from these individuals have been shown to be highly resistant to infection with HIV-1. As yet no individuals with the homozygous mutant have been found to be HIV infected. Approximately 1% of the Caucasian population is homozygous and 20% are heterozygous. Mutations in CCR-5 have not been found in African subjects. Heterozygosity is thought to provide partial protection, i.e. individuals with one mutant copy of the CCR-5 gene appear to progress more slowly to AIDS than individuals without this mutation. Mutations in the CCR-5 chemokine receptor have thus been shown to be a mechanism of HIV resistance in a small number of persons. Many highly exposed seronegative individuals, however, do not have a CCR-5 mutation and the explanation for their resistance still needs to be elucidated.
When the CD8+ cells effectively make a large quantity of the chemokines, they may fill up and block the ‘doorway’ for infection provided by the CCR5 protein.17, 20, 21, 29 Conversely, when levels of the chemokines are low or absent for any reason, the virus is free to infect cells more easily because the CCR5 receptor protein is readily available to it. Collectively, the back-to-back discoveries of the role of the chemokines and the CCR5 receptor site shed important new light on how HIV infects cells and may explain why the disease process differs from person to person.
Certainly there are people with an inherited deletion who have progressed to AIDS and died. CCR5 deletion or not, monitoring health, making wise treatment choices and keeping the virus in check is critical to managing HIV disease. There are hundreds of people who have been categorized as long-term nonprogressors who have fully intact CCR5 genes. For example, there were a number of reports of identified individuals who had inherited the deletion in the CCR5 gene from both parents and were nonetheless infected with HIV. According to previous reports and identified cases, those who inherited the gene from both parents were assumed to have some natural immunity to HIV infection. To the contrary, rather than being infected with the most common strain of HIV, the individuals were infected with the type of virus typically only found in some people with advanced stages of HIV disease, that rely on the CXCR4 protein to support entry into the immune cell. This type of virus is associated with dramatic and rapid loss of CD4+ cells and more rapid progression of disease. In line with this, these reported individuals suffered a very rapid course of the disease.30
PROTECTION AGAINST HIV INFECTION RELATED TO THE IMMUNE SYSTEM
Protection in infants
Approximately 25% of infants born to HIV-infected mothers are persistently infected with HIV and progress to AIDS. Of the remaining 75%, many are uninfected because an inadequate dose of virus crosses the placenta. However, investigations of fetuses from HIV-positive mothers who were electively aborted in early pregnancy have shown that the majority have HIV-DNA sequences in cells from various organs.31, 32, 33 From these studies, it would therefore appear that the majority of infected fetuses are able to overcome and clear the infection. The mechanism, however, is still unclear as the immune system of the fetus is immature and ineffective in clearing most transplacentally transmitted viruses.
Further evidence of protection related to the immune system in the infant are the manifestations of lymphocyte reactivity to HIV, which are found in seronegative infants. Some 40% of uninfected infants demonstrated lymphocyte reactivity (by IL-2 production) in their cord blood and peripheral blood lymphocytes in response to several HIV peptides including GP160 and nef proteins.34, 35 Even more convincing is the finding of HIV-specific cytotoxic T-lymphocytes implying continuous antigenic stimulation and therefore viral replication in the host.34, 35
The most striking and widely publicized illustration of the ability of an infant to overcome HIV infection is that of a child in Los Angeles born to an HIV positive mother, who had negative HIV cultures of blood at birth, but then produced sub-sequent positive cultures at 19 days and 51 days.36, 37 However, numerous follow-up blood cultures were negative and the child has remained sero-negative and clinically well afterwards. Several other cases of sero-reverting infants have also been described.34, 38 The exact mechanism for sero-reverting is not yet completely understood.
Protection in adults
Sero-negative adults who have been exposed to HIV also have demonstrated an immune response to the virus as shown by lymphocyte reactivity in response to HIV antigens. These individuals include those having unprotected sexual intercourse with multiple HIV infected partners, intravenous drug abusers, prostitutes, recipients of blood or blood products contaminated with HIV and healthcare workers who have been exposed to HIV through needle injuries with HIV infected blood.39, 40 Some of the possible mechanisms could include exposure to virus-free antigens of HIV or defective strains of HIV. However, the finding of CD8+ cytotoxic T-lymphocytes, which were MHC class-I restricted, would indicate at least one round of viral replication and suggest that the immune system was able to overcome and clear the infection without seroconversion (the production of antibodies).
MALE CIRCUMCISION
Circumcised males are less likely than their uncircumcised peers to acquire sexually transmitted infection and circumcision may reduce the risk of acquiring and spreading such infections by > 50%.41 Adult male circumcision has been recognized to provide protection against HIV infection.42 Adult male circumcision has been found to provide > 60% protective effect against infection with HIV in a randomized trial of >3,000 sexually active, heterosexual men in South Africa.43 In this trial, subjects willing to be circumcised were randomized to receive immediate circumcision or to wait 20 months for the procedure. In the intervention group, medical physicians removed the penile foreskin after administering local anesthetic and using sterile surgical procedures. At 21 months, the researchers diagnosed 69 new HIV cases, 51 in the control group that had yet to be circumcised, and 18 in the group that had been circumcised. This translates into a 65% protective effect (with a 95% confidence interval of 40% to 80%).
Furthermore, other trials have been stopped due to dramatic preliminary results indicating that medical circumcision reduces the risk of men getting HIV during heterosexual intercourse by > 50%. Results from these randomized controlled trials bolster previous evidence from more than 40 observational epidemiological studies and clinical trials that showed an association between male circumcision and a reduced risk of HIV infection.
OTHER MOLECULES
Other molecules have been implicated in the resistance and susceptibility to HIV infection. They include the human leukocyte antigens (HLA) (see below), beta chemokines and defensins, polymorphism in the cytokine genes, configuration on chromosome-22, beta 2 microglobulin,44, 45 HIV-2,46 etc.
MECHANISMS OF RESISTANCE TOWARDS PROGRESSION TO AIDS
INDIVIDUALS WHO SEEM TO MOUNT A PARTICULARLY VIGOROUS AND EFFECTIVE IMMUNE RESPONSE
It has been reported that over a hundred people did not become infected with HIV despite hundreds of exposures through sexual contact or intravenous (IV) drug sharing. It has been hypothesised that they had a unique immunological ability to marshal the response of CD8+ lymphocytes.47 As in the general population, the CD4+ lymphocytes of these people were susceptible to HIV infection. It was only in the presence of the CD8+ cells that viral replication was stopped. The CD8+ cells of non-HIV exposed individuals studied did not mount this response. It is believed that this immunity results from exposure to low amounts of virus, which is enough to get the cellular immune antiviral response going. Each subsequent exposure then acts as a kind of booster. Researchers do not know why a strong CD8+ cell antiviral response occurs in some people and not in others.
It was found that the antiviral action of CD8+ cells was amplified when exposed to macrophages expressing a CD86 molecule on their surface. Macrophages play a role in increasing CD8+ cell function. Researchers had previously suspected that the macrophages stimulate CD8+ cells through the CD80 molecule, rather than the CD86 molecule. The researchers demonstrated the role of the CD86 molecule by blocking the interaction of the CD28 molecule on CD8+ cells with the CD86 molecule found on macrophages, and showed that the macrophages’ enhancing effects were abrogated. When they exposed macrophages to anti-CD86 antibodies, the CD8+ cells were not stimulated. In contrast, anti-CD80 blocking antibodies had little effect on the ability of macrophages to enhance CD8+ cell antiviral response. Moreover, the researchers were able to reverse the decreased antiviral response that followed treatment of macrophages with anti-CD86 neutralizing antibodies by exposing the CD8+ cells to anti-CD28 antibodies, which are able to carry out the same function as the CD86 molecule. These studies indicate that engagement of the CD28 molecule on CD8+ cells with the CD86 molecule on macrophages is critical for optimal suppression of HIV replication by the CD8+ cells.47
LONG-TERM NON-PROGRESSORS (LTNP)
These HIV-positive individuals have not been on antiviral therapy yet have remained clinically well and have had a reasonable immune function with high and stable CD4+ lymphocyte counts for many years without showing signs of immune compromise or evidence of progression to AIDS.48
There is a wide range of clinical responses to HIV infection in humans. In about 80% of HIV infected individuals AIDS, develops within the median time of 12 years. Individuals with this clinical course are referred to as typical progressors. In 10% of HIV infected subjects, AIDS develops within 3 years after infection; these individuals are referred to as rapid progressors. On the other side of the spectrum, about 10% of HIV infected persons remain asymptomatic for at least 15 years and often up to 20 years after HIV infection, despite not being on anti-retroviral therapy. Their immune function is relatively well maintained with CD4+ lymphocyte counts above 600/mm3 and low plasma levels of HIV-1 RNA. Biopsies of their lymph nodes confirm the non-progressive nature of their infection with little evidence of the hyperplastic and involuted changes or lymphocyte depletion seen in lymph node tissue from subjects with progressive disease. These individuals are referred to as long-term non-progressors (LTNP) or long-term survivors. Both viral as well as host factors play a role in long-term non-progression of HIV.49
Factors related to the virus
Culture of HIV from the blood of LTNPs is difficult and often unsuccessful because of the lower plasma viral load. Molecular characterization of cultured isolates, have revealed the presence of a number of genetic defects which could be the basis for the attenuation of the virus.35, 50 Occasionally, genetic defects such as in the NfkB or Sp1 site within the long terminal repeats of the virus have been demonstrated. However, the best-documented and studied genetic lesions associated with virus attenuation in LTNPs are the nef deletion mutants. The nef gene is a crucial regulatory gene of HIV-1. Monkeys experimentally inoculated with simian immunodeficiency virus (SIV) which have deletions in their nef gene, show no signs of disease and have low viral loads in the plasma and normal CD4+ lymphocyte counts.51 In humans, nef deleted mutants of HIV-1 have also been shown to play a role in the genesis of long-term non-progression. An HIV infected male homosexual donated blood and infected some 7 recipients over a period of 3 years (in the era before blood was routinely tested for HIV). Surprisingly, neither the donor nor any of the recipients developed any symptoms and had remained healthy (with the exception of 2 who died from unrelated causes). Molecular studies on isolates from 4 of the 8 subjects demonstrated defects in the nef gene. Although nef deletion mutants still only account for a very small number of LTNPs, they have aroused great scientific interest because of their potential usefulness in the development of an HIV vaccine.
Factors related to the host
From studies of a number of cohorts of LTNPs, a pattern appears to be emerging characterizing these individuals as having immune responses which are quantitatively and qualitatively more potent and more effective in controlling HIV infection.52 Vigorous virus-specific humoral and cell mediated immune responses have been demonstrated in these subjects. High titres of potent neutralizing antibodies to a wide spectrum of HIV isolates have been shown to be present in the sera of LTNPs. In addition, there are strong CD8+ cytotoxic lymphocyte responses in these individuals, reflecting long-standing stimulation of the immune system by continuing viral replication.53 The resulting immune response is able effectively to suppress viral replication and thus relatively low viral loads are found in their plasma and high CD4+ lymphocyte counts are maintained in the blood. The lymph node architecture remains intact and the degree of virus trapping in the follicular dendritic network in the lymph nodes is considerably lower.
Precisely why some individuals respond with a more favorable immune response than others is still not clear. It is also uncertain whether the more effective immune response is a cause of lower viral loads or whether the more effective immune response is the consequence of infection with more attenuated viral variants.
HLA MOLECULES AND RESISTANCE TO HIV INFECTION AND DISEASE PROGRESSION
Evidence for acquired resistance to HIV infection54 reflects previous immunological priming with a low sub-infective dose of virus. Individuals who appear to be naturally protected against HIV infection or from disease progression could be intrinsically resistant through genetic host factors such as genes coding for co-receptors e.g. CCR5, 17 discussed earlier, and their HLA genotypes.55, 56, 57 AIDS pathogenesis would appear to involve multigene systems such as the HLA complex and the derivative immune response genes of the T cell receptor (TCR). These and other cofactors (see Table 1), such as psychosocial factors and nutrition, are believed to influence disease progression.55 There are two types of studies, in vitro and in vivo, on HLA and susceptibility or resistance to HIV infection.55
Table 1:
List of some of the factors that can affect AIDS progression
| Appropriate drug treatment (eg. HAART) |
| Non-adherence to drug therapy |
| Stress |
| Nutrition and malnutrition |
| Genetic make up of the individual |
| Age |
| Gender |
| Pregnancy |
| Environmental factors |
| Special habits |
| Effects of other diseases (sexual transmitted diseases, STD) |
| The degree and frequency of virus exposure |
| Co-infection with other viruses |
| Risk activity (behavior) |
| Additional immunosuppressive factors |
| Ethnic background |
| Drug addiction |
| Hemophiliacs |
| Autoimmune diseases |
| Exercise |
In vitro type studies
We have shown that certain HLA molecules correlate, with in vitro high or low HIV virus replication.55, 57–59 Our own results showed clearly that certain HLA specificities were significantly correlated in vitro with infectivity by both HIV-1 and HIV-2 isolates and that each isolate had its own correlation with HIV infection.55, 58 This may partly explain the differences in the in vivo results on the association of HLA with certain features of AIDS and the difficulty in confirming such correlation. However, more studies in vitro on a large scale and on groups who are at high risk or low risk need to be addressed with the identification of the HIV isolate that is predominant in each individual patient. These studies will characterize better those HLA specificities that are important for infectivity by HIV-1 and HIV-2. The existence of a significant HLA association with all the known HIV-1 and all HIV-2 isolates infecting human populations may lead to the identification of important immunodominant epitopes that can be useful in vaccine development against the different clades (subtypes) of HIVs. There is a possibility that HLA are not the actual genes responsible for susceptibility or resistance to HIV infection, rather other, as yet unidentified genes closely linked to HLA, or masked by the HLA, could be the real genes determining susceptibility or resistance to HIV.60, 61 This can be revealed only by intensive future research work and by better knowledge and understanding of the genes that are linked to the HLA molecules.55
Many reports have already suggested the existence of an association between progression to full blown AIDS, or certain features of AIDS such as AIDS related Kaposi’s sarcoma, with some HLA haplotypes.56, 62–67 HLA-B35 has been shown by many researchers to correlate with susceptibility to faster progression towards AIDS. However, the HLA haplotypes associated either with progression, or with a defined AIDS related syndrome, are not identical in different studies. Because of the nature of HIV-1 infection and the associated disease, it would have been surprising not to find some correlates with HLA haplotypes in studies of an infected population. In order to understand a potentially complex disease, we have shown that the HLA genotype of the individual may determine the rate of virus production, and possibly susceptibility to disease in HIV infected populations.58 Not all of the known HLA alleles are detected in the populations studied. Relatively homogenous populations express a restricted range of HLA alleles.56 In general, it is difficult to confirm an association between HLA and disease, as frequencies of HLA alleles between different populations can vary greatly, and more than one HLA allele may be associated with susceptibility to infection or to disease progression.55
Our own in vitro results showed clearly that HLAB70 and also B58 alleles are indeed correlated with high viral replication to HIV-2 infection of peripheral blood mononuclear cells (PBMCs). The correlation was HIV-2 isolate dependent with the HLA-B70 allele correlating with the growth of the HIV-2-ROD strain and the B58 allele with the HIV-2-CBL-20 strain. In addition, HLA-B44 seems to be important for resistance to HIV-1 infection.55, 58 Previous HLA-HIV correlations usually do not take into account the type of HIV isolate(s) within a particular cohort or a group of patients. This may partly explain the lack of agreement between studies on the involvement of a particular HLA haplotype in the HIV-1 disease process.55 Our results show that viral replication in different donor PBMCs depends both on the HIV strain, and also on the HLA type of the PBMCs. The only two alleles, which were shown, to be significantly correlated with high viral replication, were both from the HLA class I B locus and may indicate an important role for this locus in controlling HIV-2 replication.58
We have demonstrated that the rates of HIV-1 and 2 production in vitro vary greatly between individuals, even when other variables such as the degree of lymphocyte activation and culture conditions are carefully controlled.59 Quantitative differences in virus production can differ by >3000 fold between the highest and the lowest virus producers. Both host cellular factors and viral properties may influence the level of viral replication in any given virus-cell combination. High and low producers, in the case of HIV-1, can be predicted from the examination of how the HLA-B locus, present in the individual, binds the GP120 peptide of HIV in comparison with the binding of HLA-DR beta chain peptide fragment.68 Although we do not know the exact mechanism accounting for our observed data at present, the mimicry of HLA by HIV may theoretically provide the T-cell activation essential for virus replication.68 The amount of viral replication in culture, which depends upon the degree of T-cell activation, would depend on the degree of mimicry between the infecting HIV strain and the HLA of the host.
In vivo (Cohort type) studies
Perhaps the most compelling evidence for the role of HLA genes in susceptibility/resistance to HIV-1 infection comes from the investigation of cohorts of high-risk individuals with chronic or repetitive exposure.66, 67, 69–72 Resistance to HIV infection in a cohort of highly exposed, persistently negative female sex workers in Thailand was associated with an increased frequency of HLA-B18.73 In a cohort of female sex workers in Kenya, a decreased risk for HIV infection was associated with a cluster of related class I alleles-HLA-A2/6802.65 Moreover, resistance was consistently demonstrated to be independently linked with HLA-DRB1*01. Increased susceptibility to HIV was linked with HLA-A*2301 in the same cohort. The HLA-A2/6802 cluster/supertype was also associated with a decreased risk of perinatal transmission in mothers of the same ethnic group. These sero-negative women have strong cytotoxic T cell responses to conserved HIV peptides presented by this HLA-A2/6802 supertype.
Studies with several viral systems, including HIV, suggest that there are families of closely related HLA alleles, like the cluster described above, that may present the same or highly similar peptide antigens. These have been termed HLA super-types and the targeted viral epitopes, supertopes. Super-types may be represented in as much as 50% of the general population and, therefore, may be desirable targets for broadly effective vaccines.74 Combinations will certainly be necessary, and there are many potential limitations. Population-based epitope/HLA studies have often been conducted in cohorts from developed countries with Caucasian majorities. Recent emphasis has been on generating similar comprehensive databases for communities and ethnic groups that vaccine efforts will target. Identification of HLA alleles linked with natural resistance to HIV infectivity is critical for the design of peptide-based vaccines.
LONG-TERM AIDS SURVIVORS [AFTER SYMPTOMS APPEARS (CD4 < 200 CELLS/MM3)]
The main goal of antiretroviral therapy is to reduce viral load to below detectable levels and maintain patients free of symptoms. Not all individuals undergoing highly active antiretroviral therapy (HAART) respond positively to their treatment and not all AIDS patients with symptoms will progress to death at the same rate. This could be due to many factors [Table 1] including non-adherence to the antiretroviral therapy and the genetic makeup of the individual.75 A group of AIDS patients exist who do not respond positively to antiretroviral treatment and therefore die much earlier than those who respond positively. The survival rate of other AIDS patients, who survive longer after their CD4+ counts are < 200 cells/mm3, is either due to good response to treatment or due to other factors. This group of patients can be considered as long-term AIDS survivors. In fact, not much research has been done on this group of patients and future studies may address this issue critically.
FACTORS THAT MAY AFFECT AIDS PROGRESSION
Many factors are known to affect AIDS progression; these include life style, stress, nutrition and malnutrition, environmental factors and so on.55 Table 1 shows some of these factors. It is beyond the scope of this review article to discuss these factors in depth. If some of these factors are adequately controlled, they have important implications for patient care and the onset of AIDS can be delayed.
IMMUNE RESPONSE AND NATURAL RESISTANCE TO HIV INFECTION
The natural resistance to HIV infection and its relation to the different elements of the immune response, although being mentioned in different sections above, will be briefly discussed below.
INNATE IMMUNITY AND RESISTANCE TO HIV INFECTION
Innate immunity during HIV infection has been extensively studied, but mainly with the complement system, interferon, natural killer (NK) cells and gamma/delta T cells. Many other areas of how innate immunity functions during HIV infection remain relatively unexplored.
The complement system is highly activated during HIV infection, as determined by high levels of complement breakdown products in blood.76, 77,78 Some of this activation is probably due to microorganisms associated with opportunistic infections. However, at least a portion of the complement activation during HIV infection is due to the interaction of HIV or HIV-infected cells with complement since the virus in infected individuals is coated with complement activation products, notably C3 fragments.79, 80, 81 Primary isolates of HIV are much more resistant to destruction by complement, probably due to both the relatively low amount of antibody that binds to primary isolate virions82 and incorporation of different types of complement control proteins.83
Interferons (IFN) have been known to play a role in the host defense against viral infections. IFN have been categorized as type-I (including IFN-α, IFN-β, IFN-ω)84 and type 2 (IFN-γ). Type 1 IFN have been generally associated with natural host defenses, while type 2 IFN-γ is, among other sources, a principal product of activated T helper cell (Th)-1 biased CD4+ T cells participating in the adaptive immune system and NK cells. IFN-γ has been known as an important macrophage activating cytokine, necessary for the final effector mechanisms of cellular immunity.85 IFN have been known to be involved in resistance to HIV; antibodies to IFN were incorporated into the initial culture systems utilized for the isolation of HIV (lymphadenopathy-associated virus).1 IFN-α, used to treat Kaposi’s sarcoma, had an effect on HIV viremia, although notably only in patients having sufficiently high numbers of CD4+ T cells.86 Their involvement in the pathogenesis of AIDS was recognized by the presence of IFN in serum during the latter stages of HIV infection.87,88 These IFN were considered acid-labile alpha-IFN because, despite their neutralization with anti-alpha antisera, they were destroyed by acidification, a characteristic of IFN-γ.
Notably, the long-term non-progressors had significantly higher numbers and functions of plasmacytoid dendritic cells (pDC) compared not only with patients with progressive HIV infection, but also with uninfected controls.89 The correlation noted between virus burden and pDC suggests that pDC function is, perhaps not surprisingly, involved in the suppression of HIV itself, either directly by IFN production acting on infected cells, or through a Th-1 mechanism. Soumelis, et al., (2001)89 showed that development of susceptibility to both OI and active Kaposi’s sarcoma depends on the simultaneous depression of both CD4+ cell counts and pDC numbers below certain critical levels.90
ACQUIRED IMMUNITY
Humoral immunity
Some of the earliest studies evaluated the generation of humoral immunity in the form of broadly neutralizing circulating or mucosal antibody. Its potential usefulness is based, in part, on vertical and horizontal transmission studies in which neutralizing antibody in the donor (positive partner or mother) was associated with reduced transmission. Antibody responses observed to date in the natural setting or in laboratory or vaccine models have generally been strain-specific or non-protective. However, these responses may not be comparable or relevant to neutralizing antibody in the uninfected partner. In animal models, neutralizing antibody can prevent a simian virus-HIV chimeric infection, but the amount of neutralizing antibody required was far in excess of that found in humans.91 The primary role of neutralizing antibody in natural resistance to HIV is not clear. In addition, the passive administration of antibody is not feasible in the general, at-risk population. There have been surprisingly few studies on circulating neutralizing IgG or IgA antibody.
Cell mediated immunity
Studies on naturally resistant populations revealed that few studies analyzed more than one protective immune defense,92 although it is difficult to compare studies on natural resistance because most have measured only one or two defenses and techniques vary substantially. The most frequently studied natural defenses implicated in effective responses against HIV are: CTL effector response; CD4+ T lymphocyte helper responses; non-cytolytic CD8+ HIV suppression and CD8+ beta chemokine production. It is beyond the scope of this review article to discuss these in details, but interested readers may be directed to recent articles on these topics.
CONCLUSION
The vast amount of evidence suggests that there is a natural resistance to HIV infection and to progression to AIDS. However, the exact mechanisms accounting for such resistance are not yet completely elucidated. Future research may reveal the exact mechanisms by which this natural resistance operates, with the potential of developing appropriate therapeutic interventions and vaccines. Long-term non-progression, as well as resistance to viral infections, is a relatively uncommon phenomenon. Adequately controlling factors that contribute to fast progression to AIDS can delay the onset of the symptoms and provide a better life style for those patients. At the present time, however, and for the majority of HIV infected individuals, antiretroviral therapy remains the only effective way of controlling HIV infection.
REFERENCES
- 1.Barre-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS) Science. 1983;220:868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- 2.Popovic M, Sarngadharan MG, Read E, Gallo RC. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre AIDS. Science. 1984;244:497–500. doi: 10.1126/science.6200935. [DOI] [PubMed] [Google Scholar]
- 3.Levy JA, Hoffman AD, Kramer SM, Landis JA, Shimabukuro JM, Shiro LS. Isolation of lymphocytopathic retroviruses from San Francisco patients with AIDS. Science. 1984;225:840–842. doi: 10.1126/science.6206563. [DOI] [PubMed] [Google Scholar]
- 4.Levy JA. Pathogenesis of HIV infection. Microbiol Rev. 1993;57:183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron DW, Simonsen JN, D’Costa LJ, Ronald AR, Maitha GM, Gakinya MN, et al. Female to male transmission of human immunodeficiency virus type 1: Risk factors for seroconversion in men. Lancet. 1989;ii:403–407. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 6.Padian NS, Shiboski SC, Jewell NP. Female to male transmission of Human Immunodeficiency Virus. JAMA. 1991;266:1664–1667. [PubMed] [Google Scholar]
- 7.Minkoff H, Nanda D, Menez R, Fikrig S. Pregnancies resulting in infants with Acquired Immunodeficiency Syndrome or AIDS Related Complex. Obst Gynecol. 1987;69:285–287. [PubMed] [Google Scholar]
- 8.Minkoff H, Nanda D, Menez R, Fikrig S. Pregnancies resulting in infants with Acquired Immunodeficiency Syndrome or AIDS-related complex: follow up of mothers, children, and subsequently born siblings. Obst Gynecol. 1987;69:288–291. [PubMed] [Google Scholar]
- 9.Cloyd MW, Moore BE. Spectrum of biological properties of Human Immunodeficiency virus (HIV-1) isolates. Virology. 1990;174:103–116. doi: 10.1016/0042-6822(90)90059-z. [DOI] [PubMed] [Google Scholar]
- 10.CDC Classification system for human T-lymphotropic virus type III/lymphadenopathy-associated virus infections. Morbid Mortal Wkly Rep. 1986;35:334–339. [PubMed] [Google Scholar]
- 11.CDC Revised classification system for HIV infection and expanded surveillance of definition for AIDS among adolescents and adults. Morbid Mortal Wkly Rep. 1993;41:17. [PubMed] [Google Scholar]
- 12.Rutherford GW, Lifson AR, Hessol NA, Darrow WW, O’Malley PM, Buchbinder SP, et al. Course of HIV-1 infection in a cohort of homosexual and bisexual men: an 11 year follow up study. BMJ. 1990;301:1183–1188. doi: 10.1136/bmj.301.6762.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Detels R, Liu Z, Hennessy K, Kan J, Visscher BR, Taylor JM, et al. Resistance to HIV-1 infection. Multicenter AIDS cohort study. J AIDS. 1994;7:1263–1269. [PubMed] [Google Scholar]
- 14.Paxton WA, Martin SR, Tse D, O’Brien TR, Skurnick J, Vandevanter NL, et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposures. Nature Med. 1996;2:412–417. doi: 10.1038/nm0496-412. [DOI] [PubMed] [Google Scholar]
- 15.Rowland-Jones S, Sutton J, Ariyoshi K, Dong T, Gotch F, McAdam S, et al. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nature Med. 1995;1:59–64. doi: 10.1038/nm0195-59. [DOI] [PubMed] [Google Scholar]
- 16.Dalgleish AG, Beverley PC, Clapham PR, Crawford DH, Greaves MF, Weiss RA. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984;312:763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- 17.Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 18.Al-Jabri AA. How Does HIV-1 Infect a Susceptible Human Cell? Current thinking! Sultan Qaboos Univ J Sci Res Med Sci. 2003;5:31–44. [PMC free article] [PubMed] [Google Scholar]
- 19.Bowers M. Chemokines and HIV. BETA. 1997;3:22–27. [PubMed] [Google Scholar]
- 20.Feng Y, Border CC, Kennedy PA, Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–876. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 21.Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 22.Chesebro B, Wehrly K, Nishio J, Perryman S. Macrophage-tropic human immunodeficiency virus isolates from different patients exhibit unusual V3 envelope sequence homogeneity in comparison with T-cell tropic isolates: definition of critical amino acids involved in cell tropism. J Virol. 1992;66:6547–6554. doi: 10.1128/jvi.66.11.6547-6554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier M, Arenzana-Seisdedos F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 24.Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, et al. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;82:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 25.Cocchi F, DeVico AL, Garzino-Demo A, Arya SK, Gallo RC, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T Cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 26.Cornelissen M, Mulder-Kampinga G, Veenstra J, Zorgdrager F, Kuiken C, Hartman S, et al. Syncytium-inducing (SI) phenotype suppression at seroconversion after intramuscular inoculation of a non-syncytium-inducing/SI phenotypically mixed human immunodeficiency virus population. J Virol. 1995;69:1810–1818. doi: 10.1128/jvi.69.3.1810-1818.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schuitemaker H, Koot M, Kootstra NA, Derksen M, de Goede REY, Van Steenwijk P, et al. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T- cell-tropic virus populations. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koot M, Keet IPM, Vos AHV, DeGoede REY, Roos MTL, Coutinho RA, et al. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann Intern Med. 1993;118:681–688. doi: 10.7326/0003-4819-118-9-199305010-00004. [DOI] [PubMed] [Google Scholar]
- 29.Arenzana-Seisdedos F, Virelizier JL, Rousset D, Clark-Lewis I, Loetscher P, Moser B, et al. HIV blocked by chemokine antagonist. Nature. 1996;383:400. doi: 10.1038/383400a0. [DOI] [PubMed] [Google Scholar]
- 30.Moore JP. Coreceptors: Implications for HIV Pathogenesis and Therapy. Science. 1997;276:51–52. doi: 10.1126/science.276.5309.51. [DOI] [PubMed] [Google Scholar]
- 31.Roques PA, Gras G, Parnet-Mathieu F, Mabondzo AM, Dollfus C, Narwa R, et al. Clearance of HIV infection in 12 perinatally infected children: clinical, virological and immunological data. AIDS. 1995;9:F19–F26. [PubMed] [Google Scholar]
- 32.Joshi VV, Oleske JM, Minnefor AB, Singh R, Bokharit T, Rapkin RH. Pathology of suspected acquired immune deficiency syndrome in children: a study of eight cases. Paediatr Pathol. 1984;2:71–87. doi: 10.3109/15513818409041189. [DOI] [PubMed] [Google Scholar]
- 33.Just J, Louie L, Abramst E, Nicholast SW, Warat D, Steins Z, et al. Genetic risk factors for perinatally acquired HIV-1 infection. Paediatr Perinat Epidem. 1992;6:215–224. doi: 10.1111/j.1365-3016.1992.tb00762.x. [DOI] [PubMed] [Google Scholar]
- 34.Baur A, Schwharz N, Ellinger S, Ellinger S, Korn K, Harrer T, et al. Continuous clearance of HIV in a vertically infected child. Lancet. 1989;ii:1045. doi: 10.1016/s0140-6736(89)91061-1. [DOI] [PubMed] [Google Scholar]
- 35.Baur AS, Sawai ET, Dazin P, Fantl WJ, Cheng MC, Peterlin BM. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localisation. Immunity. 1994;1:373–384. doi: 10.1016/1074-7613(94)90068-x. [DOI] [PubMed] [Google Scholar]
- 36.Bryson Y, Chen ISI. HIV clearance in an infant? Nature. 1995;375:637–638. doi: 10.1038/375637a0. [DOI] [PubMed] [Google Scholar]
- 37.Bryson YJ, Pang S, Wei LS, Dickover R, Diagne A, Chen ISI. Clearance of HIV infection in a perinatally infected infant. N Engl J Med. 1995;332:833–838. doi: 10.1056/NEJM199503303321301. [DOI] [PubMed] [Google Scholar]
- 38.Frenkel LM, Nichols JE, Wagner LE, Haase AT, Roberts NJ., Jr Loss of HIV-1 viremia with persistent specific immunity in a women and her child. J Cellular Biochem. 1995;21:234. [Google Scholar]
- 39.Perrin LH, Zubler R, Hirschel B, Martin JL, Salomon D, Saurat JH, et al. Reversal of positive serology for human immunodeficiency virus (HIV). A propose of 2 case reports. Schweiz Med Wochenschr. 1988;118:1641–1644. [PubMed] [Google Scholar]
- 40.Taylor R. Quiet clues to HIV-1 immunity: Do some people resist infection? J NIH Res. 1994;6:29–31. [Google Scholar]
- 41.Williams BG, Lloyd-Smith JO, Gouws E, Hankins C, Getz WM, Hargrove J, et al. The potential impact of male circumcision on HIV in Sub-Saharan Africa. PLoS Med. 2006;3:e262. doi: 10.1371/journal.pmed.0030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Vincenzi I, Mertens T. Male circumcision: a role in HIV prevention? AIDS. 1994;8:153–160. [PubMed] [Google Scholar]
- 43.Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lifson AR, Hessol NA, Buchbinder SP, O’Malley PM, Barnhart L, Segal M, et al. Serum Beta-2-Microglobulin and prediction of progression to AIDS in HIV infection. Lancet. 1992;339:1436–1440. doi: 10.1016/0140-6736(92)92030-j. [DOI] [PubMed] [Google Scholar]
- 45.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, et al. Homozygous Defect in HIV-1 Coreceptor Accounts for Resistance of Some Multiply-Exposed Individuals to HIV-1 Infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 46.Travers K, Mboup S, Marlink R, Gueye-Ndiaye A, Siby T, Thior I, et al. Natural protection against HIV-1 infection provided by HIV-2. Science. 1995;268:1612–1615. doi: 10.1126/science.7539936. [DOI] [PubMed] [Google Scholar]
- 47.Westby M, Manca F, Dalgleish AG. The role of host immune responses in determining the outcome of HIV infection. Immunol Today. 1996;17:120–126. doi: 10.1016/0167-5699(96)80603-7. [DOI] [PubMed] [Google Scholar]
- 48.Lemp GF, Hirozawa AM, Cohen JB, Derish PA, McKinney KC, Hernandez SR. Survival for women and men with AIDS. J Infect Dis. 1992;166:74–79. doi: 10.1093/infdis/166.1.74. [DOI] [PubMed] [Google Scholar]
- 49.Dean M, Carrington M, Winkler C, Huttley GA, Smith MW, Allikmets R, et al. “Genetic Restricion of HIV-1 Infection and Progression to AIDS by a deletion Allele of the CKR-5 Structural gene”. Science. 1996;273:1856–1862. doi: 10.1126/science.273.5283.1856. [DOI] [PubMed] [Google Scholar]
- 50.Ahmed N, Venkatesan S. Nef protein of HIV-1 is a transcriptional repressor of HIV-1 LTR. Science. 1988;241:1481–1485. doi: 10.1126/science.3262235. [DOI] [PubMed] [Google Scholar]
- 51.Kestler HW, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez CE, Desco M, Montes MG, Natividad L, Gonzalez B, Zabay JM. Immunological and serological markers predictive of progression to AIDS in a cohort of HIV infected drug users. AIDS. 1990;4:987–994. doi: 10.1097/00002030-199010000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Gao Y, Qing L, Zhang LQ, Safrit JT, Ho DD. Virological and immunological characterization of long-term survivors of HIV-1 infection. N Eng J Med. 1994;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 54.Steel CM, Ludlam CA, Beatson D, Peutherer JF, Cuthbert RJG, Simmonds P, et al. HLA haplotype A1, B8, DR3 as a risk factors for HIV related disease. Lancet. 1988;i:1185–1188. doi: 10.1016/s0140-6736(88)92009-0. [DOI] [PubMed] [Google Scholar]
- 55.Al-Jabri AA. HLA and in vitro susceptibility to HIV infection. Mol Immunol. 2002;38:959–967. doi: 10.1016/s0161-5890(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 56.Roe DL, Lewis RE, Cruse JM. Association of HLA-DQ and –DR alleles with protection from or infection with HIV-1. Exp Mol Pathol. 2000;68:21–28. doi: 10.1006/exmp.1999.2287. [DOI] [PubMed] [Google Scholar]
- 57.Al-Jabri AA, Bottazo GF, Mccloskey D, Oxford JS. Evidence for Resistance Among PBMCS from Healthy Bangladeshis to Infection by Certain Isolates of HIV-1 & 2. Abstr P5/1, First European Meeting of Virology; Wurzburg, Germany. September 1995; pp. 10–13. [Google Scholar]
- 58.Al-Jabri AA, Mccloskey D, Addawee M, Bottazzo FG, Sachs J, Oxford JS. In Vitro Correlation Between Human Leukocyte Antigen Class I and II Phenotype and HIV Infectivity of Activated Peripheral Blood Mononuclear Cell Cultures. AIDS. 1998;12:217–8. [PubMed] [Google Scholar]
- 59.Al-Jabri AA, Wigg MD, Oxford JS. Initial in vitro screening of drug candidates for their potential antiviral activities. In: Kangro H, Mahy B, editors. Virology Methods Manual. London: Academic Press; 1996. pp. 293–308. [Google Scholar]
- 60.Cameron PU, Cobain TJ, Zhang WJ, Kay PH, Dawkins RL. Influence of C4 null genes on infection with human immunodeficiency virus. BMJ. 1988;296:1627–1628. doi: 10.1136/bmj.296.6637.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cameron PU, Mallal SA, French MAH, Dawkins RL. Major histocompatibility complex genes influence the outcome of HIV infection, Ancestral haplotypes with C4 null alleles explain diverse HLA associations. Hum Immunol. 1990;29:282–295. doi: 10.1016/0198-8859(90)90042-n. [DOI] [PubMed] [Google Scholar]
- 62.Fabio G, Scorza R, Lazzarin A, Marchini M, Zarantonello M, D’Arminio A, et al. HLA associated susceptibility to HIV-1 infection. Clin Exp Immunol. 1992;87:20–23. doi: 10.1111/j.1365-2249.1992.tb06407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carrington M, Nelson GW, Martin MP, Kissner T, Vlahov D, Goedert JJ, et al. HLA and HIV-1: heterozygote advantage and B*35-Cw*04 disadvantage. Science. 1999;283:1748–1752. doi: 10.1126/science.283.5408.1748. [DOI] [PubMed] [Google Scholar]
- 64.de Sorrentino AH, Marinic K, Motta P, Sorrentino A, Lopez R, Illiovich E. HLA class I alleles associated with susceptibility or resistance to human immunodeficiency virus type 1 infection among a population in Chac province, Argentina. J Infect Dis. 2000;182:1523–1526. doi: 10.1086/315854. [DOI] [PubMed] [Google Scholar]
- 65.MacDonald KS, Embree JE, Nagelkerke NJ, Castillo J, Ramhadin S, Njenga S, et al. The HLA A2/6802 supertype is associated with reduced risk of perinatal human immunodeficiency virus type 1 transmission. J Infect Dis. 2001;183:503–506. doi: 10.1086/318092. [DOI] [PubMed] [Google Scholar]
- 66.Mann D, Carrington M, Kroner BL. The human major histocompatibility complex and HIV-1 pathogenesis. AIDS. 1994;8:S53–S60. [Google Scholar]
- 67.McNeil AJ, Yap PL, Gore SM, Brettle RP, McColl M, Wyld R, et al. Association of HLA types A1-B8-DR3 and B27 with rapid and slow progression of HIV disease. Q J Med. 1996;89:177–185. doi: 10.1093/qjmed/89.3.177. [DOI] [PubMed] [Google Scholar]
- 68.Habeshaw JA, Wilson SE, Hounsell EF, Oxford JS. How HIV-1 lentivirus causes immune deficiency disease. Med Hypoth. 1999;52:59–67. doi: 10.1054/mehy.1997.0632. [DOI] [PubMed] [Google Scholar]
- 69.Itescu S, Mathur-Wagh U, Skovron ML, Brancato LJ, Marmor M, Zeleniuch-Jacquotte A, et al. HLA-B35 is associated with accelerated progression to AIDS. J IDS. 1991;5:37–45. [PubMed] [Google Scholar]
- 70.Sahmoud T, Laurian Y, Gazengel C, Sultan Y, Gautreau C, Costagliola D. Progression to AIDS in French haemophiliacs: association with HLA-B35. AIDS. 1993;7:497–500. doi: 10.1097/00002030-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 71.Scorza SR, Fabio G, Lazzarin A, Eisera N, Foppa Ulberti C, Moroni M, et al. HLA associated susceptibility to AIDS: HLA B35 is a major risk factor for Italian HIV infected intravenous drug addicts. Hum Immunol. 1988;22:73–79. doi: 10.1016/0198-8859(88)90038-9. [DOI] [PubMed] [Google Scholar]
- 72.Scorza SR, Fabio G, Lazzarin A, Eisera NB, Moroni M, Zanussi C. HLA associated susceptibility to Acquired Immunodeficiency Syndrome in Italian patients with Human Immunodeficiency Virus infection. Lancet. 1986;ii:1187–1189. doi: 10.1016/s0140-6736(86)92197-5. [DOI] [PubMed] [Google Scholar]
- 73.Palumbo Paul, Skurnick Joan, Rohowsky-Kochan Christine, Louria Donald. Natural resistance to HIV: Is the evidence good enough to design an effective vaccine. AIDS Science. 2002;2:11. [Google Scholar]
- 74.Altfeld MA, Livingston B, Reshamwala N, Nguyen PT, Addo MM, Shea A, et al. Identification of Novel HLAA2-Restricted Human Immunodeficiency Virus Type 1-Specific Cytotoxic T-Lymphocyte Epitopes Predicted by the HLA-A2 Supertype Peptide-Binding Motif. J Virol. 2001;75:1301–1311. doi: 10.1128/JVI.75.3.1301-1311.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Louie LG, Newman B, King M-C. Influence of host genotype on progression to AIDS among HIV infected men. AIDS. 1991;4:814–818. [PubMed] [Google Scholar]
- 76.Spear GT. Interaction of non-antibody factors with HIV in plasma. AIDS. 1993;7:1149–1157. doi: 10.1097/00002030-199309000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Spear GT, Sullivan BL, Landay AL, Lint TF. Neutralization of human immunodeficiency virus type 1 by complement occurs by viral lysis. J Virol. 1990;64:5869–5873. doi: 10.1128/jvi.64.12.5869-5873.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moir S, Malaspina A, Li Y, et al. B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J Exp Med. 2000;192:637–646. doi: 10.1084/jem.192.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sullivan BL, Knopoff EJ, Saifuddin M, et al. Susceptibility of HIV-1 plasma virus to complement-mediated lysis. Evidence for a role in clearance of virus in vivo. J Immunol. 1996;157:1791–1798. [PubMed] [Google Scholar]
- 80.Sullivan BL, Takefman DM, Spear GT. Complement can neutralize HIV-1 plasma virus by a C5-independent mechanism. Virology. 1998;248:173–181. doi: 10.1006/viro.1998.9289. [DOI] [PubMed] [Google Scholar]
- 81.Kacani L, Prodinger WM, Sprinzl GM, et al. Detachment of human immunodeficiency virus type 1 from germinal centers by blocking complement receptor type 2. J Virol. 2000;74:7997–8002. doi: 10.1128/jvi.74.17.7997-8002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takefman DM, Sullivan BL, Sha BE, Spear GT. Mechanisms of resistance of HIV-1 primary isolates to complement-mediated lysis. Virology. 1998;246:370–378. doi: 10.1006/viro.1998.9205. [DOI] [PubMed] [Google Scholar]
- 83.Spear GT, Hart M, Olinger GG, Hashemi FB, Saifuddin M. The role of the complement system in virus infections. Curr Top Microbiol Immunol. 2001;260:229–245. doi: 10.1007/978-3-662-05783-4_12. [DOI] [PubMed] [Google Scholar]
- 84.Bogdan C. The function of type I interferons in antimicrobial immunity. Curr Opin Immunol. 2000;12:419–424. doi: 10.1016/s0952-7915(00)00111-4. [DOI] [PubMed] [Google Scholar]
- 85.Cousens LP, Peterson R, Hsu S, Dorner A, Altman JD, Ahmed R, et al. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J Exp Med. 1999;189:1315–1328. doi: 10.1084/jem.189.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krown SE. Interferon-alpha: evolving therapy for AIDS-associated Kaposi’s sarcoma. J Interferon Cytokine Res. 1998;18:209–214. doi: 10.1089/jir.1998.18.209. [DOI] [PubMed] [Google Scholar]
- 87.DeStefano E, Friedman RM, Friedman-Kien AE, et al. Acid-labile human leukocyte interferon in homosexual men with Kaposi’s sarcomaand lymphadenopathy. J Infect Dis. 1982;146:451–459. doi: 10.1093/infdis/146.4.451. [DOI] [PubMed] [Google Scholar]
- 88.Ferbas J, Navratil J, Logar A, Rinaldo C. Selective decrease in human immunodeficiency virus type 1 (HIV-1)-induced alpha interferon production by peripheral blood mononuclear cells during HIV infection. Clin Diagn Lab Immunol. 1995;2:138–142. doi: 10.1128/cdli.2.2.138-142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 90.Siegal FP, Lopez C, Fitzgerald PA, Shah K, Baron P, Leiderman IZ, et al. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J Clin Invest. 1986;78:115–123. doi: 10.1172/JCI112539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith SM. HIV vaccine development in the nonhuman primate model of AIDS. J Biomed Sci. 2002;9:100–11. doi: 10.1007/BF02256020. [DOI] [PubMed] [Google Scholar]
- 92.Haynes BF, Pantaleo G, Fauci AS. Toward an understanding of the correlates of protective immunity to HIV infection. Science. 1996;271:324–328. doi: 10.1126/science.271.5247.324. [DOI] [PubMed] [Google Scholar]