Abstract
Endothelial progenitor cells (EPCs) are mobilized into the vascular space and home to damaged tissues, where they promote repair in part through a process of angiogenesis. Neuregulins (NRGs) are ligands in the epidermal growth factor family that signal through type I receptor tyrosine kinases in the erbB family (erbB2, erbB3, and erbB4) and regulate endothelial cell biology, promoting angiogenesis. Stimuli such as ischemia and exercise that promote EPC mobilization also induce cleavage and release of transmembrane NRG from cardiac microvascular endothelial cells (CMECs). We hypothesized that NRG/erbB signaling may regulate EPC biology. Using an embryonic (e)EPC cell line that homes to and repairs injured myocardium, we were able to detect erbB2 and erbB3 transcripts. Identical receptor expression was found in EPCs isolated from rat bone marrow and human whole blood. NRG treatment of eEPCs induces phosphorylation of kinases including Akt, GSK-3β, and Erk1/2 and the nuclear accumulation and transcriptional activation of β-catenin. NRG does not induce eEPC proliferation or migration but does protect eEPCs against serum deprivation-induced apoptosis. These results suggest a role for tissue-derived NRG in the regulation of EPC survival.
Keywords: erbB2, erbB3, apoptosis
endothelial progenitor cells (EPCs) are an adult stem cell population originating in the bone marrow (BM) that can be recruited to sites of active angiogenesis, where they promote tissue repair (2, 14, 31, 45). Recent efforts have focused on a number of important factors that regulate EPC mobilization and homing, including vascular endothelial growth factor (VEGF) and stromal cell-derived factor 1 (SDF-1). The potential role of other growth factors has not been systematically examined. Neuregulins (NRGs) are ligands in the epidermal growth factor family that mediate cell-cell interactions in various tissues including the heart, breast, and nervous system. NRGs signal through type I receptor tyrosine kinases in the erbB family (erbB2, erbB3, and erbB4), which can form homo- and heterodimers upon ligand binding. In the heart NRG expression is restricted to endothelial cells (ECs) in the endocardial and myocardial capillaries (5, 46), where it acts in a paracrine and juxtacrine manner to regulate cardiac myocyte survival and hypertrophy (3, 24, 46).
In the context of the cardiovascular system, much research has focused on NRG's effects on cardiac myocytes (30). Recent studies suggest an important role for NRG/erbB signaling in regulating the vasculature (35). Trastuzumab, a humanized monoclonal antibody targeting the erbB2 receptor used to treat breast cancer, has been shown to induce regression of tumor vasculature in a transplanted mouse model (16). Independently, NRG/erbB signaling has been reported to regulate tumor angiogenesis via induction of VEGF expression (22, 40, 43). Although upregulation of VEGF expression may, in part, explain the NRG-dependent angiogenic effect, there is evidence for a VEGF-independent effect of NRG on angiogenesis (35). In human brain microvascular ECs NRG treatment protects against oxidant injury, indicating that NRG regulates endothelial homeostasis in “normal” (e.g., noncancerous) tissues as well (27). These data indicate a potential role for NRG/erbB signaling in regulation of vascular homeostasis.
Interestingly, several stimuli known to modulate EPC mobilization and recruitment also induce processing of transmembrane NRG to release soluble NRG (20). Ischemic injury is a well-documented stimulus leading to EPC mobilization and recruitment (15, 19, 35). In intact, Langendorff-perfused mouse hearts, ischemia-reperfusion leads to proteolytic processing of full-length NRG and subsequent phosphorylation of erbB4 receptors in the myocardium (20). Exercise, another potent inducer of EPC mobilization (21, 33, 42), also activates NRG processing and erbB receptor phosphorylation in skeletal muscle (23). These data together with the known role of NRG as a paracrine/endocrine signal originating from mature microvascular ECs led us to hypothesize that NRG/erbB signaling modulates EPC function. To test this hypothesis, we have utilized a clonal mouse embryonic (e)EPC cell line. These cells have been shown to express EC markers (13) and incorporate into vasculature (13) and attenuate ischemia-reperfusion in animal models (18, 19). We demonstrate a role for NRG/erbB signaling in regulation of EPC survival.
MATERIALS AND METHODS
Culture and characterization of murine embryonic endothelial progenitor cells.
eEPCs used in this study (T19B line) were thawed from an aliquot of cells frozen close to the initial time of cell line isolation. Detailed isolation procedures are described in the original article by Hatzopoulos et al. (13). Cells were maintained on tissue culture plates precoated with 0.1% gelatin solution in water (gelatin from porcine skin, type A; Sigma catalog no. G2500). Growth medium consisted of high-glucose DMEM supplemented with 10% fetal calf serum (FCS), HEPES (25 mM), l-glutamine (2 mM), antibiotics (penicillin-streptomycin), nonessential amino acids, and l β-mercaptoethanol (100 μM). For experiments, cells were treated either in 0.5% FCS or in minimum essential medium with Earle's balanced salt solution (MEM-EBSS; Hyclone catalog no. SH30244.01). Recombinant human NRG-1β extracellular domain (R&D Systems catalog no. 377-HB-050) was used at 50 ng/ml unless otherwise noted. The erbB2 kinase inhibitor GW572016 (aka lapatinib, di-p-toluenesulfonate salt; LC Laboratories) was dissolved in DMSO and diluted to 1 μM, unless otherwise noted.
The ability of these cells to adhere to ischemic tissue was assessed in the isolated mouse heart subjected to ischemia and reperfusion. This protocol was approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center. Mouse hearts were isolated and perfused as described previously (20) and subjected to 15 min of ischemia. At 20 min of reperfusion, 5 × 105 eEPCs, fluorescently labeled with the lipophilic tracer DiIC18 (19), were injected in a side port over the course of 30 s. Hearts were perfused for an additional 10 min and then snap frozen in liquid nitrogen for cryosectioning. Sections were mounted in DAPI-containing mounting medium, and eEPCs were quantified by fluorescence microscopy.
Detection of erbB receptors and NRG mRNA by real-time RT-PCR.
Total mRNA was isolated with TRIzol reagent according to the manufacturer's instructions. One-step real-time RT-PCR was performed with the QuantiTect SYBR Green Kit (Qiagen) on a Cepheid SmartCycler. Presynthesized primer pairs for erbB2, erbB3, erbB4, and NRG-1 were purchased from Qiagen (QuantiTect Primer Assays). RNA concentrations were determined by absorbance measured at 260 nm in duplicate before dilution. Samples were normalized by including primers targeting the 18S ribosome protein L32 (actggaattcgctgccctccggcct and gcataagctttcggtctgactggtg). Human erbB receptor mRNA levels were quantified with the following primers: erbB2 forward: CCCTCTGACGTCCATCATCT, erbB2 reverse: TGATGAGGATCCCAAAGACC; erbB3 forward: AGGAACCCTGTGTCCTTGTG, erbB3 reverse: TCCCAAAATGCTGGGATTAC; erbB4 forward: TGATGTCAGCTCAGACTGTGG, erbB4 reverse: CATTGTAAGGGTCCCCATGA.
Detection of protein expression and protein phosphorylation.
eEPCs were lysed in either modified RIPA buffer containing Tris·HCl (50 mM, pH 7.5), NaCl (150 mM), EDTA (0.5 mM), NP-40 (1%), and 0.25% deoxycholate or CelLytic M Cell Lysis Reagent (Sigma, catalog no. C2978). Protease and phosphatase inhibitors were added to both lysis buffer solutions. Protease inhibitor cocktail solution (Sigma, catalog no. P8340) was diluted 1:100; tyrosine phosphatase inhibitor (Sigma, catalog no. P5726) and serine/threonine phosphatase inhibitor (Sigma, catalog no. P2850) solutions were diluted 1:200. Before loading, samples were normalized to protein concentration as determined by DC protein assay (Bio-Rad, catalog no. 500-0016). Samples were then resolved on 4–20% Tris·HCl gels (Bio-Rad) and transferred to polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Total erbB2 and erbB3 antibodies were purchased from Santa Cruz Biotechnology (catalog nos. sc-284, sc-285). The following antibodies were purchased from Cell Signaling: phospho (p)-Akt(Ser473) (catalog no. 4058), p-p42/44(Thr202/Tyr204) (catalog no. 4376), p42/44 (catalog no. 9102), p-Stat3(Ser727) catalog no. 9134, p-glycogen synthetase kinase (GSK)-3α/β(Ser21/9) (catalog no. 9331), p-MDM2(Ser166) (catalog no. 3521), and p-BAD(Ser136) (catalog no. 9295). p-erbB2(Tyr1248) antibody was purchased from Millipore (catalog no. 06-229). Total Akt antibody was purchased from BD Biosciences (catalog no. 610860), and mouse β-actin antibody was purchased from Sigma (catalog no. A1978).
DNA ladder assay.
eEPCs were plated on 100-mm plates and grown to ∼70% confluence in growth medium (10% FCS). Plates were then washed in PBS, and serum-free medium containing experimental treatments was added. After 18-h incubation at 37°C, genomic DNA was isolated as directed by instructions included in the Wizard Genomic DNA Purification Kit (Promega, catalog no. A1120). Equal volumes of purified genomic DNA were resolved on a 1.3% agarose gel and visualized by ethidium bromide staining.
Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling assay.
Approximately 1 × 105 cells were placed in growth medium on a 12-well plate and allowed to grow overnight. Medium was changed to serum-free medium containing experimental treatments and incubated for an additional 18 h. Manufacturer's instructions included with the Roche In-Situ Cell Death Detection Kit (Roche, catalog no. 11684795910) were followed. Briefly, cells were fixed for 1 h in 4% paraformaldehyde-PBS, washed in PBS, and then permeabilized for 2 min in 0.2% Triton X-100-PBS. After permeabilization, cells were again washed two times in PBS; 100 μl of terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) reaction mixture was then added per well, and the plate was incubated at 37°C for 1 h. After incubation, cells were washed in PBS and 20 μl of DAPI-containing mounting medium was added to each well. A coverslip was applied, and TUNEL-positive cells were quantified by counting under fluorescence microscopy. Percent TUNEL positive cells represents the number of TUNEL-positive nuclei in 300 total nuclei.
Cell cycle analysis by fluorescence-activated cell sorting.
Cells were grown to 70% confluence and treated overnight in 0.5% FCS. After treatment, cells were trypsinized and counted. Cell number was standardized across all conditions (∼2 × 106 total cells). After resuspension in 3 ml of PBS, cells were fixed by addition of 7 ml of ice-cold ethanol dropwise while vortexing and incubation overnight at 4°C. Fixed cells were washed once with 5 ml of PBS and resuspended in propidium iodide staining buffer (200 μg/ml propidium iodide, 0.1% Triton X-100 in PBS). Staining was done at room temperature for 1–2 h. Cells were analyzed on a three-laser flow cytometer (LSRII), and propidium iodide content was used to determine cell cycle stage.
Bromodeoxyuridine proliferation assay.
Instructions provided with the bromodeoxyuridine (BrdU) Cell Proliferation Assay (Calbiochem, catalog no. QIA58) were followed. Briefly, ∼10,000 cells/well were plated in a 96-well culture dish. Cells were allowed to attach overnight in 0.5% FCS medium. After changing to fresh 0.5% FCS, the BrdU label was diluted as described in the instructions and 20 μl of BrdU working solution was added to each well. Plates were returned to the incubator overnight. The following day, contents of the wells were removed by inverting the plates. Cells were then fixed on the plate for 30 min with the fixative/denaturing solution provided. After removal of the fixative solution, a solution containing diluted anti-BrdU antibody was added and the plates were incubated at room temperature for 1 h. Plates were then washed three times with wash buffer, followed by addition of diluted goat anti-mouse IgG horseradish peroxidase (HRP) conjugate. After 30-min incubation, plates were again washed three times and 100 μl/well substrate solution was added. Color development was allowed to proceed for 15 min in the dark, at which point the reaction was stopped by addition of stop solution. Absorbance at 450 nm was measured.
TOP-FLASH luciferase reporter assay.
Approximately 100,000 cells were plated in each well of a 12-well plate in 0.5% FCS. Cells were allowed to attach overnight. The following day, 4 μg of TOP-FLASH (T-cell factor optimal promoter) reporter plasmid DNA and 1.5 μg of pCDNA3.1-LacZ were mixed with the transfection reagent Lipofectamine (Invitrogen) in 13 ml of MEM-EBSS. After aspiration of the 0.5% FCS medium and washing of the plate with PBS, 1 ml of this transfection mixture was added to each well. Cells were returned to a 37°C incubator for 7 h, at which point the transfection mixture was aspirated and cells were returned to 0.5% FCS and treated for an additional 18 h. For each experiment, all treatment conditions were done in triplicate (3 wells per condition). Cells were lysed and luciferase and LacZ activity were determined as indicated in the instructions included with the Dual-Light Luciferase Assay Kit (Applied Biosystems) with a Monolight 2010 luminometer.
eEPC adhesion.
Adhesion experiments were carried out on non-tissue culture-treated 96-well plates (Costar, catalog no. 9107). Plates were coated overnight with either human fibronectin (Sigma, catalog no. F2006) or a mixture of fibronectin and NRG (50 ng/ml final concentration) and then washed two times with 200 μl/well PBS before plating of cells. eEPCs were trypsinized and resuspended in serum-free medium (MEM-EBSS) containing 100 μg/ml soybean trypsin inhibitor (Calbiochem, catalog no. 65035). Cells were centrifuged and resuspended at ∼250,000 cells/ml in serum-free medium; 200 μl of this cell suspension was plated per well (∼50,000 cells/well), and plates were incubated at 37°C for 1 h. After incubation, medium was decanted and the plate was washed three times with 200 μl/well PBS. To assess adherent cell number, 100 μl of acid phosphatase reaction buffer [100 mM sodium acetate pH 5.5, 0.1% Triton X-100, 10 mM p-nitrophenyl phosphate (New England Biolabs, catalog no. P0757)] was added per well and the plate was returned to the incubator for an additional hour. The reaction was stopped by adding 30 μl/well 1 N NaOH, and absorbance was measured at 410 nm.
Transwell migration.
Twenty-four-well Transwell inserts (8.0-μm pore size; BD Biosciences, catalog no. 353097) were coated with 0.1% gelatin for 1 h and then transferred to 24-well plates containing 1 ml 0.5% FCS ± experimental treatments per well. eEPCs were trypsinized and resuspended in 0.5% FCS. Cell suspension (1 ml, 50,000 cells/ml) was added to the top portion of the Transwell insert, and the plate was incubated at 37°C for 6 h. After incubation, Transwell inserts were removed and cells were fixed by submerging inserts in ice-cold methanol for 30 min. Cells adherent to the top portion of the Transwell insert were removed with a cotton-tipped applicator swab. To stain cells on the bottom portion, the insert was submerged in Trypan blue stain (GIBCO, catalog no. 15250-61) for 30 min. After washing in PBS the insert was excised and mounted on a slide in DAPI-containing mounting medium (VectaShield, Vector Labs). Cells in five ×20 magnification fields were counted for each condition.
Isolation of rat bone marrow EPCs.
This study protocol was approved by the Institutional Animal Care and Use Committee of Vanderbilt University Medical Center. Marrow was isolated from the femurs and tibias of Sprague-Dawley rats. Mononuclear cells were then separated by density gradient centrifugation with Lympholyte Cell Separation Medium (Cedarlane Labs, catalog no. CL5041). After lysis of red blood cells (RBCs) (BD Pharm Lyse lysing buffer; BD Biosciences, catalog no. 555899), the remaining mononuclear cells were washed in PBS and resuspended in EGM-2 medium supplemented with 2.5% fetal bovine serum, human epidermal growth factor, VEGF, human fibroblast growth factor-2, insulin-like growth factor-1, ascorbic acid, and heparin according to the instructions included with the EGM-2 Bullet Kit (Clonetics, catalog no. CC-3162). Resuspended cells were plated on 60-cm plates precoated overnight with 10 μg/ml human fibronectin (Sigma, catalog no. F2006) in PBS. Medium was changed at 3 days, and adherent cells were maintained in culture for an additional 3 days before endothelial identity was confirmed by assessing diacetylated LDL uptake and BS-1 lectin staining.
Isolation and identification of human peripheral blood-derived EPCs.
This study protocol was approved by the Institutional Review Board at Vanderbilt University Medical Center. Peripheral blood mononuclear cells were isolated by density gradient centrifugation. Four milliliters of peripheral blood was carefully layered onto four milliliters of Histopaque 1077 density gradient solution (Sigma, catalog no. 10771) and spun at 500 g for 30 min with the centrifuge rotor brake disabled. The mononuclear cell layer was transferred to a fresh 15-ml tube and washed with 10 ml of PBS. The wash step was repeated once more, and cells were resuspended in EGM-2 medium supplemented with 2.5% fetal bovine serum, human epidermal growth factor, VEGF, human fibroblast growth factor-2, insulin-like growth factor-1, ascorbic acid, and heparin (see Isolation of rat bone marrow EPCs) at ∼2 × 106 cells/ml on plates coated overnight with a 10 μg/ml fibronectin solution in PBS (fibronectin from human plasma; Sigma, catalog no. F2006). Adherent cells were further characterized by assessing their ability to take up diacetylated LDL and to bind BS-1 lectin. Acetylated LDL was added (10 ng/ml final concn) (Biomedical Technologies catalog no. BT 902) to the growth medium, and cells were incubated overnight. The following day cells were washed, fixed in 4% paraformaldehyde, and stained with BS-1 lectin FITC conjugate (Sigma, catalog no. L9381; final concn 10 μg/ml). Finally, cell surface expression of CD34 and VEGF receptor (VEGFR)2 was assessed by fluorescence-activated cell sorting (FACS) analysis. Briefly, peripheral blood was isolated by standard venipuncture techniques. Blood (0.5 ml) was removed from an EDTA-anticoagulated purple-top tube and transferred to a 2-ml tube. RBCs were lysed by addition of 1 ml of 1× BD Pharmlyse solution (BD Biosciences, catalog no. 555899) and then tumbling the tube for 5 min at room temperature. The RBC lysis step was repeated once more, and white blood cells (WBCs) were spun down and then washed twice in FACS buffer (1% FCS, 0.1% sodium azide in PBS). WBCs were counted, and cell concentration was adjusted to 2–3 × 106 cells/ml. One milliliter of this cell suspension (2–3 × 106 total cells) was then stained for FACS analysis. Cells were stained with flurophore-conjugated antibodies that recognize VEGFR2 and CD34 (anti-hVEGFR2-phycoerythrin, R&D Systems, catalog no. FAB357P; anti-human CD34-FITC, BD Biosciences, catalog no. 555821). Twenty microliters of each antibody was added to the cell suspension, and cells were tumbled in the dark at room temperature for 30–45 min. After staining, cells were washed once in FACS buffer, resuspended in 1 ml of FACS buffer, and analyzed on a three-laser flow cytometer.
Statistical analysis.
Densitometry was performed with ImageJ software. Statistical analyses were carried out with a paired Student's t-test. Differences between groups were considered significant at P < 0.05. Data are represented as means ± SE.
RESULTS
eEPCs express erbB receptors.
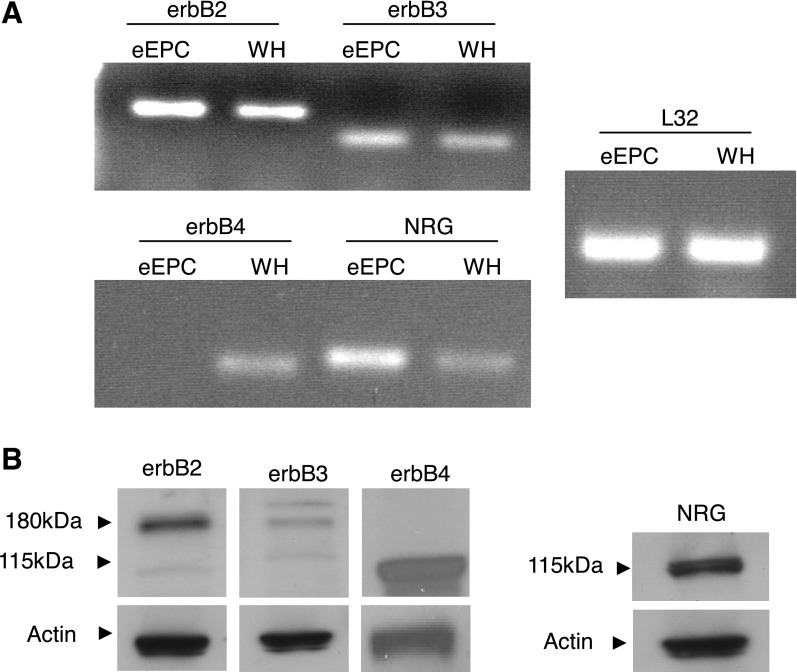
To investigate the role of NRG in EPC biology, we first examined the expression of NRG and NRG receptors in eEPCs. Quantitative real-time PCR demonstrated high mRNA expression of erbB2 and erbB3 receptors, while erbB4 transcripts were not detected (Fig. 1A). We observed an identical pattern of erbB gene expression in rat BM-derived and human peripheral blood-derived EPCs (Supplemental Fig. S1).1 Western blot of eEPC lysates confirmed 185-kDa erbB2 and erbB3, but not erbB4, expression (Fig. 1B), and this expression pattern was recapitulated in primary rat BM-derived EPCs (Supplemental Fig S2). The erbB receptor complement expressed by both murine eEPCs and rat BM-derived EPCs (erbB2+/erbB3+/erbB4−) is consistent with that of mature cardiac microvascular ECs (8). Interestingly, NRG-1 ligand itself was highly expressed at both the mRNA and protein levels in murine eEPCs and rat BM-derived EPCs (Supplemental Figs. S1 and S2).
Fig. 1.
Embryonic endothelial progenitor cells (eEPCs) express erbB receptors. A: RT-PCR analysis demonstrating expression of erbB2 and erbB3 receptors as well as neuregulin (Nrg)-1 ligand. Mouse whole heart tissue (WH) was used as a positive control, and ribosomal L32 RNA was amplified as a loading control. B: Western blot analysis of eEPC lysate showing expression of erbB2, erbB3, and NRG-1 proteins. Images are representative of at least 3 experiments.
NRG induces receptor phosphorylation and intracellular signaling in eEPCs.
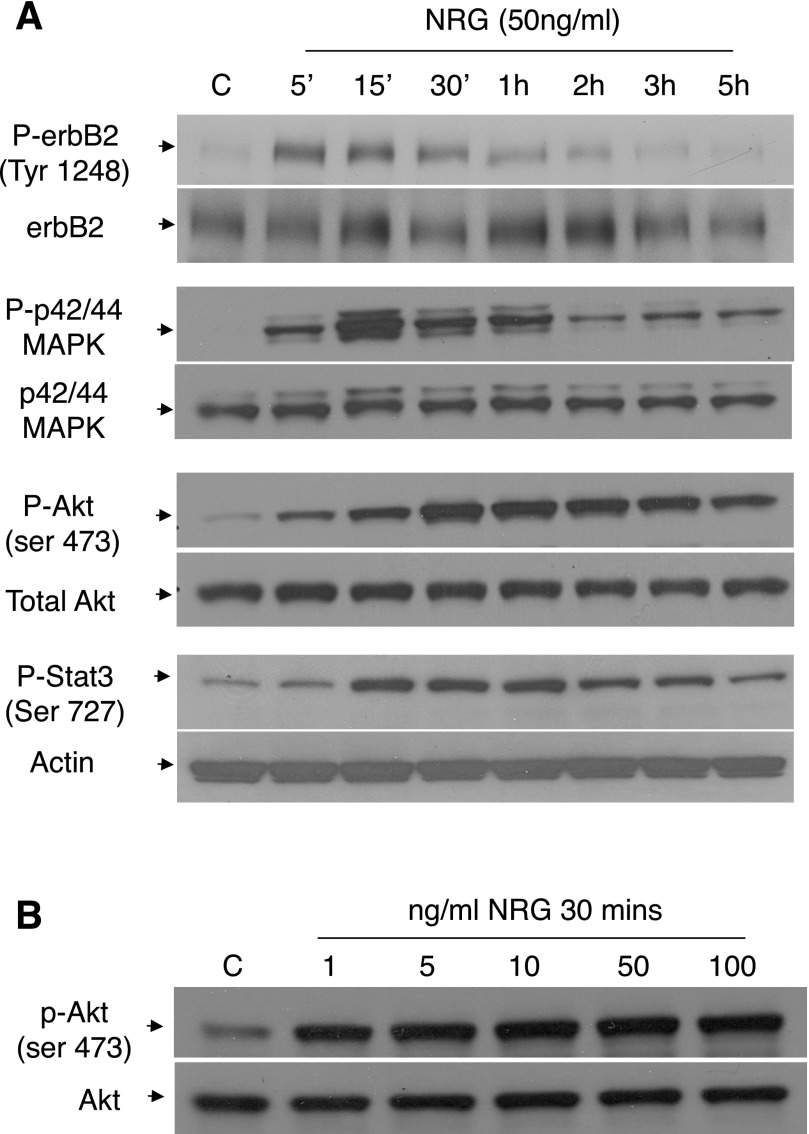
Recombinant human NRG has been shown by several groups, including ours, to regulate both rat and mouse EC biology (3, 4, 12). To test whether the erbB receptors are functional in eEPCs, we exposed the cells to NRG and analyzed receptor phosphorylation. NRG-1β treatment of murine eEPCs led to a time-dependent increase in erbB2 phosphorylation (Fig. 2A). Similarly, Akt phosphorylation (Ser473) increased after NRG treatment, peaking ∼30 min after treatment with persistent activation detectable for up to 5 h (Fig. 2A). Several other kinases, known to act downstream of erbB receptors, were activated in response to NRG treatment including p42/44 (ERK1/2) and Stat3, suggesting a robust induction of signaling pathways modulated by NRG activation of erbB receptors. Phosphorylation of both Erk1/2 and Stat3 peaked at 15 min after treatment and were at baseline by 5 h. Rat BM-derived EPCs responded similarly, with phosphorylation of Akt in response to NRG treatment (Supplemental Fig. S2).
Fig. 2.
NRG-induced signaling in eEPCs. A: cells were serum starved for 3 h and then treated with NRG-1β (50 ng/ml) for the indicated times. Lysates were resolved on a 4–20% gel, transferred to polyvinylidene difluoride (PVDF) membranes, and probed with the indicated antibodies. Images are representative of at least 3 experiments. B: cells were serum starved for 3 h and treated for 30 min with the indicated NRG-1β concentrations. Western blotting was performed as described in materials and methods. Image is representative of at least 3 experiments. C, control.
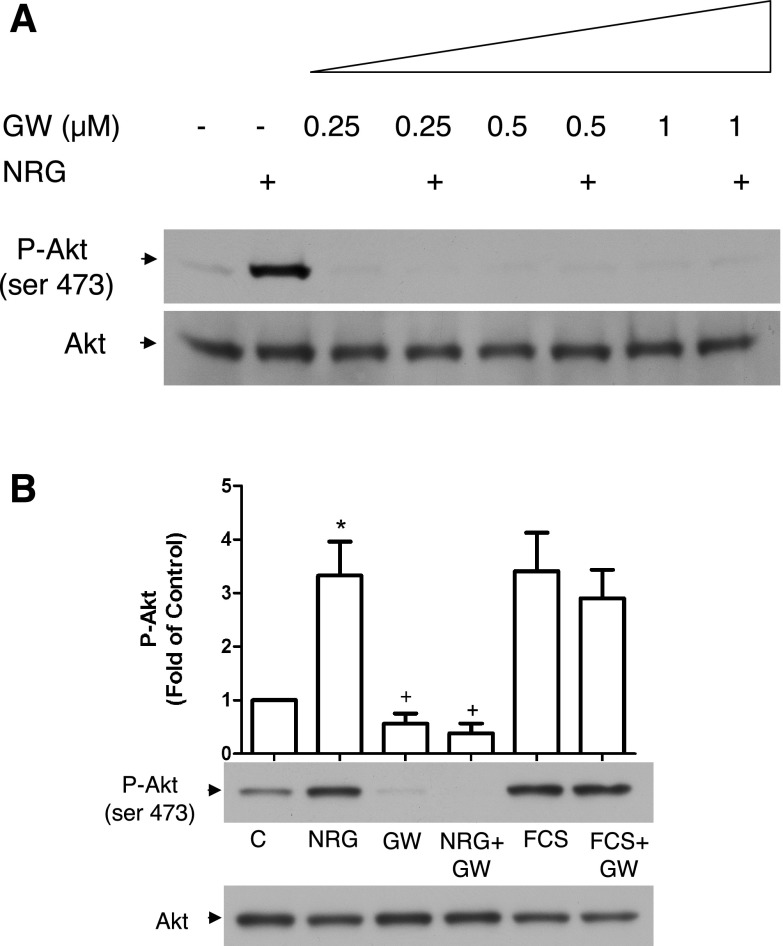
The receptor tyrosine kinase inhibitor GW572016 (aka lapatinib) is a dual-specificity inhibitor targeting erbB1 (EGFR) and erbB2 recently approved to treat metastatic breast cancer. GW572016 effectively abolished NRG-induced Akt phosphorylation in eEPCs (Fig. 3) as well as erbB2 and ERK phosphorylation (data not shown).
Fig. 3.
GW572016 is an effective inhibitor of NRG-activated signaling in eEPCs. A: eEPCs were serum starved for 3 h, pretreated with the indicated concentrations of GW572016 (GW) for 30 min, and then treated with NRG-1β (50 ng/ml) for an additional 30 min. Lysates were resolved on a 4–20% gel, transferred to PVDF membranes, and probed with the indicated antibodies. B: cells were serum starved for 3 h, pretreated with GW572016 for 30 min, and then treated with either NRG-1β or fetal calf serum (FCS) for 30 min. Akt phosphorylation was then assessed by Western blot ([GW572016] = 1 μM, FCS = 10%; n = 3). *P < 0.05 vs. C; +P < 0.05 vs. NRG.
NRG promotes phosphorylation of GSK-3 and β-catenin transcriptional activity.
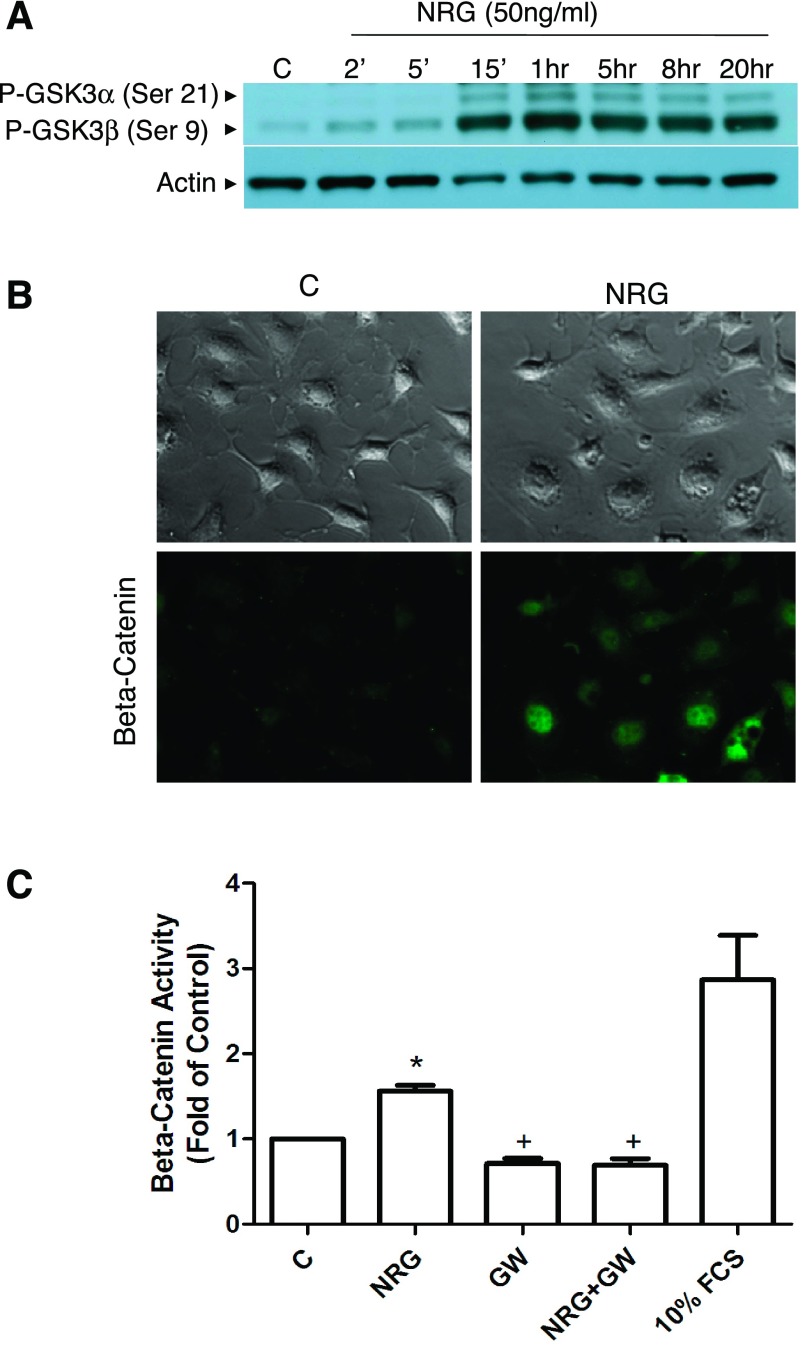
NRG treatment of eEPCs promoted time-dependent phosphorylation of GSK-3α and β as well as accumulation and transcriptional activation of β-catenin. GSK-3 phosphorylation occurred within 15 min of treatment with NRG and persisted for at least 20 h (Fig. 4A). Cytoplasmic and nuclear accumulation of β-catenin was also observed after NRG treatment (Fig. 4B).
Fig. 4.
NRG induces glycogen synthetase kinase (GSK)-3 phosphorylation and β-catenin accumulation and transcriptional activity. A: immunoblot of phospho (p)-GSK-3α/β. Cells were serum starved for 3 h and then treated with NRG-1β (50 ng/ml) for the indicated times. Images are representative of at least 3 independent experiments. B: immunofluorescence detection of intracellular β-catenin. Cells were serum starved for 3 h and then treated with NRG-1β (50 ng/ml) for an additional 3 h before being fixed and probed for β-catenin. Representative of n = 2. C: luciferase reporter assay. Cells were transfected with the TOP-FLASH β-catenin reporter plasmid as well as a LacZ control plasmid. After transfection, cells were pretreated with GW572016 for 1 h, followed by treatment with NRG-1β (50 ng/ml) or 10% FCS for 18 h. Protein lysates were prepared, and luciferase and LacZ activity were assessed. Luciferase activity was normalized to LacZ activity (n = 3). *P < 0.05 vs. control; +P < 0.05 vs. NRG-1β treated.
To assess β-catenin transcriptional activity, cells were transfected with a construct expressing the β-catenin responsive T-cell factor/lymphoid enhancer factor (TCF/LEF) promoter upstream of the luciferase reporter gene. NRG treatment increased activity of the TCF/LEF promoter, and this effect was abolished by the erbB2 kinase inhibitor GW572016 (Fig. 4C). Taken together these results suggest that NRG treatment induces β-catenin transcriptional activity in EPCs via GSK-3α/β phosphorylation.
NRG/erbB signaling regulates eEPC survival.
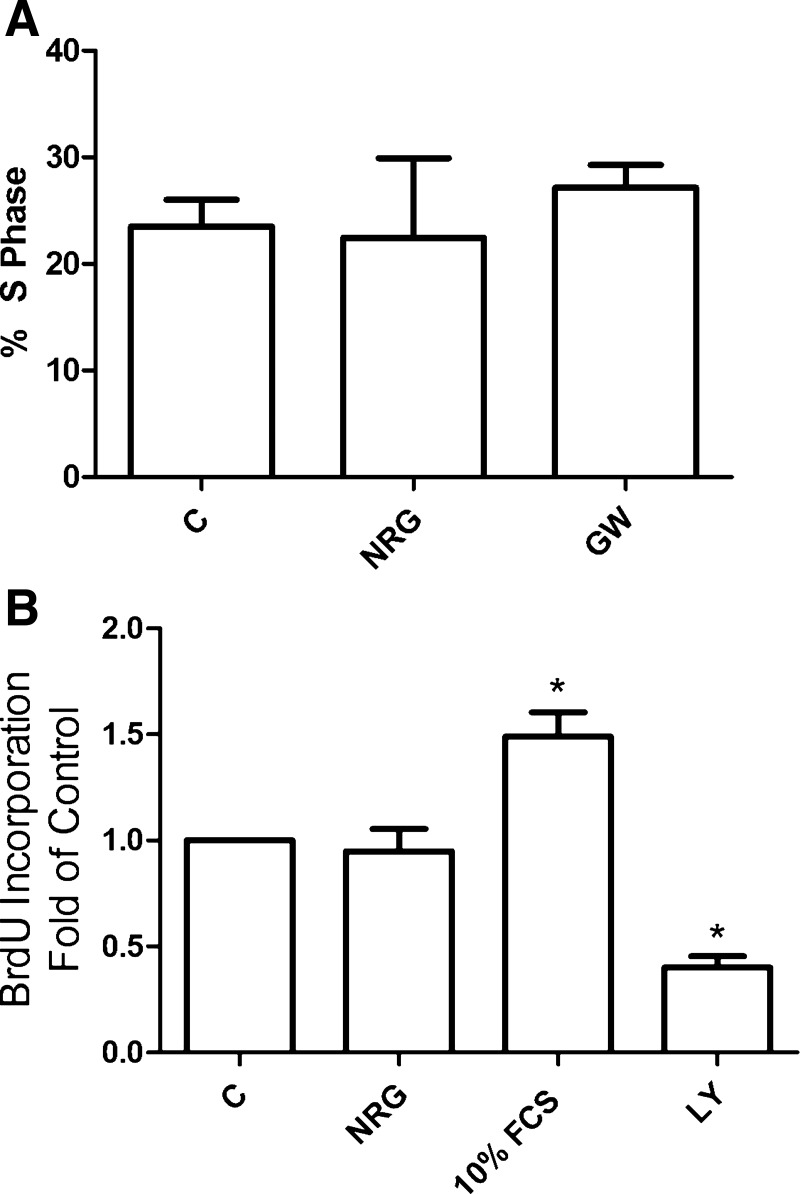
In light of NRG's role in other tissues, we tested the hypothesis that NRG/erbB signaling regulates eEPC proliferation, differentiation, survival, and migration/adhesion. NRG treatment had no effect on eEPC proliferation (Fig. 5), nor did NRG seem to affect eEPC differentiation as assessed by gross cell morphology and mRNA expression profile. We assessed mRNA levels of platelet endothelial cell adhesion molecule (PECAM), von Willebrand factor (vWF), VEGFR2, and AC133 by real time RT-PCR as well as by preliminary microarray analysis and were unable to demonstrate consistent transcript level changes following NRG treatment (data not shown). In addition, we assessed NRG's effect on eEPC adhesion and migration. Neither adhesion nor migration was affected by NRG treatment (Supplemental Fig. S3).
Fig. 5.
NRG does not regulate eEPC proliferation. A: fluorescence-activated cell sorting (FACS) analysis of cell cycle. Cells were treated with either 50 ng/ml NRG-1β or 1 μM GW572016 overnight in 0.5% FCS. After fixing, cells were stained with propidium iodide and analyzed by flow cytometry (n = 3). B: bromodeoxyuridine (BrdU) incorporation. Cells were treated as in A and counted as described in materials and methods ([NRG-1β] = 50 ng/ml, [LY-294002] = 10 μM; n = 3). *P < 0.05 vs. control.
NRG inhibits serum deprivation-induced eEPC apoptosis.
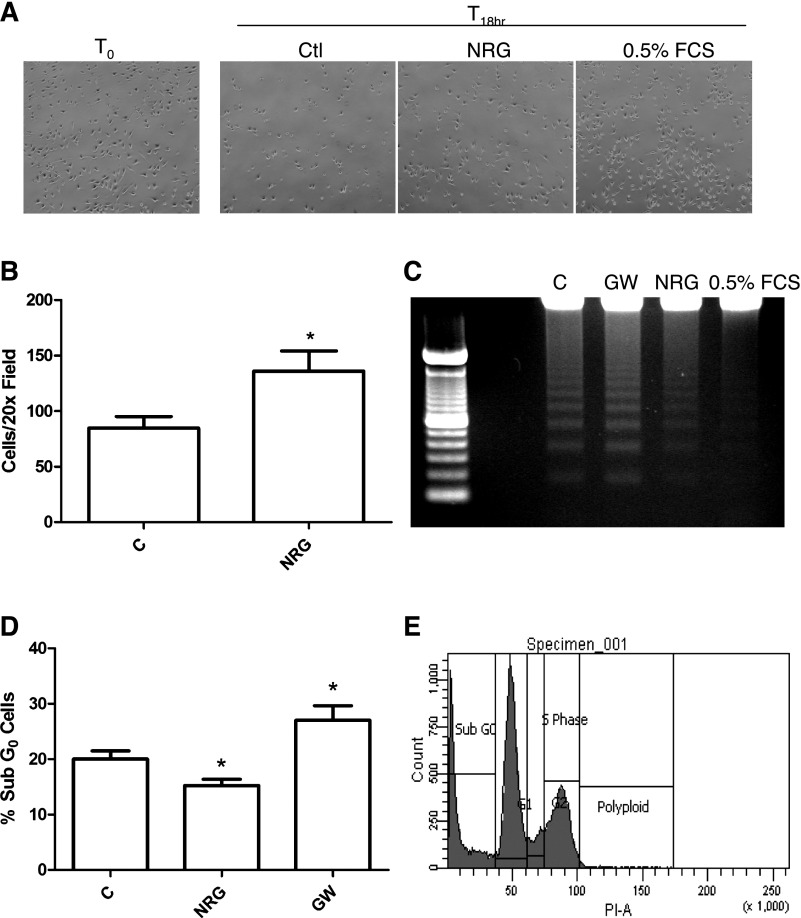
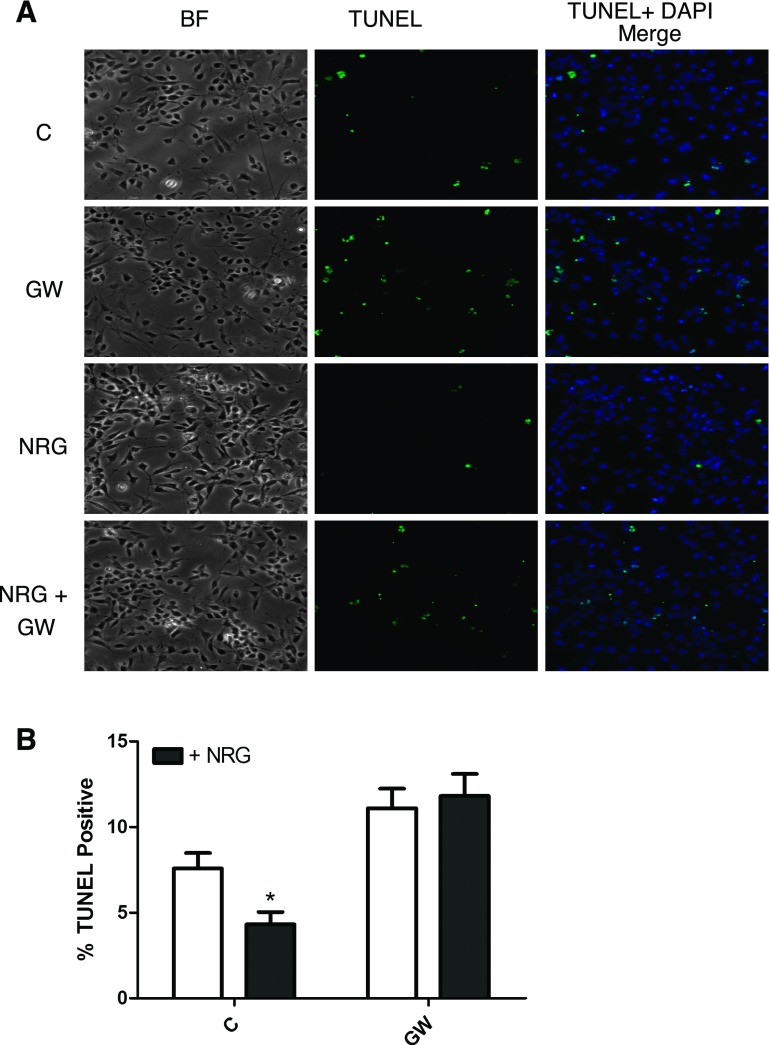
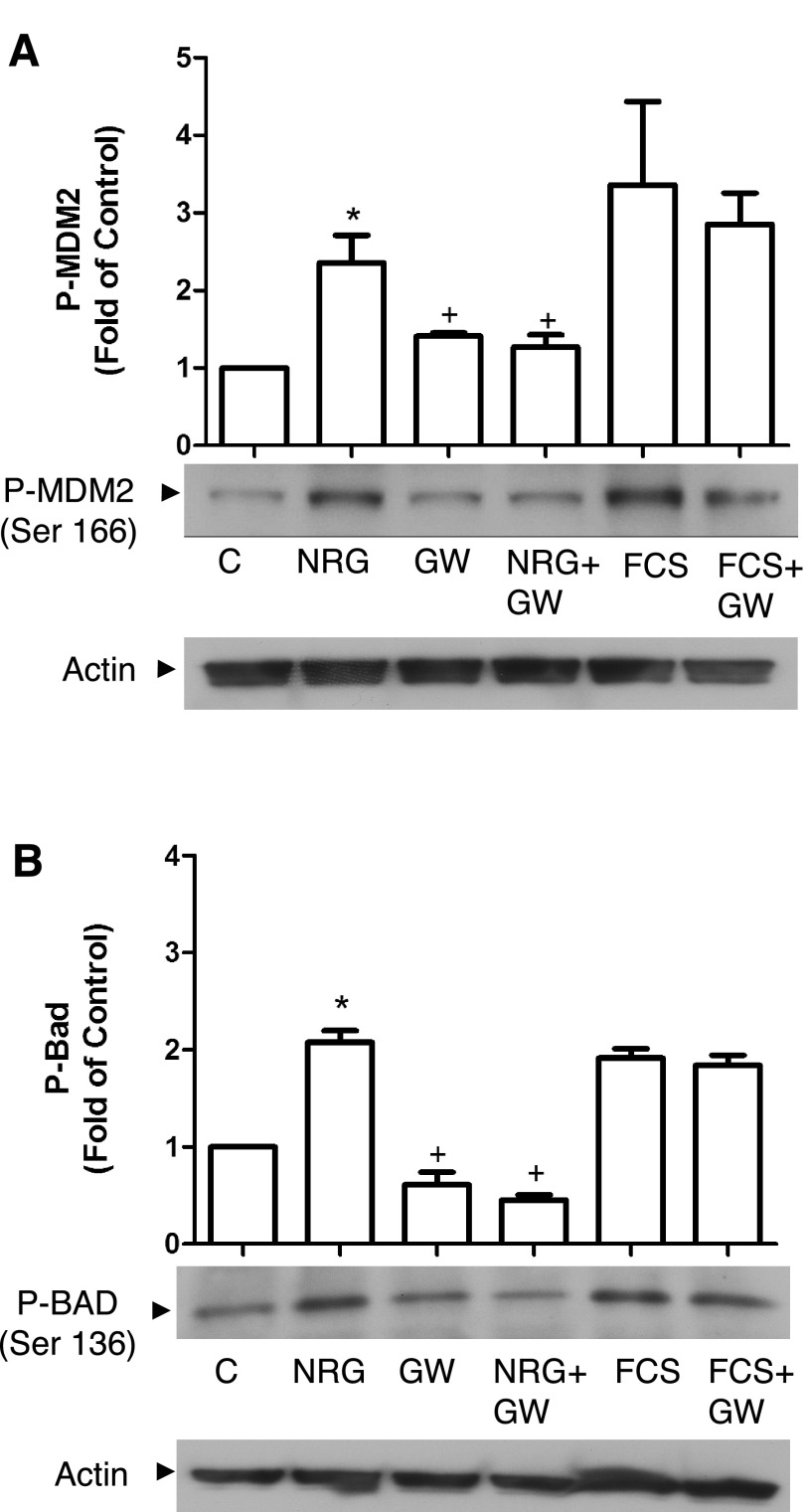
When eEPCs were deprived of serum for 18 h, eEPC apoptosis could be detected by DNA ladder and TUNEL assay as well as FACS analysis of cell cycle. NRG treatment decreased serum deprivation-induced apoptosis as assessed by DNA laddering (Fig. 6B) and FACS analysis of sub-G0 cells (Fig. 6C) and significantly decreased the number of TUNEL-positive nuclei (Fig. 7). The antiapoptotic effect of NRG was prevented by treatment with the erbB2 inhibitor GW572016. To further characterize NRG's affect on eEPC apoptosis we assessed apoptosis regulator proteins downstream of Akt. NRG treatment significantly increased phosphorylation of both MDM2 and Bad, and this effect was inhibited by GW572016 (Fig. 8).
Fig. 6.
NRG promotes eEPC survival. A: cells were transferred to either serum-free medium ± NRG-1β (50 ng/ml) or 0.5% FCS-containing medium for 18 h (T18hr). T0, time 0; Ctl, control. B: total adherent cell no. per ×2 field (n = 4). *P < 0.05. C: treated as in A. Genomic DNA was isolated and resolved on a 1.5% agarose gel. D: cells were treated as in A and then trypsinized, fixed, and stained with propidium iodide. %Apoptotic cells represents sub-G0 cells as determined by FACS (n = 3). *P < 0.05. E: FACS raw data with representative cell cycle gate settings.
Fig. 7.
NRG promotes eEPC survival. A: cells were treated overnight in serum-free medium containing NRG-1β (50 ng/ml), GW572016 (1 μM), or both and then fixed and stained by terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL). BF, bright field. B: quantification of TUNEL-positive cells was performed as described in materials and methods (n = 3). *P < 0.05.
Fig. 8.
NRG induces phosphorylation of apoptosis regulators. After 3 h of serum starvation cells were pretreated with GW572016 (1 μM) for 30 min and then treated with NRG-1β (50 ng/ml) or FCS (10%) for an additional 30 min. Lysates were probed with antibodies to p-MDM2 (A) and p-Bad (B). Blots were quantified by densitometry as described in materials and methods (n = 3). *P < 0.05 vs. C; +P < 0.05 vs. NRG.
DISCUSSION
To our knowledge the present work is the first to demonstrate a role for NRG/erbB signaling in EPC biology. These data add a new dimension to NRG/erbB signaling in the cardiovascular system, and they raise several interesting questions regarding the regulation of EPCs in tissue homeostasis. In addition, these observations may bear upon both the therapeutic and off-target effects of erbB2-targeted therapy.
The mouse eEPC line recapitulates many important functional characteristics of primary EPCs. In vitro differentiation of these cells leads to expression of endothelial specific genes including PECAM, vWF, and VEGFR-2 and downregulation of stem cell markers such as AC133/prominin (13). Furthermore, eEPCs have been shown to incorporate into developing neovasculature in the setting of physiological morphogenesis (13) as well as tumor angiogenesis (39). Importantly, these cells can also home to ischemic areas (Supplemental Fig. S4) and have been shown to decrease infarct size in in vivo models of myocardial ischemia (18, 19). NRG treatment has been shown to induce phosphorylation of Akt, Erk1/2, and Stat3 and to regulate diverse functions including proliferation, differentiation, and survival in multiple cell types from diverse tissues (11, 26, 41, 46). Therefore, we assessed these kinases in EPCs after NRG treatment. While NRG did induce phosphorylation of Akt, Erk1/2, and Stat3, EPC survival but not proliferation was positively affected. Although no clear NRG-dependent effect on EPC proliferation was observed, it is possible that we missed a modest effect under the experimental conditions used. Proliferation experiments were carried out in low-serum medium (0.5% FCS); however, even under these conditions, a significant percentage of cells were observed in S phase (Fig. 5A) in the untreated condition. Cells were not quiescent at baseline; therefore a modest NRG-dependent effect on proliferation may have been obscured by baseline proliferation. Nor did NRG have a direct effect on eEPC differentiation. It remains possible that NRG plays a permissive role either by potentiating the prodifferentiation effects of other growth factors or simply by promoting eEPC survival long enough for differentiation to occur. A number of ligands have been shown to regulate EPC apoptosis. In culture, estrogen treatment of mouse BM-derived EPCs decreased TNF-α-induced EPC apoptosis, and this effect was mediated by inhibition of caspase 8 activity (36). Similarly, angiotensin II has been to shown to promote rat BM-derived EPC survival in vitro (44). While few ligands have been described, some kinases have been shown to regulate EPC survival. Choi et al. (7) demonstrated increased survival of early EPCs in vitro after transduction with catalytically inactive GSK-3β. Others have demonstrated that inhibition of mammalian target of rapamycin (mTOR) with rapamycin leads to EPC death (28). mTOR is involved in growth factor signaling, especially those growth factors that signal through phosphatidylinositol 3-kinase (PI3K)/Akt (10). We have demonstrated that NRG treatment of eEPCs induces robust phosphorylation of both GSK-3β and Akt. The apoptosis regulators MDM2 and BAD are known Akt substrates, and the PI3K/Akt/BAD axis mediates NRG-dependent survival in other cell types (11, 25). Our data support a similar role for these proteins in mediating NRG's antiapoptotic effect on EPCs.
To further support the notion that results obtained with the eEPC line are generalizable to other EPC populations, we found the same complement of erbB receptor expression in murine embryonic EPCs and rat BM-derived and human peripheral blood-derived CD34+/VEGFR2+ EPCs (Supplemental Fig. S1). The erbB receptor complement of EPCs (erbB2+/erbB3+/erbB4−) is consistent with that of mature ECs (8, 27). Another interesting similarity is that eEPCs, like mature ECs, express NRG-1 ligand. In addition to contributing directly to vasculogenesis, release of growth factors is an important mechanism by which EPCs promote tissue repair (32, 38). EPC expression of NRG ligand raises the possibility of EPC-mediated autocrine or paracrine signaling that is felt to be an important mechanism for neoangiogenesis, and organ repair is mediated by this ligand.
It is interesting that stimuli such as ischemia or exercise, known to promote EPC mobilization (1, 15, 33), also promote cleavage and release of NRG (20, 23). In healthy subjects circulating NRG correlates with both circulating NRG and fitness (29). These observations support the hypothesis that circulating NRG is a mediator of EPC biology. In light of the present findings that eEPC survival is increased by NRG, it is plausible that NRG/erbB signaling preserves EPC survival either in the adverse milieu of the injured target tissue or during transit from source to target tissues.
Mechanisms for the effects of erbB2-targeted cancer therapies should be considered in light of our findings that NRG/erbB signaling regulates EPC survival. One would predict that patients undergoing treatment with GW572016 would have decreased circulating EPCs. Given the role of EPCs in tumor vasculature (34, 39), this is likely to slow tumor angiogenesis. Indeed, regression of tumor vasculature has been observed following treatment with the anti-erbB2 therapy trastuzumab (16). In addition, an effect of erbB2-targeted therapy on the survival of EPCs would be expected to reduce cardiac repair after injury. Perhaps this accounts, at least in part, for trastuzumab- and lapatinib-associated decreases in cardiac function. These hypotheses are currently being examined in a prospective study in breast cancer patients undergoing treatment with erbB2-targeted therapies.
GRANTS
This work was supported by National Institutes of Health Grants HL-068144 (D. B. Sawyer), HL-083958 (A. K. Hatzopoulos), HL-100398 (A. K. Hatzopoulos and D. B. Sawyer), and K01-AG-024056 (C. C. Lim) and an Established Investigator Award from the American Heart Association to D. B. Sawyer. This work was supported in part by the Vanderbilt Clinical and Translational Science Awards (CTSA) Grant UL1-RR-024975 from NCRR/NIH.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Tommy Wang and Vasan Ramachandran for helpful conversations. We thank Dr. Pampee Young for consultation over isolation of bone marrow-derived EPCs.
Footnotes
Supplemental Material for this article is available online at the Journal website.
REFERENCES
- 1. Adams V, Lenk K, Linke A, Lenz D, Erbs S, Sandri M, Tarnok A, Gielen S, Emmrich F, Schuler G, Hambrecht R. Increase of circulating endothelial progenitor cells in patients with coronary artery disease after exercise-induced ischemia. Arterioscler Thromb Vasc Biol 24: 684– 690, 2004. [DOI] [PubMed] [Google Scholar]
- 2. Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol 287: C572– C579, 2004. [DOI] [PubMed] [Google Scholar]
- 3. Baliga R, Pimental D, Zhao Y, Simmons W, Marchionni M, Sawyer DB, Kelly RA. NRG-1-induced cardiomyocyte hypertrophy: role of PI-3-kinase, p70S6K, and MEK-MAPK-RSK. Am J Physiol Heart Circ Physiol 277: H2026– H2037, 1999. [DOI] [PubMed] [Google Scholar]
- 4. Bersell KS, Arab B, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell 138: 257– 270, 2009. [DOI] [PubMed] [Google Scholar]
- 5. Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83: 59– 115, 2003. [DOI] [PubMed] [Google Scholar]
- 6. Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res 284: 66– 77, 2003. [DOI] [PubMed] [Google Scholar]
- 7. Choi JH, Hur J, Yoon CH, Kim JH, Lee CS, Youn SW, Oh IY, Skurk C, Murohara T, Park YB, Walsh K, Kim HS. Augmentation of therapeutic angiogenesis using genetically modified human endothelial progenitor cells with altered glycogen synthase kinase-3beta activity. J Biol Chem 279: 49430– 49438, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Cote G, Miller T, Lebrasseur N, Kuramochi Y, Sawyer DB. Neuregulin-1alpha and beta isoform expression in cardiac microvascular endothelial cells and function in cardiac myocytes in vitro. Exp Cell Res 311: 135– 146, 2005. [DOI] [PubMed] [Google Scholar]
- 9. DeFalco E, Porcelli D, Torella A, Straino S, Iachininoto M, Orlandi A, Truffa S, Biglioli P, Napolitano M, Capogrossi M, Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 104: 3472– 3482, 2004. [DOI] [PubMed] [Google Scholar]
- 10. Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23: 3151– 3171, 2004. [DOI] [PubMed] [Google Scholar]
- 11. Flores A, Mallon B, Matsui T, Ogawa W, Rosenzweig A, Okamoto T, Macklin W. Akt-mediated survival of oligodendrocytes induced by neuregulins. J Neurosci 20: 7622– 7630, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fukazawa R, Miller T, Kuramochi Y, Frantz S, Kim Y, Marchionni M, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol 35: 1473– 1479, 2003. [DOI] [PubMed] [Google Scholar]
- 13. Hatzopoulos A, Folkman J, Vasile E, Eiselen G, Rosenberg R. Isolation and characterization of endothelial progenitor cells from mouse embryos. Development 125: 1457– 1468, 1998. [DOI] [PubMed] [Google Scholar]
- 14. Hristov M, Erl W, Weber PC. Endothelial progenitor cells: mobilization, differentiation, and homing. Arterioscler Thromb Vasc Biol 23: 1185– 1189, 2003. [DOI] [PubMed] [Google Scholar]
- 15. Ii M, Nishimura H, Iwakura A, Wecker A, Eaton E, Asahara T, Losordo DW. Endothelial progenitor cells are rapidly recruited to myocardium and mediate protective effect of ischemic preconditioning via “imported” nitric oxide synthase activity. Circulation 111: 1114– 1120, 2005. [DOI] [PubMed] [Google Scholar]
- 16. Izumi Y, Xu L, DiTomaso E, Fukumura D, Jain R. Tumour biology: Herceptin acts as an anti-angiogenic cocktail. Nature 416: 279– 280, 2002. [DOI] [PubMed] [Google Scholar]
- 17. Junttila T, Sundvall M, Maatta J, Elenius K. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc Med 10: 304– 310, 2000. [DOI] [PubMed] [Google Scholar]
- 18. Kupatt C, Horktkotte J, Vlastos G, Pfosser A, Lebherz C, Semisch M, Thalgott M, Buttner K, Browarzyk C, Mages J, Hoffman R, Deten A, Lamparter M, Muller F, Beck H, Buning H, Boekstegers P, Hatzopoulos A. Embryonic endothelial progenitor cells expressing a broad range of proangiogenic and remodeling factors enhance vascularization and tissue recovery in acute and chronic ischemia. FASEB J 19: 1576– 1578, 2005. [DOI] [PubMed] [Google Scholar]
- 19. Kupatt C, Hinkerl R, Lamparter M, von Bruhl M, Pohl T, Horskotte J, Beck H, Muller S, Delker S, Gildehaus F, Buning H, Hatzopoulos A, Boekstegers P. Retroinfusion of embryonic endothelial progenitor cells attenuates ischemia-reperfusion injury in pigs: role of phosphatidylinositol 3-kinase/AKT kinase. Circulation 112: I117– I122, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Kuramochi Y, Cote G, Guo X, Lebrasseur N, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem 279: 51141– 51147, 2004. [DOI] [PubMed] [Google Scholar]
- 21. Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation 109: 220– 226, 2004. [DOI] [PubMed] [Google Scholar]
- 22. Laughner E, Taghavi P, Chiles K, Mahon P, Semenza G. HER2 (neu) signaling increases the rate of hypoxia-inducible factor 1alpha (HIF-1alpha) synthesis: novel mechanism for HIF-1-mediated vascular endothelial growth factor expression. Mol Cell Biol 21: 3995– 4004, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lebrasseur N, Cote G, Miller T, Fielding R, Sawyer DB. Regulation of neuregulin/ErbB signaling by contractile activity in skeletal muscle. Am J Physiol Cell Physiol 284: C1149– C1155, 2003. [DOI] [PubMed] [Google Scholar]
- 24. Lemmens K, Segers V, Demold M, DeKeulenaer G. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem 281: 19469– 19477, 2006. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Tennekoon G, Birnbaum M, Marchionni M, Rutkowski J. Neuregulin signaling through a PI3K/Akt/Bad pathway in Schwann cell survival. Mol Cell Neurosci 17: 761– 767, 2001. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Kern JA. Neuregulin-1 activates the JAK-STAT pathway and regulates lung epithelial cell proliferation. Am J Respir Cell Mol Biol 27: 306– 313, 2002. [DOI] [PubMed] [Google Scholar]
- 27. Lok J, Sardi S, Guo S, Besancon E, Ha D, Rosell A, Kim W, Corfas G, Lo E. Neuregulin-1 signaling in brain endothelial cells. J Cereb Blood Flow Metab 29: 39– 43, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miriuka S, Rao V, Peterson M, Tumiati L, Delgado D, Mohan R, Ramzy D, Stewart D, Ross H, Waddell T. mTOR inhibition induces endothelial progenitor cell death. Am J Transplant 6: 2069– 2079, 2006. [DOI] [PubMed] [Google Scholar]
- 29. Moondra V, Sarma S, Buxton T, Safa R, Cote G, Storerm T, Lebrasseur N, Sawyer DB. Serum neuregulin-1beta as a biomarker of cardiovascular fitness. Open Biomark J 2: 1– 5, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pentassuglia L, Graf M, Lane H, Kuramochi Y, Cote G, Timolati F, Sawyer DB, Zuppinger C, Suter T. Inhibition of ErbB2 by receptor tyrosine kinase inhibitors causes myofibrillar structural damage without cell death in adult rat cardiomyocytes. Exp Cell Res 315: 1302– 1312, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rafii S, Lyden D. Therapeutic stem and progenitor cell transplantation for organ vascularization and regeneration. Nat Med 9: 702– 712, 2003. [DOI] [PubMed] [Google Scholar]
- 32. Rehman J, Li J, Orschell C, March K. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107: 1164– 1169, 2003. [DOI] [PubMed] [Google Scholar]
- 33. Rehman J, Li J, Pravathaneni L, Karlsson G, Panchal V, Temm C, Mahenthiran J, March K. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol 43: 2314– 2318, 2004. [DOI] [PubMed] [Google Scholar]
- 34. Ribatti D. The involvement of endothelial progenitor cells in tumor angiogenesis. J Cell Mol Med 8: 294– 300, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Russell KS, Stern D, Polverini P, Bender J. Neuregulin activation of ErbB receptors in vascular endothelium leads to angiogenesis. Am J Physiol Heart Circ Physiol 277: H2205– H2211, 1999. [DOI] [PubMed] [Google Scholar]
- 36. Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Piller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation 107: 3059– 3065, 2003. [DOI] [PubMed] [Google Scholar]
- 37. Takahashi T, Kalka C, Masuda H, Silver M, Kearney M, Magner M, Isner J, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5: 434– 438, 1999. [DOI] [PubMed] [Google Scholar]
- 38. Urbich C, Aicher A, Heeschen C, Dernbach E, Hormann W, Zeiher A, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol 39: 733– 742, 2005. [DOI] [PubMed] [Google Scholar]
- 39. Vajkoczy P, Blum S, Lamparter M, Mailhammer R, Erber R, Engelhardt B, Vestweber D, Hatzopoulos A. Multistep nature of microvascular recruitment of ex vivo-expanded embryonic endothelial progenitor cells during tumor angiogenesis. J Exp Med 197: 1755– 1765, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiong S, Grijalve R, Zhang L, Nguyen N, Posters P, Pollock R, Yu D. Up-regulation of vascular endothelial growth factor in breast cancer cells by the heregulin-beta1-activated p38 signaling pathway enhances endothelial cell migration. Cancer Res 61: 1727– 1732, 2001. [PubMed] [Google Scholar]
- 41. Yang C, Klein E, Assoian R, Kazanietz M. Heregulin beta1 promotes breast cancer cell proliferation through Rac/ERK-dependent induction of cyclin D1 and p21Cip1. Biochem J 410: 167– 175, 2008. [DOI] [PubMed] [Google Scholar]
- 42. Yang Z, Wang J, Chen L, Luo C, Tang A, Tao J. Acute exercise-induced nitric oxide production contributes to upregulation of circulating endothelial progenitor cells in healthy subjects. J Hum Hypertens 21: 452– 460, 2007. [DOI] [PubMed] [Google Scholar]
- 43. Yen L, Benilmame N, Nie Z, Xiao D, Wang T, AlMoustafa A, Esumi H, Milanini J, Hynes N, Pages G, Alaoui-Jamali M. Differential regulation of tumor angiogenesis by distinct ErbB homo- and heterodimers. Mol Biol Cell 13: 4029– 4044, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yin T, Ma X, Zhao L, Cheng K, Wang H. Angiotensin II promotes NO production, inhibits apoptosis and enhances adhesion potential of bone marrow-derived endothelial progenitor cells. Cell Res 18: 792– 799, 2008. [DOI] [PubMed] [Google Scholar]
- 45. Young PP, Vaughan DE, Hatzopoulos AK. Biologic properties of endothelial progenitor cells and their potential for cell therapy. Prog Cardiovasc Dis 49: 421– 429, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhao YY, Sawyer D, Baliga R, Opel D, Han X, Marchionni M, Kelly R. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem 273: 10261– 10269, 1998. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.