Abstract
Purpose
The Study of Theories about Myopia Progression (STAMP) is a two-year, double-masked, randomized clinical trial of myopic children 6 to 11 years old. STAMP will evaluate the one-year effect of progressive addition lenses (PALs) compared to single vision lenses (SVLs) on central refraction, peripheral refraction in four quadrants, and accommodative response and convergence. STAMP will also evaluate any changes one year after discontinuing PALs. Baseline characteristics of enrolled children are reported.
Methods
Eligible children had a high accommodative lag and either: (1) low myopia (less myopic than −2.25 D spherical equivalent) or (2) high myopia (more myopic that −2.25 D spherical equivalent) and esophoria at near. Children were randomly assigned to wear either PALs or SVLs for one year to determine the difference in myopia progression in the PAL group relative to the SVL group. All children will then wear SVLs for the second year to evaluate the permanence of any treatment effect. Complete ocular biometric data are collected at six-month intervals.
Results
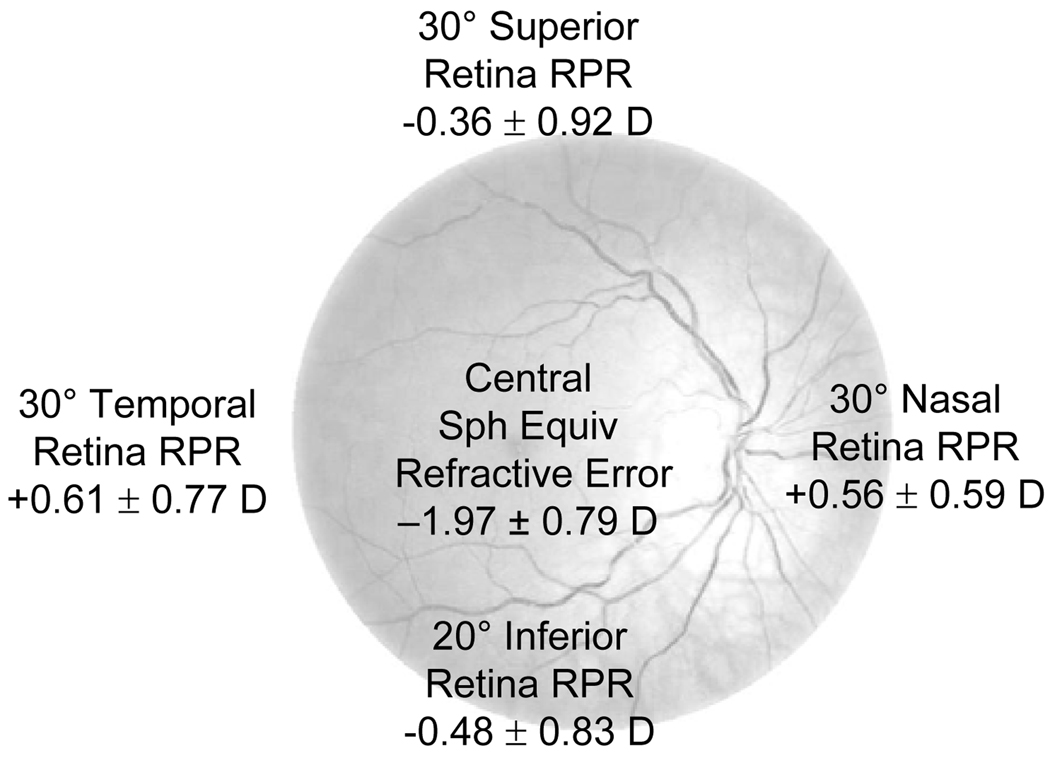
Over 17 months, 192 children were screened, and 85 (44%) were eligible and enrolled. Of these 85 children, 44 (52%) were female, and 54 (64%) were esophoric at near. The mean age (± SD) was 9.8 ± 1.3 years. The right eye mean cycloplegic spherical equivalent refractive error was −1.95 ± 0.78 D. Horizontal relative peripheral hyperopia (30° nasal retina +0.56 ± 0.59 D; 30° temporal retina +0.61 ± 0.77 D) and vertical relative peripheral myopia (30° superior retina −0.36 ± 0.92 D; 20° inferior retina −0.48 ± 0.83 D) were found.
Conclusions
The baseline data for STAMP are reported. Asymmetry between vertical and horizontal meridian relative peripheral refraction was found. STAMP will utilize the ocular biometric changes associated with the PAL-treatment effect to attempt to elucidate the mechanism responsible for the treatment effect.
Keywords: children, juvenile-onset myopia, progressive addition lenses, single vision lenses, myopia progression, randomized clinical trial
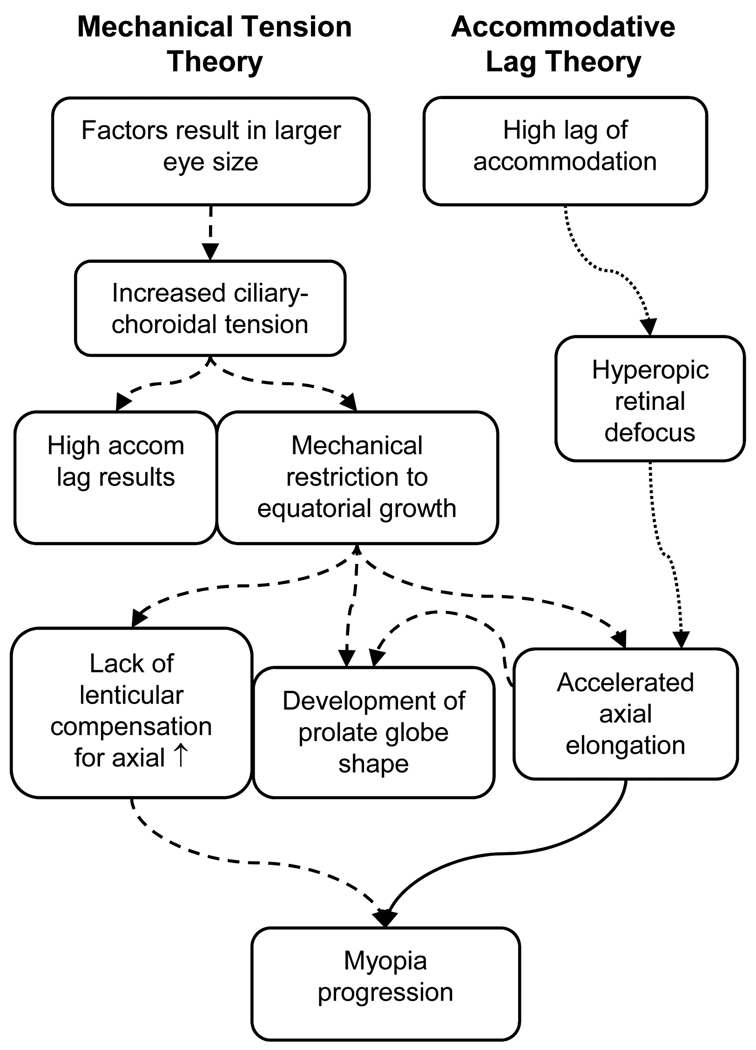
One in three adults in the United States is myopic,1 and the prevalence of myopia is increasing.2 A better understanding of the mechanism responsible for myopic progression could help develop effective treatments to reduce or prevent the progression of juvenile-onset myopia. Two current theories of juvenile-onset myopia progression rely on different mechanisms (Figure 1) with each theory pointing to a different therapeutic approach.
Figure 1.
Accommodative lag and mechanical tension theories of myopia progression. (Dashed lines = Mechanical Tension Theory; Dotted lines = Accommodative Lag Theory; Solid line = common to both theories)
The first theory is based on the hypothesis that hyperopic retinal blur caused by a high lag of accommodation during near work activities induces abnormal axial growth of the eye.3–5 This theory is supported by observations that myopes have a reduced accommodative response compared to emmetropes6–9 and thus an insufficient accommodative response to blur. The Correction of Myopia Evaluation Trial (COMET) finding that progressive addition lenses (PALs) are more effective at slowing myopia progression in children with a high accommodative lag supports the accommodative lag rationale;10 however, there has been only little to moderate success when myopic children with all levels of accommodative lag were included in interventions attempting to reduce progression using bifocals or PALs.11–14 Interestingly, COMET also found that the treatment effect produced by reducing hyperopic retinal blur with PALs occurred mainly during the first year of treatment.13 If PALs reduce myopic progression by decreasing hyperopic retinal blur during near work, there should be an accumulating PAL-treatment effect over the three-year study period because the myopigenic stimulus was decreased in the treatment group throughout the study.
A role for hyperopic defocus is strongly supported by the finding that the eyes of young animals respond to minus lens wear with faster axial elongation.15–19 On the other hand, brief periods of clear vision completely negate the “grow” signal produced by otherwise constant hyperopic retinal blur across animal models.20–22 Because children would have to do constant near work to prevent exposure to the potent “stop” signal provided by brief periods of clear vision when viewing an object at distance, these results in animals question the importance of transient hyperopic retinal defocus due to accommodative lag during near work. There are also controversies over whether accommodative lag in children is elevated prior to the onset of myopia23, 24 and whether accommodative lag is associated with myopic progression.25–27
A second theory has been proposed based on longitudinal ocular growth data from emmetropic and myopic children. This theory asserts that mechanical tension created by the crystalline lens or ciliary body restricts equatorial ocular expansion and causes accelerated axial elongation.28, 29 The mechanical tension theory proposes that there are factors that produce a larger than normal eye size in children at risk for myopia (Figure 1). Ciliary-choroidal tension in the anterior portion of the globe reaches a critical point where proportional expansion of the globe during eye growth is no longer possible. Once equatorial growth is restricted, there is accelerated axial growth. Myopia results from excess elongation that is uncompensated because the crystalline lens can no longer decrease in power by thinning and stretching.28, 29 A second consequence of reaching this critical point is that the tension results in an increase in the amount of effort required to accommodate, thereby producing an increased accommodative lag23 and AC/A ratio.30 In this theory, high accommodative lag is a by-product of myopia, and so lag is correlated with, but does not cause, myopic progression. A study in marmosets finding that high accommodative lag appears to be a consequence of myopia as opposed to being the cause provides additional support for this hypothesis.31 Ciliary-choroidal tension restricting equatorial growth may be the trigger for myopia onset and could explain why one longitudinal study found that increased accommodative lag accompanies, rather than precedes, the onset of myopia.23
The mechanical tension model of equatorial restriction is also consistent with the distortion in eye shape that takes place in myopia. Peripheral refractive error (i.e., the difference between the spherical equivalent refractive error of a peripheral retinal location and the central retina) has been used as a surrogate for characterizing ocular shape.32–35 Although asymmetries in peripheral refraction have been reported,32, 36, 37 measurements in adults and children have consistently shown that myopic eyes are relatively more hyperopic in the periphery of the horizontal meridian compared to the fovea. This relative peripheral hyperopia suggests that the myopic retina has a more prolate (less oblate) shape than emmetropic or hyperopic eyes.32–36, 38, 39 A decrease in the oblate shape of the horizontal meridian of the globe (or increase in relative prolate shape) as myopia increases has also been evident in MRI data.40 Thinner crystalline lenses have been associated with more hyperopic relative peripheral refractions in children,33 providing support for the hypothesis that ciliary-choroidal tension causes restricted equatorial growth with accelerated axial growth that leads to the more prolate eye shape of myopes. The finding that accommodation causes the eye to become more prolate provides evidence that ciliary-choroidal tension is capable of influencing ocular shape.41 Longitudinal data from children who ultimately become myopic have also shown a significant increase in relative peripheral hyperopia (relatively more prolate shape) two years prior to the onset of their myopia,38 which could be explained by increasing ciliary-choroidal tension that begins to slow equatorial expansion without impeding axial growth.
Previous clinical trials to evaluate whether bifocal spectacles or PALs reduce myopia progression included various biometric measurements.11–14 No previous study collected complete biometric data and optical characteristics of the eye because they were appropriately designed to evaluate the effectiveness of the intervention; however, comprehensive measurements that describe fully the changes in all ocular components and optics of the eye may be needed to explain the treatment’s underlying mechanism. Measurements of accommodative lag through the bifocal add are needed to determine the reduction in accommodative lag caused by PALs. Data describing treatment effect permanence after discontinuing PAL wear in a controlled, randomized sample have not been reported to date; therefore, prevention versus a simple delay of myopia progression cannot be distinguished. Work involving peripheral visual manipulation in chicks,42 and more recent work in infant rhesus monkeys,43 provides convincing evidence for a significant role of the peripheral visual experience in emmetropization, demonstrating the importance of also measuring peripheral retinal blur and considering the peripheral image shell induced by spectacle lenses. Comprehensive ocular measurements, both biometric and optical, will help determine the most appropriate model of juvenile myopia progression.
The purpose of the Study of Theories about Myopia Progression (STAMP) is to elucidate the mechanism responsible for the ocular biometric changes associated with the PAL-treatment effect and to determine whether this effect persists. Determining the most appropriate model of myopia progression will help guide whether future research should focus on treatments that alter the environment, such as bifocals or peripheral image manipulation, or on non-environmental treatments, such as pharmacological and genetic therapies. The two theories of myopia progression being evaluated may not be mutually exclusive, and some combination of the theories is a possible finding. This paper presents the STAMP baseline data.
METHODS
STAMP is a two-year, double-masked, randomized clinical trial. All children were randomly assigned to wear either single vision lenses (SVLs) or PALs with a +2.00-D add (Varilux Ellipse, Essilor of America) during the first year of the study, and all children will wear SVLs for the second year of the study. By randomizing children to either SVLs or PALs during the first year, it will be possible to detect the presence of the PAL treatment effect during the first year of the study. By then having all children wear SVLs in the second year, any significant rebound of the PAL treatment effect can be detected.
Eligible children were enrolled, randomized, and are being followed at six-month intervals for two years. Refractive error, axial length, peripheral ocular shape, central and peripheral aberrations, accommodation, corneal shape, anterior chamber depth, crystalline lens thickness and curvatures, and distance and near phoria are measured at six-month intervals. A survey of near work and outdoor activity is also administered at each visit (see Survey, Supplemental Digital Content 1, which shows the survey). At visits after randomization, regional spectacle lens measurements are made with an aberrometer at points that correspond with the peripheral ocular shape and aberration measurements to determine the defocus experienced at each retinal location.
Enrollment Criteria
The STAMP enrollment criteria are listed in Table 1. Age at the time of enrollment was restricted to ensure that the children would be younger when they completed the study than the average age at which myopia progression has been reported to cease.44 The upper limit of myopia was set at −4.50 D in each meridian to prevent inclusion of children with high, progressive myopia, which often has different genetic markers than common myopia.45 The lower limit of myopia was set at −0.75 D in each meridian to ensure that the child’s uncorrected visual acuity was reduced enough to promote full-time spectacle wear.
Table 1.
Study enrollment criteria.
| • 6 to 11 years of age |
| • ≥ 1.30 D accommodative lag (4D stimulus) without correction for lens effectivity |
| • Esophoria at near if more than −2.25 D spherical equivalent |
| • At least −0.75 D myopia in each meridian measured with cycloplegic autorefraction but not more than −4.50 D in each meridian in each eye |
| • Astigmatism ≤2.00 DC in each eye |
| • Anisometropia ≤2.00 D |
| • No strabismus |
| • No history of contact lens wear |
| • No previous bifocal wear |
| • Best corrected VA of at least 20/32 logMAR equivalent |
| • Birth weight ≥ 1250g by parental report |
| • No diabetes mellitus |
Children were enrolled with high accommodative lag and either: (1) low myopia (−2.25 D spherical equivalent or less) or (2) high myopia (more myopic than −2.25 D spherical equivalent) and esophoria at near. High accommodative lag was defined as greater than a median split of data, the same criterion used to define high accommodative lag in COMET. The median level of 1.30 D for a 4-D Badal stimulus was chosen from myopic children in the Collaborative Longitudinal Evaluation of Ethnicity and Refractive Error (CLEERE) Study because the CLEERE protocol is the one used to measure lag in STAMP.23 Myopic children with a high accommodative lag and near esophoria had the greatest one and three-year treatment effect in COMET (0.39 and 0.64 D), and children with high accommodative lag and low myopia (−2.25 D or less spherical equivalent myopia) had significant one and three-year treatment effects in COMET of 0.28 and 0.48 D, respectively.10 These two subgroups of myopic children were chosen to increase the likelihood of replicating a significant treatment effect after one year of wearing PALs while maximizing the generalizability of the results to myopic children.
Prior to assessing eligibility, parents provided informed consent and children provided verbal assent. The protocol was approved by the Biomedical Sciences Institutional Review Board at The Ohio State University. All children who met the eligibility criteria were enrolled in the study and were randomized to a treatment group after the baseline examination was completed. A web portal was used to confirm eligibility and administer the randomization, and the group assignment could only be accessed after entering all outcome data. Block randomization using random, even block sizes ensured that an equal number of children were randomized to each group while preventing the examiner from predicting the next assignment. Randomization was stratified by esophoria at near measured with full spectacle correction. After randomization, all outcome data were collected by an examiner masked to the treatment assignment.
Sample Size
The mean one-year progression rate calculated for the subgroups of children in COMET who wore SVLs and had a high accommodative lag with either low myopia or high myopia with esophoria at near was −0.69 D.10 A sample size of 36 children per group is sufficient to detect both a clinically significant PAL treatment effect in year one and a rebound effect in year two of 0.25 D with 80% power and α = 0.05 based on an average progression rate of −0.69 D ± 0.37 D per year. A rebound effect in year two is defined as a loss of the year-one treatment effect in the PAL group after being switched to SVLs in year two because of an increased rate of myopia progression compared to the SVL-only control group. The standard deviation of the rate of myopia progression used in the sample size calculation was estimated using reported annual rates of myopia progression in children (−0.50 D per year12, 13, 44 with a standard deviation of ±0.25 D44, 46, 47) and data from the Contact Lens and Myopia Progression (CLAMP) Study (J. Walline and L. Jones-Jordan, personal communication). After adjusting for an estimated loss to follow-up of 15%, it was necessary to enroll at least 84 children (42 in each of the two groups).
Variables Measured
Central Refractive Error (Primary Outcome)
Central spherical equivalent refractive error was measured with autorefraction under cycloplegia using the Grand Seiko WV-500 autorefractor (Grand Seiko Co., Hiroshima, Japan) 30 minutes after instilling 0.5% proparacaine and the first of two drops of 1% tropicamide, separated by 5 minutes. To ensure that accommodation was not stimulated, a Badal lens was used to simulate distance viewing. A reduced Snellen chart was moved inward from beyond the far point until the letters were clear and then moved to a slightly blurred position to ensure relaxation of accommodation. Ten valid autorefractor readings were obtained and averaged using the Thibos power vector method.48 Spherical equivalent refractive error measured under cycloplegic conditions with the Grand Seiko autorefractor has been reported to agree with cycloplegic subjective refraction in children.49 When measuring spherical equivalent refractive error under cycloplegic conditions, the between-session 95% limits of agreement for the Grand Seiko is reported to be ±0.24 D.50
Phoria
The modified Thorington method was used to assess phoria in this study.51 To check for a strabismus, unilateral cover test was performed at distance with best-correction in place and at near with best correction, with and without a +2.00-D add.
Accommodative Lag and Response AC/A
Measurements of accommodative response (lag of accommodation) were made monocularly using the Grand Seiko WV-500 autorefractor. At each visit, accommodative response of the right eye through the habitual correction was measured at three stimulus levels: 0.00 D, 2.00 D and 4.00 D. The child fixated a letter target (4 by 4 letter grid; 20/155 Snellen equivalent) viewed through a Badal lens with the right eye while the left eye was occluded with an infrared patch. The letter target was moved to the appropriate location on the Badal track for each demand level, and five readings were made at each accommodative demand. The position of the left eye was measured simultaneously through an accessory camera mounted on the Grand Seiko. This camera photographed the positions of Purkinje images I and IV as a measure of eye position at the three levels of accommodation. The change in eye position per unit change in accommodative response yielded the AC/A ratio. Each subject was calibrated by measuring the change in the positions of these images for a 10° eye movement prior to measurement.
Additional measurements of accommodative response to a 4.00-D stimulus were also made at each visit using the same experimental setup. At the baseline visit, in addition to measuring accommodation through the habitual correction, measurements of accommodative response were made through the manifest refraction determined at that visit and through the manifest refraction with a +2.00-D add. At the 6-month and 12-month visits, accommodative response measurements were made through the child’s habitual correction with and without a +2.00-D add as well as the manifest refraction with and without a +2.00-D add. At the 18-month and 24-month visits, accommodative response was measured through the habitual and manifest corrections only.
Corneal Curvature and Thickness
The Humphrey Atlas Corneal Topography System Model 993 (Carl Zeiss Meditec, Dublin, CA) was used to measure corneal topographical data. 52, 53 The Visante anterior segment Optical Coherence Tomographer (Carl Zeiss Meditec, Dublin, CA) measured full corneal pachymetry.54
Intraocular Pressure (IOP)
IOP was measured using a hand-held Tonopen applanation tonometer (Reichert, Depew, NY) after instillation of 0.5% proparacaine.
Ocular Shape and Relative Peripheral Defocus
The Complete Ophthalmic Analysis System for Vision Research (COAS-VR; AMO WaveFront Sciences, Albuquerque, NM), an open field aberrometer, was used to measure central and peripheral aberrations after cycloplegia.55 Nine central measurements of the right eye were made while the child viewed a fixation target. The child then turned his or her head to view fixation targets located 30°nasally and 30° temporally. The child turned his or her eye to view targets located 30° superiorly and 20°inferiorly.
For the purpose of calculating relative peripheral refraction, central and peripheral refractive error values were obtained from each child’s cycloplegic aberrometry measurements. Relative peripheral refraction was determined by calculating the difference in spherical equivalent refractive error between the peripheral and central spherical equivalent.37 At all visits after enrollment, regional aberrometry measurements of each child’s right spectacle lens were made while mounted in front of a model eye. These spectacle-only measurements were combined with the child’s corresponding central and peripheral cycloplegic refractive error measurements to determine the relative peripheral refraction experienced by the child when wearing his or her STAMP glasses.
Crystalline Lens Radii of Curvature
Video phakometry was performed using a custom system56 to calculate the radii of curvature of the crystalline lens. The calculations also yield an individual equivalent index of refraction for the crystalline lens. This measurement was made after cycloplegia with 1% tropicamide.57 One eye was occluded while the other fixated a red LED. Video recordings of Purkinje images I, III, and IV are digitized by frame-grabbing software and image processing software determines the distance between each of the Purkinje images.
Axial Dimensions
Anterior chamber depth and crystalline lens thickness were measured using A-scan ultrasonography (Humphrey Model 820, Humphrey Instruments).58 This procedure requires corneal anesthesia, cycloplegia, and mydriasis for accurate measurement. The between-session 95% limits of agreement for ultrasound measurements of crystalline lens thickness is reported to be ±0.20 mm.58 Axial length was measured using the Zeiss IOLMaster (Carl Zeiss Meditec, Dublin, CA), which is more repeatable than A-scan ultrasonography.59, 60 Five measurements were made and averaged using each instrument. The repeatability of axial length measured by the IOLMaster (i.e., between-session 95% limits of agreement) is reported to be between ±0.04 mm and ±0.06 mm.59–61
Near Work and Outdoor Activity Assessment
A determination of the amount of near work and outdoor activity that each child typically does outside of school was assessed at each visit using a survey completed by the parent or guardian accompanying the child to the visit (see Survey, Supplemental Digital Content 1). Diopter hours is a composite variable that weights near activities by the assumed accommodative demand for the task. Diopter hours is calculated as follows: 3 × (hours studying + hours reading for pleasure + hours playing handheld electronic games) + 2 × (hours playing video games + computer hours) + (hours watching television). The survey data for a child are not reported if the total number of hours per week reported across all questions totaled more than 82 hours because this was deemed to be unlikely given the number of hours available per week outside of school.
Data Management
All data were dual-entered by the Optometry Coordinating Center at The Ohio State University. The baseline summary statistics were performed using STATA 9.2 (StataCorp; College Station, TX) and SPSS 16.0 (SPSS, Inc.; Chicago, IL). A chi-square test was used to determine whether there was balance between treatment groups in the number of children who were and were not esophoric at near. Comparisons of relative peripheral refraction by retinal location were performed using a repeated measures ANOVA. When appropriate, post-hoc t-test comparisons were performed using the method described by Tukey and the appropriate mean square error from the ANOVA.
RESULTS
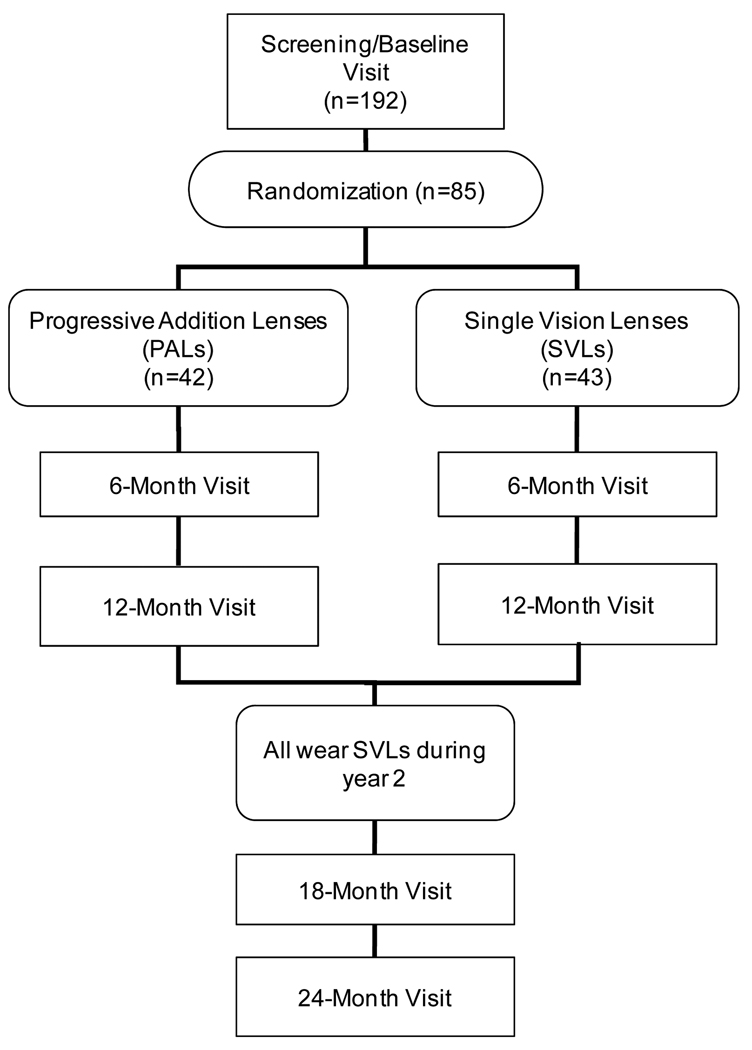
Enrollment began in December 2006 and was completed in May 2008. In total, 192 children were screened, and 85 (44%) were eligible and enrolled (Figure 2). Of the children enrolled, 42 were randomly assigned to wear PALs, and 43 were assigned to wear SVLs.
Figure 2.
Flowchart of STAMP visits and randomization.
The age of children at the enrollment visit ranged from 6 to 11 years with a mean (± SD) of 9.8 ± 1.3 years, and 44 (52%) were female. The mean cycloplegic spherical equivalent refractive error as determine by autorefraction using the Grand Seiko for the right eye was −1.95 ± 0.78 D, and the mean axial length was 24.17 ± 0.80 mm. The mean (± SD) accommodative lag after correcting for lens effectivity for a 4-D Badal stimulus measured with full manifest correction was 1.71 ± 0.37 D and ranged from 1.18 D to 2.85 D. Accommodative lag values were not corrected for lens effectivity when determining eligibility; therefore, the minimum effectivity-corrected lag value is less than 1.30 D. Of the 85 children enrolled, 10 (11.8 %) children had an accommodative lag value less than 1.30 D after correcting for lens effectivity, and the ten children were split equally between the treatment groups (i.e., five in the PAL group and five in the SVL group).
Of the children enrolled, 54 (64%) were esophoric at near at the baseline visit. Randomization was stratified by whether the child was esophoric at near. Of the 54 children with esophoria at near, 28 were assigned to SVLs, and 26 were assigned to PALs. Of the 31 children who were not esophoric at near, 15 were assigned to SVLs, and 16 were assigned to PALs. There was no imbalance between treatment groups in the number of children with and without esophoria at near (p = 0.76; chi-square test).
The race and ethnicity distribution of the children is shown in Table 2. Overall, 68.2% of the children were white, and 20.0% were African-American. Only 5.9% were Hispanic.
Table 2.
Race and ethnicity distribution of children enrolled in STAMP.
| Hispanic | Non-Hispanic | Total | |
|---|---|---|---|
| African American | 1 | 16 | 17 |
| White | 2 | 56 | 58 |
| Asian | 0 | 6 | 6 |
| Other | 2 | 2 | 4 |
| Total | 5 | 80 | 85 |
With the exception of survey outcomes, baseline summary statistics for the primary and secondary outcomes are shown in Table 3. Summary statistics for the near work and outdoor activity survey are shown in Table 4.
Table 3.
Summary statistics at baseline. (n = 85 unless noted otherwise.)
| Mean ± SD | Minimum | Maximum | |
|---|---|---|---|
| OD M (Spherical Equivalent; D) | −1.95 D ± 0.78 | −4.02 | −0.83 |
| OD J0 (D) | +0.08 D ± 0.21 | −0.42 | 0.89 |
| OD J45 (D) | −0.14 D ± 0.16 | −0.51 | +0.23 |
| OS M (Spherical Equivalent; D) | −1.99 D ± 0.78 | −4.20 | −0.91 |
| OS J0 (D) | +0.10 D ± 0.20 | −0.40 | +0.85 |
| OS J45 (D) | −0.17 D ± 0.18 | −0.60 | +0.19 |
| Accommodative Lag (D) (4-D Stim w/ full Manifest) |
1.71 D ± 0.37 | 1.18 | 2.85 |
| Axial Length OD (mm) | 24.17 ± 0.80 | 22.36 | 27.36 |
| Near Phoria (Δ; + = eso) | 0.72 ± 4.23 | −16 | 17 |
| AC/A ratio^ (Δ/D) | 8.83 ± 3.41 | 3.12 | 19.40 |
| Flat Meridian Keratometry (D) | 43.45 D ± 1.61 | 39.69 | 47.16 |
| Steep Meridian Keratometry (D) | 44.20 D ± 1.61 | 39.90 | 47.56 |
| Intraocular pressure (mm Hg) | 16.9 ± 2.9 | 11 | 24 |
| Corneal thickness (µm) | 536.3 ± 31.3 | 446.3 | 622.7 |
| Crystalline lens ϕ | |||
| Thickness (mm) | 3.36 ± 0.15 | 3.03 | 3.71 |
| Index of refraction | 1.428 ± 0.008 | 1.414 | 1.450 |
| Radius of curvature | |||
| Anterior (mm) | 12.28 ± 1.17 | 9.31 | 14.87 |
| Posterior (mm) | 6.44 ± 0.54 | 5.37 | 7.96 |
| Relative peripheral refraction (D) | |||
| 30° nasal retina | +0.56 D ± 0.59 | −0.48 | +2.20 |
| 30° temporal retina | +0.61 D ± 0.77 | −1.53 | +2.98 |
| 30° superior retina | −0.36 D ± 0.92 | −1.99 | +2.39 |
| 20° inferior retina | −0.48 D ± 0.84 | −3.14 | +2.04 |
AC/A ratio values were censored if the accommodative response was less than 1 diopter for a 4-D stimulus and if the AC/A ratio was greater than 20 Δ/D (n = 69; data from 16 children censored)
Phakometry data missing on two children (n = 83)
Table 4.
Summary statistics for near work and outdoor activity at baseline.
| Hours per week outside of school* |
Mean ± SD | Minimum | Maximum |
|---|---|---|---|
| Studies or reads for school | 4.75 ± 3.81 | 0 | 25 |
| Reads for pleasure | 4.17 ± 3.72 | 0 | 21 |
| Watches television | 8.90 ± 5.96 | 0 | 30 |
| Uses a computer | 4.12 ± 4.10 | 0 | 25 |
| Plays video games | 2.34 ± 3.35 | 0 | 16 |
| Plays handheld electronic games | 1.92 ± 2.73 | 0 | 11 |
| Engages in outdoor activities | 8.91 ± 6.38 | 0 | 28 |
| Diopter hours (near work composite) |
53.85 ± 23.24 | 9.5 | 105 |
Near work and outdoor activity data are not reported for three subjects because the total number of hours per week outside of school reported for all activities was greater than 82 hours, which was considered to be unlikely. (n = 82)
Differences in relative peripheral refraction were found that depended on the retinal location (Figure 3; repeated-measures ANOVA; p < 0.001). There were no asymmetries in relative peripheral refraction in the vertical meridian (superior and inferior retina) or in the horizontal meridian (nasal and temporal retina) (all p > 0.05; Tukey’s HSD); however, relative peripheral refraction values in the horizontal meridian were more hyperopic than in the vertical meridian (all p < 0.05; Tukey’s HSD). In the horizontal meridian, eyes of myopic children on average had relative peripheral hyperopia (i.e., a relatively more prolate shape). In the vertical meridian, myopic eyes on average had relative peripheral myopia (i.e., a relatively more oblate shape).
Figure 3.
Mean (± SD) relative peripheral refraction (RPR) at baseline for all children. (sph equiv = spherical equivalent)
DISCUSSION
Baseline Data
The mean age (9.8 years), spherical equivalent amount of myopia (−1.95 D), and axial length (24.17 mm) reflect the enrollment criteria for STAMP. The elevated mean lag of accommodation for a 4-D stimulus (1.71 D) reflects the eligibility criterion that children had to have a high lag of accommodation. The baseline characteristics of children in STAMP are similar to the baseline characteristics of children who participated in COMET, the most recent clinical trial using PALs in the United States. In COMET, at study entry, the mean (± SD) age was 9.3 ± 1.3 years,13 spherical equivalent refractive error was −2.38 ± 0.81 D, and axial length was 24.1 ± 0.7 mm.62 The baseline refractive error in STAMP was slightly less myopic than in COMET (p < 0.0001; t-test), which likely reflects the requirement that children with higher myopia in STAMP also be esophoric at near.
Primary Expected Outcomes
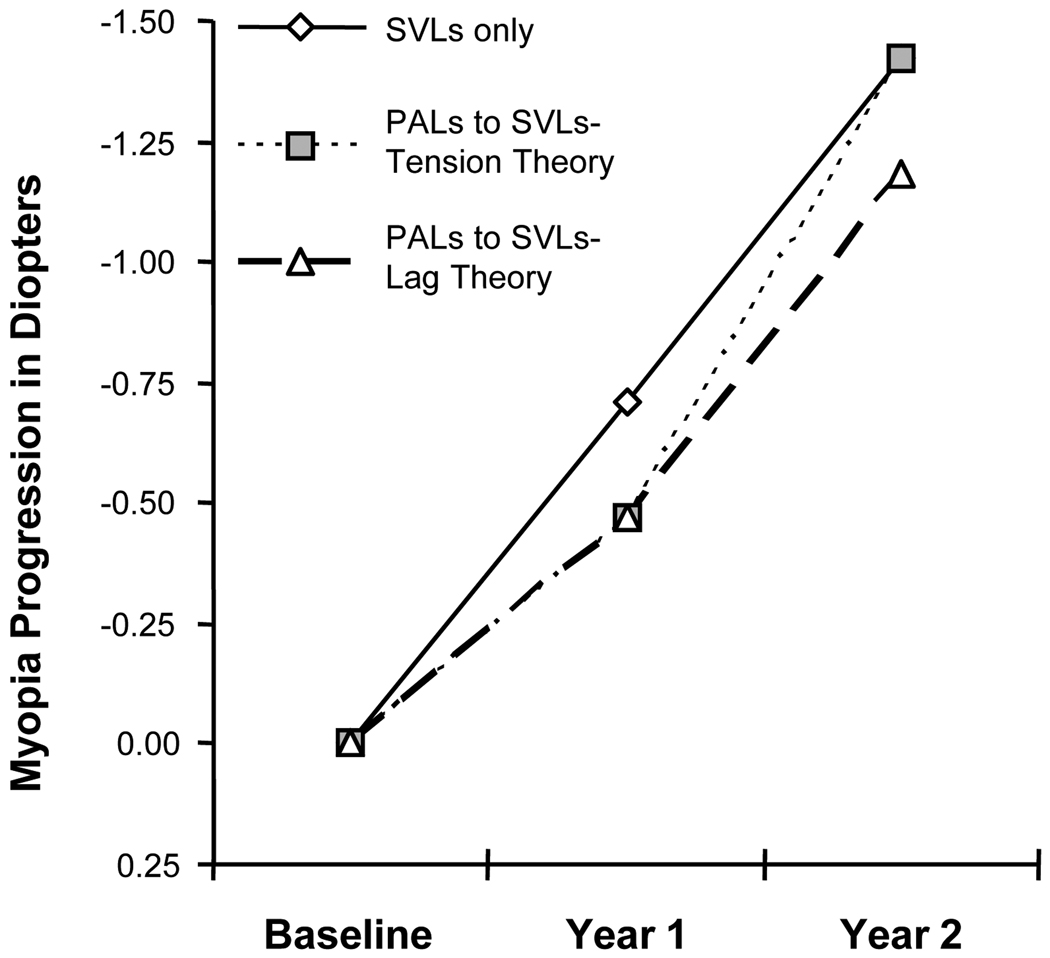
A reduction in myopia progression is expected in the PAL group after the first year based on both theories of myopia progression (Figure 4). During the second year when both groups wear SVLs, the accommodative lag theory predicts equal rates of myopia progression (i.e., no loss of the treatment effect) for both the PAL and SVL groups. The mechanical tension theory predicts a loss of the treatment effect in the PAL group after being switched to SVLs (i.e., a greater progression rate in the PAL group than in the SVL group during the second year). A loss of treatment effect would suggest that an increase in ciliary-choroidal tension was present once accommodative effort was restored to its normal state when the PALs were removed; however, refractive error alone is not sufficient to determine the mechanism behind the PAL treatment effect and myopia progression. Accommodative lag measurements made through the child’s actual near correction will assess whether accommodative lag returned to its pretreatment level after discontinuing PALs and whether the amount of hyperopic retinal blur during near work (i.e., accommodative lag) is related to the observed myopic progression.
Figure 4.
Hypothesized progression of myopia based on the mechanical tension theory and the accommodative lag theory.
Secondary Expected Outcomes
In addition to accommodative lag, to understand fully whether mechanical tension or hyperopic defocus due to accommodative lag contributes to myopia progression, the complete ocular component measurements must be considered. Both models of myopia etiology predict that axial length will increase less in the PAL group compared to the SVL group after one year, consistent with previous studies.13 Under the accommodative lag theory, the reduction in axial elongation is due to decreased hyperopic retinal blur on the retina during near work, which results in a decreased stimulus to grow. The mechanical tension theory predicts a reduction in axial elongation due to decreased ciliary-choroidal tension because less effort is needed to accommodate when wearing PALs. With this tension reduced, more proportional expansion of the globe is possible, which yields less rapid axial elongation. Recall that the mechanical tension theory hypothesizes that the accelerated axial elongation observed in myopia is due to ciliary-choroidal tension preventing proportional expansion of the globe in children with factors that produce a larger than normal eye size.
During the second year when both groups wear SVLs, the accommodative lag theory predicts equal amounts of axial length growth (i.e., no loss of the treatment effect) for both the PAL and SVL groups. Equal axial elongation is expected between the two groups because hyperopic retinal blur during near work is expected to be equal between the two groups once children wearing PALs are returned to SVLs. Measurements of accommodative lag at each visit will be used to verify whether lag returned to an equal level between groups after discontinuing PAL wear. The mechanical tension theory predicts a loss of the treatment effect in the PAL group after being switched to SVLs (i.e., greater axial length growth in the PAL group during the second year) because of an increased amount of ciliary-choroidal tension. Once PALs are removed, the additional equatorial expansion that occurred during PAL wear is expected to result in more accommodative effort being necessary to achieve the same accommodative response when compared to the SVL control group. The increased accommodative effort in turn increases ciliary-choroidal tension in the PAL group relative to the SVL group and causes accelerated axial elongation due to the greater amount of equatorial restriction in the PAL group. This more rapid axial elongation in the PAL group after being switched to SVLs results in a loss of the PAL treatment effect.
Retinal shape measurements combined with phakometry, and lens thickness measurements will determine if there is a decrease in mechanical tension when PALs are worn and if mechanical tension increases after the PAL group is switched to SVLs during year two of the study. In the mechanical tension theory, because PALs decrease accommodative effort, ciliary-choroidal tension created by accommodative effort will decrease. If the source of equatorial restriction is primarily from ciliary-choroidal tension, the crystalline lens should be able to stretch as the equator expands, causing flattening and thinning of the lens at the end of year one in the PAL group. In this case, the crystalline lens would remain thinner and flatter at the end of year two in the PAL group after being switched to SVLs because of the additional equatorial expansion during the first year of the study. The possible changes in the crystalline lens are based on the assumption that the source of tension is primarily the ciliary muscle; however, because COMET did not find a significant change in crystalline lens thickness during myopia progression in either SVLs or PALs,13 it is possible that either the mechanical tension theory is wrong, or that lens thickness changes, if they exist, are too small to be reliably measured by A-scan ultrasonography. Therefore, it will be important to also evaluate other biometric measurements, such as retinal shape and the peripheral image shell, which can aid in the determination of whether mechanical tension or retinal blur cause the PAL treatment effect.
Expected retinal shape changes differ between the two theories. In the accommodative lag theory, either no change in retinal shape will be found in the PAL group compared to the SVL group, or else there will be a superior/inferior refraction asymmetry. Both chicks and monkeys reared with different regional retinal defocus have been shown to have asymmetric retinal growth.63, 64 Therefore, if hyperopic retinal blur induces elongation as proposed in the lag theory, it may be possible to detect less relative myopia in the superior retina compared to the inferior retina in the PAL group because the bifocal add reduces hyperopic defocus on the superior retina. This type of asymmetry would support theories involving retinal defocus. In the mechanical tension theory, relieving accommodative effort with a PAL is expected to result in less restriction to equatorial growth and to create a relatively more oblate shape when compared to the control group after the first year of PAL wear. Once the PAL group is switched to SVLs, this theory predicts a relative increase in the prolate shape of the retina because restoring accommodative effort will again result in equatorial restriction to growth.
Baseline Relative Peripheral Refraction Asymmetry
The STAMP baseline relative peripheral refraction findings are of particular interest. The majority of studies that have measured relative peripheral refraction have done so in the horizontal meridian of the eye and found that myopic eyes of both adults and children have relative peripheral hyperopia (i.e., a relatively more prolate ocular shape).32–36, 38, 39 Consistent with other reports in the literature, relative peripheral hyperopia in the horizontal meridian was found in the myopic children enrolled in STAMP; however, relative peripheral myopia was found in the vertical meridian. We are aware of three studies that have reported relative peripheral refraction in the vertical meridian of the eye – two in adults35, 36 and one in children.39 Our finding of relative peripheral myopia in the vertical meridian of myopic eyes is consistent with a study of adults that found relative peripheral myopia across all refractive errors when measuring the superior and inferior retina of 43 hyperopic, emmetropic, and myopic eyes.36 Another study of 18 myopic adult eyes also reported relative peripheral myopia in the superior retina; however, relative peripheral hyperopia was reported in the inferior retina.35 Consistent with our results in children, relative peripheral myopia was reported in the superior and inferior retina of all eyes in a study that included 18 hyperopic, 21 emmetropic, and 10 myopic children. The myopic children had relatively less peripheral myopia than emmetropic and hyperopic children.39
The finding of relative peripheral myopic defocus in the vertical meridian of myopic eyes is intriguing. It was recently reported that local retinal regions respond to local defocus in emmetropizing infant monkeys,64 and it is well established across animal models that brief periods of exposure to either plus lenses or unrestricted vision prevent axial elongation in response to an otherwise constant stimulus to grow from a minus lens.17, 20–22 Given these results, theories proposing that peripheral retinal defocus causes the onset of myopia in children should also be able to account for the relative peripheral myopic defocus found in the vertical meridian of the myopic eye, which should act as a potent “stop” signal to growth. While the hyperopic defocus found in the horizontal meridian of myopic eyes could act as a stimulus for axial growth, this “grow” signal in the horizontal meridian of the eye would have to outweigh the “stop” signal created in the vertical meridian by myopic defocus. Given the evidence for local retinal control of eye growth across several animal models,64–67 a differential role for the horizontal and vertical retina seems unlikely.
Because spectacle correction can alter peripheral defocus,68 determining the effect of myopic correction on peripheral defocus in both the vertical and horizontal meridian of the eye is warranted. The relative peripheral refraction data collected over time in STAMP will evaluate the effects of both horizontal and vertical retinal defocus on myopia progression. Regional lensometry with each child’s spectacle correction is being performed at locations on the spectacle lens that correspond to the location of each relative peripheral refraction measurement; therefore, it will be possible to determine the peripheral defocus experienced by each child when wearing his or her spectacles. These data will be used to determine whether the peripheral defocus induced by single vision and progressive addition lenses results in corresponding changes in ocular shape.
CONCLUSIONS
In summary, the complete biometric and optical data collected over the course of this two-year clinical trial will help differentiate between the mechanisms resulting in the reduction in myopia progression observed when children wear PALs. While no single measurement will provide conclusive support for either theory, the combined results will allow us to determine which model best explains the observed changes in the ocular components. The baseline and one-year data will be used to determine whether a PAL treatment effect is present after the first year of the study. STAMP will then determine whether a treatment effect rebound occurs after the second year when all children wear single vision lenses. Particular attention will be paid to changes in accommodation, ocular shape, and peripheral retinal defocus by treatment group. Because peripheral refraction describes retinal shape and changes in ocular tension, this ocular component variable will allow us to evaluate the mechanical tension theory. Because accommodative lag describes the amount of hyperopic retinal blur, this variable will allow us to evaluate the accommodative lag theory. By measuring the peripheral image shell produced by the spectacle correction, it will also be possible to determine whether peripheral blur is associated with myopia progression in this cohort.
Supplementary Material
Supplemental Digital Content 1. File that shows the survey of near work and outdoor activity completed by the parent/guardian at each visit. .pdf
ACKNOWLEDGMENTS
This study was supported by NIH/NEI grant K12-EY015447, Essilor of America Inc., and an AOF Presidents Circle Ezell Fellowship (to DAB). Presented, in part, at the 2008 American Academy of Optometry Annual Meeting, Anaheim, CA.
The Data and Safety Monitoring Committee comprises Mark A. Bullimore, MCOptom, PhD (chair); Leslie Hyman, PhD; and Melvin L. Moeschberger, PhD.
Masked examiners: Bradley Dougherty, OD MS (2007-present), Kerri McTigue (2008-present), Donald O. Mutti, OD PhD (2008-present), Kathryn Richdale, OD MS (2007-present), Eric Ritchey, OD MS (2007-present), and Aaron Zimmerman, OD MS (2007–2008).
Opticians: Melissa Button (2007-present), Aaron Chapman (2006–2007), Melissa Hill (2006–2008), Brandy Knight (2008-present), Scott Motley (2007–2009), and Jeff Rohlf (2006-present).
Optometry Coordinating Center: Lisa Jones-Jordan, PhD (Director, 2005-present), G. Lynn Mitchell, MAS (Biostatistician, 2005 - present), Loraine Sinnott, PhD (Biostatistician, 2005 - present), Linda Barrett (Data Entry; 2005–2007), Austen Tanner (Data Entry, 2005-present), Melanie Schray (Database Management, 2005 - present)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Vitale S, Ellwein L, Cotch MF, Ferris FL, 3rd, Sperduto R. Prevalence of refractive error in the United States, 1999–2004. Arch Ophthalmol. 2008;126:1111–1119. doi: 10.1001/archopht.126.8.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 3.Charman WN. Near vision, lags of accommodation and myopia. Ophthalmic Physiol Opt. 1999;19:126–133. doi: 10.1046/j.1475-1313.1999.00414.x. [DOI] [PubMed] [Google Scholar]
- 4.Goss DA, Hampton MJ, Wickham MG. Selected review on genetic factors in myopia. J Am Optom Assoc. 1988;59:875–884. [PubMed] [Google Scholar]
- 5.Goss DA, Rainey BB. Relationship of accommodative response and nearpoint phoria in a sample of myopic children. Optom Vis Sci. 1999;76:292–294. doi: 10.1097/00006324-199905000-00016. [DOI] [PubMed] [Google Scholar]
- 6.Bullimore MA, Gilmartin B, Royston JM. Steady-state accommodation and ocular biometry in late-onset myopia. Doc Ophthalmol. 1992;80:143–155. doi: 10.1007/BF00161240. [DOI] [PubMed] [Google Scholar]
- 7.Gwiazda J, Thorn F, Bauer J, Held R. Myopic children show insufficient accommodative response to blur. Invest Ophthalmol Vis Sci. 1993;34:690–694. [PubMed] [Google Scholar]
- 8.McBrien NA, Millodot M. The effect of refractive error on the accommodative response gradient. Ophthalmic Physiol Opt. 1986;6:145–149. [PubMed] [Google Scholar]
- 9.Abbott ML, Schmid KL, Strang NC. Differences in the accommodation stimulus response curves of adult myopes and emmetropes. Ophthalmic Physiol Opt. 1998;18:13–20. [PubMed] [Google Scholar]
- 10.Gwiazda JE, Hyman L, Norton TT, Hussein ME, Marsh-Tootle W, Manny R, Wang Y, Everett D. Accommodation and related risk factors associated with myopia progression and their interaction with treatment in COMET children. Invest Ophthalmol Vis Sci. 2004;45:2143–2151. doi: 10.1167/iovs.03-1306. [DOI] [PubMed] [Google Scholar]
- 11.Edwards MH, Li RW, Lam CS, Lew JK, Yu BS. The Hong Kong progressive lens myopia control study: study design and main findings. Invest Ophthalmol Vis Sci. 2002;43:2852–2858. [PubMed] [Google Scholar]
- 12.Fulk GW, Cyert LA, Parker DE. A randomized trial of the effect of single-vision vs. bifocal lenses on myopia progression in children with esophoria. Optom Vis Sci. 2000;77:395–401. doi: 10.1097/00006324-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Gwiazda J, Hyman L, Hussein M, Everett D, Norton TT, Kurtz D, Leske MC, Manny R, Marsh-Tootle W, Scheiman M. A randomized clinical trial of progressive addition lenses versus single vision lenses on the progression of myopia in children. Invest Ophthalmol Vis Sci. 2003;44:1492–1500. doi: 10.1167/iovs.02-0816. [DOI] [PubMed] [Google Scholar]
- 14.Hasebe S, Ohtsuki H, Nonaka T, Nakatsuka C, Miyata M, Hamasaki I, Kimura S. Effect of progressive addition lenses on myopia progression in Japanese children: a prospective, randomized, double-masked, crossover trial. Invest Ophthalmol Vis Sci. 2008;49:2781–2789. doi: 10.1167/iovs.07-0385. [DOI] [PubMed] [Google Scholar]
- 15.Hung LF, Crawford ML, Smith EL., 3rd Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995;1:761–765. doi: 10.1038/nm0895-761. [DOI] [PubMed] [Google Scholar]
- 16.Schaeffel F, Glasser A, Howland HC. Accommodation, refractive error and eye growth in chickens. Vision Res. 1988;28:639–657. doi: 10.1016/0042-6989(88)90113-7. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh AW, Siegwart JT, Jr, Norton TT. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom Vis Sci. 1999;76:308–315. doi: 10.1097/00006324-199905000-00019. [DOI] [PubMed] [Google Scholar]
- 18.Whatham AR, Judge SJ. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001;41:267–273. doi: 10.1016/s0042-6989(00)00250-9. [DOI] [PubMed] [Google Scholar]
- 19.Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992;12:448–456. [PubMed] [Google Scholar]
- 20.Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996;36:1023–1036. doi: 10.1016/0042-6989(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhu X, Winawer JA, Wallman J. Potency of myopic defocus in spectacle lens compensation. Invest Ophthalmol Vis Sci. 2003;44:2818–2827. doi: 10.1167/iovs.02-0606. [DOI] [PubMed] [Google Scholar]
- 22.Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–4699. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mutti DO, Mitchell GL, Hayes JR, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Accommodative lag before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2006;47:837–846. doi: 10.1167/iovs.05-0888. [DOI] [PubMed] [Google Scholar]
- 24.Gwiazda J, Thorn F, Held R. Accommodation, accommodative convergence, and response AC/A ratios before and at the onset of myopia in children. Optom Vis Sci. 2005;82:273–278. doi: 10.1097/01.opx.0000159363.07082.7d. [DOI] [PubMed] [Google Scholar]
- 25.Weizhong L, Zhikuan Y, Wen L, Xiang C, Jian G. A longitudinal study on the relationship between myopia development and near accommodation lag in myopic children. Ophthalmic Physiol Opt. 2008;28:57–61. doi: 10.1111/j.1475-1313.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 26.Allen PM, O'Leary DJ. Accommodation functions: co-dependency and relationship to refractive error. Vision Res. 2006;46:491–505. doi: 10.1016/j.visres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 27.Rosenfield M, Desai R, Portello JK. Do progressing myopes show reduced accommodative responses? Optom Vis Sci. 2002;79:268–273. doi: 10.1097/00006324-200204000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Mutti DO, Zadnik K, Fusaro RE, Friedman NE, Sholtz RI, Adams AJ. Optical and structural development of the crystalline lens in childhood. Invest Ophthalmol Vis Sci. 1998;39:120–133. [PubMed] [Google Scholar]
- 29.Zadnik K, Mutti DO, Fusaro RE, Adams AJ. Longitudinal evidence of crystalline lens thinning in children. Invest Ophthalmol Vis Sci. 1995;36:1581–1587. [PubMed] [Google Scholar]
- 30.Mutti DO, Jones LA, Moeschberger ML, Zadnik K. AC/A ratio, age, and refractive error in children. Invest Ophthalmol Vis Sci. 2000;41:2469–2478. [PubMed] [Google Scholar]
- 31.Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007;47:1228–1244. doi: 10.1016/j.visres.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millodot M. Effect of ametropia on peripheral refraction. Am J Optom Physiol Opt. 1981;58:691–695. doi: 10.1097/00006324-198109000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Mutti DO, Sholtz RI, Friedman NE, Zadnik K. Peripheral refraction and ocular shape in children. Invest Ophthalmol Vis Sci. 2000;41:1022–1030. [PubMed] [Google Scholar]
- 34.Logan NS, Gilmartin B, Wildsoet CF, Dunne MC. Posterior retinal contour in adult human anisomyopia. Invest Ophthalmol Vis Sci. 2004;45:2152–2162. doi: 10.1167/iovs.03-0875. [DOI] [PubMed] [Google Scholar]
- 35.Seidemann A, Schaeffel F, Guirao A, Lopez-Gil N, Artal P. Peripheral refractive errors in myopic, emmetropic, and hyperopic young subjects. J Opt Soc Am (A) 2002;19:2363–2373. doi: 10.1364/josaa.19.002363. [DOI] [PubMed] [Google Scholar]
- 36.Atchison DA, Pritchard N, Schmid KL. Peripheral refraction along the horizontal and vertical visual fields in myopia. Vision Res. 2006;46:1450–1458. doi: 10.1016/j.visres.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 37.Berntsen DA, Mutti DO, Zadnik K. Validation of aberrometry-based relative peripheral refraction measurements. Ophthalmic Physiol Opt. 2008;28:83–90. doi: 10.1111/j.1475-1313.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 38.Mutti DO, Hayes JR, Mitchell GL, Jones LA, Moeschberger ML, Cotter SA, Kleinstein RN, Manny RE, Twelker JD, Zadnik K. Refractive error, axial length, and relative peripheral refractive error before and after the onset of myopia. Invest Ophthalmol Vis Sci. 2007;48:2510–2519. doi: 10.1167/iovs.06-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmid GF. Variability of retinal steepness at the posterior pole in children 7–15 years of age. Curr Eye Res. 2003;27:61–68. doi: 10.1076/ceyr.27.2.61.15454. [DOI] [PubMed] [Google Scholar]
- 40.Atchison DA, Pritchard N, Schmid KL, Scott DH, Jones CE, Pope JM. Shape of the retinal surface in emmetropia and myopia. Invest Ophthalmol Vis Sci. 2005;46:2698–2707. doi: 10.1167/iovs.04-1506. [DOI] [PubMed] [Google Scholar]
- 41.Walker TW, Mutti DO. The effect of accommodation on ocular shape. Optom Vis Sci. 2002;79:424–430. doi: 10.1097/00006324-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 42.Irving EL, Callender MG, Sivak JG. Inducing ametropias in hatchling chicks by defocus--aperture effects and cylindrical lenses. Vision Res. 1995;35:1165–1174. doi: 10.1016/0042-6989(94)00235-e. [DOI] [PubMed] [Google Scholar]
- 43.Smith EL, 3rd, Hung LF, Huang J. Relative peripheral hyperopic defocus alters central refractive development in infant monkeys. Vision Res. 2009;49:2386–2392. doi: 10.1016/j.visres.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goss DA, Winkler RL. Progression of myopia in youth: age of cessation. Am J Optom Physiol Opt. 1983;60:651–658. doi: 10.1097/00006324-198308000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Ibay G, Doan B, Reider L, Dana D, Schlifka M, Hu H, Holmes T, O'Neill J, Owens R, Ciner E, Bailey-Wilson JE, Stambolian D. Candidate high myopia loci on chromosomes 18p and 12q do not play a major role in susceptibility to common myopia. BMC Med Genet. 2004;5:20. doi: 10.1186/1471-2350-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goss DA, Cox VD. Trends in the change of clinical refractive error in myopes. J Am Optom Assoc. 1985;56:608–613. [PubMed] [Google Scholar]
- 47.Goss DA. Effect of bifocal lenses on the rate of childhood myopia progression. Am J Optom Physiol Opt. 1986;63:135–141. doi: 10.1097/00006324-198602000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Thibos LN, Wheeler W, Horner D. Power vectors: an application of Fourier analysis to the description and statistical analysis of refractive error. Optom Vis Sci. 1997;74:367–375. doi: 10.1097/00006324-199706000-00019. [DOI] [PubMed] [Google Scholar]
- 49.Choong YF, Chen AH, Goh PP. A comparison of autorefraction and subjective refraction with and without cycloplegia in primary school children. Am J Ophthalmol. 2006;142:68–74. doi: 10.1016/j.ajo.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 50.Bailey MD, Twa MD, Mitchell GL, Dhaliwal DK, Jones LA, McMahon TT. Repeatability of autorefraction and axial length measurements after laser in situ keratomileusis. J Cataract Refract Surg. 2005;31:1025–1034. doi: 10.1016/j.jcrs.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 51.Rainey BB, Schroeder TL, Goss DA, Grosvenor TP. Inter-examiner repeatability of heterophoria tests. Optom Vis Sci. 1998;75:719–726. doi: 10.1097/00006324-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 52.Chui WS, Cho P. A comparative study of the performance of different corneal topographers on children with respect to orthokeratology practice. Optom Vis Sci. 2005;82:420–427. doi: 10.1097/01.opx.0000162642.24885.71. [DOI] [PubMed] [Google Scholar]
- 53.Jeandervin M, Barr J. Comparison of repeat videokeratography: repeatability and accuracy. Optom Vis Sci. 1998;75:663–669. doi: 10.1097/00006324-199809000-00021. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed S, Lee GK, Rao SK, Wong AL, Cheng AC, Li EY, Chi SC, Lam DS. Repeatability and reproducibility of pachymetric mapping with Visante anterior segment-optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:5499–5504. doi: 10.1167/iovs.07-0591. [DOI] [PubMed] [Google Scholar]
- 55.Cheng X, Himebaugh NL, Kollbaum PS, Thibos LN, Bradley A. Validation of a clinical Shack-Hartmann aberrometer. Optom Vis Sci. 2003;80:587–595. doi: 10.1097/00006324-200308000-00013. [DOI] [PubMed] [Google Scholar]
- 56.Mutti DO, Zadnik K, Adams AJ. A video technique for phakometry of the human crystalline lens. Invest Ophthalmol Vis Sci. 1992;33:1771–1782. [PubMed] [Google Scholar]
- 57.Mutti DO, Zadnik K, Egashira S, Kish L, Twelker JD, Adams AJ. The effect of cycloplegia on measurement of the ocular components. Invest Ophthalmol Vis Sci. 1994;35:515–527. [PubMed] [Google Scholar]
- 58.Zadnik K, Mutti DO, Adams AJ. The repeatability of measurement of the ocular components. Invest Ophthalmol Vis Sci. 1992;33:2325–2333. [PubMed] [Google Scholar]
- 59.Carkeet A, Saw SM, Gazzard G, Tang W, Tan DT. Repeatability of IOLMaster biometry in children. Optom Vis Sci. 2004;81:829–834. doi: 10.1097/01.opx.0000145020.33250.c0. [DOI] [PubMed] [Google Scholar]
- 60.Sheng H, Bottjer CA, Bullimore MA. Ocular component measurement using the Zeiss IOLMaster. Optom Vis Sci. 2004;81:27–34. doi: 10.1097/00006324-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Hussin HM, Spry PG, Majid MA, Gouws P. Reliability and validity of the partial coherence interferometry for measurement of ocular axial length in children. Eye (Lond) 2006;20:1021–1024. doi: 10.1038/sj.eye.6702069. [DOI] [PubMed] [Google Scholar]
- 62.Gwiazda J, Marsh-Tootle WL, Hyman L, Hussein M, Norton TT. Baseline refractive and ocular component measures of children enrolled in the correction of myopia evaluation trial (COMET) Invest Ophthalmol Vis Sci. 2002;43:314–321. [PubMed] [Google Scholar]
- 63.Miles FA, Wallman J. Local ocular compensation for imposed local refractive error. Vision Res. 1990;30:339–349. doi: 10.1016/0042-6989(90)90076-w. [DOI] [PubMed] [Google Scholar]
- 64.Smith EL, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Optical defocus influences refractive development in monkeys via local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010 Mar 10; doi: 10.1167/iovs.09-4969. Epub ahead of print 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997;37:659–668. doi: 10.1016/s0042-6989(96)00224-6. [DOI] [PubMed] [Google Scholar]
- 66.Norton TT, Siegwart JT., Jr Animal models of emmetropization: matching axial length to the focal plane. J Am Optom Assoc. 1995;66:405–414. [PubMed] [Google Scholar]
- 67.Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987;237:73–77. doi: 10.1126/science.3603011. [DOI] [PubMed] [Google Scholar]
- 68.Lin Z, Martinez A, Chen X, Li L, Sankaridurg P, Holden BA, Ge J. Peripheral defocus with single-vision spectacle lenses in myopic children. Optom Vis Sci. 87:4–9. doi: 10.1097/OPX.0b013e3181c078f1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. File that shows the survey of near work and outdoor activity completed by the parent/guardian at each visit. .pdf