Abstract
It has previously been established that ventral spinocerebellar tract (VSCT) neurons and dorsal spinocerebellar tract neurons located in Clarke's column (CC DSCT neurons) forward information on actions of premotor interneurons in reflex pathways from muscle afferents on α-motoneurons. Whether DSCT neurons located in the dorsal horn (dh DSCT neurons) and spinocervical tract (SCT) neurons are involved in forwarding similar feedback information has not yet been investigated. The aim of the present study was therefore to examine the input from premotor interneurons to these neurons. Electrical stimuli were applied within major hindlimb motor nuclei to activate axon-collaterals of interneurons projecting to these nuclei, and intracellular records were obtained from dh DSCT and SCT neurons. Direct actions of the stimulated interneurons were differentiated from indirect actions by latencies of postsynaptic potentials evoked by intraspinal stimuli and by the absence or presence of temporal facilitation. Direct actions of premotor interneurons were found in a smaller proportion of dh DSCT than of CC DSCT neurons. However, they were evoked by both excitatory and inhibitory interneurons, whereas only inhibitory premotor interneurons were previously found to affect CC DSCT neurons [as indicated by monosynaptic excitatory postsynaptic potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) in dh DSCT and only IPSPs in CC DSCT neurons]. No effects of premotor interneurons were found in SCT neurons, since monosynaptic EPSPs or IPSPs were only evoked in them by stimuli applied outside motor nuclei. The study thus reveals a considerable differentiation of feedback information provided by different populations of ascending tract neurons.
Keywords: spinal cord, negative feedback
ascending tract neurons forward various kinds of information to their supraspinal target cells, including information on stimuli acting on our body, on descending commands sent to spinal neurons (the efference copy), on operation of spinal neuronal networks mediating responses to both peripheral stimuli and descending commands, and on the results of these responses. Monitoring of peripheral events has been analyzed in a number of studies and with respect to all categories of ascending tract neurons, including spinocerebellar (Arshavsky et al. 1978a, Arshavsky et al. 1972, Bosco and Poppele 2001, Lindström 1973, Lundberg 1971, Oscarsson 1973), spinoreticular (Maunz et al. 1978, Menetrey et al. 1980, Oscarsson and Sjolund 1977), or spinothalamic (Brown 1981a, Willis and Westlund 1997) neurons. The role of feedback information on the results of the movements has been extensively discussed in the context of the general theory of movement, in particular in the context of corrections based on this feedback (see Ito 2006). Monitoring of the descending commands has been much less explored, and it was discussed primarily with respect to spinocerebellar neurons (Arshavsky et al. 1978b, Arshavsky et al. 1978c, Bosco and Poppele 2001, Hammar et al. 2011). Monitoring of the spinal neuronal activity, the subject of the present study, has likewise attracted less attention. The most detailed studies focused to date on two subpopulations of ascending tract neurons: ventral spinocerebellar tract (VSCT) neurons and dorsal spinocerebellar tract (DSCT) neurons located in Clarke's column. They revealed that both of these may monitor actions of inhibitory premotor interneurons on spinal motoneurons (Burke et al. 1971, Hongo et al. 1983a and 1983b, Jankowska and Puczynska 2008, Jankowska et al. 2010, Lindström and Schomburg 1974, Lundberg 1971, Lundberg and Weight 1971). By acting on these spinocerebellar neurons, inhibitory interneurons in pathways between primary afferents and hindlimb motoneurons may thus supply the cerebellum with information on the degree of inhibition that they contribute to spinal reflexes. Based on this information cerebellar neurons may adjust both the reflex actions and the descending commands sent to motoneurons to optimize motor performance. However, distinct populations of spinocerebellar neurons may be specialized in processing feedback information of different kinds. The first aim of the present study was therefore to investigate whether another population of DSCT neurons [dorsal horn DSCT (dh DSCT) neurons located in the dorsal horn] (Edgley and Jankowska 1988, Edgley and Gallimore 1988) likewise forward information provided by premotor interneurons (represented by cells X in Fig. 1A). If not, dh DSCT neurons might be only indirectly affected by interneurons acting on motoneurons (represented by cells Y in Fig. 1B) or by interneurons that do not project to motor nuclei (represented by cells Z and ZZ in Fig. 1C). Our second aim was to evaluate the specificity of feed-back information supplied by spinocerebellar neurons by comparing the sources of collateral information forwarded by spinal interneurons to spinocerebellar tract neurons with that to spinocervical tract neurons (SCT) that might be indirectly providing the thalamus with such information (Brown 1981b, Downie et al. 1988). The comparison between dh DSCT neurons and SCT neurons is particularly relevant in this context because neurons of the two populations receive similar input from primary afferents (monosynaptic input from both group II and skin afferents) (Hammar et al. 1994, Harrison and Jankowska 1984) and are located within the same areas of the dorsal horn.
Fig. 1.
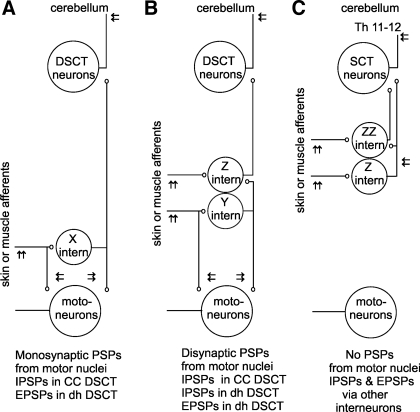
Diagram of possible relays of actions of peripheral afferents on dorsal spinocerebellar tract (DSCT) and spinocervical tract (SCT) neurons. Large circles represent DSCT neurons projecting to the cerebellum, SCT neurons terminating in the lateral cervical nucleus in the C3–4 segments and hindlimb motoneurons. Smaller circles represent interneurons that might mediate disynaptic or trisynaptic actions evoked by stimulation of peripheral nerves. A: neuronal pathways via interneurons X (excitatory or inhibitory) exerting direct actions on hindlimb motoneurons and on ascending tract neurons. B: neuronal pathways via interneurons Y (excitatory) acting directly on motoneurons but only indirectly on ascending tract neurons, via interneurons Z (excitatory or inhibitory). C: neuronal pathways via interneurons Z and ZZ (excitatory and inhibitory) that do not project to motor nuclei but might relay disynaptic and trisynaptic effects of primary afferents, being excited by the afferents either directly or indirectly. Arrows indicate sites of application of stimuli to the peripheral nerves, in the motor nuclei or dorsal to them, to the lateral funiculus at the Th 11–12 level and within the cerebellum, as indicated. PSPs evoked via these pathways are indicated at the bottom.
These questions were addressed by comparing postsynaptic potentials evoked in dh DSCT and SCT neurons with those evoked in previously investigated DSCT located in Clarke's column (CC DSCT) and VSCT neurons by intraspinal stimuli applied within major hindlimb motor nuclei. Monosynaptically evoked EPSPs and IPSPs were used as a measure of actions of premotor interneurons and oligosynaptic EPSPs and IPSPs as a measure of actions of other interneurons, as in the studies of Hongo et al. (1983a) and Jankowska et al. (2010).
METHODS
Preparation.
The experiments were performed on 10 deeply anesthetized cats weighing 2.4–3.6 kg. Anesthesia was induced with pentobarbital sodium (Apoteksbolaget, Sweden; 40–44 mg/kg ip) and maintained with intermittent doses of α-chloralose (Rhône-Poulenc Santé, France; 5 mg/kg; administered intravenously every 1 to 2 h up to 50 mg/kg). Additional doses of α-chloralose were given when increases in continuously monitored blood pressure or heart rate occurred during surgery or any of the experimental procedures. During recordings, neuromuscular transmission was blocked by pancuronium bromide (Pavulon, Organon, Sweden; about 0.2 mg/kg/h iv) and the animals were artificially ventilated. The effectiveness of synaptic transmission was increased by intravenous application of 4-aminopyridine (4-AP; Sigma) in doses 0.1–0.2 mg /kg iv. Mean blood pressure was kept at 100–140 mmHg and the end-tidal concentration of CO2 at about 4% by adjusting the parameters of artificial ventilation and the rate of a continuous infusion of a bicarbonate buffer solution with 5% glucose (1 to 2 ml·h−1·kg−1). Core body temperature was kept at about 38°C by servo-controlled infrared lamps. The experiments were terminated by a lethal dose of anesthetic. All these procedures were approved by the local Ethics Committee (Göteborgs djurförsöksetiska nämnd) and followed NIH and EU guidelines for animal care.
The spinal cord was exposed by laminectomy from the third to the sixth lumbar (L3-L6) segments and at the level of the low thoracic (Th10-Th12) segments. Records from dh DSCT neurons were obtained in the L4 and L5 segments, from CC DSCT neurons analyzed for comparison in the L3 and L4 segments, and from SCT neurons in the L5 and L6 segments. The dura mater was left intact, except for small holes (about 1 mm2) over the dorsal columns through which both the stimulating and recording electrodes were inserted, at the sites of lesions of the dorsal columns (see results) and/or at the level of the L7/S1 segments when the stimulating electrode was inserted to the motor nuclei via the lateral funiculus.
The caudal part of the cerebellum was exposed to allow insertion of an electrode used to stimulate axons of DSCT neurons to activate them antidromically. The cerebellar stimulation sites were at locations from which distinct descending volleys were evoked by stimuli of 20–50 μA. They were just rostral to, or within, the ipsilateral nucleus interpositus (at Horsley-Clarke coordinates about P 7, L 3.0–3.5, H 0 to −1).
Several left hindlimb nerves were dissected free, transected, and mounted on stimulating electrodes. They included quadriceps (Q) and sartorius (Sart) branches of the femoral nerve mounted in subcutaneous cuff electrodes, the posterior biceps and semitendinosus (PBST), anterior biceps and semimembranosus (ABSM), sural (Sur), gastrocnemius-soleus (GS), plantaris (PL), flexor digitorum and hallucis longus (FDL), deep peroneal (DP) including extensor digitorum longus and tibialis anterior nerves and superficial peroneal (SP).
Stimulation and recording.
Constant voltage stimuli were applied to peripheral nerves at intensities expressed in multiples of threshold (T) for the activation of the most excitable fibers. Axons of DSCT and SCT neurons within the ipsilateral lateral funiculus at the Th12 level were stimulated extradurally, with two silver ball electrodes in contact with its surface, using 0.2-ms long constant current pulses of 100–200 μA. Intracerebellar axonal branches of DSCT neurons were stimulated by using similar current pulses at intensities <100 μA applied through a tungsten electrode (0.3 mm diameter, electrolytically sharpened, insulated except for the tip, with 30–200 KOhm impedance). Intraspinal stimuli were applied through similar but thinner tungsten electrodes (0.1 mm diameter, with 100–300 KOhm impedance). The intraspinal tungsten electrode was introduced only after the location of gastrocnemius-soleus and biceps-semitendinosus motor nuclei had been defined using records from a glass micropipette filled with 2M NaCl, as illustrated in Fig. 2.
Fig. 2.
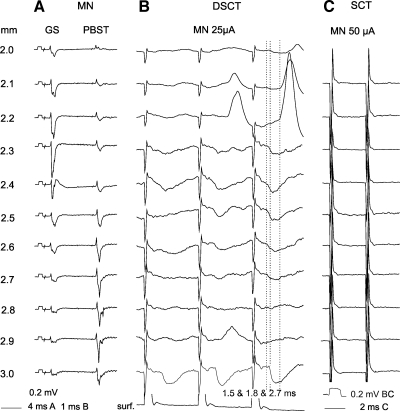
Comparison of effects of stimuli applied at different depths along an electrode track traversing motor nuclei (MN) on DSCT and SCT neurons. A: antidromic field potentials evoked along an electrode track across gastrocnemius-soleus (GS) and posterior biceps-semitendinosus motor nuclei (PBST); they defined the location of the stimulating electrode. Averages of 10 single records are shown. B: records of potentials evoked in a dorsal horn DSCT (dh DSCT) neuron by stimuli applied along this track, with the bottom record from the surface of the spinal cord. Averages of 20 records are shown. C: illustration of failures to evoke similar potentials in a SCT neuron by stimuli applied along the same electrode track; note that these stimuli were ineffective despite their higher intensity. Records from the illustrated DSCT neuron, including its identification, are also shown in Fig. 3. Depths at which the stimuli were applied are indicated to the left of the records in A and their intensities above records in B and C. Dotted lines indicate onset of the earliest EPSPs and IPSPs. Time and voltage calibrations are as indicated in A and C. In this and the following figures the negativity in the microelectrode records is downward and in records from the cord dorsum upward. The largest shock artefacts are truncated.
Effects of any interneurons projecting to motor nuclei were tested by applying stimuli of 20–50 μA at several depths in the areas within, which antidromic field potentials were evoked by stimulation of the gastrocnemius-soleus and biceps-semitendinosus motor nuclei nerves. Lack of effects was verified by using stimuli up to 100 μA. These motor nuclei were selected for purposes of this study because disynaptic IPSPs and EPSPs from groups I and II afferents are evoked in high proportions of gastrocnemius-soleus and biceps-semotendinosus motoneurons in parallel with disynaptic PSPs from these afferents in VSCT and CC DSCT neurons (Hongo et al. 1983b, Jankowska and Puczynska 2008, Lindström and Schomburg 1974, Lundberg and Weight 1970).
Both intracerebellar and intraspinal stimulation sites were marked by electrolytic lesions made at the end of the experiments and reconstructed from serial frozen sections cut in the plane of insertion of the electrodes and reconstructed histologically, with examples in Fig. 3E.
Fig. 3.
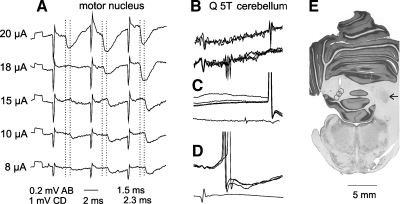
Comparison of IPSPs evoked in a dh DSCT neuron by stimuli of different intensities. A: IPSPs evoked in the dh DSCT neuron illustrated in Fig. 2B by changing intensity of stimuli applied in the posterior biceps-semitendinosus (PBST) motor nucleus at the depth of 2.4 mm. Averages of 10 records are shown. Dotted lines indicate onset of IPSPs evoked by the third 20 and 10 μA stimuli. The figures below the records indicate their latencies; note difference of 0.8 ms. B–D: records identifying this neuron as the spinocerebellar neuron. B: records obtained just before the penetration illustrating collision between spike potentials evoked by stimulation of the cerebellum and of the quadriceps (Q) nerve; spikes from the cerebellum are seen to the right (top traces) only when they were not preceded by the synaptically evoked spikes (those in the middle of the lower traces). C and D: intracellularly evoked antidromic spike potentials following stimulation of the cerebellum and synaptically evoked spikes induced in the same neuron by stimulation of Q. E: reconstruction of 3 stimulation sites in the cerebellum in approximately the same plane; the arrow indicates the right nucleus interpositus.
Glass micropipettes used for extracellular and intracellular recording (against the reference electrode inserted in a back muscle) had an impedance of about 2 to 3 MOhms and 4–6 MOhms, respectively. They were broken to about 2 or 1 μm and filled with a solution of 2M NaCl or KCitrate, respectively. For further details of the experimental procedures see Jankowska et al. (2010).
Analysis.
Both original data and averages of 10–30 single records (with the time resolution of 30 μs per address) were stored online using software for sampling and analysis developed by E. Eide, T. Holmström, and N. Pihlgren (Göteborg University). Latencies of postsynaptic potentials evoked by stimulation of peripheral nerves were measured from afferent volleys recorded from the cord dorsum close to the recording electrode penetration site, whereas those evoked by intraspinal stimuli were measured from stimulus artefacts. The reason for relating the latencies to the intraspinal stimuli was that the ensuing nerve volleys were most often superimposed on shock artefacts and therefore difficult to monitor. However, when the volleys were distinct, their positive phase occurred at about 0.5 ms latency from the onset of the stimulus, depending on the distance from the motor nuclei. Latencies of synaptic effects with respect to the volleys would thus be about 0.5 ms shorter than those measured from the stimuli.
Differences between data sets were assessed for statistical significance by using Student's t-test (for unpaired or paired samples assuming equal variances and the 2-tail distribution).
Samples.
Intracellular records were obtained from 33 dh DSCT neurons located within the most lateral part of the dorsal horn in the L4/5 segments at locations where the largest focal field potentials were evoked from quadriceps group II muscle afferents. Of these neurons, 25 were identified by antidromic activation from within the region of the left nucleus interpositus in the cerebellum, at locations indicated in Fig. 3E; by stimuli applied to the ipsilateral lateral funiculus at the Th11–12 level (at thresholds of about 100 μA); and by monosynaptic EPSPs from both skin and group II muscle afferents (Edgley and Jankowska 1988). An additional eight neurons recorded in preparations in which monosynaptic actions of peripheral afferents on these neurons were eliminated by transection of dorsal columns (see below and results) were identified by only the first two of these criteria.
CC DSCT neurons (21 total) analyzed for comparison were identified by antidromic activation from the cerebellum (similarly from within the region of the ipsilateral nucleus interpositus), by stimuli applied to the ipsilateral lateral funiculus, and by their location within the most medial part of the dorsal horn in the L3/4 segments. The first few cells in each experiment were also identified by monosynaptic input from the lowest threshold muscle afferents, but this information was lacking for the cells analyzed after the transection of the dorsal columns caudal to Clarke's column, made to eliminate monosynaptic actions of group I afferents upon them (see Hongo et al. 1983a and 1983b). The transections were made because discharges of CC DSCT neurons following the arrival of nerve impulses in fibers running in the dorsal columns greatly interfered with the detection of small PSPs evoked by interneurons activated by stimuli applied in motor nuclei. Another reason was that with the dorsal columns intact it would be impossible to distinguish monosynaptic EPSPs evoked by stimulation of group Ia and II afferents from EPSPs evoked by stimulation of terminal axonal branches of excitatory premotor interneurons. Fortunately axons of premotor interneurons ascend in the lateral column and are not affected by the transection of the dorsal columns.
SCT neurons (33 total) were identified by their antidromic activation from the ipsilateral lateral funiculus at the Th11–12 level but not from the cerebellum; by location within the most lateral part of the dorsal horn in the L4-L5 segments, within the regions where largest focal field potentials were evoked from the saphenus and/or superficial peroneal nerves; and by monosynaptic input from these cutaneous nerves. The sample was restricted to neurons in which monosynaptic EPSPs were evoked from skin but not muscle nerves, although a certain proportion of SCT neurons are coexcited by group II afferents (Hammar et al. 1994, Harrison and Jankowska 1984), to decrease the risk of including dh DSCT neurons coexcited by group II and cutaneous afferents that were not antidromically activated from the stimulated region of the cerebellum.
When spike potentials of neurons to be investigated were recorded extracellularly, a collision test was used to ensure that the shortest latency spike potentials were indeed evoked antidromically and not synaptically (via fibers coexcited by the same stimuli); this procedure was particularly important when the antidromic activation was evoked by stimulation of the lateral funiculus. The spikes were classified as evoked antidromically when they were collided by spike potentials evoked by stimulation of a peripheral nerve at a critical time interval [about twice conduction time from the Th segment plus refractory period of the axons (see Asif and Edgley 1992, Fuller and Schlag 1976, Krutki et al. 2003, Lipski 1981)]. Criteria of antidromic activation in intracellular records were used short latency of the spikes, usually coinciding with the descending volleys, or following them within about 0.5 ms, and the appearance of these spikes at an all-or-none fashion.
RESULTS
Figure 1A outlines pathways via which disynaptic EPSPs and IPSPs from muscle or cutaneous afferents could be evoked in DSCT neurons via interneurons denoted X, acting in parallel on motoneurons and on these ascending tract neurons and therefore be evoked monosynaptically following stimulation of terminal branches of these interneurons in motor nuclei. Figure 1, B and C, shows that disynaptic EPSPs and IPSPs could also be evoked by interneurons that do not project to motor nuclei (Z and ZZ) and trisynaptic EPSPs and IPSPs via either premotor interneurons (Y) and interneurons (Z) or via interneurons Z and ZZ.
Considering these possibilities, we examined whether stimuli applied in motor nuclei evoked postsynaptic potentials in dh DSCT and SCT neurons. If disynaptic EPSPs or IPSPs evoked from peripheral afferents were mediated by interneurons that inhibit motoneurons (X in Fig. 1), stimuli applied within motor nuclei should stimulate terminal branches of these interneurons and the ensuing nerve impulses conducted along their ascending axonal branches should evoke monosynaptic EPSPs or IPSPs in the tested neurons. Under these conditions effects of intraspinal stimuli should be evoked at similar minimal latencies as in VSCT neurons, i.e., ≤1.4 ms from the stimuli (see Jankowska et al. 2010), and follow both single and brief trains of stimuli. However, although monosynaptic IPSPs could be unequivocally attributed to inhibitory premotor interneurons, monosynaptic EPSPs that were potentially evoked by excitatory premotor interneurons had to be differentiated from monosynaptic EPSPs induced by collaterals of primary afferents stimulated within motor nuclei. The latter could be expected in CC DSCT neurons with input from group Ia afferents contacting motoneurons, but also in dh DSCT neurons with input from group II afferents in view of projections of some such afferents to the ventral horn (Hongo 1992, Stauffer et al. 1976). To prevent monosynaptic EPSPs from primary afferents to be evoked in these neurons, records from all CC DSCT and from some dh DSCT neurons were obtained in preparations in which the axonal branches of peripheral afferents ascending within dorsal columns were transected between the L4 and L5 segments (Hongo et al. 1983a and 1983b).
If disynaptic postsynaptic potentials evoked in ascending tract neurons were evoked by interneurons that do not contact motoneurons (Z or ZZ), these interneurons could likewise be activated by stimuli applied in motor nuclei, but indirectly, i.e., by collaterals of afferents that project to motor nuclei, or by excitatory premotor interneurons (Y in Fig. 1B). In such cases stimuli applied in motor nuclei should only be followed by longer latency potentials. These potentials would be evoked at latencies corresponding to segmental latencies of disynaptic potentials evoked from primary afferents and display strong temporal facilitation (see Jankowska et al. 2003 and appendix). However, interneurons Z and ZZ could also be activated by afferents that do not project to motor nuclei and are not coexcited by premotor interneurons as outlined in Fig. 1C. If so, stimuli applied in motor nuclei should not affect them and such stimuli should not be followed by any postsynaptic potentials in ascending tract neurons.
The results revealed that stimuli applied in motor nuclei evoke both EPSPs and IPSPs in dh DSCT neurons and that the features of these postsynaptic potentials are compatible with disynaptic rather than monosynaptic coupling. One of their common features was that that they often failed to be evoked after the first stimulus but appeared after the second or third stimulus (e.g., at depths 2.2, 2.5, 2.7, or 2.9 mm in records in Fig. 2B) or were considerably larger after the second or third than after the first stimuli in a train (at depths 2.3 and 2.6 ms). They thus displayed potent temporal facilitation characteristic for oligosynaptically evoked postsynaptic potentials, which was even more marked when the stimulus intensity was lowered, as shown in Fig. 3A. Furthermore, even when single stimuli were effective, e.g., from the depths 2.4 and 3.0 mm in the neuron illustrated in Fig. 2B, they were followed by postsynaptic potentials evoked only after the second or third stimuli.
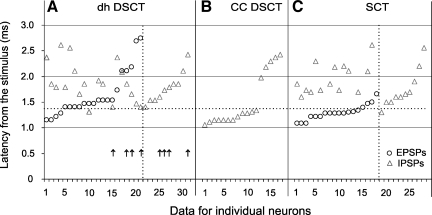
Latencies of potentials illustrated in Figs. 2 and 3 were likewise more compatible with latencies of disynaptically than monosynaptically evoked postsynaptic potentials. Taking 1.4 ms from the stimuli as the lowest limit of latencies of disynaptically evoked postsynaptic potentials (Jankowska et al. 2010) only the latencies of the four earliest EPSPs fell within the range of monosynaptic EPSPs evoked in dh DSCT neurons plotted in Fig. 4A, and latencies of seven EPSPs were only 0.1 ms longer than the 1.4-ms borderline. However, the fact that seven of these eleven earliest EPSPs appeared only after the second stimulus speaks against the possibility that they were evoked monosynaptically.
Fig. 4.
Comparison of latencies of the earliest components of EPSP and IPSP evoked in dh DSCT and CC DSCT neurons from gastrocnemius-soleus (GS) and biceps-semotendinosus motor nuclei and in SCT neurons from the intermediate zone or the dorsal horn. A: latencies of the earliest EPSPs and IPSPs evoked in dh DSCT neurons after 50 or 100 μA stimuli applied at the depths corresponding to the location of the gastrocnemius-soleus and biceps-semotendinosus motor nuclei. The 2 longest latency IPSPs (4.93 and 5.76 ms) have not been included. Arrows in A indicate PSPs recorded in 8 dh DSCT neurons after a lesion of the dorsal columns caudal to the recording site. B: latencies of IPSPs evoked by 20–75 μA stimuli from the GS or PBST motor nuclei in CC DSCT neurons. C: latencies of the earliest EPSPs and IPSPs evoked in SCT neurons by stimuli of 50 μA applied in laminae V-VII. Continuous lines separate data for dh DSCT, DSCT located in Clarke's column (CC DSCT), and SCT neurons. Circles and triangles are for EPSPs and IPSPs, respectively. The latencies are ranked from the shortest to the longest for EPSPs in neurons in which both EPSPs and IPSPs were evoked and for IPSPs in neurons in which only IPSPs were found (the 2 sets of data are separated by vertical dotted lines). Latencies below the horizontal dotted line fulfill criteria of latencies of monosynaptically evoked PSPs. Those above are compatible with di- and trisynaptic coupling (see discussion) but might also include longest latencies of monosynaptically evoked PSPs.
The latencies of the great majority of IPSPs were even longer. Only four IPSPs were evoked at latencies that were at, or below, the 1.4-ms borderline, but none of these were evoked by single stimuli. We therefore cannot claim that the same interneurons mediate disynaptic inhibition of motoneurons and of dh DSCT neurons. This is in contrast with IPSPs evoked in VSCT neurons, which could be unequivocally classified as evoked monosynaptically; see examples of IPSPs evoked by the same stimuli that induced only longer latency PSPs in DSCT neurons in Fig. 5, C and D, and the data for the whole population of VSCT neurons analyzed by Jankowska et al. (2010).
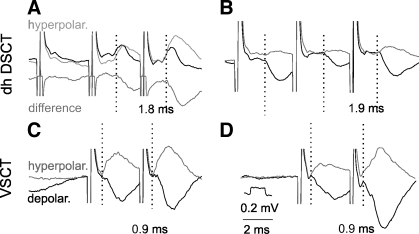
Fig. 5.
Comparison of latencies of IPSPs evoked from motor nuclei in dh DSCT and ventral spinocerebellar tract (VSCT) neurons. Records from 2 DSCT and 2 VSCT neurons recorded in the same experiment to compare synaptic effects evoked by stimuli applied at the same locations within the gray matter (in PBST motor nucleus). The stimuli were 100 μA in A and B and 50 μA in C and D. Black and gray traces are averaged records obtained during depolarization (depolar.) and hyperpolarization of the neurons, respectively (10 and 10 nA in A, 4 and 30 nA in B, 20 and 15 nA in C, and 20 and 20 nA in D). The polarization was used to define points of deviation (indicated by dotted lines) of potentials recorded when the neurons were depolarized and reversed by hyperpolarization and thus the latencies of the IPSPs. The latencies are indicated below the records. Bottom trace in A: difference between the PSPs recorded during the depolarization and the hyperpolarization. Time and voltage calibrations in D are for all records. This procedure revealed an earlier onset of the IPSPs than defined otherwise but never as early as of IPSPs recorded in VSCT neurons recorded in the same experiment, shown for comparison in C and D. Shock artefacts are truncated.
One of the reasons why latencies of IPSPs evoked in dh DSCT neurons were longer than of EPSPs might be that they were overestimated, especially when they were preceded by shorter latency EPSPs. However, even when IPSPs followed EPSPs, as in Fig. 5A, the onset of IPSPs could be made more distinct after the DSCT neurons had been depolarized and/or hyperpolarized, and by using the point of deviation between the original IPSPs and reversed IPSPs for the measurements, as in Fig. 5, A and B.
Effects of stimuli applied in gastrocnemius-soleus and biceps-semitendinosus nuclei did not show marked differences. Both EPSPs and IPSPs were evoked from these nuclei (EPSPs in 20 and 14 of the 33 neurons tested and IPSPs in 27 and 17). In only two neurons none were evoked from these nuclei. Furthermore, comparison of latencies of EPSPs evoked within the range of 0.7–3.0 ms from the gastrocnemius-soleus and biceps-semitendinosus motor nuclei (1.75 ± 0.10 ms and 1.67 ± 0.12 ms, respectively) did not indicate statistically significant differences between them (Student's t-test, P > 0.2). No statistically significantly differences were found between latencies of IPSPs evoked from the gastrocnemius-soleus and biceps-semitendinosus motor nuclei (2.05 ± 0.17 and 2.39 ± 0.21 ms, respectively).
Effects of stimuli applied in motor nuclei on dh DSCT neurons thus appear to differ from those on CC DSCT neurons described by Hongo et al. (1983a) in two respects. One is that both EPSPs and IPSPs were evoked in dh DSCT neurons, whereas only IPSPs were evoked in CC DSCT neurons (see Table 1 and Fig. 4B). Another difference appears to be in the coupling of IPSPs. We have not found sufficient grounds to classify IPSPs evoked in dh DSCT neurons as evoked monosynaptically, whereas IPSPs in CC DSCT neurons were compatible with direct actions of premotor inhibitory interneurons, i.e., evoked monosynaptically. Two series of control experiments described in the following sections were therefore made to account for these differences.
Table 1.
Comparison of proportions of dh DSCT, CC DSCT, and SCT neurons with proportions of VSCT neurons in which PSPs were evoked from motor nuclei
| From Within Motor Nuclei |
|||||
|---|---|---|---|---|---|
| VSCT | dh DSCT | CC DSCT | SCT | From Outside SCT | |
| n | 133 | 33 | 22 | 28 | 20 |
| IPSPs latency ranges (ms) (%) | |||||
| 0.7–3.0 | 88 | 88 | 81 | 0 | 86 |
| 0.7–1.4 | 73 | 3 | 54 | 0 | 3 |
| 1.4–3.0 | 65 | 85 | 23 | 0 | 70 |
| EPSPs latency ranges (ms) (%) | |||||
| 0.7–3.0 | 65 | 64 | 0 | 0 | 55 |
| 0.7–1.4 | 48 | 12 | 0 | 0 | 42 |
| 1.4–3.0 | 43 | 55 | 0 | 0 | 12 |
PSPs evoked by stimuli applied in motor nuclei are grouped depending on the latencies of their earliest components. The range of 0.7–1.4 ms is compatible with monosynaptic coupling, whereas PSPs evoked at 1.4–3.0 ms would include those evoked di- and trisynaptically but possibly also some monosynaptically (see discussion). The data are for PSPs evoked by stimuli ≤50 μA but negative results were verified using stimuli of 100 μA. For details of data on VSCT neurons see Jankowska et al. (2010). VSCT, ventral spinocerebellar tract; DSCT, dorsal spinocerebellar tract dh DSCT, dorsal horn DSCT; CC DSCT, DSCT located in Clarke's column; SCT, spinocervical tract.
Reassessment of disynaptic excitatory input to dh DSCT neurons.
Oligosynaptic actions of group I and II afferents on CC DSCT neurons recorded after transection of the dorsal columns just caudal to Clarke's column were inhibitory, with the exception of EPSPs seen in two neurons recorded in a preparation in which the transection was not complete (Hongo et al. 1983b, Jankowska and Puczynska 2008). They would thus be compatible with exclusive actions of inhibitory interneurons located caudal to the border between the L4 and L5 segments (where dorsal columns were transected) and any excitatory interneurons located more rostrally (see Hongo et al. 1983b).
We therefore examined effects of stimuli applied in motor nuclei in dh DSCT neurons recorded under conditions matching conditions of recording from CC DSCT neurons, i.e., after transection of the dorsal columns. The lesion of the dorsal columns was performed within the most rostral part of the L5 segment; its completeness was verified by checking that no responses were recorded in peripheral nerves to stimuli applied rostral to the lesion. Under these conditions stimuli applied in motor nuclei evoked EPSPs in 4/8 of the dh DSCT neurons, as compared with 17/25 of dh DSCT neurons with dorsal columns intact, i.e., in a not much smaller proportion. They are indicated by arrows in Fig. 4A. Furthermore, records in Fig. 6 show that EPSPs evoked after transection of the dorsal columns appeared at latencies compatible with disynaptic coupling (2.27 ± 0.18 ms), any differences between them and latencies of IPSPs being not statistically significant (P > 0.2). These observations lead thus to the conclusion that any EPSPs evoked in dh DSCT neurons in preparations with dorsal columns intact at latencies <1.4 ms (Fig. 4A) were more likely evoked by ascending collaterals of group II afferents stimulated in the motor nuclei than by excitatory premotor interneurons. They indicate also that at least some disynaptic EPSPs are evoked in dh DSCT neurons by interneurons located in the L5 or more caudal segments that do not act on CC DSCT neurons. They are thus in support of differences in interneuronally mediated excitatory input to dh DSCT and CC DSCT neurons.
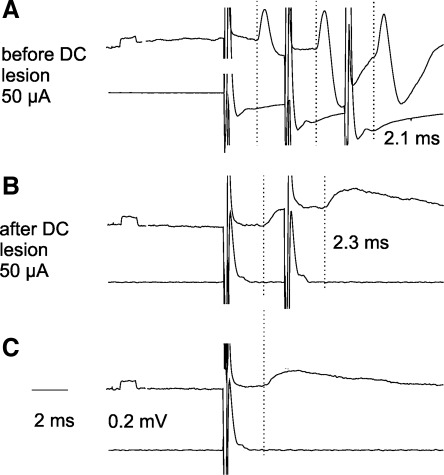
Fig. 6.
Examples of PSPs recorded in dh DSCT neurons before and after transection of the dorsal columns. Upper traces are from 2 dh DSCT neurons, 1 recorded from before (A) and another one after (B and C) the DC lesion in the same experiment. PSPs were evoked in these neurons by triple, double, or single stimuli applied in the GS motor nucleus. Lower traces are from the surface of the spinal cord above the lesion. Averages of 30 records are shown. Dotted lines indicate onset of EPSPs. Note similar latencies of EPSPs evoked before and after transection of the dorsal columns, showing that both were compatible with disynaptic coupling. Note also greater amplitude of EPSPs evoked by the second stimulus in B than by single stimuli in C, i.e., their temporal facilitation. Shock artefacts are truncated.
Reassessment of coupling between inhibitory neurons projecting to motor nuclei and to Clarke's column.
The aim of the second series of control experiments was to verify that IPSPs evoked from motor nuclei in CC DSCT neurons are compatible with monosynaptically evoked IPSPs not only on the basis of previously used arguments (Hongo et al. 1983a) but also with respect to the criterion of lack, or only marginal temporal facilitation. The difference between temporal facilitation of monosynaptically and disynaptically evoked actions of stimuli applied in motor nuclei turned out to be much less reliable than in previous studies (e.g., Jankowska et al. 2003). The reasons were changes in excitability of fibers terminating in motor nuclei following successive stimuli, as described in the appendix. Despite this, temporal facilitation of disynaptically evoked PSPs was expected to exceed that of any monosynaptic actions.
Effects of triple stimuli applied in motor nuclei were analyzed in 22 neurons in Clarke's column. As in the study of Hongo et al. (1983a) only IPSPs were evoked in them. In addition, these IPSPs were most often evoked by single stimuli (in 17/22 neurons). The range of latencies of these IPSPs was also similar (1.02–2.37 ms), and minimal latencies of ≤1.4 ms from the stimuli in the majority of these CC DSCT neurons (Fig. 4B) corresponded to latencies of about 0.9 ms or less from nerve volleys induced by these stimuli. They would therefore be compatible with direct actions of inhibitory interneurons projecting to motor nuclei but not with indirectly induced IPSPs. As illustrated in Fig. 7, A and C, IPSPs evoked at such latencies displayed either moderate facilitation (120–151%) or no temporal facilitation. Furthermore, small increases in peak amplitudes of IPSPs evoked by successive stimuli at ≤1.4-ms latencies would be attributable to increased effectiveness of the stimuli rather than to temporal facilitation of activation of interneurons relying less directly on evoked IPSPs (see appendix). In contrast, increases in peak amplitudes of later components of these IPSPs and of IPSPs evoked at latencies ≥1.4 ms were significantly larger (148–219%), especially after the third stimuli, with an example in Fig. 7, B and D. In addition data points for the shorter and longer latency IPSPs in the whole sample of CC DSCT neurons plotted in Fig. 7E showed clear-cut clustering, and the later components of the early IPSPs and some longer latency IPSPs were evoked only by the second or third stimuli.
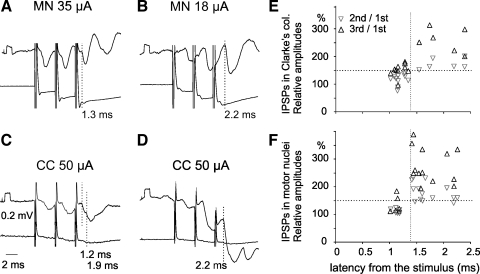
Fig. 7.
Comparison of latencies and amplitudes of IPSPs evoked in Clarke's column DSCT neurons from hindlimb motor nuclei and in motoneurons from the Clarke's column. A and B: records of IPSPs from 2 CC DSCT neurons (upper traces) and from cord dorsum (lower traces) evoked by stimuli applied within gastrocnemius-soleus and biceps-semitendinosus motor nuclei (at depths 2.8 mm and 3.4 mm, respectively). C and D: records of IPSPs evoked in anterior biceps-semimembranosus (C) and posterior biceps-semitendinosus (D) motoneurons by stimulation of Clarke's column. Vertical dotted lines indicate onsets of early components of IPSPs in A (latency 1.3 ms), of early and later components in C (latency 1.2 and 1.9 ms), and of longer latency (2.1 ms) IPSPs in B and D. Note that amplitudes of IPSPs evoked at short latencies were similar after the 3 stimuli, whereas amplitudes of longer latency IPSPs increased considerably. E and F: plots of increases in peak amplitudes of IPSPs (ordinate) evoked in 17 CC DSCT neurons (E) and 20 motoneurons (F) as a function of their latencies (abscissa). Gray and black triangles indicate increases after the second and third stimuli, respectively. Note clustering of data points in the left bottom and right top quadrants separated by dotted lines and that temporal facilitation of IPSPs evoked at latencies ≤1.4 ms was weaker. Differences between mean values of facilitation of short- and long-latency IPSPs (to the left and right of the vertical dotted lines in E and F) were highly statistically significant (P < 0.001, Student's t-test). MN, motor nucleus. Shock artefacts are truncated.
Similar effects were found in 20/24 motoneurons, including gastrocnemius-soleus, biceps, semitendinosus, or semimembranosus motoneurons following triple stimuli applied in Clarke's column, which were expected to activate the rostrally projecting axon collaterals of the same inhibitory interneurons (X in Fig. 1). These are illustrated in Fig. 7, C, D, and F.
Present results are therefore in support of the original conclusion that some premotor interneurons inhibit in parallel both motoneurons and CC DSCT neurons. IPSPs attributable to such interneurons were found in 12/22 CC DSCT neurons and 6/24 motoneurons, or totally in 39% of their total sample, which would be an even higher proportion than that of 21% of neurons in which IPSPs were evoked at latencies not exceeding 1.5 ms in the sample of Hongo et al. (1983a) [see Hongo et al. (1983a) Fig. 7]. Thereby, they also show a marked difference in the coupling of inhibitory interneurons with dh DSCT and CC DSCT neurons reflected in different proportions of neurons with shorter and longer latencies of IPSPs evoked from motor nuclei (see Table 1). Provided that more marked temporal facilitation of longer latency than of shorter latency IPSPs defines the former IPSPs as evoked disynaptically, our observations lead also to the conclusion that IPSPs evoked in a considerable proportion of CC DSCT neurons might be induced disynaptically. This would mean that they are evoked by some afferents or excitatory interneurons that activate inhibitory interneurons, the feature they would share with dh DSCT neurons.
Stimuli applied in gastrocnemius-soleus and biceps-semitendinosus motor nuclei failed to evoke synaptic potentials in any of the 28 SCT neurons tested, even when the intensity of these stimuli was twice that needed to evoke PSPs in dh DSCT neurons recorded in the same experiment, or up to 100 μA, as illustrated in Fig. 2E.
In contrast, stimuli applied dorsal to motor nuclei, in laminae V-VII, evoked PSPs in all but one of the SCT neurons. Both EPSPs and IPSPs were recorded in 14 neurons (with examples in Fig. 8C), only EPSPs in three and only IPSPs in 10. EPSPs were evoked at latencies 1.37 ± 0.08 ms and IPSPs at latencies 1.88 ± 0.06 ms from the onset of the stimuli (Fig. 4C). The differences between latencies of EPSPs and IPSPs were statistically significant (Student's t-test; P < 0.001). Latencies of EPSPs evoked in 13 SCT neurons were 0.9–1.34 ms and, since all these EPSPs were evoked by single stimuli, they fulfilled criteria of monosynaptically induced PSPs. Longer latencies of the remaining EPSPs and of all but one IPSP would on the other hand be consistent with disynaptic coupling. However, as in spinocerebellar neurons, not only the IPSPs and longer latency EPSPs but also the shortest latency EPSPs showed some temporal facilitation (those evoked by the first and second stimuli in Fig. 8C).
Fig. 8.
An example of postsynaptic potentials evoked from outside but not from within motor nuclei in SCT neurons. A: an electrode track along which stimuli were applied while recording from SCT neurons. Upper circle shows the location of an electrolytic lesion made at the depth at which maximal antidromic field potentials were evoked from the gastrocnemius-soleus nerve. Lower circle shows the area from which PSPs were most efficiently evoked. B: records of field potentials evoked at different depths (indicated to the left) by stimulation of gastrocnemius-soleus (GS) and posterior biceps-semitendinosus (PBST) nerves. C: intracellular records from a SCT neuron in which EPSPs and IPSPs were evoked by 100 μA from the depths 2.3–2.8 mm but not from within motor nuclei. Note that the most effective stimuli were those applied at the 2.3–2.4 mm depths, as judged both by the slopes of the rising face of the IPSPs and effects of not only the second but also the first stimuli. Bottom records in B and C are from the surface of the spinal cord. Top record in C is from just outside the neuron. Dotted lines in C indicate onset of EPSPs (latency 1.1–1.2 ms) and IPSPs (latency 1.5–1.6 ms).
DISCUSSION
Direct or indirect input to spinocerebellar neurons via interneurons terminating in motor nuclei.
The results of this study show marked differences in the coupling between premotor interneurons and the two populations of DSCT neurons and even more marked differences between DSCT neurons and SCT neurons, as well as the two populations of VSCT neurons described by Jankowska et al. (2010). However, the interpretation of these results depends to a great extent on the reliability of differentiation between monosynaptically (directly) and disynaptically (via additional interneurons) evoked postsynaptic actions of stimuli applied in motor nuclei. They are differentiated taking into account both their latencies and either the absence or only moderate degree of temporal facilitation of postsynaptic potentials evoked by these stimuli, albeit none of these criteria appear to differentiate in an unequivocal way monosynaptic actions evoked at longer than minimal latencies from disynaptic actions.
By generalization from shortest segmental latencies of disynaptic IPSPs from primary afferents (about 1.1 ms for IPSPs evoked from group I afferents in Q), the earliest disynaptic actions evoked from motor nuclei would require about 1.5 ms from the stimuli including about 1.1 ms from the volleys that themselves are evoked at latencies of 0.4–0.5 ms from the stimuli. Postsynaptic potentials evoked at latencies ≤1.4 ms from the stimuli may thus reasonably safely be classified as evoked monosynaptically. However, the upper limit of latencies of EPSPs evoked monosynaptically by very slowly conducting collaterals of either afferents or interneurons could not be predicted, and they might overlap with the shortest latencies of disynaptically evoked EPSPs. PSPs evoked at latencies >1.4 ms could thus be evoked either mono- or disynaptically. The probability that latencies >1.4 ms included latencies of monosynaptically evoked PSPs would nevertheless be low in populations of neurons in which no unquestionable monosynaptic PSPs were found, or were exceptional, as in the case of IPSPs from motor nuclei in dh DSCT neurons (see Fig. 4A).
The occurrence or lack of temporal facilitation was found to be a very convenient means to differentiate between disynaptic and monosynaptic actions evoked in other neurons (see Jankowska et al. 2003). It was therefore a complicating factor when nerve volleys in fibers stimulated in motor nuclei were found to be larger after the second and third than after the first stimuli. As shown in the appendix, this was in contrast with the same, or even decaying, amplitudes of volleys evoked by successive stimuli applied to either dorsal or ventral roots. There might be several nonexcluding explanations for this finding. One might be longer lasting residual higher excitability of terminal axon collaterals than of myelinated stem axons of primary afferents following stimulation, resulting in a greater number of fibers activated by successive stimuli (see appendix). Another might be the depolarization of terminals of group Ia afferents by GABAergic interneurons forming axo-axonic synapses in motor nuclei by intraspinal stimuli. Whatever the explanation, this phenomenon made the use of temporal facilitation to differentiate between disynaptically and monosynaptically evoked PSPs of limited value, in particular when the PSPs were evoked by the first as well as the second and third stimuli. Temporal facilitation was nevertheless considered as a reasonably strong indication for disynaptic coupling when the PSPs evoked at latencies ≥1.4 ms appeared only after the second or third stimuli (see data points indicated by open symbols in Fig. 4) and when temporal facilitation was prominent. As shown in Fig. 7, E and F, temporal facilitation of longer latency PSPs was very marked, e.g., by 200% or more, whereas that of shorter latency PSPs was as a rule less than 150%.
Facilitation of synaptic transmission in spinal reflex pathways by 4-AP might be due to broadening of action potentials in the stimulated nerve fibers and the resulting increase in the amount of transmitter released from terminals of these fibers, as well as to more direct actions of 4-AP on voltage-activated calcium channels (Qian and Saggau 1999, Wheeler et al. 1996, Wu et al. 2009). Whatever their mechanisms, effects of 4-AP were expected to be more marked on polysynaptic than monosynaptic actions. 4-AP should primarily increase the amplitudes of monosynaptically evoked EPSPs and IPSPs and thereby make them easier to detect. However, effects of 4-AP might be critical for disynaptic excitation or inhibition of these neurons mediated via other neurons, by increasing amplitudes of EPSPs evoked in neurons mediating polysynaptic actions otherwise depressed by anesthesia. Whether the probability of disclosing di- and trisynaptic EPSPs and IPSPs under these conditions approached that in nonanesthetized preparations cannot be estimated.
Differences in input from spinal interneurons to dh DSCT, CC DSCT, and SCT neurons.
The results of this study reveal marked differences between interneuronally relayed input to DSCT neurons located in Clarke's column and in the dorsal horn and to SCT neurons located in the same region as dh DSCT neurons and with similar input from peripheral afferents. The main differences are as follows. First, direct actions of premotor inhibitory interneurons have been found in a high proportion of CC DSCT neurons, extending the original evidence of Hongo et al. 1983a, whereas only occasional short latency IPSPs were evoked from motor nuclei in dh DSCT neurons, indicating that any direct actions of premotor inhibitory interneurons on dh DSCT neurons would be much weaker. Furthermore, previous studies demonstrated that IPSPs evoked in both dh DSCT and CC DSCT neurons are mediated by interneurons mediating reflex actions of group Ib and group II afferents but not by group Ia inhibitory interneurons (Edgley and Jankowska 1988, Hongo et al. 1983a and 1983b, Jankowska and Edgley 2010). The morphology and immunocytochemistry of these interneurons, including their axonal projections and transmitter content, has been recently investigated in detail (Bannatyne et al. 2009, Liu et al. 2010), and the relationships between their subpopulations and subpopulations of genetically identified interneurons are discussed (Jankowska and Edgley 2010). Second, disynaptic excitation following stimuli applied in motor nuclei was evoked in a high proportion of dh DSCT neurons but in none of the CC DSCT neurons. Third, disynaptic inhibition following stimuli applied in motor nuclei was evoked in a higher proportion of dh DSCT than of CC DSCT neurons. Finally, stimuli applied in motor nuclei failed to evoke any effects in SCT neurons.
Because negative results are generally less convincing than positive ones, our failure to reveal directly evoked effects of stimuli applied in motor nuclei in SCT neurons and in the majority of dh DSCT neurons requires some comments. It may therefore be pointed out that when the intensity of such ineffective stimuli was increased to 100 μA they would excite nerve fibers within a radius of about 1 mm (Gustafsson and Jankowska 1976). Because premotor interneurons branch profusely within their terminal projection areas (see Bannatyne et al. 2003, Bannatyne et al. 2009, Bras et al. 1989, Czarkowska et al. 1976, Tkacs and Wurster 1991) and because numerous interneurons contact individual motoneurons, stimuli applied at any of the stimulation sites should excite a considerable proportion of interneurons terminating within the gastrocnemius-soleus and biceps-semitendinosus and the neighboring motor nuclei. Furthermore, even weaker stimuli, 10–50 μA stimuli, applied in these motor nuclei evoked IPSPs fulfilling criteria of monosynaptic IPSPs in a high proportion of CC DSCT neurons (Hongo et al. 1983a; present study) and VSCT neurons (Jankowska et al. 2010).
The most plausible explanation for the differences found in this study is thus that different effects of stimuli applied in motor nuclei do indeed reflect differences in connections between spinal interneurons and the various spinocerebellar neurons. Because CC DSCT neurons are inhibited by interneurons in reflex pathways from both group Ib and II afferents (Hongo et al. 1983b, Jankowska and Puczynska 2008), monosynaptic IPSPs following stimuli applied in gastrocnemius-soleus and biceps-semitendinosus motor nuclei in CC DSCT neurons are in good agreement with parallel collateral actions of these interneurons on motoneurons and CC DSCT neurons. The same interneurons might also mediate disynaptic IPSPs evoked from motor nuclei because input to these interneurons is provided not only from group Ib and II afferents but also from group Ia afferents (for references see Jankowska 1992, Jankowska and Edgley 2010) and both group Ia and II afferents would be stimulated in motor nuclei. The situation would be different in the case of EPSPs and IPSPs in dh DSCT neurons with excitatory and inhibitory input from group II muscle and skin afferents but not from group I afferents (Edgley and Jankowska 1988). They should not be mediated by intermediate zone interneurons coexcited by group I and II afferents that project to motor nuclei (Bannatyne et al. 2006, Bannatyne et al. 2009, Bras et al. 1989, Cavallari et al. 1987, Edgley and Jankowska 1987). However, they could be mediated by dorsal horn interneurons with input from group II and skin afferents (Edgley and Jankowska 1987), some of which have been found to project to motor nuclei (Bannatyne et al. 2006) and might mediate the rare, possibly monosynaptic IPSPs evoked by stimuli applied in these nuclei. Both inhibitory and excitatory dorsal horn interneurons of this population could also be activated by stimulation of group II afferents projecting to motor nuclei and contribute to disynaptic EPSPs as well as IPSPs. It should also be noted that dorsal horn interneurons with input from group II and cutaneous afferents are likely to provide input to not only dh DSCT neurons but also SCT neurons (Hammar et al. 1994, Harrison and Jankowska 1984). The failures of stimuli applied in motor nuclei to evoke any postsynaptic potentials in SCT neurons would therefore indicate that different subpopulations of dorsal horn interneurons provide input to dh DSCT and SCT neurons.
Functional consequences of differences in information provided to spinocerebellar neurons by premotor interneurons.
The reported differences in patterns of actions of spinal interneurons on dh DSCT and CC DSCT neurons add to the previously found differences in the peripheral input to spinocerebellar neurons. In particular, actions of excitatory and inhibitory interneurons on dh DSCT neurons would serve to either increase or decrease the probability of activation of these neurons, whereas the predominant inhibitory actions on CC DSCT neurons would weaken the activation probability. Information forwarded in this way would concern reflex actions mediated by both premotor interneurons (represented by cells X and Y in Fig. 1, A and B; via VSCT and CC DSCT neurons) and interneurons activated by either primary afferents or by premotor interneurons (represented by cells Z in Fig. 1B; via dh DSCT neurons, albeit also VSCT and CC DSCT neurons). However, the modulation of activity of spinocerebellar neurons might also be used as an indicator of the likely spinal effects of descending commands, as discussed by Jankowska et al. (2010) and Hantman and Jessell (2010), and VSCT, CC DSCT, and dh DSCT might be specialized in forwarding different aspects of such information to the cerebellum. Since the most potent actions of premotor interneurons on VSCT and CC DSCT neurons are inhibitory, relatively frequent excitation of dh DSCT might be of particular interest in this context.
The different effects of intraspinal stimuli on SCT and spinocerebellar neurons show in addition that information on modulatory actions of premotor interneurons must be much more essential for neurons in the cerebellum than for thalamic target cells of SCT neurons, despite the similar information forwarded by SCT and dh DSCT neurons on peripheral events (Edgley and Jankowska 1988, Hammar et al. 1994). In this context the existence of some highly specific cerebellar projection areas of CC DSCT neurons, dh DSCT neurons, and the spinal border cell subpopulation of VSCT neurons (Matsushita and Ikeda 1980, Matsushita and Yaginuma 1989) is particularly striking and suggests that distinct cerebellar neurons process information provided by these neurons.
GRANTS
The study was supported by grants from NINDS/NIH (R01 NS040863) and from the Swedish Research Council (15393-01).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
ACKNOWLEDGMENTS
We thank Rauni Larsson for invaluable assistance and I. Hammar for comments. Present address of S. Jelen: Institute of Anatomy, Åarhus University, 8000 & 197, Arhus C, Denmark.
APPENDIX
Two mechanisms of temporal facilitation of effects of a train of stimuli applied in motor nuclei.
Records of nerve volleys following stimuli applied in motor nuclei, such as in Fig. 7A or Fig. 8C, suggested that their amplitudes were larger after the second and third stimuli than after the first stimuli. If this were the case, a certain increase in PSPs evoked by successive stimuli could be due to an increase in the number of stimulated fibers rather than to temporal facilitation of transmission between these fibers and their target cells and more effective activation of these cells. Implications of temporal facilitation following stimuli applied in motor nuclei would thus become less straight forward than in other cases where they provide strong indications of indirect synaptic actions.
It is well known that electrical stimuli applied to peripheral nerves or to axons of central neurons may result in both decrease and increase in their excitability (see Gardner-Medwin 1972, Kress and Mennerick 2009, Swadlow and Waxman 1976, Tkacs and Wurster 1991, Waxman 2000, Waxman and Swadlow 1977, Wigstrom and Gustafsson 1981). A decrease in excitability occurs during the refractory period following activation of the fibers but also after a long-lasting (e.g., for 10 min) repetitive stimulation with gradual recovery to control levels over 15–20 min (Kiernan et al. 2004). However, tetanic stimulation may also evoke a postactivation hyperexcitability related to K+ accumulation in the restricted diffusion space under the myelin sheath (Kiernan et al. 1997) or to other mechanisms (Kress and Mennerick 2009, Wigstrom and Gustafsson 1981). In axon terminals and nonmyelinated fibers, albeit usually not in preterminal nerve branches, such changes were found even after single volleys (see Tkacs and Wurster 1991, Zucker 1974). However, in some preparations a pronounced supernormal period following a single conditioning volley was also seen in preterminal fibers (nonmyelinated parallel fibers of the cerebellar cortex) (Malenka et al. 1983).
Since fibers stimulated in motor nuclei under our experimental conditions would include terminal branches of fibers targeting motoneurons, it was conceivable that their excitability could likewise be increased by single stimuli and result in an increase in the number of fibers reaching threshold following successive stimuli. This possibility could not be tested on terminals of interneurons projecting to motor nuclei, but it was verified on muscle afferents. To this end we compared amplitudes of nerve volleys induced in a muscle nerve following stimuli at the same parameters as those used to test their effects on spinocerebellar neurons.
In four experiments in which this was done, nerve volleys in the gastrocnemius-soleus nerve were significantly larger when they were evoked by the second and third than after the first stimuli. As illustrated in Fig. 9A, this was more marked when the stimuli were applied within the dorsal or ventral parts of the nucleus, i.e., where the density of the terminals would be lower, than within its center. This marked amplitude increase stands in contrast with effects of stimuli applied to distal stumps of either dorsal or ventral roots (Fig. 9, D, E, I, and J) or to effects of stimuli applied within descending tracts (medullary pyramid or the medial longitudinal fascicle in previous studies), where much smaller increases in volleys following the second and third stimuli occurred, and less frequently. They are seen, e.g., in Fig. 2 in Jankowska et al. (2003), Fig. 3 in Matsuyama and Jankowska (2004), Fig. 2, E and F, in Stecina et al. (2008), and Fig. 5 in Landgren et al. (1962) but not in Fig. 1 in Edgley et al. (1997), Fig. 1 in Krutki et al. (2003), or Fig. 3 in Edgley et al. (2004).
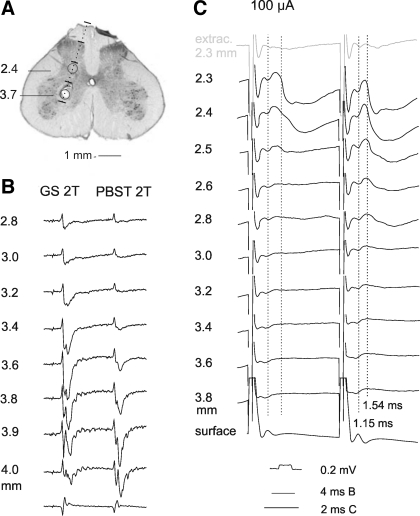
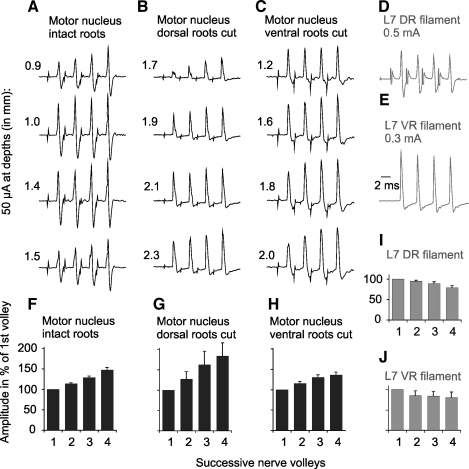
Fig. 9.
Comparison of nerve volleys evoked by stimuli applied in motor nuclei (incrementing) and by stimulation of dorsal or ventral root fibers (decrementing). All records are from the gastrocnemius-soleus muscle nerve (averages of 10 or 20 single records) A: examples of nerve volleys evoked by 50 μA stimuli applied along electrode tracks crossing gastrocnemius-soleus and biceps-semitendinosus motor nuclei in which maximal antidromic field potentials from the gastrocnemius-soleus nerve were evoked at 1.3 mm depth, in preparation with both ventral and dorsal roots intact. B and C: as in A but in preparations in which dorsal (B) or ventral (C) L7 and S1 roots were transected. Maximal antidromic field potentials from the gastrocnemius-soleus nerve were evoked in them at depths 1.9 and 1.8 mm, respectively. D and E: nerve volleys evoked by stimulation of the L7 dorsal and ventral roots, respectively. F–J: relative changes in peak amplitudes of nerve volleys illustrated in A–E (mean values ± SE). Shock artefacts in D are truncated.
However, the excitability of terminals of primary afferents could be increased not only by the above mentioned mechanisms but also by their depolarization by GABAergic interneurons, such interneurons being likely activated by intraspinal stimuli. To estimate effects of primary afferent depolarization on volleys evoked from the motor nuclei we have therefore compared effects of intraspinal stimuli in preparations in which volleys in afferent fibers were eliminated by transection of the L7 and S1 dorsal roots (Fig. 9B), and in preparations in which the dorsal roots remained intact but the L7 and S1 ventral roots were transected (Fig. 9C). Because similar increases in nerve volleys recorded from the gastrocnemius-soleus nerve were found in these preparations, the contribution of the primary afferent depolarization to effects of successive stimuli did not appear to be significant.
Increases in amplitudes of volleys evoked in the GS nerve by the second, third, and fourth stimuli applied in motor nuclei varied between 102% and 137%, 102% and 166%, or 103% and 201%, with respect to those evoked by the first stimuli (Fig. 9, F–H). If terminals of interneurons (represented by interneurons X and Y in Fig. 1) were affected in the same way, increases in the numbers of these terminals would explain increasing amplitudes of monosynaptic IPSPs evoked in CC DSCT neurons. This would justify the classification as evoked monosynaptically of all IPSPs evoked at latencies compatible with direct actions of premotor inhibitory interneurons (at ≤1.4 m from the stimuli; see above) despite increases in amplitude after successive stimuli. However, accrued effects of successive stimuli would be expected to have an even greater impact on synaptic activation of other interneurons (represented by interneurons Z in Fig. 1) and on disynaptically evoked excitation and/or inhibition of DSCT neurons on the basis of both temporal and spacial facilitation of synaptic transmission between the stimulated fibers and these neurons. They would therefore explain the much more marked temporal facilitation of PSPs evoked at longer latencies, and therefore be more compatible with a disynaptic coupling, illustrated in Fig. 7, E and F. However, because temporal facilitation of effects of successive stimuli applied in motor nuclei would depend on increases in the excitability of fibers stimulated as well as more efficient synaptic actions of these fibers on their target cells, this would restrict the value of temporal facilitation as a means to differentiate between longer latency monosynaptically and disynaptically evoked effects of stimuli applied in motor nuclei.
We report these observations as a separate appendix because they are relevant not only for the present study but also for the interpretation of effects of tetanic stimulation in other central structures, including effects of intracortical stimuli, or of stimuli applied in various subcortical nuclei.
REFERENCES
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res 151: 493–506, 1978a [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by descending tracts during scratching in the cat. I. Activity of vestibulospinal neurons. Brain Res 159: 99–110, 1978b [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Orlovsky GN, Pavlova GA, Perret C. Messages conveyed by descending tracts during scratching in the cat. II. Activity of rubrospinal neurons. Brain Res 159: 111–123, 1978c [DOI] [PubMed] [Google Scholar]
- Arshavsky YI, Berkinblit MB, Fukson OI, Gelfand IM, Orlovsky GN. Origin of modulation in neurones of the ventral spinocerebellar tract during locomotion. Brain Res 43: 276–279, 1972 [DOI] [PubMed] [Google Scholar]
- Asif M, Edgley SA. Projections of group II-activated midlumbar spinocerebellar tract neurones to the region of nucleus Z in the cat. J Physiol (Lond) 448: 565–578, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Networks of inhibitory and excitatory commissural interneurons mediating crossed reticulospinal actions. Eur J Neurosci 18: 2273–2284, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Edgley SA, Hammar I, Jankowska E, Maxwell DJ. Differential projections of excitatory and inhibitory dorsal horn interneurons relaying information from group II muscle afferents in the cat spinal cord. J Neurosci 26: 2871–2880, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne BA, Liu TT, Hammar I, Stecina K, Jankowska E, Maxwell DJ. Excitatory and inhibitory intermediate zone interneurons in pathways from feline group I and II afferents: differences in axonal projections and input. J Physiol (Lond) 587: 379–399, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco G, Poppele RE. Proprioception from a spinocerebellar perspective. Physiol Rev 81: 539–568, 2001 [DOI] [PubMed] [Google Scholar]
- Bras H, Cavallari P, Jankowska E, Kubin L. Morphology of midlumbar interneurones relaying information from group II muscle afferents in the cat spinal cord. J Comp Neurol 290: 1–15, 1989 [DOI] [PubMed] [Google Scholar]
- Brown AG. Organization in the Spinal Cord: The Anatomy and Physiology of Identified Neurones. Berlin: Springer, 1981a [Google Scholar]
- Brown AG. The spinocervical tract. Prog Neurobiol 17: 59–96, 1981b [DOI] [PubMed] [Google Scholar]
- Burke R, Lundberg A, Weight F. Spinal border cell origin of the ventral spinocerebellar tract. Exp Brain Res 12: 283–294, 1971 [DOI] [PubMed] [Google Scholar]
- Cavallari P, Edgley SA, Jankowska E. Post-synaptic actions of midlumbar interneurones on motoneurones of hind-limb muscles in the cat. J Physiol (Lond) 389: 675–689, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarkowska J, Jankowska E, Sybirska E. Axonal projections of spinal interneurones excited by group I afferents in the cat, revealed by intracellular staining with horseradish peroxidase. Brain Res 118: 115–118, 1976 [DOI] [PubMed] [Google Scholar]
- Downie JW, Ferrington DG, Sorkin LS, Willis WD., Jr The primate spinocervicothalamic pathway: responses of cells of the lateral cervical nucleus and spinocervical tract to innocuous and noxious stimuli. J Neurophysiol 59: 861–885, 1988 [DOI] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. An interneuronal relay for group I and II muscle afferents in the midlumbar segments of the cat spinal cord. J Physiol (Lond) 389: 647–674, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E. Information processed by dorsal horn spinocerebellar tract neurones in the cat. J Physiol (Lond) 397: 81–97, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Gallimore CM. The morphology and projections of dorsal horn spinocerebellar tract neurones in the cat. J Physiol (Lond) 397: 99–111, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Hammar I. Ipsilateral actions of feline corticospinal tract neurons on limb motoneurons. J Neurosci 24: 7804–7813, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Eyre JA, Lemon RN, Miller S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain 120: 839–853, 1997 [DOI] [PubMed] [Google Scholar]
- Fuller JH, Schlag JD. Determination of antidromic excitation by the collision test: problems of interpretation. Brain Res 112: 283–298, 1976 [DOI] [PubMed] [Google Scholar]
- Gardner-Medwin AR. An extreme supernormal period in cerebellar parallel fibres. J Physiol (Lond) 222: 357–371, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Jankowska E. Direct and indirect activation of nerve cells by electrical pulses applied extracellularly. J Physiol (Lond) 258: 33–61, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammar I, Lackberg ZS, Jankowska E. New observations on input to spino-cervical tract neurons from muscle afferents. Exp Brain Res 100: 1–6, 1994 [DOI] [PubMed] [Google Scholar]
- Hammar I, Krutki P, Drzymala-Celichowska H, Nilsson E, Jankowska E. A trans-spinal loop between neurones in the reticular formation and in the cerebellum. J Physiol (Lond) 589: 653–665, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantman AW, Jessell TM. Clarke's column neurons as the focus of a corticospinal corollary circuit. Nat Neurosci 13: 1233–1239, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E. An intracellular study of descending and non-cutaneous afferent input to spinocervical tract neurones in the cat. J Physiol (Lond) 356: 245–261, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Muscle Afferents and Spinal Control of Movement, edited by Jami L, Pierrot-Deseilligny E, Zytnicki D. Oxford, New York, Seoul, Tokyo: Pergamon Press, 1992, pp. 389–394 [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. The same interneurones mediate inhibition of dorsal spinocerebellar tract cells and lumbar motoneurones in the cat. J Physiol (Lond) 342: 161–180, 1983a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Ohno T, Sasaki S, Yamashita M, Yoshida K. Inhibition of dorsal spinocerebellar tract cells by interneurones in upper and lower lumbar segments in the cat. J Physiol (London) 342: 145–159, 1983b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78: 272–303, 2006 [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progr Neurobiol 38: 335–378, 1992 [DOI] [PubMed] [Google Scholar]
- Jankowska E, Puczynska A. Interneuronal activity in reflex pathways from group II muscle afferents is monitored by dorsal spinocerebellar tract neurons in the cat. J Neurosci 28: 3615–3622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Edgley SA. Functional subdivision of feline spinal interneurons in reflex pathways from group Ib and II muscle afferents; an update. Eur J Neurosci 32: 881–893, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Krutki P, Hammar I. Collateral actions of premotor interneurones on ventral spinocerebellar tract neurones in the cat. J Neurophysiol 104: 1872–1883, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation upon feline hindlimb motoneurons. J Neurosci 23: 1867–1878, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Lin CS, Burke D. Differences in activity-dependent hyperpolarization in human sensory and motor axons. J Physiol (Lond) 558: 341–349, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Hales JP, Gracies JM, Mogyoros I, Burke D. Paraesthesiae induced by prolonged high frequency stimulation of human cutaneous afferents. J Physiol (Lond) 501: 461–471, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress GJ, Mennerick S. Action potential initiation and propagation: upstream influences on neurotransmission. Neuroscience 158: 211–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krutki P, Jankowska E, Edgley SA. Are crossed actions of reticulospinal and vestibulospinal neurons on feline motoneurons mediated by the same or separate commissural neurons? J Neurosci 23: 8041–8050, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgren S, Phillips CG, Porter R. Minimal synaptic actions of pyramidal impulses on some alpha motoneurones of the baboon's hand and forearm. J Physiol (Lond) 161: 91–111, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindström S, Schomburg ED. Group I inhibition in Ib excited ventral spinocerebellar tract neurones. Acta Physiol Scand 90: 166–185, 1974 [DOI] [PubMed] [Google Scholar]
- Lindström S. Recurrent control from motor axon collaterals of Ia inhibitory pathways in the spinal cord of the cat. Acta Physiol Scand 89, Suppl 392: 1–43, 1973 [PubMed] [Google Scholar]
- Lipski J. Antidromic activation of neurones as an analytic tool in the study of the central nervous system. J Neurosci Methods 4: 1–32, 1981 [DOI] [PubMed] [Google Scholar]
- Liu TT, Bannatyne BA, Jankowska E, Maxwell DJ. Properties of axon terminals contacting intermediate zone excitatory and inhibitory premotor interneurons with monosynaptic input from group I and II muscle afferents. J Physiol (Lond) 588: 4217–4233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Exp Brain Res 12: 317–330, 1971 [DOI] [PubMed] [Google Scholar]
- Lundberg A, Weight F. Signalling of reciprocal Ia inhibition by the ventral spinocerebellar tract. Brain Research 23: 109–111, 1970 [DOI] [PubMed] [Google Scholar]
- Lundberg A, Weight F. Functional organization of connexions to the ventral spinocerebellar tract. Exp Brain Res 12: 295–316, 1971 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kocsis JD, Waxman SG. The supernormal period of the cerebellar parallel fibers effects of Ca++ and K+. Pflügers Arch 397: 176–183, 1983 [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M. Spinocerebellar projections to the vermis of the posterior lobe and the paramedian lobule in the cat, as studied by retrograde transport of horseradish peroxidase. J Comp Neurol 192: 143–162, 1980 [DOI] [PubMed] [Google Scholar]
- Matsushita M, Yaginuma H. Spinocerebellar projections from spinal border cells in the cat as studied by anterograde transport of wheat germ agglutinin-horseradish peroxidase. J Comp Neurol 288: 19–38, 1989 [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Jankowska E. Coupling between feline cerebellum (fastigial neurons) and motoneurons innervating hindlimb muscles. J Neurophysiol 91: 1183–1192, 2004 [DOI] [PubMed] [Google Scholar]
- Maunz RA, Pitts NG, Peterson BW. Cat spinoreticular neurons: locations, responses and changes in responses during repetitive stimulation. Brain Res 148: 365–379, 1978 [DOI] [PubMed] [Google Scholar]
- Menetrey D, Chaouch A, Besson JM. Location and properties of dorsal horn neurons at origin of spinoreticular tract in lumbar enlargement of the rat. J Neurophysiol 44: 862–877, 1980 [DOI] [PubMed] [Google Scholar]
- Oscarsson O. Functional organization of spinocerebellar paths. In: Handbook of Sensory Physiology, edited by Iggo I. Berlin: Springer Verlag, 1973, pp. 339– 862–380 [Google Scholar]
- Oscarsson O, Sjolund B. The ventral spino-olivocerebellar system in the cat. III. Functional characteristics of the five paths. Exp Brain Res 28: 505–520, 1977 [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Activity-dependent modulation of K+ currents at presynaptic terminals of mammalian central synapses. J Physiol (Lond) 519: 427–437, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer EK, Watt DG, Taylor A, Reinking RM, Stuart DG. Analysis of muscle receptor connections by spike-triggered averaging. 2. Spindle group II afferents. J Neurophysiol 39: 1393–1402, 1976 [DOI] [PubMed] [Google Scholar]
- Stecina K, Slawinska U, Jankowska E. Ipsilateral actions from the feline red nucleus on hindlimb motoneurones. J Physiol (Lond) 586: 5865–5884, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swadlow HA, Waxman SG. Variations in conduction velocity and excitability following single and multiple impulses of visual callosal axons in the rabbit. Exp Neurol 53: 128–150, 1976 [DOI] [PubMed] [Google Scholar]
- Tkacs NC, Wurster RD. Strength-duration and activity-dependent excitability properties of frog afferent axons and their intraspinal projections. J Neurophysiol 65: 468–476, 1991 [DOI] [PubMed] [Google Scholar]
- Waxman SG. The neuron as a dynamic electrogenic machine: modulation of sodium-channel expression as a basis for functional plasticity in neurons. Philos Trans R Soc Lond B Biol Sci 355: 199–213, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman SG, Swadlow HA. The conduction properties of axons in central white matter. Prog Neurobiol 8: 297–324, 1977 [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci 16: 2226–2237, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Increased excitability of hippocampal unmyelinated fibres following conditioning stimulation. Brain Res 229: 507–513, 1981 [DOI] [PubMed] [Google Scholar]
- Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14: 2–31, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZZ, Li DP, Chen SR, Pan HL. Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit. J Biol Chem 284: 36453–36461, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Excitability changes in crayfish motor neurone terminals. J Physiol 241: 111–126, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]