Abstract
While progress in conventional treatments is making steady and incremental gains to reduce mortality associated with heart failure, there remains a need to explore potentially new therapeutic approaches. Heart failure induced by different etiologies such as coronary artery disease, hypertension, diabetes, infection, or inflammation results generally in calcium cycling dysregulation at the myocyte level. Recent advances in understanding of the molecular basis of these calcium cycling abnormalities, together with the evolution of increasingly efficient gene transfer technology, has placed heart failure within reach of gene-based therapy. Furthermore, the recent successful completion of a Phase 2 trial targeting the sarcoplasmic reticulum calcium pump (SERCA2a) ushers in a new era for gene therapy for the treatment of heart failure.
Keywords: Calcium, Heart failure, Sarcoplasmic Reticulum, SERCA2a
Introduction
End-stage congestive heart failure (CHF) has a poor prognosis, and therapeutic choices are still limited. Congestive heart failure (CHF) is a clinical syndrome in which the heart is unable to pump enough blood to meet the metabolic demands of various organs in the body. CHF is a leading cause of death and morbidity in modern societies and its incidence continues to increase especially as the population ages [1]. Recent treatments for CHF, have focused on blocking neurohormonal pathways and have resulted in an increase in survival, however, these therapeutic approaches do not completely prevent the progression of CHF. This has resulted in many investigators exploring molecular targets that can improve excitation–contraction (E–C) coupling which is deficient in CHF [2, 3].
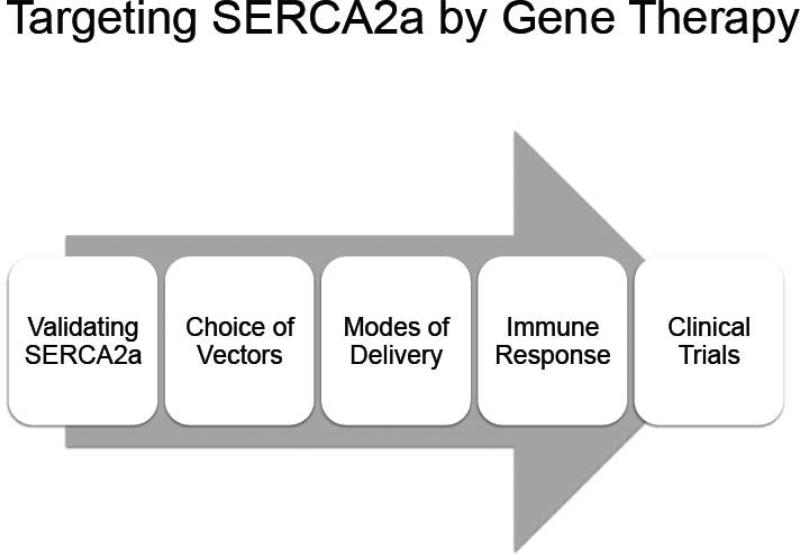
In this review, we will describe how our group has targeted a specific pathway in congestive heart failure, namely the impaired re-uptake of Ca2+ by the SR which is mainly caused by low expression of cardiac Sarco/endoplasmic reticulum Ca2+ ATPase (SERCA2a) pump and also its dysfunction using gene therapy. As shown in Figure 1, we will review the validation of the target, the choice of vectors used, the immune response and to AAV vectors, the delivery method and pre-clinical data obtained in large animals, and finally clinical trials using gene therapy approaches.
Figure 1.
Various steps from target validation to clinical trials in gene therapy
Excitation-Contraction Coupling
Each cell type expresses a unique set of Ca2+-regulatory components that modulate and regulates intracellular Ca2+-signaling yielding different spatial and temporal properties depending on the cell type [4]. Ca2+ is a highly versatile intracellular signaling molecule that regulates many different cellular processes, from fertilization to cell death. At any moment in time, the level of intracellular Ca2+ is tightly regulated by a balance between the release of Ca2+ into the cytosol and the removal of Ca2+ by the combined action of buffers, pumps and ion channels. Figure 2 represents the various components of SR Ca2+ cycling in the heart. During excitation-contraction (EC) coupling, Ca2+ entry through the L-type Ca2+ channels (LTCC) triggers Ca2+ release from the SR through ryanodine receptors (i.e. RyR2). The combination of Ca2+ influx and release raises free intracellular Ca2+ concentration from a resting or diastolic calcium concentration in the nanomolar range to the micromolar range thereby allowing Ca2+ to associate to troponin C a myofilament protein that is part of the troponin complex resulting in sarcomere shortening and muscle contraction. Subsequent muscle relaxation is initiated by RyR2 closure accompanied by Ca2+ dissociation from the troponin complex. Ca2+ reuptake into the SR is catalyzed by SERCA2a. Calcium is also partially extruded to the external medium through the action of plasma-membrane Ca2+ ATPase (PMCA). In humans, SERCA2a removes 70% of Ca2+ and the rest is removed by the Na2+/Ca2+ exchanger (NCX, 28%) and PMCA (2%) [5]. The Na2+/Ca2+ has low affinity but high capacity whereas the PMCA has high affinity and low capacity. Several protein kinases and phosphosphatases are also associated with SR Ca2+ cycling such as protein kinase A (PKA), Ca2+/calmodulin kinase (CaMKII), and protein phosphatase type 1 (PP1).
Figure 2. Excitation-Contraction Coupling.
Solid lines indicate the Ca2+ cycling in the cardiomyocyte. Upon a depolarizing signal, extracellular Ca2+ enters the cytosol via the LTTC, which triggers induces the release of a greater amount of Ca2+ through the RYR2 which initiates contraction at the myofilaments. Removal of Ca2+ during diastole is primarily facilitated via SERCA2a and to a lesser extent by the NCX, and PMCA. Increase the cytosolic Ca2+ concentration activates CaMKII. CaMKII phosphorylates the LTTC, RyR2, and PLN; Long dash dot lines. Dash lines delineate β-adrenergic stimulation, AC is activated, which leads to production of cAMP and PKA activation. PKA then phosphorylates the RyR2, TnI, and PLN, which augments contractility. Also, PKA phosphorylates I-1, and then I-1 activate PP1, which cause hypophosphorylation of PLN. AC, adenylyl cyclase; β-AR, β-adrenergic receptor; CASQ, calsequestrin; CaMKII, Ca2+/calmodulin-denpendent protein kinase; GαS, GTP binding protein; HRC, histidin-rich Ca2+ binding protein; I-1, inhibitor-1; LTTC, L-type Ca2+ channel; NCX, Na+/Ca2+ exchanger; PKA, protein kinase A; PLN, phospholamban; PMCA, plasma-membrane Ca2+-ATPase; RyR2: ryanodine receptor 2; SERCA2a, SR Ca+-ATPase; TnI: troponin I.
First Report of Calcium Cycling in Experimental and Human Heart Failure
In the early 1980s the bioluminescent indicator aequorin was used in multicellular preparations[6]. Intracellular Ca2+ transients were recorded with aequorin during isometric contraction of myocardium from ferrets with pressure overload hypertrophy and from patients with end-stage heart failure by Gwathmey and colleagues[7, 8]. In contrast to controls, contractions and Ca2+ transients of muscles from failing hearts were markedly prolonged, and the Ca2+ transients in human heart failure exhibited 2 distinct components. Muscles from failing hearts showed a diminished capacity to restore low resting Ca2+ levels during diastole. These experiments provide the first direct evidence from actively contracting human myocardium that intracellular Ca2+ handling is abnormal and may cause systolic and diastolic dysfunction in heart failure. [8, 9] [10]. In single cardiomyocytes isolated from left ventricular myocardium of patients with cardiac failure caused by dilated or ischemic cardiomyopathy undergoing transplantation, there were three distinct changes reported by Beuckelmann et al: 1) a decrease in systolic calcium released, 2) a significant prolongation of calcium and 3) an increase in diastolic calcium [11]. These abnormalities in intracellular calcium signaling pointed towards abnormalities in SR function but also to electrical remodeling in human heart failure. [12]
SR Ca2+ handling proteins in Heart Failure
Defective intracellular Ca2+ homeostasis through alterations of Ca2+ handling proteins has been investigated in both experimental and human HF. A slowed relaxation rate or impaired diastolic function in HF is associated with a a slower rate of decay of Ca2+ transient and increased diastolic intracellular Ca2+ concentration and diastolic stiffness [13]. These defects have been linked to at least three factors: (i) decreased Ca2+ re-uptake into the SR, (ii) decreased Ca2+ storage of the SR, and (iii) Ca2+ leak from the SR. Recently several proteins have been reported as major regulators.
SR Ca2+ load
SR Ca2+ uptake is mainly regulated by the SERCA2a Ca2+ pump. SERCA2a activity is increased via phospholamban (PLN) phosphorylation by reducing its affinity for Ca2+. In mammals, three different genes (ATP2A1-3) encode at least two different SERCA protein isoforms. SERCA2 is the predominant variant of all SERCA isoforms and phylogenetically the oldest. Three different splice transcripts are reported, SERCA2a, SERCA2b, and SERCA2c, which differ at the C-terminus. SERCA2a is mainly expressed in the heart.Low amounts of SERCA2b [14] and possibly even lower amounts of SERCA2c have also been detected. Functional differences between the isoforms SERCA2a and SERCA2b have been demonstrated in studies of transgenic animals. Long-term expression of SERCA2a in transgenic animals improves contractility without harmful effects, but substitution of SERCA2a with the SERCA2b isoform causes cardiac dysfunction and hypertrophy [15, 16]. A number of studies collectively have shown significantly reduced SERCA2a expression and activity in failing human and animal myocardium. Several studies in samples obtained from failing human hearts received at the time of cardiac transplanation suggest that SR Ca2+ transport function is altered in end-stage human heart failure. A decrease in the level of SERCA2a pump (mRNA, protein, or activity) has been strongly correlated with decreased myocardial function and impaired force-frequency responses [17, 18]. Impaired re-uptake of Ca2+ into the SR, reflected by a reduced SERCA2a protein level, would at least partially explain the lower SR Ca2+ concentration, elevated diastolic calcium concentration, and decreased intracellular Ca2+ transients at higher frequencies of contraction.
As an endogenous regulator of SERCA2a, PLN controls the affinity of SERCA2a for Ca2+. With an unchanged PLN level in HF, the PLN/SERCA2a ratio increases, diminishing Ca2+ affinity of SERCA2a. Moreover, decreased PLN phosphorylation by PKA and CaMKII at Ser16 and Thr17, respectively, which relieves SERCA2a inhibition is a further complicating factor that would contribute to the impaired cardiac contractility of the failing heart [19, 20]. Dephosphorylation of PLN is also controlled by protein phosphatase 1 (PP1) activity which is increased in HF. However, PP1 is itself inhibited by its endogenous modulator, inhibitor-1 (I-1) which can be activated by PKA and inhibited by PKC [21].
SR Ca2+ store
During a regular Ca2+ transient only 40~60% of the total SR Ca2+ content is released thereby leaving a sizable Ca2+ reserve within the SR. This implies that a number of mechanisms are activated to terminate the Ca2+ release process before the SR Ca2+ store is emptied.
Calsequestrin (CASQ), high-capacity Ca2+ binding protein in the SR lumen, has been shown to play a major role as a SR Ca2+ storage protein which was bound to approximately 50~75% of the Ca2+ in the SR lumen. Changes in calsequestrin levels have been shown to be associated with cardiac remodeling and ventricular arrhythmias in rodent models [22].
Another SR luminal protein, histidine rich Ca2+ binding protein (HRC) has been reported to be another important SR Ca2+ storage protein, which binds to SERCA2a and triadin. Decreased HRC levels have been detected in both HF patients and animal model of HF [23].
SR Ca2+ release
RyR2 channels are part of a massive macromolecular signaling complex, which play a crucial role in excitation-contraction coupling in the heart. During diastolic phase, the resting state of the cardiac cycle, RyR2 channels must lock tightly otherwise Ca2+ will leak uncontrollably into the cytosol from the SR. Thus, opening and closing of the tetrameric channel requires strict regulation by several inhibitory and activating accessory proteins. Recently, the phosphorylation state of RyR2 has been considered as the major trigger factor. In HF, PKA-mediated hyperphoshporylation of RyR2 at S2809 has been suggested to induce diastolic release of Ca2+ through dissociation of the channel-stabilizing protein, FKBP12.6 [24]. More recently, however other groups have shown that PKA-mediated hyperphosphorylation of RyR-S2809 plays no role in SR Ca2+ leak [25]. Currently, CAMKII phosphorylation of RYR2 has emerged as an important modulator of the channel function in cardiovascular diseases. [26]. Another protein of interest is S100A1 which binds to the RyR2 and increases its open probability during systole while open probability of the RyR2 is reduced due to S100A1 at diastolic cytosolic Ca2+ concentrations. In addition, S100A1 enhances SERCA2a activity. In HF. S100A1 is decreased and its rescue has been shown to improve contractile function in heart failure[27, 28].
Therapeutic targets for HF
Restoring proper SR Ca2+ mobilization has been the focus of sustained investigations for innovative therapeutic approaches in the past few years to rescue HF. Among the major SR proteins that have been investigated, altering SERCA2a and/or PLN levels or activity to restore disturbed Ca2+ uptake into the SR and preventing SR Ca2+ leak have been proposed as a promising therapeutic strategies for HF.
SERCA2a restoration
Transgenic animal models have been developed to define the role of the SERCA pump in Ca2+ homeostasis and cardiac physiology. Transgenic (TG) mice overexpressing SERCA2a by 1.5-fold resulted in increased SR Ca2+ uptake and release and enhanced myocardial contractility [29, 30]. No cardiac pathology has been observed in these animals, suggesting that SERCA2a overexpression (albeit at low levels) can be tolerated by the heart. On the other hand, disruption of the SERCA2 allele is lethal.Homozygous null (SERCA2−/−) mice die early in development. Heterozygous (SERCA2+/−) mice showed 65% of SERCA2 protein levels compared with wild type hearts. Although no cardiac pathology was exhibited, when stressed by pressure overload, they developed heart failure much more quickly than WT control mice [31]. In addition, these mice showed impaired intracellular Ca2+ homeostasis and decreased rates of myocardial contractility [31, 32]. Thus, these studies employing TG and gene knockout animal models showed that SERCA pump level is a critical determinant of SR function and changes in its level can modify contractility.
The search for SERCA2a modulators by pharmacological means has not yielded so far any specific or non-toxic agents. This has led to the consideration of gene therapy as a method to specifically enhance SERCA2a activity. Even though a number of vectors were used to deliver SERCA2a gene to the heart, recombinant adeno-associated vectors have proven to have the essential features for long term expression.
Ancillary effects of SERCA2a overexpression
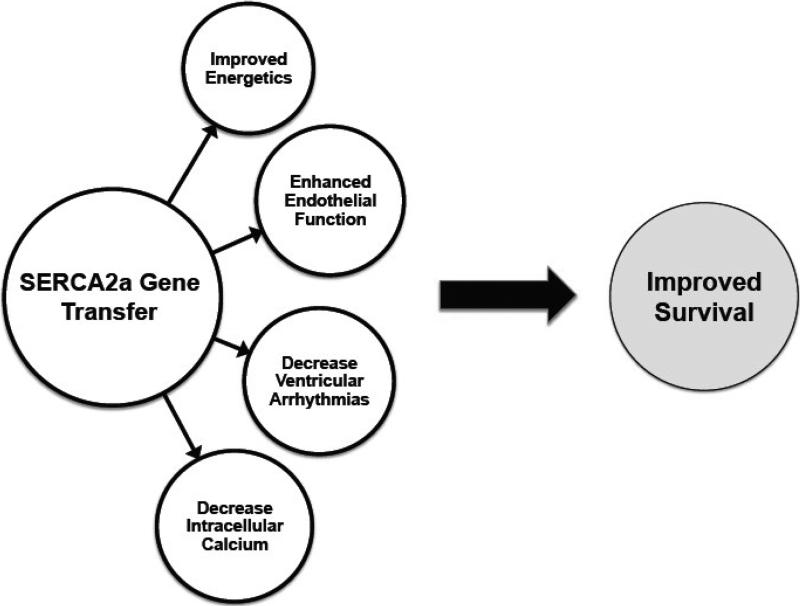
Beyond its effects on myocardial contractility, SERCA2a overexpression has specific effects on other cardiovascular targets that in combination improve its therapeutic effects in HF as depicted in figure 3. These include
Figure 3.
Various effects of SERCA2a on the cardiovascular system.
Mechanoenergetics
In pre-clinical HF models including rodents, porcine, and sheep, AAV-mediated SERCA2a overexpression significantly improved cardiac metabolism (as measured by the ratio of creatine phosphate to ATP ratio), and energy utilization (as measured by restoration of the O2 consumption to Emax relationship to normal levels) even when the underlying pathophysiology or insult (eg, mitral valve rupture or pacing induced heart failure) was not fixed [33-35]. The restoration of myocardial energetics is an important issue because increased myocardial contractility is associated with greater energy demands. These studies showed a beneficial remodeling following SERCA2a overexpression within cardiomyocytes restoring the metabolic machinery to normal.
Endothelial effects
We have demonstrated that in a heart failure model coronary flow increased following AAV1.SERCA2a gene transfer. We found that eNOS expression and the activity of eNOS promoter were greater in SERCA2a overexpressing human endothelial cells. We also demonstrated that in SERCA2a overexpressing cells, Ser1177eNOS phosphorylation was increased in basal state. Furthermore, we demonstrated by co-immunoprecipitation that SERCA2a and eNOS are associated in functional protein-protein complex. This indicates that both proteins are localized in a similar calcium environment and also suggests that SERCA2a may directly control eNOS activity. Our results suggest that increased coronary flow occurring after intracoronary SERCA2a gene transfer in a HF model may be due to increased eNOS expression and activity in coronary artery EC.
Arrhythmias
One of the early concerns with SERCA2a overexpression was that with additional SERCA2a pumps, the SR would trigger calcium release especially with leaky Ryanodine receptors [36]. In addition, the inhomogeneous expression of SERCA2a can induce pro-arrhythmogenic effects in this setting. We and other investigators embarked on an extensive evaluation of whether SERCA2a overexpression can induce ventricular arrhythmias. In a rodent model of ischemia-reperfusion, we found that SERCA2a overexpression decreased ventricular arrhythmias [37]. These results were further corroborated in a large animal model of ischemia reperfusion [38]. More recently, another group found that overexpression of SERCA2a suppresses electrical alternans [39] interrupting an important pathway leading to cardiac fibrillation.
Cellular Remodeling
The decrease in intracellular calcium and its redistribution within the SR decreases the activation of various kinases and proteases that are involved in the hypertrophic response [40]. This improvement in calcium cycling induces transcriptional and proteomic changes as the cell remodels.
The effects of SERCA2a on contractility along with the ancillary effects have led to the overall improvement in not only overall cardiac function but also in survival in the experimental models. Furthermore, SERCA2a overexpression in these animal models led to “by-stander” effects so that isolated cardiomyocytes which were not infected in vivo had improved contractile function. This phenomenon was also observed in other studies with gene therapy targeting S100A1 [27] In addition, large animal studies showed that there was a clear gene dosing effects whereby an increase in SERCA2a delivered resulted in an improvement in overall function [35]. These dose-gene effects were critical in validating the biological significance of enhancing SERCA2a function in the setting of heart failure.
Choice of Vectors
A large number of viral vectors have been used in the cardiovascular field mostly on an experimental basis. Gene delivery systems are classified into two main categories, the non-viral and the recombinant viral vectors [41, 42]. In this review we will focus on viral vectors. Briefly, non-viral vectors include plasmid DNA, liposome-DNA complexes and polymer-DNA complexes [43]. The advantages of non-viral vectors include the ease of vector production, the reduced limitation on the expression cassette size and the relatively minimal biosafety risks. Their limitations include low transfection efficiency and transient effect due to their intracellular degradation. This makes them more suitable for transient, short lived expression of particular genes in certain disease states, such as angiopathies where transient expression of angiogenic factors by a modest number of gene modified cells is sufficient to obtain a desired phenotypic effect. On the other hand, certain cardiovascular disease states, such as heart failure require widespread and sustained transgene expression in order to achieve the desired effect. For these disease states viral vectors are the most suitable, as they offer a relatively high gene transfer efficiency and long term transgene expression. Their limitations include the limited packaging capacity, inconsistencies in bioactivity and purity between vector stocks, and biosafety risks. Moreover, the presence of neutralizing antibodies against viral vectors makes it more challenging and poses the demand to engineer or design a viral vector that can escape the immune response and at the same time that can transduce the largest number of cardiac cells. Viral vectors most commonly used in cardiovascular disease states are adenoviral vectors, adeno-associated virus vectors and lentivirus vectors.
An important component of the vector system is the promoter cassette used to drive and regulate the expression of the gene. Regulation of a therapeutic gene expression may be necessary in vivo. A regulatable gene-expression system can be designed which may be responsive to specific transactivators [44, 45]. Systems exploiting physiological regulation can be designed to incorporate promoters with transcriptional activity that is contingent upon signals provided by the pathophysiology of interest. Hypoxia, intravascular shear stress and left ventricular strain have all been used in models of this type of regulation. In the setting of heart failure regulatable system would be important if the gene of interest needs to be turned on or off for a short period of time. This would include genes driving angiogenesis or stem cell recruitment or expansion.
Adenoviral Vectors
Recombinant adenoviral vectors can transduce all major cardiac cell types both in vitro and in vivo. It is the most commonly used vector in preclinical gene therapy models and in clinical cardiovascular gene therapy protocols due to the ease of their production and the broad cell tropism, particularly within the cardiovascular system [46]. However, in vivo, the pattern of myocardial transduction reflects the method of vector delivery. Direct myocardial injection results in intense transduction at the site of injection [47], whereas intracoronary delivery results in more widespread transduction in the distribution of the injected vessel [48]. A number of maneuvers have been shown to enhance adenoviral myocardial transduction efficiency during intracoronary injection. Those maneuvers include physical approaches, such as increase in transcoronary pressure gradient [49] or the application of ultrasound energy to disperse circulating vector as it traverses the myocardium [50, 51]. Chemical approaches include the use of vasodilatory agents that facilitate vector passage from the vascular lumen to the myocardium [52-54]. Translation of this vector system from research to clinical settings is going to be challenging for a number of reasons as the adenovirus vectors can evoke an intense immune and inflammatory reaction, beside the presence of neutralizing antibodies and the de novo development of these antibodies precludes readministration of the same vector serotype. Regarding their biosafety, a number of approaches have been pursued. These include the production of adenoviral vectors with reduced amounts of viral parental genes. The gutted adenoviral vectors are devoid of almost all of the viral parental genes. Another approach to reduce vector associated immunological responses is to modify vector tropism by engineering fiber coat proteins. This avoids transduction of unintended cells, such as antigen presenting cells, and increases gene transfer efficiency to target cells.
Lentivirus Vector
Lentivirus vectors transduce mitotically quiescent cells through genomic integration, particularly within the cardiovascular system. Unfortunately, they are less clinically interesting for the fear regarding the pothogenicity of the parental virus which is based on the human immunodeficiency virus type 1 (HIV-1) [55]. In order to become suitable for clinical use, vector modifications are needed such as deletion of all accessory proteins from the packaging system, separation of packaging elements into multiple plasmids and the use of a chimeric 5’-long term repeat (LTR) and a self inactivating 3’-LTR in the vector plasmid [56, 57]. Modifications to the vector backbone to optimize performance include the incorporation of a central polypurine tract (cPPT) and a posttranscriptional regulatory element PRE) to enhance nuclear import and mRNA translation respectively [58]. These vector modifications have the potential to improve in vivo transduction efficiency of the myocardium [59]. The strengths of this system include the ability to confer long term stable transgene expression and an increased packaging capacity compared with rAAV. However the major limitation of this vector system relates to its high biosafety risk.
Recombinant Adeno-Associated Virus vector
Recombinant adeno-associated virus (rAAV) vectors belong to the family of the dependant parvovirus AAV type 2 [60, 61], a naturally occuring, non-pathogenic, non-integrating and non-replicating virus that depends on a helper virus for replication [62, 63]. When wild type AAV infects a cell in the absence of a helper virus, the AAV persists in a latent form within the cell until superinfection with a helper virus results in productive replication of the AAV along with the helper. The wtAAV is a 4.7 kb single-stranded molecule comprised of a single open-reading frame (ORF) encoding the four replication (Rep) proteins and a single ORF for the three structural capsid (cap) proteins [64]. These protein coding regions are flanked on either side by paired 145 nucleotide inverted terminal repeat (ITR) sequences. The ITRs are the critical elements directing replication, viral packaging and host cell integration of the DNA sequences that they flank. The majority of integration events occur in a specific site, called AAVS1, in the q arm of the human chromosome 19 [65, 66]. There are 12 different AAV serotypes, each one of them has a different tissue tropism [67]. AAV1 has been shown to transduce cardiac and skeletal muscle efficiently. Recently, AAV8 and AAV9 have shown strong tropism toward cardiac tissue which makes them suitable for gene therapy in the cardiovascular system [68-71]. rAAV were produced in the mid 1980's by creating ITR deleted helper plasmids to supply rep and cap function and as a result the property of site-specific integration into chromosome 19q is lost in rAAV vectors, which lack the AAV Rep78 and Rep68 proteins [72]. rAAV2 vector plasmids were constructed in which ITR sequences flanked the therapeutic gene of interest, along with an appropriate promoter and a polyadenylation signal. This vector has a number of clinically favorable attributes over the adenoviral vector. First, it lacks parental agent pathogenicity and vector related cytotoxicity and as a result does not induce a host immune response. rAAV vectors have been used to deliver genes to over 200 study subjects by various routes of administration for potential treatment of genetic disorders such as cystic fibrosis, haemophilia, hereditary emphysema due to α1-antitrypsin deficiency and other neurologic disorders such as Canavan disease, Batten disease, Parkinson and Alzheimer disease without any significant safety concerns [73, 74]. Second, it has the capacity for stable long term gene expression through random genomic integration and or stable extra-chromosomal (episomal) sequences maintenance in non-dividing or slowly dividing transduced cells such as the striated muscles and the cardiac myocytes [60, 61, 75, 76]. Large animal models of single-administration of rAAV2 injection into the muscle [77, 78] and the liver have demonstrated persistent expression for more than 3 years. Results from human trials suggest that repeated delivery might be required, which heightens the interest in developing alternative serotypes and understanding their potential for cross-neutralization. Studies evaluating onset, peak and persistence of expression with nonAAV2 serotypes in vivo are at an early stage [79]. A pseudotype AAV2/1 vector has been designed to effectively transduce cardiac myocytes. AAV2/1 vector has the capsid of AAV1 vector that efficiently transduces cardiac myocytes, while its ITRs are derived from the AAV2 vector as they have been used in many previous clinical trials and their safety profile is established. The time from vector administration to peak of therapeutic protein levels with rAAV2 vectors in vivo is much longer than with most other viral or non-viral gene therapeutics. The onset of transgenic protein expression is often detectable within two weeks of treatment. The plateau of expression occurs 4-8 weeks post viral injection into the muscle [77, 78]. The delay of peak expression is likely due to the requirement for input single-stranded rAAV transgene conversion to a double-stranded molecule that can serve as a template for DNA transcription within the target cell [80, 81]. One strategy to overcome this is to design a double-stranded template when delivering a small gene [82]. Major limitations of the rAAV vector systems include the production of high titer vector stocks of consistent purity and bioactivity, a limited packaging capacity of 4.8 kb and the potential for pre-existing neutralizing antibodies in human populations.
Delivery Methods
A number of delivery methods have been described that are translatable to the clinical setting. Surgical techniques have been used effectively. Developing delivery methods that can provide us with the highest transduction efficiency to the largest number of cardiomyocytes when targeting molecular pathways involved in the pathophysiology of heart failure become very important. A number of delivery methods exist, each one of which has different transduction efficiency and hence will be suitable for specific therapies. Also the mode of delivery differs between rodents or small animals and large animals in vivo. In small animals the mode of delivery includes direct intramyocardial injection, tail vein injection and a catheter based technique that we have developed [49]. In this approach, a catheter is inserted in the left ventricular apex and is advanced beyond the aortic valve. A high concentration of adenoviral vector is infused through the catheter while the aorta and the pulmonary artery are clamped simultaneously distal to the tip of the catheter for a period of approximately 30 sec. This method achieves grossly homogeneous transduction of cardiac myocytes throughout the left and the right ventricles of the heart and hence can produce dramatic transgene specific physiological effects in vivo [49]. Maurice et al [83] used this technique to express β2 receptors in rabbit hearts; however, they only clamped the aorta and achieved predominantly epicardial gene expression. By clamping the pulmonary artery and the aorta simultaneously, we reduced left ventricle preload and as a consequence the left ventricle end-diastolic pressure which allows perfusion of the virus at relatively low pressure and the endocardium can be efficiently transduced. On the other hand, tail vein delivery, offers a high transduction efficiency of cardiac myocytes in small animals by certain rAAV serotypes, such as rAAV8-9 serotypes [84]. Tail vein injection is applied to alter the expression of different genes in animal models of pressure overload hypertrophy and heart failure, where a second survival surgery would not be feasible in such sick animals.
The mode of gene delivery in large animal models include catheter based myocardial delivery, pericardial delivery and surgical delivery.
Pericardial delivery offers a low transduction efficiency that is mainly distributed to the epicardium. The transduction efficiency can be improved by the co-administration of proteolytic enzymes that disrupts the pericardial cellular and extracellular barriers to the myocardium [85]. Surgical delivery is the most invasive where direct injection of vector into the myocardium can be achieved via the transthoracic or subxiphisternal approaches. This approach is mostly applied in small animals rather than large animals [47]. The transduction efficiency with this method is mainly restricted to the local myocardium.
A novel technique, the V-Focus system, is a minimally invasive procedure that enables a closed circuit to be percutaneously established between the coronary arteries and the coronary sinus, hence allowing efficient vector delivery by isolating the coronary circulation from the general circulation, with minimal damage to the heart [86]. The advantage of this technique is that it allows high transduction efficiency to the myocardium with minimal systemic vector delivery. Only 10% of the perfused blood escapes the closed circuit through the thebesian veins. Adequate oxygenation of the myocardium is maintained in part by circulating the perfusate through an extracorporeal membrane oxygenation system. Once the circuit is established, the vector is directly injected into the circuit and circulates through the myocardium for approximately 10 min [86]. This technique has allowed a widespread transduction of SERCA2a in an ovine model of heart failure with a substantial increase in contractility both with adenoviral and rAAV gene transfer.
Surgical gene transfer methods have also been tried. Animals have been placed on bypass and antegrade injection of vector has been performed with high efficiency of gene transfer [87]. More recently, investigator used a complete surgical isolation of the heart in situ with retrograde (through the coronary venous circulation) administration of both vector and endothelial permeabilizing agents to increase myocyte transduction efficiency [88]. This technique involves clamping both vena cavae and all pulmonary veins. On cardiopulmonary bypass, the aorta and pulmonary artery are crossclamped, and the heart isolated. Viral solution is then infused retrograde into the coronary sinus and recirculated for a total of 30 minutes. Even though this novel cardiac surgical technique of cardiac isolation and retrograde delivery of vector through the coronary sinus results in efficient myocyte transduction in an adult large animal in vivo, its translation to patients with severe heart failure may be limited [88].
Percutaneous catheter based gene delivery to the myocardium in vivo can be achieved by the intracoronary route, by endocardial delivery or by retrograde infusion of the coronary veins. Intracoronary delivery using a slow infusion as compared to bolus infusion is superior and offers on average 50% transduction efficiency which is affected by a number of factors such as animal species, pharmacological agents used to permeabilize vasculature and vector related titer variability. In contrast, percutaneous endocardial delivery offers focal gene delivery to the myocardium and is suitable for therapies involving angiogenesis [89] and to a lesser extent to focal arrhythmia therapy [90]. Retrograde infusion of vector via the coronary veins constitutes a novel catheter based technique for myocardial gene delivery and in studies pioneered by Boekstegers et al it was shown to be superior to antegrade coronary injections [91]. However other studies showed that antegrade coronary injections was superior to retrograde injections [92]. The retrograde infusion has the advantage over the intracoronary method that it bypasses diseased arteries that are likely to impede vector delivery, which makes it a clinically attractive mode of gene delivery. However it requires stoppage of antegrade flow which may not be tolerated in patients with severe heart failure. More recently, we developed a percutaneous and clinically applicable catheter based gene delivery method that allowed slow selective antegrade myocardial gene transfer and a transduction efficiency of ~60% [34]. As shown in figure 4, this method is quite easily translatable in the human cardiac catheterization laboratory and was the one chosen for the SERCA2a gene therapy trials [93, 94].
Figure 4.
Method of gene transfer using slow infusion into the coronary arteries.
Immune Response
One of the challenges with viral gene transfer is the pre-existence of neutralizing antibodies [95, 96]. In different studies, it has been shown that a significant proportion of adults are seropositive for AAV2 (up to ~80%)% AAV1 (~50%), AAV5 (~40%) and AAV6 (~30%) [97] [98] [99]. The presence of pre-existing antibodies can have considerable implications for cardiovascular gene therapy because it has been shown in several studies that these AAV specific antibodies are neutralizing [100-103] and can severely compromise the utility of a gene therapy approach using AAV vectors. These results highlight the necessity to determine neutralizing antibody titers against the specific vector used in a patient population.
Another issue that can occur is that cells may transiently express AAV capsid protein on their cell surface. T-cell response could occur in any organ but greatest concern has been focused on the liver and heart (site of injection). This response is dose dependent and in clinical trials to treat hemophilia or liportein lipase deficiency, higher doses (>1013 viral genomes) were associated with activation of capsid specific T cells and elimination of the transgene [104] [105]. In the clinical trials, to evaluate potential development of a T-cell response, it is important to use an ELISPOT assay to detect anti-AAV capsid T cell responses (IFN-γ release when patient's peripheral blood monocytes are exposed to capsid peptide).
Pharmacological Methods to Enhance Uptake
Independent of the delivery methods used, pahrmacological approaches which include the use of vasodilatory and permeabilizing agents have been added to facilitate transfer of vector form the vascular lumen to the myocardium [54, 106, 107]. In fact a number of agents that increase the permeability of the vascular bed have been used in preclinical trials including nitroglycerin, nitroprusside, serotonin, bradykinin, histamine, substance P, and VEGF (Vascular endothelial growth factor). Clinically and in the setting of heart failure, these agents must be used in caution as not to decrease systemic blood pressure. In the clinical trial of AAV1.SERCA2a gene transfer in patients with heart failure, intravenous nitroglycerin was used.
Clinical Trials
Following the large amount of data gathered in large animals and the toxicology studies that were performed to examine the safety profile of expressing SERCA2a, a first-inhuman, phase 1 trial (CUPID) was recently conducted restoring the levels of this key enzyme in HF patients by gene transfer of the SERCA2a cDNA via a recombinant AAV vector (AAV1.SERCA2a). Because there was concern that inhomogeneous SERCA2a overexpresson may lead to a pro-arrhythmogenic state, all patients in the phase 1 (and also phase 2) trial were required to have Implantable Cardioverter Defibrillators (ICDs). It was critical that the assessment of the efficacy of gene therapy trials be robust enough to detect biological signals. In pre-clinical trials and large animal studies, investigators have relied mainly on hemodynamic measurements coupled with non-invasive techniques such as echocardiography and MRI (Magnetic resonance Imaging). MRIs, which provide the most detailed structural measurements of the heart cannot be routinely used in advanced heart failure patients since a majority of them would have ICDs (Implantable cardiac defibrillators) or biventricular pacemakers. Clinically, invasive monitoring is obviously difficult to perform, however there are a number of clinical measures that can be followed. These include endpoints such as death, LVAD (Left ventricular assist device), transplant, and heart failure hospitalizations. In addition, other parameters such echocardiographic measures, Quality of life assessments, biomarkers, six minute walk tests and oxygen consumption tests are routinely uses in heart failure trials as secondary end-points.
The results of the phase 1 study demonstrated an acceptable safety profile, and improvements at 6 months across symptomatic, functional, biomarker and LV function/remodeling parameters [93, 94]. However, even though the phase 1 trial showed biological efficacy, the phase 1 trial was open label and it is hard to interpret these results. The safety profile of phase 1, let to the approval of a double-blind placebo controlled randomized trial of AAV1.SERCA2a [94]. The phase 2 trial enrolled 39 patients with advanced HF who were randomized to receive intracoronary adeno-associated virus 1 (AAV1) mediated SERCA2a gene delivery (in one of 3 doses (low dose - 6 × 1011 DNAse Resistant Particles (DRP), middle dose - 3 × 1012 DRP and high dose - 1 × 1013 DRP) versus placebo and was finally completed. At six months, the AAV1.SERCA2a treated patients, demonstrated improvement or stabilization in NYHA class, MLWHFQ, 6MWT, VO2 max, NT-proBNP levels, and LV end-systolic volumes. Significant increases in time to adjudicated CV events, and a decreased frequency of CV events per patient were observed on AAV1.SERCA2a. No increases in adverse events, disease-related events, laboratory abnormalities or arrhythmias were observed in AAV1.SERCA2a treated patients compared to placebo. Even though larger studies are needed to establish AAV.SERCA2a as a treatment modality for advanced HF, the CUPID trial demonstrated that SERCA2a is a critical target in the pathogenesis of HF.
Two other clinical trials targeting SERCA2a are currently enrolling patients. The first trial is in patients with advanced heart failure having received left ventricular assist devices at least one month prior to treatment and who will receive either AAV6.SERCA2a or saline. This trial is being conducted in the United Kingdom. A second Phase 2 monocenter double-blind randomized placebo-controled, parallel study will be held in the Institut of Cardiology Pitié-Salpêtrière, Paris, France with the primary objective to investigate the impact of AAV6-CMV-SERCA2a on cardiac remodeling parameters in patients with severe heart failure.
Conclusion
SR calcium cycling is crucial to the process of excitation–contraction coupling. Dysfunction of several calcium cycling proteins have been strongly associated with the pathogenesis of human heart failure. A large body of experimental findings to date have clearly demonstrated the concept that reversal of impaired calcium handling can prevent and/or partially reverse the progression of heart failure. The clinical trial of gene delivery of AAV1.SERCA2a in patients with heart failure, with its positive biological signals, has clearly demonstrated that SERCA2a is an important therapeutic target. New molecular based strategies for restoring calcium cycling may provide a new therapeutic paradigm for patients with severe heart failure.
Acknowledgements
This work is supported by Leducq Foundation through the Caerus network (RJH), NIH R44HL068354 (JKG), NIH HL093183, HL088434, HL071763, HL080498, HL083156, and HL100396 (RJH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. Feb 23;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Gwathmey JK, Slawsky MT, Hajjar RJ, Briggs GM, Morgan JP. Role of intracellular calcium handling in force-interval relationships of human ventricular myocardium. J Clin Invest. 1990 May;85(5):1599–613. doi: 10.1172/JCI114611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002 Aug;34(8):951–69. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 4.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol. 2003 Jul;4(7):517–29. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 5.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002 Jan 10;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 6.Blinks JR, Wier WG, Hess P, Prendergast FG. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- 7.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circ Res. 1987 Jul;61(1):70–6. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 8.Gwathmey JK, Morgan JP. Altered calcium handling in experimental pressure-overload hypertrophy in the ferret. Circ Res. 1985 Dec;57(6):836–43. doi: 10.1161/01.res.57.6.836. [DOI] [PubMed] [Google Scholar]
- 9.Gwathmey JK, Morgan JP. The effects of milrinone and piroximone on intracellular calcium handling in working myocardium from the ferret. Br J Pharmacol. 1985 May;85(1):97–108. doi: 10.1111/j.1476-5381.1985.tb08835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwathmey JK, Copelas L, MacKinnon R, Schoen FJ, Feldman MD, Grossman W, et al. Abnormal intracellular calcium handling in myocardium from patients with end-stage heart failure. Circulation Research. 1987;61(1):70–6. doi: 10.1161/01.res.61.1.70. [DOI] [PubMed] [Google Scholar]
- 11.Beuckelmann DJ, Nabauer M, Erdmann E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992 Mar;85(3):1046–55. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- 12.Akar FG, Tomaselli GF. Ion channels as novel therapeutic targets in heart failure. Ann Med. 2005;37(1):44–54. doi: 10.1080/07853890510007214. [DOI] [PubMed] [Google Scholar]
- 13.Morgan JP, Erny RE, Allen PD, Grossman W, Gwathmey JK. Abnormal intracellular calcium handling, a major cause of systolic and diastolic dysfunction in ventricular myocardium from patients with heart failure. Circulation. 1990 Feb;81(2 Suppl):III21–32. [PubMed] [Google Scholar]
- 14.Vangheluwe P, Louch WE, Ver Heyen M, Sipido K, Raeymaekers L, Wuytack F. Ca2+ transport ATPase isoforms SERCA2a and SERCA2b are targeted to the same sites in the murine heart. Cell Calcium. 2003 Dec;34(6):457–64. doi: 10.1016/s0143-4160(03)00126-x. [DOI] [PubMed] [Google Scholar]
- 15.He H, Giordano FJ, Hilal-Dandan R, Choi DJ, Rockman HA, McDonough PM, et al. Overexpression of the rat sarcoplasmic reticulum Ca2+ ATPase gene in the heart of transgenic mice accelerates calcium transients and cardiac relaxation. J Clin Invest. 1997 Jul 15;100(2):380–9. doi: 10.1172/JCI119544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker DL, Hashimoto K, Grupp IL, Ji Y, Reed T, Loukianov E, et al. Targeted overexpression of the sarcoplasmic reticulum Ca2+-ATPase increases cardiaccontractility in transgenic mouse hearts. Circ Res. 1998 Dec 14-28;83(12):1205–14. doi: 10.1161/01.res.83.12.1205. [DOI] [PubMed] [Google Scholar]
- 17.Bavendiek U, Brixius K, Munch G, Zobel C, Muller-Ehmsen J, Schwinger RH. Effect of inotropic interventions on the force-frequency relation in the human heart. Basic Res Cardiol. 1998;93(Suppl 1):76–85. doi: 10.1007/s003950050224. [DOI] [PubMed] [Google Scholar]
- 18.Arai M, Alpert NR, MacLennan DH, Barton P, Periasamy M. Alterations in sarcoplasmic reticulum gene expression in human heart failure. A possible mechanism for alterations in systolic and diastolic properties of the failing myocardium. Circ Res. 1993 Feb;72(2):463–9. doi: 10.1161/01.res.72.2.463. [DOI] [PubMed] [Google Scholar]
- 19.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J Mol Cell Cardiol. 1999 Mar;31(3):479–91. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 20.Haghighi K, Gregory KN, Kranias EG. Sarcoplasmic reticulum Ca-ATPase-phospholamban interactions and dilated cardiomyopathy. Biochem Biophys Res Commun. 2004 Oct 1;322(4):1214–22. doi: 10.1016/j.bbrc.2004.07.164. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez P, Mitton B, Nicolaou P, Chen G, Kranias EG. Phosphorylation of human inhibitor-1 at Ser67 and/or Thr75 attenuates stimulatory effects of protein kinase A signaling in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2007 Jul;293(1):H762–9. doi: 10.1152/ajpheart.00104.2007. [DOI] [PubMed] [Google Scholar]
- 22.Chopra N, Kannankeril PJ, Yang T, Hlaing T, Holinstat I, Ettensohn K, et al. Modest reductions of cardiac calsequestrin increase sarcoplasmic reticulum Ca2+ leak independent of luminal Ca2+ and trigger ventricular arrhythmias in mice. Circ Res. 2007 Sep 14;101(6):617–26. doi: 10.1161/CIRCRESAHA.107.157552. [DOI] [PubMed] [Google Scholar]
- 23.Arvanitis DA, Vafiadaki E, Fan GC, Mitton BA, Gregory KN, Del Monte F, et al. Histidine-rich Ca-binding protein interacts with sarcoplasmic reticulum Ca-ATPase. Am J Physiol Heart Circ Physiol. 2007 Sep;293(3):H1581–9. doi: 10.1152/ajpheart.00278.2007. [DOI] [PubMed] [Google Scholar]
- 24.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008 Jan 15;77(2):245–55. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 25.Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, et al. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res. 2010 Jun 11;106(11):1743–52. doi: 10.1161/CIRCRESAHA.110.219816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009 Jul;119(7):1940–51. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pleger ST, Most P, Boucher M, Soltys S, Chuprun JK, Pleger W, et al. Stable myocardial-specific AAV6-S100A1 gene therapy results in chronic functional heart failure rescue. Circulation. 2007 May 15;115(19):2506–15. doi: 10.1161/CIRCULATIONAHA.106.671701. [DOI] [PubMed] [Google Scholar]
- 28.Most P, Pleger ST, Volkers M, Heidt B, Boerries M, Weichenhan D, et al. Cardiac adenoviral S100A1 gene delivery rescues failing myocardium. J Clin Invest. 2004 Dec;114(11):1550–63. doi: 10.1172/JCI21454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loukianov E, Ji Y, Grupp IL, Kirkpatrick DL, Baker DL, Loukianova T, et al. Enhanced myocardial contractility and increased Ca2+ transport function in transgenic hearts expressing the fast-twitch skeletal muscle sarcoplasmic reticulum Ca2+-ATPase. Circ Res. 1998 Nov 2;83(9):889–97. doi: 10.1161/01.res.83.9.889. [DOI] [PubMed] [Google Scholar]
- 30.Mork HK, Sjaastad I, Sande JB, Periasamy M, Sejersted OM, Louch WE. Increased cardiomyocyte function and Ca2+ transients in mice during early congestive heart failure. J Mol Cell Cardiol. 2007 Aug;43(2):177–86. doi: 10.1016/j.yjmcc.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Periasamy M, Reed TD, Liu LH, Ji Y, Loukianov E, Paul RJ, et al. Impaired cardiac performance in heterozygous mice with a null mutation in the sarco(endo)plasmic reticulum Ca2+-ATPase isoform 2 (SERCA2) gene. J Biol Chem. 1999 Jan 22;274(4):2556–62. doi: 10.1074/jbc.274.4.2556. [DOI] [PubMed] [Google Scholar]
- 32.Schultz Jel J, Glascock BJ, Witt SA, Nieman ML, Nattamai KJ, Liu LH, et al. Accelerated onset of heart failure in mice during pressure overload with chronically decreased SERCA2 calcium pump activity. Am J Physiol Heart Circ Physiol. 2004 Mar;286(3):H1146–53. doi: 10.1152/ajpheart.00720.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sakata S, Lebeche D, Sakata N, Sakata Y, Chemaly ER, Liang LF, et al. Restoration of mechanical and energetic function in failing aortic-banded rat hearts by gene transfer of calcium cycling proteins. J Mol Cell Cardiol. 2007 Apr;42(4):852–61. doi: 10.1016/j.yjmcc.2007.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H, et al. Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure. J Am Coll Cardiol. 2008 Mar 18;51(11):1112–9. doi: 10.1016/j.jacc.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 35.Byrne MJ, Power JM, Preovolos A, Mariani JA, Hajjar RJ, Kaye DM. Recirculating cardiac delivery of AAV2/1SERCA2a improves myocardial function in an experimental model of heart failure in large animals. Gene Ther. 2008 Dec;15(23):1550–7. doi: 10.1038/gt.2008.120. [DOI] [PubMed] [Google Scholar]
- 36.Davia K, Bernobich E, Ranu HK, del Monte F, Terracciano CM, MacLeod KT, et al. SERCA2A overexpression decreases the incidence of aftercontractions in adult rabbit ventricular myocytes. J Mol Cell Cardiol. 2001 May;33(5):1005–15. doi: 10.1006/jmcc.2001.1368. [DOI] [PubMed] [Google Scholar]
- 37.del Monte F, Lebeche D, Guerrero JL, Tsuji T, Doye AA, Gwathmey JK, et al. Abrogation of ventricular arrhythmias in a model of ischemia and reperfusion by targeting myocardial calcium cycling. Proc Natl Acad Sci U S A. 2004 Apr 13;101(15):5622–7. doi: 10.1073/pnas.0305778101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prunier F, Kawase Y, Gianni D, Scapin C, Danik SB, Ellinor PT, et al. Prevention of ventricular arrhythmias with sarcoplasmic reticulum Ca2+ ATPase pump overexpression in a porcine model of ischemia reperfusion. Circulation. 2008 Aug 5;118(6):614–24. doi: 10.1161/CIRCULATIONAHA.108.770883. [DOI] [PubMed] [Google Scholar]
- 39.Cutler MJ, Wan X, Laurita KR, Hajjar RJ, Rosenbaum DS. Targeted SERCA2a gene expression identifies molecular mechanism and therapeutic target for arrhythmogenic cardiac alternans. Circ Arrhythm Electrophysiol. 2009 Dec;2(6):686–94. doi: 10.1161/CIRCEP.109.863118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Del Monte F, Dalal R, Tabchy A, Couget J, Bloch KD, Peterson R, et al. Transcriptional changes following restoration of SERCA2a levels in failing rat hearts. Faseb J. 2004 Sep;18(12):1474–6. doi: 10.1096/fj.04-1714fje. [DOI] [PubMed] [Google Scholar]
- 41.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001 Jan;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 42.Nishikawa M, Huang L. Nonviral vectors in the new millennium: delivery barriers in gene transfer. Hum Gene Ther. 2001 May 20;12(8):861–70. doi: 10.1089/104303401750195836. [DOI] [PubMed] [Google Scholar]
- 43.Felgner PL. Nonviral strategies for gene therapy. Sci Am. 1997 Jun;276(6):102–6. doi: 10.1038/scientificamerican0697-102. [DOI] [PubMed] [Google Scholar]
- 44.Agha-Mohammadi S, Lotze MT. Regulatable systems: applications in gene therapy and replicating viruses. J Clin Invest. 2000 May;105(9):1177–83. doi: 10.1172/JCI10027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Agha-Mohammadi S, Lotze MT. Immunomodulation of cancer: potential use of selectively replicating agents. J Clin Invest. 2000 May;105(9):1173–6. doi: 10.1172/JCI10026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isner JM. Myocardial gene therapy. Nature. 2002 Jan 10;415(6868):234–9. doi: 10.1038/415234a. [DOI] [PubMed] [Google Scholar]
- 47.Guzman RJ, Lemarchand P, Crystal RG, Epstein SE, Finkel T. Efficient and selective adenovirus-mediated gene transfer into vascular neointima. Circulation. 1993 Dec;88(6):2838–48. doi: 10.1161/01.cir.88.6.2838. [DOI] [PubMed] [Google Scholar]
- 48.Barr E, Carroll J, Kalynych AM, Tripathy SK, Kozarsky K, Wilson JM, et al. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994 Jan;1(1):51–8. [PubMed] [Google Scholar]
- 49.Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK, et al. Modulation of ventricular function through gene transfer in vivo. Proc Natl Acad Sci U S A. 1998 Apr 28;95(9):5251–6. doi: 10.1073/pnas.95.9.5251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, et al. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000 Jun 6;101(22):2554–6. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 51.Beeri R, Guerrero JL, Supple G, Sullivan S, Levine RA, Hajjar RJ. New efficient catheter-based system for myocardial gene delivery. Circulation. 2002 Oct 1;106(14):1756–9. doi: 10.1161/01.cir.0000035240.92015.e4. [DOI] [PubMed] [Google Scholar]
- 52.Donahue JK, Kikkawa K, Johns DC, Marban E, Lawrence JH. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci U S A. 1997 Apr 29;94(9):4664–8. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donahue JK, Kikkawa K, Thomas AD, Marban E, Lawrence JH. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998 May;5(5):630–4. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- 54.Nagata K, Marban E, Lawrence JH, Donahue JK. Phosphodiesterase inhibitor-mediated potentiation of adenovirus delivery to myocardium. J Mol Cell Cardiol. 2001 Mar;33(3):575–80. doi: 10.1006/jmcc.2000.1322. [DOI] [PubMed] [Google Scholar]
- 55.Klages N, Zufferey R, Trono D. A stable system for the high-titer production of multiply attenuated lentiviral vectors. Mol Ther. 2000 Aug;2(2):170–6. doi: 10.1006/mthe.2000.0103. [DOI] [PubMed] [Google Scholar]
- 56.Galimi F, Noll M, Kanazawa Y, Lax T, Chen C, Grompe M, et al. Gene therapy of Fanconi anemia: preclinical efficacy using lentiviral vectors. Blood. 2002 Oct 15;100(8):2732–6. doi: 10.1182/blood-2002-04-1245. [DOI] [PubMed] [Google Scholar]
- 57.Galimi F, Verma IM. Opportunities for the use of lentiviral vectors in human gene therapy. Curr Top Microbiol Immunol. 2002;261:245–54. doi: 10.1007/978-3-642-56114-6_13. [DOI] [PubMed] [Google Scholar]
- 58.Sirven A, Pflumio F, Zennou V, Titeux M, Vainchenker W, Coulombel L, et al. The human immunodeficiency virus type-1 central DNA flap is a crucial determinant for lentiviral vector nuclear import and gene transduction of human hematopoietic stem cells. Blood. 2000 Dec 15;96(13):4103–10. [PubMed] [Google Scholar]
- 59.Bonci D, Cittadini A, Latronico MV, Borello U, Aycock JK, Drusco A, et al. ‘Advanced’ generation lentiviruses as efficient vectors for cardiomyocyte gene transduction in vitro and in vivo. Gene Ther. 2003 Apr;10(8):630–6. doi: 10.1038/sj.gt.3301936. [DOI] [PubMed] [Google Scholar]
- 60.Monahan PE, Samulski RJ. AAV vectors: is clinical success on the horizon? Gene Ther. 2000 Jan;7(1):24–30. doi: 10.1038/sj.gt.3301109. [DOI] [PubMed] [Google Scholar]
- 61.Monahan PE, Samulski RJ, Tazelaar J, Xiao X, Nichols TC, Bellinger DA, et al. Direct intramuscular injection with recombinant AAV vectors results in sustained expression in a dog model of hemophilia. Gene Ther. 1998 Jan;5(1):40–9. doi: 10.1038/sj.gt.3300548. [DOI] [PubMed] [Google Scholar]
- 62.Flotte TR, Carter BJ. Adeno-associated virus vectors for gene therapy. Gene Ther. 1995 Aug;2(6):357–62. [PubMed] [Google Scholar]
- 63.Schnepp BC, Jensen RL, Chen CL, Johnson PR, Clark KR. Characterization of adeno-associated virus genomes isolated from human tissues. J Virol. 2005 Dec;79(23):14793–803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Monahan PE, Jooss K, Sands MS. Safety of adeno-associated virus gene therapy vectors: a current evaluation. Expert Opin Drug Saf. 2002 May;1(1):79–91. doi: 10.1517/14740338.1.1.79. [DOI] [PubMed] [Google Scholar]
- 65.Dutheil N, Shi F, Dupressoir T, Linden RM. Adeno-associated virus site-specifically integrates into a muscle-specific DNA region. Proc Natl Acad Sci U S A. 2000 Apr 25;97(9):4862–6. doi: 10.1073/pnas.080079397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Young SM, Jr., Samulski RJ. Adeno-associated virus (AAV) site-specific recombination does not require a Rep-dependent origin of replication within the AAV terminal repeat. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13525–30. doi: 10.1073/pnas.241508998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carter PJ, Samulski RJ. Adeno-associated viral vectors as gene delivery vehicles. Int J Mol Med. 2000 Jul;6(1):17–27. doi: 10.3892/ijmm.6.1.17. [DOI] [PubMed] [Google Scholar]
- 68.Inagaki K, Fuess S, Storm TA, Gibson GA, McTiernan CF, Kay MA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006 Jul;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pacak CA, Mah CS, Thattaliyath BD, Conlon TJ, Lewis MA, Cloutier DE, et al. Recombinant adeno-associated virus serotype 9 leads to preferential cardiac transduction in vivo. Circ Res. 2006 Aug 18;99(4):e3–9. doi: 10.1161/01.RES.0000237661.18885.f6. [DOI] [PubMed] [Google Scholar]
- 70.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005 Mar;23(3):321–8. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 71.Zhu T, Zhou L, Mori S, Wang Z, McTiernan CF, Qiao C, et al. Sustained whole-body functional rescue in congestive heart failure and muscular dystrophy hamsters by systemic gene transfer. Circulation. 2005 Oct 25;112(17):2650–9. doi: 10.1161/CIRCULATIONAHA.105.565598. [DOI] [PubMed] [Google Scholar]
- 72.Tratschin JD, Miller IL, Smith MG, Carter BJ. Adeno-associated virus vector for high-frequency integration, expression, and rescue of genes in mammalian cells. Mol Cell Biol. 1985 Nov;5(11):3251–60. doi: 10.1128/mcb.5.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005 May;16(5):541–50. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 74.Flotte TR. Adeno-associated virus-based gene therapy for inherited disorders. Pediatr Res. 2005 Dec;58(6):1143–7. doi: 10.1203/01.pdr.0000189226.03684.fe. [DOI] [PubMed] [Google Scholar]
- 75.Monahan PE, Samulski RJ. Adeno-associated virus vectors for gene therapy: more pros than cons? Mol Med Today. 2000 Nov;6(11):433–40. doi: 10.1016/s1357-4310(00)01810-4. [DOI] [PubMed] [Google Scholar]
- 76.Schnepp BC, Clark KR, Klemanski DL, Pacak CA, Johnson PR. Genetic fate of recombinant adeno-associated virus vector genomes in muscle. J Virol. 2003 Mar;77(6):3495–504. doi: 10.1128/JVI.77.6.3495-3504.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snyder RO, Miao C, Meuse L, Tubb J, Donahue BA, Lin HF, et al. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat Med. 1999 Jan;5(1):64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 78.Kessler PD, Podsakoff GM, Chen X, McQuiston SA, Colosi PC, Matelis LA, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci U S A. 1996 Nov 26;93(24):14082–7. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, et al. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol. 2002 Jan;76(2):791–801. doi: 10.1128/JVI.76.2.791-801.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ferrari FK, Samulski T, Shenk T, Samulski RJ. Second-strand synthesis is a rate-limiting step for efficient transduction by recombinant adeno-associated virus vectors. J Virol. 1996 May;70(5):3227–34. doi: 10.1128/jvi.70.5.3227-3234.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Malik AK, Monahan PE, Allen DL, Chen BG, Samulski RJ, Kurachi K. Kinetics of recombinant adeno-associated virus-mediated gene transfer. J Virol. 2000 Apr;74(8):3555–65. doi: 10.1128/jvi.74.8.3555-3565.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nakai H, Storm TA, Kay MA. Recruitment of single-stranded recombinant adeno-associated virus vector genomes and intermolecular recombination are responsible for stable transduction of liver in vivo. J Virol. 2000 Oct;74(20):9451–63. doi: 10.1128/jvi.74.20.9451-9463.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maurice JP, Hata JA, Shah AS, White DC, McDonald PH, Dolber PC, et al. Enhancement of cardiac function after adenoviral-mediated in vivo intracoronary beta2-adrenergic receptor gene delivery. J Clin Invest. 1999 Jul;104(1):21–9. doi: 10.1172/JCI6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008 Jun;16(6):1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 85.Fromes Y, Salmon A, Wang X, Collin H, Rouche A, Hagege A, et al. Gene delivery to the myocardium by intrapericardial injection. Gene Ther. 1999 Apr;6(4):683–8. doi: 10.1038/sj.gt.3300853. [DOI] [PubMed] [Google Scholar]
- 86.Preovolos AC, Mennen MT, Bilney A, Mariani J, Kaye DM, Power JM. Development of a novel perfusion technique to allow targeted delivery of gene therapy--the V-Focus system. J Extra Corpor Technol. 2006 Mar;38(1):51–2. [PMC free article] [PubMed] [Google Scholar]
- 87.Bridges CR, Burkman JM, Malekan R, Konig SM, Chen H, Yarnall CB, et al. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002 Jun;73(6):1939–46. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- 88.Bridges CR, Gopal K, Holt DE, Yarnall C, Cole S, Anderson RB, et al. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J Thorac Cardiovasc Surg. 2005 Nov;130(5):1364. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 89.Koransky ML, Robbins RC, Blau HM. VEGF gene delivery for treatment of ischemic cardiovascular disease. Trends Cardiovasc Med. 2002 Apr;12(3):108–14. doi: 10.1016/s1050-1738(01)00158-x. [DOI] [PubMed] [Google Scholar]
- 90.Edelberg JM, Huang DT, Josephson ME, Rosenberg RD. Molecular enhancement of porcine cardiac chronotropy. Heart. 2001 Nov;86(5):559–62. doi: 10.1136/heart.86.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, et al. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000 Feb;7(3):232–40. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 92.Hoshino K, Kimura T, De Grand AM, Yoneyama R, Kawase Y, Houser S, et al. Three catheter-based strategies for cardiac delivery of therapeutic gelatin microspheres. Gene Ther. 2006 Sep;13(18):1320–7. doi: 10.1038/sj.gt.3302793. [DOI] [PubMed] [Google Scholar]
- 93.Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A, et al. Design of a phase 1/2 trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure. J Card Fail. 2008 Jun;14(5):355–67. doi: 10.1016/j.cardfail.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 94.Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B, et al. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009 Apr;15(3):171–81. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kwon I, Schaffer DV. Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer. Pharm Res. 2008 Mar;25(3):489–99. doi: 10.1007/s11095-007-9431-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zaiss AK, Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008 Jun;15(11):808–16. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- 97.Halbert CL, Miller AD, McNamara S, Emerson J, Gibson RL, Ramsey B, et al. Prevalence of neutralizing antibodies against adeno-associated virus (AAV) types 2, 5, and 6 in cystic fibrosis and normal populations: Implications for gene therapy using AAV vectors. Hum Gene Ther. 2006 Apr;17(4):440–7. doi: 10.1089/hum.2006.17.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009 Feb 1;199(3):381–90. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boutin S, Monteilhet V, Veron P, Leborgne C, Benveniste O, Montus MF, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010 Jun;21(6):704–12. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 100.Buning H, Ried MU, Perabo L, Gerner FM, Huttner NA, Enssle J, et al. Receptor targeting of adeno-associated virus vectors. Gene Ther. 2003 Jul;10(14):1142–51. doi: 10.1038/sj.gt.3301976. [DOI] [PubMed] [Google Scholar]
- 101.Cottard V, Valvason C, Falgarone G, Lutomski D, Boissier MC, Bessis N. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol. 2004 Mar;24(2):162–9. doi: 10.1023/B:JOCI.0000019781.64421.5c. [DOI] [PubMed] [Google Scholar]
- 102.Erles K, Rohde V, Thaele M, Roth S, Edler L, Schlehofer JR. DNA of adeno-associated virus (AAV) in testicular tissue and in abnormal semen samples. Hum Reprod. 2001 Nov;16(11):2333–7. doi: 10.1093/humrep/16.11.2333. [DOI] [PubMed] [Google Scholar]
- 103.Moskalenko M, Chen L, van Roey M, Donahue BA, Snyder RO, McArthur JG, et al. Epitope mapping of human anti-adeno-associated virus type 2 neutralizing antibodies: implications for gene therapy and virus structure. J Virol. 2000 Feb;74(4):1761–6. doi: 10.1128/jvi.74.4.1761-1766.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009 Sep 3;114(10):2077–86. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mingozzi F, Maus MV, Hui DJ, Sabatino DE, Murphy SL, Rasko JE, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007 Apr;13(4):419–22. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 106.Donahue JK, Kikkawa K, Johns DC, Marban E, J. L. Ultrarapid highly efficient viral gene transfer to the heart. Proceedings of the National Academy of Sciences. 1997;94:4664–8. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Donahue JK, Kikkawa K, Thomas AD, Marban E, Lawrence JH. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Therapy. 1998;5(5):630–4. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]