Abstract
During yeast sporulation, internal membrane synthesis ensures that each haploid nucleus is packaged into a spore. Prospore membrane formation requires Spo14p, a phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2]-stimulated phospholipase D (PLD), which hydrolyzes phosphatidylcholine (PtdCho) to phosphatidic acid (PtdOH) and choline. We found that both meiosis and spore formation also require the phosphatidylinositol (PtdIns)/PtdCho transport protein Sec14p. Specific ablation of the PtdIns transport activity of Sec14p was sufficient to impair spore formation but not meiosis. Overexpression of Pik1p, a PtdIns 4-kinase, suppressed the sec14-1 meiosis and spore formation defects; conversely, pik1-ts diploids failed to undergo meiosis and spore formation. The PtdIns(4)P 5-kinase, Mss4p, also is essential for spore formation. Use of phosphoinositide-specific GFP-PH domain reporters confirmed that PtdIns(4,5)P2 is enriched in prospore membranes. sec14, pik1, and mss4 mutants displayed decreased Spo14p PLD activity, whereas absence of Spo14p did not affect phosphoinositide levels in vivo, suggesting that formation of PtdIns(4,5)P2 is important for Spo14p activity. Spo14p-generated PtdOH appears to have an essential role in sporulation, because treatment of cells with 1-butanol, which supports Spo14p-catalyzed PtdCho breakdown but leads to production of Cho and Ptd-butanol, blocks spore formation at concentrations where the inert isomer, 2-butanol, has little effect. Thus, rather than a role for PtdOH in stimulating PtdIns(4,5)P2 formation, our findings indicate that during sporulation, Spo14p-mediated PtdOH production functions downstream of Sec14p-, Pik1p-, and Mss4p-dependent PtdIns(4,5)P2 synthesis.
INTRODUCTION
Sporulation in Saccharomyces cerevisiae is a form of cellular differentiation analogous to gametogenesis in metazoans. In sporulation, the chromosomes in a MATa/MATα diploid are duplicated and then segregated, via the meiotic divisions, into four haploid sets, which are then packaged into spores (reviewed in Kupiec et al., 1997). Spore formation requires synthesis of a membrane, known as the prospore membrane, around each haploid nucleus (Byers, 1981). Prospore membrane formation is thought to require rerouting of exocytic post-Golgi vesicles to the spindle pole bodies (SPBs; Neiman, 1998). These vesicles then fuse, initiating formation of the prospore membrane, which then spreads, by further vesicle fusion, until it surrounds each haploid nucleus. How the secretory apparatus is modified to carry out these membrane trafficking events is not well understood, although considerable evidence suggests that signaling by phospholipids is involved.
SPO14 (also known as PLD1) encodes the major phospholipase D (PLD) activity in yeast. Although additional PLD activities have been detected and can hydrolyze phosphatidylcholine (PtdCho) as well as other phospholipids (reviewed in Rudge and Engebrecht, 1999), Spo14p is the only yeast member of the highly conserved eukaryotic PtdCho-specific PLD family; these enzymes hydrolyze PtdCho to phosphatidic acid (PtdOH) and choline. Spo14p action is essential for the formation of the prospore membrane (Rudge et al., 1998b) and for increased PtdOH synthesis during sporulation (Rudge et al., 2001). In contrast, under normal physiological conditions, Spo14p is dispensable for secretion and hence is not essential for vegetative growth (Sreenivas et al., 1998; Xie et al., 1998). Thus, Spo14p plays a pivotal role in distinguishing between constitutive and developmentally regulated membrane trafficking events (Rudge et al., 2001; Rudge et al., 2002). However, the specific function of Spo14p action in prospore membrane biogenesis has not yet been elucidated.
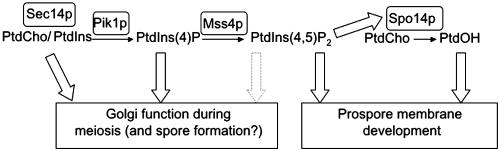
It has been amply demonstrated that mammalian PLD is potently stimulated by phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2] (Brown et al., 1995). Likewise, PtdIns(4,5)P2 stimulates Spo14p in vitro (Rose et al., 1995), and genetic analyses indicate that PtdIns(4,5)P2 is essential for activating the function of Spo14p in vivo (Sciorra et al., 1999, 2002). These observations suggest that PtdIns(4,5)P2 generation is necessary to activate Spo14p for its role in prospore membrane formation. However, in mammalian cells, PtdOH also stimulates PtdIns(4,5)P2 production because PtdOH activates certain PtdIns(4)P 5-kinase isoforms (Honda et al., 1999). The sole PtdIns(4)P 5-kinase in S. cerevisiae, Mss4p, is highly homologous to its mammalian counterparts (Desriviéres et al., 1998; Homma et al., 1998), and there is some evidence that it is also stimulated by PtdOH (Homma et al., 1998). Hence it is possible that PtdIns(4,5)P2 is a lipid critical for prospore membrane biogenesis and spore coat deposition, in analogy to the demonstrated role of phosphoinositides in serving as recognition determinants for the recruitment of many classes of proteins to biological membranes (Cullen et al., 2001; Thorner, 2001) and that the essential role of Spo14p is to generate PtdOH to ensure that PtdIns(4,5)P2 synthesis occurs at a sufficiently high rate. Thus, central to understanding Spo14p/PLD function is delineating the relationship between PtdOH and PtdIns(4,5)P2 in vivo.
Exit of secretory proteins from the Golgi requires the function of Sec14p, a PtdIns/PtdCho transfer protein (Bankaitis et al., 1989, 1990), and Pik1p, a PtdIns 4-kinase, which phosphorylates PtdIns to synthesize PtdIns(4)P (Hama et al., 1999; Walch-Solimena and Novick, 1999; Audhya et al., 2000). Consequently, SEC14 and PIK1 are essential genes in vegetatively growing cells. A second PtdIns 4-kinase, Stt4p (Yoshida et al., 1994), generates a distinct and essential pool of PtdIns(4)P (Audhya et al., 2000), whereas a third PtdIns 4-kinase (Lsb6p) has no essential function and is not required for sporulation (Han et al., 2002). There is substantial evidence that Sec14p contributes significantly to the pool of PtdIns that is converted to PtdIns(4)P in the Golgi via the action of Pik1p (Hama et al., 1999; Rivas et al., 1999).
It has been postulated that phosphatidylinositol transfer proteins (PITPs) such as Sec14p stimulate PLD function by promoting synthesis of PtdIns(4,5)P2 (Liscovitch and Cantley, 1995). Consistent with this suggestion, increased expression of Shf2p and Shf4p, yeast PITPs that display PtdIns-but not PtdCho-transfer activity in vitro, can rescue the growth and secretory defects of sec14 mutants and requires the function of Spo14p to do so (Li et al., 2000). However, Sec14p itself exhibits both PtdCho and PtdIns transfer activity in vitro (Bankaitis et al., 1990), and the results of genetic analyses have not supported a model in which the essential vegetative function of Sec14p is to provide for activation of Spo14p (Sreenivas et al., 1998; Xie et al., 1998), because unlike sec14Δ mutants, spo14Δ mutants are viable (Honigberg et al., 1992; Rose et al., 1995). Moreover, homozygous sfh2Δ and sfh4Δ diploids sporulate at wild-type frequencies (Rabitsch et al., 2001). Because Spo14p does have an essential function in sporulation, we wanted to determine if, under these circumstances, Sec14p might be required to supply the PtdIns necessary for PtdIns(4,5)P2 production and Spo14p/PLD activation. Our analysis supports a model whereby Spo14p-generated PtdOH acts downstream of Sec14p-, Pik1p-, and Mss4p-dependent PtdIns(4,5)P2 synthesis during sporulation.
MATERIALS AND METHODS
Yeast Strains and Media
Routine growth and manipulation of S. cerevisiae strains were performed as described by Rose et al. (1990). Yeast strains used in this study are listed in Table 1. CTY159 (sec14-1ts) (obtained from Vytas Bankaitis, University of North Carolina, Chapel Hill; Xie et al., 1998), YES47 (pik1-11) and YES102 (pik1-83) (Hendricks et al., 1999), and SD102 (mss4-2) (obtained from Michael Hall, University of Basel; Desrivieres et al., 1998) were backcrossed six times against the SK-1 strain background (Fast, 1973). Pairs of haploid segregants of opposite mating type from the final backcrosses were mated to generate homozygous sec14-1 (Y3242), pik1-11 (Y3637), pik1-83 (Y3732), and mss4-2 (Y4350) diploids. Derivatives of these strains were used for the experiments described here (Table 1).
Table 1.
Genotypes of yeast strains
| Strain | Genotype |
|---|---|
| NH144a | 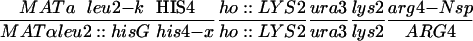 |
| Y433 | NH144 homozygous spo14 :: URA3 |
| Y598 | Y433, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4048 | NH144, plus pRS426 GFP-PH(FAPP1) |
| Y4049 | NH144, plus pRS426 GFP-2xPH(PLCδ) |
| Y3242 | 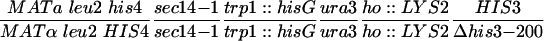 |
| Y3247 | Y3242, plus pUN55 (URA3 CEN4) |
| Y3248 | Y3242, plus pME1878 (SEC14 URA3 CEN4) |
| Y3351 | Y3242 homozygous kes1 :: URA3 |
| Y3352 | Y3242 homozygous cki1 :: LEU2 |
| Y3404 | Y3242, plus Yep24-CSR1/SFH2 (SFH2 URA3 2μ) |
| Y3408 | Y3242, plus pME1308 (MSS4 URA3 2μ) |
| Y3437 | Y3351 homozygous spo14 :: LEU2 |
| Y3449 | Y3437, plus pUN15 (TRP1 CEN4) |
| Y3450 | Y3437, plus pME1753 (spo14S-11 TRP1 CEN4) |
| Y3451 | Y3437, plus pME1761 (SPO14 TRP1 CEN4) |
| Y3528 | Y3242, plus pME1946 (sec14(K66A K239A) URA3 CEN4) |
| Y3553 | Y3242, plus pME2062 (sec14(K66A K239A) URA3 2μ) |
| Y3587 | Y3242, plus pGALHA-STT4-8 (HA-STT4 URA3 2μ) |
| Y3645 | Y3242, plus pME1854 (SFH4 URA3 2μ) |
| Y3663 | Y3242, plus YEp352 PIK1 (PIK1 URA3 2μ) |
| Y3753 | Y3242 homozygous spo14::LEU2 |
| Y3756 | Y3753, plus pUN55 (URA3 CEN4) |
| Y3757 | Y3753, plus pME1878 (SEC14 URA3 CEN4) |
| Y4278 | Y3247, plus pUN105 (LEU2 CEN4) |
| Y4279 | Y3247, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4281 | Y3248, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4297 | Y3528, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4509 | Y3753, plus pME1854 (SFH4 URA3 2μ) |
| Y4510 | Y3753, plus YEp352 PIK1 (PIK1 URA3 2μ) |
| Y4513 | Y3528, plus pME936 (SPO14 TRP1 2μ) |
| Y4514 | Y3528, plus pYO1966 (MSS4 TRP1 2μ) |
| Y4515 | Y3753, plus Yep24-CSR1/SFH2 (SFH2 URA3 2μ) |
| Y315b | 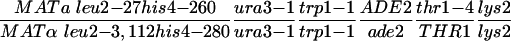 |
| Y1483 | Y315 homozygous kes1::URA3 |
| Y3537 | Y1483 homozygous sec14::LEU2 |
| Y1838 | Y315 homozygous spo14-S11 |
| Y1925 | Y315 homozygous spo14::LEU2 |
| Y3825 | Y1838, plus YEp352 (URA3 2μ) and pME856 (TRP1 2μ) |
| Y3826 | Y1838, plus Yep352 (URA3 2μ) and pYO1966 (MSS4 TRP1 2μ) |
| Y3827 | Y1838, plus YEp352 PIK1 (PIK1 URA3 2μ) and pME856 (TRP1 2μ) |
| Y3828 | Y1838, plus YEp352 PIK1 (PIK1 URA3 2μ) and pYO1966 (MSS4 TRP1 2μ) |
| Y4516 | Y1925, plus YEp352 (URA3 2μ) and pME856 (TRP1 2μ) |
| Y4517 | Y1925, plus YEp352 PIK1 (PIK1 URA3 2μ) and pME856 (TRP1 2μ) |
| Y4518 | Y1925, plus pGALHA-STT4-8 (HA-STT4 URA3 2μ) and pME856 (TRP1 2μ) |
| Y4519 | Y1925, plus Yep352 (URA3 2μ) and pYO1966 (MSS4 TRP1 2μ) |
| Y4520 | Y1925, plus YEp352 PIK1 (PIK1 URA3 2μ) and pYO1966 (MSS4 TRP1 2μ) |
| Y4521 | Y1925, plus pGALHA-STT4-8 (HA-STT4 URA3 2μ) and pYO1966 (MSS4 TRP1 2μ) |
| Y4522 | Y1838, plus pGALHA-STT4-8 (HA-STT4 URA3 2μ) and pME856 (TRP1 2μ) |
| Y4523 | Y1838, plus pGALHA-STT4-8 (HA-STT4 URA3 2μ) and pYO1966 (MSS4 TRP1 2μ) |
| Y3637 |  |
| Y3654 | Y3637, plus pUN55 (URA3 CEN4) |
| Y3655 | Y3637, plus pME1963 (PIK1 URA3 CEN4) |
| Y3890 | Y3637 homozygous spo14::LEU2 |
| Y4076 | Y3637, plus pME1881 (SEC14 URA3 2μ) |
| Y4077 | Y3637, plus pKR577 (SPO14 URA3 2μ) |
| Y4288 | Y3654, plus pUN105 (LEU2 CEN4) |
| Y4289 | Y3654, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4291 | Y3655, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y3732 |  |
| Y3723 | Y3732, plus pUN55 (URA3 CEN4) |
| Y3724 | Y3732, plus pME1963 (PIK1 URA3 CEN4) |
| Y3907 | Y3732 homozygous spo14::LEU2 |
| Y4078 | Y3732, plus pME1881 (SEC14 URA3 2μ) |
| Y4079 | Y3732, plus pKR577 (SPO14 URA3 2μ) |
| Y4284 | Y3723, plus pUN105 (LEU2 CEN4) |
| Y4285 | Y3723, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4287 | Y3724, plus pME940 (HA-SPO14 LEU2 CEN4) |
| Y4350 | 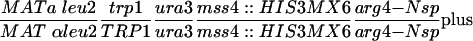 |
| YCplac111::mss4-2ts (LEU2 CEN) | |
| Y4354 | Y4350, plus pUN55 (URA3 CEN4) |
| Y4355 | Y4350, plus pME2188 (MSS4 URA3 CEN4) |
| Y4359 | Y4350, plus pKR577 (SPO14 URA3 2μ) |
| Y4360 | Y4350, plus YEp352 PIK1 (PIK1 URA3 2μ) |
| Y4361 | Y4350, plus pME1881 (SEC14 URA3 2μ) |
| Y4409 | Y4350 homozygous spo14::URA3 |
DNA-mediated transformation of yeast cells used the lithium acetate procedure (Ito et al., 1983). Gene replacement and disruptions (see below) were performed by the one-step method (Rothstein, 1983) and were confirmed using the PCR and appropriate synthetic oligonucleotide primers.
Plasmids
Low-copy number (CEN) plasmids (pUN series; Elledge and Davis, 1988) expressing wild-type SPO14 (pME1761) or the mutant allele spo14-S11 (pME1753) are described in detail elsewhere (Rudge et al., 2001). pKR577 (URA3) and pME936 (TRP1) are high-copy number plasmids (2 μ) containing SPO14 (Rose et al., 1995). SPO14 tagged with the influenza virus hemagglutinin (HA) epitope and expressed from its own promoter (pME940) is fully functional and was characterized previously (Rudge et al., 1998b). pKR466 (spo14::URA3, which removes 4014 base pairs of the 5052-base pair ORF) and pME913 (spo14::LEU2, which removes 4187 base pairs) were used to generate chromosomal spo14 disruption/deletions and have been described (Rose et al., 1995; Rudge et al., 1998a).
pME1878 was isolated by complementation of the sec14-1 mutation from a genomic library in a CEN vector (Rose et al., 1987) and contains SEC14 coding sequences. Site-directed mutagenesis was performed to generate the double mutant sec14(K66A K239A) (pME1946), using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) as recommended by the manufacturer. The mutagenic primers were identical to those described by Phillips et al. (1999). SEC14 and sec14(K66A K239A) were each subcloned into a multicopy (2 μ DNA-based) yeast vector, YEp352 (Hill et al., 1986), as ∼3.5-kb XbaI-HindIII fragments to generate pME1881 and pME2062, respectively.
The BamHI-SacI fragment containing the PIK1 gene from pRS314PIK1 (Flanagan et al., 1993) was moved into the corresponding sites of pUN55 (Elledge and Davis, 1988) to create pME1963. The BamHI-XhoI fragment containing the MSS4 gene from pYO1966 (Homma et al., 1998; generous gift of Yoshikazu Ohya, University of Tokyo, Tokyo, Japan) was moved into the BamHI and XhoI sites of pUN55 and YEp352 to generate pME2188 and pME1308, respectively. The ClaI-, whose ends had been filled in with the Klenow fragment of DNA polymerase, SacI fragment of PDR17/SFH4 from pBVH1402 (van den Hazel et al., 1999; generous gift of Maria Adelaide do Valle Matta, Universite Catholique de Louvain, Louvain-la-Neuve, Belgium), was moved into the SmaI and SacI sites of YEp352 to generate pME1854. YEp352 PIK1 (Flanagan et al., 1993), pGALHA-STT4-8 (Cutler et al., 1997; generous gift of Maria Cardenas, Duke University Medical Center, Durham, NC), and YEp24-CSR1/SFH2 (Santos and Snyder, 2000; generous gift of Beatriz Santos, Universidad de Salamanca, Salamanca, Spain) are all described in detail in the references cited. pRE352 (kes1::URA3, which removes the N-terminal 262 amino acids of Kes1p; Fang et al., 1996), and pME1827 (cki1::LEU2, which removes 757 base pairs in the middle of the 1749-base pair ORF; Rudge et al., 2001) were used to generate chromosomal disruption/deletions of kes1 and cki1, respectively. Finally, plasmids encoding in vivo phosphoinositide-specific reporters, pRS426GFP-PH(FAPP1) and pRS426GFP-2xPH(PLCδ), are described in detail elsewhere (Stefan et al., 2002; generous gift of Scott Emr and Christopher Stefan, University of California at San Diego, La Jolla, CA).
Analysis of Meiosis and Sporulation
Cells were grown in YP-acetate medium and induced to undergo sporulation as previously described (Krisak et al., 1994). In experiments in which sec14, pik1, or mss4 conditional mutants were shifted to restrictive temperature, the culture was induced for 1 h at room temperature to ensure initiation of the sporulation program before the shift to 34.5°C. To drive expression of pGALHA-STT4, 0.03% galactose was added to the sporulation medium (McCarroll and Esposito, 1994). For analysis of meiotic division, samples from triplicate cultures were removed and fixed with 3.7% formaldehyde. Fixed cells were then stained with 4′6-diamindino-2-phenyl-indole (DAPI), examined under a fluorescence microscope, and scored as described before (Rose et al., 1995). For each time point, a minimum of 300 cells was examined from each of three independent cultures. The frequency of spore formation was monitored as the formation of two-, three-, or four-spored asci by phase microscopy; a minimum of 600 cells from each culture was examined.
In Vivo BODIPY-PtdCho Analysis
BODIPY-PtdCho (4 μM final concentration; Molecular Probes, Eugene, OR) was added directly to cultures (∼2 × 108 cells) 4 h after induction of sporulation (and thus 3 h after shift to 34.5°C). Cells were harvested 3 h later, and lipids were extracted as previously described (Rudge et al., 2001). All assays were performed in triplicate. The percentage conversion of intracellular BODIPY-PtdCho to BODIPY-PtdOH was determined from arbitrary fluorescence units obtained from TLC plates using a FluorImager 595 (Molecular Dynamics, Sunnyvale, CA) operating at excitation and emission wavelengths of 488 and 530 nm, respectively. Data values were recorded using ImageQuant 5.1 software (Molecular Dynamics). The amount of BODIPY-PtdOH measured was an underestimation, because Spo14p-derived BODIPY-PtdOH was further metabolized to lyso-BODIPY-PtdOH and BODIPY-diacylglycerol (Rudge et al., 2001).
Immunoblot Analysis
Cell extracts were prepared as described (Rudge et al., 2001). A total of 10 μg of each protein extract was subjected to SDS-PAGE on 5% SDS-polyacrylamide gels. Proteins were electrophoretically transferred onto nitrocellulose membranes (pore size, 0.45 μm; Bio-Rad Laboratories, Hercules, CA), and antibody detection was performed as previously described (Rudge et al., 1998b) using enhanced chemiluminescence detection on preflashed film.
Assays of PLD Activity in Immunoprecipitates
To measure PLD activity in vitro, HA-Spo14p was expressed and immunoprecipitated from lysates of sporulating cells using the mAb 12CA5 (BabCO, Richmond, CA) as described previously (Rudge et al., 1998b). PLD activity was then measured using vesicles containing BODIPY-PtdCho as the substrate, and the product BODIPY-PtdOH was quantitated as a percentage of the input BODIPY-PtdCho, as described before (Rudge et al., 1998b). At least two independent trials of all assays, each performed in triplicate, were carried out.
Fluoresence Microscopy
To visualize the distribution of PtdIns(4)P and PtdIns(4,5)P2 in live cells in real time during sporulation, diploids expressing GFP-2xPH(PLCδ) or GFP-PH(FAPP1) were induced to sporulate, concentrated, and examined by fluorescence microscopy using an Axiovert S1002TV inverted fluorescence microscope (Carl Zeiss, Thornwood, NY). Images were acquired and stored digitally using a Delta Vision deconvolution system (Applied Precision, Seattle, WA).
In Vivo Phosphoinositide Analysis
Analysis of phosphoinositide levels in wild-type and homozygous spo14 diploids was performed by labeling cells with 100 μCi myo-[2-3H]inositol (Nycomed Amersham, Princeton, NJ) for 6 h after transfer to sporulation medium. Cells were harvested by centrifugation and resuspended in 1 ml ice-cold 4.5% perchloric acid (Whiteford et al., 1996). After 15 min on ice, 0.5 g of acid-washed glass beads were added and the cells were lysed by vortexing for 5 min at maximum speed. The cell extracts were centrifuged at 14,000 rpm for 10 min at 4°C. The pellets were washed with 1 ml of 100 mM EDTA, centrifuged as described above, and resuspended in 50 μl of sterile distilled deionized water.
The phospholipids contained within each cell pellet were deacylated by treatment with methylamine (Hawkins et al., 1986). Briefly, 0.5 ml of methylamine reagent (10.7% methylamine, 45.7% methanol, 11.4% 1-butanol) was added to each cell pellet and samples were incubated in a 53°C heat block for 50 min. Unreacted methylamine was then removed in vacuo, and the dried pellet was resuspended in 300 μl of sterile water. After a second sequence of drying in vacuo and resuspension in 300 μl of sterile water, an equal volume of 1-butanol/ethyl ether/formic acid ethyl ester (20:4:1) was added to the pellet suspension. Each sample was vortexed for 5 min and centrifuged at 14,000 rpm for 2 min. The aqueous phase containing the [3H]glycero-phosphoinositides was transferred to a new tube, and the extraction was repeated once more with 1-butanol/ethyl ether/formic acid ethyl ester (20:4:1). Finally, the aqueous phase was collected and dried in vacuo.
Samples were resuspended in sterile water, and equal cpm quantities of wild-type and spo14 diploid [3H]glycero-phosphoinositides were analyzed using an anion exchange PartisphereSAX (Whatman Inc., Clifton, NJ) column coupled to a System Gold HPLC (Beckman Coulter, Fullerton, CA) system and an on-line radiomatic detector (Packard Instruments, Meriden, CT). The column was developed with a gradient of 1 M (NH4)2HPO4, pH 3.8 (pH adjusted with phosphoric acid): 1% for 5 min, 1-20% >44 min, 20-50% >3.75 min, and 50% for 8 min more; the flow rate used was 1.0 ml/min (Stack et al., 1995).
The total cpm values of the [3H]glycero-phosphoinositides extracted from wild-type and spo14 diploids were comparable, and for comparison of the levels of the individual species within each sample, the raw cpms in each peak were expressed as a percentage of the total cpms eluted.
RESULTS
Sec14p Is Required for Meiosis and Spore Formation
To determine if Sec14p might play a role in delivering lipids that contribute to the ability of Spo14p to carry out its essential function during sporulation, we generated a sec14-1/sec14-1 homozygous diploid in the SK-1 strain background, in which sporulation is ordinarily rapid and efficient, even at high temperatures (Fast, 1973). This strain was sporulation proficient at the permissive temperature (25°C) but was unable to form spores at the restrictive temperature of 34.5°C (Table 2, line 1). This defect was due solely to the lack of Sec14p function, because it was fully rescued by a SEC14 plasmid (Table 2, line 2). To determine the stage at which sporulation stalled, we monitored the meiotic divisions. In the absence of Sec14p function, very few cells completed the meiotic divisions (Table 2, lines 1 and 2). In contrast, a spo14 mutant, although totally unable to form spores, did undergo the meiotic divisions, albeit with reduced efficiency (Table 2, line 3; Honigberg et al., 1992; Rose et al., 1995). As expected, a sec14-1 spo14 double mutant was severely defective in both meiosis and spore formation (Table 2, line 4). These data indicate that the Sec14p-dependent processes required for sporulation are not all mediated by Spo14p.
Table 2.
Meiotic progression and spore formation by sec14 mutants
| % Spore formationc
|
|||||
|---|---|---|---|---|---|
| Strain | Relevant genotypea | % Meiotic divisionb | 25°C | 34.5°C | |
| 1. Y3247 | sec14-1ts + CEN | 3 ± 1 | 55 ± 5 | 0.2 ± 0.5 | |
| 2. Y3248 | sec14-1ts + SEC14 CEN | 75 ± 8 | 81 ± 12 | 71 ± 10 | |
| 3. Y3757 | sec14-1ts spo14Δ + SEC14 CEN | 42 ± 5 | <0.1 | <0.1 | |
| 4. Y3756 | sec14-1ts spo14Δ + CEN | 2 ± 1 | <0.1 | <0.1 | |
| 5. Y3351 | sec14-1ts kes1Δ | 62 ± 6 | 75 ± 12 | 55 ± 8 | |
| 6. Y3352 | sec14-1ts cki1 Δ | 45 ± 8 | 73 ± 9 | 41 ± 7 | |
| 7. Y3449 | sec14-1ts kes1Δ spo14Δ + CEN | 4 ± 2 | <0.1 | <0.1 | |
| 8. Y3451 | sec14-1ts kes1Δ spo14Δ + SPO14 CEN | 58 ± 9 | 76 ± 12 | 56 ± 6 | |
| 9. Y315d | SEC14 KES1 | 65 ± 6 | 59 ± 4 | ||
| 10. Y1483d | SEC14 kes1Δ | 68 ± 6 | 63 ± 6 | ||
| 11. Y3537d | sec14Δ kes1Δ | 63 ± 5 | 55 ± 5 | ||
| 12. Y3450 | sec14-1ts kes1Δspo14Δ + spo14S-11 CEN | 37 ± 4 | <0.1 | <0.1 | |
| 13. Y3528 | sec14-1ts + sec14(K66A K239A)CEN | 48 ± 8 | 59 ± 7 | 5 ± 1 | |
| 14. Y3553 | sec14-1ts + sec14(K66A K239A) 2μ | 68 ± 7 | 77 ± 1 | 64 ± 5 | |
| 15. Y3404 | sec14-1ts + SFH2 2μ | ND | 78 ± 5 | 65 ± 3 | |
| 16. Y4515 | sec14-1ts spo14Δ + SFH2 2μ | 2 ± 1 | <0.1 | <0.1 | |
| 17. Y3645 | sec14-1ts + SFH4 2μ | ND | 75 ± 4 | 56 ± 4 | |
| 18. Y4509 | sec14-1ts spo14Δ + SFH4 2μ | 3 ± 1 | <0.1 | <0.1 | |
| 19. Y3663 | sec14-1ts + PIK1 2μ | 52 ± 6 | 77 ± 6 | 52 ± 4 | |
| 20. Y4510 | sec14-1ts spo14Δ + PIK1 2μ | 3 ± 2 | <0.1 | <0.1 | |
| 21. Y3587 | sec14-1ts + STT4 2μ | 6 ± 2 | 55 ± 5 | <0.1 | |
| 22. Y3408 | sec14-1ts + MSS4 2μ | 2 ± 1 | 55 ± 5 | <0.1 | |
| 23. Y4514 | sec14-1ts + sec14(K66A K239A)CEN + MSS4 2μ | ND | 62 ± 6 | 4 ± 1 | |
| 24. Y4513 | sec14-1ts + sec14(K66A K239A)CEN + SPO14 2μ | ND | 58 ± 7 | 6 ± 2 | |
CEN refers to a low-copy-number plasmid, whereas 2μ refers to a high-copy-number plasmid
The meiotic divisions at 34.5°C were monitored by DAPI fluorescence (see MATERIALS AND METHODS); cells that had completed Meiosis I (2 DAPI-staining bodies) or Meiosis II (3 or 4 DAPI-staining bodies) were scored. No significant differences in success in completing Meiosis I vs. Meiosis II were observed. Values are means ± SD
Frequencies of spore formation at the indicated temperatures were determined as described in MATERIALS AND METHODS; values are means ± SD
S288C strain background. Meiosis and spore formation were monitored at 30°C because strains of this background do not sporulate well at 34.5°C
The failure of the sec14-1 cells to undergo the meiotic divisions might mean that meiosis was not initiated in these strains. To address this issue, we examined the expression of HOP1, a gene that is not expressed in vegetative cells but is specifically induced in prophase of Meiosis I (Hollingsworth et al., 1990; Vershon et al., 1992). Using a plasmid-borne HOP1-lacZ gene fusion as a reporter (Vershon et al., 1992), we found that no activity was expressed in vegetatively growing sec14-1 cells, as expected, but that expression was induced upon transfer to sporulation medium at both permissive (25°C) and restrictive (34.5°C) temperatures (unpublished data). Thus, the sec14-1 cells do appear to initiate the sporulation program.
The Requirement for Sec14p in Meiosis Can Be Bypassed
In vegetative cells, the requirement for Sec14p can be alleviated by the inactivation of any of several genes, including CKI1, an enzyme in the CDP-choline pathway for PtdCho biosynthesis (Cleves et al., 1991), and KES1, a PtdIns-binding protein that antagonizes Sec14p function (Li et al., 2002). This phenomenon is referred to as “Sec14 bypass” (Cleves et al., 1991); under these circumstances, Spo14p is essential (Sreenivas et al., 1998; Xie et al., 1998; Li et al., 2002). To determine whether the requirement for Sec14p in sporulation could also be relieved by such mutations, we constructed the appropriate double mutants. The sec14-1 kes1Δ and the sec14-1 cki1Δ cells underwent meiosis and sporulated at essentially wild-type efficiency (Table 2, lines 5-6), and, as in vegetative cells, this suppression required the presence of functional Spo14p (Table 2, lines 7-8; unpublished data). Because the 34.5°C used in the sporulation experiments is close to the minimum restrictive temperature for the sec14-1 allele (Bankaitis et al., 1989), it seemed possible that kes1 and cki1 mutations permit sec14-1 diploids to sporulate simply by reducing the severity of the sec14-1 allele. However, a sec14Δ kes1Δ strain was both viable, as reported previously (Cleves et al., 1991), and sporulated well (Table 2, lines 9-11).
We previously identified the spo14S (S for separation-of-function) mutant alleles, which permit vegetative growth of sec14 cells carrying bypass mutations but fail to support spore formation in an otherwise wild-type background (Rudge et al., 2001). When these spo14S alleles were expressed as the sole source of Spo14p in sec14-1 kes1Δcells, they again were unable to support spore formation (Table 2, line 12; our unpublished results). However, these strains were able to complete meiosis with reasonable efficiency. Thus, these findings suggest that Sec14p, or under Sec14-bypass conditions Spo14p, is adequate for supporting meiosis, but that Spo14p function satisfies an additional requirement in prospore membrane formation.
PtdIns Binding/Transport Activity of Sec14p is Required for Maximal Spo14p Activity and for Spore Formation
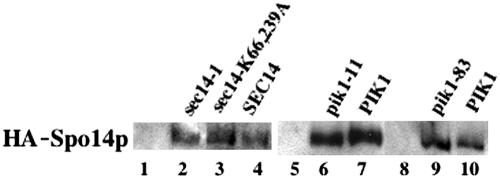
To determine whether inactivation of Sec14p had any effect on Spo14p function in sporulating cells, we monitored Spo14p PLD activity by measuring conversion of internalized BODIPY-PtdCho to BODIPY-PtdOH. As previously reported, hydrolysis of BODIPY-PtdCho occurs almost exclusively by the action of Spo14p, as spo14Δ strains did not generate any appreciable BODIPY-PtdOH (Table 3, lines 1-2; Rudge et al., 2001). Inactivation of Sec14p resulted in a decrease in Spo14p-dependent generation of BODIPY-Pt-dOH (Table 3, lines 3-4). This did not appear to be due to reduced synthesis of Spo14p, because steady state levels of HA-Spo14p were not significantly altered in the sec14-1 mutant (Figure 1; lanes 2 and 4). Thus, in contrast to what is observed in vegetative cells, where Spo14p activity is stimulated in the absence of Sec14p (Sreenivas et al., 1998), Spo14p activity in sporulating cells appears to be Sec14p dependent. From these data alone, it is not clear whether this effect reflects a direct role of Sec14p in promoting Spo14p activity or the early blockage of the sporulation program (at a point before Spo14p activity is maximal) by the sec14-1 lesion.
Table 3.
Spo14p-catalyzed hydrolysis of internalized BODIPY-Ptd-Choa
| Strain | Relevant genotype | % BODIPY-PtdOH |
|---|---|---|
| 1. Y3757 | spo14Δ | 0.41 ± 0.05 |
| 2. Y3248 | SPO14 SEC14 | 5.1 ± 0.7 |
| 3. Y3247 | sec14-1 | 2.0 ± 0.5 |
| 4. Y3756 | sec14-1 spo14 Δ | 0.51 ± 0.10 |
| 5. Y3528 | sec14(K66A K239A) | 1.5 ± 0.2 |
| 6. Y4283 | sec14(K66A K239A) spo14 Δ | 0.43 ± 0.08 |
| 7. Y3655 | PIK1 | 5.0 ± 0.6 |
| 8. Y3654 | pik1-11 | 2.0 ± 0.4 |
| 9. Y3890 | pik1-11 spo14Δ | 0.48 ± 0.10 |
| 10. Y3724 | PIK1 | 5.9 ± 0.7 |
| 11. Y3723 | pik1-83 | 1.9 ± 0.1 |
| 12. Y3907 | pik1-83 spo14Δ | 0.45 ± 0.08 |
| 13. Y4355 | MSS4 | 5.3 ± 1.0 |
| 14. Y4354 | mss4-2 | 1.9 ± 0.4 |
| 15. Y4409 | mss4 spo14Δ | 0.43 ± 0.10 |
The percentage conversion of intracellular BODIPY-PtdCho to BODIPY-PtdOH was determined in cells sporulating at 34.5°C as described in MATERIALS AND METHODS. Values are means ± SD from three independent experiments
Figure 1.
HA-Spo14p in sec14 and pik1 mutants. Immunoblot of HA-Spo14p in (1) sec14-1 (Y4278); (2) sec14-1 + HA-SPO14 (Y4279); (3) sec14(K66A K239A) + HA-SPO14 (Y4297); (4) SEC14 + HA-SPO14 (Y4281); (5) pik1-11 (Y4288); (6) pik1-11 + HA-SPO14 (Y4289); (7) PIK1 + HA-SPO14 (Y4291); (8) pik1-83 (Y4284); (9) pik1-83 + HA-SPO14 (Y4285); and (10) PIK1 + HA-SPO14 (Y4287).
Sec14p possesses both PtdCho and PtdIns transfer activity (Bankaitis et al., 1990). Thus, the reduction in Spo14p activity observed in sec14-1 cells could be due to the loss of Sec14p-dependent transport of the substrate, PtdCho, to the cellular compartment(s) where Spo14p functions, to loss of Sec14p-dependent transport of PtdIns and the consequent failure to synthesize the PLD activator PtdIns(4,5)P2, or both. To explore the relative contributions of PtdIns and PtdCho binding/transport by Sec14p, we took advantage of a mutant, sec14(K66A K239A), that retains the ability to bind and transport PtdCho but is specifically defective in PtdIns binding and transport (Phillips et al., 1999). This mutant supports the essential function of Sec14p for growth and secretion in vegetative cells (Phillips et al., 1999). However, when expressed in sec14-1 cells at near-normal levels from a lowcopy-number plasmid, this mutant supported only very low levels of spore formation at the nonpermissive temperature (Table 2, line 13). Remarkably, however, the mutant restored the ability to complete meiosis to 64% of the level seen in cells expressing wild-type SEC14 (Table 2, lines 2 and 13). These observations suggest that Sec14p function (and its PtdIns-transport role specifically) plays an essential role in spore formation that is independent of the Sec14p role in meiosis.
Moreover, when we assayed Spo14p activity in the sec14(K66A K239A) mutant, it was reduced as in the sec14-1 strain (Table 3, lines 5 and 6), even though the sec14(K66A K239A) mutation also did not appear to affect Spo14p protein levels (Figure 1, lanes 3 and 4). These data suggest strongly that the reduction in Spo14p activity in the absence of Sec14p function does not simply reflect an early block in the sporulation program. Instead, the PtdIns transport activity of Sec14p appears to be necessary to obtain maximal Spo14p activity, perhaps explaining, at least in part, its importance for spore formation. A plausible hypothesis is that PtdIns transport by Sec14p is necessary for maximal synthesis of the Spo14p activator PtdIns(4,5)P2.
The observation that sec14(K66A K239A) increased the sporulation proficiency of a sec14-1 strain significantly (from ∼0.2 to ∼5%) might mean that the mutant protein possesses residual PtdIns transport activity and/or the delivery or removal of PtdCho to or from a membrane compartment also contributes to the efficiency of sporulation. Consistent with either alternative, expression of sec14(K66A K239A) from a high-copy-number plasmid increased sporulation proficiency to near normal levels at both permissive and restrictive temperatures (Table 2, line 14).
Suppression of sec14 Sporulation Defect by Overexpression of Sfh2p, Sfh4p, or Pik1p, but not Mss4p or Spo14p
A number of Sec14p homologues exist in yeast that possess PtdIns, but not PtdCho, transport activities. In vegetative cells, two of these, Sfh2/Csr1p (Li et al., 2000; Santos and Snyder, 2000) and Sfh4/Pdr17p (van den Hazel et al., 1999; Li et al., 2000), potently suppress the sec14-1 lethality when overexpressed, and this suppression is dependent on Spo14p (Li et al., 2000). Likewise, these proteins suppressed the sec14-1 meiosis and spore-formation defects (Table 2, lines 15 and 17), and the suppression of the meiotic defect was dependent on Spo14p function (Table 2, lines 16 and 18). These results support the conclusions that Spo14p can compensate for loss of Sec14p function in meiosis and that PtdIns transport is important for spore formation.
In vegetative sec14(K66A K239A) cells, the synthesis of PtdIns(4)P is reduced (Phillips et al., 1999), and overexpression of the PtdIns 4-kinase Pik1p suppresses the growth defect of sec14-1 cells at semirestrictive temperature (Hama et al., 1999). Similarly, we found that overexpression of PIK1 could restore the sporulation of sec14-1 diploids to near wild-type frequency, and suppression of the meiotic defect was dependent on Spo14p (Table 2, lines 19-20). In contrast, overexpression of the other PtdIns 4-kinase isoform, Stt4p, which supplies PtdIns(4)P primarily at the plasma membrane (Audhya et al., 2000), was unable to suppress the sporulation defect of sec14-1 cells (Table 2, line 21). These results support the hypothesis that PtdIns delivery is an essential function of Sec14p for spore formation and suggest that it is specifically the pool of PtdIns(4)P available at the Golgi that is critical.
In contrast, overexpression of the PtdIns(4)P 5-kinase Mss4p was unable to rescue the sec14-1 or sec14(K66A K239A) sporulation defect (Table 2, lines 22-23). Superficially, these results suggest that the critical lipid for spore formation is PtdIns(4)P and not PtdIns(4,5)P2. However, in the absence of an elevated supply of its immediate substrate, overexpressed Mss4p may be unable to generate a marked elevation in PtdIns(4,5)P2, as suggested by the observations of Desrivières et al. (1998). We favor this interpretation because our other observations (see below) suggest that PtdIns(4,5)P2 does have an important role(s) in spore formation. Alternatively, both PtdIns(4)P and PtdIns(4,5)P2 could be important for sporulation.
Finally, overexpression of Spo14p was unable to rescue the sec14(K66A K239A) spore-formation defect (Table 2, line 24). This result suggests that when phosphoinositides are limiting, additional copies of Spo14p do not provide more PLD activity, that phosphoinositides play roles in spore formation in addition to activating Spo14p, or both.
PIK1 Function Is Required for Sporulation
Like sec14-1 cells, diploids homozygous for either of two pik1-ts alleles (Hendricks et al., 1999) were defective for both meiosis and spore formation at the nonpermissive temperature (Table 4, line 1, and unpublished data). These defects were fully rescued by a low-copy-number PIK1 plasmid (Table 4, line 2). As expected, pik1 spo14Δ double mutants were severely defective in both meiosis and spore formation (Table 4, line 3). Consistent with the hypothesis that Sec14p provides precursor for Pik1p (see above), overexpression of SEC14 could not suppress the pik1 defect (Table 4, line 4). These observations suggest that PtdIns(4)P synthesis by Pik1p is required for meiosis for reasons independent of any possible PtdIns(4,5)P2-mediated stimulation of Spo14p. Consistent with this conclusion, overexpression of SPO14 also could not suppress the sporulation defect of pik1-ts cells (Table 4, line 5). Nonetheless, as measured by the BODIPY-PtdCho assay, Spo14p activity was reduced when Pik1p was inactivated (Table 3, lines 7-12), although Spo14p levels were not significantly altered (Figure 1, lanes 6-10). Thus, although Pik1p appears to have a role in meiosis that is independent of Spo14p, it also appears to be necessary for full activation of Spo14p.
Table 4.
Meiotic progression and sporulation proficiency of pik1 and mss4 mutantsa
| % Spore formationb
|
||||
|---|---|---|---|---|
| Strain | Relevant genotype | Meiotic divisionb | 25°C | 34.5°C |
| 1. Y3654 | pik1-11 + CEN | 4 ± 2 | 75 ± 3 | 1 ± 1 |
| 2. Y3655 | pik1-11 + PIK1 CEN | 72 ± 8 | 80 ± 5 | 72 ± 3 |
| 3. Y3890 | pik1-11 spo14Δ | 2 ± 1 | <0.1 | <0.1 |
| 4. Y4076 | pik1-11 + SEC14 2μ | 2 ± 1 | 75 ± 3 | 1 ± 1 |
| 5. Y4077 | pik1-11 + SPO14 2μ | 2 ± 1 | 75 ± 3 | 1 ± 1 |
| 6. Y4354 | mss4-2 + CEN | 30.3 ± 6.3 | 75 ± 3 | 8.3 ± 1.5 |
| 7. Y4355 | mss4-2 + MSS4 CEN | 80 ± 4.4 | 80 ± 5 | 72 ± 3 |
| 8. Y4409 | mss4-2 spo14Δ | 35 ± 5 | <0.1 | <0.1 |
| 9. Y4361 | mss4-2 + SEC14 2μ | 37 ± 5 | 75 ± 3 | 9.1 ± 1 |
| 10. Y4360 | mss4-2 + PIK1 2μ | 29 ± 5 | 75 ± 3 | 7.8 ± 1 |
| 11. Y4359 | mss4-2 + SPO14 2μ | 35 ± 5 | 79 ± 6 | 8.6 ± 1 |
Data for pik1-11 are shown; data for pik1-83 were essentially identical
Meiotic divisions at 34.5°C and spore formation were monitored as described in Table 2
PtdIns(4,5)P2 Synthesis is Essential for Sporulation
If sec14 and pik1 mutants display decreased Spo14p activity because of failure to generate adequate levels of PtdIns(4,5)P2, then the PtdIns4(P) 5-kinase Mss4p should also be required for spore formation and maximal Spo14p PLD activity. Indeed, an mss4-2 strain was defective for spore formation at the nonpermissive temperature although many cells progressed through meiosis (Table 4, lines 6-7). As expected, deletion of SPO14 eliminated the residual sporulation of the mss4-2 mutant (Table 4; line 8).
Overexpression of SEC14 or PIK1 did not suppress the mss4-2 defect (Table 4, lines 9-10), suggesting that increasing the Mss4p substrate, PtdIns(4)P, did not increase PtdIns(4,5)P2 levels. Furthermore, as measured by the BODIPY-PtdCho assay, Spo14p activity was reduced when Mss4p was inactivated (Table 3, lines 13-15), suggesting that Mss4p function might be required for spore formation at least in part through activation of Spo14p. However, a complication for this simple model is that overexpression of SPO14 did not suppress the mss4-2 defect (Table 4, line 11).
PtdIns(4,5)P2 Is a Specific Activator of the PLD Activity of Spo14p
Given the effects of the sec14(K66A K239A) and pik1 mutations on spore formation and Spo14p activity, it seemed possible that PtdIns and/or PtdIns(4)P, in addition to PtdIns(4,5)P2 (Rose et al., 1995; Sciorra et al., 1999, 2002), might directly modulate Spo14p activity. However, when epitope-tagged Spo14p was expressed in sporulating cells, immunoprecipitated, and assayed for PLD activity, only PtdIns(4,5)P2 had a significant effect on PLD activity (Table 5). Thus, PtdIns and PtdIns(4)P would presumably need to be converted in the cell to PtdIns(4,5)P2 to influence the PLD activity of Spo14p.
Table 5.
Phosphoinositide specificity of Spo14p activation
| Phosphoinositide | BODIPY-PtdOHa |
|---|---|
| No phosphoinositide | 30 ± 7 |
| PtdIns | 36 ± 11 |
| PtdIns(4)P | 35 ± 6 |
| PtdIns(4,5)P2 | 246 ± 17 |
BODIPY-PtdOH formation is expressed as nM per input of HA-Spo14p. HA-Spo14p was immunoprecipitated from extracts of sporulating cells of strain Y598 and its PLD activity was assayed as described in MATERIALS AND METHODS. PtdIns, PtdIns(4)P, or PtdIns(4,5)P2 was added at 5 μM, the concentration of Ptdlns(4,5)P2 known to produce maximum stimulation of Spo14p activity (Rose et al., 1995). Values are means± SD of three independent trials, each performed in duplicate
PtdIns(4,5)P2 Localizes to the Prospore Membrane during Sporulation
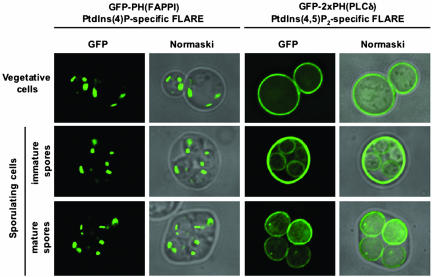
To explore further the apparent requirement for PtdIns(4,5)P2 during sporulation, we used fluorescent probes for localizing specific phosphoinositides to individual cellular membranes in yeast (Stefan et al., 2002). In these probes, green fluorescent protein (GFP) is fused to pleckstrin homology (PH) domains specific for either PtdIns(4)P [PH(FAPPI)] (Dowler et al., 2000) or PtdIns(4,5)P2 [PH(PLCδ)] (Kavran et al., 1998). In vegetatively growing cells, these probes indicate that PtdIns(4)P is enriched at the Golgi, whereas PtdIns(4,5)P2 is concentrated at the plasma membrane (Figure 2, top panels; Levine and Munro, 2002; Stefan et al., 2002). In sporulating cells, the PtdIns(4)P-specific probe decorated defined patches within each spore, similar to the pattern seen in vegetative cells (Figure 2, left panels). In striking contrast, the PtdIns(4,5)P2-specific probe was confined essentially to the plasma membrane and/or prospore membranes depending on the stage of sporulation examined (Figure 2, right panels). Thus, both Spo14p (Rudge et al., 1998b) and PtdIns(4,5)P2 localize to the prospore membrane, consistent with the hypothesis that production of PtdIns(4,5)P2 for activation of Spo14p is a key role of Sec14p, Pik1p, and Mss4p in spore formation. A complication is that we did not observe a defined intracellular signal for the PtdIns(4,5)P2-specific probe before prospore membrane closure, although a spo14Δ mutant blocks early in prospore membrane formation. However, this may be because most of the PtdIns(4,5)P2 is in the plasma membrane of the mother cell and a certain critical mass of prospore membrane is required to observe the internal pools of PtdIns(4,5)P2. In addition, we cannot exclude the possibility that there are small pools of PtdIns(4)P at the prospore membranes that are not detectable by the PtdIns(4)P-specific probe, perhaps because they are rapidly phosphorylated to PtdIns(4,5)P2 by Mss4p.
Figure 2.
Prospore membranes are enriched in PtdIns(4,5)P2. The steady state localizations of PtdIns(4)P and PtdIns(4,5)P2 were visualized in vegetative and sporulating wild-type cells (strains Y4048 and Y4049) as described in MATERIALS AND METHODS. Samples were taken 8 and 12 h after the induction of sporulation. Fluorescence (left panels) and Normarski (right panels) images of the same cells are shown.
Dependence of Spore Formation on Spo14p-derived PtdOH
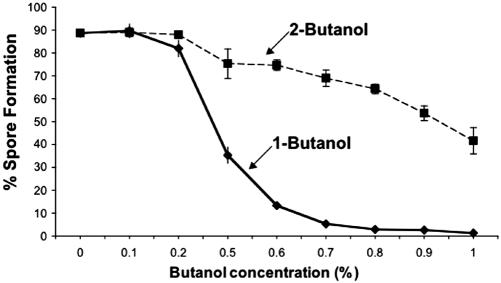
Spo14p action simultaneously reduces PtdCho and increases PtdOH and choline. To determine which of these effects represents the essential role of Spo14p in spore formation, we made use of an enzymatic property of the PtdCho-specific PLDs, the transphosphatidylation reaction, which is specific to Spo14p in yeast (Rose et al., 1995; Rudge and Engebrecht, 1999; Rudge et al., 2001). In the presence of primary alcohols, these enzymes cleave PtdCho and release choline, but generate a biologically inert phosphatidylalcohol lipid rather than PtdOH. Thus, we monitored the effect of addition of either 1-butanol (which is a substrate for the transphosphatidylation reaction) or of 2-butanol (which is not a substrate) on sporulation. Spore formation, but not meiosis, was significantly inhibited by 1-butantol at concentrations at which 2-butanol had little effect (Figure 3), suggesting that PtdOH is the essential product of Spo14p action during sporulation.
Figure 3.
Dependence of spore formation on Spo14p-generated PtdOH. Spore formation by wild-type strain NH144 in the presence of various concentrations of 1-butanol or 2-butanol. 75± 7 and 62± 5% of the cells underwent the meiotic divisions in the presence of 0.6 and 0.7% of 1-butanol, respectively.
Spo14p Does Not Appear to Function Upstream of Mss4p during Spore Formation
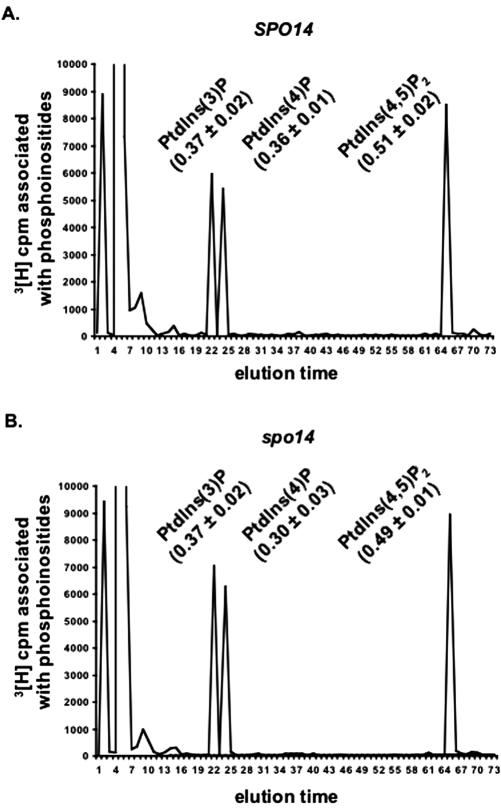
Given the apparent stimulation of Mss4p by PtdOH (Homma et al., 1998) and the observation that the mss4-2 mutation affected spore formation more strongly than it did meiosis (Table 4, line 6), it seemed possible that a major role of Spo14p in sporulation is to ensure that PtdIns(4,5)P2 synthesis occurs at a sufficiently high rate. In this case, PtdIns(4,5)P2 synthesis would presumably be compromised in sporulating spo14Δ cells, and overexpression of the phosphoinositide kinases might rescue spo14 mutants. To explore these possibilities, we first analyzed the glycero-phosphoinositides derived from [3H]inositol-labeled sporulating cells. The results indicated that the levels of PtdIns(3)P, PtdIns(4)P, and PtdIns(4,5)P2 were similar in spo14Δ and normal cells (Figure 4). (PtdIns(3,5)P2 was undetectable in all [3H]inositol-labeled sporulating cells tested.) We then examined whether overexpression of PIK1, STT, and/or MSS4 could suppress the spore formation defect of spo14Δcells or of cells containing either the spo14S-11 or conditional spo14(S251P) allele (Rudge et al., 2002). No one plasmid, or pair of plasmids, allowed spo14 mutants to produce spores (Table 6 and unpublished data).
Figure 4.
Spo14p does not regulate PtdIns(4,5)P2 levels during sporulation. Phosphoinositide levels in wild-type (A) and homozygous spo14Δ diploids (strains NH144 and Y433; B) were determined by HPLC analysis of sporulating cells as described in MATERIALS AND METHODS. The numbers in parentheses represent the total cpm in that peak expressed as a percentage of the total cpm eluted from the column; means± ranges of two independent experiments are shown. (Note that glycero-phosphatidylinositol [the material eluted at 4-7 min] represents ∼97% of the total glycero-phosphoinositides extracted.)
Table 6.
Sporulation proficiency of spo14 mutants
| Strain | Relevant genotype | % Sporulationa |
|---|---|---|
| Y4516 | spo14Δ + 2μ | <0.1 |
| Y4517 | spo14Δ + PIK1 2μ | <0.1 |
| Y4518 | spo14Δ + STT4 2μ | <0.1 |
| Y4519 | spo14Δ + MSS4 2μ | <0.1 |
| Y4520 | spo14Δ + PIK1 2μ + MSS4 2μ | <0.1 |
| Y4521 | spo14Δ + STT4 2μ + MSS4 2μ | <0.1 |
| Y3825 | spo14-S11 + 2μ | <0.1 |
| Y3827 | spo14-S11 + PIK1 2μ | <0.1 |
| Y4522 | spo14-S11 + STT4 2μ | <0.1 |
| Y3826 | spo14-S11 + MSS4 2μ | <0.1 |
| Y3828 | spo14-S11 + PIK1 2μ + MSS4 2μ | <0.1 |
| Y4523 | spo14-S11 + STT4 2μ + MSS4 2μ | <0.1 |
Spore formation was monitored 30°C as described in Table 2
Taken together, these data argue against the hypothesis that a major role of Spo14p in sporulation is to generate PtdOH to stimulate PtdIns(4,5)P2 synthesis. Instead, it appears that Spo14p-generated PtdOH (and/or products derived from it, such as diacylglycerol) plays an essential role in prospore membrane formation that is distinct from the role(s) of phosphoinositides.
DISCUSSION
Phosphoinositide Function during Meiosis
In this study, we have demonstrated that the PtdIns/PtdCho transfer protein Sec14p and the PtdIns 4-kinase Pik1p are both required for meiotic progression. In contrast, although the PLD Spo14p is required for spore formation, its activity is not necessary for reasonably efficient initiation and completion of Meiosis I and Meiosis II (Honigberg et al., 1992; Rose et al., 1995). Thus, although our data suggest that Sec14p-regulated synthesis of PtdIns(4,5)P2 is essential for activation of Spo14p, as discussed further below, phosphoinositides clearly play additional roles during sporulation (Figure 5). In particular, it seems likely that PtdIns(4)P is important for meiosis because pik1 mutants are severely defective in meiotic division, and overexpression of Pik1p, but not of the PtdIns(4)P 5-kinase, Mss4p, potently suppresses the sec14-1 meiotic defect. In contrast to the sec14-1 and pik1 mutants, the mss4-2 mutant was able to undergo meiosis with reasonable efficiency but failed to form spores. This indicates either that the synthesis of PtdIns(4)P, but not PtdIns(4,5)P2, is important for meiosis or that the synthesis of PtdIns(4,5)P2 is important but that the mss4-2 allele is not a null mutation at the temperature sporulation was examined (34.5°C). In support of the latter possibility, the restrictive temperature for the mss4-2 allele in vegetative cells is 37°C (Desrivières et al., 1998), and even at 34.5°C there was a partial defect in the completion of meiosis.
Figure 5.
Model for the possible relationships between the phosphoinositides [PtdIns, PtdIns(4)P and PtdIns(4,5)P2] and Spo14p-generated PtdOH and their functions in meiosis and spore formation. See text for details. The dashed arrow indicates uncertainty about the role of PtdIns(4,5)P2 in meiosis, probably due to leakiness of the mss4 allele used.
Although Sec14p function is important for meiotic progression, its exact role(s) remain unknown. The ability of the sec14(K66A K239A) mutant, which is specifically defective in PtdIns transport (Phillips et al., 1999), to undergo the meiotic divisions at first sight suggests that it is only the PtdCho transport activity, and not the PtdIns transport activity, of Sec14p that is important for meiosis. However, the finding that overexpression of Sfh2p or Sfh4p, PITPs that lack PtdCho transport activity, suppresses the sec14-1 sporulation defect, implies that PtdIns transport is indeed important for meiosis. Thus, presumably, either Sec14(K66A K239A)p has residual PtdIns transport activity or normal levels of Sfh2p and Sfh4p can provide sufficient PtdIns transport for meiosis in a sec14(K66A K239A) background. That the suppression by SFH2 or SFH4 is dependent on Spo14p indicates that Spo14p PLD activity can substitute for Sec14p under these conditions. This may be due to activation of Spo14p through enhanced PtdIns(4,5)P2 generation, as observed in Sec14 bypass conditions (Li et al., 2000), and may be a consequence of Spo14p-dependent PtdCho cleavage mimicking Sec14p-PtdCho transport function.
What is the role of phosphoinositides in meiosis? Substantial evidence demonstrates that both sec14 (Bankaitis et al., 1989) and pik1 (Hama et al., 1999; Walch-Solimena and Novick, 1999) mutations block vesicle-mediated transport from the Golgi. That both sec14-1 and pik1-ts diploids are unable to carry out meiosis suggests that trafficking through the secretory pathway is required for meiotic progression. Alternatively, these proteins and the phosphoinositides generated by their action may play additional roles during meiosis. Consistent with the idea that trafficking is important for meiosis, gsg1 mutants are defective in meiotic division (Engebrecht et al., 1998), and recent work has indicated that Gsg1p is the high-molecular-weight subunit of both the TRAPP I complex, involved in vesicle-mediated transport from the ER to the Golgi, and the TRAPP II complex, involved in intra-Golgi trafficking (Sacher et al., 2000, 2001). Mutations in SPO15/VPS1, which encodes a dynamin-like protein required for sorting of proteins from the Golgi to the vacuole, also fail to undergo meiotic division (Yeh et al., 1991). Thus, it seems that successful meiotic progression requires the function of much of the normal secretory machinery.
Phosphoinositides and Spo14p-generated PtdOH in Spore Formation
In addition to their involvement in meiosis, phosphoinositides, and PtdIns(4,5)P2 in particular, appear to have a distinct role(s) in spore formation (Figure 5). This conclusion is supported by several lines of evidence, including 1) the completion of meiosis but not spore formation by the sec14(K66A K239A) and mss4-2 mutants; 2) the suppression of the sec14-1 spore-formation (as well as meiosis) defect by overexpression of PIK1, SFH2, or SFH4; 3) the dependence of spore formation on Spo14p and its product PtdOH; and 4) the dependence of Spo14p activity on Sec14p-, Pik1p-, and Mss4p-dependent generation of PtdIns(4,5)P2. Not surprisingly, it does not appear that activation of Spo14p is the only target of phosphoinositides in spore formation, because overexpression of Spo14p was unable to relieve the sporulation defect of either the sec14(K66A K239A) or mss4-2 mutant.
What is the relationship between PtdIns(4,5)P2 and PtdOH during spore formation? One possibility is that PtdOH derived from Spo14p-mediated hydrolysis of PtdCho is important for stimulating Mss4p (Homma et al., 1998), thereby providing for a positive feedback loop that promotes optimal synthesis of PtdIns(4,5)P2 and supports those processes that depend uniquely and vitally on this phosphoinositide. However, the stimulation of PtdIns(4)P 5-kinases by PtdOH has been observed in vitro, and the physiological relevance of these observations is not known. Because Spo14p action is a major pathway for regulated PtdOH formation in S. cerevisiae (which lacks any recognizable diacylglycerol kinase; Kearns et al., 1997), this organism is a good system to investigate the relationship between PtdOH levels and PtdIns(4,5)P2 synthesis. Our finding that PtdIns(4,5)P2 levels are not detectably reduced in the absence of Spo14p, whereas PtdOH levels are reduced (Rudge et al., 2001), indicates that PtdOH does not markedly stimulate PtdIns(4,5)P2 synthesis. However, we cannot exclude the possibility that Spo14p-dependent PtdOH stimulates small and highly localized changes in PtdIns(4,5)P2 concentration that are especially critical for sporulation.
Hence, it seems that both PtdIns(4,5)P2 and PtdOH generation contribute to establishing a lipid environment critical to the function of proteins necessary for the membrane trafficking events required for prospore membrane synthesis. Recently, it has been reported that Gcs1p and Age2p, two Golgi-associated GTPase-activating proteins (GAPs) for the Arf family of small GTPases, which have an essential role in the formation of coated vesicles for both exocytic and endocytic transport, are activated by PtdOH and diacylglycerol (which can be derived from PtdOH), inhibited by PtdCho, and probably recruited to membranes via a PH domain that binds phosphoinositides (Yanagisawa et al., 2002). These Arf-GAPs, and perhaps other proteins, may act as integrators that decipher the relative levels of PtdCho, PtdOH, and phosphoinositides and thereby adjust the rate of vesicle-mediated membrane trafficking accordingly. In support of these ideas is the requirement for Arf1p in sporulation, which occurs downstream of Spo14p-mediated PtdOH synthesis (Rudge et al., 1998a) and perhaps explains why yeast do not contain an Arf-activated PLD (Rudge et al., 1998a; Rudge and Engebrecht, 1999). Once vesicles are generated, there may be a specific requirement for Spo14p-generated PtdOH in vesicle fusion at the SPBs (our unpublished results).
Although our study was in progress, it was found that the spo20+ gene of the fission yeast Schizosaccharomyces pombe, which was identified originally on the basis of its requirement for prospore membrane formation (Hirata and Shimoda, 1992), encodes a homolog of S. cerevisiae Sec14p (Nakase et al., 2001). Unlike sec14-1 cells, which we found were unable to carry out meiosis, the spo20 mutant S. pombe cells progressed through meiosis. This difference is probably attributable to a partial retention of function by the spo20 allele used, based on its other phenotypes. In addition, the PtdIns-binding-deficient Sec14(K66A K239A)p, when expressed from a strong inducible promoter on a high-copy plasmid, fully compensated for the loss of Spo20p function during S. pombe sporulation (Nakase et al., 2001). However, these investigators did not examine lower levels of expression. As we have shown here, when expressed at a more physiological level, Sec14(K66A K239A)p is unable to support S. cerevisiae spore formation. It would be interesting to determine if the S. pombe homolog of Spo14p (Rudge and Engebrecht, 1999) is essential for prospore membrane formation and/or if Spo20p is required for the maximal activity of that PLD. Alternatively, the requirements for PtdIns transport activity in sporulation may be different in the two yeasts.
In conclusion, our results provide the first in vivo evidence that Sec14p positively contributes to Spo14p/PLD function through its role in the synthesis of PtdIns(4,5)P2. Furthermore, our findings indicate that both phosphoinositides and Spo14p-dependent PtdOH synthesis are essential for sporulation in S. cerevisiae. We propose that the action of Spo14p in generating PtdOH lies downstream of a Sec14p-Pik1p-Mss4p-dependent pathway of PtdIns(4,5)P2 synthesis that is essential for the formation and/or delivery of post-Golgi vesicles to, or their fusion at, the meiotic plaques of the SPBs, where prospore membrane formation begins.
Acknowledgments
We thank Maria Cardenas, Maria Adelaide do Valle Matta, Yoshikazu Ohya, Beatriz Santos, Michael Hall, Vytas Bankaitis, and Scott Emr for the generous gifts of yeast strains and plasmids; Christopher Stefan for advice on the use of his fluorescent PIP-FLARES; Jaime Connolly, Eric Sawey, and Aimee Jaramillo-Lambert for technical assistance; and Anjon Audhya for helpful discussions. We are especially indebted to Scott D. Emr (Howard Hughes Medical Institute, University of California at San Diego, La Jolla, CA) for material support (to S.A.R. and V.A.S.) during the completion of this work. This work was supported by National Institutes of Health (NIH) Research Grant GM21841 and a grant from the Lowe Syndrome Association (to J.T.), by NIH Research Grants GM54388 and CA12451 (to A.J.M.), and by NIH Research Grant GM66124 (to J.E.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-04-0245. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-04-0245.
References
- Audhya, A., Foti, M., and Emr, S.D. (2000). Distinct roles for the yeast phosphatidylinositol 4-kinases, Stt4p and Pik1p, in secretion, cell growth, and organelle membrane dynamics. Mol. Biol. Cell 11, 2673-2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankaitis, V.A., Aitken, J.R., Cleves, A.E., and Dowhan, W. (1990). An essential role for a phospholipid transfer protein in yeast Golgi function. Nature 347, 561-562. [DOI] [PubMed] [Google Scholar]
- Bankaitis, V.A., Malehorn, D.E., Emr, S.D., and Green, R. (1989). The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J. Cell Biol. 108, 1271-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, H.A., Gutowski, S., Kahn, R.A., and Sternweis, P.C. (1995). Partial purification and characterization of Arf-sensitive phospholipase D from porcine brain. J. Biol. Chem. 270, 14935-14943. [DOI] [PubMed] [Google Scholar]
- Byers, B. (1981). Cytology of the yeast life cycle. In: The Molecular Biology of the Yeast Saccharomyces: Life cycle and Inheritance, ed. J.N. Strathern, E.W. Jones, and J.R. Broach, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 59-96.
- Cleves, A.E., McGee, T.P., Whitters, E.A., Champion, K.M., Aitken, J.R., Dowhan, W., Goebl, M., and Bankaitis, V.A. (1991). Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell 64, 789-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen, P.J., Cozier, G.E., Banting, G., and Mellor, H. (2001). Modular phosphoinositide-binding domains—their role in signalling and membrane trafficking. Curr. Biol. 11, R882-R893. [DOI] [PubMed] [Google Scholar]
- Cutler, N.S., Heitman, J., and Cardenas, M.E. (1997). STT4 is an essential phosphatidylinositol 4-kinase that is a target of wortmannin in Saccharomyces cerevisiae. J. Biol. Chem. 272, 27671-27677. [DOI] [PubMed] [Google Scholar]
- Desrivières, S., Cooke, F.R., Parker, P.J., and Hall, M.N. (1998). MSS4, a phosphaditylinositol-4-phosphate 5-kinase required for organization of the actin cyoskeleton in Saccharomyces cerevisiae. J. Biol. Chem. 273, 15787-15793. [DOI] [PubMed] [Google Scholar]
- Dowler, S., Currie, R.A., Campbell, D.G., Deak, M., Kular, G., Downes, C.P., and Alessi, D.R. (2000). Identification of pleckstrin-homology-domain-containing proteins with novel phosphoinositide-binding specificities. Biochem. J. 351, 19-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge, S.J., and Davis, R.W. (1988). A family of versatile centromeric vectors designed for use in the sectoring-shuffle mutagenesis assay in Saccharomyces cerevisiae. Gene 70, 303-312. [DOI] [PubMed] [Google Scholar]
- Engebrecht, J., Masse, S., Davis, L., Rose, K., and Kessel, T. (1998). Yeast meiotic mutants proficient for the induction of ectopic recombination. Genetics 148, 581-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, M., Kearns, B.G., Gedvilaite, A., Kagiwada, S., Kearns, M., Fung, M.K., and Bankaitis, V.A. (1996). Kes1p shares homology with human oxysterol binding protein and participates in a novel regulatory pathway for yeast Golgi-derived transport vesicle biogenesis. EMBO J. 15, 6447-6459. [PMC free article] [PubMed] [Google Scholar]
- Fast, D. (1973). Sporulation synchrony of Saccharomyces cerevisiae grown in various carbon sources. J. Bacteriol. 116, 925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan, C.A., Schnieders, E.A., Emerick, A.W., Kunisawa, R., Admon, A., and Thorner, J. (1993). Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science 262, 1444-1448. [DOI] [PubMed] [Google Scholar]
- Hama, H., Schnieders, E.A., Thorner, J., Takemoto, J.Y., and DeWald, D.B. (1999). Direct involvement of phosphatidylinositol 4-phosphate in secretion in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 274, 34294-34300. [DOI] [PubMed] [Google Scholar]
- Han, G.S., Audhya, A., Markley, D.J., Emr, S.D., and Carman, G.M. (2002). The Saccharomyces cerevisiae LSB6 gene encodes PI 4-kinase activity. J. Biol. Chem. 277, 47709-47718. [DOI] [PubMed] [Google Scholar]
- Hawkins, P.T., Stephens, L., and Downes, C.P. (1986). Rapid formation of inositol 1, 3, 4, 5-tetrakisphosphate and inositol 1, 3, 4-trisphosphate in rat parotid glands may both result indirectly from receptor-stimulated release of inositol 1, 4, 5-trisphosphate from phosphatidylinositol 4, 5-bisphosphate. Biochem. J. 238, 507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks, K.B., Wang, B.Q., Schnieders, E.A., and Thorner, J. (1999). Yeast homologue of neuronal frequenin is a regulator of phosphatidylinositol-4-OH kinase. Nat. Cell Biol. 1, 234-241. [DOI] [PubMed] [Google Scholar]
- Hill, J.E., Myers, A.M., Koerner, T.J., and Tzagoloff, A. (1986). Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2, 163-167. [DOI] [PubMed] [Google Scholar]
- Hirata, A., and Shimoda, C. (1992). Electron microscopic examination of sporulation-deficient mutants of the fission yeast Schizosaccharomyces pombe. Arch. Microbiol. 158, 249-255. [DOI] [PubMed] [Google Scholar]
- Hollingsworth, N.M., Goetsch, L., and Byers, B. (1990). The HOP1 gene encodes a meiosis-specific component of yeast chromosomes. Cell 61, 73-84. [DOI] [PubMed] [Google Scholar]
- Hollingsworth, N.M., Ponte, L., and Halsey, C. (1995). MSH5, a novel MutS homolog, facilitates meiotic reciprocal recombination between homologs in Saccharomyces cerevisiae but not mismatch repair. Genes Dev. 9, 1728-1739. [DOI] [PubMed] [Google Scholar]
- Homma, K., Terui, S., Minemura, M., Qadota, H., Anraku, Y., Kanaho, Y., and Ohya, Y. (1998). Phosphatidylinositol-4-phosphate 5-kinase localized on the plasma membrane is essential for yeast cell morphogenesis. J. Biol. Chem. 273, 15779-15786. [DOI] [PubMed] [Google Scholar]
- Honda, A. et al. (1999). Phosphatidylinositol 4-phosphate 5-kinase is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell 99, 521-532. [DOI] [PubMed] [Google Scholar]
- Honigberg, S.M., Conicella, C., and Esposito, R.E. (1992). Commitment to meiosis in Saccharomyces cerevisiae: involvement of the SPO14 gene. Genetics 130, 703-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, H., Fukada, Y., Murata, K., and Kimura, A. (1983). Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153, 163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavran, J.M., Klein, D.E., Lee, A., Falasca, M., Isakoff, S.J., Skolnik, E.Y., and Lemmon, M.A. (1998). Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J. Biol. Chem. 273, 30497-30508. [DOI] [PubMed] [Google Scholar]
- Kearns, B.G., McGee, T.P., Mayinger, P., Gedvilaite, A., Phillips, S.E., Kagiwada, S., and Bankaitis, V.A. (1997). Essential role for diacylglycerol in protein transport from the yeast Golgi complex. Nature 387, 101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisak, L., Strich, R., Winters, R.S., Hall, J.P., Mallory, M.J., Kreitzer, D., Tuan, R.S., and Winter, E. (1994). SMK1, a developmentally regulated MAP kinase, is required for spore wall assembly in Saccharomyces cerevisiae. Genes Dev. 8, 2151-2161. [DOI] [PubMed] [Google Scholar]
- Kupiec, M., Byers, B., Esposito, R.E., and Mitchell, A.P. (1997). Meiosis and sporulation in Saccharomyces cerevisiae. In: The Molecular and Cellular Biology of the yeast Saccharomyces. Cell Cycle and Cell Biology, ed. J.R. Pringle, J.R. Broach, and E.W. Jones, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press, 889-1036.
- Levine, T.P., and Munro, S. (2002). Targeting of Golgi-specific pleckstrin homology domains involves both PtdIns 4-kinase-dependent and -independent components. Curr. Biol. 12, 695-704. [DOI] [PubMed] [Google Scholar]
- Li, X., Rivas, M.P., Fang, M., Marchena, J., Mehrotra, B., Chaudhary, A., Feng, L., Prestwich, G.D., and Bankaitis, V.A. (2002). Analysis of oxysterol binding protein homologue Kes1p function in regulation of Sec14p-dependent protein transport from the yeast Golgi complex. J. Cell Biol. 157, 63-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X., Routt, S.M., Xie, Z., Cui, X., Fang, M., Kearns, M.A., Bard, M., Kirsch, D.R., and Bankaitis, V.A. (2000). Identification of a novel family of nonclassical yeast PITPs whose function modulates phospholipase D activity and Sec14p-independent cell growth. Mol. Biol. Cell 11, 1989-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch, M., and Cantley, L.C. (1995). Signal transduction and membrane traffic: the PITP/Phosphoinositide connection. Cell 81, 659-662. [DOI] [PubMed] [Google Scholar]
- McCarroll, R.M., and Esposito, R.E. (1994). SPO13 negatively regulates the progression of mitotic and meiotic nuclear division in Saccharomyces cerevisiae. Genetics 138, 47-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakase, Y., Nakamura, T., Hirata, A., Routt, S.M., Skinner, H.B., Bankaitis, V.A., and Shimoda, C. (2001). The Schizosaccharomyces pombe spo20+ gene encoding a homologue of Saccharomyces cerevisiae Sec14 plays an important role in forespore membrane formation. Mol. Biol. Cell 12, 901-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiman, A.M. (1998). Prospore membrane formation defines a developmentally regulated branch of the secretory pathway in yeast. J. Cell Biol. 140, 29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, S.E. et al. (1999). Yeast Sec14p deficient in phosphatidylinositol transfer activity is functional in vivo. Mol. Cell 4, 187-197. [DOI] [PubMed] [Google Scholar]
- Rabitsch, K.P. et al. (2001). A screen for genes required for meiosis and spore formation based on whole-genome expression. Curr. Biol. 11, 1001-1009. [DOI] [PubMed] [Google Scholar]
- Rivas, M.P., Kearns, B.G., Xie, Z., Guo, S., Sekar, M.C., Hosaka, K., Kagiwada, S., York, J.D., and Bankaitis, V.A. (1999). Pleiotropic alterations in lipid metabolism in yeast sac1 mutants: relationship to “bypass Sec14p” and inositol auxotrophy. Mol. Biol. Cell 10, 2235-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, K., Rudge, S.A., Frohman, M.A., Morris, A.J., and Engebrecht, J. (1995). Phospholipase D signaling is essential for meiosis. Proc. Natl. Acad. Sci. USA 92, 12151-12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, M.D., Novick, P., Thomas, J.H., Botstein, D., and Fink, G.R. (1987). A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene 60, 237-243. [DOI] [PubMed] [Google Scholar]
- Rose, M.D., Winston, F., and Hieter, P. (1990). Methods in Yeast Genetics, A Laboratory Course Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Rothstein, R. (1983). One step gene disruption in yeast. Methods Enzymol. 101, 202-211. [DOI] [PubMed] [Google Scholar]
- Rudge, S.A., and Engebrecht, J. (1999). Regulation and function of PLDs in yeast. Biochim. Biophys. Acta 1439, 167-174. [DOI] [PubMed] [Google Scholar]
- Rudge, S.A., Zhou, C., and Engebrecht, J. (2002). Differential regulation of Saccharomyces cerevisiae phospholipase D in sporulation and Sec14-independent secretion. Genetics 160, 1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge, S.A., Cavenagh, M.M., Kamath, R., Sciorra, V.A., Morris, A.J., Kahn, R.A., and Engebrecht, J. (1998a). ADP-Ribosylation factors do not activate yeast phospholipase D, but are required for sporulation. Mol. Biol. Cell 9, 2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge, S.A., Morris, A.J., and Engebrecht, J. (1998b). Relocalization of phospholipase D activity mediates membrane formation during meiosis. J. Cell Biol. 140, 2025-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge, S.A., Pettitt, T.R., Zhou, C., Wakelam, M.J., and Engebrecht, J. (2001). SPO14 separation-of-function mutations define unique roles for phospholipase D in secretion and cellular differentiation in Saccharomyces cerevisiae. Genetics 158, 1431-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher, M., Barrowman, J., Schieltz, D., Yates, J.R., 3rd, and Ferro-Novick, S. (2000). Identification and characterization of five new subunits of TRAPP. Eur. J. Cell Biol. 79, 71-80. [DOI] [PubMed] [Google Scholar]
- Sacher, M., Barrowman, J., Wang, W., Horecka, J., Zhang, Y., Pypaert, M., and Ferro-Novick, S. (2001). TRAPP I implicated in the specificity of tethering in ER-to-Golgi transport. Mol. Cell 7, 433-442. [DOI] [PubMed] [Google Scholar]
- Santos, B., and Snyder, M. (2000). Sbe2p and Sbe22p, two homologous Golgi proteins involved in yeast cell wall formation. Mol. Biol. Cell 11, 435-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra, V.A., Rudge, S.A., Prestwich, G.D., Frohman, M.A., Engebrecht, J., and Morris, A.J. (1999). Identification of a phosphoinositide binding motif that mediates activation of mammalian and yeast phospholipase D isoenzymes. EMBO J. 20, 5911-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciorra, V.A., Rudge, S.A., Wang, J., McLaughlin, S., Engebrecht, J., and Morris, A.J. (2002). Dual role for phosphoinositides in regulation of yeast and mammalian phospholipase D enzymes. J. Cell Biol. 159, 1039-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreenivas, A., Patton-Vogt, J.L., Bruno, V., Griac, P., and Henry, S.A. (1998). A role for phospholipase D (Pld1p) in growth, secretion and regulation of membrane lipid synthesis in yeast. J. Biol. Chem. 273, 16635-16638. [DOI] [PubMed] [Google Scholar]
- Stack, J.H., DeWald, D.B., Takegawa, K., and Emr, S.D. (1995). Vesicle-mediated protein transport: regulatory interactions between the Vps15 protein kinase and the Vps34 PtdIns 3-kinase essential for protein sorting to the vacuole in yeast. J. Cell Biol. 129, 321-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan, C.J., Audhya, A., and Emr, S.D. (2002). The yeast synaptojanin-like proteins control the cellular distribution of phosphatidylinositol (4, 5)-bisphosphate. Mol. Biol. Cell 13, 542-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner, J.W. (2001). Greasing the wheels of secretory transport. Nat. Cell Biol. 3, E196-E198. [DOI] [PubMed] [Google Scholar]
- van den Hazel, H.B., Pichler, H., do Valle Matta, M.A., Leitner, E., Goffeau, A., and Daum, G. (1999). PDR16 and PDR17, two homologous genes of Saccharomyces cerevisiae, affect lipid biosynthesis and resistance to multiple drugs. J. Biol. Chem. 274, 1934-1941. [DOI] [PubMed] [Google Scholar]
- Vershon, A.K., Hollingsworth, N.M., and Johnson, A.D. (1992). Meiotic induction of the yeast HOP1 gene is controlled by positive and negative regulatory sites. Mol. Cell. Biol. 12, 3706-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena, C., and Novick, P. (1999). The yeast phosphatidylinositol-4-OH kinase Pik1 regulates secretion at the Golgi. Nat. Cell Biol. 1, 523-525. [DOI] [PubMed] [Google Scholar]
- Whiteford, C.C., Best, C., Kazlauskas, A., and Ulug, E.T. (1996). D-3 phosphoinositide metabolism in cells treated with platelet-derived growth factor. Biochem. J. 319, 851-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, Z., Fang, M., Rivas, M.P., Faulkner, A.J., Sternweis, P.C., Engebrecht, J., and Bankaitis, V.A. (1998). Phospholipase D activity is required for suppression of yeast phosphatidylinositol transfer protein defects. Proc. Natl. Acad. Sci. USA 95, 12346-12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa, L.L., Marchena, J., Xie, Z., Li, X., Poon, P.P., Singer, R.A., Johnston, G.C., Randazzo, P.A., and Bankaitis, V.A. (2002). Activity of specific lipid-regulated ADP ribosylation factor-GTPase-activation proteins is required for Sec14p-dependent Golgi secretory function in yeast. Mol. Biol. Cell 13, 2193-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, E., Driscoll, R., Coltrera, M., Olins, A., and Bloom, K. (1991). A dynamin-like protein encoded by the yeast sporulation gene SPO15. Nature 349, 713-715. [DOI] [PubMed] [Google Scholar]
- Yoshida, S., Ohya, Y., Goebl, M., Nakano, A., and Anraku, Y. (1994). A novel gene, STT4, encodes a phosphatiylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J. Biol. Chem. 269, 1166-1171. [PubMed] [Google Scholar]