Abstract
Catestatin, a neuroendocrine peptide with effects on human autonomic function, has recently been found to be a cutaneous antimicrobial peptide. Human catestatin exhibits three single nucleotide polymorphisms: Gly364Ser, Pro370Leu and Arg374Gln. Given reports indicating that antimicrobial peptides and neuropeptides induce mast cell activation, we postulated that catestatin might stimulate numerous functions of human mast cells, thereby participating in the regulation of skin inflammatory responses. Catestatin and its naturally occurring variants caused the human mast cell line LAD2 and peripheral blood-derived mast cells to migrate, degranulate and release leukotriene C4 and prostaglandins D2 and E2. Moreover, catestatins increased intracellular Ca2+ mobilization in mast cells, and induced the production of pro-inflammatory cytokines/chemokines such as granulocyte–macrophage colony-stimulating factor, monocyte chemotactic protein-1/CCL2, macrophage inflammatory protein-1α/CCL3 and macrophage inflammatory protein-1β/CCL4. Our evaluation of possible cellular mechanisms suggested that G-proteins, phospholipase C and the mitogen-activated protein kinase/extracellular signal-regulated kinase (ERK) are involved in catestatin-induced mast cell activation as evidenced by the inhibitory effects of pertussis toxin (G-protein inhibitor), U-73122 (phospholipase C inhibitor) and U0126 (ERK inhibitor), respectively. We also found that human mast cells express the α7 subunit of the nicotinic acetylcholine receptor at both the mRNA and protein levels. Given that silencing the α7 receptor mRNA and an α7-specific inhibitor did not affect catestatin-mediated activation of mast cells, however, we concluded that this receptor is not likely to be functional in human mast cell stimulation by catestatins. Our finding that the neuroendocrine antimicrobial peptide catestatin activates human mast cells suggests that this peptide might have immunomodulatory functions, and provides a new link between neuroendocrine and cutaneous immune systems.
Keywords: activation, basophils, disease, mast cells, signal transduction, signalling, skin (dermatology) immunology
Introduction
The cutaneous immune system involves both innate and adaptive immunity. Antimicrobial peptides (AMPs) form an innate epithelial chemical barrier and have direct microbicidal effects on numerous bacteria, fungi and enveloped viruses.1 Moreover, multiple components of the innate and adaptive immune systems are thought to be coordinated by AMPs.2 In addition to their microbicidal activities, AMPs exhibit a variety of activities, including endotoxin neutralization, pro- and anti-apoptotic effects, chemoattraction, wound repair, angiogenesis, tumour surveillance, and enhancement of the production of cytokines and chemokines.1,2 Among the numerous AMPs discovered so far in human skin, diverse properties have been reported for human β-defensins, cathelicidin LL-37 and S100 proteins.1 Recently, catestatin, a neuroendocrine peptide derived from the pro-hormone chromogranin A,3 has been demonstrated to be an AMP in human skin.4 Beyond its microbicidal properties, however, the immunomodulatory activities of catestatin in cutaneous tissue remain unknown.
The neuroendocrine protein chromogranin A is a member of the granin family found in the secretory granules of endocrine, neuroendocrine and neuronal cells.5 Upon proteolytic cleavage, chromogranin A can give rise to biologically active peptides such as pancreastatin, β-granin, vasostatin, parastatin and catestatin.3 Catestatin is a 21-amino acid residue, cationic and hydrophobic peptide that affects human autonomic function as a catecholamine release inhibitor, via non-competitive inhibition of nicotinic acetylcholine receptors (nAChRs).6 Catestatin occurs in normal human skin,4 and is reported to exhibit antimicrobial activity against a wide array of skin pathogens, including bacteria, yeast and fungi.4,7 Catestatin is also a potent vasodilator, given its ability to induce in vivo histamine release in rats,8 and a chemotactic factor for human monocytes.9 The expression of catestatin in human skin has been detected in keratinocytes, and can be increased in response to injury or infection in murine skin.4 The human catestatin exhibits three naturally occurring single nucleotide polymorphisms, Gly364Ser, Pro370Leu and Arg374Gln, which are estimated to occur in ∼ 4% of the population.10 These polymorphisms show different potencies in terms of their inhibition of catecholamine secretion, with a rank order of Pro370Leu > wild-type catestatin > Gly364Ser > Arg374Gln.11
Mast cells are frequently present in areas with close proximity to epithelial surfaces. They are important effector cells of the innate immune system and participate in allergy, inflammation, immune surveillance and sensitization to allergens.12 Moreover, their numbers in local tissues increase under conditions such as wound healing and inflammatory and allergic diseases.12,13 Among the various mast cell stimulants, AMPs (e.g. human β-defensins and cathelicidin LL-37) and neuropeptides (e.g. substance P and vasoactive intestinal polypeptide) have both been reported.14–18 Therefore, we postulated that the neuroendocrine AMP catestatin might also activate diverse functions of human mast cells.
Our findings demonstrated that catestatin and its variants caused mast cells to migrate, degranulate and release inflammatory mediators such as leukotriene C4 (LTC4), prostaglandin D2 (PGD2) and PGE2. In addition, catestatins induced the production of cytokines and chemokines, and catestatin-mediated mast cell activation was regulated by G-proteins, phospholipase C (PLC), and the mitogen-activated protein kinase extracellular signal-regulated kinase (MAPK ERK). We also found that human mast cells express the α7 subunit of the nAChR; however, this receptor is not likely to function in catestatin-caused mast cell activation. Our finding that the skin-derived AMP catestatin activates various functions of human mast cells suggests that this peptide may have an immunomodulatory role, and supports the hypothesis of a link between the neuroendocrine and cutaneous immune systems.
Materials and methods
Reagents
Human wild-type catestatin (SSMKLSFRARAYGFRGPGPQL), catestatin natural variants Gly364Ser (SSMKLSFRARAYSFRGPGPQL), Pro370Leu (SSMKLSFRARAYGFRGPGLQL), and Arg374Gln (SSMKLSFRARAYGFRGPGPQLRQGWRPSSREDSLEAGLPLQVRGYPEE), and a scrambled form of catestatin sCst (MKLSSSFRAYARGFRGPGPQL) were synthesized using a solid-phase method on a peptide synthesizer (model PSSM-8; Shimadzu, Kyoto, Japan) by fluoroenylmethoxycarbonyl (Fmoc) chemistry, and their molecular masses were confirmed using a mass spectrometer (model TSQ 700; Thermo Quest Finnigan, Manchester, UK). Compound 48/80 was purchased from Sigma-Aldrich (St Louis, MO). Enzyme immunoassay (EIA) kits for LTC4, PGD2 and PGE2 were purchased from Cayman Chemical Company (Ann Arbor, MI), and cytokine and chemokine ELISA kits were obtained from R&D Systems (Minneapolis, MN). Rabbit polyclonal antibodies against phosphorylated p38, ERK and jun N-terminal kinase (JNK), in addition to unphosphorylated p38, ERK and JNK, were from Cell Signaling Technology (Beverly, MA). The G-protein inhibitor pertussis toxin, ERK inhibitor U0126, JNK inhibitor II SP600125, PLC inhibitor U-73122, and PLC inhibitor inactive control U-73343 were obtained from Calbiochem (La Jolla, CA). The nAChR primers used were from Invitrogen (Camarillo, CA), and small interfering RNA (siRNA) targeting the α7 nAChR and control siRNA were purchased from Applied Biosystems (Branchburg, NJ).
Cell culture
The LAD2 cell line isolated from the bone marrow of a patient with mast cell leukaemia was a kind gift from Dr Arnold Kirshenbaum (National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, MD).19 These cells were grown in Stem Pro-34 medium containing nutrient supplements (Invitrogen), supplemented with 2 mm l-glutamine (Invitrogen), 100 IU/ml penicillin and 100 μg/ml streptomycin (Meiji Seika, Tokyo, Japan), and 100 ng/ml human stem cell factor (SCF) (Wako, Osaka, Japan). Cell culture medium was hemi-depleted every week with fresh medium. Human peripheral blood-derived cultured mast cells were obtained using previously described methods with some modifications.16 Briefly, granulocyte colony-stimulating factor (G-CSF) -mobilized human peripheral bloods CD34+ cells (Veritas Corporation, Tokyo, Japan) were cultured in serum-free Iscove's methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) containing 200 ng/ml SCF, 50 ng/ml interleukin-6 (IL-6) and 2·5 ng/ml IL-3 (PeproTech, London, UK), 100 IU/ml penicillin and 100 μg/ml streptomycin (Gibco, Grand Island, NY). At 6 weeks, the methylcellulose medium was dissolved in PBS, and the cells were then resuspended and cultured in Iscove's modified Dulbecco's medium supplemented with 100 ng/ml SCF, 50 ng/ml IL-6, 5% fetal calf serum, 55 μm 2-mercaptoethanol, 100 IU/ml penicillin and 100 μg/ml streptomycin. Hemi-depletions of media were performed weekly by adding fresh media. The final purity of mast cells always exceeded 95%.
β-Hexosaminidase release assay
Mast cells (2 × 105 cells/well) were suspended in Tyrode's buffer [10 mm HEPES buffer (pH 7·4), 130 mm NaCl, 5 mm KCl and 5·6 mm glucose] containing 0·1% BSA, 1 mm CaCl2 and 0·6 mm MgCl2, then stimulated with various concentrations of catestatin peptides or diluent (0·01% acetic acid) for 40 min at 37°. The β-hexosaminidase levels in the supernatants and total cell lysates solubilized with Triton X-100 were quantified by hydrolysis of p-nitrophenyl-N-acetyl-β-d-glucopyranoside in 0·1 m sodium citrate buffer for 90 min at 37°. The percentage of β-hexosaminidase release was calculated as reported previously.15 In some experiments, inhibitors were added 2 hr before stimulation, and β-hexosaminidase release was measured as described above.
EIA and ELISA
Mast cells (1 × 106 cells) were incubated with catestatins at the indicated concentrations for 0·5–24 hr at 37°. After stimulation, the cells were centrifuged, and the cell-free supernatants from cultures of stimulated mast cells or non-stimulated control cells were used for LTC4, PGD2 and PGE2 quantification by an EIA, while granulocyte macrophage colony-stimulating factor (GM-CSF), monocyte chemotactic protein (MCP-1)/CCL2, macrophage inflammatory protein 1α (MIP-1α/CCL3 and MIP-1β/CCL4 were measured using appropriate ELISA kits according to the manufacturer's instructions. In some experiments, inhibitors were added 2 hr before stimulation, and the EIA or ELISA quantification was performed as described above.
Total RNA extraction, quantitative real-time PCR and reverse trancription PCR
Total RNA was extracted from mast cells using an RNeasy Micro kit (Qiagen, Venlo, the Netherlands). First-strand cDNA was then synthesized from 2 μg total RNA using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer's instructions. Quantitative real-time PCR was performed as reported previously,16 using TaqMan Universal PCR Master Mix (Applied Biosystems). Amplification and detection of mRNA were analysed using a 7500 Real-Time PCR System (Applied Biosystems) according to the manufacturer's instructions.
The mRNA expression of the α3, α4, α7 and α9 subunits of the nAChRs in mast cells was examined by reverse transcription (RT-) PCR using an Advantage 2 PCR Enzyme System (Clontech Laboratories, Palo Alto, CA) according to the manufacturer's instructions. The primers used were: α3 subunit (401 bp), sense primer CCATGTCTCAGCTGGTG, antisense primer GTCCTTGAGGTTCATGGA; α4 subunit (346 bp), sense primer TGGGTGAAGCAGGAGTGG, antisense primer AGTCCAGCTGGTCCACG; α7 subunit (414 bp), sense primer CCTGGCCAGTGTGGAG, antisense primer TACGCAAAGTCTTTGGACAC; α9 subunit (403 bp), sense primer GTCCAGGGTCTTGTTTGT, antisense primer ATCCGCTCTTGCTATGAT; glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 447 bp), sense primer ACCACAGTCCATGCCATCAC, antisense primer TCCACCACCCTGTTGCTGTA. The PCR amplification was carried out for 35 cycles (1 min at 95°, 30 seconds at 95° and 1 min at 68° repeated for 34 cycles, and 1 min at 68°). Aliquots of the PCR products were run on 2% agarose gels and visualized by ethidium bromide staining.
Treatment with pertussis toxin, U-73122 and MAPK inhibitors
The effects of pertussis toxin, U-73122, U0126 and SP600125 on various mast cell functions induced by catestatin and its variants were evaluated by pre-treating mast cells with pertussis toxin (1000 ng/ml), U-73122 or its inactive control U-73343 (10 μm each), U0126 (10 μm), or SP600125 (20 μm) for 2 hr at 37° in StemPro-34 medium. The cells were then stimulated with wild-type catestatin and its variants for indicated time periods, and appropriate assays were performed as described above.
Western blot analysis
Mast cells (1 × 106 cells) were stimulated with 5 μm catestatins for 5 min to 1 hr. After stimulation, cell lysates were obtained by lysing cells in lysis buffer (50 mm Tris–HCl (pH 8), 150 mm NaCl, 0·02% NaN3, 0·1% SDS, 1% Nonidet P-40 containing a protease inhibitor cocktail, Phosphatase Inhibitor Cocktail 1 and Cocktail 2 (Sigma-Aldrich) prepared according to the manufacturer's instructions. The amount of total protein was determined using a BCA Protein Assay (Pierce Chemical, Rockford, IL), and equal amounts of total protein were subjected to 12·5% SDS–PAGE analysis. After the non-specific binding sites were blocked, the blots were incubated with polyclonal antibodies against phosphorylated or unphosphorylated p38, ERK and JNK overnight. The membranes were developed using an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Measurements of intracellular Ca2+ mobilization
Intracellular Ca2+ mobilization was measured by a no-washing method using a FLIPR Calcium Assay kit (Molecular Devices, Sunnyvale, CA). Cells (100 μl) were seeded at a density of 2 × 105 cells per well into 96-well, black-walled, clear-bottom microtitre plates coated with poly-d-lysine (Becton-Dickinson, NJ), and then loaded for 1 hr at 37° in an equivalent volume of Hanks' balanced salt solution containing 20 mm HEPES, 2·5 mm probenecid (Sigma-Aldrich), and Calcium 3 Reagent (Molecular Devices, Menlo Park, CA), pH 7·4, prepared according to the manufacturer's instructions. To form a uniform monolayer of cells on the bottoms of the wells, the microplate was gently centrifuged for 5 min with low acceleration and without brake. The cell-containing plate was placed into a FlexStation II (Molecular Devices), and a volume of 50 μl catestatins diluted in assay buffer was added to each well to achieve the final indicated concentrations. The maximum change in fluorescence over baseline was quantified using softmax pro (version 5) software (Molecular Devices).
Chemotaxis assay
The chemotaxis assay was performed using a 48-well chemotaxis micro-chamber (Neuroprobe, Cabin John, MD). Mast cells (50 μl of 3 × 106 cells/ml) were added to the upper wells separated from the lower wells containing chemoattractants by a polycarbonate membrane with pores 8 μm in diameter. After 3 hr of incubation, the mast cells that migrated and adhered to the underside of the filter were fixed and stained with DiffQuick. The membrane was mounted, and the cells that migrated were counted under a light microscope in three randomly chosen high-power fields. In some experiments, inhibitors were added 2 hr before the assay, and chemotaxis was evaluated as described above.
FACS analysis of α7 nAChR expression in mast cells
Mast cells (1 × 106 cells) were suspended in BD Cytofix/Cytoperm solution (BD Biosciences Pharmingen, San Diego, CA) for 20 min according to the manufacturer's instructions. Following one wash with BD Perm/Wash buffer, an antibody against the α7 nAChR (Santa Cruz Biotechnology, Santa Cruz, CA) or an isotype control rat IgG1κ antibody (BD Biosciences) was added for 30 min. The expression of the α7 nAChR was evaluated by FACS after staining with FITC-conjugated goat anti-rat IgG (BD Biosciences).
RNA interference with α7 nAChR siRNA
Mast cells (100 μl at a density of 3 × 107 cells/ml) were transfected with 400 nmα7 nAChR siRNA or control siRNA (Applied Biosystems) using the Amaxa Cell Line Nucleofector Kit V, programme T-030 (Lonza Bio, Cologne, Germany), according to the manufacturer's instructions. Gene silencing was carried out for at least 24 hr, and the efficacy of knockdown was confirmed by quantitative real-time PCR using α7 nAChR-specific primers/probes. Following transfection, the cells were stimulated with catestatin peptides, and an assessment of degranulation or cytokine/chemokine production was carried out as described above.
Statistical analysis
Statistical analysis was performed using one-way analysis of variance with a multiple comparison test or Student's t-test (Prism 4; GraphPad Software, San Diego, CA), and P < 0·05 was considered to be significant. The results are shown as the mean ± SD.
Results
Effects of catestatins on mast cell degranulation
The β-hexosaminidase enzyme is released in combination with histamine and, therefore, is a marker of mast cell degranulation.20 As shown in Fig. 1(a), wild-type catestatin and its variants markedly induced β-hexosaminidase release from LAD2 cells at 2·5 μm, whereas nanomolar concentrations (100 and 500 nm) did not cause mast cell degranulation. Wild-type catestatin, Gly364Ser and Pro370Leu displayed nearly identical potencies, whereas Arg374Gln showed lower activity. Scrambled catestatin used as a control peptide had no effect on mast cell degranulation, suggesting that catestatin-mediated human mast cell activation is specific. Compound 48/80 was used as a positive control.
Figure 1.
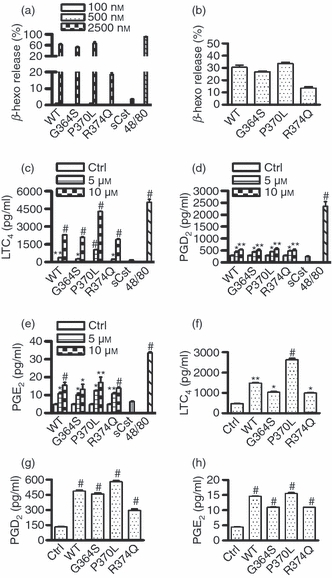
Catestatins induce human mast cell degranulation and release of leukotriene (LT) C4, prostaglandin (PG) D2 and PGE2. (a) LAD2 cells (2 × 105 cells) were incubated with 100–2500 nm wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L) or Arg374Gln (R374Q), 2500 nm scrambled catestatin (sCst), 5 μg/ml compound 48/80 (48/80), or diluent 0·01% acetic acid. After 40 min incubation at 37°, β-hexosaminidase (β-Hexo) release was measured in the supernatants as described in the Materials and methods section. Values are the mean ± SD of five separate experiments. (b) Peripheral blood-derived mast cells (2 × 105 cells) were incubated with 5 μm WT catestatin, G364S, P370L or R374Q and β-Hexo release was measured. Values are the mean ± SD of three separate experiments. (c–e) LAD2 cells (1 × 106 cells) were stimulated for 30 min at 37° with 5–10 μm WT catestatin, G364S, P370L or R374Q, 10 μm sCst, 5 μg/ml compound 48/80 (48/80), or diluent 0·01% acetic acid (Ctrl, control). LTC4, PGD2 and PGE2 released into the supernatants were quantified by an enzyme immunoassay. (f–h) Furthermore, peripheral blood-derived mast cells (1 × 106 cells) were incubated 10 μm WT catestatin, G364S, P370L or R374Q, or diluent 0·01% acetic acid (Ctrl, control) as above, and LTC4, PGD2 and PGE2 release was quantified. Values are shown as the mean ± SD of three to six separate experiments, and in comparison with untreated cells (Ctrl, control), respectively. *P < 0·05, **P < 0·01, #P < 0·001.
Catestatins also notably caused degranulation of peripheral blood-derived mast cells (Fig. 1b); however, these cells had a weaker response to wild-type catestatin and its variants when compared with LAD2 cells (5 μm for peripheral blood mast cells versus 2·5 μm for LAD2 cells), implying different characteristics of these two cell types. The doses of catestatin peptides used in this study were not toxic to mast cells, as evaluated by trypan blue dye exclusion, and lactate dehydrogenase activity (data not shown).
Catestatins induce the release of lipid mediators LTC4, PGD2 and PGE2
When stimulated, mast cells undergo degranulation and release of various eicosanoids in inflammatory or allergic diseases.21 Therefore, given that catestatin peptides induced mast cell degranulation, we investigated their ability to cause the release of LTs and PGs from human mast cells. In support of our hypothesis, wild-type catestatin and its mutants noticeably enhanced LTC4, PGD2 and PGE2 release from LAD2 cells in a dose-dependent manner. Scrambled catestatin had no effect, and compound 48/80 was a positive control (Fig. 1c–e). We also confirmed that wild-type catestatin and its variants significantly augmented LTC4, PGD2 and PGE2 release from peripheral blood-derived mast cells (Fig. 1f–h). Although catestatin peptides increased LTC4 release by approximately 100-fold, the release of PGD2 and PGE2 was only increased two- to three-fold. We verified that longer stimulation (3–12 hr) of the cells did not further increase the amounts of LTC4, PGD2 and PGE2 released (data not shown).
Catestatins stimulate gene expression and protein production of cytokines and chemokines by mast cells
As a number of AMPs and neuropeptides known to induce mast cell degranulation have been reported to increase chemokine and cytokine production,16,17 we next tested whether catestatin peptides would also activate mast cells to generate pro-inflammatory cytokines and chemokines, including GM-CSF, IL-4, IL-5, IL-8, TNF-α, MCP-1/CCL2, MIP-1α/CCL3 and MIP-1β/CCL4. Following 1 hr of stimulation, we observed that wild-type catestatin and its variants noticeably enhanced the mRNA expression levels of the above-mentioned cytokines and chemokines in a dose-dependent manner (Fig. 2). We chose to stimulate the cells for 1 hr because in preliminary experiments the highest mRNA expression levels were observed after 1 hr of a 1–24 hr stimulation.
Figure 2.
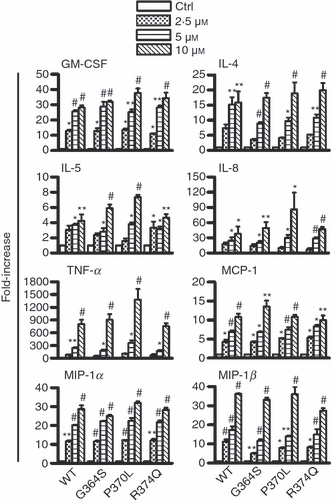
Enhancement of the mRNA expressions of various cytokines and chemokines. LAD2 cells (1 × 106 cells) were incubated with 2·5–10 μm wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L) or Arg374Gln (R374Q), or diluent 0·01% acetic acid (Ctrl, control) for 1 hr. Following incubation, total RNA was extracted and converted into cDNA, and quantitative real-time PCR was performed to analyse changes in the gene expression of granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin-4 (IL-4), IL-5, IL-8, tumour necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3) and MIP-1β/CCL4. Each bar shows the mean ± SD of three different experiments. Values represent fold increases in gene expression above untreated cells (Ctrl, control). *P < 0·05, **P < 0·01, #P < 0·001.
After observing enhanced mRNA expression of various cytokines and chemokines, the stimulatory effects of catestatin peptides on the production of the respective cytokine and chemokine proteins by mast cells were evaluated using an ELISA. Among the cytokines and chemokines tested, wild-type catestatin and its variants, but not scrambled catestatin, only selectively increased the production of GM-CSF, MCP-1/CCL2, MIP-1α/CCL3 and MIP-1β/CCL4 (Fig. 3), and this effect was dose-dependent. The production of cytokines and chemokines was highest after 6 hr of stimulation.
Figure 3.
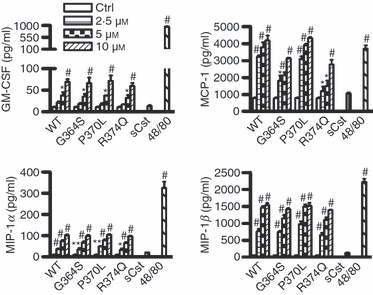
Stimulatory effects of catestatins on the production of cytokines and chemokines by mast cells. LAD2 cells (1 × 106 cells) were treated with the indicated concentrations of wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L) or Arg374Gln (R374Q), 10 μm scrambled catestatin (sCst), 5 μg/ml compound 48/80 (48/80), or diluent 0·01% acetic acid (Ctrl, control) for 6 hr, and the amounts of granulocyte–macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3) and MIP-1β/CCL4 released into the culture supernatants were determined by an ELISA. Values were compared between stimulated and untreated cells (Ctrl, control). *P < 0·05, **P < 0·01, #P < 0·001. Each bar represents the mean ± SD of three separate experiments.
Induction of intracellular Ca2+ mobilization
Given that calcium is critically involved in the regulation of numerous functions of mast cells,22 we tested whether catestatin peptides have the ability to mobilize intracellular Ca2+ in mast cells, thereby leading to cell activation. As expected, wild-type catestatin and its variants induced considerable increases of intracellular Ca2+ mobilization in human mast cells. These Ca2+ increases were dose-dependent, and catestatin concentrations as low as 1·25 μm caused large amounts of Ca2+ influx, reaching a peak at around 50 seconds after the addition of catestatin peptides (Fig. 4a).
Figure 4.

Catestatins mediate intracellular Ca2+ mobilization and mast cell chemotaxis. (a) LAD2 cells (2 × 105 cells) were incubated for 1 hr at 37° in Hanks' balanced salt solution containing HEPES, probenecid and Calcium 3 Reagent, and then stimulated with 1·25–5 μm wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L) or Arg374Gln (R374Q), or diluent 0·01% acetic acid (Ctrl, control). The data presented are representative of four separate experiments and are shown as the changes in fluorescence. (b) LAD2 cells (50 μl from 3 × 106 cells/ml) were placed in the upper wells of a chemotaxis micro-chamber and allowed to migrate towards 0·02–1·25 μm WT catestatin, G364S, P370L or R374Q, 0·32 μm scrambled catestatin (sCst), or diluent 0·01% acetic acid (Ctrl, control) for 3 hr at 37°. Chemotaxis was assessed by counting under a light microscope the number of cells that migrated through the polycarbonate membrane with 8-μm diameter pores, in three randomly chosen high-power fields. Values were compared between stimulated and non-stimulated cells (Ctrl, control). *P < 0·05, **P < 0·01, #P < 0·001. Each bar represents the mean ± SD of nine separate experiments. (c) Peripheral blood-derived mast cells (50 μl from 3 × 106 cells/ml) were allowed to migrate towards 0·32 μm WT catestatin, G364S, P370L or R374Q, or diluent 0·01% acetic acid (Ctrl, control), and chemotaxis assay was performed as above. Values were compared between stimulated and untreated cells (Ctrl, control). *P < 0·001. Each bar represents the mean ± SD of three separate experiments.
Catestatins induce mast cell chemotaxis
Because catestatin is a potent chemoattractant for monocytes,9 we evaluated whether this peptide would also chemoattract human mast cells. In support of our hypothesis, wild-type catestatin and its variants induced mast cell chemotaxis, and the dose-dependence of this effect gave a bell-shaped curve. The optimal chemotactic concentration was as low as 0·32 μm, whereas higher concentrations of catestatin peptides resulted in the inhibition of cell migration. Scrambled catestatin had no effect on LAD2 mast cell migration (Fig. 4b). Similar results with 0·32 μm wild-type catestatin and its variants were observed in human peripheral blood-derived cultured mast cells (Fig. 4c).
Inhibitory effects of pertussis toxin and U-73122
To evaluate the cellular mechanisms by which catestatins activate human mast cells, we investigated whether the G-protein and PLC pathways were involved in catestatin-mediated human mast cell activation by using the specific inhibitors, pertussis toxin and U-73122, respectively. Prior treatment of the mast cells with pertussis toxin or U-73122 significantly suppressed the mast cell degranulation and release of LTC4, PGD2 and PGE2 induced by wild-type catestatin and its variants (Fig. 5a–d). In addition, both inhibitors markedly suppressed mast cell chemotaxis, intracellular Ca2+ mobilization, and the production of cytokines and chemokines (Fig. 5e–j). U-73122 was more potent than pertussis toxin, and its inactive control, U-73343, had no effect on mast cell activation.
Figure 5.
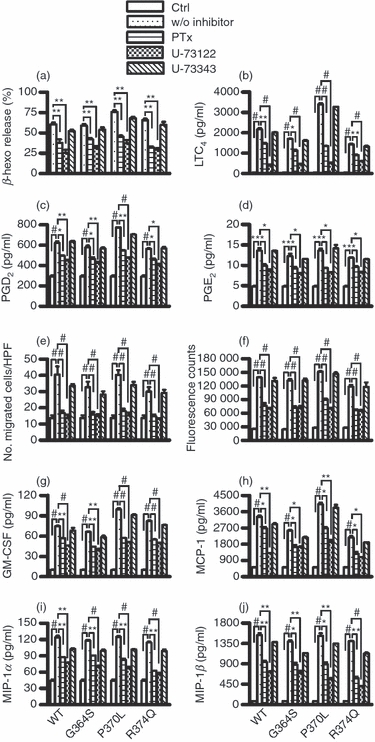
Inhibitory effects of pertussis toxin and U-73122 on catestatin-induced mast cell activation. Cells were pre-treated with 1000 ng/ml pertussis toxin (PTx), 10 μm U-73122, 10 μm U-73343 or 0·1% DMSO for 2 hr. (a) Pre-treated cells (2 × 105 cells) were stimulated with 2·5 μm wild-type catestatin (WT), Gly364Ser (G364S) or Pro370Leu (P370L), 5 μm Arg374Gln (R374Q), or diluent 0·01% acetic acid (Ctrl, control) for 40 min, and β-hexosaminidase (β-Hexo) release was measured. (b–d) Pre-treated cells (1 × 106 cells) were also stimulated with 10 μm WT catestatin, G364S, P370L, R374Q, or 0·01% acetic acid (Ctrl, control) for 30 min, and the release of leukotriene (LT) C4, prostaglandin (PG) D2, and PGE2 was assessed by an enzyme immunoassay. (e) In addition, pre-treated cells were incubated with 0·32 μm WT catestatin, G364S, P370L, R374Q, or 0·01% acetic acid (Ctrl, control) for 3 hr, and the chemotaxis assay was then performed. (f) Cells were also evaluated for intracellular Ca2+ mobilization following stimulation with 5 μm WT catestatin, G364S, P370L, R374Q, or 0·01% acetic acid (Ctrl, control). Furthermore, cells were stimulated for 6 hr with 10 μm WT catestatin, G364S, P370L, R374Q, or 0·01% acetic acid (Ctrl, control), and the levels of (g) granulocyte–macrophage colony-stimulating factor (GM-CSF), (h) monocyte chemoattractant protein-1 (MCP-1/CCL2), (i) macrophage inflammatory protein-1α (MIP-1α/CCL3) and (j) MIP-1β/CCL4 released into the supernatants were determined by an ELISA. Values are the mean ± SD of four to nine separate experiments. **P < 0·01 and #P < 0·001 for comparisons between untreated cells (Ctrl, control) and stimulated groups without inhibitor (w/o inhibitor). *P < 0·05, **P < 0·01, and #P < 0·001 for comparisons between the presence and absence of inhibitors.
Phosphorylation of the MAPKs is mediated by catestatin peptides
To further understand the signalling pathways of catestatin peptides in human mast cells, we also examined whether these peptides could activate MAPK pathways. The MAPK pathway was a likely candidate because it has been reported to be responsible for AMP-mediated activation of mast cells,1,15 and because catestatin induces human monocyte migration via MAPK activation.9 As shown in Fig. 6(a), wild-type catestatin and its variants almost identically enhanced phosphorylation of ERK and JNK, but not p38 in mast cells, as observed after 5 min of stimulation with catestatin peptides. Scrambled catestatin had no effect on MAPK phosphorylation. Notably, longer exposure of mast cells to catestatin peptides, up to 60 min, did not lead to enhanced p38 phosphorylation (data not shown).
Figure 6.
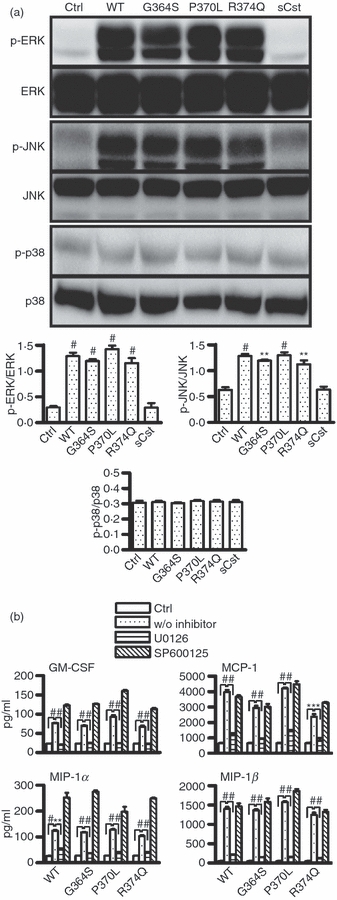
Involvement of the mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK). (a) Catestatins induce the phosphorylation of ERK and JNK. LAD2 cells (1 × 106 cells) were stimulated with 5 μm wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L), Arg374Gln (R374Q), and scrambled catestatin (sCst), or diluent 0·01% acetic acid (Ctrl, control) for 5 min, and the levels of phosphorylated ERK (p-ERK), JNK (p-JNK), and p38 (p-p38), and unphosphorylated ERK, JNK, and p38 in the cellular lysates were determined by Western blot analysis. Upper panel: representative of three separate experiments with similar results. Lower panel: Bands were quantified by densitometry using the software program Image Gauge (LAS-4000plus) to allow correction for protein loading. Data represent the ratio of the intensity of each phosphorylated protein (p-ERK, p-JNK or p-p38) divided by the amount of the respective unphosphorylated protein (ERK, JNK or p38). Values are the mean ± SD of three independent experiments. *P < 0·05 as compared between stimulated and untreated cells (Ctrl, control). (b) Inhibitory effects of ERK and JNK inhibitors. LAD2 cells (1 × 106 cells) were pre-treated with 10 μm U0126, 20 μm SP600125 or 0·1% DMSO for 2 hr, and the cells were then exposed to 10 μm wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L), Arg374Gln (R374Q) or diluent 0·01% acetic acid (Ctrl, control) for 6 hr. The concentrations of granulocyte–macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3) and MIP-1β/CCL4 in the culture supernatants were measured by an ELISA. Values are the mean ± SD of four separate experiments and were compared between untreated cells (Ctrl, control) and stimulated cells without inhibitor (w/o inhibitor), **P < 0·01, #P < 0·001, and between cells with the presence and absence of ERK inhibitor. *P < 0·05, **P < 0·01, #P < 0·001.
The requirement for MAPK signalling pathways in catestatin-induced mast cell stimulation was evaluated by pre-treating mast cells with specific inhibitors for ERK and JNK: U0126 and SP600125, respectively. As shown in Fig. 6(b), U0126 almost completely abolished the production of cytokines and chemokines mediated by wild-type catestatin and its variants, whereas SP600125 had no inhibitory effect. Similarly, other inhibitors specific to JNK did not reduce the stimulatory effects of catestatin peptides (data not shown). We confirmed that both U0126 and SP600125 suppressed ERK and JNK phosphorylation, respectively (data not shown), suggesting that only ERK is required for catestatin-induced stimulation of human mast cells.
Expression of the α7 nAChR
Given that the activation of G-proteins may imply the presence of functional receptors, we next assessed the possibility that catestatin peptides might activate human mast cells via specific receptors. Catestatin inhibits catecholamine release through nAChR activation;6 therefore, we envisaged that nAChRs might be involved in catestatin-induced mast cell stimulation. Among the nAChRs tested, including α3, α4, α7 and α9, we observed that only the α7 subunit mRNA was expressed in human mast cells as shown by RT-PCR (Fig. 7a). To confirm the presence of the α7 nAChR in mast cells at the protein level, we performed FACS analysis. As shown in Fig. 7(b), staining human mast cells with an α7 nAChR-specific antibody showed increased expression of the α7 nAChR compared with staining with a control IgG.
Figure 7.
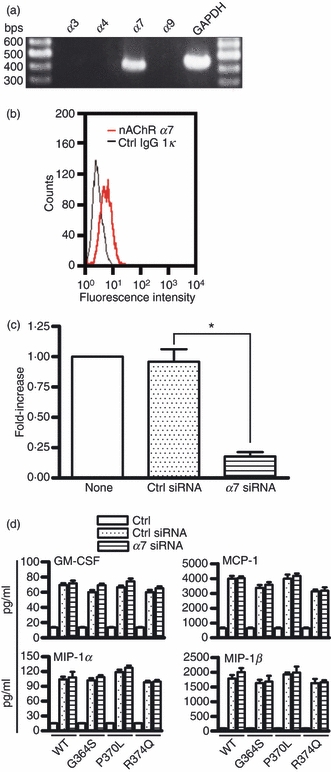
Expression of the α7 nicotinic acetylcholine receptor (nAChR) on mast cells and its involvement in catestatin-induced activation of mast cells. (a) Total RNA (2 μg) isolated from LAD2 cells was analysed for expression of the α3, α4, α7 and α9 subunits of nAChR by RT-PCR. Aliquots of the PCR products were run on 2% agarose gels and visualized by ethidium bromide staining. One representative of three separate experiments is shown. (b) Expression of the α7 nAChR protein in mast cells. Cells (1 × 106 cells) were incubated with an α7 nAChR antibody (nAChR α7) or isotype control rat IgG1κ antibody (Ctrl IgG1κ) for 30 min, and following staining with FITC-conjugated goat anti-rat IgG, the expression of the α7 nAChR was evaluated by FACS. (c) Cells (3 × 106 cells) were transfected with 400 nmα7 nAChR siRNA or control siRNA using the Amaxa Cell Line Nucleofector kit V, program T-030. After 24 hr of gene silencing, α7 nAChR mRNA expression was evaluated by quantitative real-time PCR. Values are the mean ± SD of three separate experiments and were compared between α7 nAChR siRNA-transfected (α7 siRNA) and control siRNA-transfected cells (Ctrl siRNA). None: non-transfected mast cells. *P < 0·001. (d) In addition, cells were transfected with 400 nmα7 nAChR siRNA or control siRNA for 48 hr. Transfected cells (1 × 106 cells) were stimulated with 10 μm wild-type catestatin (WT), Gly364Ser (G364S), Pro370Leu (P370L), Arg374Gln (R374Q), or diluent 0·01% acetic acid (Ctrl, control) for 6 hr, and the amounts of granulocyte–macrophage colony-stimulating factor (GM-CSF), monocyte chemoattractant protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1α (MIP-1α/CCL3) and MIP-1β/CCL4 released into the culture supernatants were determined by an ELISA. Each bar represents the mean ± SD of three separate experiments.
To determine whether the α7 nAChR is used functionally by catestatin peptides to activate human mast cells, we performed α7 nAChR gene silencing by transfecting the mast cells with α7 nAChR siRNA, and used these transfected cells to assess the possible involvement of the α7 nAChR in catestatin-induced mast cell degranulation and production of cytokines and chemokines. As seen in Fig. 7(c), silencing the α7 nAChR for 24 hr almost completely suppressed α7 nAChR mRNA expression, compared with cells transfected with the control siRNA. Our experiments using these α7 nAChR siRNA-transfected mast cells, however, failed to show that the α7 nAChR is indeed functional in catestatin-mediated mast cell activation, as there were no significant differences in the production of cytokines and chemokines (Fig. 7d), and degranulation (data not shown) between mast cells transfected with the α7 nAChR siRNA and the control siRNA. Longer gene silencing of the α7 nAChR (48–96 hr) did not modify the stimulatory effects of wild-type catestatin and its variants on human mast cells (data not shown). This result was supported by the observation that inhibitors specific to the α7 nAChR such as α-bungarotoxin also had no effect on catestatin-mediated mast cell stimulation (data not shown). Hence, the α7 nAChR is not likely to be involved in catestatin-induced human mast cell activation.
Discussion
In the present study, we investigated the roles of the neuroendocrine AMP catestatin in immune responses based on its stimulatory effects on human mast cells. We demonstrated that wild-type catestatin and its naturally occurring variants induce mast cell migration and degranulation, release of lipid mediators such as PGs and LTs, and production of cytokines and chemokines. Catestatin-mediated mast cell activation was shown to be under the control of G-proteins, PLC and the MAPK/ERK pathways. Although the α7 nAChR was expressed in human mast cells, this receptor is not likely to be functional in catestatin-induced mast cell activation. Although catestatin has been shown to stimulate rat mast cell release of histamine,23 to our knowledge, this is the first study demonstrating multiple functions of wild-type catestatin and its variants in human mast cells. Our findings suggest a new role for catestatin peptides in immunoregulation of the cutaneous immune system via mast cell activation.
Eicosanoids and histamine are mainly secreted by activated mast cells, and are mediators of inflammatory reactions.21 Both LTs and PGs are critically involved in inflammatory and allergic conditions, and PGD2 and PGE2 are abundant in allergic skin inflammation such as contact hypersensitivity.24–26 Furthermore, intracellular Ca2+ is thought to play a key role in mast cell activation, including chemotaxis and release of histamine and eicosanoids.27,28 In this report, wild-type catestatin and its variants increased intracellular Ca2+ mobilization in mast cells and caused them to migrate, degranulate, and release inflammatory mediators. These observations suggest that catestatin peptides might participate in inflammatory reactions via mast cell activation. Overall, wild-type catestatin and its variants had almost equal potencies in activating human mast cells, except for the strongest activity of Pro370Leu in inducing LTC4 release, and the least stimulatory capacity of Arg374Gln in degranulating mast cells. This observation partially contradicts the literature relating to catestatin peptides, where wild-type catestatin and its variants display differential potencies in inhibiting catecholamine release and in inducing monocyte migration.9,11 This was not the result of artificial effects of catestatin peptides, because a control peptide had no effect on mast activation. Hence, the potencies of wild-type catestatin and its variants might vary following their specific activities, and between cell types.
Mast cells accumulate and become activated at sites of inflammation, and their numbers significantly increase during wounding,29 where the levels of catestatin have been found to be enhanced.4 Although the amount of catestatin has been estimated to 20 μm in normal murine skin,4 the precise concentration of an active catestatin in human skin is not yet known. However, because the levels of catestatin increase during skin injury or inflammatory conditions,4 one could expect that catestatin might reach its optimal levels at inflammatory sites or wound sites. In this study, the concentrations used for catestatin peptides ranged from 0·02 to 10 μm, doses that have been reported to display antimicrobial activities against skin pathogens4 and Plasmodium falciparum.30 However, because circulating concentrations of catestatin are in the nanomolar range,31 this suggests that in vitro mast cell activation by catestatin peptides might require higher doses than those expected in vivo. Catestatin has been detected in suprabasal and granular keratinocytes and, to a lesser extent, in the dermis.4 Given that catestatin expression is markedly increased during cutaneous inflammation or skin injury where mast cells accumulate,29 direct contact may occur between catestatin and mast cells, resulting in mast cell activation.
We also herein demonstrated that wild-type catestatin and its variants caused significant increases in the mRNA expression levels of various cytokines and chemokines, but only enhanced the protein levels of GM-CSF, MCP-1/CCL2, MIP-1α/CCL3 and MIP-1β/CCL4. This implies that catestatin-induced human mast cell stimulation may be selective for a limited number of inflammatory mediators. Indeed, there are numerous reports highlighting the inflammatory roles of GM-CSF, MCP-1/CCL2, MIP-1α/CCL3 and MIP-1β/CCL4. It is know that GM-CSF is involved in allergic diseases via its promotion of the antigen-processing activity of Langerhans and dendritic cells, and takes part in the maintenance of the chronic inflammatory process in atopic dermatitis.32 The chemokines MIP-1α/CCL3 and MIP-1β/CCL4 are regarded as markers of local skin inflammatory responses,33 and are critical in both acute inflammation and chronic inflammatory diseases.34,35 Furthermore, MIP-1α/CCL3 enhances the migration of T cells, macrophages, eosinophils and neutrophils in human skin.36 As for MCP-1/CCL2, it displays chemoattractant activity for numerous inflammatory and immune cells, and participates in the pathogenesis of systemic sclerosis and fibrotic processes.36,37 In addition, MCP-1/CCL2 is up-regulated in the epidermis of the chronic lesional skin of atopic dermatitis and psoriasis patients.38 Taken together, our results suggest that in addition to histamine and eicosanoid release, catestatins may also participate in the regulation of cutaneous inflammatory processes by promoting the production of inflammatory cytokines and chemokines by mast cells.
To understand the molecular mechanisms underlying the activities of catestatin peptides, we investigated the requirement for G-proteins and PLC, as their roles in mast cell activation have been reported previously,15,16 and involvement of G-protein pathway has been claimed in catestatin-stimulated rat mast cells and human monocytes.9,23 The G-protein inhibitor pertussis toxin and the PLC inhibitor U-73122 showed inhibitory effects on all catestatin-mediated mast cell functions, implying that catestatins act via G-protein and PLC pathways to exert their stimulatory effects on human mast cells. Although both pertussis toxin and U-73122 had significant inhibitory effects on catestatin activity, the inhibition was not complete, suggesting the presence of additional pathways such as another activating receptor or transactivation. Therefore, we attempted to identify a functional receptor for catestatin in mast cells. Catestatin reportedly inhibits catecholamine release via nAChRs so these receptors were chosen as candidates for our investigation of possible catestatin receptors in human mast cells.6 Among nAChRs examined, we only found the α7 subunit to be expressed in human mast cells, and unexpectedly this receptor was not likely to be used by catestatin peptides because neither α7 nAChR gene silencing nor the α7 nAChR antagonist α-bungarotoxin inhibited catestatin-induced activation of mast cells. This was not consistent with the studies by Kageyama-Yahara et al.39 reporting the expression of α4, α7 and β2 nAChRs in mouse bone-marrow-derived mast cells, and by Mishra et al.40 demonstrating the expression of α7, α9 and α10 nAChRs in a rat mast/basophil cell line (RBL-2H3). However, as there are important functional differences between rodent and human mast cells,41 and because there is a marked heterogeneity in mast cell responses both between species and from different tissues within the same species,42 one could not conclude that the presence of the α7 subunit in human mast cells in our study was irrelevant. The αnAChR has also been detected in another human mast cell line (HMC-1), in basophils, macrophages, epithelial cells and endothelial cells;43–45 however, the role of the α7 receptor in inflammation is not yet known. Although the presence of non-functional α7 receptor in human mast cells does not exclude the existence of other still unidentified catestatin receptors, it is noteworthy that as catestatin is a cationic peptide, it might act either at some non-selective membrane receptors or might directly bind to and activate G proteins sensitive to pertussis toxin and coupled to PLC, as has been shown for most basic secretagogues of mast cells.46 This is supported by a previous report that catestatin probably elicits its histamine releasing activity from rat mast cells via a receptor-independent activation of the pertussis toxin-sensitive pathway.23
In the course of evaluating the downstream cellular mechanisms involved in mast cell activation by catestatin, we focused on MAPK cascades, which participate in different activities such as cell survival and proliferation, and expression of pro-inflammatory cytokines and chemokines.47,48 Catestatin peptides induced the phosphorylation of ERK and JNK, but not p38. Given that the ERK-specific inhibitor U0126 showed an almost complete inhibition of catestatin-stimulated cytokine and chemokine production, we concluded that only ERK was involved in catestatin-mediated mast cell activation. Notably, although JNK phosphorylation was increased by catestatin peptides, the inhibition of JNK did not affect the ability of catestatin to stimulate mast cells, implying that the JNK pathway might not be required for mast cell activation by wild-type catestatin and its variants.
Neuropeptides and the neuroendocrine system have previously been thought to be regulators of cutaneous immunity.49,50 Moreover, the influence of neural and neuroendocrine factors on mast cell activities has been demonstrated by the fact that surgical denervation of sensory nerve fibres impairs mast cell-induced cutaneous anaphylaxis.51 The current study reveals one more link between the immune and neuroendocrine systems in which the neuroendocrine AMP catestatin activates human mast cells, and may exert immunomodulatory effects on the cutaneous immune system. Further studies are needed for investigation of the pathophysiological roles of catestatin peptides in tissues where mast cells are abundantly present.
Acknowledgments
Our sincere thanks go to Dr Arnold Kirshenbaum (National Institutes of Health, National Institute of Allergy and Infectious Diseases, Bethesda, MD) for kindly providing the LAD2 cell line. We thank the members of the Atopy (Allergy) Research Center and the Department of Immunology of Juntendo University School of Medicine for their encouragement and critical comments, and Ms Michiyo Matsumoto for secretarial assistance. We are also deeply indebted to Dr Mukesh Pasupuleti (University of British Columbia, Vancouver, Canada) for his contribution in designing the catestatin scrambled peptide. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Atopy (Allergy) Research Center, Juntendo University, Tokyo, Japan; and Japan International Cooperation Agency (JICA).
Glossary
Abbreviations
- AMP
antimicrobial peptide
- EIA
enzyme immunoassay
- ERK
extracellular signal-regulated kinase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- IL-6
interleukin-6
- JNK
Jun N-terminal kinase
- LT
leukotriene
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemotactic protein
- MIP
macrophage inflammatory protein
- nAChR
nicotinic acetylcholine receptor
- PG
prostaglandin
- PLC
phospholipase C
- SCF
stem cell factor
- siRNA
small interfering RNA
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Niyonsaba F, Nagaoka I, Ogawa H, Okumura K. Multifunctional antimicrobial proteins and peptides: natural activators of immune systems. Curr Pharm Des. 2009;15:2393–413. doi: 10.2174/138161209788682271. [DOI] [PubMed] [Google Scholar]
- 2.Schauber J, Gallo RL. Antimicrobial peptides and the skin immune defense system. J Allergy Clin Immunol. 2008;122:261–6. doi: 10.1016/j.jaci.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor CV, Taupenot L, Mahata SK, et al. Formation of the catecholamine release-inhibitory peptide catestatin from chromogranin A. Determination of proteolytic cleavage sites in hormone storage granules. J Biol Chem. 2000;275:22905–15. doi: 10.1074/jbc.M001232200. [DOI] [PubMed] [Google Scholar]
- 4.Radek KA, Lopez-Garcia B, Hupe M, et al. The neuroendocrine peptide catestatin is a cutaneous antimicrobial and induced in the skin after injury. J Invest Dermatol. 2008;128:1525–34. doi: 10.1038/sj.jid.5701225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taupenot L, Harper KL, O'Connor DT. The chromogranin–secretogranin family. N Engl J Med. 2003;348:1134–49. doi: 10.1056/NEJMra021405. [DOI] [PubMed] [Google Scholar]
- 6.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, Gill BM, Parmer RJ. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–33. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briolat J, Wu SD, Mahata SK, et al. New antimicrobial activity for the catecholamine release-inhibitory peptide from chromogranin A. Cell Mol Life Sci. 2005;62:377–85. doi: 10.1007/s00018-004-4461-9. [DOI] [PubMed] [Google Scholar]
- 8.Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–8. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 9.Egger M, Beer AG, Theurl M, et al. Monocyte migration: a novel effect and signaling pathways of catestatin. Eur J Pharmacol. 2008;598:104–11. doi: 10.1016/j.ejphar.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 10.Wen G, Mahata SK, Cadman P, et al. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, O'Connor DT. The catecholamine release-inhibitory catestatin fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–91. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- 12.Rothe MJ, Nowak M, Kerdel FA. The mast cell in health and disease. J Am Acad Dermatol. 1990;23:615–24. doi: 10.1016/0190-9622(90)70264-i. [DOI] [PubMed] [Google Scholar]
- 13.McLachlan JB, Shelburne CP, Hart JP, Pizzo SV, Goyal R, Brooking-Dixon R, Staats HF, Abraham SN. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–41. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- 14.Niyonsaba F, Someya A, Hirata M, Ogawa H, Nagaoka I. Evaluation of the effects of peptide antibiotics human β-defensins-1/-2 and LL-37 on histamine release and prostaglandin D2 production from mast cells. Eur J Immunol. 2001;31:1066–75. doi: 10.1002/1521-4141(200104)31:4<1066::aid-immu1066>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Niyonsaba F, Ushio H, et al. Antimicrobial peptides human β-defensin (hBD)-3 and hBD-4 activate mast cells and increase skin vascular permeability. Eur J Immunol. 2007;37:434–44. doi: 10.1002/eji.200636379. [DOI] [PubMed] [Google Scholar]
- 16.Niyonsaba F, Ushio H, Hara M, et al. Antimicrobial peptides human β-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. 2010;184:3526–34. doi: 10.4049/jimmunol.0900712. [DOI] [PubMed] [Google Scholar]
- 17.Kulka M, Sheen CH, Tancowny BP, Grammer LC, Schleimer RP. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schiemann F, Brandt E, Gross R, Lindner B, Mittelstädt J, Sommerhoff CP, Schulmistrat J, Petersen F. The cathelicidin LL-37 activates human mast cells and is degraded by mast cell tryptase: counter-regulation by CXCL4. J Immunol. 2009;183:2223–31. doi: 10.4049/jimmunol.0803587. [DOI] [PubMed] [Google Scholar]
- 19.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk Res. 2003;27:677–82. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz LB, Austen KF, Wasserman SI. Immunologic release of β-hexosaminidase and β-glucuronidase from purified rat serosal mast cells. J Immunol. 1979;123:1445–50. [PubMed] [Google Scholar]
- 21.Galli SJ. New concepts about the mast cell. N Engl J Med. 1993;328:257–65. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- 22.Di Capite J, Parekh AB. CRAC channels and Ca2+ signaling in mast cells. Immunol Rev. 2009;231:45–58. doi: 10.1111/j.1600-065X.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 23.Krüger PG, Mahata SK, Helle KB. Catestatin (CgA344-364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regul Pept. 2003;114:29–35. doi: 10.1016/s0167-0115(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 24.Oguma T, Asano K, Ishizaka A. Role of prostaglandin D2 and its receptors in the pathophysiology of asthma. Allergol Int. 2008;57:307–12. doi: 10.2332/allergolint.08-RAI-0033. [DOI] [PubMed] [Google Scholar]
- 25.Steinke JW, Bradley D, Arango P, Crouse CD, Frierson H, Kountakis SE, Kraft M, Borish L. Cysteinyl leukotriene expression in chronic hyperplastic sinusitis-nasal polyposis: importance to eosinophilia and asthma. J Allergy Clin Immunol. 2003;111:342–9. doi: 10.1067/mai.2003.67. [DOI] [PubMed] [Google Scholar]
- 26.Ruzicka T, Printz MP. Arachidonic acid metabolism in skin: experimental contact dermatitis in guinea pigs. Int Arch Allergy Appl Immunol. 1982;69:347–52. doi: 10.1159/000233198. [DOI] [PubMed] [Google Scholar]
- 27.Jung ID, Lee HS, Lee HY, Choi OH. FcεRI-mediated mast cell migration: signaling pathways and dependence on cytosolic free Ca2+ concentration. Cell Signal. 2009;21:1698–705. doi: 10.1016/j.cellsig.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawabe H, Hayashi H, Hayaishi O. Differential calcium effects on prostaglandin D2 generation and histamine release from isolated rat peritoneal mast cells. Biochem Biophys Res Commun. 1987;143:467–74. doi: 10.1016/0006-291x(87)91377-5. [DOI] [PubMed] [Google Scholar]
- 29.Pereira MC, de Pinho CB, Medrado AR, Andrade Zde A, Reis SR. Influence of 670 nm low-level laser therapy on mast cells and vascular response of cutaneous injuries. J Photochem Photobiol B. 2010;98:188–92. doi: 10.1016/j.jphotobiol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Akaddar A, Doderer-Lang C, Marzahn MR, et al. Catestatin, an endogenous chromogranin A-derived peptide, inhibits in vitro growth of Plasmodium falciparum. Cell Mol Life Sci. 2010;67:1005–15. doi: 10.1007/s00018-009-0235-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–45. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 32.Esche C, de Benedetto A, Beck LA. Keratinocytes in atopic dermatitis: inflammatory signals. Curr Allergy Asthma Rep. 2004;4:276–84. doi: 10.1007/s11882-004-0071-8. [DOI] [PubMed] [Google Scholar]
- 33.Stojadinovic A, Elster EA, Anam K, Tadaki D, Amare M, Zins S, Davis TA. Angiogenic response to extracorporeal shock wave treatment in murine skin isografts. Angiogenesis. 2008;11:369–80. doi: 10.1007/s10456-008-9120-6. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh CH, Frink M, Hsieh YC, Kan WH, Hsu JT, Schwacha MG, Choudhry MA, Chaudry IH. The role of MIP-1α in the development of systemic inflammatory response and organ injury following trauma hemorrhage. J Immunol. 2008;181:2806–12. doi: 10.4049/jimmunol.181.4.2806. [DOI] [PubMed] [Google Scholar]
- 35.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–6. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 36.Gaga M, Ong YE, Benyahia F, Aizen M, Barkans J, Kay AB. Skin reactivity and local cell recruitment in human atopic and nonatopic subjects by CCL2/MCP-1 and CCL3/MIP-1α. Allergy. 2008;63:703–11. doi: 10.1111/j.1398-9995.2007.01578.x. [DOI] [PubMed] [Google Scholar]
- 37.Karrer S, Bosserhoff AK, Weiderer P, et al. The-2518 promotor polymorphism in the MCP-1 gene is associated with systemic sclerosis. J Invest Dermatol. 2005;124:92–8. doi: 10.1111/j.0022-202X.2004.23512.x. [DOI] [PubMed] [Google Scholar]
- 38.Giustizieri ML, Mascia F, Frezzolini A, De Pità O, Chinni LM, Giannetti A, Girolomoni G, Pastore S. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell-derived cytokines. J Allergy Clin Immunol. 2001;107:871–7. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- 39.Kageyama-Yahara N, Suehiro Y, Yamamoto T, Kadowaki M. IgE-induced degranulation of mucosal mast cells is negatively regulated via nicotinic acetylcholine receptors. Biochem Biophys Res Commun. 2008;377:321–5. doi: 10.1016/j.bbrc.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Mishra NC, Rir-sima-ah J, Boyd RT, Singh SP, Gundavarapu S, Langley RJ, Razani-Boroujerdi S, Sopori ML. Nicotine inhibits Fcε RI-induced cysteinyl leukotrienes and cytokine production without affecting mast cell degranulation through α7/α9/α10-nicotinic receptors. J Immunol. 2010;185:588–96. doi: 10.4049/jimmunol.0902227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffy SM, Lawley WJ, Conley EC, Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol. 2001;167:4261–70. doi: 10.4049/jimmunol.167.8.4261. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi J, Kuroda E, Yamashita U. Strain difference of murine bone marrow-derived mast cell functions. J Leukoc Biol. 2005;78:605–11. doi: 10.1189/jlb.1104676. [DOI] [PubMed] [Google Scholar]
- 43.Sudheer PS, Hall JE, Donev R, Read G, Rowbottom A, Williams PE. Nicotinic acetylcholine receptors on basophils and mast cells. Anaesthesia. 2006;61:1170–4. doi: 10.1111/j.1365-2044.2006.04870.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor α7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–8. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, Pereira EF, Maus AD, Ostlie NS, Navaneetham D, Lei S, Albuquerque EX, Conti-Fine BM. Human bronchial epithelial and endothelial cells express α7 nicotinic acetylcholine receptors. Mol Pharmacol. 2001;60:1201–9. doi: 10.1124/mol.60.6.1201. [DOI] [PubMed] [Google Scholar]
- 46.Ferry X, Brehin S, Kamel R, Landry Y. G protein-dependent activation of mast cell by peptides and basic secretagogues. Peptides. 2002;23:1507–15. doi: 10.1016/s0196-9781(02)00090-6. [DOI] [PubMed] [Google Scholar]
- 47.Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- 48.Chang L, Karin M. Mammalian MAP kinase signaling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 49.Ding W, Wagner JA, Granstein RD. CGRP, PACAP, and VIP modulate Langerhans cell function by inhibiting NF-κB activation. J Invest Dermatol. 2007;127:2357–67. doi: 10.1038/sj.jid.5700858. [DOI] [PubMed] [Google Scholar]
- 50.Fox FE, Kubin M, Cassin M, et al. Calcitonin gene-related peptide inhibits proliferation and antigen presentation by human peripheral blood mononuclear cells: effects on B7, interleukin 10, and interleukin 12. J Invest Dermatol. 1997;108:43–8. doi: 10.1111/1523-1747.ep12285627. [DOI] [PubMed] [Google Scholar]
- 51.Siebenhaar F, Magerl M, Peters EM, Hendrix S, Metz M, Maurer M. Mast cell-driven skin inflammation is impaired in the absence of sensory nerves. J Allergy Clin Immunol. 2008;121:955–61. doi: 10.1016/j.jaci.2007.11.013. [DOI] [PubMed] [Google Scholar]


