Summary
Enthusiasm for therapeutic cancer vaccines has been rejuvenated with the recent completion of several large, randomized phase III clinical trials that in some cases have reported an improvement in progression free or overall survival. However, an honest appraisal of their efficacy reveals modest clinical benefit and a frequent requirement for patients with relatively indolent cancers and minimal or no measurable disease. Experience with adoptive cell transfer-based immunotherapies unequivocally establishes that T cells can mediate durable complete responses, even in the setting of advanced metastatic disease. Further, these findings reveal that the successful vaccines of the future must confront (i) a corrupted tumor microenvironment containing regulatory T cells and aberrantly matured myeloid cells, (ii) a tumor-specific T-cell repertoire that is prone to immunologic exhaustion and senescence, and (iii) highly mutable tumor targets capable of antigen loss and immune evasion. Future progress may come from innovations in the development of selective preparative regimens that eliminate or neutralize suppressive cellular populations, more effective immunologic adjuvants, and further refinement of agents capable of antagonizing immune check-point blockade pathways.
Keywords: cell intrinsic modulators of metabolism and memory formation, Toll-like receptor agonist, CTLA-4, PD-1, Wnt/β-catenin, mTOR
Introduction
‘Even the longest journey must begin where you stand.’
- Lao-tzu, Chinese Philosopher
There is inherent intellectual, conceptual, and methodological appeal to the idea of actively immunizing patients with cancer against their disease. Prophylactic vaccines against infectious agents have unquestionably been a major contributor to improved life expectancy in both the developed and developing worlds over the last two centuries (1). More recently, the development of vaccines that induce protective immunity against viral pathogens known to induce cancer, such as high risk serotypes of the human papillomavirus (HPV) and the hepatitis B virus (HBV), have entered routine clinical practice (2–5). Inspired by these successes, great effort, expense, and the precious resource of patient volunteers have been employed in an attempt to develop therapeutic cancer vaccines. The path thus far has not been an easy one.
Nearly seven years ago, we summarized our experience in the Surgery Branch of the National Cancer Institute, U.S.A. and that of a wide range of international investigators who had performed well designed and methodologically sound clinical trials of therapeutic cancer vaccines using standard oncologic assessment criteria as a study end point (6). The conclusions drawn from this critical analysis were as sobering as they were controversial: the overwhelming majority (>96%) of patients in the studies evaluated who received vaccine therapy for their underlying cancer did not exhibit objective evidence of cancer regression using standard oncologic reporting criteria. In hindsight, such a poor overall response rate was perhaps predictable as all early phase oncology clinical trials, when considered together, have response rates on the order of 3.8% to 11%, depending on whether the specific trial included an approved reagent with known anti-tumor activity (7, 8).
Following this publication, some investigators have advocated for modification of current World Health Organization (WHO) and Response Evaluation Criteria in Solid Tumors (RECIST) response criteria specifically for immunotherapy trials in an attempt to avoid possible underestimation of clinical benefit an experimental immunotherapy may provide (9–12). Arguments in favor of modifying these criteria have in part been based on the observation that a proportion of melanoma patients treated with ipilimumab, a blocking monoclonal antibody targeting the negative co-stimulatory molecule cytotoxic T lymphocyte-associated protein 4 (CTLA-4) (CD152), experienced prolonged stabilization of disease or even transient progression of disease before achieving an objective response consistent with current assessment criteria (12–14). Additionally, in a recently completed phase III clinical trial using the same reagent, only a modest proportion of patients (about 11%) experienced tumor regression consistent with WHO criteria despite having an overall survival benefit compared with the control arm (15). However, as has correctly been pointed out by others (16, 17), ad hoc modification of current response criteria may just as likely lead to the risk of overestimation of benefit, thereby allowing patients to continue on an inactive and potentially toxic regimen without the opportunity to transition to other clinical trials.
This latter point has become increasingly important in diseases such as melanoma, where we have gratifyingly transitioned from a paucity of efficacious treatment options to a number of approaches that in early phase trials have significant anti-tumor activity. Specifically, the adoptive cell adoptive cell transfer (ACT) of tumor-infiltrating lymphocytes (TILs) into lympho-depleted patients or the use of potent inhibitors of the BRAF V600E oncogene mutation in the roughly 50% of patients harboring this mutation (18) have very high objective response rates ranging from 50% to as much as high as 81% (19–21). It is for these reasons that groups such as ours have remained committed to adhering to standardized oncologic response criteria and evaluation of overall survival as primary end points in cancer immunotherapy trials until well-validated surrogate end points are prospectively established in an effort to allow for meaningful and objective comparisons between studies (16, 22, 23). Regardless, there is broad consensus in the oncology and immunotherapy communities that randomized clinical studies using overall survival as a primary endpoint can (i) provide definitive evidence on whether immune-based interventions for the treatment of cancer are truly providing benefit to patients, defined strictly as extending longevity, and (ii) allow for the validation of surrogate end points or response criteria that may be incorporated into the design of future clinical trials (24–26).
Since we last summarized the state of therapeutic cancer vaccines in 2004 (6), several such phase III trials have matured and reported their findings either in peer reviewed journals or in abstract form. While some of these trials did not reach their predefined primary study end points, others have reported positive results. In one notable case, the data from the trial led to the approval of sipuleucel-T by the United States Food and Drug Administration (FDA) as the first therapeutic cancer vaccine in humans (27). Additionally, beyond large phase III clinical trials, numerous early phase clinical studies of therapeutic cancer vaccines testing new vaccine modalities or targeting novel antigens continue to be initiated and reported. Armed with these findings, we feel it is time for the cancer immunotherapy community to once again take pause, reflect, and ask the question: ‘has the era of efficacious therapeutic cancer vaccines finally arrived?’
In this review, we provide an updated critical re-assessment of the state of therapeutic cancer vaccines. While significant technical and scientific progress has been achieved in the fields of vaccinology and immunobiology and although the important bench mark of positive randomized phase III immunotherapy clinical trials has finally been reached (27–29), much remains to be accomplished both in terms of efficacy and applicability. As we discuss below, current and future therapeutic vaccines must overcome multiple barriers to achieve success: (i) a corrupted tumor microenvironment containing regulatory T cells (Tregs) and aberrantly matured myeloid cells with suppressive properties (MDSC), (ii) a tumor-specific T-cell repertoire that is prone to immunologic exhaustion and senescence, and (iii) highly mutable tumor targets capable of antigen loss and immune evasion. We conclude by offering our perspective on a rational path forward to improving immunotherapies for the treatment of metastatic cancer. These include a renewed investment in the development of effective immunologic adjuvants, consideration for the use of pharmacologic modulators of critical metabolic and developmental pathways (such the mTOR and Wnt/β-catenin pathways) which have shown promise in enhancing T-cell quality and function in pre-clinical studies, ongoing development of checkpoint blockade inhibitors, and finally combining active immunization or its surrogates with the adoptive transfer of tumor-reactive T cells.
A critical re-assessment of contemporary therapeutic cancer vaccine trials
Defining the metrics of success
There are multiple metrics that may be used to assess the relative success or failure of a given therapeutic cancer vaccine candidate. The most robust, and arguably the most meaningful, clinical end-point in therapeutic oncology clinical trials is the assessment of overall survival --the extent to which an experimental intervention actually extends a patient’s life. As standard therapeutic and best supportive care practice patterns continuously evolve, a meaningful assessment of overall survival can only be performed when the survival of a group of patients receiving an experimental intervention is directly and contemporaneously compared to a control arm that does not receive the intervention. Although this randomized design can provide definitive results if performed correctly, it also has a number of insufficiencies. The ethical issues associated with this clinical trial design have been discussed elsewhere and can be of concern where some control patient cohorts receive treatments known to be ineffective or inferior (30). In addition, the accurate determination of overall survival in this manner often takes a large patient cohort followed over a period defined in many months if not years. Thus, there remains a critical need for surrogate end points which could allow investigators to determine early on and with a relatively small number of patients whether an intervention is worth developing further.
An intuitive and commonly used surrogate end point in immunotherapy trials is measurement of changes in the immune system (11, 31). This may be accomplished by assessment of antigen-specific cytotoxicity or T-cell proliferation, determination of T-cell reactivity by enzyme-linked immunospot assay (ELISpot) or intracellular cytokine assays, enumeration of tetramer-reactive T cells, or quantification of antigen-specific antibodies compared before and after vaccination (32). It is now clear, however, that the mere induction of very high levels of tumor-reactive T cells in circulation is often inadequate to offer protection from cancer recurrence or mediate cancer regression in realistic animal models or in human patients. For example, in pmel-1 T-cell receptor (TCR) transgenic mice, where greater than 95% of all CD8+ T cells are reactive against a major histocompatibility complex (MHC) class I-restricted epitope derived from the shared melanoma/melanocyte antigen gp100, tumor growth is identical in transgenic mice and in non-transgenic littermate controls (33, 34). Similarly in humans, very high levels (in excess of 10% of all peripheral blood CD8+ T cells) have been raised against an HLA-A*0201 restricted gp100-epitope through prime-boosting in adjuvant melanoma patients with no objective evidence of disease (35). Despite these impressively high levels of circulating anti-tumor T cells, there was no apparent difference in the rates at which patients experienced recurrence of their disease. Taken together, these data suggest that while measurement of precursor frequency provides some immunological data, including whether or not a vaccine is sufficient to cause immune activation and expansion, currently available assays are not necessarily predictive of patient benefit.
A second surrogate end point often used in immunotherapy trials is determination of the response rate, an objective and standardized measurement of tumor shrinkage. For both classic cytotoxic chemotherapy agents and newer, so-called molecularly targeted agents, a higher overall objective response rate appears to be reasonably well correlated with the likelihood that a particular agent will obtain regulatory approval (36, 37) and, more importantly, extend overall survival (38, 39). Additionally, determination of the response rate is relatively facile and reproducible between institutions using common radiographic instruments, such as computed tomography (CT) scanners. Finally, in contrast with ‘stable disease’ measured over an arbitrary time frame, assessment of objective responses minimizes the possibility of false positive results because inactive agents are expected to have a response rate that approaches zero and spontaneous regression in diseases like melanoma is exceedingly rare (40). It is for these reasons that we continue to believe there is important value in assessing and comparing the objective response rate mediated by different immunotherapy regimens, particularly in the setting of early phase clinical trials.
A re-appraisal of early phase cancer vaccines trials since 2004
To determine whether progress has been made in the efficacy of early phase therapeutic cancer vaccines since 2004, the year of our last published overview (6), we selected a sample of therapeutic vaccine trials including 856 patients representing multiple types of solid cancers and which explicitly used standardized oncologic response criteria (41–43) as a study endpoint (Table 1). Although the sheer number of cancer vaccine trials precluded analysis of every trial that was performed in this interval, we sought to include a wide variety of studies we felt were representative of the field as a whole, including trials evaluating tumors considered to be immunogenic and non- immunogenic and which evaluated a diverse array of vaccine platforms. Trials that included compounds with known anti-tumor activity were specifically excluded. As discussed above, given the poor predictive value of current immuno-monitoring correlates with clinical efficacy, combined with the low false positive rate of anti-tumor activity when objective responses are observed, we used response rates as a measure of a positive outcome. While stabilization of disease, particularly when strictly defined and prolonged in duration, may very well translate into improved survival for conventional chemotherapies (44), molecularly targeted therapies (45, 46), and immunotherapies (15), we chose not assess this parameter as it is difficult to make sense of in what are generally small single arm studies. Consequently, our present analysis may underestimate the benefit of any given vaccine regimen.
Table 1.
Results of selected early phase therapeutic vaccine trials in patients with metastatic solid cancers since 2004
| Vaccine type | Reference | Cancer type | Vaccine | Total patients | Patients responding |
|---|---|---|---|---|---|
| Peptide | 158 | Melanoma | NY-ESO-1 + CpG | 8 | 0 |
| 159 | Melanoma | Multi-epitope vaccine alone | 17 | 2 (PR) | |
| 160 | Melanoma | gp100 + IFA +/- GM-CSF | 28 | 0 | |
| 161 | Melanoma | NY-ESO-1 + IFA | 37 | 1 (PR) | |
| 162 | Kidney | Multi-epitope vaccine + IFA | 10 | 0 | |
| 163 | Kidney | CA9 multi-epitope vaccine + IFA | 23 | 3 (PR) | |
| 164 | Lung | Multi-epitope vaccine + IFA | 63 | 1 (CR)/1 (PR) | |
| 165 | Lung | Telomerase peptides + IFA | 22 | 0 | |
| 166 | Breast | Survivin peptide +/− IFA | 17 | 0 | |
| 167 | Brain | WT1 + IFA | 21 | 2 (PR) | |
| 168 | Esophagus | CHP-NY-ESO-1 and CHP-Her2 peptides + OK-432 | 8 | 0 | |
| 169 | Esophagus | Multi-epitope vaccine + IFA | 10 | 1 (CR) | |
| 170 | Urothelial | Survivin peptide + IFA | 9 | 0 | |
| 171 | Gynecologic | WT1 peptide + IFA | 12 | 0 | |
| 172 | Multiple | Chimeric Her2 peptide + ISA 720 | 24 | 1 (PR) | |
| 173 | Multiple | CHP-Her2 +/− GM-CSF or OK-432 | 9 | 0 | |
| 174 | Multiple | WT1 + IFA | 10 | 1 (PR) | |
| 175 | Multiple | Telomerase peptides + IFA | 19 | 0 | |
| Response rate: | 13/347 (3.7%) | ||||
| Dendritic cell | 176 | Melanoma | Transduced with MART-1 | 17 | 0 |
| 177 | Melanoma | Pulsed with allogeneic tumor lysate | 7 | 0 | |
| 178 | Melanoma | Pulsed with allogeneic tumor lysate | 20 | 1 (CR)/1 (PR) | |
| 179 | Melanoma | Pulsed with autologous tumor lysate | 46 | 3 (CR)/3 (PR) | |
| 180 | Melanoma | Transfected with RNA | 20 | 0 | |
| 181 | Melanoma | Pulsed with peptides | 12 | 0 | |
| 182 | Kidney | Pulsed with peptides | 30 | 0 | |
| 183 | Kidney | Allogeneic DC fused with autologous tumor | 20 | 2 (PR) | |
| 184 | Breast | Pulsed with peptides | 26 | 0 | |
| 185 | Thyroid | Pulsed with allogeneic tumor lysate | 10 | 0 | |
| 186 | Multiple | Pulsed with CEA peptide | 9 | 0 | |
| 187 | Multiple | Pulsed with mannan-MUC1 fusion protein | 10 | 0 | |
| 188 | Multiple | Modified with pox-virus encoding CEA + TRICOM | 13 | 0 | |
| Response rate: | 10/240 (4.2%) | ||||
| Virus | 189 | Melanoma | Heterologous prime-boost poxvirus-tyrosinase | 13 | 0 |
| 190 | Kidney | Poxvirus-encoded 5T4 | 13 | 0 | |
| 191 | Prostate | Heterologous prime-boost poxvirus-PSA + TRICOM | 7 | 0 | |
| 192 | Colorectal | Poxvirus-encoded 5T4 | 17 | 0 | |
| 193 | Multiple | Poxvirus-encoded CEA + TRICOM | 58 | 1 | |
| Response rate: | 1/108 (0.9%) | ||||
| Protein | 194 | Melanoma | NY-ESO-1 protein + ISCOMATRIX | 27 | 0 |
| PBMC | 195 | Lung | αGalCer-pulsed PBMC cultured with IL-2 + GM-CSF | 17 | 0 |
| Ganglioside | 196 | Melanoma | Ganglioside + IFA | 22 | 1 (PR) |
| Endothelial cell | 197 | Multiple | Fixed human umbilical vein endothelial cells | 9 | 1 (CR)/2 (PR) |
| RNA | 198 | Melanoma | Autologous tumor mRNA | 8 | 0 |
| Response rate: | 4/83 (4.8%) | ||||
| Tumor cells | 199 | Lung | Allogeneic tumor line transduced with antisense TGF-β2 | 21 | 0 |
| 200 | Lung | Autologous tumor mixed with allogeneic GM-CSF transduced tumor | 49 | 0 | |
| 201 | Mesothelioma | Autologous tumor + GM-CSF | 22 | 0 | |
| 202 | Brain | Autologous tumor transduced with antisense TGF-β2 | 6 | 2 (PR) | |
| Response rate: | 2/98 (2.0%) | ||||
| Plasmid DNA | 203 | Melanoma | MART-1 and Tyrosinase | 19 | 0 |
| 204 | Melanoma | gp100 +/− GM-CSF | 8 | 0 | |
| 205 | Head and neck | Hsp65 | 21 | 4 (PR) | |
| 206 | Multiple | NY-ESO-1 | 3 | 0 | |
| Response rate: | 4/51 (7.8%) | ||||
| Overall response rate: | 34/927 (3.7%) |
Some trials contain fewer reported total numbers of patients than in the primary report as only patients with evaluable disease at the time of enrollment are included on this table.
Thirty-three objective responses were reported for an overall objective response rate of 3.7% across all of the trials evaluated. There were 13 (3.7%) responses in 347 patients receiving peptide vaccines, 10 (4.2%) in 240 patients receiving dendritic cell vaccines, 1 (0.9%) response in 108 patients receiving recombinant viral vaccines, 2 (2%) responses in 98 patients receiving tumor cell vaccines, and 4 (7.8%) responses in 51 patients receiving DNA plasmid vaccines. Four (4.8%) responses were seen in 83 total patients that received either full-length protein, peripheral blood mononuclear cell (PBMC), ganglioside, or RNA-based vaccines. These results are essentially identical to our findings in 2004, where we found our own vaccine trials had a response rate of only 1.9%, with only a 3.8% response rate observed by extra-mural and international investigators (6). Taken together, these data suggest that when considered as a whole and when using objective response rate as the metric of success, there has not been a significant improvement in the efficacy of early phase therapeutic cancer vaccine trials since 2004.
Overall survival in randomized therapeutic cancer vaccine trials
As noted above, survival is arguably the most important and least subjective study endpoint in therapeutic oncology trials. Therefore, in addition to surveying early phase therapeutic vaccine trials which reported objective response rates, we also assessed the efficacy of a series of large, randomized and controlled clinical trials of therapeutic cancer vaccines where overall survival was a study endpoint (Table 2). Trials that focused exclusively on vaccination in the setting of no observable disease (adjuvant setting) were excluded from our analysis.
Table 2.
Overall survival results of randomized controlled trials of therapeutic cancer vaccines in patients with solid cancers.
| Cancer Type | Phase | Total patients | Trial design | Clinical setting | Survival | Reference |
|---|---|---|---|---|---|---|
| Melanoma | 3 | 322 | Hsp-96 Vs. physician’s choice | Stage IV | No improvement in overall survival (P = 0.32) | 207 |
| Melanoma | 3 | 185 | gp100:209–217(210M) peptide + high dose IL-2 Vs. high dose IL-2 | Stage IV | No improvement in overall survival (P = 0.11) | 28 |
| Kidney | 3 | 733 | Poxvirus-encoded 5T4 + SOC Vs. SOC only | Stage III/IV | No improvement in overall survival (P = 0.55) | 208 |
| Lung | 3 | 515 | Bec2/BCG Vs. observation | Limited-stage SCLC after major response to chemo-radiation induction therapy | No improvement in overall survival (P = 0.28) | 209 |
| Lung | 2 | 171 | Liposomal BLP25 + Cyclophosphamide + BSC Vs. BSC alone | Stage IIIB/IV NSCLC | No improvement in overall survival (P = 0.11) | 210 |
| Lung | 2 | 80 | EGF protein + IFA + cyclophosphamide Vs. BSC | Stage IIIB/IV NSCLC | No improvement in overall survival (P = 0.10) | 211 |
| Prostate | 3 | 512 | Sipuleucel-T Vs. placebo | Asymptomatic or minimally symptomatic metastatic CRPC, any Gleason score | 4.1 month improvement in median overall survival (P = 0.02) | 27 |
| Prostate | 3 | 127 | Sipuleucel-T Vs. placebo | Asymptomatic metastatic CRPC, any Gleason score | 4.5 month improvement in median overall survival (P = 0.01) | 48 |
| Prostate | 3 | 98 | Sipuleucel-T Vs. placebo | Asymptomatic metastatic CRPC, any Gleason score | No improvement in overall survival (P = 0.33) | 50 |
| Prostate | 3 | 621 | Prostate GVAX Vs. docetaxel + prednisone | Asymptomatic or minimally symptomatic metastatic CRPC, chemotherapy naive, any Gleason score | No improvement in overall survival (P = 0.78) | 212 |
| Prostate | 3 | 114 | Prostate GVAX + docetaxel + prednisone Vs. docetaxel + prednisone | Metastatic CRPC, chemotherapy naive, any Gleason score | Study terminated early due to excessive deaths in vaccine arm (P = 0.01) | 213 |
| Prostate | 2 | 125 | Heterologous prime-boost poxvirus-PSA + TRICOM Vs. control vector | Asymptomatic or minimally symptomatic metastatic CRPC, Gleason score ≤ 7 | 8.5 month improvement in median overall survival (P = 0.001) | 49 |
SOC = standard of care; BSC = best supportive care.
Unfortunately, as is the case with many oncologic therapies in late-stage testing (47), the majority of these trials did not reach their primary endpoint or failed to find differences in overall survival. In most cases, this was despite promising early phase clinical data with the very same agents. Some vaccine trials did, however, demonstrate a significant improvement in overall survival (27, 48, 49). For example, 2 out of 3 nearly identically designed phase III trials evaluating sipuleucel-T in men with metastatic castrastion-resistant prostate cancer (CRPC) reported an improvement in overall survival on the order of approximately 4 months in the group randomized to receive vaccination over the placebo group (27, 48, 50). Of note, only a single objective partial response was recorded among the 330 patients who received vaccine in the one trial that explicitly reported radiographic responses, and there were no significant differences in time to progression in any of the 3 trials. Further, declines in PSA values of 50% or more were observed in only 13 of 536 patients (2.4%) randomized to the sipuleucel-T arms across all 3 trials.
This pattern of increased survival in the absence of objective responses or a delay in time to progression runs counter to previous experience with conventional cytotoxic chemotherapies where either disease regression or stabilization of disease was correlated with improvements in overall survival (38, 39). As such, these findings have been met with caution and reserve among some investigators (51). Some have suggested that a flaw in the trial design or imbalances between the study groups (52) may have accounted for apparent differences in survival between treatment groups in the absence of demonstrable anti-tumor effects. Indeed, patients randomized to the vaccine arm received docetaxel, a chemotherapy known to improve overall survival in this patient population (53, 54), both earlier and with greater frequency than patients randomized to the placebo arm. A recent subgroup analysis of the TAX327 study, one of the registration trials for docetaxel in metastatic CRPC patients, suggests that men who were asymptomatic or minimally symptomatic had a median overall survival of 25.6 months (55). This value is very close to the 25.8 month survival observed in patients randomized to the sipuleucel T arm of the study. To correct for this potential confounding factor, the authors performed a post hoc analysis censoring patients at the time they initiated docetaxel therapy. However, as the authors correctly point out, there is no universally accepted method to correct for the impact of subsequent treatment.
In a separate prostate cancer vaccine trial using a heterlogous prime-boosting regimen with recombinant pox-viruses encoding PSA, a significant improvement in overall survival was also observed, although the study’s primary end-point of improved time to progression was not met (49). However, several confounding factors with this trial have been brought to attention (56). Specifically, there were apparent imbalances between the two groups such that survival in the control arm was far less than would be predicted based on established nomograms (57). Additionally, no information regarding subsequent therapies such as docetaxel was reported, limiting the ability to determine the extent to which differences in outcome may be attributed to the effects of the experimental vaccines versus those of established therapies. A planned phase III trial using this same pox-virus based regimen should help resolve these questions more definitively and may help add further weight to the concept of improved overall survival in the absence of overt anti-tumor responses (58).
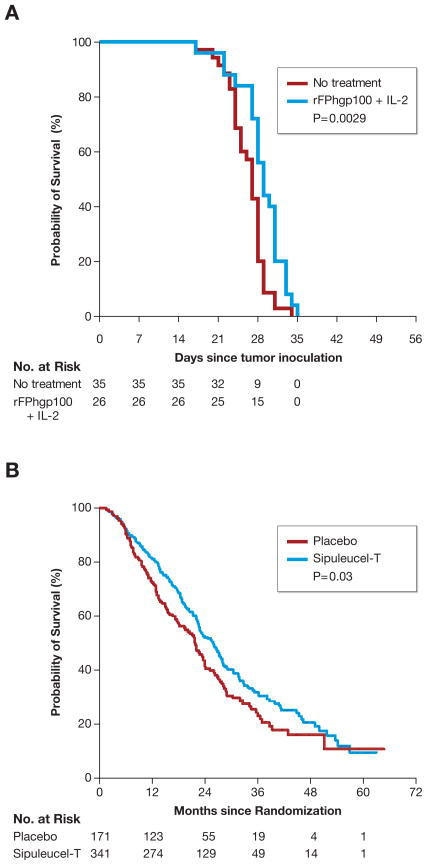
Even in these positive trials, no tail in survival was observed, implying that nearly all patients would ultimately die as a direct consequence of their underlying malignancy. This critical limitation of active immunization for the treatment of cancer, as presently conceived and employed, was predicted in mouse models (Fig. 1). Clearly improvement in the treatment of cancer often comes in small, incremental waves. It is abundantly clear, however, that immunotherapy is capable of causing durable and complete tumor regressions, albeit in a minority of patients at present (59) and especially after intensive host conditioning followed by ACT (19, 20). It therefore seems reasonable that cancer vaccinologists may aspire to such a goal of more robust and durable responses to vaccine therapy by adapting principles gained through experience with successful adoptive T-cell therapies. Promising pre-clinical and early clinical work offers some hope of how to rationally move forward towards this goal.
Fig. 1. Efficacy of therapeutic cancer vaccines in mouse and human.
Realistic animal models for the treatment of human cancers have been developed in an attempt to test, improve, and refine immunotherapies for possible clinical translation. In many cases, results in mice have predicted both limitations as well as promising new therapies in man. (A) Treatment of established B16 melanoma in mice with a recombinant viral vector encoding the shared self/tumor antigen gp100 plus recombinant IL-2 causes a statistically significant (P = 0.0029) but modest improvement in overall survival. All mice ultimately expired from uncontrolled tumor growth. (B) Treatment of metastatic castration resistant prostate cancer in humans with autologous peripheral-blood mononuclear cells activated with a recombinant fusion protein consisting of GM-CSF and the self/tumor-associated antigen prostatic acid phoshatase causes a statistically significant (P =0.03) but modest improvement in overall survival. The vast majority of patients expired from uncontrolled tumor growth. Data reproduced with permission from Kantoff P, et al, NEJM 2010.
A critical need for better immunologic adjuvants
Adjuvants, substances added to vaccines that trigger components of the innate immune system to increase or modify a subsequent adaptive immune response, have been known to be a critical determinant of the success or failure of vaccines for over 90 years (60). Despite this fact, the mechanisms of action and even a precise knowledge of the constituents of many vaccine adjuvants have remained poorly characterized until quite recently (61, 62). It is for this reason that the late Charlie Janeway famously and poignantly described the nature of adjuvants as ‘the immunologist’s dirty little secret’ (63). Some current and previously tested cancer vaccine formulations, such as recombinant viral vectors, functionally contain or are designed to express their own adjuvant (64, 65). Many others, such as peptide-based vaccines, do not and therefore require co-administration of adjuvant in order to induce a productive immune response.
Historically, the choice of adjuvants available for human application has been extremely limited, in part due to concerns regarding toxicity but also due to significant regulatory and financial hurdles (66). In fact, only aluminum salt-based adjuvants and a combination of aluminum hydroxide with monophosphoryl lipid A (MPL), a natural glycolipid derived from Salmonella cell membranes that functions as a Toll-like receptor 4 (TLR4) agonist, have been licensed for human use in the United States. Although there is generally a greater acceptance of possible side effects and toxicity related to vaccine adjuvants in potentially life-threatening diseases, such as human immunodeficiency virus (HIV) infection and cancer, our current armamentarium of adjuvants remains woefully inadequate. Several recent examples illustrate this point.
Based in part on pre-clinical data that demonstrated enhanced protection from tumor re-challenge using irradiated tumor lines retrovirally transduced to secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) (67), this cytokine has frequently been incorporated into many preventative and therapeutic cancer vaccines. Until recently, however, the impact of adding GM-CSF to cancer vaccines in patients had not been systematically evaluated in a controlled fashion.
Two randomized trials comparing active immunization either with or without co-administration of GM-CSF in adjuvant melanoma patients demonstrated inferior immunologic and patient outcomes in the groups randomized to receive GM-CSF (68, 69). In one trial, patients were given a combination of 12 different class I-restricted melanoma-associated epitopes in conjunction with a class II-restricted tetanus ‘helper’ epitope with or without GM-CSF (68). Immune responses against many of the class I-restricted epitopes, as measured by both ELIspot and frequency of tetramer-reactive cells, were significantly lower in the GM-CSF arm. In addition, a trend towards inferior survival was observed in the GM-CSF arm. In a second trial, an allogeneic, irradiated, whole-cell melanoma vaccine derived from three different melanoma cell lines was administered alone or in combination with GM-CSF (69). All study participants received BCG as an additional form of adjuvant. Patients in the GM-CSF arm had an impaired cell-mediated immune response, as evidenced by reduced delayed-type hypersensitivity responses to low doses of the immunizing cell lines, compared with the control arm. Of greater concern, this trial also observed a trend towards worse overall survival in those patients who received GM-CSF. These findings mirror results from earlier clinical studies where either reduced immunogenicity or a failure to augment immunogenicity was seen in vaccine regimens using GM-CSF as an adjuvant compared to a control arm that did not receive the cytokine (70–73). The mechanism behind the reduced immune-responsiveness observed in the presence of exogenous GM-CSF remains to be completely determined. However, it is intriguing to note that GM-CSF was found to inhibit, rather than potentiate, vaccine-induced immunity in pre-clinical as well as a recent clinical study by expanding a population of myeloid cells with suppressive properties (termed MDSC) (74–76). Some authors have suggested that lower-doses of GM-CSF (<80 μg per day) may enhance rather than attenuate cell-mediated immunity; however, this hypothesis requires further evaluation in patients (67).
A second recent example of the critical deficiency in effective vaccine adjuvants involves our experience and that of others with Montanide ISA 51 incomplete Freund’s adjuvant (IFA), an oil-in-water emulsion frequently used in peptide vaccine clinical trials. In 2006, the formulation of Montanide ISA 51 IFA was altered at the mandate of regulatory agencies so that the oleic acid component would be derived from olives, rather than beef tallow, due to theoretical concerns regarding transmission of animal-borne illnesses such as prion disease. This change impacted several ongoing clinical trials, in effect creating an unplanned experiment where sequential cohorts of patients would receive either IFA containing animal derivatives (IFA AD) or IFA derived from vegetable material (IFA VG) (77, 78). This change was not without apparent consequence.
In a retrospective analysis of immune responses generated in sequential adjuvant clinical trials using gp100:209–217 (210M), an anchor-modified epitope of the shared melanoma/melanocyte antigen gp100 (79), we found that immune responses were significantly reduced following the transition from an animal-derived to a vegetable-derived source of IFA (76). Using animal-derived IFA, we found that the vast majority (89%) of patients could be immunized against gp100 using a multi-course vaccination regimen with tetramer-positive frequencies reaching as high as 42% of all circulating CD8+ T cells (80, 81). Of note, a subset of patients who received peptide in saline alone did not exhibit any evidence of immunization (81). Following the transition to IFA VG; however, we discovered that antigen-specific immune responses, as measured by ELISpot and in vitro sensitization assays, as well as local skin reactions were all dramatically impaired. Although some groups did not find any apparent differences in the ability to immunize patients in the interval spanning the transition from IFA AD to IFA VG, these trials incorporated a class II-restricted helper epitope in their vaccine regimen, potentially mitigating the loss of any ‘contaminant’ in the animal-derived product that may have boosted the adjuvanticity of the compound (78). In pre-clinical studies, peptide immunization using IFA VG caused antigen-specific CD8+ T cells to undergo a primary proliferative response but entirely abrogated their ability to mediate a recall response on boosting. Further, peptide/IFA mixtures resulted in long-term sequestration of antigen-specific CD8+ T cells at the vaccination site, in part due to continuous antigen presentation by dendritic cells and B cells (82). Whether this is a specific property of IFA VG or all oil-in-water emulsions remains to be determined. Taken together, these examples demonstrate our critical need to develop more potent and consistent molecularly defined adjuvants.
Cell-intrinsic modulation of T-cell differentiation status using pharmacologic modulators of metabolism
Memory CD4+ and CD8+ T cells are diverse with respect to phenotype, function, and anatomic partitioning. Classically, memory T cells have been divided into two major subsets, termed central memory T cells (Tcm) and effector memory T cells (Tem), based in part on the surface expression of the lymph node homing molecules CD62L and CCR7 as well as the ability to secrete IL-2 (83). More recently a third T memory subset, termed T memory stem cell (Tscm), has been described in mice that display robust self-renewal and the multi-potent capacity to generate Tcm, Tem, and effector T cells (84, 85). Experiments in viral, bacterial, and tumor models have revealed that Tcm are more efficient than Temcells in clearing pathogens and treating tumors due to their enhanced proliferative capacity, persistence, and poly-functionality (86, 87). Additional experiments have demonstrated that Tscm are even more potent yet than Tcm cells in their recall capacity and ability to mediate tumor regression (85). Thus, the selective generation of highly functional Tcm and Tscm subsets in vivo following vaccination has been a sought after goal in vaccinology. Several recent reports suggest that such selectivity in T-cell memory subset formation may indeed be possible through the pharmacologic modulation of the metabolic fitness of responding T cells.
Complementing immunologic adjuvants, which act in a cell-extrinisic manner to enhance immunologic function, has been the recent development of a class of reagents which we have termed cell-intrinsic modulators of metabolism and memory formation (CIMMs) (88). Through the modulation of key metabolic and developmental pathways, such as the adenosine monophosphate-activated kinase (AMPK)/mammalian target of rapamycin (mTOR) and Wnt/β-catenin pathways, CIMMs have been shown to enhance both the quality and quantity of memory CD8+ T cells generated in mice (85, 89–91) and non-human primates (89). Importantly, many candidate CIMMs are already available for human use, potentially allowing for the expeditious translation into human vaccine studies in the near future (88).
Araki and coworkers (89) demonstrated that pharmacologic inhibition of the mTOR pathway using the FDA-approved immune-modulator rapamycin either during the priming or contraction phases of a primary CD8+ T-cell response could enhance T-memory formation. Specifically, the authors found that rapamycin administration during T-cell priming and expansion augmented the absolute number of both Tcm and Tem compared with mice given a vehicle control due to enrichment in IL-7RαhighKLRG1low memory precursors at the peak of the immune response. By contrast, administration of rapamycin during the contraction phase caused an accelerated accumulation of Tcm cells. Similarly, rapamycin during in vitro priming of CD8+ T cells under type 1 conditions with IL-12 was also found to augment memory formation and anti-tumor immunity following adoptive cell transfer (91). In a related study, Pearce and colleagues (90) demonstrated that provision of the commonly used diabetes drug metformin, an AMPK-agonist, during the contraction phase of a primary immune response enhanced subsequent CD8+ T cell memory recall responses and augmented protection tumor from tumor challenge.
The canonical Wnt/β-catenin pathway (92) has recently been found to be a key developmental pathway necessary for the generation of Tscm (85) and the persistence of CD8+ T memory cells in general (93–95). By potentiating Wnt/β-catenin signaling through inhibition of glycogen synthase kinase-β, a component of a multimeric complex that normally targets β-catenin for proteosomal degradation, an increased frequency of tumor-reactive Tscm and Tcm was observed in response to vaccination with a recombinant pox-virus vector. Notably, this enhancement in desirable memory subsets occurred without compromising the absolute number of responding antigen-specific CD8+ T cells.
Immunologic checkpoint blockade antagonists and the removal of suppressive cell populations
The paradigm for a prototypical naive T-cell response to an antigenic challenge, such as an acute viral infection, is that responding T cells undergo a multi-log clonal expansion, acquire effector functions and permeate peripheral tissues, clear the instigating pathogen, and subsequently contract to form a durable population of antigen-experienced memory T cells (96). In cases where the target antigen remains persistent and is not cleared rapidly, such as the tumor-bearing state or in chronic viral infections, this orderly sequence of events can become profoundly disrupted. Consequently, T cells may be generated that are progressively impaired or ‘exhausted’ with respect to their functional and proliferative capabilities (97, 98). Such exhaustion is likely to be a leading, if not primary, reason for the relative ineffectiveness of the majority of therapeutic cancer vaccines. Correlated with the exhausted T-cell phenotype is the surface expression of a diverse array of negative regulatory molecules, including CTLA-4, programmed cell death-1 (PD-1) (CD279), lymphocyte-activation gene 3 (LAG3) (CD223), and T-cell Ig- and mucin-domain-containing molecule-3 (TIM-3), among numerous others (99). Besides being markers of T-cell exhaustion, these molecules actively contribute to suppression of cytokine secretion and proliferative potential (100). Thus, they can represent ideal targets for therapeutic blockade to restore T-cell function in the tumor-bearing state, particularly when combined with concurrent immune stimulation such as vaccination.
CTLA-4 is a type I transmembrane glycoprotein that possesses close homology to the costimulatory molecule CD28 (101). Like CD28, CTLA-4 binds both B7-1 (CD80) and B7-2 (CD86); however, it does so with much higher affinity. Consequently, under limiting conditions, CTLA-4 may function as a B7 sink (102, 103). In contrast to CD28, which delivers pro-proliferative and anti-apoptosis signals to T cells, ligation of CTLA-4 functions to restrict T-cell activation. Beyond serving as a counter-regulatory factor for T-cell activation, CTLA-4 has also been found to be progressively upregulated on chronically stimulated and exhausted T cells (104). Further, CTLA-4 is constitutively expressed on the surface of Tregs, where it contributes to the ability of these cells to antagonize the effector functions of responding T cells (105), including anti-tumor T cells (106).
Treatment with the CTLA-4 blocking antibody ipilimumab has moderate single-agent efficacy in mediating cancer regression in melanoma (15, 107–110) and renal cell carcinoma patients (111). More importantly, ipilimumab has recently been demonstrated to significantly extend overall survival of previously treated melanoma patients in a recently completed randomized phase III clinical trial (15). Interestingly, although the addition of a gp100 peptide vaccine with ipilimumab had no significant impact on overall survival compared with ipilimumab alone, the combination did cause a significant reduction in the overall response rate. These results contrast with the recent experience of combining the same gp100 peptide with bolus IL-2 where an increased frequency of objective responses and a significant improvement in progression free survival were observed compared with IL-2 administration alone (28). The reason why peptide vaccination appears to enhance IL-2 mediated responses while potentially limiting ipilimumab responses remains unclear. However, given that overall survival was not impaired in the combined vaccine plus ipilimumab arm, combined with pre-clinical data suggesting that vaccination may augment the anti-tumor function of CTLA-4 (112). Trials combining alternative vaccine regimens with blockade of CTLA-4, using other checkpoint mediators such as PD-1 (113), or combinations of checkpoint inhibitors may show promise.
Combining ACT with vaccination or vaccine surrogates
In contrast with experience from most therapeutic cancer vaccines, adoptive T-cell therapy can mediate objective tumor regression consistent with standard assessment criteria and, more importantly, durable long term complete responses in a reproducible minority of melanoma patients (19, 20, 114–116). It is this latter point in particular, the ability to mediate complete and sustained eradication of all observable cancer even in the setting of advanced disease, which appears to distinguish ACT from other standard and experimental treatment modalities for metastatic melanoma. Thus, any intervention which could increase the frequency of durable complete responses with ACT could have tremendous clinical impact. One such intervention may be the combination of ACT with concurrent antigen-specific vaccination or a mimetic for vaccination.
Studies in mice using multiple different tumor models have demonstrated that antigen-specific vaccination in combination with adoptively transferred CD4+ or CD8+ tumor-reactive T cells can potently enhance the magnitude of the anti-tumor immune response raised against established tumors (117–123). The mechanisms underlying these improved responses with concurrent vaccination following ACT are multi-factorial. However, key components include induction of a multi-log clonal expansion of adoptively transferred T cells (118), facilitation of T-cell entry into peripheral sites throughout the body where tumors may reside (34, 121), and the release of cytokines, such as IFNγ, which allow T cells to scan and detect target tissues expressing cognate antigen (121, 124, 125). Preliminary clinical experience using either adoptive cell transfer of TIL in combination with concurrent vaccination or transfer of EBV-reactive T cells genetically engineered to express a receptor against a tumor-associated antigen into EBV-positive patients suggest that in vivo antigen re-stimulation may also augment antitumor T-cell function in humans (126, 127).
From a practical standpoint, co-administration of an antigen-specific vaccine in combination with ACT requires knowledge of the reactivity contained in the transferred T-cell population. This may be possible when the transferred T cells are mono-specific by design, such as T cells genetically engineered to express a single exogenous T-cell receptor (TCR) (128, 129) or a cloned population of T cells raised against a defined epitope (115, 130, 131). In the case of TILs, however, provision of an antigen-specific vaccine at the time of cell infusion can prove technically challenging, if not impossible. In general, TILs contain reactivity against a multitude of tumor antigens rather than possessing a single dominant specificity. Further complicating matters, more than 50% of TIL cultures contain reactivity that is autologous in nature (19, 20, 132), precluding the ability to administer an ‘off the shelf’ vaccine reagent targeting these antigens. This is an especially critical problem, as optimization of the in vivo function of TILs remains a high priority. In contrast with other sources of T cells for ACT, such as cloned T cells (115, 130, 131, 133) or T cells engineered to express exogenous TCRs reactive against shared melanoma/melanocyte antigens (128, 129), TILs appear to be capable of mediating durable cancer regression in a larger frequency of patients with relatively less toxicity to host tissues (19, 20). Recent experiments in mice have demonstrated at least two potential solutions to solve this problem by providing mimetics for vaccination that act in an antigen-independent manner.
Ablative host conditioning with HSC rescue can mimic vaccination
One approach has been to increase the intensity of host pre-conditioning prior to cell infusion to the point that rescue of hematologic function through the transfer of hemaptopoetic stem cells (HSCs) is required (134, 135). In mice, it has long been known that host conditioning with a sub-lethal, non-myeloablative dose of radiation or provision of immune-depleting chemotherapy prior to T-cell infusion can potently enhance the anti-tumor function of transferred T cells (136–140). The mechanisms underlying this augmented tumor immunity have recently been resolved (141). These include removal of antigen-irrelevant CD4+ and CD8+ T cells as well as NK cells which function as homeostatic cytokine sinks for the cytokines IL-7 and IL-15 (140, 142), a temporary depletion of suppressive cell populations such as CD4+CD25+FOXP3+ T regulatory cells (Tregs) and MDSCs (135, 143), and mucosal injury liberating systemic levels of the TLR4 agonist LPS into circulation which is capable of maturing splenic dendritic cells (144).
Building on these findings, experiments have subsequently been undertaken to determine the impact of intensifying host conditioning to a fully myeloablative state. In addition to providing for a deeper and more durable reduction in potentially suppressive cell populations, including Tregs, MDSCs, and NKT cells (135), myeloablative host-conditioning unexpectedly also induced a profound expansion of transferred T cells in a HSC-dependent manner (134). Significantly, this expansion occurred in the absence of an exogenously provided antigen-specific vaccine. Perhaps most importantly, myeloablative host conditioning followed by HSC rescue provided equivalent tumor treatment to ACT combined with concurrent vaccination in myeloablated and non-myeloablated hosts. Thus, myeloablative conditioning with HSC rescue can replace the requirement for antigen-specific vaccination to achieve optimal tumor treatment. High intensity host conditioning has been translated to TIL transfer in patients with an apparent increase in the frequency of objective responses (20), including a relatively high rate of durable complete responses, compared with patients who have been treated with TILs alone or TILs with non-myeloablative pre-conditioning (145).
Programming tumor-reactive T cells prior to cell transfer can replace vaccination
A second approach to provide the benefits of concurrent vaccination with ACT in an antigen-independent manner has been to ‘program’ T cells with a short-term stimulus immediately prior to cell infusion. Work pioneered in the Pamer, Ahmed, and Schoenberger (146–149) laboratories has demonstrated that provision of a brief, TCR-focused programming stimulus to naive CD8+ T cells is sufficient to induce clonal proliferation, acquisition of effector functions, and entry into the memory pool in the absence of additional stimulation. Adapting this approach to antigen-experienced cells, we and others (125, 150) have shown in mice that tumor-reactive, memory-like CD8+ T cells can be programmed in an antigen-specific manner with irradiated peptide-pulsed APCs or in an entirely non-specific manner using combined anti-CD3/anti-CD28 stimulation. Programmed tumor-reactive T cells expanded vigorously in vivo upon transfer, gained effector functions, and killed tumor in an IFNγ-dependent manner, all in the absence of a systemically administered cancer vaccine. By contrast, transfer of non re-stimulated T cells resulted in only a marginal delay in tumor growth compared with untreated controls. Translation of the programming approach to patients may be accomplished through bead-based delivery of costimulation signals (151), stimulation of T cells with adherent or removable artificial antigen presenting cells (152), or by subjecting T cells to a rapid expansion protocol with anti-CD3, IL-2, and allogeneic irradiated feeder cells immediately prior to cell transfer (153).
Conclusions
The era of effective immune-based therapies for the treatment of advanced solid cancers is upon us. A nearly 25 year experience with bolus IL-2 has shown that a reproducible minority of patients with advanced metastatic cancer can enjoy durable, long-term complete responses which may even represent cures (154, 155). More recently, immune check-point blockade using antibodies to CTLA-4 has demonstrably improved overall survival in previously treated melanoma patients (15). Further, the adoptive transfer of tumor-reactive T cells into lympho-depleted hosts has shown that a significant percentage of treated patients can experience immune-mediated cancer regression across multiple institutions (19, 156, 157). The latest results from cancer vaccine trials may indicate a prolongation in survival in the absence of evidence of objective tumor regression or significant prolongation in time to progression. The seeming dissociation of clear antitumor activity with improvements in survival will require new hypotheses, new mechanistic inquiries, and new exploratory techniques to validate these observations. Nevertheless, despite apparent progress in the field of cancer vaccines, it is clear from all of the available evidence using the current therapeutic cancer vaccines in the setting of metastatic disease that patients almost universally exit treatment with viable and hence ultimately lethal tumors. These findings stand in stark contrast with immunotherapies based on the adoptive transfer of tumor-specific T cells after immunodepleting regimens, where complete and long-lasting tumor regression is incontrovertibly observed (20). It is important to note, however, that any shortcomings in the current cancer vaccines should not be viewed as an experimental dead end but rather as an opportunity to continue to explore the development of enhanced immune adjuvants, the possible incorporation of CIMMs into clinical trials, and an incentive to continue innovative combinations of vaccines with immune checkpoint blockade and adoptive T-cell transfer-based immunotherapies.
Acknowledgments
This work was supported by the Intramural Research Program of the U.S. National Institutes of Health, National Cancer Institute, Center for Cancer Research. The authors thank Christopher Scott and Megan Bachinski for reading and editing of the manuscript.
Reference List
- 1.Plotkin SA. Vaccines: past, present and future. Nat Med. 2005;11:S5–11. doi: 10.1038/nm1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–1927. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–2170. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 4.Ault KA. Effect of prophylactic human papillomavirus L1 virus-like-particle vaccine on risk of cervical intraepithelial neoplasia grade 2, grade 3, and adenocarcinoma in situ: a combined analysis of four randomised clinical trials. Lancet. 2007;369:1861–1868. doi: 10.1016/S0140-6736(07)60852-6. [DOI] [PubMed] [Google Scholar]
- 5.Chang MH, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855–1859. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Horstmann E, et al. Risks and benefits of phase 1 oncology trials, 1991 through 2002. N Engl J Med. 2005;352:895–904. doi: 10.1056/NEJMsa042220. [DOI] [PubMed] [Google Scholar]
- 8.Roberts TG, Jr, et al. Trends in the risks and benefits to patients with cancer participating in phase 1 clinical trials. JAMA. 2004;292:2130–2140. doi: 10.1001/jama.292.17.2130. [DOI] [PubMed] [Google Scholar]
- 9.Weber J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol Immunother. 2009;58:823–830. doi: 10.1007/s00262-008-0653-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolchok JD, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 11.Hoos A, Parmiani G, Hege K, et al. A clinical development paradigm for cancer vaccines and related biologics. J Immunother. 2007;30:1–15. doi: 10.1097/01.cji.0000211341.88835.ae. [DOI] [PubMed] [Google Scholar]
- 12.Hoos A, Eggermont AM, Janetzki S, et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst. 2010;102:1388–1397. doi: 10.1093/jnci/djq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saenger YM, Wolchok JD. The heterogeneity of the kinetics of response to ipilimumab in metastatic melanoma: patient cases. Cancer Immun. 2008;8:1. [PMC free article] [PubMed] [Google Scholar]
- 14.Weber JS, et al. Phase I/II study of ipilimumab for patients with metastatic melanoma. J Clin Oncol. 2008;26:5950–5956. doi: 10.1200/JCO.2008.16.1927. [DOI] [PubMed] [Google Scholar]
- 15.Hodi FS, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ribas A, Chmielowski B, Glaspy JA. Do we need a different set of response assessment criteria for tumor immunotherapy? Clin Cancer Res. 2009;15:7116–7118. doi: 10.1158/1078-0432.CCR-09-2376. [DOI] [PubMed] [Google Scholar]
- 17.Berry DA. The hazards of endpoints. J Natl Cancer Inst. 2010;102:1376–1377. doi: 10.1093/jnci/djq334. [DOI] [PubMed] [Google Scholar]
- 18.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 19.Dudley ME, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N EngJ Med. 2010;363:809–819. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Restifo NP, Rosenberg SA. Use of standard criteria for assessment of cancer vaccines. Lancet Oncol. 2005;6:3–4. doi: 10.1016/S1470-2045(04)01693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang JC. Vitespen: a vaccine for renal cancer? Lancet. 2008;372:92–93. doi: 10.1016/S0140-6736(08)60698-4. [DOI] [PubMed] [Google Scholar]
- 24.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic Cancer Vaccines in Prostate Cancer: The Paradox of Improved Survival Without Changes in Time to Progression. Oncologist. 2010 doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Drake CG. Prostate cancer as a model for tumour immunotherapy. Nat Rev Immunol. 2010;10:580–593. doi: 10.1038/nri2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Driscoll JJ, Rixe O. Overall survival: still the gold standard: why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009;15:401–405. doi: 10.1097/PPO.0b013e3181bdc2e0. [DOI] [PubMed] [Google Scholar]
- 27.Kantoff PW, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 28.Schwartzentruber DJ, et al. A phase III multi-institutional randomized study of immunization with the gp100: 209–217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. J Clin Oncol. 2009:27. [Google Scholar]
- 29.Schuster SJ, et al. Idiotype vaccine therapy (BiovaxID) in follicular lymphoma in first complete remission: Phase III clinical trial results. J Clin Oncol. 2009:27. [Google Scholar]
- 30.Hellman S, Hellman DS. Of mice but not men. Problems of the randomized clinical trial. N Engl J Med. 1991;324:1585–1589. doi: 10.1056/NEJM199105303242208. [DOI] [PubMed] [Google Scholar]
- 31.Janetzki S, et al. “MIATA”-minimal information about T cell assays. Immunity. 2009;31:527–528. doi: 10.1016/j.immuni.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keilholz U, Martus P, Scheibenbogen C. Immune monitoring of T-cell responses in cancer vaccine development. Clin Cancer Res. 2006;12:2346s–2352s. doi: 10.1158/1078-0432.CCR-05-2540. [DOI] [PubMed] [Google Scholar]
- 33.Overwijk WW, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer DC, Balasubramaniam S, Hanada K, et al. Vaccine-stimulated, adoptively transferred CD8+ T cells traffic indiscriminately and ubiquitously while mediating specific tumor destruction. J Immunol. 2004;173:7209–7216. doi: 10.4049/jimmunol.173.12.7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 36.Goffin J, Baral S, Tu D, Nomikos D, Seymour L. Objective responses in patients with malignant melanoma or renal cell cancer in early clinical studies do not predict regulatory approval. Clin Cancer Res. 2005;11:5928–5934. doi: 10.1158/1078-0432.CCR-05-0130. [DOI] [PubMed] [Google Scholar]
- 37.El-Maraghi RH, Eisenhauer EA. Review of phase II trial designs used in studies of molecular targeted agents: outcomes and predictors of success in phase III. J Clin Oncol. 2008;26:1346–1354. doi: 10.1200/JCO.2007.13.5913. [DOI] [PubMed] [Google Scholar]
- 38.Paesmans M, et al. Response to chemotherapy has predictive value for further survival of patients with advanced non-small cell lung cancer: 10 years experience of the European Lung Cancer Working Party. Eur J Cancer. 1997;33:2326–2332. doi: 10.1016/s0959-8049(97)00325-0. [DOI] [PubMed] [Google Scholar]
- 39.Buyse M, et al. Relation between tumour response to first-line chemotherapy and survival in advanced colorectal cancer: a meta-analysis. Meta-Analysis Group in Cancer. Lancet. 2000;356:373–378. doi: 10.1016/s0140-6736(00)02528-9. [DOI] [PubMed] [Google Scholar]
- 40.Kalialis LV, Drzewiecki KT, Mohammadi M, Mehlsen AB, Klyver H. Spontaneous regression of metastases from malignant melanoma: a case report. Melanoma Res. 2008;18:279–283. doi: 10.1097/CMR.0b013e328307ee4c. [DOI] [PubMed] [Google Scholar]
- 41.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 43.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Murray N, Coppin C, Coldman A, Pater J, Rapp E. Drug delivery analysis of the Canadian multicenter trial in non-small-cell lung cancer. J Clin Oncol. 1994;12:2333–2339. doi: 10.1200/JCO.1994.12.11.2333. [DOI] [PubMed] [Google Scholar]
- 45.Hudes G, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 46.Llovet JM, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 47.Adjei AA, Christian M, Ivy P. Novel designs and end points for phase II clinical trials. Clin Cancer Res. 2009;15:1866–1872. doi: 10.1158/1078-0432.CCR-08-2035. [DOI] [PubMed] [Google Scholar]
- 48.Small EJ, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 49.Kantoff PW, et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J Clin Oncol. 2010;28:1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Higano CS, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115:3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 51.DeFrancesco L. Landmark approval for Dendreon's cancer vaccine. Nat Biotechnol. 2010;28:531–532. doi: 10.1038/nbt0610-531. [DOI] [PubMed] [Google Scholar]
- 52.Longo DL. New therapies for castration-resistant prostate cancer. N Engl J Med. 2010;363:479–481. doi: 10.1056/NEJMe1006300. [DOI] [PubMed] [Google Scholar]
- 53.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 54.Tannock IF, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 55.Berthold DR, Pond GR, Roessner M, et al. Treatment of hormone-refractory prostate cancer with docetaxel or mitoxantrone: relationships between prostate-specific antigen, pain, and quality of life response and survival in the TAX-327 study. Clin Cancer Res. 2008;14:2763–2767. doi: 10.1158/1078-0432.CCR-07-0944. [DOI] [PubMed] [Google Scholar]
- 56.Small EJ, Fong L. Developing immunotherapy as legitimate therapy for patients with prostate cancer. J Clin Oncol. 2010;28:1085–1087. doi: 10.1200/JCO.2009.26.3483. [DOI] [PubMed] [Google Scholar]
- 57.Halabi S, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 58.Madan RA, Gulley JL, Fojo T, Dahut WL. Therapeutic cancer vaccines in prostate cancer: the paradox of improved survival without changes in time to progression. Oncologist. 2010 doi: 10.1634/theoncologist.2010-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith FO, et al. Treatment of metastatic melanoma using interleukin-2 alone or inconjunction with vaccines. Clin Cancer Res. 2008;14:5610–5618. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Petrovsky N, Aguilar JC. Vaccine adjuvants: current state and future trends. Immunol Cell Biol. 2004;82:488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- 61.Pashine A, Valiante NM, Ulmer JB. Targeting the innate immune response with improved vaccine adjuvants. Nat Med. 2005;11:S63–S68. doi: 10.1038/nm1210. [DOI] [PubMed] [Google Scholar]
- 62.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54:1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 64.Rosenberg SA, et al. Recombinant fowlpox viruses encoding the anchor-modified gp100 melanoma antigen can generate antitumor immune responses in patients with metastatic melanoma. ClinCancer Res. 2003;9:2973–2980. [PMC free article] [PubMed] [Google Scholar]
- 65.Marshall JL, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 66.Dubensky TW, Jr, Reed SG. Adjuvants for cancer vaccines. Semin Immunol. 2010;22:155–161. doi: 10.1016/j.smim.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Dranoff G, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slingluff CL, Jr, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. 2009;15:7036–7044. doi: 10.1158/1078-0432.CCR-09-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. 2009;15:7029–7035. doi: 10.1158/1078-0432.CCR-09-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenberg SA, et al. Impact of cytokine administration on the generation of antitumor reactivity in patients with metastatic melanoma receiving a peptide vaccine. J Immunol. 1999;163:1690–1695. [PMC free article] [PubMed] [Google Scholar]
- 71.von MM, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–1191. [PubMed] [Google Scholar]
- 72.Simmons SJ, et al. GM-CSF as a systemic adjuvant in a phase II prostate cancer vaccine trial. Prostate. 1999;39:291–297. doi: 10.1002/(sici)1097-0045(19990601)39:4<291::aid-pros10>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 73.Kirkwood JM, et al. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/-granulocyte-monocyte colony-stimulating factor and/or IFN-alpha2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin Cancer Res. 2009;15:1443–1451. doi: 10.1158/1078-0432.CCR-08-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bronte V, et al. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–5737. [PMC free article] [PubMed] [Google Scholar]
- 75.Serafini P, et al. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–6343. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 76.Filipazzi P, et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 77.Rosenberg SA, Yang JC, Kammula US, et al. Different adjuvanticity of incomplete freund's adjuvant derived from beef or vegetable components in melanoma patients immunized with a peptide vaccine. J Immunother. 2010;33:626–629. doi: 10.1097/CJI.0b013e3181dac9de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Slingluff CL, et al. Immunogenicity for CD8+ and CD4+ T cells of 2 formulations of an incomplete freund's adjuvant for multipeptide melanoma vaccines. J Immunother. 2010;33:630–638. doi: 10.1097/CJI.0b013e3181e311ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Parkhurst MR, et al. Improved induction of melanoma-reactive CTL with peptides from the melanoma antigen gp100 modified at HLA-A*0201-binding residues. J Immunol. 1996;157:2539–2548. [PubMed] [Google Scholar]
- 80.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rosenberg SA, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–6176. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 82.Overwijk W, Hailemuchael Y, Dai ZM, Jaffarzad N, Hwu P. Peptide/Incomplete Freund Adjuvant Emulsion Depots are a Graveyard for Tumor Antigen-specific CD8(+) T Cells. J Immunother. 2009;32:971. [Google Scholar]
- 83.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 84.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–1305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 85.Gattinoni L, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 87.Klebanoff CA, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci USA. 2005;102:9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gattinoni L, Klebanoff CA, Restifo NP. Pharmacologic induction of CD8+ T cell memory: better living through chemistry. Sci Transl Med. 2009;1:11ps12. doi: 10.1126/scitranslmed.3000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Araki K, et al. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR kinase determines effector versus memory CD8+ T cell fate by regulating the expression of transcription factors T-bet and Eomesodermin. Immunity. 2010;32:67–78. doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gattinoni L, Ji Y, Restifo NP. Wnt/{beta}-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao DM, et al. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jeannet G, et al. Essential role of the Wnt pathway effector Tcf-1 for the establishment of functional CD8 T cell memory. Proc Natl Acad Sci USA. 2010;107:9777–9782. doi: 10.1073/pnas.0914127107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou X, et al. Differentiation and persistence of memory CD8(+) T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 97.Gattinoni L, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–1626. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klebanoff CA, Gattinoni L, Restifo NP. CD8+ T-cell memory in tumor immunology and immunotherapy. Immunol Rev. 2006;211:214–224. doi: 10.1111/j.0105-2896.2006.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pentcheva-Hoang T, Corse E, Allison JP. Negative regulators of T-cell activation: potential targets for therapeutic intervention in cancer, autoimmune disease, and persistent infections. Immunol Rev. 2009;229:67–87. doi: 10.1111/j.1600-065X.2009.00763.x. [DOI] [PubMed] [Google Scholar]
- 101.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 102.Masteller EL, Chuang E, Mullen AC, Reiner SL, Thompson CB. Structural analysis of CTLA-4 function in vivo. J Immunol. 2000;164:5319–5327. doi: 10.4049/jimmunol.164.10.5319. [DOI] [PubMed] [Google Scholar]
- 103.Engelhardt JJ, Sullivan TJ, Allison JP. CTLA-4 overexpression inhibits T cell responses through a CD28-B7-dependent mechanism. J Immunol. 2006;177:1052–1061. doi: 10.4049/jimmunol.177.2.1052. [DOI] [PubMed] [Google Scholar]
- 104.Wherry EJ, et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 105.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 106.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Downey SG, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clin Cancer Res. 2007;13:6681–6688. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Weber J, et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–5598. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 109.Wolchok JD, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–164. doi: 10.1016/S1470-2045(09)70334-1. [DOI] [PubMed] [Google Scholar]
- 110.O'Day SJ, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010;21:1712–1717. doi: 10.1093/annonc/mdq013. [DOI] [PubMed] [Google Scholar]
- 111.Yang JC, et al. Ipilimumab (anti-CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother. 2007;30:825–830. doi: 10.1097/CJI.0b013e318156e47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.van EA, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28:3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Besser MJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 115.Hunder NN, et al. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–2703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dudley ME, et al. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Overwijk WW, Theoret MR, Finkelstein SE, et al. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hwang LN, Yu Z, Palmer DC, Restifo NP. The in vivo expansion rate of properly stimulated transferred CD8+ T cells exceeds that of an aggressively growing mouse tumor. Cancer Res. 2006;66:1132–1138. doi: 10.1158/0008-5472.CAN-05-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lou Y, Wang G, Lizee G, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–6790. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Muranski P, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Witte MA, et al. TCR gene therapy of spontaneous prostate carcinoma requires in vivo T cell activation. J Immunol. 2008;181:2563–2571. doi: 10.4049/jimmunol.181.4.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ugel S, et al. Autoimmune B-cell lymphopenia after successful adoptive therapy with telomerase-specific T lymphocytes. Blood. 2010;115:1374–1384. doi: 10.1182/blood-2009-07-233270. [DOI] [PubMed] [Google Scholar]
- 123.Koya RC, Mok S, Comin-Anduix B, et al. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc Natl Acad Sci USA. 2010;107:14286–14291. doi: 10.1073/pnas.1008300107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Palmer DC, Chan CC, Gattinoni L, et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc Natl Acad Sci USA. 2008;105:8061–8066. doi: 10.1073/pnas.0710929105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Klebanoff CA, Yu Z, Hwang LN, et al. Programming tumor-reactive effector memory CD8+ T cells in vitro obviates the requirement for in vivo vaccination. Blood. 2009;114:1776–1783. doi: 10.1182/blood-2008-12-192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith FO, Klapper JA, Wunderlich JR, Rosenberg SA, Dudley ME. Impact of a recombinant fowlpox vaccine on the efficacy of adoptive cell therapy with tumor infiltrating lymphocytes in a patient with metastatic melanoma. J Immunother. 2009;32:870–874. doi: 10.1097/CJI.0b013e3181b36b69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pule MA, Savoldo B, Myers GD, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Johnson LA, Morgan RA, Dudley ME, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dudley ME, Wunderlich J, Nishimura MI, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 131.Mackensen A, Meidenbauer N, Vogl S, et al. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 132.Goff SL, Smith FO, Klapper JA, et al. Tumor infiltrating lymphocyte therapy for metastatic melanoma: analysis of tumors resected for TIL. J Immunother. 2010;33:840–847. doi: 10.1097/CJI.0b013e3181f05b91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wrzesinski C, Paulos CM, Gattinoni L, et al. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wrzesinski C, Paulos CM, Kaiser A, et al. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010;33:1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berenson JR, Einstein AB, Jr, Fefer A. Syngeneic adoptive immunotherapy and chemoimmunotherapy of a Friend leukemia: requirement for T cells. J Immunol. 1975;115:234–238. [PubMed] [Google Scholar]
- 137.Cheever MA, Greenberg PD, Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J Immunol. 1980;125:711–714. [PubMed] [Google Scholar]
- 138.North RJ. Cyclophosphamide-facilitated adoptive immunotherapy of an established tumor depends on elimination of tumor induced suppressor T cells. J Exp Med. 1982;155:1063–1074. doi: 10.1084/jem.155.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dummer W, et al. T cell homeostatic proliferation elicits effective antitumor autoimmunity. J Clin Invest. 2002;110:185–192. doi: 10.1172/JCI15175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gattinoni L, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Klebanoff CA, Khong HT, Antony PA, Palmer DC, Restifo NP. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Klebanoff CA, et al. IL-15 enhances the in vivo antitumor activity of tumor-reactive CD8+ T cells. Proc Natl Acad Sci USA. 2004;101:1969–1974. doi: 10.1073/pnas.0307298101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Antony PA, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Paulos CM, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rosenberg SA, Dudley ME. Adoptive cell therapy for the treatment of patients with metastatic melanoma. Curr Opin Immunol. 2009;21:233–240. doi: 10.1016/j.coi.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mercado R, et al. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–6839. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 147.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggersa developmental program in naive cells. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 149.van Stipdonk MJ, et al. Dynamic programming of CD8+ T lymphocyte responses. Nat Immunol. 2003;4:361–365. doi: 10.1038/ni912. [DOI] [PubMed] [Google Scholar]
- 150.Lin GH, et al. Evaluating the cellular targets of anti-4-1BB agonist antibody during immunotherapy of a pre-established tumor in mice. PLoS One. 2010;5:e11003. doi: 10.1371/journal.pone.0011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rapoport AP, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of Costimulated autologous T cells. Clin Cancer Res. 2009;15:4499–4507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Turtle CJ, Riddell SR. Artificial antigen-presenting cells for use in adoptive immunotherapy. Cancer J. 2010;16:374–381. doi: 10.1097/PPO.0b013e3181eb33a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Riddell SR, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 154.Atkins MB, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 155.Klapper JA, et al. High-dose interleukin-2 for the treatment of metastatic renal cell carcinoma: a retrospective analysis of response and survival in patients treated in the surgery branch at the National Cancer Institute between 1986 and 2006. Cancer. 2008;113:293–301. doi: 10.1002/cncr.23552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Besser MJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16:2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 157.Radvanyi L, et al. Adoptive T-cell therapy at the MID Anderson Cancer Center: early results and a promisingfuture. J Immunother. 2008;31:924–925. [Google Scholar]
- 158.Fourcade J, et al. Immunization with analog peptide in combination with CpG and montanide expands tumor antigen-specific CD8+ T cells in melanoma patients. J Immunother. 2008;31:781–791. doi: 10.1097/CJI.0b013e318183af0b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Slingluff CL, Jr, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol. 2008;26:4973–4980. doi: 10.1200/JCO.2008.17.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Celis E. Overlapping human leukocyte antigen class I/II binding peptide vaccine for the treatment of patients with stage IV melanoma: evidence of systemic immune dysfunction. Cancer. 2007;110:203–214. doi: 10.1002/cncr.22744. [DOI] [PubMed] [Google Scholar]
- 161.Khong HT, et al. Immunization of HLA-A*0201 and/or HLA-DPbeta1*04 patients with metastatic melanoma using epitopes from the NY-ESO-1 antigen. J Immunother. 2004;27:472–477. doi: 10.1097/00002371-200411000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Suekane S, et al. Phase I trial of personalized peptide vaccination for cytokine-refractory metastatic renal cell carcinoma patients. Cancer Sci. 2007;98:1965–1968. doi: 10.1111/j.1349-7006.2007.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Uemura H, et al. A phase I trial of vaccination of CA9-derived peptides for HLA-A24-positive patients with cytokine-refractory metastatic renal cell carcinoma. Clin Cancer Res. 2006;12:1768–1775. doi: 10.1158/1078-0432.CCR-05-2253. [DOI] [PubMed] [Google Scholar]
- 164.Barve M, et al. Induction of immune responses and clinical efficacy in a phase II trial of IDM-2101, a 10-epitope cytotoxic T-lymphocyte vaccine, in metastatic non-small-cell lung cancer. J Clin Oncol. 2008;26:4418–4425. doi: 10.1200/JCO.2008.16.6462. [DOI] [PubMed] [Google Scholar]
- 165.Bolonaki I, et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol. 2007;25:2727–2734. doi: 10.1200/JCO.2006.10.3465. [DOI] [PubMed] [Google Scholar]
- 166.Tsuruma T, et al. Clinical and immunological evaluation of anti-apoptosis protein, survivin-derived peptide vaccine in phase I clinical study for patients with advanced or recurrent breast cancer. J Transl Med. 2008;6:24. doi: 10.1186/1479-5876-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Izumoto S, et al. Phase II clinical trial of Wilms tumor 1 peptide vaccination for patients with recurrent glioblastoma multiforme. J Neurosurg. 2008;108:963–971. doi: 10.3171/JNS/2008/108/5/0963. [DOI] [PubMed] [Google Scholar]
- 168.Aoki M, et al. Antibody responses against NY-ESO-1 and HER2 antigens in patients vaccinated with combinations of cholesteryl pullulan (CHP)-NY-ESO-1 and CHP-HER2 with OK-432. Vaccine. 2009;27:6854–6861. doi: 10.1016/j.vaccine.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 169.Kono K, et al. Vaccination with multiple peptides derived from novel cancer-testis antigens can induce specific T-cell responses and clinical responses in advanced esophageal cancer. Cancer Sci. 2009;100:1502–1509. doi: 10.1111/j.1349-7006.2009.01200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Honma I, et al. Phase I clinical study of anti-apoptosis protein survivin-derived peptide vaccination for patients with advanced or recurrent urothelial cancer. Cancer Immunol Immunother. 2009;58:1801–1807. doi: 10.1007/s00262-009-0691-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohno S, et al. Wilms' tumor 1 (WT1) peptide immunotherapy for gynecological malignancy. Anticancer Res. 2009;29:4779–4784. [PubMed] [Google Scholar]
- 172.Kaumaya PT, et al. Phase I active immunotherapy with combination of two chimeric, human epidermal growth factor receptor 2, B-cell epitopes fused to a promiscuous T-cell epitope in patients with metastatic and/or recurrent solid tumors. J Clin Oncol. 2009;27:5270–5277. doi: 10.1200/JCO.2009.22.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kitano S, et al. HER2-specific T-cell immune responses in patients vaccinated with truncated HER2 protein complexed with nanogels of cholesteryl pullulan. Clin Cancer Res. 2006;12:7397–7405. doi: 10.1158/1078-0432.CCR-06-1546. [DOI] [PubMed] [Google Scholar]
- 174.Morita S, et al. A phase I/IItrial of a WT1 (Wilms' tumor gene) peptide vaccine in patients with solid malignancy: safety assessment based on the phase I data. Jpn J Clin Oncol. 2006;36:231–236. doi: 10.1093/jjco/hyl005. [DOI] [PubMed] [Google Scholar]
- 175.Mavroudis D, et al. A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology. 2006;70:306–314. doi: 10.1159/000096252. [DOI] [PubMed] [Google Scholar]
- 176.Butterfield LHC, et al. Adenovirus MART-1-engineered autologous dendritic cell vaccine for metastatic melanoma. J Immunother. 2008;31:294–309. doi: 10.1097/CJI.0b013e31816a8910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Von Euw EM, et al. A phase I clinical study of vaccination of melanoma patients with dendritic cells loaded with allogeneic apoptotic/necrotic melanoma cells. Analysis of toxicity and immune response to the vaccine and of IL-10 -1082 promoter genotype as predictor of disease progression. J Transl Med. 2008;6:6. doi: 10.1186/1479-5876-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Palucka AK, et al. Dendritic cells loaded with killed allogeneic melanoma cells can induce objective clinical responses and MART-1 specific CD8+ T-cell immunity. J Immunother. 2006;29:545–557. doi: 10.1097/01.cji.0000211309.90621.8b. [DOI] [PubMed] [Google Scholar]
- 179.O'Rourke MG, et al. Dendritic cell immunotherapy for stage IV melanoma. Melanoma Res. 2007;17:316–322. doi: 10.1097/CMR.0b013e3282c3a73b. [DOI] [PubMed] [Google Scholar]
- 180.Kyte JA, et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Ther. 2006;13:905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- 181.Lesimple T, et al. Immunologic and clinical effects of injecting mature peptide-loaded dendritic cells by intralymphatic and intranodal routes in metastatic melanoma patients. Clin Cancer Res. 2006;12:7380–7388. doi: 10.1158/1078-0432.CCR-06-1879. [DOI] [PubMed] [Google Scholar]
- 182.Berntsen A, et al. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma -A clinical, phase 1/2 trial. J Immunother. 2008;31:771–780. doi: 10.1097/CJI.0b013e3181833818. [DOI] [PubMed] [Google Scholar]
- 183.Avigan DE, et al. Phase I/II study of vaccination with electrofused allogeneic dendritic Cells/Autologous tumor-derived cells in patients with stage IV renal cell carcinoma. J Immunother. 2007;30:749–761. doi: 10.1097/CJI.0b013e3180de4ce8. [DOI] [PubMed] [Google Scholar]
- 184.Svane IM, et al. Vaccination with p53 peptide-pulsed dendritic cells is associated with disease stabilization in patients with p53 expressing advanced breast cancer; monitoring of serum YKL-40 and IL-6 as response biomarkers. Cancer Immunol Immunother. 2007;56:1485–1499. doi: 10.1007/s00262-007-0293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bachleitner-Hofmann T, et al. Pilot trial of autologous dendritic cells loaded with tumor lysate(s) from allogeneic tumor cell lines in patients with metastatic medullary thyroid carcinoma. Oncol Rep. 2009;21:1585–1592. doi: 10.3892/or_00000391. [DOI] [PubMed] [Google Scholar]
- 186.Babatz J, et al. Induction of cellular immune responses against carcinoembryonic antigen in patients with metastatic tumors after vaccination with altered peptide ligand-loaded dendritic cells. Cancer Immunol Immunother. 2006;55:268–276. doi: 10.1007/s00262-005-0021-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Loveland BE, et al. Mannan-MUC1-pulsed dendritic cell immunotherapy: a phase I trial in patients with adenocarcinoma. Clin Cancer Res. 2006;12:869–877. doi: 10.1158/1078-0432.CCR-05-1574. [DOI] [PubMed] [Google Scholar]
- 188.Morse MA, Clay TM, Hobeika AC, et al. Phase I study of immunization with dendritic cells modified with fowlpox encoding carcinoembryonic antigen and costimulatory molecules. Clin Cancer Res. 2005;11:3017–3024. doi: 10.1158/1078-0432.CCR-04-2172. [DOI] [PubMed] [Google Scholar]
- 189.Lindsey KR, et al. Evaluation of prime/boost regimens using recombinant poxvirus/tyrosinase vaccines for the treatment of patients with metastatic melanoma. Clin Cancer Res. 2006;12:2526–2537. doi: 10.1158/1078-0432.CCR-05-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Amato RJ, et al. Vaccination of renal cell cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) alone or administered in combination with interferon-alpha (IFN-alpha): a phase 2 trial. J Immunother. 2009;32:765–772. doi: 10.1097/CJI.0b013e3181ace876. [DOI] [PubMed] [Google Scholar]
- 191.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 192.Harrop R, et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin Cancer Res. 2006;12:3416–3424. doi: 10.1158/1078-0432.CCR-05-2732. [DOI] [PubMed] [Google Scholar]
- 193.Marshall JL, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 194.Nicholaou T, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–2173. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 195.Motohashi S, et al. A phase I-II study of alpha-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–2501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 196.Osorio M, et al. Heterophilic NeuGcGM3 ganglioside cancer vaccine in advanced melanoma patients: results of a Phase Ib/IIa study. Cancer Biol Ther. 2008;7:488–495. doi: 10.4161/cbt.7.4.5476. [DOI] [PubMed] [Google Scholar]
- 197.Okaji Y, Tsuno NH, Tanaka M, et al. Pilot study of anti-angiogenic vaccine using fixed whole endothelium in patients with progressive malignancy after failure of conventional therapy. Eur J Cancer. 2008;44:383–390. doi: 10.1016/j.ejca.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 198.Weide B, et al. Results of the first phase I/II clinical vaccination trial with direct injection of mRNA. J Immunother. 2008;31:180–188. doi: 10.1097/CJI.0b013e31815ce501. [DOI] [PubMed] [Google Scholar]
- 199.Nemunaitis J, et al. Phase II trial of Belagenpumatucel-L, a TGF-beta2 antisense gene modified allogeneic tumor vaccine in advanced non small cell lung cancer (NSCLC) patients. Cancer Gene Ther. 2009;16:620–624. doi: 10.1038/cgt.2009.15. [DOI] [PubMed] [Google Scholar]
- 200.Nemunaitis J, et al. Phase 1/2 trial of autologous tumor mixed with an allogeneic GVAX vaccine in advanced-stage non-small-cell lung cancer. Cancer Gene Ther. 2006;13:555–562. doi: 10.1038/sj.cgt.7700922. [DOI] [PubMed] [Google Scholar]
- 201.Powell A, Creaney J, Broomfield S, Van BI, Robinson B. Recombinant GM-CSF plus autologous tumor cells as a vaccine for patients with mesothelioma. Lung Cancer. 2006;52:189–197. doi: 10.1016/j.lungcan.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 202.Fakhrai H, et al. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13:1052–1060. doi: 10.1038/sj.cgt.7700975. [DOI] [PubMed] [Google Scholar]
- 203.Weber J, et al. Phase 1 trial of intranodal injection of a Melan-A/MART-1 DNA plasmid vaccine in patients with stage IV melanoma. J Immunother. 2008;31:215–223. doi: 10.1097/CJI.0b013e3181611420. [DOI] [PubMed] [Google Scholar]
- 204.Cassaday RD, et al. A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Clin Cancer Res. 2007;13:540–549. doi: 10.1158/1078-0432.CCR-06-2039. [DOI] [PubMed] [Google Scholar]
- 205.Victora GD, et al. Immune response to vaccination with DNA-Hsp65 in a phase I clinical trial with head and neck cancer patients. Cancer Gene Ther. 2009;16:598–608. doi: 10.1038/cgt.2009.9. [DOI] [PubMed] [Google Scholar]
- 206.Gnjatic S, et al. NY-ESO-1 DNA vaccine induces T-cell responses that are suppressed by regulatory T cells. Clin Cancer Res. 2009;15:2130–2139. doi: 10.1158/1078-0432.CCR-08-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Testori A, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician's choice of treatment for stage IV melanoma: The C-100-21 study group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 208.Amato RJ, et al. Vaccination of Metastatic Renal Cancer Patients with MVA-5T4: A Randomized, Double Blind, Placebo Controlled Phase III Study. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-10-2082. [DOI] [PubMed] [Google Scholar]
- 209.Giaccone G, et al. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study) J Clin Oncol. 2005;23:6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 210.Butts C, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol. 2005;23:6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 211.Neninger VE, et al. Phase II randomized controlled trial of an epidermal growth factor vaccine in advanced non-small-cell lung cancer. J Clin Oncol. 2008;26:1452–1458. doi: 10.1200/JCO.2007.11.5980. [DOI] [PubMed] [Google Scholar]
- 212.Higano C, et al. A Phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration-resistant prostate cancer (CRPC). American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium; 2009; 2009. [Google Scholar]
- 213.Small E, et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC). American Society of Clinical Oncology 2009 Genitourinary Cancers Symposium; 2009; 2009. [Google Scholar]