Abstract
The purpose of this investigation was to determine whether a nutritionally essential amino acid, l-lysine, acts like a serotonin receptor 4 (5-HT4) antagonist, and if l-lysine is beneficial in animal models of serotonin (5-HT)-induced anxiety, diarrhea, ileum contractions, and tachycardia and in stress-induced fecal excretion. The radioligand-binding assay was used to test the binding of l-lysine to various 5-HT receptors. The effects of l-lysine on 5-HT-induced contractions of isolated guinea pig ileum were studied in vitro. The effects of oral administration of l-lysine on diarrhea, stress-induced fecal excretion, and 5-HT-induced corticosterone release, tachycardia, and anxiety (an elevated plus maze paradigm) were studied in rats in vivo. l-Lysine (0.8 mmol/dl) inhibited (9.17%) binding of 5-HT to the 5-HT4 receptor, without any effect on 5-HT1A,2A,2B,2C,3 binding. l-Lysine (0.07 and 0.7 mmol/dl) blocked 5-HT-induced contractions of an isolated guinea pig ileum in vitro (P < 0.05 and P < 0.01). Orally applied l-lysine (1 g/kg of body weight) inhibited (P < 0.12) diarrhea triggered by coadministration of restraint stress and 5-hydroxytryptophane (10 mg/kg of body weight), and significantly blocked anxiety induced by the 5-HT4 receptor agonist (3.0 mmol/liter) in rats in vivo. No effects of l-lysine or the 5-HT4 receptor agonist on plasma corticosterone and heart rate were recorded. l-Lysine may be a partial 5-HT4 receptor antagonist and suppresses 5-HT4 receptor-mediated intestinal pathologies and anxiety in rats. An increase in nutritional load of l-lysine might be a useful tool in treating stress-induced anxiety and 5-HT-related diarrhea-type intestinal dysfunctions.
Stress and stress-induced anxiety play a major role in functional intestinal disorders (1). The stimulation of intestinal contraction and colonic transit are the most consistent patterns in the motility response of the intestinal tract to acute stress (2, 3); however, the stress–gut interactions are enormously complex (1). Both stress-induced anxiety and intestinal disorders are traced, from among other molecular pathologies, to the inappropriate responses of the central and/or peripheral serotonin (5-HT) system (4, 5). Among the 5-HT receptors, the 5-HT4 receptor plays a “prostress” role in the gut and throughout the body by mobilizing energy and facilitating behavioral, gastroin-testinal, and cardiovascular stress responses (6, 7).
5-HT4 receptors are located within the cells and neurons of the gastrointestinal tract (6, 8). Agonists on the 5-HT4 receptor increase cholinergic transmission (9) and thus contract the smooth muscle in the rat and guinea pig ileum† and the human colon (11). Antagonists on the 5-HT4 receptor do not affect normal, healthy gut function (12) but prevent the disturbances caused by stress (4) or high 5-HT activity (13). Thus, 5-HT4 receptor antagonists are promising targets for the treatment of diarrhea-predominant irritable bowel syndrome (12). In addition to their effect on the gastrointestinal tract, 5-HT4 antagonists block corticosteroid secretion in the adrenal cortex (14), 5-HT-induced tachycardia (15, 16), and stress-induced anxiety (17).
However, the 5-HT receptor system is heterogeneous (18), and direct receptor targeting often causes side effects, such as diarrhea, gastric bleeding, and anxiety (19). Therefore, it is not surprising that few satisfying pharmacological treatments of stress-triggered, 5-HT-mediated intestinal disorders exist and that other therapeutic approaches, namely nutritional ones, are sought (20–22). An interesting candidate to include in “nutritional management” of stress-related gastrointestinal disorders would be an essential amino acid, l-lysine (Lys). Lys blunted stress-induced anxiety in rats (23), whereas a dietary deficiency of Lys increased stress-induced colonic transit and anxiety, because of an enhanced transmission of 5-HT in the amygdala (24).
Because the effects of Lys overlap with the “antistress” effects of the 5-HT4 receptor antagonists (4), we hypothesized that dietary Lys could act as a partial antagonist on the 5-HT4 receptors. The present study examined whether the provision of oral l-lysine might result in the blockage of 5-HT-mediated pathologies linked to stress exposure and/or 5-HT sensitization in rats. Ileum contractions, stress-induced fecal excretion, diarrhea, anxiety, and tachycardia were monitored.
Materials and Methods
Radioligand-Binding Assay. The Lys-induced (lot no. 003VKB8, Ajinomoto, Tokyo) inhibition rate on the human recombinant 5-HT1A, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT3, and the guinea pig corpus striatum 5-HT4 receptor were studied. All cloned receptors were commercially obtained (Biosignal, Montreal) and diluted with assay buffer before use. Assay buffers (pH 7.4) were prepared as follows, 5-HT1A, 0.1% ascorbic acid/0.5 mmol/liter EDTA/10 mmol/liter MgSO4/50 mmol/liter Tris·HCl; 5-HT2B, 0.1% ascorbic acid/4.0 mmol/liter CaCl2/50.0 mmol/liter/Tris·HCl; 5-HT2C, 0.1% ascorbic acid/10.0 μmol/liter pargiline/50.0 mmol/liter Tris·HCl; 5-HT3, 5.0 mmol/liter MgCl2/1.0 mmol/liter EDTA/50.0 mmol/liter Tris·HCl; and 5-HT2A and 5-HT4, 50 mmol/liter Tris·HCl. Animal tissues (Funakoshi, Tokyo) were weighed (wet), homogenized in 10-fold volume of buffer on an ice bath, and centrifuged (50,000 × g, 4°C, 20 min). The fractions obtained were washed once by centrifugation with 10-fold volume of the buffer, and the microsomal fraction was stored as receptor at –80°C until use. To determine the binding degree, the inhibition rate of Lys and a positive reference substance were calculated from binding of a tracer to a receptor. Designed amounts of Lys with replacement substances (final concentration, 10 mmol/liter) or replacement substances alone (final concentration, 0.1 mmol/liter) were dissolved in dimethyl sulfoxide (Kanto Chemicals, Tokyo). The following replacement substances were used: 5-HT1A, 5-HT2B, and 5-HT4, serotonin; 5-HT2A, ketanserine; 5-HT2C, mesulergine; and 5-HT3, MDL72222.
The following tracers were used: 5-HT1A; 3H-8-OH DPAT (0.48 nmol/liter, Perkin–Elmer Life Science); 5-HT2A; 3H-8Ketanserine (4.4 nmol/liter, Perkin–Elmer Life Science); 5-HT2B, 125I-LSD (0.06 nmol/liter, Perkin–Elmer Life Science); 5-HT2C, 3H-Mesulergine (1.94 nmol/liter, Amersham Pharmacia Biosciences); 5-HT3, 3H-GR65630 (0.84 nmol/liter, Perkin–Elmer Life Science); 5-HT4, and 3H-GR113808 (0.98 nmol/liter, Amersham Pharmacia Biotech).
All determinations were performed in duplicate. Replacement substance solution (100 μl) was added to a tube designed for calculation of nonspecific binding. Dimethyl sulfoxide (100 μl) solution was added to a tube designed for calculation of specific binding. Lys solution (100 μl), or a positive reference substance solution, was added for calculation of the inhibition. Three hundred microliters of assay buffer, 100 μl of tracer solution, and 500 μl of receptor solution were added into each tube. The mixtures were incubated (M-100N, Taitec, Saitama, Japan), filtered with Cell Harvester (M-30R, Brandel, Bethesda), and washed three times with Tris·HCl solution (3 ml, 50 mmol/liter, pH 7.4). Paper filters and liquid scintillator (5 ml) were placed into vials, and the radioactivity was counted with a liquid scintillation counter (1500 or 2500TR, Packard) for 2 min. The inhibition rate was calculated from “100 – binding ratio.” The binding ratio was defined as follows, [(B – N)/(B0 – N)] × 100 (%), where B is bound radioactivity in the presence of test substance or positive reference substance, B0 is bound radioactivity in the absence of test substance or positive reference substance, and N is nonspecific bound radioactivity.
Effects of Lys on 5-HT-Induced Contraction of the Guinea Pig Ileum in Vitro. Male Hartley guinea pigs (body weight, 270–280 g, n = 3) (Japan SLC, Tokyo) were housed in bracket cages (12 h light/dark cycle; dark period, 1900–0700 hours) in a room with controlled temperature and humidity. All animals were treated in compliance with the U.S. Public Health Service policy on Human Care and Use of Laboratory Animals and the National Institutes of Health guidelines (National Research Council's Guide for the Care and Use of Laboratory Animals, available at http://oacu.od.nih.gov/regs/guide/guidex.htm). Guinea pigs consumed distilled water and chow diet ad libitum. A 2-cm-long segment of proximal ileum was isolated after killing the animals by exsanguination and suspended in a Magnus tube (100 ml) filled with a nutrient solution (Tyrode solution/137.0 mmol/liter NaCl/2.7 mmol/liter KCl/1.8 mmol/liter CaCl2/0.4 mmol/liter NaH2PO4/1.1 mmol/liter MgCl2/12.0 mmol/liter NaHCO3/5.6 mmol/liter d(+)-glucose; volume, 10 ml; temperature, 32°C), which was gassed with a mixture of 95% O2/5% CO2, with a 0.5-g load. The Tyrode solution contained methysergide (1 μmol/liter) to antagonize 5-HT1 receptors. All chemicals were obtained from Wako Pure Chemicals, Tokyo). Contractile responses of the ileum strips were recorded on an ink-writing recorder (SR-6211, Western Graphtec, Irvine, CA) by an isotonic transducer (TD-112S, Nihon Kohden, Tokyo) and an isotonic transducer input box (JD-112S, Nihon Kohden). First, the Lys (or l-leucine) solution (0.007, 0.07, or 0.7 mmol/dl) was added to the Magnus tube for 5 min to evaluate the effects of the amino acids alone. Gavaging rats with a dose of 1 g/kg Lys increased plasma Lys to tens of millimoles per deciliter (26); thus, the concentrations above were within physiological range produced in the intestinal lumen. Afterward, 5-HT solution was added to the Magnus tube. All solvents were added in a single volume (100 μl). The ileal strips were washed when their contractions induced by 5-HT showed a peak and allowed to stand until a return to the baseline was confirmed (rest period). The strips were washed at 5-min intervals during the rest period. These procedures were repeated up to the highest concentration of Lys solution. 5-HT-induced contractions observed after addition of Lys (l-leucine) solution were recorded as the percentage of the 5-HT-induced contractions (100%) observed before addition of the solvent (Fig. 1). The effect of amino acid solution was analyzed by using one-way ANOVA followed by Dunnett's test.
Fig. 1.
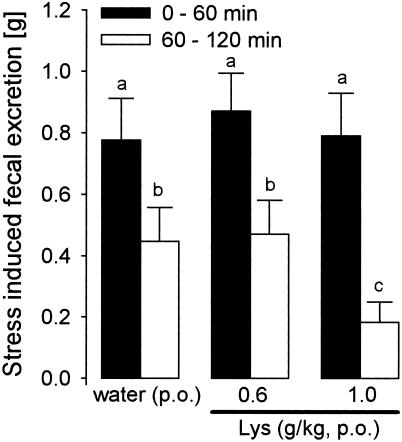
Effect of Lys on the weight of fecal pellets excreted during the first hour (filled columns) and the second hour (open columns) of wrap-restraint stress in rats. Lys or water was administered 40 min before exposure to wrap-restraint stress. Means of 20 rats + SEM are shown. The bars with different superscript letters differ significantly at P < 0.05.
Effects of Lys on Normal and Wrap-Restraint, Stress-Induced Fecal Excretion. Male Wistar rats (body weight, 250–280 g, n = 80) (Charles River Japan, Tokyo) were housed individually in conventional hanging cages (12 h light/dark cycle; dark period, 1900–0700 hours). Rats consumed distilled water and chow diet ad libitum. Rats were exposed to wrap-restraint stress as described (3). Rats were briefly and lightly anesthetized with ether, and their forepaws, upper forelimbs, and thoracic trunks were wrapped with adhesive tape. The wet weight of fecal pellets expelled by each animal was determined by an experimenter blinded to treatments at two time intervals, 60 and 120 min after stress induction.
Rats were treated with a single oral infusion of water (n = 20), Lys solution (600 mg/kg of body weight, n = 20, or 1 g/kg of body weight, n = 20) 40 min before stress exposure (stress exposure, 1400–1600 hours). The infusion volume was kept constant (5 ml). Two groups of naïve (nonstressed) rats (n = 10 per group) were infused orally with water or Lys (1 g/kg of body weight) at 1320 hours, and fecal excretion was measured at 1500 and 1600 hours. Time and treatment differences were analyzed at the end of experiments by using three-way ANOVA followed by Duncan's multiple range test.
Effects of Lys on Diarrhea Evoked by Coadministration of Restraint Stress and 5-Hydroxytryptophane (5-HTP) in Fasted Rats. Male Wistar rats (body weight, 250–280 g, n = 60) (Charles River Japan) were housed in conventional hanging cages (12-h light/dark cycle; dark period, 1900–0700 hours). Rats consumed distilled water and chow diet ad libitum, but were deprived of diet starting 24 h before testing. Rats were divided into two main groups (n = 30 per group). Members of the first group were orally infused with water (volume, 5 ml), and members of the second group were infused with a Lys solution (1 g/kg of body weight). One hour thereafter, all rats were subjected to wrap-restraint stress, as described above. Rats from each group were subdivided into two subgroups (n = 15 per subgroup) and s.c. injected with saline or 5-HTP (10.0 mg/kg injection volume, 0.5 ml; Ajinomoto, Tokyo). The incidents of diarrhea were evaluated from time 0 (s.c. injection) until 60 min later. A rat was judged by a skilled experimenter to have diarrhea if there was excretion of loose stools, and no formed feces were observed during the experimental period (60 min). The incidence of diarrhea is presented as number of rats with loose stool versus number of rats used. Treatment differences were analyzed by using two-way ANOVA.
Effects of Lys on Normal and 5-HT4 Receptor Agonist-Affected, Plasma Corticosterone, Epinephrine, and Norepinephrine. Male Wistar rats (body weight, 250–280 g, n = 20) (Charles River Japan) were housed individually in conventional hanging cages (12 h light/dark cycle; dark period, 1900–0700 hours). Rats consumed distilled water and chow diet ad libitum. After a week of daily handling (5 min/day), rats were orally infused with water (n = 12) or Lys solution (1 g/kg of body weight, n = 12). One hour thereafter, each group was subdivided into two subgroups (n = 6 per subgroup). The members of the first subgroup from each oral treatment were s.c. injected with saline, and members of the second subgroup were s.c. injected with 5-HT4 receptor agonist (RS 67333 HCl, 3.0 mmol/liter, injection volume, 0.3 ml; Tocris Cookson, St. Louis). Thirty minutes thereafter, rats were subjected to ether stress and blood samples were collected from the posterior vena cava. Blood samples were centrifuged for 20 min (3,000 rpm) and plasma was separated. Corticosterone was measured by solid-phase 125I RIA (Diagnostic Products, Los Angeles), and norepinephrine and epinephrine were measured by the HPLC method, as described in detail (25). Treatment differences were analyzed by using two-way ANOVA followed by Duncan's multiple range test.
Effects of Lys on Basal Heart Rate and Tachycardia Evoked by 5-HT. Wistar rats (body weight, 400 g, n = 7) (Charles River Japan) were divided into two groups (n = 3 and 4, respectively). Rats were anesthetized with pentobarbital (90 mg/kg i.p.), and the left femoral vein was cannulated for infusions and the left carotid artery for heart rate measurement. The core temperature was maintained at 37 ± 1°C by a heating pad. Heart rate was measured with a tachograph triggered from the blood pressure signal and recorded on an ink-writing recorder (Graphtec; SR-6211) by a carrier amplifier (AP-601G, Nihon Kohden) and a heart-rate counter (AT-601G, Nihon Kohden). After stable conditions had been observed for 15min, baseline values of mean heart rate were determined. Then the first group of rats (n = 3) received i.v. bolus injections of Lys [0.7 mmol/dl (0.52 mg/kg) and 7.0 mmol/dl (5.2 mg/kg)], given sequentially. Injection volume was kept constant at 0.2 ml throughout the experiment. Fifteen minutes after the second injection, 5-HT (Sigma) was infused (100 μg/kg i.v.). The responses to the above dose of 5-HT were observed for 10 min.
The second, separate group of rats (n = 4) received i.v. bolus injections of 5-HT4 receptor agonist (RS 67333 HCl, 100.0 μg/kg). Fifteen minutes after RS 67333 HCl, Lys (7.0 mmol/dl i.v.) was injected. Ten minutes after Lys injection, 5-HT (Sigma; 100 μg/kg i.v.) was injected. Treatment differences were analyzed by using one-way ANOVA followed by Duncan's multiple range test.
Effects of Lys on Anxiety Evoked by 5-HT4 Receptor Agonist. Male Wistar rats (body weight, 250–280 g, n = 28) (Charles River Japan) were housed individually in conventional hanging cages (12 h light/dark cycle; dark period, 1900–0700 hours). Rats consumed distilled water and chow diet ad libitum for 1 week. Thereafter, rats were divided into two groups (n = 14 per group) and were orally infused with water (5 ml) or a Lys solution (1 g/kg of body weight). One hour after the oral infusion, the two groups were further subdivided into two subgroups (n = 7 per subgroup). The first subgroup from each oral treatment was s.c. injected with saline, and the second subgroup with 5-HT4 receptor agonist (RS 67333 HCl, 3.0 mmol/liter, injection volume, 0.3 ml, Tocris Cookson). Thirty minutes after the s.c. injection, rats were exposed to an elevated plus maze constructed of dark-gray polyacrylic resin and consisting of four arms (50 cm long and 10 cm wide) arranged in a shape of a plus sign and elevated 90 cm above the floor (24). Two opposite arms were open, and 40-cm-high walls protected the other two. All arms were interconnected with a 10 × 10-cm-wide central area, designed as open (relatively unprotected area). The behavior was recorded with a computerized video camera (NTSC, Sony, Tokyo) mounted above the center of the maze. Speed, time spent in the open vs. closed arms, the total path length and number of entries into each arm were recorded by using analysis software (smart, Panlab, Barcelona). Additional measures comprised frequency scores for rearing in the closed arms (vertical movement against the side and/or end of the walls), as described (22). A curtain surrounded the whole apparatus so that the rats could not see the experimenter, who observed the animals' behavior on a computer screen. To encourage exploration in the open arms, the room was only dimly lit. The maze was cleaned thoroughly with water containing a detergent before a new rat was exposed to it. Exposure started by placing the rat on the central area facing a closed arm. All experiments were done between 1130 and 1500 hours. Rats were tested on the elevated plus maze at 10-min intervals. The first 30 s of testing was not included in the statistical analysis to discard possible neophobic responses to the elevated plus maze. Treatment differences were analyzed by using two-way ANOVA followed by Duncan's multiple range test.
Results
To determine whether Lys directly interacts with 5-HT receptors, a displacement study was conducted on six major subclasses of 5-HT receptors (Table 1). Lys (0.8 mmol/dl) selectively inhibited binding of 5-HT to the 5-HT4 receptor (inhibition rate, 9.17%). The effect of Lys was dose-dependent. At a 10-times-higher dose (8.0 mmol/dl), Lys inhibited, with comparable efficacy, the binding of 5-HT to both 5-HT4 and 5-HT1A receptors. At a 100-times higher dose (80.0 mmol/dl), comparable effects of Lys were found on 5-HT2A, 5-HT2B, 5-HT4, and 5-HT1A receptors; however, this dose of Lys is not attainable in physiological conditions, so this finding is not included into the discussion.
Table 1. l-Lysine induced inhibition on 5-HT receptors.
| Inhibition, %
|
|||
|---|---|---|---|
| Receptor | 0.8 mmol/dl | 8.0 mmol/dl | 80.0 mmol/dl |
| 5-HT1A | 1.77 | 20.7 | 34.45 |
| 5-HT2A | <1.00 | 8.32 | 23.19 |
| 5-HT2B | <1.00 | <1.00 | 37.1 |
| 5-HT2C | <1.00 | 1.12 | <1.00 |
| 5-HT3 | <1.00 | 1.39 | <1.00 |
| 5-HT4 | 9.17 | 22.52 | 41.77 |
Means of two observations.
The Lys solution did not induce contractions in isolated guinea pig ileum when applied alone (0.7 and 7.0 mmol/dl, data not shown), suggesting that it has no 5-HT agonistic effects. However, the Lys dose-dependently (0.07 and 0.7 mmol/dl) blocked the 5-HT-induced contractions (Table 2) (P < 0.05 and P < 0.01, respectively), as compared with the solvent group. These findings indicate antagonistic effects of Lys on 5-HT receptors in guinea pig ileum.
Table 2. 5-HT (10 mmol/liter) induced contractions of isolated guinea pig ileum.
| Compound | Dose, mmol/dl | Contraction rate, % |
|---|---|---|
| Before | 100 | |
| Vehicle† | 105 ± 2 | |
| l-Lysine | 0.007 | 109 ± 4 |
| 0.07 | 90 ± 3* | |
| 0.7 | 75 ± 5** | |
| l-Leucine | 0.07 | 103 ± 5 |
| 0.7 | 98 ± 6 |
Data are means ± SEM of three animals. *, P < 0.05; and **, P < 0.01 (significant difference from vehicle).
Distilled water (100 μl/10 ml).
No effect of Lys (1 g/kg, oral infusion p.o.) on the weight of fecal pellets was found in the nonstressed rats (data not shown). Wrap-restraint stress did not cause diarrhea; feces were dry and formed. The stress exposure increased the weight of fecal pellets (one-way ANOVA, P < 0.05), as compared with nonstressed controls evaluated at the same daily hour (1400–1600 hours). Two-way ANOVA proved an interaction between Lys treatment (1 g/kg p.o.) and time of stress exposure (Fig. 1, P < 0.05). No effects of Lys at a dose of 600 mg/kg p.o. were found (Fig. 1).
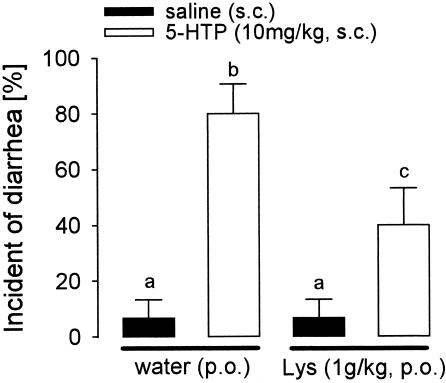
To investigate the effects of Lys on diarrhea incidence, wrap restraint stress was combined with 5-HTP in rats deprived of diet 24 h before the experiment. Because all rats in this experiment were fasted and restrained, the effects of the treatments (Lys and 5-HTP) were evaluated by two-way ANOVA. A main effect of 5-HTP (P < 0.001), together with a significant interaction between Lys and 5-HTP (Fig. 2, P < 0.05), was found.
Fig. 2.
Effect of Lys (1 g/kg p.o.) on the incident of diarrhea triggered by a combined application of wrap-restraint stress (1 h) and 5-HTP in fasted rats. Lys was infused 1 h before 5-HTP injection. Wrap-restraint stress was initiated immediately after s.c. treatment. Data are means of 10 rats + SEM. The bars with different superscript letters differ significantly at P < 0.05.
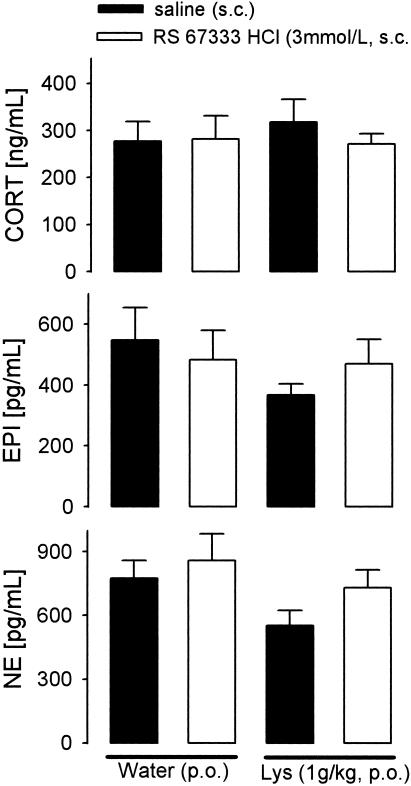
Two-way ANOVA revealed an absence of main, or combined, effects of the 5-HT4 receptor agonist (RS 67333 HCl, 3.0 mmol/liter s.c.) and Lys (1 g/kg p.o.) on plasma corticosterone, epinephrine, and norepinephrine (Fig. 3).
Fig. 3.
Effect Lys or 5-HT4 receptor agonist (RS 67333 HCl, 3.0 mmol/dl) on plasma corticosterone (CORT), epinephrine (EPI), and norepinephrine (NE) in rats. Rats were treated orally with Lys or water and 60 min later injected with RS 67333 HCl (s.c.) or saline (s.c.). Thirty minutes thereafter, rats were anesthetized with ether, and blood samples were collected from the posterior vena cava. Data are means of six rats + SEM.
Baseline heart rate in anesthetized rats, before application of any drug, was 405 ± 13 beats per min (bpm). The 5-HT4 receptor agonist (RS 67333 HCl, 100 μg/kg i.v.) did not affect basal heart rate (Δheart rate, +4.7 ± 4.6 bpm). Similarly, Lys (0.52 and 5.2 mg/kg i.v.) had no measurable effect on the baseline responses (Δheart rate, –1.8 ± 3.5 bpm and –0.7 ± 1.9 bpm, respectively). In comparison with reports (27), 5-HT (100 μg/kg i.v.) significantly (one-way ANOVA, P < 0.05) elevated heart rate (Δheart rate, +45.0 ± 5.0 bpm), and this effect was not affected by the application of Lys (5.2 mg/kg i.v.) 10 min before 5-HT (Δheart rate, +47.5 ± 12.5 bpm).
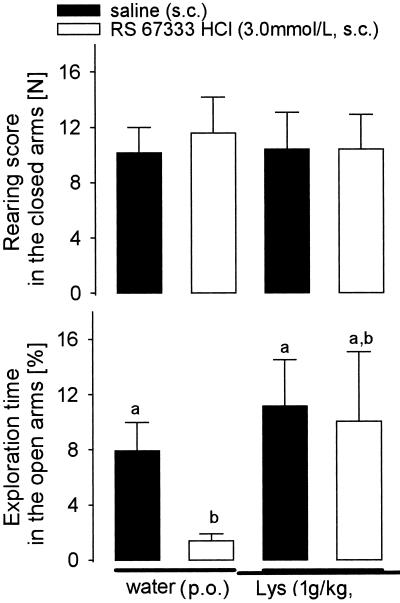
No main effect of either 5-HT4 receptor agonist (RS 67333 HCl, 3.0 mmol/liter s.c.), or Lys (1 g/kg p.o.) on speed (Lys, 40.6 ± 1.6 mm/s; RS 67333 HCl, 39.5 ± 2.6 mm/s) and distance of movement (data not shown) on the elevated plus maze was found. No changes in the rearing score in closed arms were observed (Fig. 4 Upper). Because normal exploratory behaviors of rats are directed into the closed arms, the negative results in closed arms indicate that Lys and RS 67333 HCl did not affect normal exploration. When anxiolytic behavior on open arms (23) was evaluated, two-way ANOVA revealed that the RS 67333-HCl-injected rats tended to spend less time in open arms (F ratio, 3.42; P < 0.077), as compared with saline-injected rats (Fig. 4 Lower). Moreover, the post hoc analysis showed that RS 67333 HCl rats not pretreated with Lys spent significantly (P < 0.05) less time exploring the open arms, as compared with other groups (Fig. 4 Lower).
Fig. 4.
Rearing score in the closed arms and exploration time in the open arms of the elevated plus maze. Rats were divided into two groups (n = 14 per group) and orally infused with water or Lys (1 g/kg of body weight). One hour later, rats were subdivided into four groups (n = 7 per group) and injected with 5-HT4 receptor agonist (RS 67333 HCl, 3.0 mmol/dl s.c.) or saline (s.c.). Thirty minutes thereafter, rats were tested for 10 min, as described (24). Data are means of seven rats + SEM. The bars with different superscript letters differ significantly at P < 0.05.
Discussion
It is recognized that diet has an important role in intestinal pathologies (e.g., irritable bowel syndrome), but this relationship (20–22) is complicated by symptomatic association to psychological disorders (28–31). Disturbances in gut and the brain 5-HT account for the majority of intestinal and psychological illnesses (4). However, pharmacological targeting of a single 5-HT receptor subclass does not address all accompanying symptoms and often leads to undesired side effects (28). Therefore, we reasoned that a complex treatment of irritable bowel syndrome should include a dietary plan, and we hypothesized that an enhancement of nutritional Lys load to subjects with concurrent intestinal and psychological complications could serve as a part of such a plan.
The hypothesis was based on the following indications. Increased anxiety and stress-induced fecal excretion were found in Lys-deficient rats (24). Prolonged Lys treatment had anxiolytic effects in normally fed, stressed rats (23), and the metabolic effects of Lys were stress-specific (26, 32). Moreover, a part of the Lys effect was traced to the 5-HT system (24). Therefore, the present study investigated whether Lys directly interacts with 5-HT receptor(s).
The binding study revealed that Lys significantly inhibited the binding of 5-HT to the guinea corpus striatum 5-HT4 receptor, although it was impossible to determine whether the binding was competitive or noncompetitive. Nevertheless, recent work showed that orally applied Lys, at a dose effective in the present in vivo experiments, elevated plasma Lys concentrations to tens of millimoles per deciliter (26). Thus, it is feasible that the 5-HT4 receptor binding of Lys has physiological implications, and those are presently tested in standard animal models.
Tsuyuki et al. (33) reported that 5-HT4 receptor-binding sites are present in the guinea pig ileum and that they are similar to those in the brain. It is also known that 5-HT enhances the contraction of electrically stimulated guinea pig ileum via the 5-HT4 receptor (6). Lys pretreatment reduced 5-HT-triggered contraction in an isolated guinea pig ileum at doses that were equal or even smaller than doses required to inhibit 5-HT4 receptor binding in the radioligand-binding assay. The results suggest that Lys is a partial 5-HT4 receptor antagonist in the intestines.
In the second experiment, Lys partially but significantly blocked the wrap-restraint stress-induced fecal excretion during the second hour of stress exposure. The time dependence is identical with that found in rats and humans after treatment with two chemically distinct 5-HT4 receptor antagonists (4, 12, 34, 35). Additionally, chronic Lys nutritional deficiency increased stress-induced defecation during the second, but neither the first nor the third hour of wrap-restraint stress (24). These results indicate that 5-HT4 receptor occupation might be affected by severe stress and fluctuations in Lys dietary intake. However, this hypothesis would require direct experimental proof.
Similarly to others (3), we were not able to induce diarrhea in rats by applying wrap-restraint stress only. Because the ability of stress to stimulate intestinal fluid secretion becomes more obvious in fasted rats injected with 5-HTP (4, 13, 36), we have combined the effects of wrap restraint, 24 h fasting, and the 5-HTP injections. The combined treatment induced diarrhea in 8 of 10 rats, and the severity of diarrhea was reduced by Lys. The results are compatible with those of Sanger et al. (4), who documented the efficacy of a 5-HT4 receptor antagonist in reducing the 5-HTP-induced diarrhea in mice, and Goldhill et al. (37), who proved the involvement of 5-HT4 receptor in the increase of intestinal fluid transport during stress exposure.
In conclusion, Lys blocked stress-induced fecal excretion and reduced the severity of diarrhea in a manner similar to the action of synthetic 5-HT4 receptor antagonists. However, alternative explanations are possible. First, Lys could have reduced the increased incidence of defecation by inducing constipation, because of the blockade of the intestinal 5-HT3 receptor. We argue against this possibility, because the ligand-binding results did not show any direct interactions between Lys and 5-HT3 receptors, and Lys did not affect the normal defecation pattern. Second, because secretory glycoprotein synthesis, rather than the catabolism (38–40), appears to be a major metabolic fate of nutritionally indispensable amino acids, it is possible that the protective effect of Lys was mediated by an increased rate of glycoprotein synthesis within the intestines. The intestinal mucosa is structurally protected by glycoproteins (mucins) (41), but the content and role of Lys in the core proteins of the intestinal mucins is unclear. Third, microbial synthesis of Lys was shown in the gastrointestinal tract (38). This Lys is made available through protein breakdown and its intestinal uptake, and it is possible that the high dietary Lys load blocked this protein breakdown in an unspecified way. Fourth, Lys might have affected the hypothalamo-pituitary-adrenal responsiveness to stress, as reported for synthetic 5-HT4 receptor antagonists (14), but neither Lys nor 5-HT4 receptor antagonist affected plasma corticosterone in our model. A few viable explanations exist for the lack of a hormonal effect, including the possibility that no 5-HT4 receptors exist in the rat adrenal cortex, which differs from humans (6, 14). In addition, the number of 5-HT4 receptors in the rat adrenal cortex may be too low, or their coupling efficacy might be different from the intestinal receptors (42). To test a hypothetical option of Lys-related changes in the sympathetic system, we measured plasma levels of norepinephrine and epinephrine. The results did not indicate significant interactions between dietary Lys and the tested sympathetic hormones. Last, Lys might have caused imbalance in amino acid homeostasis, and the observed in vivo effects might have resulted from this imbalance rather than from a separate action of Lys. Although Lys, at doses 10–100 times higher blocked 5-HT binding to 5-HT1 and 5-HT2 receptors, these results have little physiological meaning because no such Lys concentrations are reachable in plasma or gut lumen (26).
Because a weak anxiolytic effect of Lys (23) and a 5-HT4 receptor antagonist (17) was reported in stressed rats, it is also feasible that the gut effects were accompanied by a brain-mediated effect of Lys. Currently, we have attempted to block the anxiety induced by 5-HT4 receptor agonist by using Lys pretreatment. We have found a partial blocking effect of Lys on anxiolytic responses but not on normal behavior. This result indicates that Lys affects 5-HT4 receptors not only in the gut but in the brain as well. However, similarly to the gut, alternative explanations do exist. First, Lys is a partial agonist on the central benzodiazepine receptors (43, 44) and protects brain cells from stressful challenges by reducing the brain's metabolic rate (45). Second, the central effects of Lys might have been triggered by l-pipecolic acid, a Lys metabolite, which modulates benzodiazepine binding (46). Third, concurrent blockage of the brain 5-HT4 receptor and a stimulation of the benzodiazepine receptors might have resulted in anxiolytic effects of Lys during conditions of stress exposure (23).
The tachycardia induced by 5-HT in pigs and humans is mediated by 5-HT4 receptors (15, 16). No 5-HT4 receptor-mediated effect had yet been demonstrated in rats, a species in which the cardiovascular effects of 5-HT involve 5-HT2 receptors (27). To completely rule out the possibility that heart rate was affected by Lys in the current in vivo experiments, we evaluated the effects of Lys on basal and 5-HT-stimulated heart rate in rats. In line with reports on pithed rats (27), a 5-HT4 receptor antagonist was ineffective in changing the cardiovascular responses. Lys, tested at two doses that were equal and higher than the dose effective in the gut, also had no effect on the heart rate and did not block the 5-HT-induced heart rate increases. Similarly to the adrenal gland, the results might be interpreted by a lack of 5-HT4 receptors in the rat heart, by differences in coupling efficacy, or differences in effective Lys concentrations. Avoiding further speculations, the present results demonstrate that Lys, at doses that effectively blocked 5-HT4 receptor-mediated gut and brain pathologies, did not affect hormonal and cardiovascular responses in rat.
This demonstration of the effectiveness of a nutritional component in animal models of diarrhea-predominant intestinal disorders was previously unreported. The safety record of Lys use in humans is established (47), with most supplemental Lys being used to suppress herpes simplex (48) and to raise the biological value of dietary protein (10, 49). Although it is too early to leap from rodent studies to the implications in humans, our data propose a therapeutic use of Lys in the treatment of stress-related intestinal disorders, in which 5-HT sensitization and concurrent anxiety disorder are diagnosed.
Abbreviations: 5-HT, serotonin; 5-HTP, 5-hydroxytryptophane.
Footnotes
King, B. F. & Sanger, G. J. (1992) Br. J. Pharmacol. 107, 313P (abstr.).
References
- 1.Monnikes, H., Tebbe, J. J., Hildebrandt, M., Arck, P., Osmanoglou, E., Rose, M., Klapp, B., Wiedenmann, B. & Hyemann-Monnikes, I. (2001) Dig. Dis. 19, 201–211. [DOI] [PubMed] [Google Scholar]
- 2.Enck, P., Merlin, V., Erckenbrecht, J. F. & Weinbeck, M. (1989) Gut 30, 455–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams, C. L., Villar, R. G., Peterson, J. M. & Burks, T. F. (1988) Gastroenterology 94, 611–621. [DOI] [PubMed] [Google Scholar]
- 4.Sanger, G. J., Yoshida, M., Yahyah, M. & Kitazumi, K. (2000) Br. J. Pharmacol. 130, 706–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ressler, K. J. & Nemeroff, C. B. (2000) Depress. Anxiety 12, 2–19. [DOI] [PubMed] [Google Scholar]
- 6.Hedge, S. S. & Eglen, R. M. (1996) FASEB J. 10, 1398–1407. [DOI] [PubMed] [Google Scholar]
- 7.Eglen, R. M., Wong, E. H. F., Dumuis, A. & Bockaert, J. (1995) Trends Pharm. Sci. 16, 391–398. [DOI] [PubMed] [Google Scholar]
- 8.Tuladhar, B. R., Costall, B. & Naylor, R. J. (2002) Br. J. Pharmacol. 136, 150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilbinger, H. & Wolf, D. (1992) Naunyn-Schmiedberg's Arch. Pharmacol. 345, 270–275. [DOI] [PubMed] [Google Scholar]
- 10.Davis, T. A., Nguyen, H. V., Costa, D. P. & Reeds, P. J. (1995) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 110, 633–639. [DOI] [PubMed] [Google Scholar]
- 11.Tam, F. S. F., Hillier, K. & Bunce, K. T. (1994) Br. J. Pharmacol. 113, 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bharucha, A. E., Camilleri, M., Haydock, S., Ferber, I., Burton, D., Cooper, S., Tompson, D., Fitzpatrick, K., Higgins, R. & Zinsmeister, A. R. (2000) Gut 47, 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banner, S. E., Smith, M. I., Bywater, D., Gaster, L. M. & Sanger, G. J. (1996) Eur. J. Pharmacol. 308, 181–186. [DOI] [PubMed] [Google Scholar]
- 14.Contesse, V., Hamel, C., Delarue, C., Lefebvre, H. & Vaudry, H. (1994) Eur. J. Pharmacol. 265, 27–33. [DOI] [PubMed] [Google Scholar]
- 15.Kaumann, A. J., Sanders, L., Brown, A. M., Murray, K. J. & Brown, M. J. (1991) Naunyn-Schmiedberg's Arch. Pharmacol. 344, 50–159. [DOI] [PubMed] [Google Scholar]
- 16.Schoemaker, R. G., Du, X. Y., Bax, W. A. & Saxena, P. R. (1993) Eur. J. Pharmacol. 230, 103–105. [DOI] [PubMed] [Google Scholar]
- 17.Kennett, G. A., Bright, F., Trail, B., Blackburn, T. P. & Sanger, G. J. (1997) Neuropharmacology 36, 707–712. [DOI] [PubMed] [Google Scholar]
- 18.Pauwels, P. J. (2000) Biochem. Pharmacol. 60, 1743–1750. [DOI] [PubMed] [Google Scholar]
- 19.Kamm, K. M. (2002) Aliment. Pharmacol. Ther. 16, 343–351. [DOI] [PubMed] [Google Scholar]
- 20.Matsueda, K. (2001) Nippon Rinsho 59, 30–34. [Google Scholar]
- 21.Floch, M. H. & Narayan, R. (2002) J. Clin. Gastroenterol. 35, S45–S52. [DOI] [PubMed] [Google Scholar]
- 22.Frissora, C. L. (2002) Compr. Ther. 28, 222–231. [DOI] [PubMed] [Google Scholar]
- 23.Smriga, M. & Torii, K. (2003) Nutr. Neurosci. 6, 125–128. [DOI] [PubMed] [Google Scholar]
- 24.Smriga, M., Kameishi, M., Uneyama, H. & Torii, K. (2002) J. Nutr. 132, 3744–3746. [DOI] [PubMed] [Google Scholar]
- 25.Smriga, M., Mori, M. & Torii, K. (2000) J. Nutr. 130, 1641–1643. [DOI] [PubMed] [Google Scholar]
- 26.Smriga, M. & Torii, K. (2003) Amino Acids 24, 435–437. [DOI] [PubMed] [Google Scholar]
- 27.Centurion, D., Ortiz, M. I., Saxena, P. R. & Villalon, C. M. (2002) Br. J. Pharmacol. 135, 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.D'Souza, D. L., Levasseur, L. M., Nezamis, J., Robbins, D. K., Simms, L. & Koch, K. M. (2001) J. Clin. Pharmacol. 41, 452–454. [DOI] [PubMed] [Google Scholar]
- 29.Blanchard, E. B., Scharff, L. & Schwartz, S. P. (1990) Behav. Res. Ther. 28, 401–405. [DOI] [PubMed] [Google Scholar]
- 30.Haug, T. T., Mykletun, A. & Dahl, A. A. (2002) Scand. J. Gastroenterol. 37, 294–298. [DOI] [PubMed] [Google Scholar]
- 31.Lydiard, R. B. (1997) J. Clin. Psychiatry 58, 51–58. [PubMed] [Google Scholar]
- 32.Galili, G., Tang, G., Zhu, X. & Gakiere, B. (2001) Curr. Opin. Plant. Biol. 4, 261–266. [DOI] [PubMed] [Google Scholar]
- 33.Tsuyuki, Y., Saitoh, M. & Muramatsu, M. (1996) Life Sci. 59, 2129–2137. [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto, O., Niida, H., Tajima, K., Shirouchi, Y., Kyotani, Y., Ueda, F., Kise, M. & Kimura, K. (1998) J. Pharmacol. Exp. Ther. 287, 691–696. [PubMed] [Google Scholar]
- 35.Pichat, P., Baudot, X., Lechevalier, P. & Angel, I. (1996) Gastroenterology 110, A735. [Google Scholar]
- 36.Barclay, G. R. & Turnberg, L. A. (1987) Gastroenterology 93, 91–97. [DOI] [PubMed] [Google Scholar]
- 37.Goldhill, J., Porquet, M. F. & Angel, I. (1998) Eur. J. Pharmacol. 353, 289–296. [DOI] [PubMed] [Google Scholar]
- 38.Van der Schoor, S. R. D., Reeds, P. J., Stoll, B., Henry, J. F., Rosenberger, J. R., Burrin, D. G. & Van Goudoever, J. B. (2002) Gastroenterology 123, 1931–1940. [DOI] [PubMed] [Google Scholar]
- 39.Van Goudoever, J. B., Stoll, B., Henry, J. F., Burrin, D. G. & Reeds, P. J. (2000) Proc. Natl. Acad. Sci. USA 97, 11620–11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van der Schoor, S. R. D., Van Goudever, J. B., Stoll, B., Henry, J. F., Rosenberger, J. F., Burrin, D. G. & Reeds, P. J. (2001) Gastroenterology 121, 1167–1175. [DOI] [PubMed] [Google Scholar]
- 41.Bengmark, S. & Jeppsson, B. (1995) J. Parenter. Enteral. Nutr. 19, 410–415. [DOI] [PubMed] [Google Scholar]
- 42.Claeysen, S., Faye, P., Sebben, M., Taviaux, S., Bockaert, J. & Dumuis, A. (1998) Ann. N.Y. Acad. Sci. 861, 49–56. [DOI] [PubMed] [Google Scholar]
- 43.Chang, Y. F., Wing, Y., Cauley, R. K. & Gao, X. M. (1993) Eur. J. Pharmacol. 233, 209–217. [DOI] [PubMed] [Google Scholar]
- 44.Chang, Y. F & Gao, X. M. (1995) Neurochem. Res. 20, 931–937. [DOI] [PubMed] [Google Scholar]
- 45.Guan, H. P. & Ku, B. S. (1999) Life Sci. 65, PL19–PL25.10416829 [Google Scholar]
- 46.Feigenbaum, P. & Chang, Y. F. (1986) Brain Res. 30, 176–179. [DOI] [PubMed] [Google Scholar]
- 47.Flodin, N. W. (1997) J. Am. Coll. Nutr. 16, 7–21. [DOI] [PubMed] [Google Scholar]
- 48.Griffith, R. S., Norins, A. L. & Kagan, C. A. (1978) Dermatologica 156, 257–267. [DOI] [PubMed] [Google Scholar]
- 49.Young, V. R. & Pellett, P. L. (1991) Am. J. Clin. Nutr. 41, 1077–1090. [DOI] [PubMed] [Google Scholar]