The photosynthetic CO2-fixing enzyme Rubisco arose some 3.5 billion years ago, in an environment when CO2 was high and oxygen (O2) was low. Under these conditions, it was CO2 saturated and presumably performed well (Badger et al., 1998). However, since the advent of oxygenic photosynthesis, the levels of O2 have risen dramatically and CO2 has fallen to very low levels. This has gradually created conditions where CO2 has become limiting for Rubisco and allowed O2 to act as an alternative inhibitory substrate for the enzyme. To cope with these dramatic environmental changes, two major strategies have evolved to help Rubisco maximize its carboxylation rate at ambient levels of limiting CO2. First, the enzyme has evolved better kinetic properties, where the Km(CO2) has decreased and the ability to distinguish against O2 has increased at the expense of catalytic rate (Badger et al., 1998). Alternatively, many photosynthetic organisms, ranging from cyanobacteria to algae to land plants, have developed active CO2-concentrating mechanisms (CCMs) to turbo-charge Rubisco’s CO2 supply at a minor metabolic cost (Badger et al., 1998). Most notably, among plants this has led to the development of C4 photosynthesis (Sage, 2004).
Most of the important grain crops (rice [Oryza sativa], wheat [Triticum aestivum], barley [Hordeum vulgare], canola [Brassica napus], soybean [Glycine max]), tuber crops, and vegetable crops are C3 species and have applied the first strategy and lack any form of CCM at the leaf or chloroplast level. Much of the inherent inefficiency in C3 photosynthesis revolves around the need to gain CO2 through passive diffusion through the leaf pores (stomata), across cell walls and cytoplasm, and eventually through to the chloroplasts. Diffusive resistance to CO2 passage results in a drawdown of the effective CO2 concentration in the chloroplast, and C3 plants have adopted strategies to maximize the diffusive conductivity for CO2 by appressing chloroplasts against the intracellular airspaces and having large chloroplast surface area-to-leaf area ratios (Evans and von Caemmerer, 1996). Low chloroplast CO2 concentrations exacerbate the CO2 limitations and increase the wasteful Rubisco oxygenation reaction of ribulose 1,5-bisphosphate (RuBP) to produce phosphoglycolate, which must be recycled back to RuBP through a complex set of reactions known as the photorespiratory cycle. This is worsened by increased temperature, with the affinity for CO2 dropping and the oxygenase reaction being relatively enhanced (Kubien and Sage, 2008). To achieve acceptable high rates of photosynthetic CO2 fixation, typical C3 species invest up to 30% of soluble protein and some 25% of leaf nitrogen into Rubisco protein. Evolution of the CCM in C4 plants effectively circumvented a number of the inefficiencies, creating the present-day impetus for attempting to introduce C4 CCMs into important C3 crops such as rice (Hibberd et al., 2008).
However, while the C4 CCM is one approach to elevating CO2 around Rubisco, drawing from our knowledge of single-cell CCMs in cyanobacteria (Price et al., 2008), there are also opportunities to elevate CO2 around Rubisco at the individual leaf chloroplast level. These prospects are expanded upon below, but in brief we consider two scenarios. The first, and simplest, approach is to consider the transplantation of cyanobacterial bicarbonate transporters to the C3 chloroplasts to provide marginal but significant improvement in photosynthetic performance. The second, more elaborate, longer term objective would be to engineer a more functional cyanobacterial CCM in the chloroplast.
THE CYANOBACTERIAL CCM
Cyanobacteria have evolved an extremely efficient CCM (Fig. 1; see below), being able to concentrate CO2 around Rubisco by a factor of up to 1,000-fold. As a result, cyanobacterial CO2 fixation has been able to retain a Rubisco with a relatively high carboxylation rate, although lower selectivity between CO2 and O2, compared with the Rubisco in C3 plants (Badger et al., 1998). Cyanobacterial cells also have high nitrogen use efficiency, as less nitrogen is devoted to Rubisco than in a C3 plant (Badger et al., 1998). In addition, Rubisco within a cyanobacterium operates at near CO2 saturation due to the action of the CCM, such that wasteful photorespiration is largely eliminated.
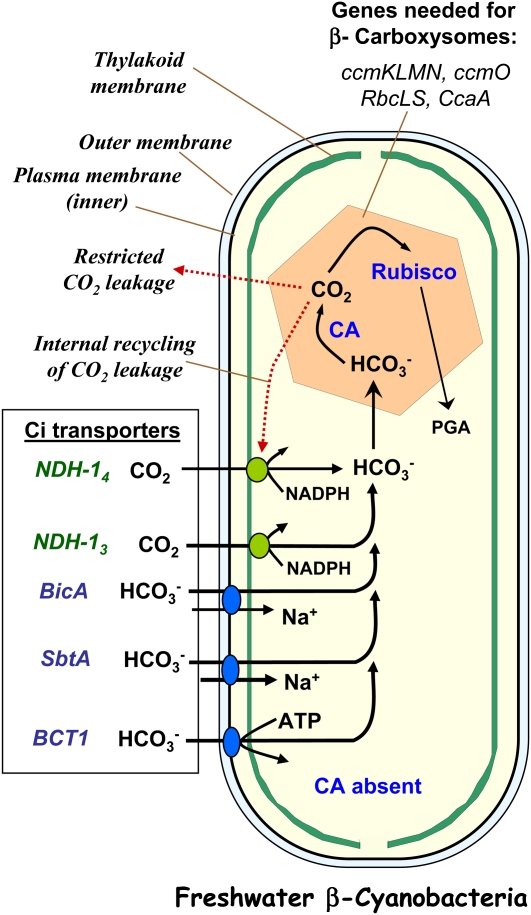
Figure 1.
The cyanobacterial CCM utilizes up to five uptake systems for DIC and the polyhedral microcompartments known as carboxysomes, which contain the cell’s complement of Rubisco and act as a localized site for the elevation of CO2 around Rubisco. A key operational feature is that all uptake systems deliver HCO3− to the general cytoplasm and that this is kept in a state of dynamic disequilibrium favoring HCO3−owing to the recycling of internally generated CO2 through the CO2 pumps and the absence of CA in the general cytoplasm. A specific, low level of CA activity is only present in the carboxysomes. In general, BicA, SbtA, BCT1, and NDH-I3 uptake systems are only induced under DIC-limiting conditions (e.g. liquid in near equilibrium with air CO2 levels or less). The carboxysomes are typically 90 to 200 nm in diameter (enlarged in this schematic), and a cell may possess five to 15 carboxysomes. By comparison, typical unicellular cyanobacteria are up to 3 μm in length, and typical C3 chloroplasts are up to 50 μm in diameter.
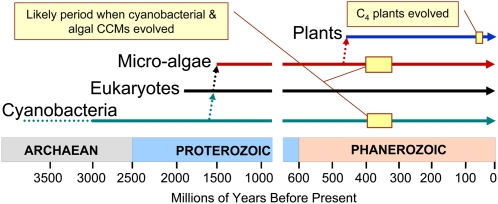
Given that an early cyanobacterial progenitor is considered to have become the original endosymbiont for chloroplast evolution in algae and land plants, the question arises as to why present-day land plants lack any apparent chloroplast-based CCM. Cyanobacterial progenitors first appeared some 2.7 billion years ago (Buick, 1992), but it is almost certain that cyanobacteria have been subjected to periods of rapid evolutionary change throughout this period. In particular, the marked drop in CO2 levels, and the rise in O2 levels, that occurred around 400 to 350 million years ago (Berner, 1990) represents a likely trigger that forced the evolution of adaptations to cope with photorespiration and low-efficiency CO2 fixation (Fig. 2). The poor availability of CO2 in water, where diffusion is 104 times slower than in air and where large unstirred layers can exist, probably provided additional evolutionary pressure. In addition, with a pKa around 6.4, CO2 is a rarer species at alkaline pH, whereas HCO3− is considerably more abundant in many aquatic environments. Evolutionary adaptations to deal with these combined pressures would have included transporters for the active uptake of dissolved inorganic carbon species (DIC; CO2 and HCO3−), the subsequent localized elevation of CO2 around Rubisco, and the partitioning of Rubisco into microcompartments known as carboxysomes (Badger et al., 2002; Price et al., 2008; see below). This may have also been the stage when microalgae developed CCMs. If, as seems likely, cyanobacteria did not evolve fully functional CCMs until 350 million years ago, then this is well after the first terrestrial plants are thought to have evolved from eukaryotic algae at around 450 million years ago (Kenrick and Crane, 1997) and long after the original endosymbiotic event that gave rise to microalgae at around 1.5 billion years ago (Dyall et al., 2004). This probably explains why present-day crop plants lack any form of chloroplast-based CCM derived from cyanobacterial or microalgal ancestors.
Figure 2.
A timeline indicating that CCMs possibly arose in cyanobacteria and microalgae at around 400 to 350 million years ago, well after the evolution of early land plants.
The cyanobacterial CCM functions to actively transport and accumulate DIC into the cell, where the accumulated HCO3− pool is utilized to generate elevated CO2 levels around Rubisco (Badger et al., 2002; Price et al., 2008). Rubisco is encapsulated in unique microcompartments known as carboxysomes that are typically 90 to 200 nm in diameter. The functional importance of these proteinaceous, icosahedral bodies that are composed of 20 equilateral triangular sides is that they act as the site of CO2 elevation within the cell, with the supply rate of CO2 from accumulated HCO3− being catalyzed by a carboxysome-located carbonic anhydrase (CA). The carboxysome shell in these cyanobacteria is composed of just six to eight proteins (Price et al., 2008), and the average unicellular cyanobacterial cells would normally possess five to 15 carboxysomes per cell. The key to the efficiency of any CCM revolves around the ability to minimize the loss of CO2 from the elevation zone. In model cyanobacteria, this is accomplished by a combination of (1) the accumulation of the ionic form of DIC, which is less membrane permeable than CO2, (2) the complete elimination of CA activity from the general cytosol to help reduce CO2 leakage out of the cell, (3) the special properties of the carboxysome protein shell acting to retard CO2 leakage, and (4) the action of the CO2 pumps in recycling CO2 leakage from the carboxysome back into the HCO3− pool (Maeda et al., 2002; Price et al., 2008).
The localization of CA, which catalyzes the reversible hydration and dehydration of CO2 and HCO3−is a key element of cyanobacterial CCMs. The absence of CA in the cytosol, and the action of the directional CO2 uptake systems that convert CO2 to HCO3− at the thylakoid membrane, allow the cell to accumulate HCO3−keep it out of rapid chemical equilibrium with CO2. This is very effective in minimizing the concentration of the diffusible CO2 molecule owing to the slow dehydration of HCO3− in the absence of CA (Walker et al., 1980). The importance of accumulating HCO3− in the cytosol, and maintaining an internal HCO3− pool out of chemical equilibrium, was shown by an experiment where human CA was expressed in the cytoplasm of a model cyanobacterium, Synechococcus elongatus PCC7942. The ectopic expression caused complete dissipation of the accumulated HCO3− pool due to the CA-mediated equilibration between CO2 and HCO3−which in turn led to increased CO2 diffusion out of the cell (Price and Badger, 1989). This is very different from the situation in C3 chloroplasts, where CA is highly abundant in the stroma in order to maximize the diffusion of CO2 across the envelope and throughout the chloroplast (Badger and Price, 1994).
Five distinct transport systems for DIC uptake have been identified in cyanobacteria (Fig. 1; Table I; for more details and related references, see Price et al., 2008). (1) BCT1, which is inducible under DIC limitation and is a high-affinity HCO3− transporter (uniporter) belonging to the traffic ATPase family. (2) SbtA, an inducible, high-affinity Na+-dependent HCO3− transporter (Price et al., 2004; Shibata et al., 2002) that apparently acts as a Na+/HCO3− symporter with relatively low flux rate. (3) BicA, a low-affinity, high-flux, Na+-dependent HCO3− transporter belonging to the widespread SulP family and related to the human SLC26 family of anion transporters (Price et al., 2004); BicA is a probable Na+/HCO3− symporter. (4) NDH-I4, a constitutive CO2 uptake system based on a specialized NADPH dehydrogenase (NDH-I) complex; this system uses NADPH as an electron donor to drive the conversion of CO2 to HCO3− during the uptake step (Price et al., 2002). Each complex is composed of 10 core subunits that are common to the respiratory NDH-I complex and three specialized subunits required for CO2 uptake. Interestingly, NDH-I-type CO2 uptake systems appear to be located on the thylakoid membranes, where they use CO2 diffusing from outside the cell or arising from leakage from the carboxysomes as a substrate for directional conversion to HCO3−. (5) NDH-I3, a second CO2 uptake system based on a modified NDH-I complex that is inducible under DIC limitation and is of higher uptake affinity than NDH-I4, located on the thylakoid membranes in Synechocystis PCC6803.
Table I. A summary of the properties of cyanobacterial DIC transporters.
| Transport Type | Mechanism | Substrate Affinity | Flux Rate | Photosynthetic Affinity (k0.5) |
| BicA | Na+-dependent HCO3− uptake | Low-medium | High | 90–170 μm HCO3− |
| SbtA | Na+-dependent HCO3− uptake | High | Low | <5 μm HCO3− |
| BCT1 | Traffic ATPase, HCO3− uptake | High | Low | 10–15 μm HCO3− |
| NDH-I4 | NADPH-driven CO2 uptake via conversion to HCO3− | Medium | High | 10–15 μm CO2 |
| NDH-I3 | NADPH-driven CO2 uptake via conversion to HCO3− | High | Low | 1–2 μm CO2 |
ADDING A HCO3− TRANSPORTER TO THE CHLOROPLAST ENVELOPE OF CROP PLANTS
With the objective of attaining a modest elevation of CO2 levels in the C3 chloroplast, the simplest approach would be to express a cyanobacterial HCO3− transporter on the inner envelope of the C3 chloroplast (Fig. 3). Single-subunit HCO3− transporters such as BicA and SbtA are the most obvious initial candidates. However, within technical restraints, the transfer of multisubunit transporters such as the BCT1 HCO3− transporter (four genes) is also possible. Additionally, the use of HCO3− transporters from microalgae such as Chlamydomonas can also be considered as viable candidates (Duanmu et al., 2009). From a technical viewpoint, the addition of DIC transporters mentioned above would be dependent on host genome transformation techniques using Agrobacterium tumefaciens, which are generally available for a range of important crop species. Chloroplast transformation techniques would not be required for this approach, and this is especially important because chloroplast transformation in crop species in not yet available. As can be seen from the associated modeling presented in this report (Fig. 4), the approach of installing BicA and/or SbtA transporters into the chloroplast inner envelope could achieve a 5% to 15% improvement in photosynthetic CO2 fixation rates at constant substomatal CO2 levels (see below).
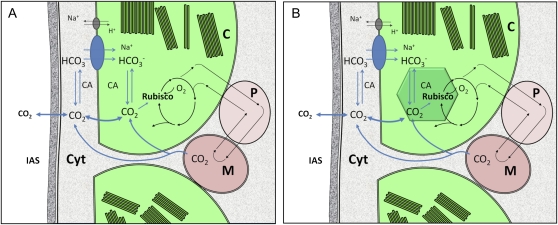
Figure 3.
Schematic representations illustrating the concepts of adding a cyanobacterial HCO3− transporter to the chloroplast envelope of a notional C3 leaf chloroplast (A) and the longer term prospect of constructing a more fully functional cyanobacterial or microalgal CCM in the C3 chloroplast (B). The diagrams show CO2 moving from the intracellular airspace (IAS; substomatal cavity) of a mesophyll leaf cell through the cell wall to the cytoplasm (Cyt) before entering the chloroplast by CO2 diffusion or via entry through a HCO3− transporter. The hexagonal structure represents the icosahedral carboxysomes that would contain the full complement of Rubisco in the chloroplast, with a specific CA partitioned to this compartment and stromal CA removed. Linkages between the carbon reduction in the chloroplast and photorespiration involving peroxisomes (P) and mitochondria (M) are also shown.
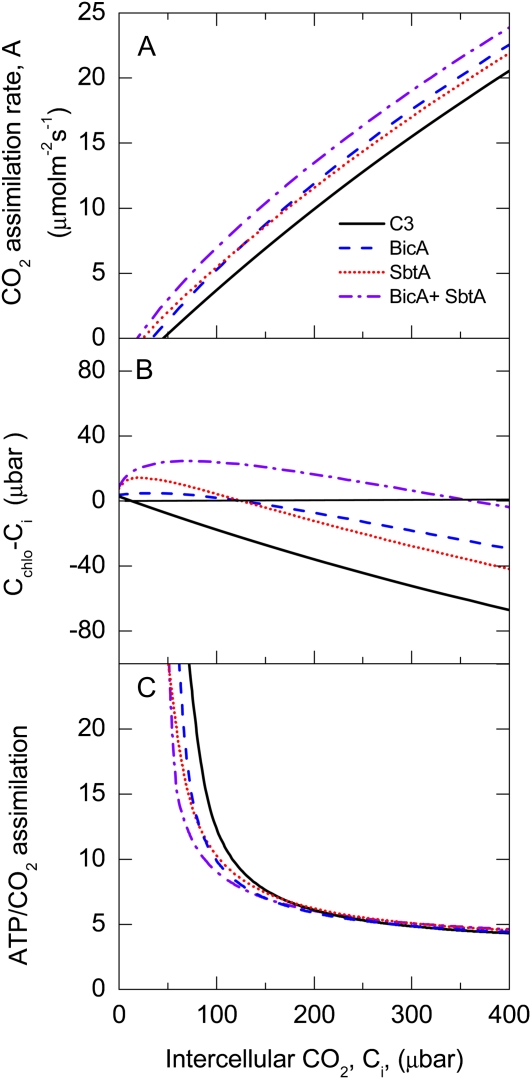
Figure 4.
A, Modeled net CO2 assimilation rate (A) as a function of intercellular CO2 partial pressure (Ci), with three different options for bicarbonate transport at the chloroplast envelope: (1) a BicA transporter with a maximum activity of 30 μmol m−2 s−1 and k1/2 for HCO3− = 90 μm (approximately 140 μbar CO2 at pH = 7.4); (2) a SbtA transporter with a maximum activity of 15 μmol m−2 s−1 and k1/2 for HCO3− = 5 μm (approximately 11 μbar CO2 at pH = 7.4); (3) with both transporters. Only light-saturated CO2 assimilation is considered, and maximal Rubisco activity was Vcmax = 100 μmol m−2 s−1. The diffusive conductance across the cell wall plasmalemma interface, and across the chloroplast envelope, were 1 and 0.5 mol m−2 s−1 bar−1, respectively. This results in a total conductance of 0.333 mol m−2 s−1 bar−1. CO2 assimilation rates are compared with Rubisco-limited C3 photosynthesis with the same maximal Rubisco activity. Other parameters and model equations used are given in the Supplemental Table S1. (The simulation becomes unrealistic at higher Ci, as it does not consider RuBP regeneration limitation.) B, The difference between chloroplast and intercellular CO2 (Cchlo − Ci) as a function of Ci for the examples given in A. C, ATP consumption per net CO2 assimilation rate for the examples given in A. Considering the proton transport required, we estimate 0.25 ATP per HCO3− transported by BicA and 0.5 ATP per HCO3− transported by SbtA.
It is clear that a CO2 diffusion gradient or drawdown exists between the CO2 level in the substomatal cavity of the leaf (Ci) and the steady-state level of CO2 in chloroplast (Cchlo), with the magnitude of this gradient sitting about 40% below Ci at high irradiance (Evans and von Caemmerer, 1996). It is important to recognize that in the first instance, the primary objective of adding a HCO3− pump would be to diminish the size of this CO2 drawdown at the chloroplast and not to elevate it significantly above the external CO2 concentration. This minimizes the risk of wasteful CO2 leakage. Such a situation is very similar to the concept of introducing a C4 cycle into C3 cells (Matsuoka et al., 2001), which has been modeled for transplantation into a typical C3 chloroplast (von Caemmerer, 2003) and found to be theoretically capable of raising the steady-state CO2 level within the chloroplast. More specific modeling data on the theoretical engineering of BicA into a chloroplast is shown in Figure 4 and discussed below. Notably, for the addition of HCO3− pumps to work, there is no need to modify CA levels in the chloroplast, since CA is needed to promote rapid equilibrium between accumulated HCO3−CO2 within the stroma.
In terms of establishing active HCO3− uptake across the chloroplast envelope, the question arises as to whether a Na+-dependent HCO3− transporter could function in a chloroplast. Estimates indicate that at least 250 μm HCO3− is present in the cytosol of a leaf cell in ambient air (Evans and von Caemmerer, 1996), and this appears to be maintained by cytosolic CA activity. The uptake affinities of SbtA (low flux rate) and BicA (high flux rate) for HCO3− in cyanobacteria are 5 to 15 μm and 90 to 170 μm, respectively (Shibata et al., 2002; Price et al., 2004) and would indicate that either transporter would operate well above its intrinsic Km. An additional concern relates to the question of energization and the involvement of Na+ gradients across the chloroplast envelope. Both SbtA and BicA require about 1 mm Na+ for half-maximal activity in the form of a standing inward gradient for Na+ (Shibata et al., 2002; Price et al., 2004). The leaf cytosol possesses 1 to 3 mm Na+ (Karley et al., 2000), and proteomic analyses have revealed that the Arabidopsis chloroplast envelope possesses several potential Na+-coupled transporters and Na+/H+ antiporters that are homologous to cyanobacterial forms (Rolland et al., 2003). Thus, there are good prospects that the chloroplast possesses and maintains an inwardly directed Na+ gradient. As a potential enhancement, the transfer of a cyanobacterial Na+/H+ antiporter (or a version from the C4 chloroplast) could also be considered if this Na+ gradient needed to be augmented, perhaps at the expense of any existing H+ gradient (proton motive force) inferred from the existence of H+-coupled transporters in the envelope (Weber et al., 2005). Any possible perturbation of osmotic and pH homeostasis in the chloroplast by elevating steady-state HCO3− levels by up to 15%, or even by as much as 25-fold relative to air-exposed leaves, would be expected to be minimal (Wagner et al., 1990).
Other problems in establishing SbtA or BicA in C3 chloroplasts would include ensuring the correct targeting to the chloroplast envelope and uncertainty about whether these transporters need to be posttranslationally activated. In the case of targeting, we expect that SbtA and BicA can be fused to the cDNAs for known envelope-targeted proteins such that details on targeting are not initially required. We have determined the membrane topology structure of BicA and SbtA as an initial step in identifying the most likely cytoplasmic regulatory domains in these transporters (Shelden et al., 2010).
It should be noted that there has been one attempt to place a putative cyanobacterial DIC transporter, IctB, in Arabidopsis and tobacco, resulting in a reported improvement in water use efficiency (WUE; the ratio of CO2 assimilation rate to transpiration rate) in Arabidopsis plants grown under dry-air conditions; in particular, a drop in the CO2 compensation point was observed (Lieman-Hurwitz et al., 2003). The basis of this improved WUE is not yet clear, since it is now known that ictB does not code for a DIC transporter (Shibata et al., 2002; Price et al., 2008), and its role in cyanobacteria is still unclear.
MODELING THE ADDITION OF BICARBONATE PUMPS TO THE C3 CHLOROPLAST
Our modeling of the consequences of taking the first step of adding one or two cyanobacterial HCO3− transporters to a C3 chloroplast is based on previous approaches used to consider the theoretical addition of a CO2 pump of single-cell C4 type (von Caemmerer, 2003; von Caemmerer and Furbank, 2003); equations and parameters used in the simulations shown in Figure 4 are detailed in the Supplemental Equations S1 and Supplemental Table S1. Much of the discussion of the benefits of introduction of single-cell C4 photosynthesis into a C3 leaf applies to the introduction of bicarbonate transporters (von Caemmerer, 2003). The key point issuing from the modeling is that the addition of either HCO3− transporter, BicA or SbtA, can lead to an increase in the rate of light-saturated CO2 assimilation at ambient and low intercellular CO2 partial pressures (Ci). The magnitude of the increase will be very much dependent on the kinetic properties of the transporters and the conductance to CO2 diffusion of the chloroplast envelope (von Caemmerer, 2003). The introduction of a transporter elevates chloroplast CO2 partial pressures (Cchlo) above Ci at low Ci values, resulting in a reduced CO2 compensation point. The addition of the high-affinity SbtA transporter is more effective at reducing the compensation point than the BicA transporter because of its lower Km, and introduction of both can be more effective again. At higher Ci levels, transporters serve to reduce the drawdown in CO2 between intercellular CO2 and the chloroplast (Fig. 4). At a constant Ci level of 250 μbar, a theoretical transgenic plant, with the assumed activities of both HCO3− uptake systems, could display an indicative assimilation rate greater than 15% higher than wild-type C3.
One of the disadvantages of C4 photosynthesis is the requirement of two ATPs per CO2 fixed in the C4 cycle, which makes the introduction of a C4 cycle into current C3 leaves energetically inefficient (von Caemmerer, 2003), although there are examples of a number of single-cell C4 species that have overcome the anatomical limitations inherent in C3 leaves (Edwards et al., 2004). It is likely that bicarbonate transport is less expensive. Considering the negative electrogenicity of HCO3− uptake in cyanobacteria (Ritchie et al., 1996) and the likely Na+:proton equivalence, we estimate a required 0.25 ATP per HCO3− transported by BicA and 0.5 ATP per HCO3− for SbtA. On this basis, the ATP requirement per net CO2 assimilation rate drops with increasing Ci during C3 photosynthesis as the cost of photorespiration decreases (Fig. 4) The introduction of bicarbonate transporters reduces the ATP cost at low Ci below that normally experienced during C3 photosynthesis and increases marginally above the C3 requirement at higher Ci (Fig. 4).
We have only considered the implications for light-saturated photosynthesis here. For healthy crops, about half the canopy will be operating under these conditions, which should make the introduction of bicarbonate transporters a productive strategy. Enhancing leaf photosynthetic rates also has the potential of increasing leaf WUE, depending on stomatal responses. Bicarbonate transporters provide the largest benefit of a low Ci, and the stomata of a plant with HCO3− pump enhancement could afford to be less open while providing the same rate of assimilation, thereby resulting in less loss of water from the leaf stomates. The SbtA transporter could be capable of improving WUE under dry-air conditions more effectively than BicA, and the addition of both transporters is likely to be even better. If SbtA or SbtA + BicA can be successfully introduced into C3 plants, such species might be able to better survive transient episodes of high water deficit under high light and moderate temperature stress.
ADDING A MORE ELABORATE CYANOBACTERIAL CCM TO THE CHLOROPLAST
A longer term objective, involving the greater technical difficulty of the introduction of multiple genes, could be to establish a more elaborate form of the cyanobacterial CCM in the chloroplast (Fig. 3). This could involve the transfer of one or two functionally active HCO3− transporters to the inner envelope membrane combined with the transfer of a CO2 uptake system to the thylakoid membranes and a Rubisco microcompartment such as the carboxysomes. The C3 chloroplast would also need to be, like the cyanobacterial cytosol, converted to a HCO3−-accumulating organelle where the HCO3− pool is held in a state of slow chemical interconversion. To do this, it would be necessary to reorganize chloroplastic C3 Rubisco into effective carboxysome structures and devise an effective means of removing the highly abundant chloroplastic CA so that HCO3− accumulation can be optimized. Certainly, it has been possible to remove up to 99% of chloroplastic CA activity in tobacco leaves by antisense RNA approaches (Price et al., 1994). Ideally, a complete removal of CA from the stroma would be more desirable, except for retaining the critically important CA in transferred carboxysomes. One of the most significant uncertainties relates to the conductance of the envelope to CO2 diffusion, with a range of estimates available (Flexas et al., 2008). Aquaporins seem to play a role in CO2 conductance; thus, a useful enhancement might be to reduce aquaporin levels in the envelope by RNA interference technology, since lower conductance would aid in reducing CO2 leakage (Flexas et al., 2006).
Our understanding of carboxysome assembly and function has improved greatly in recent years, to the point where engineering the assembly of a carboxysome in the chloroplast is approaching feasibility. This has been aided by advances in determining the crystal structures of some key components of the shell (Yeates et al., 2008) and our own efforts to identify proteins required as key Rubisco- and shell-organizing proteins (Long et al., 2007, 2010). The remarkable feature of the small shell proteins is an ability to self-assemble (Yeates et al., 2008). This property, shared with some virus coat proteins, could greatly aid the final goal of assembling functional carboxysomes within the chloroplast. The longer term objective of engineering a more potent form of the cyanobacterial CCM into the chloroplast may provide greater photosynthetic enhancements than the introduction of bicarbonate transporters alone.
CONCLUSION
Modeling indicates that the addition of cyanobacterial (or microalgal) HCO3− pumps at the chloroplast envelope of a typical C3 plant could provide a significant boost to the photosynthetic performance of leaf photosynthesis, either as increased assimilation rate or as improved WUE. A next research focus is to target BicA and SbtA transporters to the chloroplast of a model C3 plant and to extend our understanding of activation and energization processes for cyanobacterial HCO3− transporters. However, it should also be clear that parallel transgenic strategies, such as improving the performance of Rubisco or raising capacities for RuBP generation or light interception, would provide complementary improvements to crop performance, as discussed in other articles in this issue.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Equations S1. Modeling equations used in generation of data in Figure 4.
Supplemental Table S1. Photosynthetic parameters used in the model simulation.
Supplementary Material
References
- Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD. (1998) The diversity and coevolution of Rubisco, plastids, pyrenoids, and chloroplast-based CO2-concentrating mechanisms in algae. Can J Bot 76: 1052–1071 [Google Scholar]
- Badger MR, Hanson D, Price GD. (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol 29: 161–173 [DOI] [PubMed] [Google Scholar]
- Badger MR, Price GD. (1994) The role of carbonic anhydrase in photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 45: 369–392 [Google Scholar]
- Berner RA. (1990) Atmospheric carbon dioxide levels over phanerozoic time. Science 249: 1382–1386 [DOI] [PubMed] [Google Scholar]
- Buick R. (1992) The antiquity of oxygenic photosynthesis: evidence from stromatolites in sulphate-deficient Archaean lakes. Science 255: 74–77 [DOI] [PubMed] [Google Scholar]
- Duanmu D, Miller AR, Horken KM, Weeks DP, Spalding MH. (2009) Knockdown of limiting-CO2-induced gene HLA3 decreases HCO3− transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 106: 5990–5995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall SD, Brown MT, Johnson PJ. (2004) Ancient invasions: from endosymbionts to organelles. Science 304: 253–257 [DOI] [PubMed] [Google Scholar]
- Edwards GE, Franceschi VR, Voznesenskaya EV. (2004) Single-cell C(4) photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55: 173–196 [DOI] [PubMed] [Google Scholar]
- Evans JR, von Caemmerer S. (1996) Carbon dioxide diffusion inside leaves. Plant Physiol 110: 339–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Diaz-Espejo A, Galmés J, Medrano H. (2008) Mesophyll conductance to CO2: current knowledge and future prospects. Plant Cell Environ 31: 602–621 [DOI] [PubMed] [Google Scholar]
- Flexas J, Ribas-Carbó M, Hanson DT, Bota J, Otto B, Cifre J, McDowell N, Medrano H, Kaldenhoff R. (2006) Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. Plant J 48: 427–439 [DOI] [PubMed] [Google Scholar]
- Hibberd JM, Sheehy JE, Langdale JA. (2008) Using C4 photosynthesis to increase the yield of rice: rationale and feasibility. Curr Opin Plant Biol 11: 228–231 [DOI] [PubMed] [Google Scholar]
- Karley AJ, Leigh RA, Sanders D. (2000) Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends Plant Sci 5: 465–470 [DOI] [PubMed] [Google Scholar]
- Kenrick P, Crane PR. (1997) The origin and early evolution of plants on land. Nature 389: 33–39 [Google Scholar]
- Kubien DS, Sage RF. (2008) The temperature response of photosynthesis in tobacco with reduced amounts of Rubisco. Plant Cell Environ 31: 407–418 [DOI] [PubMed] [Google Scholar]
- Lieman-Hurwitz J, Rachmilevitch S, Mittler R, Marcus Y, Kaplan A. (2003) Enhanced photosynthesis and growth of transgenic plants that express ictB, a gene involved in HCO3− accumulation in cyanobacteria. Plant Biotechnol J 1: 43–50 [DOI] [PubMed] [Google Scholar]
- Long BM, Badger MR, Whitney SM, Price GD. (2007) Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA. J Biol Chem 282: 29323–29335 [DOI] [PubMed] [Google Scholar]
- Long BM, Tucker L, Badger MR, Price GD. (2010) Functional cyanobacterial beta-carboxysomes have an absolute requirement for both long and short forms of the CcmM protein. Plant Physiol 153: 285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda S, Badger MR, Price GD. (2002) Novel gene products associated with NdhD3/D4-containing NDH-1 complexes are involved in photosynthetic CO2 hydration in the cyanobacterium, Synechococcus sp. PCC7942. Mol Microbiol 43: 425–435 [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Furbank RT, Fukayama H, Miyao M. (2001) Molecular engineering of C-4 photosynthesis. Annu Rev Plant Physiol Plant Mol Biol 52: 297–314 [DOI] [PubMed] [Google Scholar]
- Price GD, Badger MR. (1989) Expression of human carbonic anhydrase in the cyanobacterium Synechococcus PCC7942 creates a high CO2-requiring phenotype: evidence for a central role for carboxysomes in the CO2 concentrating mechanism. Plant Physiol 91: 505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price GD, Badger MR, Woodger FJ, Long BM. (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59: 1441–1461 [DOI] [PubMed] [Google Scholar]
- Price GD, Maeda S, Omata T, Badger MR. (2002) Modes of active inorganic carbon uptake in the cyanobacterium, Synechococcus sp PCC7942. Funct Plant Biol 29: 131–149 [DOI] [PubMed] [Google Scholar]
- Price GD, von Caemmerer S, Evans JR, Yu JW, Lloyd J, Oja V, Kell P, Harrison K, Gallagher A, Badger MR. (1994) Specific reduction of chloroplast carbonic anhydrase activity by antisense RNA in transgenic tobacco plants has a minor effect on photosynthetic CO2 assimilation. Planta 193: 331–340 [Google Scholar]
- Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L. (2004) Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci USA 101: 18228–18233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie RJ, Nadolny C, Larkum AWD. (1996) Driving forces for bicarbonate transport in the cyanobacterium Synechococcus R2 (PCC 7942). Plant Physiol 112: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland N, Ferro M, Seigneurin-Berny D, Garin J, Douce R, Joyard J. (2003) Proteomics of chloroplast envelope membranes. Photosynth Res 78: 205–230 [DOI] [PubMed] [Google Scholar]
- Sage RF. (2004) The evolution of C-4 photosynthesis. New Phytol 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Shelden MC, Howitt SM, Price GD. (2010) Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Mol Membr Biol 27: 12–23 [DOI] [PubMed] [Google Scholar]
- Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, Fukuzawa H, Kaplan A, Ogawa T. (2002) Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: function and phylogenetic analysis. J Biol Chem 277: 18658–18664 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. (2003) C4 photosynthesis in a single C3 cell is theoretically inefficient but may ameliorate internal CO2 diffusion limitations of C3 leaves. Plant Cell Environ 26: 1191–1197 [Google Scholar]
- von Caemmerer S, Furbank RT. (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77: 191–207 [DOI] [PubMed] [Google Scholar]
- Wagner U, Kolbowski J, Oja V, Laisk A, Heber U. (1990) pH homeostasis of the chloroplast stroma can protect photosynthesis of leaves during the influx of potentially acidic gases. Biochim Biophys Acta 1016: 115–120 [Google Scholar]
- Walker NA, Smith FA, Cathers IR. (1980) Bicarbonate assimilation by freshwater charophytes and higher plants. 1. Membrane transport of bicarbonate ions is not proven. J Membr Biol 57: 51–58 [Google Scholar]
- Weber APM, Schwacke R, Flügge UI. (2005) Solute transporters of the plastid envelope membrane. Annu Rev Plant Biol 56: 133–164 [DOI] [PubMed] [Google Scholar]
- Yeates TO, Kerfeld CA, Heinhorst S, Cannon GC, Shively JM. (2008) Protein-based organelles in bacteria: carboxysomes and related microcompartments. Nat Rev Microbiol 6: 681–691 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.