Abstract
Aluminum (Al) is a harmful element that rapidly inhibits the elongation of plant roots in acidic soils. The release of organic anions explains Al resistance in annual crops, but the mechanisms that are responsible for superior Al resistance in some woody plants remain unclear. We examined cell properties at the surface layer of the root apex in the camphor tree (Cinnamomum camphora) to understand its high Al resistance mechanism. Exposure to 500 μm Al for 8 d, more than 20-fold higher concentration and longer duration than what soybean (Glycine max) can tolerate, only reduced root elongation in the camphor tree to 64% of the control despite the slight induction of citrate release. In addition, Al content in the root apices was maintained at low levels. Histochemical profiling revealed that proanthocyanidin (PA)-accumulating cells were present at the adjacent outer layer of epidermis cells at the root apex, having distinctive zones for cell division and the early phase of cell expansion. Then the PA cells were gradually detached off the root, leaving thin debris behind, and the root surface was replaced with the elongating epidermis cells at the 3- to 4-mm region behind the tip. Al did not affect the proliferation of PA cells or epidermis cells, except for the delay in the start of expansion and the accelerated detachment of the former. In soybean roots, the innermost lateral root cap cells were absent in both PA accumulation and active cell division and failed to protect the epidermal cell expansion at 25 μm Al. These results suggest that transient proliferation and detachment of PA cells may facilitate the expansion of epidermis cells away from Al during root elongation in camphor tree.
Acidic soil is a common soil type that accounts for one-third of the available terrestrial lands in the world. The extensive distribution of acidic soils is specific to boreal regions and the tropics, which corresponds with the present location of forest vegetation. In strongly acidic soils (e.g. oxisols, podsols, and acid sulfate soils), a soil pH below 5.0 affects plant growth via the leaching of essential ions and toxicity caused by excess protons and metal ions. Ionic aluminum (Al) is one of the critical factors that inhibit root growth in acidic soils, thereby reducing their aboveground production (Kochian et al., 2004). As the third most abundant element in the Earth’s crust, Al exists in soil particles as oxides, hydroxides, and nonexchangeable forms at neutral pH. However, as soil pH decreases, ionic forms of Al such as Al(OH)2+ and Al(OH)2+ increase in soil solution. At pH ≤ 5.0, trivalent Al ion (Al3+) becomes predominant as the most toxic form for plant roots. In acidic soils, leaching of base cations further enhances Al toxicity.
Al inhibits root elongation within hours by affecting the cell expansion zone (Ryan et al., 1993; Sivaguru et al., 1999). Al mainly accumulates in the apoplast of the epidermal layer of root apices (Horst, 1995; Taylor et al., 2000) and induces oxidative stress in the root apex (Yamamoto et al., 2001). Meanwhile, the excretion of organic anions such as citrate, malate, or oxalate is one of the most prevalent Al resistance mechanisms in many plants (Delhaize et al., 1993; Ma et al., 2001; Ryan et al., 2001). Recently, the molecular basis of organic anion release was identified in several plants including wheat (Triticum aestivum), barley (Hordeum vulgare), and sorghum (Sorghum bicolor; Delhaize et al., 2004; Sasaki et al., 2004; Furukawa et al., 2007; Magalhaes et al., 2007). However, it remains unclear whether organic anion release is effective in resisting high Al concentrations (more than 50 μm) for extended periods. Al-inducible release of malate is attenuated within a few days in the roots of wheat or oilseed rape (Brassica napus; Zheng et al., 1998). Jones et al. (1996) demonstrated that soil microorganisms rapidly decompose malate, indicating that this anion may play a lesser role in detoxifying Al in soils. Seedlings of signalgrass (Brachiaria decumbens), belonging to the Poaceae family, show high Al tolerance without apparent induction of organic anions (Wenzl et al., 2001).
Proanthocyanidins (PAs) are a class of flavonoid polymer composed of flavan-3-ols. They accumulate in the bark, fruit skin, and seed testa. Because of their high antioxidative properties, PAs are thought to play protective roles against biotic stresses, such as attacks from pathogens, insects, and mammals. In seed testa, PAs are involved in the control of seed germination through light permeation or the transportation of water and hormones (Debeaujon et al., 2000). A R2R3-type MYB transcription factor, AtTT2 (Arabidopsis thaliana TRANSPARENT TESTA2), regulates the late steps of PA biosynthesis (Nesi et al., 2001). Inducible accumulation of PA occurs in hairy roots of Medicago truncatula that heterologously express AtTT2 (Pang et al., 2008) or of grapevine (Vitis vinifera) that express VvMybPA2 (Terrier et al., 2009). Studies with Arabidopsis (A. thaliana) mutants defective in flavonoid biosynthesis have revealed that some PA precursors (e.g. flavanones and flavanols) are involved in root functions, including auxin transport (Peer and Murphy, 2007), lateral root formation (Rashotte et al., 2000), and nematode-induced gall formation (Wasson et al., 2009). However, it remains less understood whether PAs accumulate in normal plant roots or whether they play any role in root growth and development.
Camphor tree (Cinnamomum camphora) is an evergreen tree that often reaches over 20 m in height and was originally distributed in subtropic regions of East and Southeast Asia. Camphor tree was introduced for camphor production in Japan and is commonly used as a shrine or ornamental tree. A long-term experiment revealed no growth inhibition of camphor tree seedlings after 4 months of exposure to 5 mm Al in a nutrient solution (Oda and Yamamoto, 2002). Meanwhile, studies of the multipurpose trees Acacia mangium and Melaleuca cajuputi, both of which are used for afforesting degraded lands in the tropics, support the idea that some woody plants exhibit extraordinary Al tolerance with a modest release of organic anions (Tahara et al., 2005; Osawa and Kojima, 2006). A better understanding of high Al tolerance mechanisms in woody plants could provide the basis for introducing highly Al-tolerant plants to marginal lands with strongly acidic soils.
To understand the high Al tolerance mechanism in camphor tree, we evaluated the roles of organic anion release and the developmental properties of the surface cells in the roots by comparing these properties with those of soybean (Glycine max), a representative citrate-releasing plant. We found that a 20-fold higher Al resistance in camphor tree than in soybean is less dependent on citrate release and that the unique proliferation of PA-accumulating cells outside the epidermal cells in camphor tree may play a protective role in excluding Al. A novel, high Al resistance mechanism that is less dependent on organic anion release is discussed.
RESULTS
Root Elongation
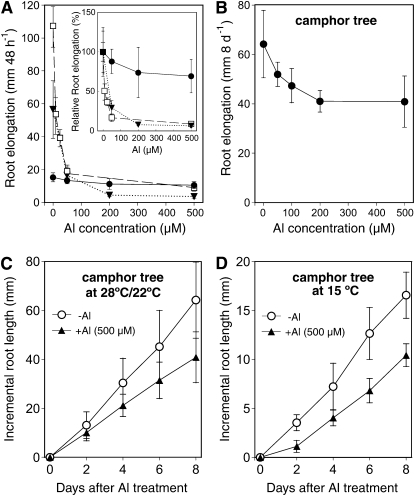
To determine the exact Al tolerance level of camphor tree, we measured root elongation at different concentrations of Al in 500 μm calcium chloride solution (pH 4.5). For comparison, we also measured root elongation of the Al-resistant soybean cv Wase-hakucho, which is a sister line of the Al-resistant cv Shiratorihakucho (Yang et al., 2000) and of the Al-resistant wheat cv Atlas 66. During 48 h of exposure, the root elongation in camphor tree was only inhibited by 10% at 50 μm Al and was inhibited by 30% at 500 μm Al, whereas those for Al-resistant cultivars of soybean and wheat were completely inhibited by a concentration of 50 μm (Fig. 1A). In the absence of Al, the root elongation rate in camphor tree (15.3 ± 2.7 mm 48 h−1; mean ± sd, n = 7) was 3.7 and seven times slower than that in wheat (56.5 ± 17.5 mm 48 h−1) and soybean (106.8 ± 12.4 mm 48 h−1), respectively. For the comparison of Al tolerance based on similar root growth, the root elongation in camphor tree was measured under a longer Al-exposure period. After 8 d of treatment without Al, the root of camphor tree elongated 64.2 ± 13.7 mm (n = 7) in length, an increment that was almost comparable to the root growth of the two annual crops for 48 h. Meanwhile, the root elongation of camphor tree at 500 μm Al reached 40.9 ± 10.4 mm, which was 64% of the level of elongation without Al (Fig. 1B). Furthermore, a constant 70% level of relative root elongation in camphor tree was maintained over 60 d of exposure to 500 μm Al every 2 d (Osawa et al., 2009). These results indicate that camphor tree roots are highly tolerant to Al over the threshold concentrations that arrest root elongation in most Al-resistant annual crops.
Figure 1.
Characteristics of highly Al-tolerant root elongation in camphor tree. A, Effect of 48-h Al exposure on root elongation in camphor tree (black circles), Al-resistant soybean cv Wase-hakucho (white squares), and Al-resistant wheat cv Atlas (black triangles). The inset represents their relative root lengths based on respective root elongation at 0 μm Al. B, Effect of 8 d of exposure to Al on root elongation in camphor tree. C, Time course of change in root elongation of camphor tree at 28°C (light) and 22°C (dark). D, Time course of change in root elongation of camphor tree at 15°C constant. In all experiments, roots were exposed to 500 μm calcium chloride solution (pH 4.5) containing different concentrations of Al; each treatment solution was renewed every 2 d. Data represent means ± sd (n = 5). Symbols without bars indicate that the sd is smaller than the symbol size.
We further measured root elongation in camphor tree at a low temperature. Without Al, the root elongation at 15°C was about one-fourth of that at 25°C. However, compared with the control growth levels at each temperature, there were no differences in relative root elongation at 500 μm Al between 15°C and 25°C (Fig. 1, C and D), confirming the Al tolerance in camphor tree is not overestimated by their slow growth rate.
Spatial Elongation Zone
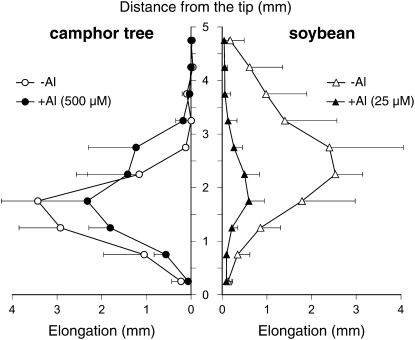
To understand how cell proliferation drives root elongation in the two species, their spatial elongation was measured by the shifts of India ink marks applied to the root surface at 0.5-mm intervals. Al concentrations and exposure periods were set to 500 μm Al and 48 h for camphor tree and 25 μm and 8 h for soybean, respectively. In camphor tree roots, the root elongation at 0 μm Al showed a maximum increment between 1.0 and 2.0 mm away from the tip, followed by a sharp decline in the sections 2.0 to 3.0 mm away from the tip (Fig. 2). In the presence of Al, the maximum zone increment at the 1.0- to 2.0-mm sections was slightly decreased, and the spatial elongation pattern was shifted to the root basal direction by 0.5 mm. In soybean root, the spatial root elongation in the absence of Al had a bell shape distribution over the entire 5-mm region, with a median peak between the 2.0- and 3.0-mm sections. Meanwhile, the treatment with 25 μm Al for 8 h suppressed the root elongation in all the sections, especially between the 3.0- and 5.0-mm sections behind the tip.
Figure 2.
Spatial distribution of root elongation in camphor tree and soybean. Camphor tree roots were exposed to 500 μm calcium chloride solution without (white circles) or with 500 μm Al (black circles) for 48 h. Soybean roots were exposed to 500 μm calcium chloride solution without (white triangles) or with 25 μm Al (black triangles) for 8 h. The increments of each 0.5-mm interval marked with Indian ink were measured. Data represent means ± sd (n = 4).
Effect of PA Accumulation in Tolerance to Other Ions
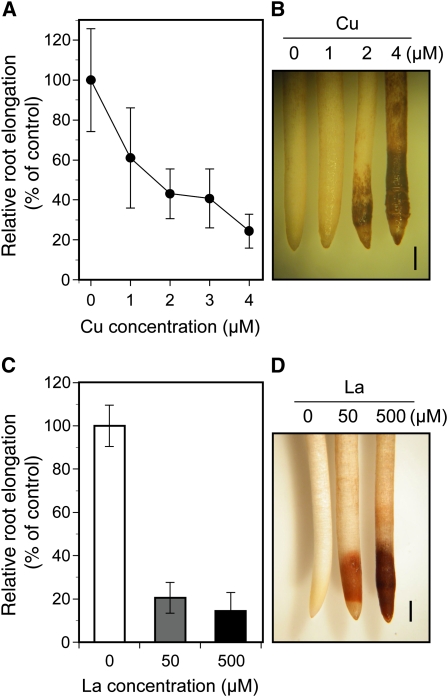
To evaluate whether Al tolerance in camphor tree is based on general mechanisms available in other metal stress, the root elongation in camphor tree in the presence of copper ion (Cu) or lanthanum ion (La) was investigated. Exposure to 4 μm Cu, which was less than 2-fold higher than the lethal concentrations for root elongation in wheat and soybean, caused a 76% reduction in camphor tree root elongation (Fig. 3A). Similarly, exposure to 50 μm La, equivalent to the concentration inhibiting the root elongation in wheat cv Atlas, caused a more than 80% reduction in root elongation (Fig. 3C). An intensive root browning in the entire 1- to 3-mm region was observed in a manner dependent on concentrations of both Cu and La (Fig. 3, B and D). These results suggest that the roots of camphor tree can mediate high levels of tolerance that is specific for Al.
Figure 3.
Growth inhibition of camphor tree roots by Cu or La. A, Effect of a 48-h exposure to Cu on root elongation in camphor tree. B, Root browning after a 48-h exposure to Cu. C, Effect of a 48-h exposure to La on camphor tree root elongation. D, Root browning after a 48-h exposure to La. Root elongation in the presence of Cu or La is expressed as relative root elongation based on the root elongation at 0 μm Cu or La, respectively. Data represent means ± sd (n = 6). Bars in B and D = 1 mm.
Organic Anion Efflux
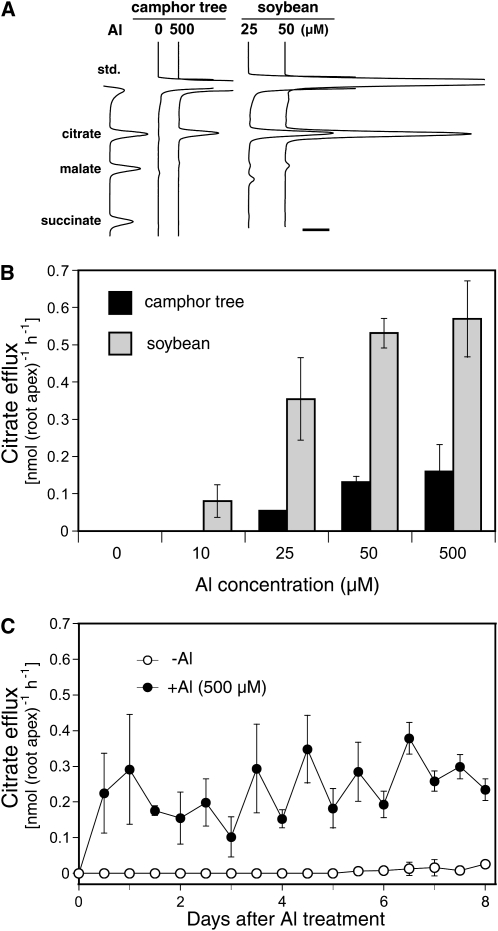
Organic anion release from camphor tree roots was evaluated to understand its role in high Al resistance. Within the first 12 h of exposure, camphor tree roots started citrate release in a manner dependent on Al concentration (Fig. 4, A and B). Induction of the citrate release at 500 μm Al was maintained throughout 8 d with a diurnal fluctuation (Fig. 4C). The citrate release in camphor tree was compared with that in soybean cv Wase-hakucho. A Hakucho descendent line, Shiratorihakucho, inductively releases citrate against Al (Yang et al., 2000). On the basis of the number of root tips, the amount of citrate release in camphor tree at 500 μm Al was 6.5, 4.1, or 3.6 times smaller than that in soybean at 25, 50, or 500 μm Al, respectively (Fig. 4, A and B). The amount of citrate release on the basis of root surface areas at the 0- to 5-mm tip region was also no larger in camphor tree than in soybean (data not shown). These results suggest that citrate release may play a less important role in the high Al resistance mechanism in camphor tree. Interestingly, even though the root elongation in soybean was completely inhibited at 50 or 500 μm Al within 12 h, their citrate release peaked at 24 to 36 h (data not shown) and the highest levels among the different Al concentrations up to 500 μm Al (Fig. 4B). In both species, no organic anion other than citrate in the root-bathing solution was detected during all periods (data not shown), and no increase in organic anion contents in the root apices was observed at 24 h of Al treatment (Supplemental Fig. S1). These results support the idea that Al-induced citrate release is not attributable to membrane leakage but to a specific transport system.
Figure 4.
Patterns of Al-induced citrate efflux in camphor tree. A, Representative chromatographs of organic anions released from roots in camphor tree and soybean during the first 12 h of Al treatment. The bar represents 1 mV S−1. B, Al concentration-dependent increase in citrate efflux from roots in camphor tree (black bars) and soybean (gray bars). Citrate efflux in the first 12 h of exposure to Al is shown. C, Time course change in citrate efflux rate from camphor tree roots after exposure to 500 μm calcium chloride solution without (white circles) or with 500 μm Al (black circles). Data represent means ± sd (n = 3). Symbols without bars indicate that the sd is smaller than the symbol size.
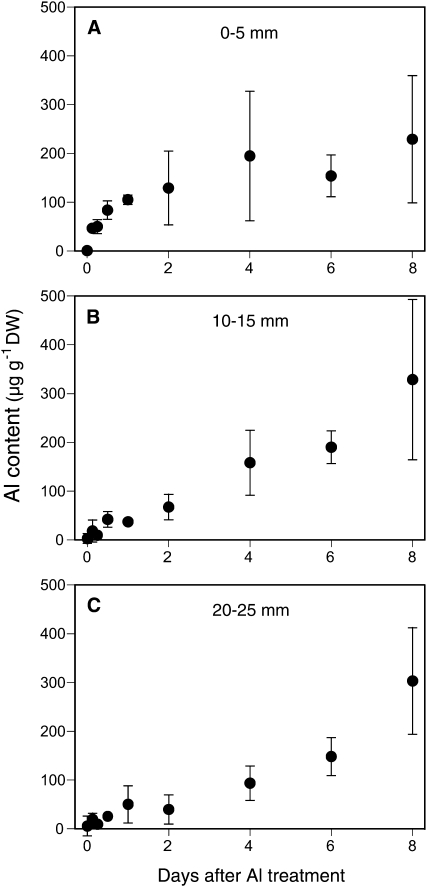
Al Accumulation
To evaluate whether an Al-exclusion mechanism is involved in the high Al tolerance in camphor tree, we determined changes in Al content in three spatial sections (0–5, 10–15, and 20–25 mm) of the root apex after exposure to 500 μm Al for 8 d. The Al contents in both 10- to 15-mm and 20- to 25-mm sections increased with time during the Al exposure period; whereas the Al content in the 0- to 5-mm section appeared to reach a stationary state in the first 48 h and remained relatively constant over the periods between days 2 and 8 (Fig. 5). The Al content in camphor tree (129 ± 76 μg g−1 dry weight) after 48 h of exposure to 500 μm Al was 3.5 times lower than that in soybean (452 ± 61 μg g−1 dry weight) after 8 h of exposure to 25 μm Al (data not shown). Histochemical staining of camphor tree roots at 24 h of Al treatment revealed that Al detectable with hematoxylin or morin was localized to the outer layer of root cap cells, especially in the region 0 to 2 mm away from the tip (Supplemental Fig. S2). These results suggest that a low Al accumulation in the root tip is involved in a high Al resistance mechanism in camphor tree.
Figure 5.
Al accumulation pattern in the roots of camphor tree. Roots were exposed to 500 μm calcium chloride solution (pH 4.5) containing 500 μm Al at the indicated times. After washing the roots with the calcium chloride solution containing 10 mm citric acid for 10 min, the roots were excised into three segments (A, 0–5 mm; B, 10–15 mm; C, 20–25 mm from the tip). Al concentration in wet-ashed samples was determined with a graphite furnace atomic absorption spectrophotometer. Data represent means ± sd (n = 3). Symbols without bars indicate that the sd is smaller than the symbol size. DW, Dry weight.
PA Accumulation in Roots
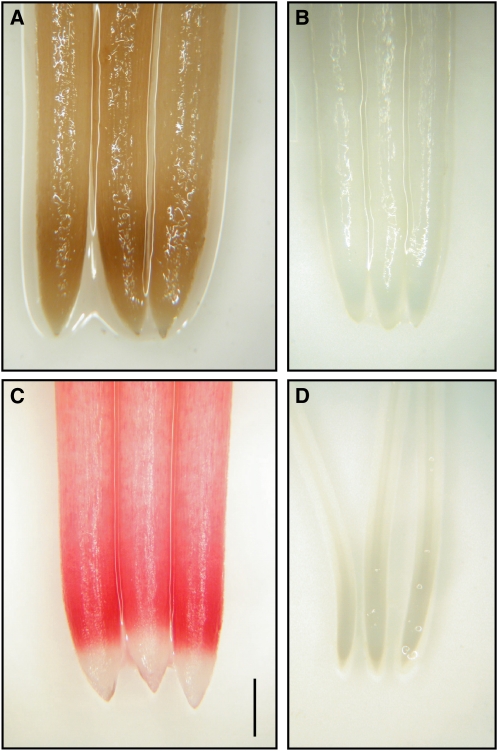
Al rapidly inhibits root elongation through the disturbance of cell expansion at the surface layer. As a first step to understand the pattern of cell expansion in high Al resistance in the roots of camphor tree, we analyzed cell length at the outer radial layer by using longitudinal sections of roots fixed with FAA (formaldehyde, acetic acid, and 50% [v/v] ethanol, 1:1:18 [v/v/v]). When fixing the camphor tree roots with FAA, we noticed that a band-like root browning developed within the region located 1 to 3 mm behind the tip (Fig. 6A). This browning presumably resulted from the aldol condensation of phenolic compounds, since the application of FAA lacking formaldehyde failed to cause browning (data not shown). The (+)-catechin, one of the major components of PAs, is vulnerable to formaldehyde-induced aldol condensation (Takano et al., 2008). We stained the roots with vanillin-HCl solution to determine whether PAs are involved in the FAA-induced browning. Within 2 min after exposure to 1% (w/v) vanillin in 6 n HCl solution, a dark red color developed at the 1- to 3-mm region behind the tip; with a delay of seconds, a light red color also developed behind the 3-mm region (Fig. 6C). Staining the roots with ρ-dimethylaminocinnamaldehyde (DMACA) reagent, which is four times more sensitive in detecting PAs than vanillin-HCl solution, resulted in blue colorimetric development in the camphor tree roots with an obscure distinction between the 1- to 3-mm region and the 3- to 10-mm region (Supplemental Fig. S3). The patterns of vanillin staining and FAA-induced browning in camphor tree roots that were exposed to 500 μm Al were similar to those of the control. In soybean, no root color was changed even after 12 h of exposure to FAA or 1 h of exposure to vanillin (Fig. 6, B and D).
Figure 6.
Identification of PA accumulation in root apices of camphor tree. A, Camphor tree roots fixed with FAA overnight. B, Soybean roots fixed with FAA overnight. C, Camphor tree roots stained with 1% (w/v) vanillin in 6 n HCl for 2 min. D, Soybean roots stained with vanillin-HCl solution for 60 min. The roots of camphor tree and soybean were exposed to 500 μm calcium chloride solution for 48 and 8 h, respectively. Bar = 1 mm.
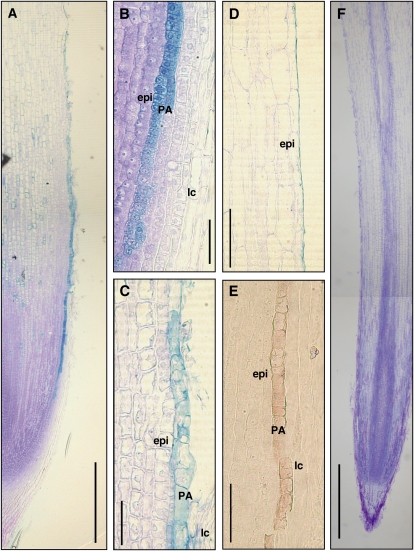
To reveal the tissue localization of PAs, we stained the fixed section of camphor tree root apices with 1% (w/v) toluidine blue O (TBO) because both vanillin and DMACA staining have poor reactivity with formaldehyde-condensed PAs. In a root exposed to 500 μm Al for 48 h, TBO staining developed a distinct light-blue color in a single layer of the innermost lateral root cap between 1 and 3 mm from the tip (Fig. 7A). The lineage of the cell accumulating TBO-reactive phenolic compounds stemmed from a periclinally divided cell at the 1-mm region, including several transverse junctions due to periclinal cell division and many phragmosome cells with a flat shape in the 1- to 2-mm region (Fig. 7B). TBO-reactive phenolic compounds accumulated in the entire cell in the region 1 to 2 mm away from the tip, whereas they accumulated less in the vacuoles of the expanding cells in the 2- to 3.5-mm region (Fig. 7, B and C). Other lateral root cap cells, whose lengths were more than 50 μm in the 1- to 2-mm region, were minimally stained with TBO. Staining with vanillin-HCl developed a faint but distinct reddish color in the same layer of the cells accumulating TBO-reactive phenolic compounds (Fig. 7E). Lignins or suberins were unlikely to be involved in the TBO-reactive phenolic compounds, since no UV light-excited autofluorescence was observed in the corresponding region (data not shown). TBO staining pattern in the 0- to 3-mm tip region of the roots without Al exposure was almost the same as that of the roots treated with 500 μm Al (data not shown). In the 2.5- to 4-mm tip region, the shapes of the cells containing TBO-reactive phenolic compounds were apparently disturbed and narrowed down in both roots, without or with Al treatment. In a root without Al exposure, the disturbed cells finally disappeared, leaving a thin layer of cell debris (less than 2 μm in width) on the surface of inner epidermis cells in the 5- to 10-mm region (Fig. 7D). Meanwhile, Al apparently promoted the collapse and detachment of PA cells in the 3- to 4-mm region (Fig. 7A), resulting in crack formation and sparse remaining cell debris on the root surface at the 5- to 10-mm tip region. In a soybean root exposed to 25 μm Al for 8 h, TBO staining developed a monotonous purple-blue color in all the tissues, including lateral root cap (Fig. 7F).
Figure 7.
Localization of PA-accumulating cells in camphor tree root apices. A, Longitudinal section of a camphor tree root. The root was exposed to 500 μm calcium chloride solution with 500 μm Al for 48 h and stained with 0.1% (w/v) TBO. B, Magnified image of the camphor tree root in A at 1 mm behind the tip. epi, Epidermis cell; lc, lateral root cap cell; PA, PA-accumulating cell. C, Magnified image of the camphor tree root in A at 2.5 mm behind the tip. D, Longitudinal section of an Al-untreated camphor tree root at 5.5 mm behind the tip. The root was exposed to 500 μm calcium chloride solution for 48 h. E, Neighboring section of the camphor tree root in A at 2 mm behind the tip. The section was stained with 1% (w/v) vanillin in 6 n HCl. F, Longitudinal section of a soybean root. The root was exposed to 25 μm Al for 8 h and stained with TBO. Bars = 500 μm in A and F, 50 μm in B and C, and 100 μm in D and E.
Cell Lineage
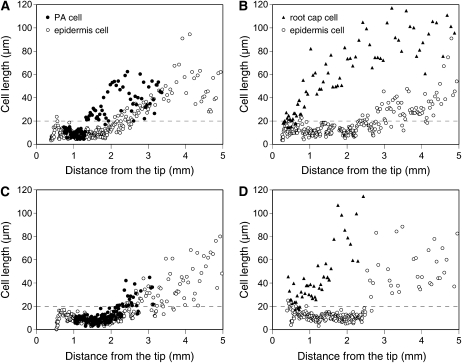
From extensive propagation followed by detachment at the root surface, we hypothesize that the PA cells may act as “pseudo”-epidermis cells in shielding the inner cells away from Al. To understand the proliferation pattern of PA cells, we analyzed the changes in the longitudinal cell length in the outer radial layers of root apices in the two species. In a camphor tree root, PA cells kept a flat shape for a while after their emergence. Until the lengths of PA cells started to increase at the 1.3-mm region behind the tip, the longitudinal lengths of PA cells (9.09 ± 2.89 μm; mean ± sd, n = 67) were almost similar to those of epidermis cells (7.80 ± 2.50 μm; n = 72) at the corresponding region (Fig. 8A). The lengths of PA cells started to increase at a 0.5-mm closer region to the tip than those of epidermis cells (Fig. 8A; Table I). By the 3-mm tip region, the lengths of PA cells reached nearly 60 μm. Because of apparent cell deformation, the lengths of PA cells were unable to be traced beyond the 4-mm region behind the tip.
Figure 8.
Cell length profiles of surface layers of root apices. Seedlings of each species were exposed to 500 μm calcium chloride solution (pH 4.5) containing respective concentrations of Al. A, Camphor tree at 0 μm Al. B, Soybean at 0 μm Al. C, Camphor tree at 500 μm Al. D, Soybean at 25 μm Al. For camphor tree, longitudinal lengths of PA cells (black circles) and epidermis cells (white circles) of the camphor tree root apex were measured. For soybean, longitudinal lengths of lateral root cap cells at the innermost layer (black triangles) and epidermis cells (white circles) were measured. The exposure periods were 48 and 8 h for camphor tree and soybean, respectively. A dashed line at 20 μm indicates an apparent distinction between cell division and cell expansion in both species. A representative profile for each treatment (n = 5 for camphor tree and n = 4 for soybean) is shown.
Table I. Profiles of root epidermal cells in the proliferation of camphor tree and soybean.
Cell numbers and distances from the tip at each origin of propagation event for PA cells and epidermis cells were summarized. The origin of cell expansion was represented by the first five neighboring cells whose lengths were increased in a consecutive order. The origin of arrest of soybean epidermis cells at 25 μm Al was represented by the first cell whose length was more than 20 μm different from its neighboring cells. Al concentrations and treatment periods for both species were the same as those described in Figure 8. Results show means ± sd (n = 5 for camphor tree; n = 4 for soybean).
| Treatment | PA Cell |
Epidermis Cell |
||||
| Total No. | Emergence | Disappearing | Expansion | Expansion | Expansion/Arrest (Soybean) | |
| mm from the tip | ||||||
| −Al | 142 ± 53 | 0.92 ± 0.18 | 3.61 ± 0.71 | 1.61 ± 0.52 | 2.08 ± 0.34 | 2.87 ± 0.34 |
| +Al | 149 ± 35 | 1.04 ± 0.18 | 2.80 ± 0.56 | 2.02 ± 0.29 | 2.30 ± 0.36 | 2.91 ± 0.24 |
When camphor tree roots were exposed to 500 μm Al for 48 h, the consecutive increase in PA cell length started at the 2-mm tip region with a 0.4-mm delay form in control, reaching 20 to 40 μm in length around the 3-mm region (Fig. 8C; Table I). Following this increase, Al-facilitated detachment of PA cells accelerated the termination of the profile at the region about 0.8 mm closer to the tip. Meanwhile, exposure to 500 μm Al almost did not change the profile of epidermis cell length compared with control. At 500 μm Al, the start of expansion of the epidermis cell around the 2.5-mm region was layered with PA cells of an undestroyed, expanding state.
In a control root of soybean, the lengths of lateral root cap cells at the innermost cap immediately exceeded over 20 μm within the 1-mm region and those of epidermis cells began to increase around the 3-mm region behind the tip (Fig. 8B). In a soybean root exposed to 25 μm Al for 8 h, the lengths of lateral root cap cells were also increased from the beginning of the lineage (Fig. 8D). Meanwhile, the exposure to Al discontinued a consecutive increase in the lengths of epidermis cells around the 3-mm region, eliminating the cell lengths between 20 and 40 μm from the profile (Fig. 8D; Table I).
PA Component Analysis
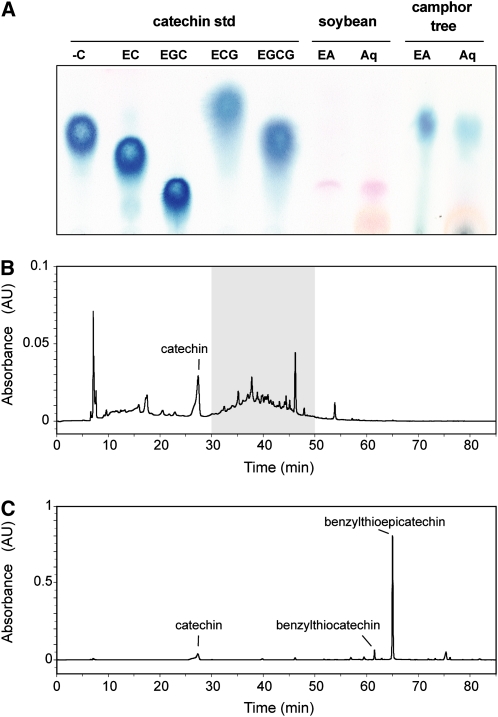
We analyzed the components of PA in the root apices (0- to 5-mm section) of camphor tree and soybean by using thin-layer chromatography (TLC). After extraction with 70% (v/v) acetone, root extracts were separated into an ethyl acetate fraction (enriched with PA monomers and oligomers) and an aqueous fraction (enriched with PA polymers). In camphor tree root apices from the 0- to 5-mm section, spraying with DMACA reagents revealed spots that share similar Rf (retardation factor) values with that of (−)-catechin in both the ethyl acetate and aqueous fractions (Fig. 9). Meanwhile, no blue color development was detected in the root apices of soybean. The exposure of the camphor tree roots to 500 μm Al for 48 h hardly changed the patterns and intensity of spots in both fractions from the 0- to 5-mm region of root apices (data not shown).
Figure 9.
Analysis of PA components in root apices. Root (0- to 5-mm section) extracts in 70% (w/v) acetone were partitioned into ethyl acetate (EA; for PA precursors and oligomers) or aqueous (Aq; for PA polymers) fractions. A, TLC of PA components in the root apices of soybean and camphor tree. The spots were detected with the DMACA reagent. Flavan-3-ol standards are indicated as follows: −C, (−)-catechin; EC, epicatechin; EGC, epigallocatechin; ECG, epicatechin gallate; EGCG, epigallocatechin gallate. B, HPLC chromatogram of products in the aqueous fraction from camphor tree root apex before thiolytic degradation. The gray area represents a baseline drift presumably due to the elution of PA polymer. C, HPLC chromatogram of degraded products in the aqueous fraction from camphor tree root apex after thiolysis. The names of peaks identified with ESI-MS are indicated.
Using liquid chromatography/electrospray ionization-mass spectrometry (LC/ESI-MS) in the negative ion mode, we further analyzed the composition of PAs in root apices of camphor tree after thiolysis degradation with benzyl mercaptan. Before the aqueous fraction of camphor tree root apices (0–5 mm) was subjected to thiolysis treatment, a major peak and a broad baseline drift were detected in the HPLC chromatogram (Fig. 9B). MS spectra and the standard confirmed that the major peak was catechin (mass-to-charge ratio [m/z] = 289). Because of its disappearance by the thiolysis treatment, the baseline drift during the elution period from 30 to 50 min was assumed to be derived from the elution of PA polymer. After the thiolysis, degraded products of PAs in camphor tree root apices provided three major peaks (Fig. 9C). The first peak was identical to catechin. MS spectra and the thiolysis degradation of a standard procyanidin B-type dimer identified that the highest peak is benzylthioepicatechin (m/z = 447) and another peak is likely to be benzylthiocatechin isomers (m/z = 447). These results indicate that the major PAs in the root apices of camphor tree were composed of epicatechin for the extension unit and catechin for the terminal unit. A value for mean degree of polymerization of PAs was 8.89 ± 0.84 (n = 2) for the 0- to 5-mm section of root apex in camphor tree, based on the molar ratio between benzylthioepicatechin and the increased amount of catechin. We also analyzed the 5- to 10-mm region of camphor tree root apices and found that the composition of PAs and its mean degree of polymerization values were largely the same between the 0- to 5-mm section and the 5- to 10-mm section (10.59 ± 1.41; n = 2).
Flavan-3-ol Bioassay
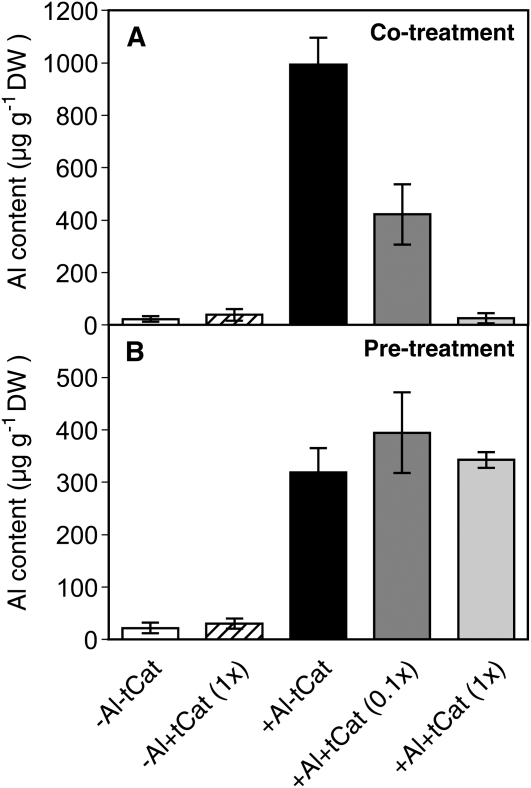
To estimate whether PAs reduce the toxicity of Al around the root, the effects of exogenous application of falvan-3-ols from tea (Camellia sinensis) leaves on the Al-induced root elongation inhibition were studied. Our preliminary experiment revealed that tea flavan-3-ols in 500 μm calcium chloride solution (pH 4.5) inhibit root elongation in soybean in a concentration-dependent manner and by 50% during 24 h of exposure at 57.5 mg L−1. This dose level was equivalent to the concentrations of each PA monomer ranging from 14.3 μm (epicatechin) to 74.2 μm (epigallocatechin gallate). The addition of a total of 57.5 mg L−1 flavan-3-ol mixtures to the calcium chloride solution (pH 4.5) containing 25 μm Al prevented the root apex from accumulating Al (Fig. 10A). The addition of tea flavan-3-ol mixtures of a 1:10 dilution also reduced the Al accumulation to almost a half level. In contrast, exposure to the flavan-3-ol mixtures prior to the exposure to 25 μm Al every 6 h had little effect in reducing Al accumulation (Fig. 10B). The addition of tea flavan-3-ol mixture to the Al solution or treatment starting with them followed by Al had little offsetting effect on the root elongation inhibition caused by either of them (Supplemental Fig. S4). These results suggest that PA monomers are effective in perturbing Al accumulation at the outside of roots.
Figure 10.
Effect of exogenous application of PA components from tea leaves on the Al accumulation in the root apex of soybean. Soybean roots were exposed to 500 μm calcium chloride solution (pH 4.5) with or without 25 μm Al and 57.5 mg L−1 of a mixture of flavan-3-ol extract. Flavan-3-ol concentrations in the extracts were equivalent to 14.3, 40.6, 17.8, and 74.2 μm for epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate, respectively. A, Al contents in soybean root apices after 24 h of treatment with Al [+Al-tCat], flavan-3-ols [−Al+tCat (1x)], or both [+Al+tCat (1x) and +Al+tCat (0.1x)]. B, Al contents in soybean root apices after 24 h of single treatment with Al [+Al-tCat] or flavan-3-ols [−Al+tCat (1x)] every 6 h or after mutual treatments with Al and flavan-3-ols [+Al+tCat (1x) and +Al+tCat (0.1x)] every 6 h. Data represent means ± sd (n = 6). DW, Dry weight.
Al-Induced Root Browning
To evaluate whether PAs are involved in oxidative stress induced by Al, the spatial distribution of root browning in camphor tree during Al treatment was investigated. After 48 h of Al treatment, root browning and surface cracks, especially between 2 and 6 mm from the tip, were developed in a manner dependent on Al concentration (Supplemental Fig. S5A). Interestingly, prolonged exposure to 500 μm Al for 10 d attenuated the novel browning formation and lightened the band formed in the early exposure period (Supplemental Fig. S5B). Meanwhile, exposure to 500 μm Al every 2 d caused a ladder-like pattern of root browning. These results suggest that the inducible root browning may be restrictive in the early response to Al.
DISCUSSION
In this study, we verified that the camphor tree roots exhibit an extraordinary Al resistance even though they release only modest amounts of citrate. Al-inducible citrate release from camphor tree at 500 μm Al is much lower than that from soybean at 25 μm Al, while root elongation is only strongly inhibited in the latter (Figs. 1 and 4). It is well known that Al rapidly inhibits root elongation in many plants, accumulating only in the surface region of the root apex. Therefore, we focused on the proliferative properties of outer radial cells at the root apex of camphor tree to understand its high Al resistance mechanism. Our histochemical analyses showed that unique PA-accumulating cells constitute the outer adjacent layer of epidermis cells in camphor tree root apices. The distribution of the PA cells is limited to a region 1 to 4 mm behind the tip, which corresponds to the entire root elongation zone (Figs. 2 and 6). The profiling of cell length revealed that the PA cell lineage has distinct zones for cell division and the early phase of cell expansion (Figs. 7 and 8). The expanding PA cells are detached in the 3- to 4-mm region behind the tip; their debris of less than 2 μm width covered the outer surface of epidermis cells at the 5-mm region thereafter (Fig. 7D). Exposure to Al at high concentration (500 μm) affects neither cell division nor early expansion in PA cells, except for a slight delay in the transition between the two phases; besides, the PA cells at the early expansion phase cover the inner epidermis cells that are transitioning from division to expansion (Fig. 8C). Although Al promotes the disturbing of PA cells at the end of rapid expansion, their susceptible region is located outside the region of root elongation in camphor tree (Figs. 2, 7, and 8). From these results and further discussion below, we propose that PA cells at the root surface could be effective in shielding the expansion of inner cells against Al. To our knowledge, this is the first detailed identification of PA accumulation in plant roots that are genetically unmodified.
In this study, we confirmed that Al inhibits root elongation in soybean, especially at the cell expansion zone (Figs. 2 and 8D). Our cell profiling in soybean root apex revealed that Al inhibits the expansion of epidermis cells through two modes of action at different steps: (1) the arrest of the phase transition in initiating cell expansion; and (2) cell disturbance after the completion of rapid expansion. In terms of step 1, our results further support the previous finding that the distal part of the cell transition zone is a critical target for Al in the inhibition of root elongation in maize (Zea mays; Sivaguru et al., 1999). The cell disturbance in step 2 is based on our finding of an irregular distribution of cell size after exposure to Al and the formation of cracks in the region 5 mm away from the tip (Fig. 7F). In contrast, cell profiling in camphor tree revealed that the proliferation of root surface cells is highly Al tolerant due to the combination of the facts that (1) the expansion of PA cells is only modestly arrested by Al at the initiation of expansion but disturbed at the end of early expansion; (2) the initiation of epidermis cell expansion is covered with expanding PA cells even in the presence of Al; and (3) the expanding epidermis cells succeed to the root surface in accordance with the Al-accelerated detachment of PA cells. Our results demonstrating that cell proliferation is followed by the immediate detachment of PA cells suggest that PA cells may have intermediate cell properties between the epidermis and lateral root cap. The synchronized proliferation of PA cells, as pseudoepidermis cells, may protect the expansion of the inner epidermis cells away from Al. Following this, the constant detachment of PA cells, akin to lateral root cap cells, could facilitate the replacement of the Al-damaged outer cells by the expanding epidermis cells. A shield effect of PA cells in the expansion of the inner epidermis cells is supported by the previous idea that crack formation in the early stage of Al toxicity is presumably caused by the differential expansion between Al-arrested epidermis cells and still-expanding cortex cells (Yamamoto et al., 2001; Motoda et al., 2010).
In this study, less Al accumulation was maintained at the root apex of camphor tree despite exposure to 500 μm Al for 8 d (Fig. 5). We also found that the coapplication of tea flavan 3-ols and Al effectively reduced Al accumulation in root apices of soybean (Fig. 10). This alleviating effect was concentration dependent, and the pretreatment starting with flavan 3-ols followed by Al failed in alleviating Al accumulation. These results support the idea that PAs may perturb the Al binding to root cells through complex formation. The monomeric catechin, one of the major PA components, has a higher stability constant for Al than the catechin dimer (Yokel, 1994; Barceló and Poschenrieder, 2002). PAs can form complexes with Al, depending on the degree of polymerization and the number of hydroxyl bases (Yoneda and Nakatsubo, 1998). Excretion of flavonoid-type phenolics, including catechin, is increased in an Al-resistant maize cultivar after treatment with both Al and silicon (Kidd et al., 2001). Root apices of the maize cultivar may also mediate Al resistance through chelating capacities of phenolic compounds such as cyclic hydroxamates and taxafolin (Poschenrieder et al., 2005; Tolrà et al., 2009). In the root apex of camphor tree, PAs are localized to the cell periphery during the cell expansion. Our HPLC analysis confirmed that neither flavan-3-ols nor PA polymers are detected in the anion-exchange resin passed through the root-bathing Al solution (data not shown), although the increased leaching of PAs from the destroyed cells is expected. PAs are known to accumulate for longer periods in seed testa, bark cork cells, and wooden xylem cells (Lepiniec et al., 2006). In this study, PAs were retained in the cell debris attached to the surface of basal roots, supporting the hypothesis that PAs might function in the apoplast for the initiation of cell expansion at high toxic levels of Al, possibly interacting with cell wall components such as pectins. Root apoplast is an exclusive site for the Al accumulation (Taylor et al., 2000), and Al accumulates in cell wall pectin, which depends on its degree of methylation (Chang et al., 1999; Ethicha et al., 2005). Previous studies also revealed that interaction of PAs with pectins (Taira et al., 1997; Kennedy et al., 2001).
As shown in Figure 10 and Supplemental Figure S4, an inhibitory concentration of tea flavan-3-ol mixture is required for the suppression of Al accumulation in the root apex of soybean. In the root apex of camphor tree, temporal accumulation and immediate discharge of PAs during cell detachment seem to be suitable for the use of the toxic substances in reducing Al accumulation at the necessary position. In addition, PA cell detachment, which begins after the start of expansion of the inner epidermis cells, could play a significant role in the removal of Al from the root surface (Figs. 7A and 8C). A slight but continuous induction of citrate release may partially contribute to the exclusion of Al in the camphor tree roots (Fig. 4C).
Our cell profiling revealed that the TBO-reactive compounds of PA cells are present in the entire region of dividing cells and then are less localized to the developing vacuoles during expansion (Fig. 7, B and C). In contrast with the previous finding that PA precursors are transported and then polymerized in vacuoles in Arabidopsis seeds (Debeaujon et al., 2001; Kitamura et al., 2004), our results showed that PAs and their components are predominant in cytosol and are less sequestered in the vacuoles during the cell proliferation in camphor tree. The AtTT12 protein, which belongs to the multidrug and toxic compound extrusion transporter family, mediates the transport of flavan-3-ol monomers into the vacuole in seed coat of Arabidopsis (Debeaujon et al., 2001). Insufficient substrate sequestration into the vacuoles or increased PA polymerization in cytosol or both may be a factor that restricts PA accumulation in the developing vacuoles at the root apices of camphor tree. Our TLC analysis revealed that the PA composition and the mean degree in polymerization of PAs are almost the same in the root sections between 0 to 5 mm and 5 to 10 mm, suggesting that PA polymerization could be a rapid response at the root apex of camphor tree.
We found that continuous root elongation of camphor tree accompanied a transient Al-induced root browning, which mainly occurs behind the root expansion zone (2–6 mm; Supplemental Fig. S5). Interestingly, we also found that prolonged exposure to Al (72 h or more) attenuates the browning formation and lightened its trace and that exposure to Al every 2 d produces a ladder-like pattern of root browning. These results suggest that PAs in the expanding cells prior to Al exposure may be a major substrate for the Al-induced root browning. Since Al intensifies the detachment of the PA cells and eliminates the coat of cell debris onto the root surface (Fig. 7A), these changes may result in the attenuation of Al-induced root browning. Vise versa, these results also support the idea that PAs are involved in the Al-induced oxidative damage during cell detachment. An intensive root browning in the entire root apices of camphor tree is induced by Cu or La at similar concentrations to the inhibitory thresholds for wheat (Fig. 3). It is unlikely that PA is effective in the universal protection of oxidative damage by metal stress; although our results do not exclude the possibility that the localization of PAs in the root surface may be spatially effective against the oxidative stress caused by Al, which is less invasive to the inside of the root than Cu or La.
In camphor tree, Al-inducible cracks are mainly formed at the 2- to 6-mm region behind the root apex (Supplemental Figs. S2 and S5), presumably due to the accelerated destruction of PA cells. The Al-induced crack formation would have less impact on the root elongation in camphor tree, since the corresponding region is almost outside of the region of root elongation and the stepwise increase in epidermis cell length is less disturbed (Figs. 2 and 8C). The Al-accelerated destruction of PA cells may be mitigated by the slow cell expansion in the 3- to 5-mm region, presumably due to the small number of cells transitioning into the expansion zone. In contrast, a moderate increase in epidermis cell length in the 3- to 5-mm region behind the tip accounted for almost half of the root elongation in soybean, implying that cells with a faster moving rate might enter into the expansion zone. Our histochemical analysis of soybean roots revealed that Al arrests the expansion of epidermis cells that are covered by at least one layer of lateral root cap cells. Apart from PA cells, most of the lateral root cap cells in camphor tree and soybean are derived from the large cells lacking apparent cell division nearest to the tip (Fig. 7, B and F). The apoplast of enlarged lateral root cap cells, whose intercellular connections are supposed to be weak for instant detachment, might be susceptible to the permeation of Al with less reduction of its toxicity. This assumption is supported by the previous finding that root cap cells play a minor role in Al-tolerance mechanisms (Ryan et al., 1993). Border cells are peripheral root cap cells that detach off the root as living cells immediately when they are exposed to solution (Hawes et al., 1998). Based on their intensive cell propagation and spatial difference in detachment, PA-accumulating cells appear to mediate a novel type of Al resistance in border-like cells, possibly different from a mechanism of Al resistance induced by pectin production in border cells of snapbean (Phaseolus vulgaris; Miyasaka and Hawes, 2001).
Our result showing that Al enhances the destruction of PA cells in the midst of expansion suggests that PA cells are likely to be highly Al tolerant until the start of expansion, although the detailed role of PAs in the initiation of cell expansion remains unclear. The Al tolerance of a forage legume species appears to be related to colocalization of Al and PAs in vacuoles (Stoutjesdijk et al., 2001). However, this possible mechanism may not account for the high Al resistance mechanism in camphor tree, since an apparent lack of PAs in vacuole sequestration is observed during cell expansion (Fig. 7C). We now have two unanswered questions: (1) the mechanism by which PA cells are able to start their expansion against high concentrations of Al; and (2) the detailed function by which PA cells protect the initiation of the expansion of inner epidermis cells away from Al. A better understanding of how Al arrests cell expansion would also help solve these questions. As seen in Figure 7A, the distribution of PA cells in camphor tree roots may spatially correspond to the region where the basipetal auxin flow occurs (Kollmeier et al., 2001). Certain PA-precursor flavonoids, including dihydrokaemphenol and naringenin, regulate root gravitropism by modulating the bapsitel auxin flow (Brown et al., 2001; Blakeslee et al., 2007; Santelia et al., 2008). We tentatively speculate that high PA accumulation in root cells might be related to the regulation of auxin flow to initiate their expansion in a sort of autonomous manner against Al. Examining other possible roles of PA in pathogen defenses and plant-microbe interactions could also help understand the detailed functions of PA in root growth.
In conclusion, we have shown that continuous root elongation of camphor tree in the presence of high Al concentrations is presumably based on the combination of cell proliferation at the two epidermal layers. Outer PA-accumulating cells that constitutively proliferate until the end of early expansion may shield the expansion of inner epidermis cells against highly toxic Al. Then, the abrupt detachment of the PA cells could help replace the Al-damaged cell surfaces with the expanding epidermis cells. The proliferation followed by the detachment of PA cells that harbor epidermis cells in root apex may develop a novel strategy for enhancing long-term and high Al resistance, both of which are essential for perennial plants to grow in nonneutralized, strongly acidic soils.
MATERIALS AND METHODS
Plant Culture
Fresh camphor tree (Cinnamomum camphora) berries were collected from under a mother tree in Kamogawa, Chiba, Japan. The seeds were removed from the fruits and stored at 4°C until use. For scarification, the endocarps were removed with a razor blade, and the seeds were sown in moistened vermiculite soil at 25°C. Three- or 4-week-old seedlings were transferred and hydroponically cultured with one-fifth-strength Hoagland solution for another 2 months. In camphor tree, the longest roots (approximately 100 mm in length) among the newly developed roots during the 2 months of solution culture were used for root elongation measurements. The camphor tree seedlings were exposed to 500 μm calcium chloride solution (pH 4.5) for 2 d before the Al treatment. Some of the camphor tree samples for the analysis of organic anion release and Al accumulation were prepared with less than 1-year-old seedlings with roots that were newly developed after the cutting of all Al-exposed roots. There were no significant differences in relative root elongation (P > 0.05, n = 21) and PA accumulation patterns at 500 μm Al between the virgin seedlings and reused ones.
Seeds of soybean (Glycine max) cv Wase-hakucho were purchased from Takii Co. The seeds were soaked in 30% (w/v) polyethylene glycol 6000 solution for 24 h before germination in wetted silica sand at 25°C. The 5-d-old seedlings were transferred to 500 μm calcium chloride solution (pH 4.5) to allow the roots to elongate for an additional 1 d. Seeds of wheat (Triticum aestivum cv Atlas) were kindly provided by Dr. Yoko Yamamoto (Okayama University). The 6-d-old wheat seedlings were prepared according to previously described conditions (Osawa and Matsumoto, 2001). All seedlings were grown in a growth room with a 14-h/10-h light/dark cycle (at 150 μmol m−2 s−1 photosynthetic photon flux density) at 28°C (light) and 22°C (dark).
Root Elongation Analysis
For Al treatment, roots of each species were exposed to different concentrations of Al in 500 μm calcium chloride solution (pH 4.5). The Al concentrations were 0, 50, 100, 200, or 500 μm Al for camphor tree, 0, 10, 25, 50, or 500 μm for soybean, and 0, 50, 200, or 500 μm for wheat. To minimize the formation of Al complexes in the treatment solution, 10 and 100 mm Al chloride solutions were freshly prepared by dissolving in 0.1 n HCl solution.
After the addition of either of the Al solutions into 500 μm calcium chloride solution (pH 4.5), the pH was readjusted to 4.5 with 0.1 n HCl or NaOH solution. Each Al solution was exchanged every 12 h for the measurement of organic anion release or every 2 d when plants were exposed to Al over 2 d. For Cu treatment, roots of camphor tree were exposed to 500 μm calcium chloride solution (pH 4.5) containing 0.1% (w/v) sodium ascorbate and 0, 1, 2, 3, or 4 μm Cu sulfate. For La, the roots were exposed to 500 μm calcium chloride solution (pH 4.5) containing 0, 50, or 500 μm La chloride. The root lengths before and after each treatment were measured with a ruler.
To determine the spatial zones for root elongation, root apices were marked with India ink at 0.5-mm intervals from the tip using a fine paintbrush with a stereomicroscope. The root apices before and after Al treatment were photographed; the length of each interval in the photograph was measured with a ruler. The growth increment for each 0.5-mm section was adjusted based on a factor of the actual length of each interval before treatment.
Measurement of Organic Anion Efflux
To collect organic anions in the root-bathing solution, 15 and 20 seedlings each for camphor tree and soybean, respectively, were transferred to a beaker containing 2 L of the calcium chloride solution (pH 4.5) with Al at the respective concentrations. At the end of every 12-h period of exposure to the root, the root-bathing solution was passed through Amberlite 500 (200–400 mesh, H+ form) and Dowex 1X8 (100–200 mesh, Cl− form) for the recovery of cations and anions, respectively. The organic anions retained in the anion-exchange resin were eluted with 8 n formic acid, evaporated to dryness with a rotary evaporator, and dissolved into distilled water. Organic anions were determined with a Shimadzu LC-6A HPLC system with an electroconductivity detector (Shimadzu) according to the analytical conditions described previously by Osawa and Kojima (2006). The number of tips in the photocopied root images was counted with image-analysis software (WinRHIZO; Regent Instrument).
Histochemical Analysis
Roots were excised in 10-mm lengths from the tip and fixed with FAA. After an exchange with 100% ethanol, roots were embedded with Technovit 7100 glycol methacrylate (Heraeus Kulzer). The embedded roots were sliced (5-μm thickness) along the root elongation direction with a Leica RM2125 rotary microtome. The sliced tissue was placed on a slide glass and stained with 1% (w/v) TBO for 10 min at 50°C and washed three times with deionized water. Root sections were photographed at a 100-fold magnification with a light microscope.
For the profiling of the PA cell and inner epidermis lineage, the length and angles of the cells from the proximal end of the quiescent center to the region 5 mm away from the tip were measured with ImageJ 1.42 software. The inclined length of each root cell at its middle axis (longitudinal direction) and its angles to the main axis of primary roots were measured; then, the cell length along the main axis of the root was calculated by multiplying the inclined length and the sine angle.
To detect PA localization, excised roots were immersed in 2% (w/v) vanillin in 6 n HCl for 2 min. The roots were photographed within minutes in the presence of 6 n HCl to avoid decolorization. For DMACA staining, excised roots were immersed in 1% (w/v) DMACA in a 3 n HCl and 50% (v/v) methanol solution for 2 min.
TLC Analysis
For the analysis of PA components, root apices were divided into two sections (0–5 and 5–10 mm) and stored at −80°C until use. Each root section was ground into powder in liquid N2 with a motor and pestle. The powder was extracted overnight with 70% (v/v) acetone containing 2% (v/v) mercaptoethanol at 4°C. After centrifugation at 3,000 rpm for 15 min, precipitates were again extracted with fresh acetone solution for 2 h. After combining each extract, a fraction enriched in both PA monomers and oligomers was partitioned with ethyl acetate, and the remaining aqueous solution was designated as the PA polymer fraction. After extraction with chloroform and then hexane, each fraction was evaporated in a vacuum centrifuge and the residues were dissolved in 100 μL of 0.37% (v/v) HCl in methanol. For TLC analysis, 10 μL of each sample was spotted onto a silica gel 60 F254 glass plate (Merck) and equilibrated with a solvent (s-butanol, water, acetic acid, and chloroform [70:20:10:10, v/v/v/v]). After drying, the plate was sprayed with a 20-fold dilution of the DMACA reagent in methanol. The plate image was obtained by using an EPSON 1680 scanner.
Thiolysis
For thiolysis analysis, dried aqueous fractions were dissolved into 70% (v/v) methanol containing 0.1% (v/v) trifluoroacetic acid (TFA) and loaded onto Sephadex LH 20 (GE Healthcare). After washing with 30% (v/v) methanol containing 0.1% TFA, PAs were eluted with 70% (v/v) acetone containing 0.1% TFA. After vacuum centrifugation, the residue was dissolved into 20 μL of 3.3% (v/v) HCl in methanol and added with 20 μL of 5% (v/v) benzyl mercaptan in methanol. After gentle vortexing, the sample mixture was incubated at 40°C for 30 min and then incubated for 10 h at room temperature. The sample mixture was kept in a freezer (−20°C) until use.
Flavan-3-ols and thiolytic degraded compounds in the reaction mixture were determined with reverse-phase HPLC in a Shimadzu QP 8000 LC-MS system with a 3-μm Cadenza CD-C18 column (Imtakt). The detection wavelength of the UV light detector was set to 280 nm. Mobile phase solutions were as follows: A, 2% (v/v) acetic acid; B, methanol; and C, 10 mm ammonium acetate. The gradient was set as follows: 0 to 2 min, 15% to 20% B; 2 to 20 min, 20% isocratic B; 20 to 70 min, 20% to 80% B; and 70 to 85 min, 80% to 15% B. A constant 4% (v/v) of C was teed just before entry into the MS detector. An ESI probe with negative ion mode was employed to identify the flavan-3-ols and degraded PA products. Purified procyanidin B1-type dimer (epicatechin-[4β-8]-catechin; the 4→8 bond in the beta position; Tokiwa Phytochemical) was used as a standard for the identification of benzylthioether flavan-3-ols. The mean degree in polymeralization of PAs in each sample was estimated from the molar ratios of catechin and benzylthioepicatechin.
Al Quantification
To quantify Al content in root apices, roots at each Al treatment period were briefly washed with 500 μm calcium chloride solution (pH 4.5) and then with 10 mm citric acid in the calcium chloride solution for 10 min. After three washes with the calcium chloride solution, the roots were sectioned into 5-mm segments from the tip. Each of the 40 root segments from the 0- to 5-mm, 10- to 15-mm, and 20- to 25-mm sections was dried at 80°C for 24 h and wet ashed in a mixture of 60% (w/v) nitric acid and 60% (w/v) hydroperchlorite solution at 140°C. Al concentrations in the diluted solutions were determined with a graphite furnace atomic absorption spectrophotometer (SIMAA 6000; Perkin-Elmer).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Contents of organic anions in root apices of camphor tree and soybean.
Supplemental Figure S2. Localization of Al in roots of camphor tree.
Supplemental Figure S3. PA accumulation in roots of camphor tree.
Supplemental Figure S4. Effect of exogenous application of PA components from tea leaves on the amelioration of Al-induced inhibition of root elongation in soybean.
Supplemental Figure S5. Al-induced root browning in camphor tree roots.
Supplementary Material
Acknowledgments
We are grateful to Hiroyuki Ikeda and Takayuki Sasaki for providing seeds of camphor tree and wheat, respectively.
References
- Barceló J, Poschenrieder C. (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48: 75–92 [Google Scholar]
- Blakeslee JJ, Bandyopadhyay A, Lee OR, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J, et al. (2007) Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 19: 131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK. (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YC, Yamamoto Y, Matsumoto H. (1999) Accumulation of aluminium in the cell wall pectin in cultured tobacco (Nicotiana tabacum L.) cells treated with a combination of aluminium and iron. Plant Cell Environ 22: 1009–1017 [Google Scholar]
- Debeaujon I, Léon-Kloosterziel KM, Koornneef M. (2000) Influence of the testa on seed dormancy, germination, and longevity in Arabidopsis. Plant Physiol 122: 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJ, Léon-Kloosterziel KM, Koornneef M. (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13: 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. (2004) Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proc Natl Acad Sci USA 101: 15249–15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Randall PJ. (1993) Aluminum tolerance in wheat (Triticum aestivum L.). II. Aluminum-stimulated excretion of malic acid from root apices. Plant Physiol 103: 695–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethicha D, Stass A, Horst WJ. (2005) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28: 1410–1420 [Google Scholar]
- Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF. (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48: 1081–1091 [DOI] [PubMed] [Google Scholar]
- Hawes MC, Brigham LA, Wen F, Woo HH, Zhu Y. (1998) Function of root border cells in plant health: pioneers in the rhizosphere. Annu Rev Phytopathol 36: 311–327 [DOI] [PubMed] [Google Scholar]
- Horst WJ. (1995) The role of the apoplast in aluminum toxicity and resistance of higher plants: a review. Z Pflanzenernahr Bodenkd 158: 419–428 [Google Scholar]
- Jones DL, Prabowo AM, Kochian LV. (1996) Kinetics of malate transport and decomposition in acid soils and isolated bacterial populations: the effect of microorganisms on root exudation of malate under Al stress. Plant Soil 182: 239–247 [Google Scholar]
- Kennedy JA, Hayasaka Y, Vidal S, Waters EJ, Jones GP. (2001) Composition of grape skin proanthocyanidins at different stages of berry development. J Agric Food Chem 49: 5348–5355 [DOI] [PubMed] [Google Scholar]
- Kidd PS, Llugany M, Poschenrieder C, Gunsé B, Barceló J. (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52: 1339–1352 [PubMed] [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A. (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37: 104–114 [DOI] [PubMed] [Google Scholar]
- Kochian LV, Hoekenga OA, Piñeros MA. (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55: 459–493 [DOI] [PubMed] [Google Scholar]
- Kollmeier M, Dietrich P, Bauer CS, Horst WJ, Hedrich R. (2001) Aluminum activates a citrate-permeable anion channel in the aluminum-sensitive zone of the maize root apex: a comparison between an aluminum-sensitive and an aluminum-resistant cultivar. Plant Physiol 126: 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M. (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57: 405–430 [DOI] [PubMed] [Google Scholar]
- Ma JF, Ryan PR, Delhaize E. (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6: 273–278 [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimarães CT, Lana UG, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, et al. (2007) A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39: 1156–1161 [DOI] [PubMed] [Google Scholar]
- Miyasaka SC, Hawes MC. (2001) Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol 125: 1978–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H. (2010) Morphological changes in the apex of pea roots during and after recovery from aluminium treatment. Plant Soil 333: 49–58 [Google Scholar]
- Nesi N, Jond C, Debeaujon I, Caboche M, Lepiniec L. (2001) The Arabidopsis TT2 gene encodes an R2R3 MYB domain protein that acts as a key determinant for proanthocyanidin accumulation in developing seed. Plant Cell 13: 2099–2114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda A, Yamamoto F. (2002) Effects of aluminum on growth and biomass allocation of hydroponically-cultured Quercus acutissima, Cinnamomum camphora and Eucalyptus viminalis seedlings. J Tree Health 6: 99–103 [Google Scholar]
- Osawa H, Kojima K. (2006) Citrate-release-mediated aluminum resistance is coupled to the inducible expression of mitochondrial citrate synthase gene in Paraserianthes falcataria. Tree Physiol 26: 565–574 [DOI] [PubMed] [Google Scholar]
- Osawa H, Matsumoto H. (2001) Possible involvement of protein phosphorylation in aluminum-responsive malate efflux from wheat root apex. Plant Physiol 126: 411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa H, Matsushima Y, Tange T. (2009) Long-term and highly aluminum-resistant root elongation in a camphor tree Cinnamomum camphora. The Proceedings of the International Plant Nutrition Colloquium XVI. http://www.escholarship.org/uc/item/5x71985r (August 28, 2009) [DOI] [PMC free article] [PubMed]
- Pang Y, Peel GJ, Sharma SB, Tang Y, Dixon RA. (2008) A transcript profiling approach reveals an epicatechin-specific glucosyltransferase expressed in the seed coat of Medicago truncatula. Proc Natl Acad Sci USA 105: 14210–14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Murphy AS. (2007) Flavonoids and auxin transport: modulators or regulators? Trends Plant Sci 12: 556–563 [DOI] [PubMed] [Google Scholar]
- Poschenrieder C, Tolrà RP, Barceló J. (2005) A role for cyclic hydroxamates in aluminium resistance in maize? J Inorg Biochem 99: 1830–1836 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK. (2000) Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiol 122: 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan PR, Delhaize E, Jones DL. (2001) Function and mechanism of organic anion exudation from plant roots. Annu Rev Plant Physiol Plant Mol Biol 52: 527–560 [DOI] [PubMed] [Google Scholar]
- Ryan PR, Ditomaso JM, Kochian LV. (1993) Aluminium toxicity in roots: an investigation of spatial sensitivity and the role of the root cap. J Exp Bot 44: 437–446 [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, et al. (2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem 283: 31218–31226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. (2004) A wheat gene encoding an aluminum-activated malate transporter. Plant J 37: 645–653 [DOI] [PubMed] [Google Scholar]
- Sivaguru M, Baluška F, Volkmann D, Felle HH, Horst WJ. (1999) Impacts of aluminum on the cytoskeleton of the maize root apex: short-term effects on the distal part of the transition zone. Plant Physiol 119: 1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoutjesdijk PA, Sale PW, Larkin PJ. (2001) Possible involvement of condensed tannins in aluminium tolerance of Lotus pedunculatus. Aust J Plant Physiol 28: 1063–1074 [Google Scholar]
- Tahara K, Norisada M, Hogetsu T, Kojima K. (2005) Aluminum tolerance and aluminum-induced deposition of callose and lignin in the root tips of Melaleuca and Eucalyptus species. J For Res 10: 325–333 [Google Scholar]
- Taira S, Ono M, Matsumoto N. (1997) Reduction of persimmon astringency by complex formation between pectin and tannins. Postharvest Biol Technol 12: 265–271 [Google Scholar]
- Takano T, Murakami T, Kamitakahara H, Nakatsubo F. (2008) Mechanism of formaldehyde adsorption of (+)-catechin. J Wood Sci 54: 329–331 [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. (2000) Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol 123: 987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier N, Torregrosa L, Ageorges A, Vialet S, Verriès C, Cheynier V, Romieu C. (2009) Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine and suggests additional targets in the pathway. Plant Physiol 149: 1028–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolrà R, Barceló J, Poschenrieder C. (2009) Constitutive and aluminium-induced patterns of phenolic compounds in two maize varieties differing in aluminium tolerance. J Inorg Biochem 103: 1486–1490 [DOI] [PubMed] [Google Scholar]
- Wasson AP, Ramsay K, Jones MG, Mathesius U. (2009) Differing requirements for flavonoids during the formation of lateral roots, nodules and root knot nematode galls in Medicago truncatula. New Phytol 183: 167–179 [DOI] [PubMed] [Google Scholar]
- Wenzl P, Patiño GM, Chaves AL, Mayer JE, Rao IM. (2001) The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol 125: 1473–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kobayashi Y, Matsumoto H. (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125: 199–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZM, Sivaguru M, Horst WJ, Matsumoto H. (2000) Aluminium tolerance is achieved by exudation of citric acid from roots of soybean (Glycine max L. Merr.). Physiol Plant 110: 72–77 [Google Scholar]
- Yokel RA. (1994) Aluminum chelation: chemistry, clinical, and experimental studies and the search for alternatives to desferrioxamine. J Toxicol Environ Health 41: 131–174 [DOI] [PubMed] [Google Scholar]
- Yoneda S, Nakatsubo F. (1998) Effects of the hydroxylation patterns and degrees of polymerization of condensed tannins on their metal-chelating capacity. J Wood Chem Technol 18: 193–205 [Google Scholar]
- Zheng S, Ma J, Matsumoto H. (1998) Continuous secretion of organic acids is related to aluminium resistance during relatively long-term exposure to aluminium stress. Physiol Plant 103: 209–214 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.