Low levels of maternal ghrelin in pregnancy program the development of the fetal uterus, compromising subsequent uterine function and the fertility of female offspring.
Abstract
Ghrelin has a well-known role in the regulation of appetite, satiety, energy metabolism, and reproduction; however ghrelin has not been implicated in reproductive tract development. We examined the effect of ghrelin deficiency on the developmental programming of female fertility. We observed that female wild-type mice born of ghrelin heterozygote dams (i.e. exposed in utero to ghrelin deficiency) had diminished fertility and produced smaller litters. We demonstrate that exposure to in utero ghrelin deficiency led to altered developmental programming of the reproductive tract. The number of ovarian follicles, corpora lutea, and embryos produced were identical in both exposed and unexposed mice. However wild-type embryos transferred to uteri of mice exposed to in utero ghrelin deficiency had a 60% reduction in the rate of embryo implantation compared with those transferred to wild-type unexposed uteri. We identified significant alterations in the uterine expression of four genes critical for implantation and a defect in uterine endometrial proliferation. Taken together, these results demonstrate that the mechanism of subfertility was abnormal endometrial function. In utero exposure to decreased levels of ghrelin led to defects in developmental programming of the uterus and subsequent subfertility in wild-type offspring.
Hormones involved in energy balance and metabolism, such as ghrelin, have been shown to regulate reproductive function in animals and humans (1, 2). Ghrelin functions as an endocrine/paracrine mediator that links energy homeostasis and physiological regulation of reproduction; however, ghrelin's role in development is not well characterized (3–5). Here we investigate the role of ghrelin in female reproductive tract developmental programming and subsequent fertility in the offspring.
Ghrelin is produced primarily in the stomach in response to hunger and starvation (6, 7). It circulates in the blood, serving as a peripheral signal to the central nervous system to stimulate feeding (2, 6–9). N-octanoyl-modified ghrelin has bioactivity whereas des-acyl ghrelin is thought to be inactive (10). Active ghrelin is an endogenous ligand for the GH secretagogue receptor type 1a (10). Production of ghrelin increases during fasting or in conditions of negative energy balance, and hence lean individuals have high serum ghrelin levels. Conversely, levels are typically low in obese individuals (11, 12). The role of ghrelin in appetite, satiety, energy metabolism, and reproduction is established; however, the impact of in utero exposure to ghrelin deficiency on the reproductive success of future generations has not previously been investigated (1).
Both ghrelin and its receptor are expressed in a wide variety of tissues, including reproductive organs such as the ovary, uterus, testes, and placenta and are thought to help regulate gonadotropin release and the timing of puberty (1, 13). Ghrelin is secreted in endometrial fluid, and levels rise in response to fasting (14). Ghrelin is expressed in both the uterus and ovaries throughout the rodent estrous cycle, reaching maximal levels in the luteal phase; ghrelin is thought to have a role in adult uterine function and endometrial decidualization (12–16). In humans, it has been established that ghrelin and its receptor are expressed in the testis, ovary, and placenta and is thought to contribute to the functional control of the reproductive axis and its integration with energy balance (1). There have been no reports describing a role for ghrelin in reproductive tract development.
Developmental programming describes the process by which a developing fetus undergoes permanent alterations or adaptations based on in utero exposures. These modifications are the mediators of gene-environment interaction in which genetic traits can be modified by an epigenetic response to the environment (17). Developmental programming provides plasticity to fetal gene expression in response to external stimuli, allowing for adaptation of fetal tissue to optimize survival after birth (18). However, adaptation to short-term adverse stimuli may alter gene expression in a way that impairs reproductive potential. Diethylstilbestrol is a well-known example of an agent affecting developmental programming of the reproductive tract; in utero exposure results in impaired reproductive potential of female offspring (19, 20).
We observed that wild-type progeny of ghrelin heterozygous dams had decreased fertility and smaller litter size when compared with wild-type progeny of wild-type dams. We hypothesized that exposure to ghrelin deficiency in utero would lead to altered developmental programming of the reproductive tract. Specifically, we determined that female offspring exposed in utero to ghrelin deficiency displayed subfertility as adults, and we isolated the defect to impairment of the uterine function. We identified epigenetic alterations in uterine gene expression that caused significant endometrial defects and impaired implantation.
Materials and Methods
Animal care
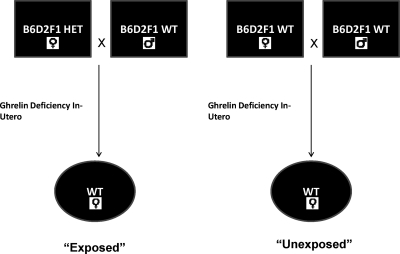
Ghrelin-deficient B6D2F1 mice and wild-type B6D2F1 were obtained from Regeneron. Phenotypic evaluation of ghrelin heterozygote and wild-type mice revealed no difference in weight, body mass index, or food intake. Adult wild-type B6D2F1 females exposed to ghrelin deficiency in utero (pups of ghrelin heterozygote dams) and wild-type females unexposed to ghrelin deficiency (pups of wild-type dams of the same strain) mice were obtained by breeding ghrelin heterozygote dams or wild-type dams, respectively, with wild-type males as demonstrated in Fig. 1 (n = 30 per group). Mice were housed in standard polypropylene cages in a temperature-controlled room (22 C) with a 14-h light, 10-h dark cycle. Food (Purina Chow, Purina Mills, Richmond, IN) and water were provided ad libitum. Wild-type female mice born from ghrelin heterozygotes dams, exposed to in utero ghrelin deficiency, are termed “exposed” (Fig. 1). They were mated with B6D2F1 male mice and analyzed for litter size. Female wild-type mice born from wild-type dams, termed “unexposed,” were similarly analyzed. All mice were genotyped after tail clipping using red extract standard genotyping protocol (Sigma Chemical Co., St. Louis, MO). All breeding was done on 6-wk-old mice, and only first pregnancy was analyzed in all studies except long-term breeding studies that were conducted over 16 months. All experiments were conducted in accordance with the Yale University Institutional Animal Care and Use Committee Guidelines.
Fig. 1.
Wild-type (WT) female mice born from ghrelin heterozygote (HET) dams, exposed to in utero ghrelin deficiency, are termed “exposed.” Female wild-type mice born from wild-type dams are termed “unexposed.”
Embryo collection and transfer
To evaluate embryo production, exposed and unexposed mice were mated with wild-type males; embryos were obtained via oviduct flushing. Separately, to evaluate term pregnancy survival rates, exposed and unexposed pseudopregnant females were mated with vasectomized males. Wild-type embryos were then transferred to both exposed and unexposed uteri. After a 17-d gestation, the mice were euthanized and litter sizes were tabulated. The birth rate per embryo transferred was calculated by comparing litter size on d 17 of pregnancy to the number of embryos that were transferred to each uterus.
Ghrelin assay
Mice were fasted for 24 h and euthanized and blood was drawn by cardiac puncture. Blood was collected in EDTA-containing tubes and kept at 4 C during processing. Samples were centrifuged at 3000 rpm for 30 min; hydrochloric acid and phenylmethylsulfonyl fluoride were immediately added to the plasma. Total and active plasma ghrelin levels were measured by RIA using kits purchased from LINCO Research (St. Charles, MO). This RIA kit uses a polyclonal antibody raised in rabbit against both octanoylated and des-octanoylated ghrelin and ghrelin as the tracer. The lower limit of detection was 80 pg/ml. Known concentrations were used to generate a standard curve. Acetylated (active) serum ghrelin levels were measured in 10 wild-type mice and 10 ghrelin heterozygotes. Because acetylated ghrelin has been widely shown to be biologically active, this study evaluated N-octanoyl-modified ghrelin.
Histological and immunohistochemical analysis
The ovaries from mice euthanized on d 4 of pregnancy were removed, fixed, sectioned, and stained with hematoxylin/eosin to evaluate the number of follicles and corpora lutea present. The complete ovary was serially sectioned from each mouse (n = 30 exposed and 30 unexposed), and the number of corpora lutea was counted using 10 random sections from each mouse. Uterine expression of proliferating cell nuclear antigen (PCNA) was assessed by immunohistochemical staining using antibody to PCNA (1:500, Chemicon, Inc., Temecula, CA). Uteri excised from exposed and unexposed mice were fixed in formalin overnight at room temperature and embedded in paraffin. A series of 5-mm sections were deparaffinized in xylene and ethanol and rinsed with 3% hydrogen peroxide. After a 1-h incubation with 1.5% normal goat blocking serum, sections were placed in primary antibody overnight at 4 C Normal goat IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as a negative control. Horse α-goat biotinylated secondary antibody (Vector Laboratories, Burlingame, CA) was applied for 1 h at 4 C. Slides were washed in 1× PBS-Tween 20, incubated in ABC Elite (Vector Laboratories) for 15 min at room temperature, washed in 1× PBS-Tween 20, and incubated for 5 min in diaminobenzidine (Vector Laboratories). A 12-sec exposure to hematoxylin was used as a counterstain. All slides were processed simultaneously for each primary antibody.
Quantitative real-time RT-PCR
Exposed and unexposed mice were mated with wild-type males. Detection of a vaginal plug was considered d 0 of pregnancy. To evaluate uteri during implantation, animals were euthanized 4 d after vaginal plug detection with carbon dioxide and cervical dislocation. Uteri were removed, and one horn was fixed in 4% formalin and embedded in paraffin for histological and immunohistochemical analysis. DNA and RNA were isolated from the other uterine horn using the DNeasy and RNeasy Mini Kits, respectively (QIAGEN, Valencia, CA) according to the manufacturer's protocol. Quantitative real-time RT-PCR was performed using the LightCycler SYBR Green RT-PCR kit from Roche (Stockholm, Sweden). Primers used are shown in Table 1. Total RNA (1 μg) was reverse transcribed in 20 μl of reaction mixture containing l0 mm each of deoxy (d) ATP, dCTP, dGTP, and dTTP; 20 pmol oligo(dT); 40 U/μl ribonuclease inhibitor, l0 U/μl avian myeloblastosis virus-reverse transcriptase, and 10× avian myeloblastosis virus-reverse transcriptase buffer for 30 min at 61 C. PCRs for endometrial receptivity genes were performed for 45 cycles of 95 C for 2 sec; 65 C for 5 sec; and 72 C for 18 sec. The increasing fluorescence of PCR products during amplification was monitored, from which a quantitative standard curve was created. Quantitation of samples was determined with the Roche LightCycler and adjusted to the quantitative expression of β-actin from these same samples. Melting curve analysis was conducted to determine the specificity of the amplified products and to ensure the absence of primer-dimer formation. All products obtained yielded the predicted melting temperature.
Table 1.
Primer sequences
| Sense 5′ → 3′ | Antisense 5′ → 3′ | |
|---|---|---|
| B-Actin | GACCTCTATGCCAACACAGT | TTGCTGATCCACATCTGCT |
| HOXA10 | GCCCTTCCGAGAGCAGCAAAG | AGGTGGACGCTGCGGCTAATCTCTA |
| Progesterone B | GGTCCCCCTTGCTTGCTTGCA | CAGGACCGAGGAAAAAGCAG |
| Progesterone AB | CTGTGCCTTACCATGTGGCA | TTCACCATGCCCGCCAGGAT |
| WNT7A | CGACAAGGAGAAGCAAGG | TCCTCCAGGATCTTCCGA |
| EMX2 | TCTGGGTCATCGCTTCCAAG | CAAAAGCGTGCTCTAGCCTTAAA |
| IGFBP1 | CCGCCACGAGCACCTTGTTCA | TGTTGGGCTGCAGCTAATCTCT |
| GHSR | AAGATGCTTGCTGTGGTG | GAGGACAAAGGACACCAG |
Bisulfite modification and methylation-specific PCR
Genomic DNA (1μg) from the uterus of mice euthanized on d 4 of pregnancy was treated with sodium bisulfite using the CpGenome DNA Modification Kit (Upstate Biotechnology, Charlottesville, VA). This process converts unmethylated cytosine residues to uracil, whereas methylated cytosines remain unchanged. Bisulfite-modified samples were aliquoted and stored at −80 C. A total of 200 ng sodium bisulfite-treated DNA was then analyzed using primer sets directed to the 5′-promoter and intron-1 regions of the bisulfite-modified Hoxa10 gene sequence. These regions contain multiple CpG islands, and alterations in methylation in this region regulate HOXA10 expression (19). DNA from CpG methylated mouse genomic DNA (New England BioLabs, Ipswich, MA) was used as a positive control for methylation. Unmethylated mouse genomic DNA (New England BioLabs) was used as a negative control for methylated genes. PCR amplification of 5 μl bisulfite-treated DNA template was performed in a 50-μl reaction containing 1.5 μl forward and reverse primers, 1.25 mmol/liter deoxynucleotide triphosphates, 25 mm Mg2+, and 0.5 μl HotStarTaq DNA polymerase (QIAGEN). All primers were synthesized by the Department of Pathology, Yale University School of Medicine. Amplification conditions were as follows: 15 min starting at 95 C; 35 cycles at 95 C for 30 sec; 59 C (methylated) or 53 C (unmethylated) for 30 sec; and 72 C for 30 sec, followed by a final extension at 72 C for 10 min. PCR products were resolved by electrophoresis on a 2% agarose gel and stained with ethidium bromide.
Bisulfite-sequencing PCR
Quantification of methylation at the 5′-promoter and intron-1 regions in the mouse was investigated via bisulfite-sequencing PCR. A total of 200 ng bisulfite-treated DNA was used in a 50-μl reaction containing 1.5 μl forward and reverse primers, 1.25 mmol/liter deoxynucleotide triphosphates, 25 mm Mg2+, and 0.5 μl HotStarTaq DNA polymerase. Amplification conditions were as follows: 15 min starting at 95 C; 35 cycles at 95 C for 30 sec; 53 C for 30 sec; and 72 C for 30 sec, followed by a final extension at 72 C for 10 min. PCR products were resolved by electrophoresis on a 2% agarose gel and stained with ethidium bromide. The appropriate-sized product bands were then isolated and excised from the gel and purified using the QIAQuick Gen Extraction Kit (QIAGEN), according to the manufacturer's protocol. The resultant products were then sequenced using the 3730xl DNA Analyzer (Applied Biosystems, Foster City, CA). For each CpG site, a C was interpreted as a methylated site, whereas a T was interpreted as an umethylated (and, thus, bisulfite-modified) site.
Statistical analysis
Litter size tabulation data are presented as mean ± sd and were analyzed using a t test. The difference in gene expression between two groups was compared by Student's t test. Differences in implantation rates were compared using χ2. The expression of PCNA protein by immunohistochemistry was quantified by two evaluators blinded to the treatment group. An H-score was determined separately for the glandular and stromal cells on each slide. Intensity of Hoxa10 nuclear staining was indicated by a value of 1, 2, or 3 (weak, moderate, or strong, respectively).
Results
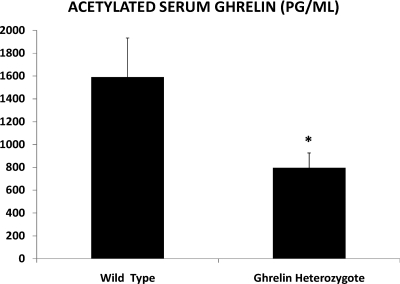
Initial experiments verified that ghrelin heterozygotes had lower serum ghrelin levels than wild-type B6D2F1 mice. After 24 h of food deprivation, serum from 10 wild-type mice and 10 ghrelin heterozygotes was collected via cardiac puncture. RIA was used to measure acetylated ghrelin levels in the serum. Heterozygotes had significantly lower serum acetylated ghrelin than the wild-type mice. Mean circulating levels of acetylated ghrelin in the heterozygotes was 796 pg/ml compared with 1159.1 pg/ml in the wild-type animals (P = 0.03) (Fig. 2). Wild-type progeny of ghrelin heterozygotes had ghrelin levels similar to unexposed wild-type controls. Food intake, weight, and weight gain in pregnancy for both wild-type and ghrelin heterozygote females are shown in Table 2. No significant differences were detected in weight, food intake, or weight gain in pregnancy (n = 30 per group).
Fig. 2.
Mean serum N-octanoyl ghrelin levels were significantly lower in ghrelin heterozygotes than in wild-type mice of the same strain, 796.0 pg/ml and 1159.1 pg/ml, respectively. (n = 5 per group; *, P = 0.02). Shown is mean ± sem.
Table 2.
Food intake, weight, and weight gain in pregnancy for wild-type and ghrelin heterozygote females
| Wild type-exposed | Wild type-unexposed | P value | |
|---|---|---|---|
| Average body weight (grams) | 16.9 | 17.1 | P = NSa |
| Average food consumption per day (grams) | 3.5 | 3.6 | P = NS |
| Average weight gain per pregnancy (until d 4)(grams) | 1.8 | 1.9 | P = NS |
NS, Nonsignificant (P > 0.05)
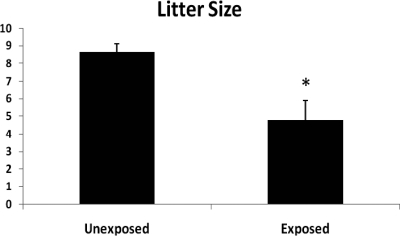
To assess the influence of maternal ghrelin deficiency on reproductive capacity in female offspring, we compared reproductive capacity of wild-type mice that developed in a ghrelin-deficient uterine environment to that of wild-type progeny of ghrelin-replete dams. Here we use the term “exposed” to refer to mice that are genetically wild-type, but are progeny of ghrelin heterozygous dams and therefore exposed to ghrelin deficiency in utero (Fig. 1). Exposed and unexposed females were mated with wild-type males over a 16-month span. As demonstrated in Fig. 3, exposed females have significantly reduced litter sizes relative to the wild-type controls. Exposed females bore an average of 4.78 pups per litter compared with 8.65 pups per litter in the unexposed group (P = 0.01). A similar difference was observed when only the first litter was analyzed. The mean number of litters was similar between the groups. The litter size of the exposed wild-type females was similar to that observed in ghrelin heterozygotes. Ghrelin (−/−) female mice infrequently conceived and rarely produced viable offspring.
Fig. 3.
Decreased mean litter size in wild-type offspring exposed to maternal ghrelin deficiency in utero (4.78) compared with wild-type unexposed to ghrelin deficiency (8.65). *, P = 0.01.
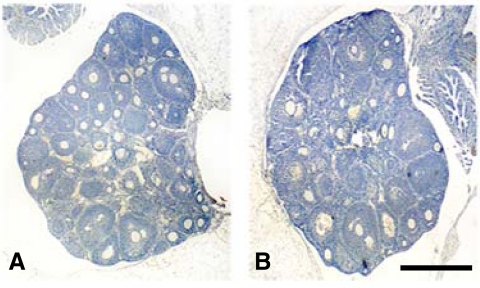
To determine whether the decreased litter sizes were related to decreased number of ovulated oocytes, 30 ovaries from each group (exposed and unexposed) were sectioned, stained with hematoxylin/eosin, and analyzed microscopically for preovulatory follicles and corpora lutea number 4 d after mating. Complete ovaries were cut into serial sections, and all follicles and corpora lutea were counted from 10 random sections per animal. Both the exposed and unexposed group contained similar numbers of follicles and corpora lutea per low-power field (Fig. 4). The mean number of corpora lutea was 14.2 in the exposed group and 13.5 in the unexposed group. Similarly, the number of embryos was assessed 4 d after detection of a vaginal plug. The mean number of embryos flushed from the uteri of exposed and unexposed mice after mating were identical (12.5 and 12.1, respectively). Normal ovarian function and embryo production suggested that the uterus, rather than ovary or embryo, was the anatomic location of the defect responsible for the observed differences in litter size.
Fig. 4.
Ovaries were obtained from exposed and unexposed mice, sectioned, and stained with hematoxylin and eosin. Similar numbers of follicles and corpora lutea were seen in the ovaries of exposed offspring (A) compared with unexposed offspring (B). (n = 30 mice per group). Representative photomicrographs are shown. Scale bar, 1 mm.
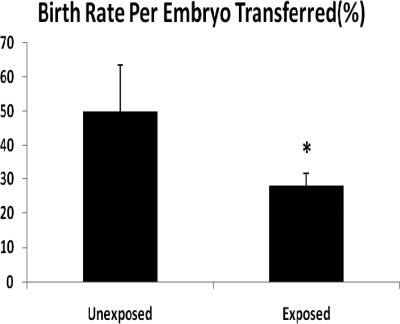
To determine whether decreased litter sizes were due to a uterine etiology, wild-type unexposed embryos were transferred to the uteri of exposed and unexposed wild-type females (Fig. 5). To calculate the term birth rate per embryo transferred, the number of embryos was compared after 17 d of gestation. In the unexposed uteri 50% of embryos implanted and produced a live born (14/28), whereas in the exposed group only 20% of embryos successfully implanted and reached maturity (6/30) (P = 0.02). Together these observations indicate that diminished ghrelin exposure in utero affects developmental programming of the uterus in exposed animals.
Fig. 5.
Birth rate of wild-type embryos transferred to exposed or unexposed uteri of pseudopregnant mice (*, P = 0.02). Birth rate per embryo transferred is the number of embryos transferred divided by the litter size on d 17.
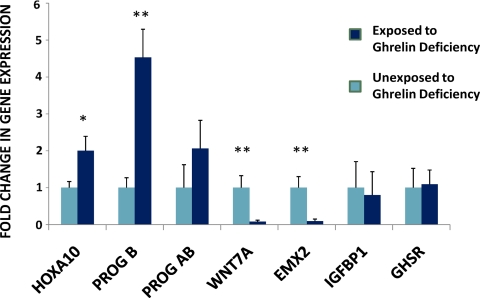
We next sought to identify the endometrial defects that accounted for the subfertility in the exposed animals. Uteri, from exposed and unexposed mice that were euthanized in the implantation window, 4 d after detection of a vaginal plug, were analyzed. Consistent with the results of the uterine embryo transfer studies, the mRNA expression of multiple genes required for uterine receptivity was altered in the uterus of the exposed mice (Fig. 6). HOXA10 and progesterone receptor B mRNA expression were significantly increased in the ghrelin-exposed mice compared with the unexposed group. WNT7A and EMX2 expression were significantly decreased in exposed mice compared with wild-type controls. Total progesterone receptor (Fig. 6, AB) expression was also moderately elevated, whereas IGF-binding protein-1 (Fig. 6, IGFBP1) expression was decreased in exposed mice, although these did not reach significance. These genes all function in both the regulation of endometrial growth and differentiation in the estrous cycle.
Fig. 6.
Exposure to ghrelin deficiency in utero alters expression of genes that are critical for endometrial receptivity and implantation. Gene expression was measured 4 d after the detection of the vaginal plug at the time of implantation. HOXA10 and progesterone B expression were significantly higher in the ghrelin-exposed group (P = 0.021 and P = 0.002, respectively). WN7A and Emx2 expression were significantly lower in the ghrelin- exposed group (P = 0.004 and P = 0.005, respectively). *, P < 0.05; **, P < 0.01.
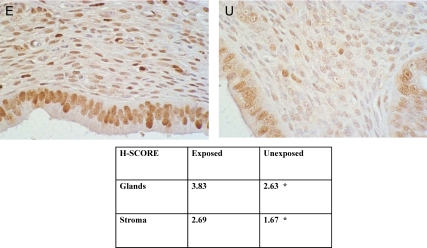
Based on the known role of Hoxa10 and Emx2 in endometrial proliferation, we investigated the ability of the exposed uterine endometrium to proliferate. Here PCNA was used as a marker of endometrial proliferation (20). In Fig. 7, we demonstrate elevated PCNA expression in exposed females relative to controls at d 4 of pregnancy. Differential intensity of PCNA staining was especially apparent in uterine stromal cells.
Fig. 7.
Immunohistochemical analysis reveals enhanced expression of PCNA in uterine cells from exposed mice. Top panel, Uterine section from exposed female (E) displayed greater stromal cell expression of PCNA at estrus than unexposed females (U). Bottom panel, H-Score was used to quantify PCNA expression in both endometrial glands and stroma (*, P < 0.03, exposed compared with unexposed).
We sought to determine the mechanism responsible for programming the expression of uterine genes after exposure to in utero ghrelin deficiency. We investigated whether the observed changes in gene expression were correlated with alterations in DNA methylation using bisulfite conversion. Twenty regions of the Hoxa10 promoter rich in CpG islands are known to epigenetically regulate this gene and were previously identified as sites responsible for in utero epigenetic regulation (19). DNA from five exposed mice and five unexposed mice were examined. The quantitative difference in methylation levels of Hoxa10 expressed as the mean percentage of methylation at each CpG island was also examined, demonstrating the extent of methylation on average for both the promoter and intronic regions. In all 20 sites, there was less than a 1% difference in methylation between exposed and unexposed mice (data not shown). Differences were not statistically significant, indicating that methylation at these sites was not responsible for alterations in gene expression.
Discussion
Successful implantation requires the synchronous development of both the embryo and the endometrium. Uterine receptivity is established by precise modulation of endometrial differentiation regulated by ovarian hormones and timed to ovulation. Multiple genes crucial for differentiation of a receptive endometrium have been described, and specifically, alteration of Hoxa10 expression dramatically affects implantation (21–26). To evaluate the peri-implantation window, we evaluated gene expression on d 4 of pregnancy. Here we show that Hoxa10 is significantly elevated after exposure to ghrelin deficiency in utero. Another marker of endometrial receptivity, Emx2, was found to be significantly decreased after exposure to ghrelin deficiency in utero. This is consistent with previous studies demonstrating that Hoxa10 directly represses Emx2 expression (27). In addition, expression of WNT7A and PR-B, markers of endometrial differentiation (28–32), were altered in exposed animals compared with controls. Coordination of endometrial differentiation with embryo maturation is critical for implantation. This aberrant gene regulation likely results in dyssynchrony between the endometrium and embryo, preventing implantation.
Precise regulation of endometrial proliferation is similarly critical for establishment of endometrial receptivity. Both Emx2 and Hoxa10 have been shown to regulate endometrial proliferation. Hoxa10-deficient mice have impaired endometrial proliferation leading to loss of implantation (25). Conversely, decreasing Emx2 expression results in increased cell proliferation and lower implantation rates (33). Here exposed mice showed elevated Hoxa10 and decreased EMX2 gene expression correlating with the observed increase in endometrial proliferation. The increased proliferation rate likely results in further uncoupling of endometrial and embryo development.
In addition to evaluating uterine receptivity in the peri-implantation window, we analyzed overall reproductive success by calculating the birth rate per embryo transferred. The mice exposed to ghrelin deficiency in utero had a decreased birth rate due to a uterine defect. We demonstrated defects in uterine gene expression in the window of implantation and suggest that these alterations lead to decreased implantation and subsequently are responsible for the observed decrease in birth rate. Additional or continued defects in uterine gene expression in the postimplantation window may further affect birth rate. The mechanism of the persistent altered gene expression is not due to DNA methylation and is a topic of current investigation.
Taken together, we have demonstrated impaired female fertility as a result of ghrelin deficiency in utero. We identified epigenetic alterations in uterine gene expression, leading to differentiation and proliferation defects and impaired embryo implantation. Exposure to ghrelin deficiency in utero impairs developmental programming and causes uterine abnormalities that result in subfertility. Ghrelin is decreased in obese individuals; these findings may have significant reproductive implications for daughters of obese women.
Acknowledgments
This work was supported by National Institutes of Health Grants HD052668, HD036887, and ES010610.
Disclosure Summary: The authors have no conflict of interest.
Footnotes
- PCNA
- Proliferating cell nuclear antigen.
References
- 1. García MC, López M, Alvarez CV, Casanueva F, Tena-Sempere M, Diéguez C. 2007. Role of ghrelin in reproduction. Reproduction 133:531–540 [DOI] [PubMed] [Google Scholar]
- 2. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. 1999. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660 [DOI] [PubMed] [Google Scholar]
- 3. Tschöp M, Smiley DL, Heiman ML. 2000. Ghrelin induces adiposity in rodents. Nature 407:908–913 [DOI] [PubMed] [Google Scholar]
- 4. Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. 2002. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346:1623–1630 [DOI] [PubMed] [Google Scholar]
- 5. Pasquali R, Pelusi C, Genghini S, Cacciari M, Gambineri A. 2003. Obesity and reproductive disorders in women. Hum Reprod Update 9:359–372 [DOI] [PubMed] [Google Scholar]
- 6. Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. 2001. A role for ghrelin in the central regulation of feeding. Nature 409:194–198 [DOI] [PubMed] [Google Scholar]
- 7. Cummings DE, Overduin J. 2007. Gastrointestinal regulation of food intake. J Clin Invest 117:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K. 2001. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86:4753–4758 [DOI] [PubMed] [Google Scholar]
- 9. Broglio F, Gottero C, Benso A, Prodam F, Volante M, Destefanis S, Gauna C, Muccioli G, Papotti M, van der Lely AJ, Ghigo E. 2003. Ghrelin and the endocrine pancreas. Endocrine 22:19–24 [DOI] [PubMed] [Google Scholar]
- 10. Kojima M, Kangawa K. 2005. Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- 11. Hansen TK, Dall R, Hosoda H, Kojima M, Kangawa K, Christiansen JS, Jørgensen JO. 2002. Weight loss increases circulating levels of ghrelin in human obesity. Clin Endocrinol (Oxf) 56:203–206 [DOI] [PubMed] [Google Scholar]
- 12. Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, Nozoe S, Hosoda H, Kangawa K, Matsukura S. 2002. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab 87:240–244 [DOI] [PubMed] [Google Scholar]
- 13. Tawadros N, Salamonsen LA, Dimitriadis E, Chen C. 2007. Facilitation of decidualization by locally produced ghrelin in the human endometrium. Mol Hum Reprod 13:483–489 [DOI] [PubMed] [Google Scholar]
- 14. Kawamura K, Sato N, Fukuda J, Kodama H, Kumagai J, Tanikawa H, Nakamura A, Honda Y, Sato T, Tanaka T. 2003. Ghrelin inhibits the development of mouse preimplantation embryos in vitro. Endocrinology 144:2623–2633 [DOI] [PubMed] [Google Scholar]
- 15. Caminos JE, Tena-Sempere M, Gaytán F, Sanchez-Criado JE, Barreiro ML, Nogueiras R, Casanueva FF, Aguilar E, Diéguez C. 2003. Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology 144:1594–1602 [DOI] [PubMed] [Google Scholar]
- 16. Tanaka K, Minoura H, Isobe T, Yonaha H, Kawato H, Wang DF, Yoshida T, Kojima M, Kangawa K, Toyoda N. 2003. Ghrelin is involved in the decidualization of human endometrial stromal cells. J Clin Endocrinol Metab 88:2335–2340 [DOI] [PubMed] [Google Scholar]
- 17. Bjornsson HT, Fallin MD, Feinberg AP. 2004. An integrated epigenetic and genetic approach to common human disease. Trends Genet 20:350–358 [DOI] [PubMed] [Google Scholar]
- 18. Greathouse KL, Cook JD, Lin K, Davis BJ, Berry TD, Bredfeldt TG, Walker CL. 2008. Identification of uterine leiomyoma genes developmentally reprogrammed by neonatal exposure to diethylstilbestrol. Reprod Sci 15:765–778 [DOI] [PubMed] [Google Scholar]
- 19. Bromer JG, Wu J, Zhou Y, Taylor HS. 2009. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150:3376–3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lai MD, Lee LR, Cheng KS, Wing LY. 2000. Expression of proliferating cell nuclear antigen in luminal epithelium during the growth and regression of rat uterus. J Endocrinol 166:87–93 [DOI] [PubMed] [Google Scholar]
- 21. Taylor HS, Vanden Heuvel GB, Igarashi P. 1997. A conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 57:1338–1345 [DOI] [PubMed] [Google Scholar]
- 22. Bagot CN, Troy PJ, Taylor HS. 2000. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther 7:1378–1384 [DOI] [PubMed] [Google Scholar]
- 23. Taylor HS, Arici A, Olive D, Igarashi P. 1998. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 101:1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Satokata I, Benson G, Maas R. 1995. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374:460–463 [DOI] [PubMed] [Google Scholar]
- 25. Yao MW, Lim H, Schust DJ, Choe SE, Farago A, Ding Y, Michaud S, Church GM, Maas RL. 2003. Gene expression profiling reveals progesterone-mediated cell cycle and immunoregulatory roles of Hoxa-10 in the preimplantation uterus. Mol Endocrinol 17:610–627 [DOI] [PubMed] [Google Scholar]
- 26. Vitiello D, Pinard R, Taylor HS. 2008. Gene expression profiling reveals putative HOXA10 downstream targets in the periimplantation mouse uterus. Reprod Sci 15:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Troy PJ, Daftary GS, Bagot CN, Taylor HS. 2003. Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol 23:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaetje R, Holtrich U, Karn T, Cikrit E, Engels K, Rody A, Kaufmann M. 2007. Characterization of WNT7A expression in human endometrium and endometriotic lesions. Fertil Steril 88:1534–1540 [DOI] [PubMed] [Google Scholar]
- 29. Parr BA, McMahon AP. 1998. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature 395:707–710 [DOI] [PubMed] [Google Scholar]
- 30. Rutanen EM, Pekonen F, Mäkinen T. 1988. Soluble 34K binding protein inhibits the binding of insulin-like growth factor I to its cell receptors in human secretory phase endometrium: evidence for autocrine/paracrine regulation of growth factor action. J Clin Endocrinol Metab 66:173–180 [DOI] [PubMed] [Google Scholar]
- 31. Fazleabas AT, Kim JJ, Strakova Z. 2004. Implantation: embryonic signals and the modulation of the uterine environment–a review. Placenta 25(Suppl A):S26–S31 [DOI] [PubMed] [Google Scholar]
- 32. Giudice LC, Dsupin BA, Jin IH, Vu TH, Hoffman AR. 1993. Differential expression of messenger ribonucleic acids encoding insulin-like growth factors and their receptors in human uterine endometrium and decidua. J Clin Endocrinol Metab 76:1115–1122 [DOI] [PubMed] [Google Scholar]
- 33. Taylor HS, Fei X. 2005. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol 19:2839–2846 [DOI] [PubMed] [Google Scholar]