Abstract
Epstein–Barr virus latent membrane protein 1 (LMP1) activation of NF-κB is critical for Epstein–Barr virus-infected B lymphocyte survival. LMP1 activates the IκB kinase complex and NF-κB through two cytoplasmic signaling domains that engage tumor necrosis factor receptor-associated factor (TRAF)1/2/3/5 or TRADD and RIP. We now use cells lacking expression of TRAF2, TRAF5, TRAF6, IKKα, IKKβ, IKKγ, TAB2, IL-1 receptor-associated kinase (IRAK)1, or IRAK4 to assess their roles in LMP1-mediated NF-κB activation. LMP1-induced RelA nuclear translocation was similar in IKKα knockout (KO) and WT murine embryo fibroblasts (MEFs) but substantially deficient in IKKβ KO MEFs. NF-κB-dependent promoter responses were also substantially deficient in IKKβ KO MEFs but were hyperactive in IKKα KO MEFs. More surprisingly, NF-κB responses were near normal in TRAF2 and TRAF5 double-KO MEFs, IKKγ KO MEFs, TAB2 KO MEFs, and IRAK4 KO MEFs but were highly deficient in TRAF6 KO MEFs and IRAK1 KO HEK293 cells. Consistent with the importance of TRAF6, LMP1-induced NF-κB activation in HEK293 cells was inhibited by expression of dominant-negative TAB2 and Ubc13 alleles. These data extend a role for IKKα in IKKβ regulation, identify an unusual IKKβ-dependent and IKKγ-independent NF-κB activation, and indicate that IRAK1 and TRAF6 are essential for LMP1-induced NF-κB activation.
Primary Epstein–Barr Virus (EBV) infection of B lymphocytes causes their long-term proliferation through expression of several proteins, including latent infection integral membrane protein 1 (LMP1). LMP1 is critical for EBV-infected cell activation, adhesion, and survival (1). EBV is causally associated with lymphoid and epithelial malignancies, including posttransplant lymphoproliferative disorders, Hodgkin's disease, anaplastic nasopharyngeal carcinoma, and gastric carcinomas; LMP1 is usually expressed in the malignant cells (2). LMP1 can transform rodent fibroblasts to anchorage, contact, and serum-independent growth and to tumorigenicity in nude mice (3). LMP1 expression in human B lymphoblasts alters cell growth, and transgenic expression in murine B cells causes hyperplasia and lymphoma (4).
LMP1-mediated NF-κB activation is essential for EBV transformed lymphoblastoid cell line survival (5). LMP1 has six transmembrane domains that cause aggregation in the plasma membrane and enable constitutive NF-κB activation through two cytoplasmic C-terminal activation regions (CTARs) (6, 7). CTARs 1 and 2 coincide with critical transformation effector sites (TES), which were defined by reverse genetic analyses of EBV genes required for B lymphocyte growth transformation. CTAR1/TES1 engages tumor necrosis factor receptor-associated factors (TRAFs) 1, 3, 2, and 5 through a consensus PXQXT motif, whereas CTAR2/TES2 engages tumor necrosis factor receptor (TNFR)-associated death domain proteins including TRADD and RIP (8, 9). Thus, LMP1 behaves like a constitutively activated TNFR, similar in repertoire of cytoplasmic interacting factors to a combination of TNFRs 1 and 2 or CD40, a critical TNFR for B lymphocyte development.
The rate-limiting step in NF-κB activation downstream of TNF, IL-1, and Toll-like receptors is the phosphorylation of IκBα by the IκB kinase (IKK) complex leading to ubiquitylation and degradation of IκBα and nuclear translocation of NF-κB. The IKK complex is composed of IKKα and IKKβ kinases bound to a scaffolding protein, IKKγ (10–13). Gene knockouts (KOs) indicate that IKKβ and IKKγ are core components for almost all nuclear translocation of RelA DNA-binding complexes, whereas IKKα is not essential (11, 14, 15). However, NF-κB-inducing kinase phosphorylates and activates IKKα, which is essential for processing p100/NF-κB2 into p52 downstream of BAFF, CD40L, lipopolysaccharide (LPS), and LTβ; RelB-containing NF-κB complexes then translocate to the nucleus (16–22).
The connections between TRAFs 1, 2, 3, and 5, TRADD, and RIP and the IKK complex are partially delineated. TRAF1/2 or TRAF3/5 heterodimeric complexes can activate NF-κB (23, 24). TRAF2 can associate with IKKα and IKKβ (25, 26), RIP can associate with IKKγ in a TNF-stimulated manner (12, 27), and TRADD can associate with TRAF2 (28). Whereas TRAF2 and TRAF5 are required for TNF-induced NF-κB activation, IL-1 and LPS signaling are normal in TRAF2/5 double-KOs (T2/5DKO) (29). In contrast, TNF signaling is normal in TRAF6 KO cells, whereas IL-1, LPS, and CD40 signaling is defective (30). Furthermore, downstream of IL-1 and LPS signaling, IL-1 receptor-associated kinase (IRAK)4 is essential for phosphorylation and activation of IRAK1 (31), which leads to activation of a TRAF6-dependent signal (32). Thus, distinct biochemical pathways exist downstream of TNF and Toll/IL-1R (TIR)-containing receptors.
The experiments reported here were initially undertaken to investigate the roles of TRAFs 2 and 5 and IKKα and IKKβ in LMP1-mediated NF-κB activation by using murine fibroblasts with specific gene KOs. The results were surprising, and they led to a broader investigation.
Materials and Methods
Plasmids. Expression vectors for F-IκBα, GFP-RelA, TAB2-C, and F-Ubc13 were kindly provided by D. Ballard (Southwestern University, Dallas), D. Thanos (Al Flemming), H. Sanjo (Osaka University), S.A., and Z. Chen (Southwestern University). The Ubc13 C87A mutant was constructed using the Stratagene QuikChange PCR mutagenesis kit. pCDNA3-LMP1 WT, pSG5-FLMP1WT, AA, and ID, pCDNA3-FIKKβ WT(Δ9) and KM(Δ34), pRK5-mycIKKα WT and kinase mutant (KM), and 3XκBL and pGK-β-gal have been described (8, 33, 34).
Cell Lines, Transfections, and Reporter Gene Assays. IKKα, IKKβ, and TAB2 KO MEFs were kindly provided by M. Karin and S.A. and were maintained in DMEM supplemented with 10% FBS (GIBCO/BRL), 2 mM l-glutamine, and antibiotics. MEFs were transfected with LF 2000 (Invitrogen). Luciferase reporter assays were normalized for transfection efficiency by cotransfecting a β-galactosidase (β-gal) expression plasmid and dividing luciferase by β-gal activity at 20–24 h posttransfection. Luciferase activities in the presence of LMP1 were plotted relative to luciferase reporter (3XκBL) in the absence of LMP1. Luciferase assays were performed as per the manufacturer's protocol (Promega). β-Gal activity was measured with the Galacton-Plus substrate system (Tropix, Bedford, MA).
Electrophoretic Mobility-Shift Assay. Briefly, 5 μg of protein from 1% Nonidet P-40 lysate was incubated with a 32P-labeled probe derived from the consensus NF-κB site from the HIV-1 LTR (35). Complexes were separated and analyzed on a 5% native PAGE followed by autoradiography. Competition with 150-fold WT versus mutant cold probe was done to verify NF-κB-specific complexes (data not shown).
GFP-RelA Nuclear Translocation. For GFP-RelA nuclear translocation assays, 105 MEFs were seeded in six-well plates and were cotransfected 24 h later with 1–1.5 μg per well GFP-RelA, 0.4–0.6 μg per well (except for 0.9 μg per well for IKKα) IκBα, and 1–1.5 μg per well LMP1 or control expression plasmids; IκBα and GFP-RelA expression was balanced so that GFP-RelA localized to the cytoplasm in the absence of LMP1. After 18–24 h, live cells were observed under a fluorescent microscope to determine the percentage of cells with GFP-RelA translocated to the nucleus.
Results
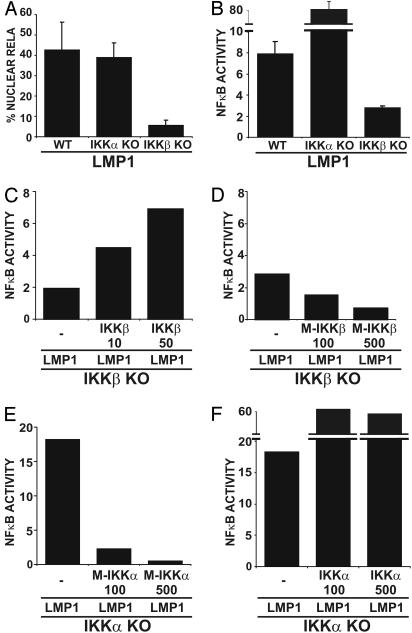
LMP1 Activation of NF-κB in MEFs Requires IKKβ but Not IKKα. The roles of IKKα and IKKβ kinases in LMP1-mediated NF-κB activation were evaluated by comparing LMP1-induced NF-κB activation in IKKα or IKKβ KO and WT MEFs. NF-κB activation was assessed by nuclear translocation of a GFP-RelA fusion protein and by an NF-κB-dependent promoter luciferase reporter assay after cotransfection with or without an LMP1 expression vector. In MEFs, GFP-RelA nuclear translocation is a qualitative measure of RelA activity, whereas the NF-κB-dependent promoter luciferase reporter assay is a quantitative measure of overall NF-κB activity and has a reasonable dynamic range conferred by the luciferase reporter. In IKKβ KO MEFs, LMP1 was markedly deficient in inducing RelA nuclear translocation (Fig. 1A) and substantially deficient in activation of a NF-κB-dependent luciferase reporter (Fig. 1B). Although low-level expression of WT IKKβ alone in IKKβ KO MEFs did not induce reporter activity, cotransfection of IKKβ KO MEFs with LMP1 and WT or KM IKKβ expression plasmids confirmed that NF-κB activation is restored by and is dependent on IKKβ kinase activity (Fig. 1 C and D and data not shown). Indeed, IKKβ KM suppressed NF-κB reporter activity below the residual level induced by LMP1 in IKKβ KO cells, consistent with dominant-negative inhibition of alternative NF-κB activators such as IKKα (Fig. 1D).
Fig. 1.
The role of IKKα and IKKβ in LMP1-mediated NF-κB activation in MEFs. (A) WT, IKKα KO, and IKKβ KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 is shown. Average values ± SD are shown from three experiments, in which LMP1 expression was similar in all transfected MEFs (not shown). (B) WT, IKKα KO, and IKKβ KO MEFs were transfected with 3XκBL and pGK-β-gal alone or with LMP1. The mean folds of NF-κB activation by LMP1 ± SE relative to β-gal activity are shown from one representative of four independent experiments performed in duplicate. (C) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of WT IKKβ expression vector in IKKβ KO MEFs are shown from a representative transfection. (D) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of catalytically inactive IKKβ (M-IKKβ) in IKKβ KO MEFs are shown from a representative transfection. (E) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of catalytically inactive IKKα (M-IKKα) in IKKα KO MEFs. (F) Folds of NF-κB activation by LMP1 alone or in the presence of increasing amounts (in ng) of WT IKKα expression vector in IKKα KO MEFs.
Although LMP1 induced GFP-RelA nuclear translocation in similar percentages of WT and IKKα KO MEFs (Fig. 1 A), NF-κB-dependent reporter activity was 10-fold higher in IKKα KO MEFs than in WT MEFs (Fig. 1B). The apparent discrepancy between the two assays is likely due to the relatively low dynamic range and strict dependence of the GFP-RelA nuclear translocation assay on IκBα levels, whereas the NF-κB-dependent reporter records the cumulative effects not only of RelA/p50 heterodimers but also RelB-, c-Rel-, and p52-containing dimers. These other NF-κB components may be induced by RelA/p50 activation by LMP1. Regardless of the underlying mechanisms, the data indicate that IKKα has an essential role in the down-modulation of LMP1-induced NF-κB activation in MEFs; IKKα may directly down-modulate the height or duration of IKKβ responses or may indirectly down-modulate NF-κB activation.
Furthermore, LMP1-induced NF-κB activation in IKKα KO MEFs reconstituted with IKKα WT or KM was surprising. IKKα KM inhibited LMP1-induced NF-κB activation at all levels of expression and with dose-dependent effects in IKKα KO MEFs (Fig. 1E and data not shown), consistent with a requirement for kinase activity for LMP1-induced IKKα/IKKβ signaling. This putative requirement dominates and precludes observation of the role of WT IKKα in IKKβ down-modulation. However, coexpression of WT IKKα and LMP1 did not prevent hyperactive NF-κB responses in IKKα KO MEFs (Fig. 1F and data not shown). Expression of even undetectable levels of WT IKKα with LMP1 in IKKα KO MEFs induced higher levels of NF-κB activity than LMP1 alone, but did not induce reporter activity without LMP1 (data not shown). This surprising result may be due to a dominant-positive effect of newly expressed IKKα, which together with a putative activation of NF-κB-inducing kinase by LMP1 may cause IKKα activation of RelB heterodimers in IKKα KO MEFs.
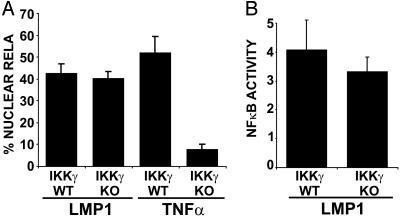
LMP1-Mediated NF-κB Activation in MEFs Does Not Require IKKγ. IKKγ is essential for TNF, IL-1, LPS, and poly(IC)-mediated NF-κB activation and was therefore expected to be critical for LMP1-induced NF-κB activation (36). However, when LMP1-mediated NF-κB activation in IKKγ KO MEFs was compared to WT MEFs, GFP-RelA nuclear translocation and NF-κB-dependent reporter activity was similar in IKKγ KO and WT MEFs (Fig. 2). In contrast, TNF induced GFP-RelA nuclear translocation in WT but not in IKKγ KO MEFs (Fig. 2 A). These data indicate that IKKγ is not essential for LMP1-mediated NF-κB activation in MEFs. However, quantitative RT-PCR analysis of endogenous gene expression induced by LMP1 in IKK KO MEFs indicated that both IKKβ-dependent/IKKγ-independent and IKKβ/IKKγ-dependent pathways existed (59). Thus, reporter activity does not reflect the complexity of endogenous gene expression where both IKKγ-dependent and -independent pathways contribute to the regulation of NF-κB target genes downstream of LMP1.
Fig. 2.
IKKγ is not essential for LMP1-mediated NF-κB activation. (A) IKKγ WT and KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid or TNF stimulation. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 or TNF is shown. Average values ± SD are shown from three experiments. LMP1 expression was similar in WT and KO MEFs (data not shown). (B) IKKγ WT and KO MEFs were transfected with reporter plasmids alone or with LMP1. Mean folds of NF-κB activation ± SD by LMP1 are shown from four experiments.
LMP1-Mediated NF-κB Activation in MEFs Does Not Require TRAF2 and TRAF5 but Does Require TRAF6. LMP1 CTAR1/TES1 constitutively binds a substantial fraction of the cytoplasmic TRAF1 and TRAF3 and a smaller fraction of TRAF2 and TRAF5, whereas LMP1 CTAR2/TES2 constitutively engages death domain-containing proteins TRADD and RIP. Signaling from TRADD and RIP may also involve TRAF2 and TRAF5 (9, 37, 38). Because TRAF2, -5, or -6 overexpression results in NF-κB activation and TRAF1 or -3 overexpression does not, TRAFs 2, 5, and 6 are implicated directly in NF-κB activation. TRAFs 2 and 5 are also implicated in TRADD signaling because TNF-induced NF-κB activation through TRADD is substantially deficient in TRAF2 and TRAF5 double-KO fibroblasts, whereas IL-1 and LPS-mediated NF-κB activation in T2/5DKO MEFs are similar to WT (29). In contrast, TRAF6 KO MEFs are substantially impaired in IL-1 and LPS-mediated NF-κB activation but not in TNF-mediated NF-κB activation (30). Based on these observations, LMP1 would be predicted to depend on TRAF2 and TRAF5 but not TRAF6.
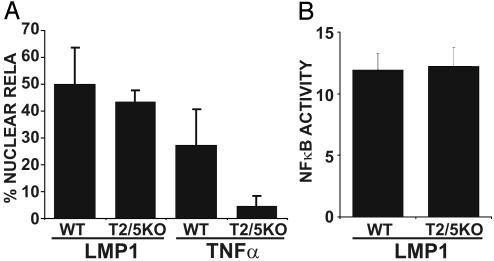
Unexpectedly, LMP1 induced similar levels of GFP-RelA nuclear translocation and NF-κB-dependent luciferase reporter activity in T2/5 DKO and WT MEFs (Fig. 3). In contrast, TNF induced GFP-RelA nuclear translocation in WT but not in T2/5 DKO MEFs (Fig. 3A). These data indicate that TRAF2 and TRAF5 are not required for LMP1-mediated NF-κB activation in MEFs.
Fig. 3.
TRAF2 and TRAF5 are not essential for LMP1-mediated NF-κB activation in MEFs. (A) WT and T2/5KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid or TNF stimulation. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 or TNF is shown. Average values ± SD are shown from three experiments, except from LMP1-mediated translocation of GFP-RelA in T2/5KO MEFs, for which five experiments were analyzed. LMP1 expression in WT and KO MEFs was similar (data not shown). (B) WT and T2/5KO MEFs were transfected with reporter plasmids alone or with an LMP1 plasmid. The mean ± SD of folds of NF-κB activation by LMP1 from four experiments is shown.
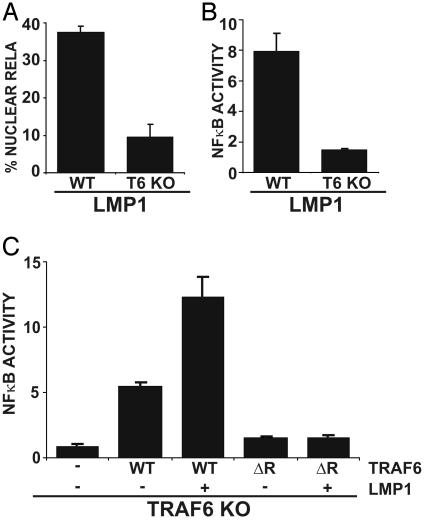
Notably, LMP1 was unable to induce GFP-RelA nuclear translocation or NF-κB-dependent luciferase reporter activity in TRAF6 KO MEFs (Fig. 4 A and B). In contrast, TNF induced similar levels of NF-κB activation in TRAF6 KO and WT MEFs (data not shown and ref. 30). To evaluate whether TRAF6 per se or a developmental defect linked to TRAF6 was responsible for the inability of TRAF6 KO MEFs to support LMP1-mediated NF-κB activation, TRAF6 KO MEFs were complemented by transient coexpression of murine TRAF6. mTRAF6 alone activated NF-κB 5-fold, and LMP1 cotransfection with mTRAF6 resulted in 12-fold activation, similar to LMP1-mediated NF-κB activation in WT MEFs, whereas no effect on LMP1 expression level was observed (Fig. 4C and data not shown). Furthermore, transfection of a mTRAF6 construct lacking the RING finger domain was unable to reconstitute LMP1-mediated NF-κB reporter activity (Fig. 4C). Thus, TRAF6 expression can rescue LMP1-mediated NF-κB activation in TRAF6 KO MEFs in a RING finger-dependent manner, and TRAF6 is essential for LMP1-mediated NF-κB activation, even when TRAF2 and TRAF5 are at WT levels.
Fig. 4.
TRAF6 is essential for LMP1-mediated NF-κB activation in MEFs. (A)WT and TRAF6 KO MEFs were transfected with GFP-RelA and IκBα-encoding plasmids in the presence or absence of an LMP1 expression plasmid. The percentage of cells with nuclear translocation of GFP-RelA induced by LMP1 is shown. Average values ± SD are shown from three experiments. LMP1 expression was similar in WT and KO MEFs (data not shown). (B) WT and TRAF6 KO MEFs were transfected with reporter plasmids alone or with an LMP1 plasmid. Mean ± SE of folds of NF-κB activation by LMP1 are shown from one representative of four experiments performed in duplicate. (C) TRAF6 KO MEFs were transfected with reporter plasmids, LMP1, and WT, or ΔRF mTRAF6 expression plasmids as indicated. Results from one representative of four similar experiments are shown as mean folds of NF-κB activation by LMP1 and/or TRAF6 ± SE.
TAB2 and Ubc13 Dominant-Negative Mutants That Associate with TRAF6 Inhibit LMP1-Mediated NF-κB Activation, but LMP1-Mediated NF-κB Activation Is at WT Levels in TAB2 KO MEFs. Because TRAF6 has an essential role in IL-1, TLR, and now LMP1-mediated NF-κB activation but not in TNFR-mediated NF-κB activation, we evaluated the possibility that LMP1 uses other IL-1 and TLR signaling components to activate NF-κB. Biochemical experiments using purified TRAF6 previously demonstrated that the TAB1/TAB2/TAK1 complex and the E2 ubiquitin-conjugating enzyme heterodimer Ubc13/Uev1A in the presence of an E1 ubiquitin-activating enzyme and ubiquitin were sufficient to induce purified IKK complexes to phosphorylate IκBα (39, 40).
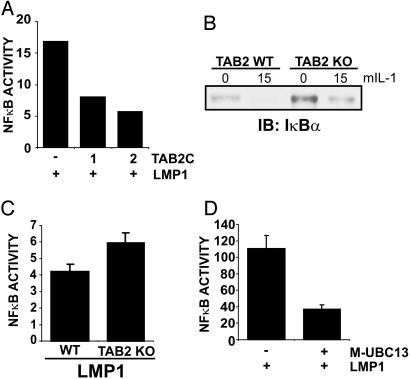
To investigate the role of the TAB1/TAB2/TAK1 complex, TAB2-C, a TAB2 dominant-negative mutant that associates with TRAF6 and TAK1 but does not enable TAK1 activation (41, 42), was coexpressed with LMP1 in 293T cells. TAB2-C markedly inhibited LMP1-mediated NF-κB activation, and the effect was dose dependent (Fig. 5A).
Fig. 5.
LMP1-mediated NF-κB activation is inhibited by TAB2-C and Ubc13-C87A but is not dependent on TAB2. (A) 293T cells were transfected with reporter plasmids alone, an LMP1 expression plasmid, or LMP1 and increasing amounts of TAB2-C expression plasmid (1 or 2 μg). Results from one representative transfection of two experiments show the mean folds of NF-κB activation by LMP1. (B)WT and TAB2 KO MEFs were treated with 100 ng/ml mIL-1 for 15 min or left untreated. Cell lysates were blotted for endogenous IκBα (Santa Cruz Biotechnology, C-21). (C) WT and TAB2 KO MEFs were transfected with reporter plasmids alone or with an LMP1 expression plasmid. Results from one representative transfection performed in triplicate show the mean ± SE of folds of NF-κB activation by LMP1. (D) An experiment similar to A was performed except that 1μg of Ubc13-C87A (M-UBC13) was cotransfected with LMP1. Results from one representative transfection of several experiments performed in duplicate show the mean NF-κB activation by LMP1. LMP1 was expressed at similar levels in the presence or absence of Ubc13-C87A (data not shown).
The physiological role of TAB2 in LMP1-mediated NF-κB activation was then assessed using TAB2 KO MEFs. TAB2 was recently shown to be nonessential for IL-1, LPS, and TNF-induced NF-κB activation (43). Thus, as expected, murine IL-1 (mIL-1) induced IκBα degradation similarly in TAB2 KO and WT MEFs (Fig. 5B). Most importantly, LMP1 induced similar levels of NF-κB activation in TAB2 KO and WT MEFs (Fig. 5C). Thus, whereas TAB2-C inhibited LMP1-induced NF-κB activation, TAB2 is not critical for LMP1-mediated NF-κB activation in MEFs, indicating a role for a TAB2-associated protein, such as TRAF6, in LMP1 signaling.
The role of Ubc13, the TRAF6-associated K63-linked ubiquitin-conjugating enzyme, in LMP1-mediated NF-κB activation was assessed by coexpressing catalytically inactive Ubc13-C87A with LMP1 in 293T cells. Ubc13-C87A reduced LMP1-mediated NF-κB activation by >50% (Fig. 5D). Somewhat surprisingly, WT Ubc13 cotransfection with LMP1 resulted in similar effects on NF-κB-dependent reporter activity (data not shown). Thus, overexpression of WT or a dominant-negative mutant Ubc13 inhibits LMP1-mediated NF-κB activation, consistent with a squelching-like effect on TRAF6 or another Ubc13 interacting protein.
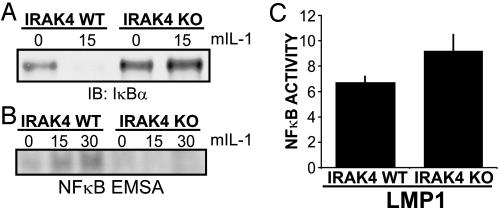
LMP1-Mediated NF-κB Activation Requires IRAK1 but Not IRAK4. Because IRAK1 and IRAK4 are key TRAF6 interacting proteins, which mediate NF-κB activation downstream of IL-1, Toll-like, and TNF receptors (44), their role in LMP1-mediated NF-κB activation was assessed. As expected, mIL-1 induced IκBα degradation and NF-κB gel shift activity in WT MEFs and did not induce IκBα degradation or NF-κB gel shift activity in IRAK4 KO MEFs (ref. 31 and Fig. 6 A and B). Surprisingly, LMP1 induced similar levels of NF-κB-dependent luciferase reporter activity in IRAK4 KO and WT MEFs (Fig. 6C). Thus, IRAK4, an essential component of IL-1 and most TLR-mediated NF-κB activation, is not critical for LMP1-mediated NF-κB activation in MEFs.
Fig. 6.
IRAK4 is not essential for LMP1-mediated NF-κB activation in MEFs. (A) IRAK4 WT and IRAK4 KO MEFs were treated with 100 ng/ml mIL-1 for 15 min or left untreated. Cell lysates were blotted for endogenous IκBα.(B) Cells were left untreated or treated with mIL-1 as in A and harvested at 15 and 30 min for electrophoretic mobility-shift assay. (C) IRAK4 WT or KO MEFs were transfected with reporter plasmids alone or a LMP1 expression plasmid. Results from one representative transfection of two performed in duplicate show the mean ± SE of folds of NF-κB activation by LMP1. LMP1 was expressed at similar levels in WT and KO MEFs (data not shown).
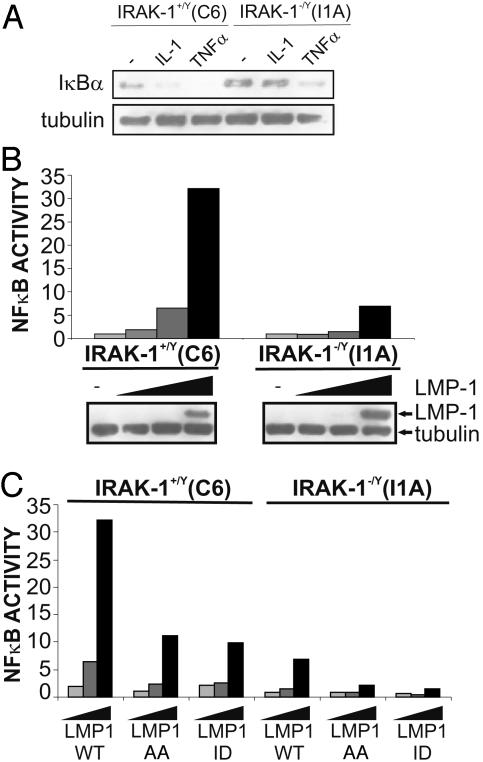
The role of IRAK1 was assessed by using I1A 293 cells (45). As expected, IL-1 and TNF induced IκBα degradation in control C6 293 cells (Fig. 7A). Similarly, as expected, IL-1 was unable to induce IκBα degradation in IRAK1-deficient I1A 293 cells, whereas TNF was relatively normal in these cells (Fig. 7A). However, whereas increasing amounts of LMP1 strongly activated NF-κB-dependent reporter activity in control cells, similar increasing amounts of LMP1 induced low level NF-κB activation in IRAK1-deficient cells (Fig. 7B). Thus, IRAK1 is critical for LMP1-mediated NF-κB activation in 293 cells.
Fig. 7.
IRAK1 is critical for LMP1-mediated NF-κB activation in 293 cells. (A) C6 or I1A cells were incubated with 100 ng/ml human TNF or human IL-1 and harvested 30 min later for immunoblotting for IκBα and tubulin (Sigma, C-2-1-5). (B) C6 and I1A cells were transfected with reporter plasmids alone or with 0, 0.1, 1, or 5 μg of an LMP1 expression plasmid and harvested 24 h later for immunoblotting and luciferase and β-gal assays. Results show NF-κB activation by LMP1 and LMP1 and tubulin expression levels. (C) Reporter assays were performed as in B except that, in addition to WT LMP1, AA and ID mutants were transfected in identical increasing amounts into C6 and I1A cells. Results show relative NF-κB activation by LMP1 alleles.
Because LMP1 activates an NF-κB-dependent reporter potently in 293 cells, the relative dependence of CTAR1/TES1 and CTAR2/TES2 on IRAK1 could be assessed. LMP1 AA (PQ204/206AA) is deficient in CTAR1/TES1 signaling, and LMP1 ID (YYD384–386ID) is deficient in CTAR2/TES2 signaling. Both induced similar levels of NF-κB activation at ≈30% of WT LMP1 levels in control C6 cells (Fig. 7C). Furthermore, both mutants were similarly reduced in NF-κB activation in IRAK1-deficient I1A cells (Fig. 7C). Thus, IRAK1 has a critical role in LMP1 CTAR1/TES1 and CTAR2/TES2-mediated NF-κB activation.
Discussion
These experiments reinforce previous understandings and provide new perspectives on the mechanisms by which LMP1 activates NF-κB. As reviewed in the introduction, LMP1 has six hydrophobic transmembrane domains that enable constitutive aggregation and signaling and two C-terminal cytoplasmic domains that mediate NF-κB activation and EBV-infected B lymphocyte proliferation. One domain binds TRAF1 and TRAF3 at a high level and TRAF2 and TRAF5 to a lesser extent, whereas TRAF6 binding is not detected. The other domain engages death domain proteins, including TRADD and RIP. Furthermore, dominant-negative IKKα or IKKβ mutant alleles inhibit LMP1-mediated NF-κB activation consistent with a model wherein LMP1 principally activates NF-κB through IKKα and IKKβ.
We now find that LMP1 activation of NF-κB can be independent of TRAF2 and TRAF5 in MEFs and surprisingly dependent on TRAF6 or IRAK1. TRAF6 can partially localize to sites of LMP1 aggregation, and overexpression of dominant-negative TRAF6 can inhibit LMP1 signaling in 293 cells (46). The role of TRAF6 is further supported by the inhibition of LMP1-mediated NF-κB activation by dominant-negative mutant TAB2 or Ubc13 alleles, which can bind to and inhibit TRAF6.
Although TRAF6 and IRAK1 dependence are hallmarks of TIR signaling, LMP1-mediated NF-κB activation in MEFs is independent of IRAK4, an essential component of TIR-associated NF-κB activation (31). LMP1 signaling to IRAK1 and TRAF6 is fundamentally different from TIR signaling not only in independence of IRAK4 but also in primarily assembling TRAF3 and TRAF1 at a high level as well as TRADD, RIP, and other death domain proteins at a low level. In contrast, TIRs initiate signals through MyD88 and other receptor-associated adaptor proteins (47).
We envisage LMP1-associated TRAFs and death domain protein complexes to be engaging TRAF6 and IRAK1 through TRAF6- or IRAK1-associated proteins. Candidates include the PKCζ-associated bridge between RIP and TRAF6, p62 (48); the IL-1 signal-dependent TRAF6-associated factors, TIFA (49) and Pellino (50); the TRAF6 mediators of a TAK1-like activation, Ubc13/Uev1a (39); and the potential scaffold for TRAF3 and TRAF6, TANK (51).
Because TRAF6 and IRAK1 have critical roles in both LMP1 and TIR signaling, activators of IKKβ downstream of TRAF6 and IRAK1, such as intermediary kinase(s) or ubiquitylation machinery, are likely to be shared by both pathways.
LMP1 differs from TIRs in that signaling from TRAF6 and IRAK1 to IKKβ can be IKKγ-independent, whereas TIR signaling is IKKγ-dependent (36). In this regard, LMP1 has hydrophobic transmembrane domains that mediate high level constitutive aggregation and signaling, and this may uniquely complement the IKKγ scaffold deficiency, or LMP1 may directly engage a potential IKKγ surrogate such as FIP2 (12).
LMP1 is unusual in the extent of hyperactivation of a MHC class I NF-κB site reporter in IKKα KO MEFs, particularly considering the critical role of IKKα in transcription through phosphorylation of p65 and histone H3 (52–54). Hyperactivation has also been observed in LTβR-induced MIP2, MIP1β, and p100 expression in mice with an IKKαAA kinase mutant allele knocked into an IKKα KO background (18). These data are consistent with a role for IKKα in IKKβ regulation and are supported by the finding of IKKβ kinase hyperactivity in basal as well as TNF-stimulated cells after expression of a kinase inactive IKKα mutant (55, 56). Moreover, IKKα KO keratinocytes exhibit prolonged TNFα-induced IκBα kinase activity, presumably attributed to IKKβ homodimers (57). Hyperactivation could also be due in part to the absence of IKKα-induced repressive transcriptional complexes (21).
Finally, we note that LMP1 is similar to the RIP-like kinase, PKK, in that PKK stimulation of NF-κB is also hyperactive in IKKα KO MEFs, is IKKβ-dependent, and is IKKγ-independent (58). LMP1 and PKK may therefore overlap in mechanisms of IKKβ activation.
Acknowledgments
We thank Drs. Dean Ballard, Michael Karin, Manolis Pasparakis, Klaus Rajewsky, James Chen, and Dimitris Thanos for cell lines and reagents and members of the Kieff, Kaye, and Wang laboratories for their helpful comments and discussion. This work was supported by an international scholarship of the Howard Hughes Medical Institute and an EMBO Young Investigator award (to G.M.), a Human Frontiers Science Program grant (to G.M. and H.N.), and National Institutes of Health Grants CA47006, CA85180, and CA87661 (to E.K.).
Abbreviations: β-gal, β-galactosidase; mIL-1, murine IL-1; CTAR, C-terminal activation regions; EBV, Epstein–Barr virus; IKK, IκB kinase; KO, knockout; LPS, lipopolysaccharide; MEF, murine embryo fibroblast; TES, transformation effector sites; TIR, Toll/IL-1R; KM, kinase mutant; TNF, tumor necrosis factor; TNFR, TNF receptor; TRAF, TNFR-associated factor; LMP, latent membrane protein; IRAK, IL-1 receptor-associated kinase.
References
- 1.Cahir-McFarland, E. D., Izumi, K. M. & Mosialos, G. (1999) Oncogene 18, 6959–6964. [DOI] [PubMed] [Google Scholar]
- 2.Rickinson, A. & Kieff, E. (2001) in Fields Virology, eds. Howley, P. M. & Knipe, D. (Lippincott, Philadelphia) pp. 2575–2627.
- 3.Wang, D., Liebowitz, D. & Kieff, E. (1985) Cell 43, 831–840. [DOI] [PubMed] [Google Scholar]
- 4.Kulwichit, W., Edwards, R. H., Davenport, E. M., Baskar, J. F., Godfrey, V. & Raab-Traub, N. (1998) Proc. Natl. Acad. Sci. USA 95, 11963–11968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cahir-McFarland, E. D., Davidson, D. M., Schauer, S. L., Duong, J. & Kieff, E. (2000) Proc. Natl. Acad. Sci. USA 97, 6055–6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang, D., Liebowitz, D., Wang, F., Gregory, C., Rickinson, A., Larson, R., Springer, T. & Kieff, E. (1988) J. Virol. 62, 4173–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang, D., Liebowitz, D. & Kieff, E. (1988) J. Virol. 62, 2337–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devergne, O., Hatzivassiliou, E., Izumi, K. M., Kaye, K. M., Kleijnen, M. F., Kieff, E. & Mosialos, G. (1996) Mol. Cell. Biol. 16, 7098–7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Izumi, K. M., Cahir McFarland, E. D., Ting, A. T., Riley, E. A., Seed, B. & Kieff, E. D. (1999) Mol. Cell. Biol. 19, 5759–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zandi, E., Rothwarf, D. M., Delhase, M., Hayakawa, M. & Karin, M. (1997) Cell 91, 243–252. [DOI] [PubMed] [Google Scholar]
- 11.Rothwarf, D. M., Zandi, E., Natoli, G. & Karin, M. (1998) Nature 395, 297–300. [DOI] [PubMed] [Google Scholar]
- 12.Li, Y., Kang, J., Friedman, J., Tarassishin, L., Ye, J., Kovalenko, A., Wallach, D. & Horwitz, M. S. (1999) Proc. Natl. Acad. Sci. USA 96, 1042–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller, B. S. & Zandi, E. (2001) J. Biol. Chem. 276, 36320–36326. [DOI] [PubMed] [Google Scholar]
- 14.Li, Q., Van Antwerp, D., Mercurio, F., Lee, K. F. & Verma, I. M. (1999) Science 284, 321–325. [DOI] [PubMed] [Google Scholar]
- 15.Li, Q., Lu, Q., Hwang, J. Y., Buscher, D., Lee, K. F., Izpisua-Belmonte, J. C. & Verma, I. M. (1999) Genes Dev. 13, 1322–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yilmaz, Z. B., Weih, D. S., Sivakumar, V. & Weih, F. (2003) EMBO J. 22, 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coope, H. J., Atkinson, P. G., Huhse, B., Belich, M., Janzen, J., Holman, M. J., Klaus, G. G., Johnston, L. H. & Ley, S. C. (2002) EMBO J. 21, 5375–5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dejardin, E., Droin, N. M., Delhase, M., Haas, E., Cao, Y., Makris, C., Li, Z. W., Karin, M., Ware, C. F. & Green, D. R. (2002) Immunity 17, 525–535. [DOI] [PubMed] [Google Scholar]
- 19.Claudio, E., Brown, K., Park, S., Wang, H. & Siebenlist, U. (2002) Nat. Immunol. 3, 958–965. [DOI] [PubMed] [Google Scholar]
- 20.Kayagaki, N., Yan, M., Seshasayee, D., Wang, H., Lee, W., French, D. M., Grewal, I. S., Cochran, A. G., Gordon, N. C., Yin, J., et al. (2002) Immunity 17, 515–524. [DOI] [PubMed] [Google Scholar]
- 21.Senftleben, U., Cao, Y., Xiao, G., Greten, F. R., Krahn, G., Bonizzi, G., Chen, Y., Hu, Y., Fong, A., Sun, S. C. & Karin, M. (2001) Science 293, 1495–1499. [DOI] [PubMed] [Google Scholar]
- 22.Xiao, G., Harhaj, E. W. & Sun, S. C. (2001) Mol. Cell 7, 401–409. [DOI] [PubMed] [Google Scholar]
- 23.Nakano, H., Oshima, H., Chung, W., Williams-Abbott, L., Ware, C. F., Yagita, H. & Okumura, K. (1996) J. Biol. Chem. 271, 14661–14664. [DOI] [PubMed] [Google Scholar]
- 24.Rothe, M., Sarma, V., Dixit, V. M. & Goeddel, D. V. (1995) Science 269, 1424–1427. [DOI] [PubMed] [Google Scholar]
- 25.Devin, A., Lin, Y., Yamaoka, S., Li, Z., Karin, M. & Liu, Z. (2001) Mol. Cell. Biol. 21, 3986–3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devin, A., Cook, A., Lin, Y., Rodriguez, Y., Kelliher, M. & Liu, Z. (2000) Immunity 12, 419–429. [DOI] [PubMed] [Google Scholar]
- 27.Zhang, S. Q., Kovalenko, A., Cantarella, G. & Wallach, D. (2000) Immunity 12, 301–311. [DOI] [PubMed] [Google Scholar]
- 28.Hsu, H., Shu, H. B., Pan, M. G. & Goeddel, D. V. (1996) Cell 84, 299–308. [DOI] [PubMed] [Google Scholar]
- 29.Tada, K., Okazaki, T., Sakon, S., Kobarai, T., Kurosawa, K., Yamaoka, S., Hashimoto, H., Mak, T. W., Yagita, H., Okumura, K., et al. (2001) J. Biol. Chem. 276, 36530–36534. [DOI] [PubMed] [Google Scholar]
- 30.Lomaga, M. A., Yeh, W. C., Sarosi, I., Duncan, G. S., Furlonger, C., Ho, A., Morony, S., Capparelli, C., Van, G., Kaufman, S., et al. (1999) Genes Dev. 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki, N., Suzuki, S., Duncan, G. S., Millar, D. G., Wada, T., Mirtsos, C., Takada, H., Wakeham, A., Itie, A., Li, S., et al. (2002) Nature 416, 750–756. [DOI] [PubMed] [Google Scholar]
- 32.Cao, Z., Xiong, J., Takeuchi, M., Kurama, T. & Goeddel, D. V. (1996) Nature 383, 443–446. [DOI] [PubMed] [Google Scholar]
- 33.Izumi, K. M. & Kieff, E. D. (1997) Proc. Natl. Acad. Sci. USA 94, 12592–12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylla, B. S., Hung, S. C., Davidson, D. M., Hatzivassiliou, E., Malinin, N. L., Wallach, D., Gilmore, T. D., Kieff, E. & Mosialos, G. (1998) Proc. Natl. Acad. Sci. USA 95, 10106–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaye, K. M., Izumi, K. M., Li, H., Johannsen, E., Davidson, D., Longnecker, R. & Kieff, E. (1999) J. Virol. 73, 10525–10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolph, D., Yeh, W. C., Wakeham, A., Rudolph, B., Nallainathan, D., Potter, J., Elia, A. J. & Mak, T. W. (2000) Genes Dev. 14, 854–862. [PMC free article] [PubMed] [Google Scholar]
- 37.Kaye, K. M., Devergne, O., Harada, J. N., Izumi, K. M., Yalamanchili, R., Kieff, E. & Mosialos, G. (1996) Proc. Natl. Acad. Sci. USA 93, 11085–11090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Izumi, K. M., Cahir-McFarland, E. D., Riley, E. A., Rizzo, D., Chen, Y. & Kieff, E. (1999) J. Virol. 73, 9908–9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deng, L., Wang, C., Spencer, E., Yang, L., Braun, A., You, J., Slaughter, C., Pickart, C. & Chen, Z. J. (2000) Cell 103, 351–361. [DOI] [PubMed] [Google Scholar]
- 40.Wang, C., Deng, L., Hong, M., Akkaraju, G. R., Inoue, J. & Chen, Z. J. (2001) Nature 412, 346–351. [DOI] [PubMed] [Google Scholar]
- 41.Mizukami, J., Takaesu, G., Akatsuka, H., Sakurai, H., Ninomiya-Tsuji, J., Matsumoto, K. & Sakurai, N. (2002) Mol. Cell. Biol. 22, 992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takaesu, G., Kishida, S., Hiyama, A., Yamaguchi, K., Shibuya, H., Irie, K., Ninomiya-Tsuji, J. & Matsumoto, K. (2000) Mol. Cell 5, 649–658. [DOI] [PubMed] [Google Scholar]
- 43.Sanjo, H., Takeda, K., Tsujimura, T., Ninomiya-Tsuji, J., Matsumoto, K. & Akira, S. (2003) Mol. Cell. Biol. 23, 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janssens, S. & Beyaert, R. (2003) Mol. Cell 11, 293–302. [DOI] [PubMed] [Google Scholar]
- 45.Li, X., Commane, M., Burns, C., Vithalani, K., Cao, Z. & Stark, G. R. (1999) Mol. Cell. Biol. 19, 4643–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schultheiss, U., Puschner, S., Kremmer, E., Mak, T. W., Engelmann, H., Hammerschmidt, W. & Kieser, A. (2001) EMBO J. 20, 5678–5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O'Neill, L. A. (2003) Biochem. Soc. Trans. 31, 643–647. [DOI] [PubMed] [Google Scholar]
- 48.Sanz, L., Diaz-Meco, M. T., Nakano, H. & Moscat, J. (2000) EMBO J. 19, 1576–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takatsuna, H., Kato, H., Gohda, J., Akiyama, T., Moriya, A., Okamoto, Y., Yamagata, Y., Otsuka, M., Umezawa, K., Semba, K. & Inoue, J. (2003) J. Biol. Chem. 278, 12144–12150. [DOI] [PubMed] [Google Scholar]
- 50.Jiang, Z., Johnson, H. J., Nie, H., Qin, J., Bird, T. A. & Li, X. (2003) J. Biol. Chem. 278, 10952–10956. [DOI] [PubMed] [Google Scholar]
- 51.Cheng, G. & Baltimore, D. (1996) Genes Dev. 10, 963–973. [DOI] [PubMed] [Google Scholar]
- 52.Anest, V., Hanson, J. L., Cogswell, P. C., Steinbrecher, K. A., Strahl, B. D. & Baldwin, A. S. (2003) Nature 423, 659–663. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto, Y., Verma, U. N., Prajapati, S., Kwak, Y. T. & Gaynor, R. B. (2003) Nature 423, 655–659. [DOI] [PubMed] [Google Scholar]
- 54.Sizemore, N., Lerner, N., Dombrowski, N., Sakurai, H. & Stark, G. R. (2002) J. Biol. Chem. 277, 3863–3869. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto, Y., Yin, M. J. & Gaynor, R. B. (2000) Mol. Cell. Biol. 20, 3655–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Mahony, A., Lin, X., Geleziunas, R. & Greene, W. C. (2000) Mol. Cell. Biol. 20, 1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu, Y., Baud, V., Oga, T., Kim, K. I., Yoshida, K. & Karin, M. (2001) Nature 410, 710–714. [DOI] [PubMed] [Google Scholar]
- 58.Muto, A., Ruland, J., McAllister-Lucas, L. M., Lucas, P. C., Yamaoka, S., Chen, F. F., Lin, A., Mak, T. W., Nunez, G. & Inohara, N. (2002) J. Biol. Chem. 277, 31871–31876. [DOI] [PubMed] [Google Scholar]
- 59.Lufig, M., Yasui, T., Soni, V., Kang, M., Jacobson, N., Cahir-McFarland, E. & Kieff, E., Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]