Abstract
Human skin equivalents (HSEs) are in vitro tissues in which a fully differentiated, stratified squamous epithelium is grown at an air–liquid interface on a Type I collagen gel harboring human dermal fibroblasts. HSEs now provide experimental human tissue models to study factors that direct re-epithelialization and epithelial–mesenchymal cross-talk following wounding. This chapter describes the fabrication of HSEs from human keratinocytes and fibroblasts and how HSEs can be modified to characterize the response of the human epithelium during wound repair. The protocols outlined first describe techniques for the generation of human tissues that closely approximate the architectural features, differentiation, and growth of human skin. This will be followed by a description of a protocol that enables HSEs to be adapted to monitor their response following wounding. These engineered human tissues provide powerful tools to study biological process in tissues that mimic the healing of human skin and of the epithelial tissue.
Keywords: Organotypic culture, Three-Dimensional model, Human skin, Wound repair, Fibroblasts
1. Introduction
1.1. Fabrication of Three-Dimensional Model of Human Skin
The development and application of tissue-engineered models that mimic human skin, known as human skin equivalents (HSEs), provide in vivo-like tissues to study epidermal biology (1, 2). We first describe methods for constructing models of human epidermis that mimic the three-dimensional tissue architecture and behavior of normal human skin. Construction of a multi-layered epithelium is accomplished by growing skin keratinocytes on the surface of a Type I collagen gel that is populated with dermal fibroblasts (Fig. 24.1). Following several days during which tissue constructs are immersed in medium, HSEs are grown at an air–liquid interface, so that tissues can fully recapitulate the in vivo-like morphologic and biochemical processes of human skin. These HSEs are amenable to manipulation of medium, substrate conditions and cellular constituents to create novel microenvironments that mimic a variety of wound conditions in cutaneous tissues.
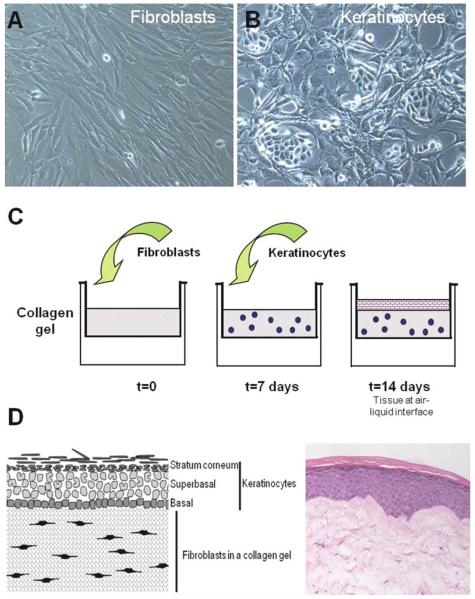
Fig. 24.1.
The three-dimensional culture technique. Cultures of human dermal fibroblasts (A) and human keratinocytes (B) are cultivated several days prior to the construction of the HSEs. Fibroblasts should be incorporated into collagen gels when they have a high number at dividing cells. To achieve this, cells are grown to confluence (A), split at 9:10 ratio and used the following day to construct collagen gels. To limit the number of differentiated cells and to increase cellular growth fraction, colonies should be relatively small in size (B). To fabricate tissues (C) fibroblasts are mixed with type I collagen and allowed to contract for 1 week prior to seeding keratinocytes. After culturing the HSEs at the air–liquid interface, a fully stratified epidermis is formed demonstrating all layers present in normal human epithelium (D).
1.2. Fabrication of Wound Healing Model
The second part of this chapter will describe how HSEs can be adapted to characterize their response during wound re-epithelialization. This protocol describes the fabrication of HSEs that are wounded and undergo re-epithelialization as keratinocytes reestablish epithelial integrity (Fig. 24.2). In vitro studies of wound re-epithelialization have often been limited by their inability to simulate wound repair as it occurs in humans. For example, “scratch” wound models using two-dimensional monolayer, keratinocyte cultures demonstrate very limited stratification, partial differentiation, and hyperproliferative growth. These cultures are helpful in studying keratinocyte migration in response to wounding, but have been of limited use in studying the complex nature of keratinocyte response and epithelial–mesenchymal cross-talk during wound repair, as monolayer cultures do not provide the tissue complexity needed to study the in vivo wound response.
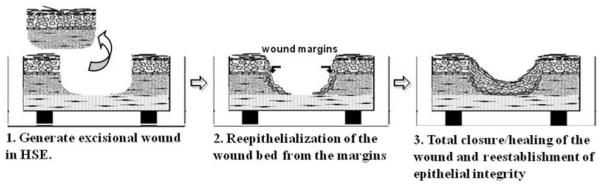
Fig. 24.2.
Diagram of the wounding of skin-equivalent cultures. (1) An excisional wound is created by removing the central portion of an HSE. (2) This tissue is layered onto a freshly contracted Type I collagen gel and migrating keratinocytes from the edge of the wound move across the wound bed to repopulate it. (3) Complete re-epithelialization leads to reestablishment of epithelial integrity as keratinocytes reconstitute a fully stratified epithelium.
HSEs can be adapted to study wound repair in human epithelium in a manner that simulates the chronology of events that occur during re-epithelialization in human skin (3, 4). These HSEs enable direct determination of the phenotypic response parameters of a wounded epithelium including cell proliferation, migration, differentiation, growth-factor response, and protease expression of epithelial and stromal cells. This wound repair model demonstrates the utility of HSEs in studying phenotypic responses that are characteristic of the switch from a normal to a regenerative epithelium upon wound re-epithelialization. This protocol describes construction of tissues that allow monitoring of the response of HSEs during re-epithelialization of wounded human skin, from the onset of keratinocyte activation and ending upon restoration of epithelial integrity. Using these protocols, HSEs are fabricated as described above and are wounded 7 days after keratinocytes are seeded onto the contracted collagen gel. One week before wounding these tissues, an additional collagen gel is fabricated that serves as a substrate onto which the wounded tissue will be transferred to monitor re-epithelialization.
1.3. Modification of Fibroblasts and Incorporation into Collagen Gels
Specialized adaptations of this HSE model that allow direct study of the role of modified fibroblasts and stromal substrate are also described. In fact, HSEs allow multiple manipulations of several components of the tissue fabrication process: the modification of the composition of the culture medium, the nature of the scaffold material, and the type of cells incorporated in the HSEs. As an example, in this protocol we describe the incorporation of phenotypically modified dermal fibroblasts and how this modification was linked to the response of wounded HSEs.
We have previously published a study using intact and wounded HSEs to test the capacity of fibroblasts passaged extensively or exposed to ECM composed of either normal collagen (NC) or denaturated collagen (DC) to modulate the phenotype of these tissues (5). We have demonstrated that dermal fibroblasts grown after extended passage on DC and incorporated into HSEs were able to modify the properties of the adjacent surface epithelium by increasing the proliferation of basal keratinocytes and significantly shortened the time needed for wounded HSEs to undergo complete re-epithelialization (Fig. 24.3). This study demonstrated that cell modifications mediated by cross-talk between dermal fibroblasts in the ECM microenvironment hold tremendous potential for novel therapeutic applications and for understanding mechanisms through which paracrine interactions between fibroblasts and keratinocytes may accelerate wound repair and reestablish tissue homeostasis.
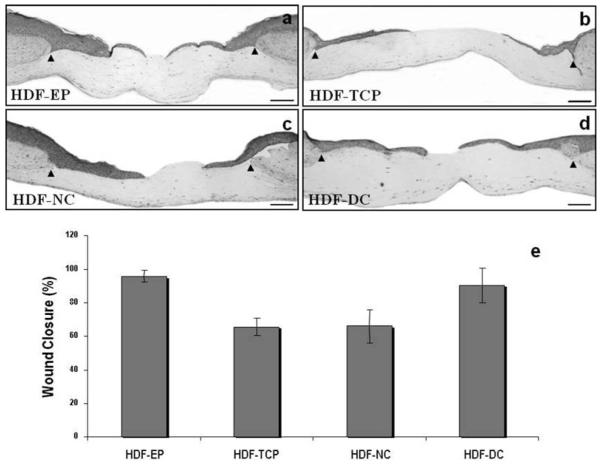
Fig. 24.3.
Fibroblasts modified by growth on denatured collagen accelerate the rate of re-epithelialization of wounded HSEs. Panels (a–d) illustrate the morphology of skin-equivalent cultures fabricated using human dermal fibroblasts early passaged (less than 7 passages on plastic culture plates) named HDF-EP cells (a) or passaged extensively (more than 15 passages) on plastic culture plates HDF-TCP (b), on plastic plates coated with normal collagen HDF-NC (c), and on plastic plates coated with heat-denaturated collagen HDF-DC (d) cells 48 hours after wounding. Arrows demarcate the initial wound edges. The progress of the wound re-epithelialization was determined by comparing the cultures immediately after wounding (time zero) with those seen 48 hours later for each condition expressed graphically as the percentage of wound closure (e). Bar=100 μm.
2. Materials
2.1. Medium Components
Water (see Note 1)
0.5 M EDTA, pH 8.0 (Invitrogen, Carlsbad, CA, cat.# 15575)
PBS (Invitrogen, Carlsbad, CA, cat.#14190)
0.25% Trypsin (Invitrogen, Carlsbad, CA, cat.# 15050)
5 mM EDTA; add 5 ml of 0.5 M EDTA to 500 ml PBS
10% EDTA/PBS; mix 50 ml of 5 mM EDTA with 450 ml PBS
0.1% Trypsin – mix 50 ml 0.25% trypsin with 75 ml PBS
50% Trypsin/EDTA – mix 50 ml of 0.1% trypsin with 50 ml of 5 mM EDTA
100× HEPES (800 mM) – dissolve 47.24 g in 250 ml ddH2O, store at −20°C for up to 1 year (Sigma, St. Louis, MO, cat.# H-4034)
100× Adenine (18 mM) – dissolve 0.972 g in 2.4 ml 4 N NaOH, q.s. to 400 ml with ddH2O, store at −20°C for up to 1 year (MP biomedicals, Solon, OH, cat.# 100190)
500× Hydrocortisone (0.25 mg/ml) – dissolve 0.0538 g in 200 ml ddH2O, store at −20°C for up to 1 year (Sigma, St. Louis, MO, cat.# H-4881
1,000× Cholera toxin (10−7 M) – dissolve 9 ng/ml in ddH2O, store at −20°C for up to 1 year (Sigma, St. Louis, MO, cat.# C-8052)
1,000× EGF (10 μg/ml) – dissolve 10 μg/ml in 0.1% BSA, store at −20°C for up to 1 year (Austral Biological, San Ramon, CA, cat.# GF-010-9)
1,000× Insulin (5 mg/ml) – dissolve 50 mg in 10 ml of 0.005 N HCl, store at −20°C for up to 1 year (Sigma, St. Louis, MO, cat.# I-2643)
10 nM triiodothyronine (T3) – add 1 ml T3 to 99 ml ddH2O for 500× stock (Sigma, cat # T-5516)
2 μM progesterone – dissolve 1 mg in 1 ml absolute ethanol, add 14.7 ml ddH2O, dilute 1 ml in 100 ml DMEM for 1,000× stock (Sigma, St. Louis, MO, cat.# P-8783)
Chelated BCS – Chelate serum by adding 10 g CHELEX 100 (Sigma, St. Louis, MO, cat.# C-7901) to 100 ml serum and stirring for 3 h at 4°C, then filter through Whatman paper, then through a sterile filter.
Transferrin (5 mg/ml) – (BioSource cat. # 352-020, 200 ml)
500× PES – contains O-phosphorylethanolamine (0.01 mM final), ethanolamine (10 μM final), and selenium (10 μg/ml final) (BioSource, cat.# P02-45-100)
2.1.1. 010 medium
43 g DME powder (JRH Biosciences, this is a special order medium base that is prepared in bulk) contains no glucose and no CaCl2
5 L ddH2O
0.5 g MgSO4
18.5 g NaHCO3
2.1.2. Keratinocyte Culture Medium
338 ml DME medium (Invitrogen, Carlsbad, CA, cat.# 11885)
112 ml F12 medium (Invitrogen, Carlsbad, CA, cat.# 11765)
25 ml FBS (Hyclone, Logan, UT, cat.# SH30071.03) (5% final)
5 ml 18 mM adenine (0.18 mM final)
3.4 ml 100× penicillin/streptomycin (Invitrogen, Carlsbad, CA, cat.# 15140-122)
5 ml 800 mM HEPES (8 mM final)
1 ml 0.25 mg/ml hydrocortisone (0.5 μg/ml final)
0.5 ml 10−7 M cholera toxin (10−10 M final) (see Note 2)
0.5 ml 10 μg/ml EGF (10 ng/ml final)
0.5 ml 5 mg/ml insulin (5 μg/ml final) Store up to 2 weeks at 4°C.
2.1.3. Fibroblast Culture Medium
500 ml DME medium (Invitrogen, Carlsbad, CA, cat.#11885)
55.6 ml FBS (Hyclone, Logan, UT, cat.# SH30071.03) (10% final)
5.6 ml 800 mM HEPES (8 mM final)
-
3.4 ml 100× Penicillin/Streptomycin (Invitrogen, Carlsbad, CA, cat.#15140-122)
Store up to 2 weeks at 4°C.
2.1.4. 3T3 Medium
500 ml DME medium (Invitrogen, Carlsbad, CA, cat.# 11885)
55.6 ml Bovine Calf Serum (Hyclone, Logan, UT, cat.# SH30072.03) (10% final)
3.4 ml 100× Penicillin/Streptomycin (Invitrogen, Carlsbad, CA, cat.#15140-122)
2.2. Fabrication of Tridimensional Tissues
2.2.1. Human Skin Equivalents
6-well tissue culture tray with 3 μm porous polycarbonate membrane inserts (Organogenesis, Canton, MA, cat.# MS-10-305)
Bovine tendon Type I collagen (Organogenesis, cat.# 200-055)
Human foreskin fibroblasts
Fibroblast culture medium (see Reagents and Solutions)
Trypsin/ethylenediaminetetraacetic acid (EDTA)
Human neonatal foreskin keratinocytes
Phosphate-buffered saline (PBS)
PBS/EDTA
Centrifuge (1,000–2,000 RPM range)
2.2.2. Fabrication of Three-Dimensional Wound Healing Model of Human Skin
6-well tissue culture tray with 3 μm porous polycarbonate membrane inserts (Organogenesis, Canton, MA, cat.# MS-10-305)
14 cm stainless steel dermatological punch (Delasco, Council Bluffs, IA, cat.# KP-14)
Dental mirror for transfer of wounded culture
Sterile scalpel with #22 blade
3. Methods
3.1. Fabrication of Collagen Matrix with Dermal Fibroblasts
Culture human foreskin fibroblasts (HFFs) in monolayer culture so that they are almost confluent one day before incorporation into the collagen.
The day before incorporation, passage fibroblasts at a 9:10 split ratio so they will be mitotically active the next day when incorporated into the collagen gel.
Passaging cells from a confluent plate ensures a higher fraction of actively dividing cells upon incorporation into collagen gels. A 9:10 passage is performed by resuspending the trypsinized cells in 10 ml of medium and adding 9 ml of the cell suspension to a new plate.
The following day, the collagen matrix is prepared by fabricating successive layers of acellular and cellular collagen onto the polycarbonate membrane. Prepare the acellular collagen as a mixture that is cooled on ice to prevent premature gelation (see Note 3). To do this, pipettes should be chilled at −20°C for 15 min before use to prevent warming of collagen when it is mixed. Avoid air bubbles when mixing. Collagen should be a straw-yellow to light pink color to ensure optimal gelation. If the color is bright yellow, add a single drop of sodium bicarbonate and triturate until a straw-yellow color is seen.
Add 1 ml of acellular collagen to each insert. Ensure that the matrix coats the entire bottom surface of the insert and allow it to gel at room temperature for 20 min. Do not move the tray while it is undergoing gelation. The color will turn pink when the collagen has fully gelled.
Trypsinize, count, and resuspend the fibroblasts to a final concentration of 3 × 105 cells/ml. A total of 5 × 105 fibroblasts will be used per 6-well tray.
Prepare the cellular collagen as a mixture that is cooled on ice (see Note 3). Fibroblasts should be added last after collagen has been neutralized so that the cells will not be damaged by the alkaline pH that exists before neutralization. Resuspend the cell/collagen suspension by gentle trituration to evenly incorporate fibroblasts into the collagen gel.
Gently triturate the cellular matrix and add 3 ml into each insert on top of the gelled acellular collagen matrix. Gently transfer the mixture to the incubator for 30 min.
When the cellular matrix has turned pink and is completely gelled (usually less than 30 min), feed the gels with 12 ml of fibroblast medium by adding 10 ml of medium to the well around the insert and 2 ml of medium directly onto the insert.
Gels are then incubated for 5–7 days to allow complete gel contraction.
During the first few days, the sides of the gel contract and will form a plateau in the center. Gels are stable between 5 and 10 days after initial construction.
3.2. Addition of Keratinocytes to the Surface of Contracted Collagen Gels
Normal human keratinocytes are cultured on a feeder layer of mitotically inactivated mouse 3T3 fibroblasts. Keratinocytes should be grown to no more than 50% confluence to minimize the number of differentiated cells seeded onto the collagen gel. Alternatively, keratinocytes can be grown in monolayer culture in low calcium and serum-free medium (see Note 4).
Remove the 3T3 feeder cells from the culture by incubating the plates in PBS/EDTA for 5 min at 37°C. 3T3’s can then be displaced by gentle pipetting so that keratinocytes will remain attached. It is important not to allow the cultures to incubate for an excessive time in PBS/EDTA, as the keratinocytes may detach from the plate as well. As soon as the 3T3’s have begun to detach, replace the PBS/EDTA with PBS, gently rinse the plate three times with PBS until all 3T3’s have been completely removed. PBS is then removed, leaving only keratinocyte colonies attached to the plate.
Trypsinize the keratinocytes with trypsin/EDTA (0.05%) for 5 min at 37°C to obtain a single cell suspension. Remove the detached cells into a 15 ml tube containing keratinocyte medium (to neutralize the trypsin) and count them. The desired number of cells is dispensed into a 15 ml tube from the tube with the trypsinized cells. Cells are then centrifuged 2,000 × g for 5 min and resuspended in a volume so that a total of 5 × 105 keratinocytes can be used per insert.
Remove all fibroblast medium from the trays with the contracted collagen 20 min before seeding keratinocytes so that keratinocytes can be seeded onto a moist collagen gel. Keratinocytes should be seeded directly onto the contracted collagen gels in an aliquot of 50 μl containing 5 × 105 cells. To modify the nature of the substrate on which keratinocytes are seeded, de-epidermalized dermis or coated polycarbonate inserts can be applied directly on top of the contracted collagen gels at this point (see Note 5).
Resuspend keratinocytes in a volume of Epidermalization I medium to a final concentration of 500,000 cells/50 μl. Carefully add the 50 μl of the cell suspension to the center of the contracted collagen gel (or onto the center of the intervening substrate placed on the collagen gel). Do not move the tray for 15 min to allow the keratinocytes to attach. Constructs are then incubated at 37°C for 30–60 min. without any medium to allow the keratinocytes to fully adhere.
Add 12 ml of Epidermalization I medium to each insert by adding 10 ml to the bottom of the well and 2 ml gently into the insert on top of the keratinocytes. Incubate at 37°C.
- Cultures are fed with medium every 2 days as follows:
- Epidermalization I medium – 12 ml per well for the first 2 days.
- Epidermalization II medium – 12 ml per well for the next 2 days.
- Cornification medium – At this point, cultures are raised to the air–liquid interface by adding 7 ml per well to the bottom of the well so that the insert just contacts the medium. Aspirate medium from the inside of the insert so that tissues can be grown at the air–liquid interface. Additional feedings with Cornification medium are done every 2 days until termination of the experiment.
3.3. Fabrication of Three-Dimensional Wound Healing Model of Human Skin
3.3.1. Tissue Wounding
HSEs to be wounded are first generated as described above. This protocol requires that an HSE with normal, primary keratinocytes and a second contracted collagen gel (onto which the wounded epithelium will be transferred) will first be simultaneously constructed.
Aspirate all medium from the HSE after 7–10 days of culture. Remove the insert from the tray and place it upside down in a sterile dish. Using a scalpel, cut away the insert membrane, and place the culture in a sterile dish right side up.
Trim the culture with the scalpel by cutting around the raised, mesa-like region to remove the part of the collagen gel not covered by keratinocytes. This will facilitate the removal and transfer of the wounded tissue from the membrane.
Cultures can be wounded with either an incisional or excisional wound. An incisional wound can be generated by incising tissues with a scalpel in a way that will allow the wound edges to be separated to generate an elliptical wound.
An excisional wound can be generated using an elliptical dermatological punch that completely penetrates the center of the tissue through the epidermis, collagen, and membrane. The excised tissue can be fixed and preserved for H&E staining in 10% formalin.
Use forceps to gently lift the edge of the wounded tissue by separating the collagen gel from the membrane. Drag the tissue onto a dental mirror while leaving the membrane behind. The transfer may be easier if the mirror is moistened with medium.
Unfold any wrinkles in the culture by gently moving the tissue back and forth on the mirror using the forceps. Once the culture is smooth, pull one side of the culture slightly over the edge of the mirror.
Carry the mirror directly over the second contracted collagen matrix so that the edge of the mirror and wounded tissue are in contact with the matrix. Slide the tissue onto the second collagen gel by teasing it gently with a closed forceps as the mirror is slowly pulled away, leaving the culture on the contracted collagen gel.
Using the forceps, tease apart the tissue wounded by incision to create an elliptical space that should be 2–3 mm at its greatest width. Smooth the tissue with the forceps to ensure that it is completely free of any folds or wrinkles.
Maintain the tissue at an air–liquid interface by adding 8 ml of Epidermalization II medium beneath the insert during re-epithelialization, change the medium every 2 days until the end of the experiment.
3.3.2. Modification of Fibroblasts and Incorporation into Collagen Gels
Phenotypically modified Human dermal fibroblasts. Cells were seeded at a density of 5 × 104 cells/ml and cultures were sequentially passaged when cell density reached confluence. Passage 8 HDFs (EP HDF) were maintained for 12 additional passages on tissue culture plastic (HDF-TCP), heated-denatured collagen films (HDF-DC), or native collagen films (HDF-NC).
Type I collagen (Roche, cat. #1179179) was dissolved at 5 mg/ml in 0.1% acetic acid and denatured by incubation at 50°C for 12 hours. These conditions were chosen based on the complete denaturation of collagen following thermal transition at 45°C.
To prepare collagen films for cell passage, 1.5 ml of collagen solution (0.5 mg/ml) was added to 35-mm tissue culture plates (Corning) and dried under vacuum.
3.4. Tissue Harvesting and Embedding for Morphological and Immunohistochemical Analysis
Remove medium from inserts and gently rinse tissues twice in PBS.
Cut away the insert membrane from the plastic insert at its base using a scalpel.
Bisect the culture and place one-half in a tissue processing cassette and immediately immerse in 10% formalin. Tissues are very thin and thus only require a short fixation (1 hour) before paraffin processing.
The other half of the tissue should be placed in a 2 M sucrose solution prepared in water. Tissue should be soaked in sucrose at 4°C for at least 1 hour, but for not more than 24 hours. Sucrose replaces water in the hydrated collagen gel and protects against freezing damage during embedding and processing.
To embed sucrose-soaked tissue for frozen preservation, make a small mold with aluminum foil using the cap or bottom of a small bottle (roughly 2 cm in diameter). Fill three-fourths of the mold with embedding medium.
Gently remove the tissue from the sucrose using narrow-tipped forceps, making sure to keep the tissue on the nylon insert membrane. Gently touch the membrane side of the tissue to a kimwipe to remove excess sucrose.
Place the tissue in the embedding medium and allow the tissue to soak for 20–30 min at room temperature.
Place a metal rack inside a Styrofoam box and fill it with liquid nitrogen to a height just under the top of the metal rack. Place the aluminum foil mold on top of the rack and stand the tissue inside to an upright position. The liquid nitrogen vapors will freeze the embedding medium and tissue in about 5 min. The tissue can then be stored at −80°C.
For histological analysis of wounded HSE: Process the tissue as described above, making sure to bisect the tissue perpendicular to the long axis of the wound (i.e., along the greatest width of the wound). Mount the tissue for sectioning en face so that the greatest width of the wound is sectioned first. It is essential to capture the first few sections as these will be the most informative sections of the wound.
3.5. Anticipated Results
Several points regarding keratinocyte behavior in HSEs should be mentioned. The first concerns the length of time during which cultures can be maintained at the air–liquid interface. In our experience, cultures can be kept at this interface for up to 17 days. After this time, the surface layer of the epithelium becomes excessively thickened due to a failure to desquamate. As a result, lower layers of the epithelium become compressed and the longevity of cultures is limited. A second and related issue concerns the growth potential of keratinocytes in HSEs. While HSEs demonstrate a basal level of proliferation that is greater than that of human skin, it has been shown that these cultures have a tremendous potential for cell growth and are very responsive to external growth stimuli.
It should be kept in mind that although keratinocytes grown in HSEs share many morphologic and biochemical features with in vivo skin, there are differences in tissue phenotype. For example, integrin receptors not normally expressed in skin may be constitutively expressed in keratinocytes grown in HSEs. This may be the result of HSEs being somewhat deficient in barrier function.
3.6. Time Considerations
Construction of HSEs requires approximately 3–4 weeks from the time HFF cells are seeded in monolayer culture until three-dimensional tissues are fully mature. HFF cells should be confluent 2 days before construction of the collagen gels. At that time, cells should be passaged at a 9:10 ratio to provide cells with a growth stimulus before incorporation into collagen gels. Complete contraction of the collagen gels requires 7–10 days, during which time the human keratinocyte cell cultures are initiated and expanded. The keratinocytes will need another week of culture before the HSEs are ready for wounding.
Footnotes
Milli-Q water should be used for preparation of all medium and supplements, and all solutions should be filtered through a 0.22 μm filter for sterility.
Cholera toxin is very toxic. Use appropriate precautions when handling stock solutions.
Fabrication of the collagen gel requires that all components be kept on ice until the gel mixture is placed into the insert. This will ensure that collagen will not prematurely precipitate from these solutions. Plastic pipettes used for collagen should be chilled before use.
The amounts listed are for a single 6-well tray scale accordingly for a smaller or larger number of inserts.
0.6 ml 10× MEM (Minimum essential medium with Earle’s salts) (Cambrex, Walkersville, MD, cat.# 12-684F)
54 μl 200 mM L-Glutamine (Invitrogen, Carlsbad, CA, cat.# 25030-081)
0.68 ml FBS (Hyclone, Logan, UT, cat.# SH30071.01)
187 μl 71.2 mg/ml NaHCO3 (Cambrex, Walkersville, MD, cat.# 17-605E)
5 ml Bovine Type I Collagen (Organogenesis, Canton, MA, cat.# 200-055) Fresh and used immediately. It should not be stored once mixed.
Keratinocyte proliferation and a high growth fraction are the most critical factors in the successful fabrication of HSEs. Most keratinocytes seeded onto HSE cultures will adhere to the connective substrate, but only replicating cells will grow after seeding. Keratinocytes that underwent a commitment to terminal differentiation while still in submerged culture will also attach to the substrate, but will not undergo further proliferation to form a well-stratified HSE. It is therefore important to grow keratinocytes so that a high growth fraction is present when monolayer cultures are seeded onto the contracted collagel gel of the HSEs. This can be accomplished by growing keratinocytes as small colonies at high clonal density in submerged cultures on 3T3 feeder layers, so that terminal differentiation will be minimized and the fraction of replicating cells will be maximized. Keratinocyte strains can be tested by screening them using a colony efficiency assay to determine those strains with the highest colony forming efficiency providing optimal morphologic differentiation and tissue architecture of HSEs.
In addition, the protocols described can be modified to allow growth of epithelial tissues on a variety of connective tissue substrates. Each of these interfaces presents an ECM that can be tailored to answer specific experimental questions. For example, tissues grown on the de-epidermalized dermis serve as an interface on which the rapid assembly of structured basement membrane occurs and tissue morphology is optimized (1). Alternatively, cultures can be grown directly on polycarbonate membranes coated with specific ECM proteins to directly study the effect of these proteins on cellular phenotype in these tissues (6).
We have found some variability in the degree to which fibroblast strains support keratinocyte growth after their incorporation into collagen gels. It appears that fibroblast support of HSE organization and growth is directly related to the degree to which fibroblasts are able to contract the collagen gel. In general, fibroblast strains demonstrating more shrinkage of the collagen gel before adding keratinocytes are better able to support keratinocyte growth. This parameter may be used to screen fibroblast strains for optimal growth support when initiating HSEs.
References
- 1.Andriani F, Margulis A, Lin N, Griffey S, Garlick JA. Analysis of micro-environmental factors contributing to basement membrane assembly and normalized epidermal phenotype. J. Invest. Dermatol. 2003;120:923–931. doi: 10.1046/j.1523-1747.2003.12235.x. [DOI] [PubMed] [Google Scholar]
- 2.Kolodka TM, Garlick JA, Taichman LB. Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus-transduced keratinocytes. Proc. Natl. Acad. Sci. USA. 1998;95:4356–4361. doi: 10.1073/pnas.95.8.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garlick JA, Taichman LB. Fate of human keratinocytes during reepithelialization in an organotypic culture model. Lab. Invest. 1994;70:916–924. [PubMed] [Google Scholar]
- 4.Garlick JA, Parks WC, Welgus HG, Taichman LB. Re-epithelialization of human oral keratinocytes in vitro. J. Dent. Res. 1996;75:912–918. doi: 10.1177/00220345960750030801. [DOI] [PubMed] [Google Scholar]
- 5.Egles C, Shamis Y, Mauney JR, Volloch V, Kaplan DL, Garlick JA. Denatured collagen modulates the phenotype of normal and wounded human skin equivalents. J. Invest. Dermatol. 2008;128:1830–1837. doi: 10.1038/sj.jid.5701240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal N, Andriani F, Pfeiffer L, Kamath P, Lin N, Satyamurthy K, Egles C, Garlick JA. The basement membrane microenvironment directs the normalization and survival of bioengineered human skin equivalents. Matrix Biol. 2008;27:163–170. doi: 10.1016/j.matbio.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]