Abstract
We recently demonstrated that human p38 mitogen-activated protein kinase (MAPK) inhibitors reduced in vitro and in vivo replication of the protozoan parasites Toxoplasma gondii and Encephalitozoon cuniculi. In this study, we assessed the efficacy of five p38 MAPK inhibitors to block the replication of Plasmodium falciparum in human erythrocytes cultured ex vivo and demonstrate that the pyridinylimidazole RWJ67657 and the pyrrolobenzimidazole RWJ68198 reduced Plasmodium falciparum replication, yielded trophozoites that were greatly diminished in size at 24 h, and that these two agents interfered with stage differentiation. Interestingly, the chloroquine-resistant strain W2 was significantly more sensitive to these drugs than was the chloroquine-sensitive strain HB3. These results suggest that pyridinylimidazoles and pyrrolobenzimidazoles designed to inhibit human p38 MAPK activation can be developed to treat malaria.
Keywords: indole-5-carboxamide, mitogen-activated protein kinase, Plasmodium falciparum, pyridinylimidazole, pyrrolobenzimidazole
1. Introduction
Protozoan parasites causing malaria (Plasmodium spp.), Chagas disease (Trypanosoma cruzi), African sleeping sickness (T. brucei), and leishmaniasis (Leishmania spp.) remain enormous global problems in endemic areas, affecting over 300 million people worldwide (Renslo and McKerrow, 2006). Only a small number of effective drugs for these diseases are available partly due to the lack of understanding of the underlying biology of these complex pathogens, with a large proportion of the proteins comprising these organisms still having no known function. Further, countries most affected by these diseases often have insufficient resources to conduct significant anti-parasitic agent research. Finally, drug resistance to first-line therapies for major protozoan diseases is increasingly widespread (Ekland and Fidock, 2007).
Mitogen-activated protein kinases (MAPKs) play pivotal roles in signal transduction and govern diverse and critical cellular processes including proliferation, differentiation, and cell survival in all eukaryotes. The high evolutionary conservation of MAPKs is remarkable, suggesting many common structural features of both metazoan and protozoan MAPKs (Lacey et al., 2007). Fifteen MAPKs have been identified in Leishmania spp. (Parsons et al., 2005, Wiese, 2007), the protozoan with the largest number of MAPKs. The deletion of the LMPK gene in L. mexicana, encoding a stage-specific MAPK, leads to growth arrest during its replication in mouse macrophages (Wiese, 1998). In contrast, only two MAPKs have been identified in Plasmodium falciparum, with Pfmap-2 playing a critical role in completing its 48-hour asexual replication cycle in human erythrocytes (Dorin-Semblat et al., 2007).
We have previously shown that several drugs designed to inhibit human p38 MAPK activation also inhibit the in vitro replication of the protozoan parasite Toxoplasma gondii (Wei et al., 2002). We have also demonstrated that the pyridinylimidazole human p38 MAPK inhibitor RWJ67657 (Fig. 1) protects mice from lethal challenge with either T. gondii or the protozoan parasite Encephalitozoon cuniculi (Wei et al., 2007), making it an excellent proof-of-principle drug. We therefore tested whether such agents could inhibit the replication of other protozoan parasites such as P. falciparum.
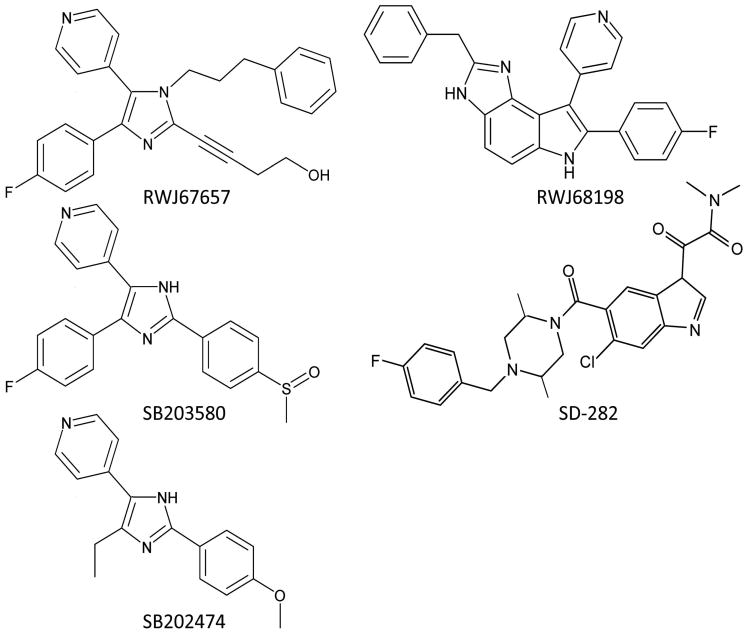
Fig. 1.
The structures of the p38 MAPK inhibitors and control pyridinylimidazole SB202474 used in this study.
2. Materials and Methods
2.1 Drugs and chemicals
The pyridinylimidazole p38 MAPK inhibitor RWJ67657 and the pyrrolobenzimidazole p38 MAPK inhibitor RWJ68198 were provided by Johnson & Johnson Pharmaceuticals (Raritan, NJ). Control pyridinylimidazole SB202474, with no inhibitory activity against human p38 MAPK (Halawani et al., 2004), and the pyridinylimidazole p38 MAPK inhibitor SB203580 were both purchased from Calbiochem (La Jolla, CA). SD-282, an indole-5-carboxamide p38 inhibitor, was obtained from Scios, Inc. (Fremont, CA). Chloroquine and mefloquine were purchased from Sigma (St. Louis, MO). Stock solutions (10 mM) of all compounds were prepared in dimethyl sulfoxide (DMSO) and frozen at -80° C until use, at which point they were diluted to a working concentration in sterile RPMI-1640 medium supplemented with 2 mM L-glutamine, sodium bicarbonate, and 20 μg/mL gentamicin (all from Sigma) and buffered with 12.5 mL of a 1 M solution of HEPES (Sigma) at a pH of 7.4, and 8% (w/v) Albumax II (Invitrogen, Carlsbad, CA). This medium is referred to as “complete RPMI-1640”.
2.2 Plasmodium falciparum
HB3 is a chloroquine-sensitive P. falciparum strain from Honduras (Bhasin and Trager, 1984) and W2 is a chloroquine-resistant P. falciparum strain from Indochina (Oduola et al., 1988). Both were obtained from the Malaria Research and Reference Reagent Resource Center (MR4; Manassas, VA) and were grown and maintained in culture in complete RPMI-1640 using the method of Trager and Jensen (Trager and Jensen, 1976) at a hematocrit of 1 – 5% and parasitemias < 5% in sealed jars under a gas mixture of 4% O2, 3% CO2 and 93% N2 at 37° C.
2.3 In vitro anti-Plasmodium assay
The Sybr green I assay was used to assess drug efficacy as previously described (Johnson et al., 2007). Stock solutions of each drug were serially diluted in 96-well plates with complete RPMI-1640 medium to produce dilutions ranging from 1 pM (in the case of mefloquine) to a maximum concentration of 200 μM (for all other human p38 inhibitors). Parasites were synchronized with 5% sorbitol to enrich for ring-stage parasites 48 h in advance of performing proliferation assays. Parasites were plated in the ring stage at 2% hematocrit and 1% parasitemia in 100 μL of each compound at defined concentrations. Drug plates were placed in sealed jars, gassed, and incubated at 37° C for 72 h. Plates were subjected to three 20-min freeze-thaw cycles. Thereafter, 100 μL of Sybr green I solution (0.2 μL of 10000× Sybr green I (Sigma) in 1 mL of lysis buffer) was added to each well of the 96-well plates, and were read on a fluorescence plate reader at excitation and emission wavelengths of 485 nm and 538 nm, respectively, after being incubated in the dark for 45 min.
The Sybr green I assay generates fluorescence counts at various concentrations of the drug as raw data. Fluorescent counts from control wells (untreated parasites) represent the maximum amount of DNA in viable parasites while those from uninfected erythrocytes represent background fluorescence. The proliferation at each drug concentration was obtained by adjusting fluorescence from drug-treated wells for background fluorescence, and then expressed as a percentage of the growth rate achieved by parasites incubated in the absence of any drug. This was plotted against corresponding concentrations of drug using Grafit software (Erithacus Software Ltd, Surrey, UK) to generate log dose-response curves from which the half-maximal inhibitory concentration (IC50) for each compound was determined. Assays were replicated 3 times to obtain the mean IC50 values for each compound. Statistical differences were assessed using the Student's two-tailed t-test. For all analyses, p values <0.05 were considered significant.
2.4 Morphological changes in P. falciparum
Ring-stage parasites were prepared exactly as described above and incubated with sub-lethal drug concentrations (1.0 μM for RWJ68198 and 7.4 μM for all other human p38 inhibitors) and grown in vitro. The indicated concentrations approximate IC50 values to obtain evidence of biological drug effects short of killing. Thin smears were prepared at 24, 48 and 72 h, stained with 1% Giemsa, and examined by phase-contrast microscopy.
3. Results and Discussion
A recent evaluation of almost two million compounds in the GlaxoSmithKline Tres Cantos antimalarial compound set (TCAMS) has identified approximately 8000 compounds with potent activity against an estimated 146 probable targets in P. falciparum (Gamo et al., 2010). Roughly half of these targets belong to the protein kinase superfamily suggesting that these proteins are largely underexploited targets for antimalarial agents (Gamo et al., 2010).
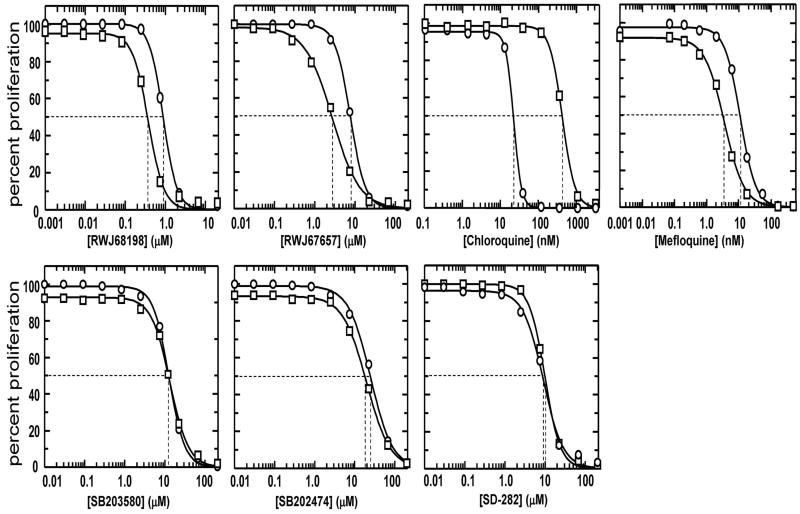
We determined the sensitivity of the five p38 MAPK inhibitors (the structures of which are shown in Fig. 1) against HB3 and W2, with dose-response curves for chloroquine and mefloquine treatments shown for comparison. Under our assay conditions, the chloroquine-sensitive strain HB3 was 19-fold more sensitive to chloroquine compared to W2, having IC50 values of 22 nM and 424 nM against the chloroquine-sensitive HB3 and chloroquine-resistant W2 strains, respectively (Fig. 2, Table 1). Mefloquine was the most potent p38 MAPK inhibitor tested against both P. falciparum strains, with IC50 values of 3.6 nM and 11.2 nM for W2 and HB3, respectively. The order of decreasing activity for the p38 MAPK inhibitors was: mefloquine > chloroquine (HB3) > RWJ68198 (W2) > chloroquine (W2) > RWJ68198 (HB3) > RWJ67657 > SD-282 > SB203580 > SB202474 (Fig. 2, Table 1). Interestingly, RWJ68198, RWJ67657, and mefloquine, were each approximately 2 – 3-fold more active against the chloroquine-resistant strain, W2 than the chloroquine-sensitive strain, HB3 (p < 0.001). In contrast, both strains have been reported to be equally sensitive to natural artemisinin, having IC50 values of 9-10 nM (Chaturvedi et al., 2009).
Fig. 2.
RWJ68198 and RWJ67657 are significantly more active against the chloroquine-resistant Plasmodium falciparum strain (W2) compared to the chloroquine-sensitive strain, HB3. Using the Sybr green I assay, the proliferation of strain HB3 (circles) was compared to strain W2 (squares) when treated with the indicated concentrations of p38 MAPK inhibitors. Dashed lines indicate the IC50 for each drug. Assays were replicated three times to obtain the mean and standard deviation IC50 values for each compound, with one representative experiment being shown.
Table 1.
Comparison of in vitro potency of drugs used in this study.
| Drug target | IC50 of drug (nM) | ||||||
|---|---|---|---|---|---|---|---|
| RWJ68198 | RWJ67657 | SD-282 | SB203580 | SB202474 | chloroquine | mefloquine | |
| P. f. W2 | 380 ± 20 | 2,710 ± 190 | 8,800 ± 260 | 13,240 ± 990 | 19,530 ± 120 | 424 ± 22 | 3.59 ± 0.46 |
| P. f. HB3 | 860 ± 60 | 7,630 ± 350 | 9,780 ± 790 | 12,610 ± 570 | 24,560 ± 330 | 22.0 ± 2 | 11.2 ± 0.90 |
| human p38αa | 22 | 1,000 | 1.6 | 7,000 | > 10 | N/Ad | N/Ad |
| T. g. TgMAPK-1b | NDc | 200 | NDc | 135 | > 10 | N/Ad | N/Ad |
IC50 data are derived as the mean and standard deviation of 3 independently derived IC50 values.
The reported IC50 value for the inhibition of purified recombinant human p38α kinase activity are taken from the following sources: RWJ68198, (Dodd et al., 2000, Rupert et al., 2003); RWJ67657, (Wadsworth et al., 1999); SB202474, (Halawani et al., 2004); SB203580, (Brumlik et al., 2004, Wadsworth et al., 1999), and SD-282 (Sweitzer et al., 2004).
The reported IC50 value for the inhibition of TgMAPK-1 autophosphorylation is taken from the following sources: SB203580, (Brumlik et al., 2004); RWJ67657 or SB202474, (Brumlik et al., unpublished observations).
ND; The IC50 value has not been determined.
N/A; Not applicable.
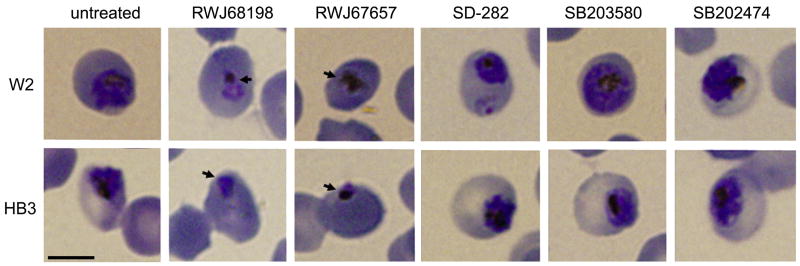
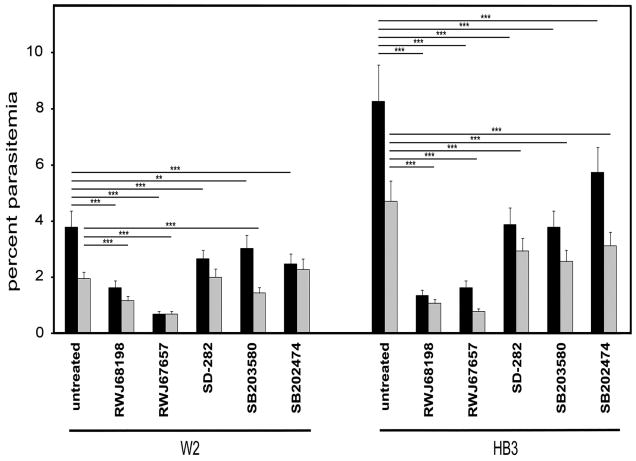
Ring-stage P. falciparum W2 or HB3 were treated with sublethal concentrations of each drug to examine the parasite stages affected. Parasites treated with RWJ68198 and RWJ67657 yielded trophozoites that were dramatically diminished in size at 24 h versus either untreated controls or parasites treated with any of other drugs (Fig. 3). In agreement with IC50 values (Table 1), in vitro cultures with HB3 treated with any of the drugs or with W2 treated with RWJ68198, RWJ67657, or SB203580 yielded significantly fewer trophozoites compared with non-treated parasite controls (Fig. 4).
Fig. 3.
Ring-stage parasites of both W2 and HB3 strains of Plasmodium falciparum were incubated with sub-lethal concentrations of all compounds (1.0 μM for RWJ68198 and 7.4 μM for all other compounds) and were grown under standard conditions of in vitro culture for 72 h. Thin blood smears were stained with 1% Giemsa stain and examined by phase-contrast microscopy at 24, 48, and 72 h, with representative images at 24 h being shown. Arrows have been placed at the periphery of representative aberrant trophozoites. Scale bar: 5 μm.
Fig. 4.
Plasmodium falciparum described in Fig. 3 were enumerated by phase-contrast microscopy following Giemsa staining at 24, 48, and 72 h, with the results at 72 h being shown. Percent parasitemia was estimated by counting the number of parasitized red blood cells (RBCs) relative to the total number of parasitized and non-parasitized RBCs in 10 fields, which was expressed as a percentage of the total number of RBCs. The mean and standard deviation is shown. Total parasites (black bars) represent the sum of all trophozoites (grey bars), schizonts, and ring-stage parasites that were enumerated. One representative experiment of at least two for each agent is shown. ***p < 0.001, **p < 0.01.
There are relatively few agents to treat medically important protozoan parasites. We hypothesized that agents developed to inhibit human p38 MAPK activation could be useful to treat Plasmodium infections based on our prior successes using p38 MAPK inhibitors to protect mice from lethal challenge with the protozoans T. gondii and E. cuniculi (Wei et al., 2007). Current data demonstrate the in vitro efficacy of agents against P. falciparum. These agents have been shown non-toxic to mammalian cells in vitro (Wei et al., 2007, Wei et al., 2002), and well-tolerated when administered to mice (Wei et al., 2007) and hamsters (Brumlik et al., unpublished observations). Interestingly, drugs showing significant activity against P. falciparum (RWJ68198, RWJ67657, and mefloquine) showed greater inhibitory activity against the chloroquine-resistant W2 strain than against chloroquine-sensitive HB3. This response is reminiscent of aryl amino alcohols such as mefloquine and halofantrine towards which chloroquine-resistant Plasmodium are hypersensitive (Witkowski et al., 2009).
The pyrrolobenzimidazole and pyridinylimidazole-based compounds and related heterocyclic compounds used in current studies are known mammalian p38 MAPK inhibitors. In addition to their known activity against p38 MAPKs, the tri-substituted pyrroles have previously been shown to have anti-parasitic activity against Apicomplexan parasites both in vitro and in vivo. However, the molecular target in these organisms was determined not to be a p38 MAPK homologue, but a parasite protein kinase G (PKG) homologue (Biftu et al., 2005). Similarity modeling of the PKG catalytic site using the crystal structure of mammalian p38α MAPK demonstrated that these tri-substituted compounds exploit a hydrophobic binding pocket that overlaps the ATP binding site, analogous to known p38 MAPK inhibitors. For most protein kinases, access to this potential pocket is blocked by bulky residues. For parasite PKG and mammalian p38 MAPK, but not for non-mammalian vertebrate PKGs or most other MAPK family members, a threonine or serine residue at the base of this pocket makes a stabilizing contact with an inhibitor fluorophenyl side chain accounting for the potency and selectivity of these compounds against p38 MAPKs. Interestingly, PKG is apparently absent in parasites such as Leishmania and Trypanosoma spp., yet these agents still exhibit potent anti-parasitic activity (Liotta and Siekierka, 2010). Thus, the molecular targets for these agents in parasites remain incompletely defined. Specific identification of their targets of action in parasites could allow development of more selective, and perhaps more efficacious anti-parasitic agents.
Although SB203580 and RWJ67657 were both considerably more potent against TgMAPK-1 relative to human p38α using in vitro kinase assays (Brumlik et al., 2004, Wadsworth et al., 1999, Wei et al., 2007) (Table 1), inhibitors developed against human p38α MAPK would be expected to exhibit differential and unpredictable potencies against protozoan MAPK homologues. The relative in vitro potencies of these drugs against recombinant mammalian and parasite target may not correlate with in vivo efficacy owing to differential drug delivery into different cell compartments (such as the parasite cytoplasm vs. the host cell cytoplasm). In our previous work (Wei et al., 2007), mice were injected intraperitoneally with 20 – 100 T. gondii RH tachyzoites, where untreated infection with a single tachyzoite is invariably fatal. All mice that survived because of treatment with clinically relevant doses of RWJ67657, then survived re-challenge with T. gondii, demonstrating that clinically relevant doses of RWJ67657 did not prevent the development of protective immunity, and are not immunosuppressive (Wei et al., 2007).
P. falciparum possesses only two MAPKs: Pfmap-1 and Pfmap-2. While the role of Pfmap-1 remains unclear (Dorin-Semblat et al., 2007), Pfmap-2 is essential for the completion of asexual reproduction, and is therefore a validated target for therapeutic intervention (Dorin-Semblat et al., 2007, Dorin et al., 2001). The inhibitory effect of human p38 MAPK inhibitor drugs against Pfmap-2 can be established by assessing the rate of recombinant Pfmap-2 autophosphorylation in vitro. Alternatively, by inducing P. falciparum resistance to human p38 MAPK inhibitors, mutations arising in pfmap-1 and/or pfmap-2 genes could help confirm which MAPK, if either, is the target of the human p38 MAPK inhibitor drugs being tested.
Our recent phylogenetic comparison of MAPKs from P. falciparum and T. gondii suggests that P. falciparum Pfmap-2 bears greatest homology to T. gondii TgMAPK-3, while Pfmap-1 bears greatest homology to T. gondii TgMAPK-2, leaving T. gondii TgMAPK-1 without an obvious homologue in P. falciparum (Lacey et al., 2007). Thus, RWJ68198 and RWJ67657 could act against different MAPK targets controlling asexual replication (Pfmap-2 in P. falciparum and TgMAPK-1 in T. gondii, respectively).
p38 MAPK inhibitors also inhibit human immunodeficiency virus replication in vitro (Shapiro et al., 1998). Nonetheless, we have failed to detect reduced replication of human hepatitis C virus replication in vitro, and saw no efficacy against several species of pathogenic fungi including Candida and Cryptococcus spp. with any of these agents (data not shown).
Pyridinylimidazoles such as SB203580 do not fully occupy the ATP binding site of their human p38α MAPK target (Tong et al., 1997). Thus structural modifications to these drugs can be made that will likely increase the specificity of these drugs for protozoan MAPKs, while simultaneously reducing their ability to target mammalian MAPKs (Brumlik et al., 2011). Likewise, the selective pyridinylimidazole RWJ67657 and the similarly sized pyrrolobenzimidazole RWJ68198 used in our studies as proof-of-principle offer a variety of inhibitor pharmacophores which can be structurally modified to increase potency and selectivity. Some human p38 MAPK inhibitors have progressed into human clinical trials for inflammatory disease, cardiovascular disease, cancer, and other indications where side effects have been manageable (Hammaker and Firestein, 2010). Thus, further evaluation of p38 MAPK inhibitors as anti-parasitic agents is warranted.
Acknowledgments
This research was supported by a Johnson & Johnson Focused Giving Award and NIH R01 AI060424 (to T.J.C.). The work at the Southwest Foundation for Biomedical Research was conducted in facilities constructed with support from Research Facilities Improvement Program Grant C06 RR013556 from the National Center for Research Resources, National Institutes of Health.
References
- Bhasin VK, Trager W. Gametocyte-forming and non-gametocyte-forming clones of Plasmodium falciparum. American Journal of Tropical Medicine and Hygiene. 1984;33:534–537. doi: 10.4269/ajtmh.1984.33.534. [DOI] [PubMed] [Google Scholar]
- Biftu T, Feng D, Ponpipom M, Girotra N, Liang GB, Qian X, Bugianesi R, Simeone J, Chang L, Gurnett A, Liberator P, Dulski P, Leavitt PS, Crumley T, Misura A, Murphy T, Rattray S, Samaras S, Tamas T, Mathew J, Brown C, Thompson D, Schmatz D, Fisher M, Wyvratt M. Synthesis and SAR of 2,3-diarylpyrrole inhibitors of parasite cGMP-dependent protein kinase as novel anticoccidial agents. Bioorganic & Medicinal Chemistry Letters. 2005;15:3296–3301. doi: 10.1016/j.bmcl.2005.04.060. [DOI] [PubMed] [Google Scholar]
- Brumlik MJ, Pandeswara S, Ludwig SM, Murthi K, Curiel TJ. Parasite mitogen-activated protein kinases as drug discovery targets to treat human protozoan pathogens. Journal of Signal Transduction. 2011 doi: 10.1155/2011/971968. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumlik MJ, Wei S, Finstad K, Nesbit J, Hyman LE, Lacey M, Burow ME, Curiel TJ. Identification of a novel mitogen-activated protein kinase in Toxoplasma gondii. International Journal For Parasitology. 2004;34:1245–1254. doi: 10.1016/j.ijpara.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG. Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chemical Society Reviews. 2009;39:435–454. doi: 10.1039/b816679j. [DOI] [PubMed] [Google Scholar]
- Dodd JH, Henry JR, Rupert KC. Substituted pyrrolobenzimidazoles for treating inflammatory diseases. Patent 6,147,096 2000
- Dorin-Semblat D, Quashie N, Halbert J, Sicard A, Doerig C, Peat E, Ranford-Cartwright L, Doerig C. Functional characterization of both MAP kinases of the human malaria parasite Plasmodium falciparum by reverse genetics. Molecular Microbiology. 2007;65:1170–1180. doi: 10.1111/j.1365-2958.2007.05859.x. [DOI] [PubMed] [Google Scholar]
- Dorin D, Le Roch K, Sallicandro P, Alano P, Parzy D, Poullet P, Meijer L, Doerig C. Pfnek-1, a NIMA-related kinase from the human malaria parasite Plasmodium falciparum Biochemical properties and possible involvement in MAPK regulation. European Journal of Biochemistry. 2001;268:2600–2608. doi: 10.1046/j.1432-1327.2001.02151.x. [DOI] [PubMed] [Google Scholar]
- Ekland EH, Fidock DA. Advances in understanding the genetic basis of antimalarial drug resistance. Current Opinions in Microbiology. 2007;10:363–370. doi: 10.1016/j.mib.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo FJ, Sanz LM, Vidal J, de Cozar C, Alvarez E, Lavandera JL, Vanderwall DE, Green DV, Kumar V, Hasan S, Brown JR, Peishoff CE, Cardon LR, Garcia-Bustos JF. Thousands of chemical starting points for antimalarial lead identification. Nature. 2010;465:305–310. doi: 10.1038/nature09107. [DOI] [PubMed] [Google Scholar]
- Halawani D, Mondeh R, Stanton LA, Beier F. p38 MAP kinase signaling is necessary for rat chondrosarcoma cell proliferation. Oncogene. 2004;23:3726–3731. doi: 10.1038/sj.onc.1207422. [DOI] [PubMed] [Google Scholar]
- Hammaker D, Firestein GS. “Go upstream, young man”: lessons learned from the p38 saga. Annals of the Rheumatic Diseases. 2010;69 1:i77–82. doi: 10.1136/ard.2009.119479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, Dennull RA, Gerena L, Lopez-Sanchez M, Roncal NE, Waters NC. Assessment and continued validation of the malaria SYBR green I-based fluorescence assay for use in malaria drug screening. Antimicrobial Agents and Chemotherapy. 2007;51:1926–1933. doi: 10.1128/AAC.01607-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MR, Brumlik MJ, Yenni RE, Burow ME, Curiel TJ. Toxoplasma gondii expresses two mitogen-activated protein kinase genes that represent distinct protozoan subfamilies. Journal of Molecular Evolution. 2007;64:4–14. doi: 10.1007/s00239-005-0197-x. [DOI] [PubMed] [Google Scholar]
- Liotta F, Siekierka JJ. Apicomplexa, trypanosoma and parasitic nematode protein kinases as antiparasitic therapeutic targets. Current Opinion in Investigational Drugs. 2010;11:147–156. [PubMed] [Google Scholar]
- Oduola AM, Milhous WK, Weatherly NF, Bowdre JH, Desjardins RE. Plasmodium falciparum: induction of resistance to mefloquine in cloned strains by continuous drug exposure in vitro. Experimental Parasitology. 1988;67:354–360. doi: 10.1016/0014-4894(88)90082-3. [DOI] [PubMed] [Google Scholar]
- Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nature Chemistry Biology. 2006;2:701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- Rupert KC, Henry JR, Dodd JH, Wadsworth SA, Cavender DE, Olini GC, Fahmy B, Siekierka JJ. Imidazopyrimidines, potent inhibitors of p38 MAP kinase. Bioorganic & Medicinal Chemistry Letters. 2003;13:347–350. doi: 10.1016/s0960-894x(02)01020-x. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Heidenreich KA, Meintzer MK, Dinarello CA. Role of p38 mitogen-activated protein kinase in HIV type 1 production in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7422–7426. doi: 10.1073/pnas.95.13.7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweitzer SM, Medicherla S, Almirez R, Dugar S, Chakravarty S, Shumilla JA, Yeomans DC, Protter AA. Antinociceptive action of a p38alpha MAPK inhibitor, SD-282, in a diabetic neuropathy model. Pain. 2004;109:409–419. doi: 10.1016/j.pain.2004.02.016. [DOI] [PubMed] [Google Scholar]
- Tong L, Pav S, White DM, Rogers S, Crane KM, Cywin CL, Brown ML, Pargellis CA. A highly specific inhibitor of human p38 MAP kinase binds in the ATP pocket. Nature Structural Biology. 1997;4:311–316. doi: 10.1038/nsb0497-311. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wadsworth SA, Cavender DE, Beers SA, Lalan P, Schafer PH, Malloy EA, Wu W, Fahmy B, Olini GC, Davis JE, Pellegrino-Gensey JL, Wachter MP, Siekierka JJ. RWJ 67657, a potent, orally active inhibitor of p38 mitogen-activated protein kinase. Journal of Pharmacology and Experimental Therapeutics. 1999;291:680–687. [PubMed] [Google Scholar]
- Wei S, Daniel BJ, Brumlik MJ, Burow ME, Zou W, Khan IA, Wadsworth S, Siekierka J, Curiel TJ. Drugs designed to inhibit human p38 mitogen-activated protein kinase activation treat Toxoplasma gondii and Encephalitozoon cuniculi infection. Antimicrobial Agents and Chemotherapy. 2007;51:4324–4328. doi: 10.1128/AAC.00680-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Marches F, Daniel B, Sonda S, Heidenreich K, Curiel T. Pyridinylimidazole p38 mitogen-activated protein kinase inhibitors block intracellular Toxoplasma gondii replication. International Journal For Parasitology. 2002;32:969–977. doi: 10.1016/s0020-7519(02)00061-9. [DOI] [PubMed] [Google Scholar]
- Wiese M. A mitogen-activated protein (MAP) kinase homologue of Leishmania mexicana is essential for parasite survival in the infected host. EMBO Journal. 1998;17:2619–2628. doi: 10.1093/emboj/17.9.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese M. Leishmania MAP kinases--familiar proteins in an unusual context. International Journal For Parasitology. 2007;37:1053–1062. doi: 10.1016/j.ijpara.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Witkowski B, Berry A, Benoit-Vical F. Resistance to antimalarial compounds: methods and applications. Drug Resistance Updates. 2009;12:42–50. doi: 10.1016/j.drup.2009.01.001. [DOI] [PubMed] [Google Scholar]