Summary
The ketogenic diet (KD) is an effective treatment for epilepsy, but its mechanisms of action are poorly understood. We investigated the hypothesis that KD inhibits mammalian target of rapamycin (mTOR) pathway signaling. The expression of pS6 and pAkt, markers of mTOR pathway activation, was reduced in hippocampus and liver of rats fed KD. In the kainate model of epilepsy, KD blocked the hippocampal pS6 elevation that occurs after status epilepticus. As mTOR signaling has been implicated in epileptogenesis, these results suggest that the KD may have anticonvulsant or antiepileptogenic actions via mTOR pathway inhibition.
Keywords: epilepsy, seizure, kainate, rat
Introduction
The ketogenic diet (KD) is an effective treatment for intractable epilepsy that appears to possess not only traditional anticonvulsant effects, but also disease-modifying and antiepileptogenic properties in humans and animal models. Patients treated with KD often have improvement in seizure control that persists long after the diet has been discontinued (Marsh et al. 2006, Patel et al. 2010). In the kainic acid (KA)-induced status epilepticus (SE) animal model of temporal lobe epilepsy, injection of KA results in SE, followed by a latent period of epileptogenesis and later development of spontaneous recurrent seizures. Early treatment with KD prevents mossy fiber sprouting and spontaneous seizures in this model, suggesting that KD can prevent epileptogenesis (Muller-Schwarze et al. 1999, Su et al. 2000). A better understanding of KD’s mechanisms could help elucidate the cascade of events involved in epileptogenesis and identify potential therapeutic targets for more effective antiepileptic agents.
The mammalian target of rapamycin (mTOR) signaling pathway has recently generated interest as an important regulator of cellular changes involved in epileptogenesis. mTOR is a protein kinase that integrates energy, nutrient and growth factor signals to regulate numerous cellular functions. mTOR is activated by phosphoinositide-3 kinase (PI3K)/Akt signaling in the presence of nutrients and growth factors, and inhibited by AMP-activated protein kinase (AMPK) in the setting of energy deprivation (Fig 1A). Dysregulated mTOR signaling has been observed in a variety of models of genetic and acquired epilepsy, including tuberous sclerosis complex (TSC) and other cortical malformations, traumatic brain injury, and pilocarpine and KA-induced SE (Wong 2010). Furthermore, the mTOR inhibitor rapamycin prevents the development of epilepsy and underlying pathophysiological mechanisms causing epileptogenesis in animal models of TSC and KA-induced SE (Zeng et al. 2008, Zeng et al. 2009). The ability of mTOR to integrate nutrient and energy signals makes it a plausible candidate for modulation by KD, which has widespread metabolic and nutritional effects on the brain and body. We therefore investigated the effects of KD on mTOR pathway signaling in normal rats, and in the KA epilepsy model.
Figure 1. Ketogenic diet inhibits mTOR signaling in hippocampus and liver of normal rats .
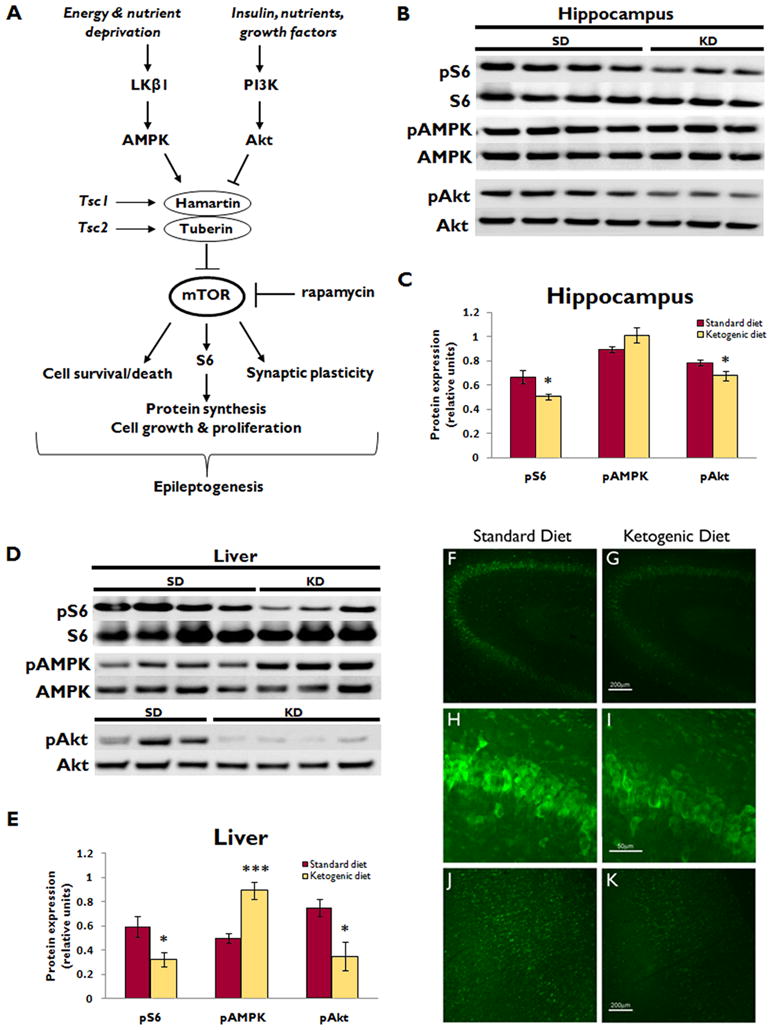
(A) Simplified schematic diagram of mTOR pathway signaling. mTOR integrates numerous upstream signals to control cellular functions that may be involved in epileptogenesis, including cell growth, proliferation, survival versus death, and synaptic plasticity. mTOR is regulated by the hamartin/tuberin complex. Insulin and other growth factors activate PI3K/Akt signaling, which inhibits the hamartin/tuberin complex, thereby relieving the inhibition of mTOR and allowing for mTOR-mediated anabolic processes. Conversely, in the setting of nutrient or energy deprivation, the AMPK pathway is activated, which augments the hamartin/tuberin inhibition of mTOR to shut off energy-requiring processes when resources are scarce. Rapamycin is the prototypic mTOR inhibitor. AMPK, AMP-activated protein kinase; PI3K, class I phosphoinositide-3 kinase; S6, ribosomal protein S6; Tsc1 and Tsc2, tuberous sclerosis complex genes 1 and 2.
(B) Western blotting shows pS6 and total S6 expression, as well as upstream pAMPK, total AMPK, pAkt, and total Akt expression in the hippocampus of normal rats after administration of either ketogenic diet (KD) or standard diet (SD) for two weeks. Each lane represents protein isolated from a single animal.
(C) Quantitative plot showing ratio of phosphorylated to total protein expression demonstrates that KD reduced hippocampal pS6 and pAkt expression (*p<0.05 for both), but did not affect pAMPK expression. (n=7 for each group)
(D) Western blotting of liver illustrates pS6 and total S6 expression, as well as upstream pAMPK, total AMPK, pAkt, and total Akt expression in normal rats after administration of KD or SD for two weeks. Each lane represents protein isolated from a single animal. Blots shown are examples from two sets of experiments, one with n=3 and one with n=4 KD fed rats, for a total of n=7 for each group.
(E) Quantitative plot showing ratio of phosphorylated to total protein expression demonstrates that KD reduced liver pS6 and pAkt expression (*p<0.05 for both), and increased pAMPK expression (***p<0.001). (n=7 for each group)
(F, G) Low power images of pS6 stained coronal sections through CA3 of the hippocampus, illustrating the reduction in pS6 expression in KD (G) compared to SD (F). Scale bar = 200μm.
(H, I) High power images of the same sections demonstrate the reduction in pS6 expression in CA3 pyramidal neurons of KD-fed rats (I) compared to SD (H). Scale bar = 50μm.
(J, K) Low power images of pS6 stained coronal sections through neocortex demonstrate a reduction in pS6 expression in KD-fed rats (K) compared to SD (J). Scale bar = 200μm.
Materials and Methods
Animals and dietary protocols
All experimental protocols were in compliance with NIH and Washington University Animal Studies Committee guidelines. For normal animal experiments, Sprague-Dawley rats (Charles River Laboratories) were given ad libitum access to KD (F3666; Bioserv) or standard diet (SD) beginning at P21. By weight, KD had a 6:1 ratio of fat to carbohydrate+protein. For the KA model, Sprague-Dawley rats were injected with KA (15mg/kg i.p., Sigma) at P35 to induce SE, and started on KD or SD after resolution of SE. Acute KA-induced seizures were monitored behaviorally using a modified Racine scale, and animals that developed stage 4 or 5 seizures for at least 3 hours were included (Zeng et al. 2009). Serum beta-hydroxybutyrate levels were assayed using KetoSite reflectance photometry (Stanbio Laboratory). For methods on western blotting, immunohistochemistry, and statistics, see supporting information (supplementary methods).
Results
In normal rats, western blot analysis demonstrated that KD reduced pS6 and pAkt expression in the hippocampus (24% and 14% respectively, p<0.05 for both, n=7 per group) and liver (45% and 54%, p<0.05 for both) (Fig. 1B–E). There was a similar trend toward decreased pS6 and pAkt with KD in neocortex, but this was not statistically significant (Fig. S1). KD increased pAMPK in liver (44%, p<0.001), but not in brain. Immunohistochemistry also demonstrated a reduction in hippocampal and neocortical pS6 expression in KD-fed rats fed (Fig. 1F–K). Baseline weights were similar in KD and SD groups at P21 (57.6±0.8g and 59.1±1.4g, respectively), but after two weeks on their respective diets, KD-fed rats had significantly lower body weight (81.0±3.1g and 174.4±9.2g, respectively, p<0.001).
To determine the potential relevance of these KD effects on mTOR signaling to epileptogenesis, the effect of KD on mTOR activation was also assessed in the KA model. In SD-fed rats, KA-induced SE increased pS6 expression in the hippocampus, but not neocortex, at 1 (77%, p<0.001, n=7, compared to control, n=8) and 7 days (38%, p<0.05, n=10) after SE, with return to baseline by 21 days (n=7) (Fig. 2). KD initiated immediately after resolution of SE did not prevent the increased pS6 expression at 1 day seen in KA-treated rats on SD, but blocked the increase in hippocampal pS6 expression at 7 days following SE. At 7 and 21 days, KD-fed rats had lower pS6 expression in both hippocampus and neocortex compared to SD-fed rats (p<0.05, except 7d hippocampus p<0.01, 7d n=10 per group, 21d n=7 per group). Correlating with the effects on pS6 expression, KD induced a significant increase in beta-hydroxybutyrate levels compared to SD at 7 (SD 0.28±0.03mM, n=5, KD 2.24±0.20mM, n=6, p<0.01) and 21 days (SD 0.24±0.03mM, n=5, KD 1.77±0.25mM, n=6, p<0.05), but not 1 day, following SE. Weight gain was reduced in rats fed KD compared to SD at 7d (SD +21.7±7.7g, KD −3.5±4.6g, p=0.01, n=10 per group) and 21d (SD +148.9±13.2g, KD +6.9±3.2g, p<0.001, n=7 per group) after SE.
Figure 2. Ketogenic diet inhibits mTOR activation after KA-induced SE.
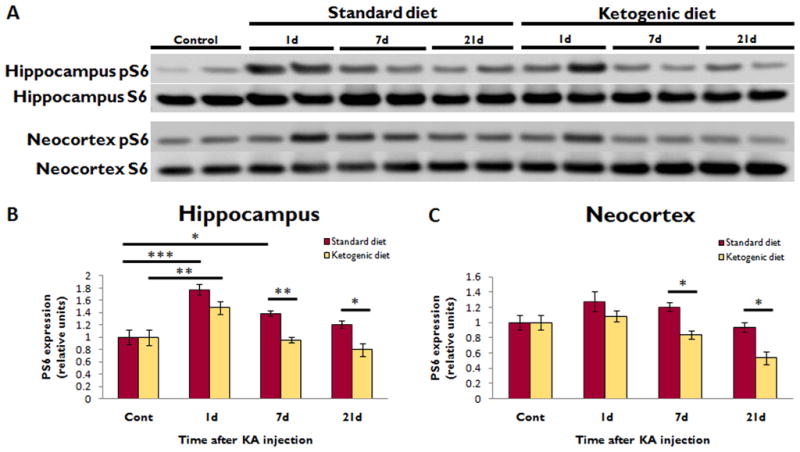
(A) Western blotting shows pS6 and total S6 expression in the hippocampus and neocortex at different time intervals after KA-induced SE in rats administered either KD or SD. Each lane represents protein isolated from a single animal.
(B) Quantitative summary of hippocampal pS6/S6 ratio, normalized to animals not treated with KA (Cont), demonstrates that KA-induced SE increased pS6 expression at 1d (***p<0.001) and 7d (*p<0.05), with return to baseline by 21d. KD did not affect the initial pS6 elevation, but completely blocked the elevation at 7d (**p<0.01).
(C) In neocortex, SE did not significantly increase pS6 expression. However, KD did reduce pS6 expression 7 and 21 days after KA-induced SE compared to SD (*p<0.05 for both). For KA experiments, n=8 for control group, n=7 for 1d SD and KD groups, n=10 for 7d SD and KD groups, and n=7 for 21d SD and KD groups.
Discussion
These results indicate that KD inhibits mTOR pathway signaling in the brain and liver of normal rats, most likely via decreased Akt signaling in both regions, as well as increased AMPK signaling in the liver. The KD has previously been shown to decrease insulin levels in rodents (Thio et al. 2006, Yamada 2008), and a reduction in insulin would be expected to inhibit pAkt and therefore mTOR signaling. Thus lower insulin levels in KD-fed animals may trigger the observed decrease in pAkt and pS6. The mechanism by which the KD increased AMPK signaling in the liver but not brain is less clear. One possibility is that the KD reduces energy and nutrient availability in liver, but not brain. Previous reports of increased brain ATP and energy stores in KD-fed animals support this explanation (Devivo et al. 1978, Bough et al. 2006). Additionally, observations that KD impairs growth in animals and children may also be explained by mTOR inhibition, given mTOR’s role in cellular growth and anabolic processes (Thio et al. 2006, Patel et al. 2010).
Alternatively, the observed mTOR inhibition may be due to other effects of the KD, including poor growth, protein restriction, or low glucose levels. Although KD-fed rats have significantly reduced growth, they have relatively preserved brain weights (Thio et al., 2006) and increased brain energy stores (Devivo et al. 1978). So while protein restriction or poor growth may contribute to mTOR inhibition in liver, it is unlikely to explain effects seen in brain. Furthermore, KD-fed rats exhibit increased caloric intake per body weight (Thio et al. 2006), which may partially compensate for low protein. Low glucose levels might also trigger mTOR inhibition, as we previously documented reduced serum glucose in KD-fed rats (Thio et al., 2006). However, low glucose and protein restriction would be expected to inhibit mTOR via AMPK activation, but we observed AMPK increases only in liver, not brain.
In this study, KD also prevented mTOR hyperactivation after KA-induced SE. Our previous work demonstrated that KA-induced SE results in biphasic mTOR activation, with a peak within 24 hours of SE and a second peak in the hippocampus 7 days after SE, a time which corresponds to the latent period of epileptogenesis (Zeng et al. 2009). Rapamycin treatment after SE blocked the second phase of hippocampal mTOR activation, decreased mossy fiber sprouting in the dentate gyrus, and reduced spontaneous seizures. This suggests that in the KA model, late mTOR hyperactivation plays a role in epileptogenesis, and pharmacological mTOR inhibition after SE is antiepileptogenic. Our demonstration of a similar blockade of late hippocampal mTOR activation with KD provides a possible mechanism for antiepileptogenic effects of KD. Although we did not assess the effect on spontaneous seizures in the present study, this mTOR pathway inhibition during the latent period of epileptogenesis may explain the previously-reported findings that KD initiation two days after KA-induced SE prevents mossy fiber sprouting and reduces spontaneous recurrent seizures, whereas KD initiation 14 days after SE has no effect on seizure frequency (Muller-Schwarze et al. 1999, Su et al. 2000). However, the ability of the KD to prevent sprouting remains controversial, as other studies found no effect of the KD on mossy fiber sprouting in dentate gyrus after KA-induced SE (Xu et al. 2006).
In summary, this study demonstrates that KD inhibits mTOR pathway signaling in the brain and liver of healthy rats, and prevents late hippocampal mTOR activation after KA-induced SE. This mTOR inhibition may underlie some of the physiological effects of KD, including growth impairment, anticonvulsant actions, and potential antiepileptogenic effects. Further studies are necessary to prove a causal relationship between mTOR inhibition and antiepileptogenic actions of the KD.
Supplementary Material
Acknowledgments
This work was supported by NIH R01 NS056872 (MW) and P30NS057105 (Washington University). None of the authors has any conflict of interest to disclose. The authors have read the Journal’s position on issues involved in ethical publications and affirm that this report is consistent with those guidelines.
References
- Bough KJ, Wetherington J, Hassel B, Pare JF, Gawryluk JW, Greene JG, Shaw R, Smith Y, Geiger JD, Dingledine JR. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol. 2006;60:223–235. doi: 10.1002/ana.20899. [DOI] [PubMed] [Google Scholar]
- Devivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- Marsh EB, Freeman JM, Kossoff EH, Vining EP, Rubenstein JE, Pyzik PL, Hemingway C. The outcome of children with intractable seizures: a 3- to 6-year follow-up of 67 children who remained on the ketogenic diet less than one year. Epilepsia. 2006;47:425–430. doi: 10.1111/j.1528-1167.2006.00439.x. [DOI] [PubMed] [Google Scholar]
- Muller-Schwarze AB, Tandon P, Liu Z, Yang Y, Holmes GL, Stafstrom CE. Ketogenic diet reduces spontaneous seizures and mossy fiber sprouting in the kainic acid model. NeuroReport. 1999;10:1517–22. doi: 10.1097/00001756-199905140-00023. [DOI] [PubMed] [Google Scholar]
- Patel A, Pyzik PL, Turner Z, Rubenstein JE, Kossoff EH. Long-term outcomes of children treated with the ketogenic diet in the past. Epilepsia. 2010;51:1277–1282. doi: 10.1111/j.1528-1167.2009.02488.x. [DOI] [PubMed] [Google Scholar]
- Su SW, Cilio MR, Sogawa Y, Silveira D, Holmes GL, Stafstrom CE. Timing of ketogenic diet initiation in an experimental epilepsy model. Dev Brain Res. 2000;125:131–138. doi: 10.1016/s0165-3806(00)00130-9. [DOI] [PubMed] [Google Scholar]
- Thio LL, Erbayat-Altay E, Rensing N, Yamada KA. Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr Res. 2006;60:413–417. doi: 10.1203/01.pdr.0000238244.54610.27. [DOI] [PubMed] [Google Scholar]
- Wong M. Mammalian target of rapamycin (mTOR) inhibition as a potential antiepileptogenic therapy: from tuberous sclerosis to common acquired epilepsies. Epilepsia. 2010;51:26–36. doi: 10.1111/j.1528-1167.2009.02341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XP, Sun RP, Jin RF. Effect of ketogenic diet on hippocampus mossy fiber sprouting and GluR5 expression in kainic acid induced rat model. Chin Med J. 2006;119:1925–1929. [PubMed] [Google Scholar]
- Yamada KA. Calorie restriction and glucose regulation. Epilepsia. 2008;49:94–96. doi: 10.1111/j.1528-1167.2008.01847.x. [DOI] [PubMed] [Google Scholar]
- Zeng LH, Rensing NR, Wong M. The mammalian target of rapamycin signaling pathway mediates epileptogenesis in a model of temporal lobe epilepsy. J Neurosci. 2009;29:6964–6972. doi: 10.1523/JNEUROSCI.0066-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng LH, Xu L, Gutmann DH, Wong M. Rapamycin prevents epilepsy in a mouse model of tuberous sclerosis complex. Ann Neurol. 2008;63:444–453. doi: 10.1002/ana.21331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


