Abstract
Background: The beneficial effects of prenatal and early postnatal intakes of omega-3 (n−3) polyunsaturated fatty acids (PUFAs) on cognitive development during infancy are well recognized. However, few studies have examined the extent to which these benefits continue to be evident in childhood.
Objective: The aim of this study was to examine the relation of n−3 PUFAs and seafood-contaminant intake with memory function in school-age children from a fish-eating community.
Design: In a prospective, longitudinal study in Arctic Quebec, we assessed Inuit children (n = 154; mean age: 11.3 y) by using a continuous visual recognition task to measure 2 event-related potential components related to recognition memory processing: the FN400 and the late positive component (LPC). Children were also examined by using 2 well-established neurobehavioral assessments of memory: the Digit span forward from Wechsler Intelligence Scales for Children, 4th edition, and the California Verbal Learning Test–Children's Version.
Results: Repeated-measures analyses of variance revealed that children with higher cord plasma concentrations of docosahexaenoic acid (DHA), which is an important n−3 PUFA, had a shorter FN400 latency and a larger LPC amplitude; and higher plasma DHA concentrations at the time of testing were associated with increased FN400 amplitude. Cord DHA–related effects were observed regardless of seafood-contaminant amounts. Multiple regression analyses also showed positive associations between cord DHA concentrations and performance on neurobehavioral assessments of memory.
Conclusion: To our knowledge, this study provides the first neurophysiologic and neurobehavioral evidence of long-term beneficial effects of n−3 PUFA intake in utero on memory function in school-age children.
INTRODUCTION
Long-chain polyunsaturated fatty acids (PUFAs) are essential for early brain development. Docosahexaenoic acid (DHA; 22:6n−3) is an n−3 fatty acid observed in large quantities in the membrane lipids of the gray matter of the cerebral cortex and in the retina. Substantial quantities are transferred to the fetus via the placenta and after birth through breastfeeding (1). Perinatal DHA intake has been shown to have significant beneficial effects on neuronal development and function (2, 3). The beneficial effects of n−3 PUFAs for early development have been studied in several randomized controlled trials. DHA supplements given to pregnant mothers have had beneficial effects on the visual function and problem-solving skills of their infants during the first year of life (4–6). Supplementing milk formulas with n−3 PUFAs in early infancy has also produced positive effects on visual acuity and cognitive development in infants born preterm (7–10) and at term (11–14).
Although the beneficial effects of early n−3 PUFA intakes on infant visual and cognitive function have been extensively investigated, very few studies have examined long-term effects of perinatal n−3 PUFA intakes beyond infancy. In one study, maternal DHA intake in pregnancy was associated with higher scores on the mental processing composite of the Kaufman Assessment Battery for Children (K-ABC) at 4 y of age (15) and on the K-ABC sequential processing index at 7 y of age (16). In another study, cord plasma DHA concentrations were related to better motor function at 7 y of age (17), but no effects were seen on the K-ABC at 4 and 7 y of age (18, 19). In both studies, cognitive assessments were limited to general intelligence measures, which provided no information on the possible benefits of DHA on specific cognitive functions, despite evidence from animal studies that suggested an important role of early DHA intake in memory (20).
Because seafood is the primary source of DHA in the human diet (21), populations that rely on fish consumption for subsistence are of special interest. The traditional diet of the Inuit is based largely on fish and marine mammals and results in relatively high PUFA concentrations compared with those in Southern populations. However, methylmercury and polychlorinated biphenyls (PCBs) are also present in the Arctic marine food web (22). These substances have been associated with adverse effects on cognitive development, including recognition memory deficits seen as early as in infancy (23–26). In a birth cohort of Inuit children from Nunavik (Arctic Quebec, Canada), we reported beneficial effects of n−3 PUFAs on visual acuity, recognition memory, and mental development during infancy (27) and on visual function at 5 and 11 y of age (28, 29). The aims of the current study were to 1) use event-related potentials (ERPs), which provide a neurophysiologic measure of cognitive function, to examine the effect of prenatal and childhood intake of DHA on performance on a continuous recognition memory task (CRT) in school-aged children from Nunavik, 2) examine the relation of DHA intake to child memory performance on well-known neurobehavioral tasks, 3) examine the relation between exposure to seafood contaminants and these ERPs and behavioral measures of memory function, and 4) assess the degree to which the adverse effects of the contaminant exposures negated or were ameliorated by any observed beneficial effects of DHA.
SUBJECTS AND METHODS
Subjects
The study participants were school-age Inuit children from Nunavik. The Nunavik region is located north of the 55th parallel, ≈1500 km from Montreal and 2000 km from the Great Lakes in the United States. For most of the participants, umbilical cord blood samples were obtained under the auspices of the Cord Blood Monitoring Program (1993–1998), which was designed to document the amounts of environmental contaminants and nutrients in newborns in Arctic Quebec (30). Three groups of Inuit mothers and their children were invited to participate in the 11-y follow-up assessment as follows: 1) children who had participated in the Environmental Contaminants and Child Development Study as infants (27, 31), 2) children who had participated in the Nunavik Preschool Study at 5 y of age (28), and 3) children for whom cord blood samples were available but had not been previously tested. In the third group, children within the highest and lowest quartiles for cord blood DHA, mercury, and PCB congener 153 (PCB 153) concentrations were recruited to participate. However, because the DHA, mercury, and PCB 153 concentrations were only moderately intercorrelated, distributions of contaminants in this group of children turned out to be very similar to those in groups 1 and 2, which had been recruited as convenience samples on the basis of their availability. Mothers were initially contacted by mail and subsequently by phone, provided with information about the study protocol, and invited to participate with their children.
Between September 2005 and April 2007, 192 children were evaluated by using the test protocol as described. Assessments were conducted in the 3 largest Nunavik villages. Participants who resided in other communities were transported by plane to one of the larger villages for testing. A maternal interview conducted during this school-age assessment documented information on demographic background, smoking, alcohol and drug use during pregnancy, as well as other maternal characteristics, and the child's diet during the preceding year. The following inclusion criteria were used: age between 10.0 and 13.0 y, birth weight ≥ 2.5 kg, gestation duration ≥35 wk, no known neurologic or clinically significant developmental disorder, and no medication use at the time of testing. Of the 192 participants assessed, one with multiple sclerosis and another treated for epilepsy were excluded. Written informed consent was obtained from a parent of each participant, and oral assent was obtained from each child. The research was approved by the Laval University and Wayne State University ethics committees and was performed in accordance with ethical standards of the 1983 Helsinki Declaration.
Continuous recognition task
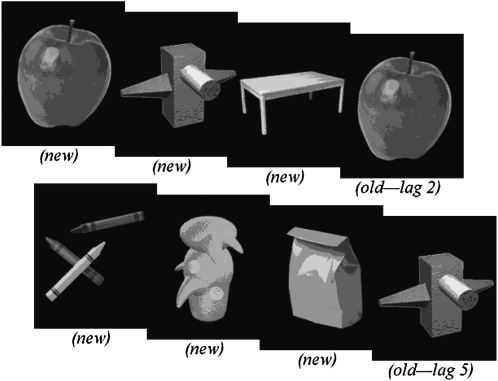
Visual recognition memory was assessed in a CRT. Each participant was tested individually in a quiet room and seated 57 cm from a 43-cm liquid crystal display monitor on which colored pictures were displayed centrally within a 7 × 7-cm space. The child was asked to press one of 2 response buttons to each individual picture depending on if she or he thought the picture had already been presented (old) or not (new). Pictures were presented in a continuous fashion over 3 blocks of 80 trials each. Old items were presented in 50% of trials at lags of 2, 5, or 10 intervening items, counterbalanced across blocks, and new items were presented in the other 50% of trials. The pictures were abstract (unnameable) and concrete (nameable) objects (Figure 1). Stimuli were presented for 500 ms with an offset-to-onset interstimulus interval of 3000 ms plus 100–300 ms stimulus-onset asynchrony (200-ms average). Correct and incorrect responses were tabulated and used to compute a discrimination accuracy measure (d′), which measured correct button presses, which were adjusted for false alarms to correct for any tendency to press the same button regardless of whether the stimulus being displayed was old or new. A null or negative d′ value suggested that the participant responded at random.
FIGURE 1.
Examples of items presented during the continuous recognition memory task. Children pressed the new button to the first presentation of an item and the old button to each subsequent presentation of that item. Lags represent the number of items intervening between 2 subsequent presentations of the same item.
Electroencephalogram recording and analyses
Electroencephalogram data acquisition was performed with a Model 15 Grass Neurodata Acquisition System (Grass Instruments, Quincy, MA). The electrooculogram was recorded with tin electrodes placed at the supraorbital ridge of one eye and the infraorbital ridge of the other eye. The electroencephalogram was recorded with 30 silver–silver chloride electrodes placed according to the international 10–20 system (32) (sites: Fz, F3, F4, F7, F8, AF3, AF4, AF7, AF8, FCz, FC1, FC2, Cz, C3, C4, T3, T4, T5, T6, Pz, P3, P4, POz, Oz, O1, O2, M1, M2, A1, and A2) referenced online to Cz, with forehead ground. The impedance was kept at <10 kΩ. Electrooculogram and electroencephalogram gains were amplified with gains of 5000 and 50,000, respectively. The digitization rate was 200 Hz.
ERPs were derived and analyzed with BrainVision Analyzer 2 software (Brain Products, Munich, Germany). Electrooculogram correction (33) was performed from the vertical electrooculogram electrode. Electroencephalogram channels were rereferenced offline to linked earlobes. High- and low-pass filters were set at 0.1 and 30 Hz, respectively. Artifact rejection (±100 μV) and baseline correction (100 ms prestimulus) were applied. ERPs were averaged for correct new and old responses separately. Responses between 100 and 2000 ms poststimulus onset were tabulated, and trials with errors (new items incorrectly identified as old and vice versa) were excluded from averaging. FN400 latency was determined automatically as the most negative peak between 300 and 500 ms. The mean amplitude was computed for the FN400 (300–500 ms) component and the late positive component (LPC) (500–800 ms). These 2 ERP components were assumed to reflect distinct processes of recognition memory: the FN400 old/new effect reflects enhanced familiarity with the old stimulus; the LPC old/new effect reflects active recollection of the old stimulus in memory (34). Analyses were performed by using the electrode site at which each ERP component reached its maximal amplitude. Participants were retained in the analyses if their behavioral performance exceeded the chance level on the task (d′ > 0) and if they had a sufficient number of acceptable ERP correct old (≥16) and correct new (≥16) trials.
Neuropsychological memory assessments
Among the neuropsychological tasks administered as part of the 11-y follow-up assessment of this cohort, 2 well-established tests that assessed different aspects of memory were selected (Digit span forward test). In the Digit span forward subtest from the Wechsler Intelligence Scales for Children, 4th edition (WISC-IV) (35), the examiner first reads sequences of numbers to the child who was asked to repeat them in the order presented. Sequences lengthened until the child failed 2 consecutive trials of the same length. The backward condition of this test was not considered in the current study because it was thought to solicit high-level executive functions that were not directly related to memory (The California Verbal Learning Test--Children's Version). The California Verbal Learning Test–Children's Version (36) assesses the child's ability to encode a 15-item list of words drawn from 3 semantic categories into episodic memory. The task was translated into Inuktitut, the local language, and the word categories and items were modified to ensure that they consisted of items that were familiar to children who grew up in an Inuit village. The task started with 5 trials, during each of which the examiner read the list of words and asked the child to repeat them back. The short-delay free recall of the initial word list was tested after a sixth trial in which the child was asked to repeat back as many words as she or he could remember from a second 15-item list of words. The long-delay free recall of the initial list was tested after a 20-min delay and followed by a word-recognition trial in which a list of 45 words was read to the child and she or he indicated which words came from the initial word list.
Biological samples
Umbilical cord and child blood samples were analyzed for concentrations of PUFAs as well as mercury, PCBs, lead, and selenium. A blood sample (30 mL) obtained from the umbilical cord was used to indicate prenatal exposure, and a venous blood sample (20 mL) obtained from each child was used to document the body burden at the time of testing. The fatty acid composition of plasma phospholipids was analyzed at the University of Guelph Lipid Analytic Laboratory (Guelph, Canada) by using capillary gas-liquid chromatography. The analytic procedure has been detailed elsewhere (27). Concentrations of DHA and eicosapentaenoic acid (EPA) were expressed as percentages of the total area of all fatty acid peaks from 14:0 to 24:1 (percentage weight).
Contaminant and selenium analyses were performed at the Centre de Toxicologie from the Institut National de Santé Publique du Québec (Quebec, Canada), which is accredited by the Canadian Association for Environmental Analytical Laboratories (Ottawa, Canada). The 14 most prevalent PCB congeners (International Union of Pure and Applied Chemistry nos. 28, 52, 99, 101, 105, 118, 128, 138, 153, 156, 170, 180, 183, 187) were measured in purified cord plasma extracts by using a high-resolution gas chromatograph (Hewlett-Packard HP5890A; Hewlett-Packard, Palo Alto, CA) with 2 capillary columns (Hewlett-Packard Ultra I and Ultra II; Hewlett-Packard) and dual Ni-63 electron capture detectors and in purified child plasma extracts by using a gas chromatograph (HP 5890 Series II Plus; Hewlett-Packard) equipped with a 30-m-long DB-5 capillary column (J&W Scientific, Folsom, CA) and HP 5890B mass spectrometer (Agilent) according to the method described by Dallaire et al (37). For the current study, PCB 153, which was expressed on a lipid basis, was used as an indicator of total PCB exposure because it is highly correlated with other PCB congeners (38) and is considered an adequate marker of exposure to environmental PCB mixtures in the Arctic (31).
Total mercury concentrations in umbilical cord blood samples were measured by using cold-vapor atomic-absorption spectrometry (Pharmacia Model 120; Pharmacia, Piscataway, NJ). Cord blood lead concentrations were measured by using graphite furnace atomic absorption with Zeeman background correction (Perkin Elmer model ZL 4100; Perkin Elmer). Cord selenium concentrations were measured by using inductively coupled plasma mass spectrometry (ICP-MS) on a Perkin Elmer Sciex Elan 6000 instrument (Perkin Elmer, Norwalk, CT). Total mercury, lead, and selenium concentrations in child blood samples were measured by ICP-MS (Perkin Elmer Sciex Elan 6000 ICP-MS instrument for lead and selenium and PE DRC II instrument for mercury; Perkin Elmer). The limits of detection (LOD) for cord-sample analyses were 0.2 μg/L for blood mercury and lead, 0.1 μmol/L for blood selenium, and 0.02 μg/L for all PCB congeners in plasma. LOD for child blood sample analyses were 0.1 μg/L for mercury, 0.002 μg/dL for lead, 0.09 μmol/L for selenium, and <0.05 μg/L for all PCB congeners except for PCB 52 (LOD: 0.15 μg/L). A value equal to one-half the limit of detection of the analytic method was entered in the database whenever a substance was not detected. Blood samples were stored at –80°C until analysis. Samples were analyzed ≤5 mo after blood sample collection for PUFAs and metals and ≤24 mo for PCBs.
Confounding variables
The following potential confounding variables were documented: age and sex of the child, whether the child was adopted, whether the child was transported by plane from a small, more remote village to larger village for the assessment, maternal age at delivery, parity and education (y), socioeconomic status (39) of the primary caregiver, maternal nonverbal reasoning abilities (Raven's progressive matrices) (40), breastfeeding duration (mo), maternal tobacco smoking during pregnancy (cigarettes/d), maternal marijuana consumption during pregnancy (yes or no), and maternal binge drinking during pregnancy (at least one episode of ≥5 standard alcohol drinks; yes or no), and lead and selenium concentrations in cord and child blood samples.
Statistical analyses
The normality of distribution was visually inspected for each variable and checked for skewness (normality range: −3.0 to 3.0). Log transformations were performed on cord and current blood EPA, mercury, PCB 153, lead, and selenium concentrations, breastfeeding duration, parity, and maternal tobacco consumption during pregnancy because these variables followed log-normal distributions. Extreme values (>3 SDs from the mean) for normally distributed variables were recoded to one point greater than the highest observed nonoutlying value according to the procedure recommended by Winer (41). The following variables were recoded by using this procedure (number of outliers in parentheses): cord and current DHA concentrations (1), maternal age (1), maternal education (9), and maternal Raven's score (1). Each of the exposure measures was dichotomized at the median for examination in the ERP analyses.
Effects of exposure variables on ERP parameters were examined in repeated-measures multivariate analyses of variance (RM-MANOVA) with the condition (old or new) as the within-subjects factor and the exposure group (split at the median) as the between-subject factor. We used dichotomous exposure measures to examine effects on ERP parameters to make it possible to visually compare the ERP waveforms of the high- compared with low-exposure groups. Any control variable that was associated (at P < 0.10) with the ERP parameters of interest in either the new or old condition was included as a covariate in the RM-MANOVA. In addition to the control variables previously listed, PCB 153 and mercury were treated as control variables in the analyses that examined the effects of DHA. Similarly, DHA and EPA were treated as control variables in the analyses that examined the effects of mercury and PCB 153. Because the data on maternal alcohol and marijuana consumption during pregnancy were missing for 31 and 32 cases, respectively, the statistical analyses were conducted with and without these variables when they satisfied the criterion for inclusion as potential confounders. Because DHA and EPA concentrations were strongly intercorrelated, results from analyses that were based on these variables were very similar. Only data for DHA are presented because DHA is believed to be more important in terms of development of the central nervous system.
The relation of each of the exposure variables (cord and current concentrations of n−3 PUFAs and seafood contaminants) to each of the behavioral task performance measures was examined by using hierarchical multiple regression analyses. Each of the control variables that were even weakly related to the outcome in question (at P < 0.10) was included in the regression analysis to control for potential confounding.
Finally, the degree to which the adverse effects of the contaminant exposures altered the beneficial effects of DHA on ERP parameters was examined by using analyses of covariance (ANCOVA) by comparing the following 4 exposure groups: low DHA and low toxicant, low DHA and high toxicant, high DHA and low toxicant, and high DHA and high toxicant (split at the median). These models included the same covariates as the RM-MANOVA. All data analyses were conducted with SPSS 12.0.1 (SPSS, Chicago, IL).
RESULTS
Descriptive statistics
A total of 154 participants (81.1% of the initial sample) were included in the final analyses. Reasons for exclusion were as follows: behavioral performance on the CRT at or below the chance level (d′ ≤ 0; n = 19), <16 artifact-free trials for either correct new or correct old trials (n = 11), technical problems that occurred during testing (n = 4), and the first block of CRT trials not completed (n = 2). Excluded participants had significantly lower current EPA concentrations (t[184] = −2.1, P = 0.04) and higher blood lead concentrations (t[185] = 2.3, P = 0.02) and tended to have higher cord EPA concentrations (t[182] = 1.7, P = 0.08) than did retained participants, but the 2 groups did not differ in other exposure variables (all P > 0.15). In excluded participants, there was a smaller proportion of children from remote villages (χ2[1]) = 3.89, P = 0.048) and a slightly (although not significantly) larger proportion of boys (χ2[1] = 3.59, P = 0.06). Although the greater proportion of boys and higher current lead concentrations in the excluded group might have suggested a greater difficulty sitting still during the task, the other group differences (ie, lower cord EPA but greater current EPA concentrations and proportionately fewer excluded children from remote villages) were difficult to interpret and probably represented a random variation. The mean number of trials included in individual averages was 80.8 for new (range: 19–117) and 71.4 for old (range: 16–116) pictures.
Descriptive statistics of the final study sample are summarized in Table 1. The mean cord plasma DHA amount (percentage of phospholipids) was ≈3 times higher than that previously reported for children from southern Quebec (42) and was more comparable with the concentrations reported by Otto et al (43) for 4 European countries and Ecuador. The mean cord blood mercury concentration was ≈20 times higher than in southern Quebec (44) and is similar to what was documented in a birth cohort from the Faroe Islands (24). Concentrations of PCBs in cord plasma samples are ≈3 times higher than in southern Quebec (31) and were similar to those reported in the Dutch PCB cohort study (45). On average, these children ate almost one marine mammal meal and 1.5 fish meals/wk, which was about one-half of the amount we reported for Nunavik Inuit women during pregnancy (46).
TABLE 1.
Descriptive statistics for the final study sample1
| n | Mean | Median | SD of mean | Range | Percentage | |
| Child age at assessment (y) | 154 | 11.3 | 11.3 | 0.6 | 10.2–12.9 | — |
| Child sex (girls) | 154 | — | — | — | — | 58.4 |
| Adoption status (yes) | 154 | — | — | — | — | 18.2 |
| Transportation by plane (yes) | 154 | — | — | — | — | 42.9 |
| Maternal characteristics | ||||||
| Age at delivery (y) | 154 | 24.1 | 22.5 | 5.9 | 15–42 | — |
| Parity | 154 | 2.0 | 2.0 | 1.8 | 0–8 | — |
| Education (y) | 154 | 8.4 | 8.5 | 2.8 | 0–16 | — |
| Socioeconomic status (Hollingshead score) | 154 | 28.3 | 27.8 | 12.5 | 8.0–59.5 | — |
| Nonverbal reasoning abilities (Raven's score) | 154 | 35.4 | 38 | 9.9 | 4–55 | — |
| Breastfeeding duration (mo) | 150 | 11.8 | 3.0 | 18.6 | 0.0–108.0 | — |
| Maternal consumption during pregnancy | ||||||
| Tobacco use, yes (cigarettes/d) | 144 | 8.1 | 7.5 | 7.8 | 0–50 | 79.9 |
| Marijuana, yes | 122 | — | — | — | — | 19.7 |
| Binge drinking, yes (≥5 standard drinks of alcohol per occasion) | 123 | — | — | — | — | 28.5 |
| n−3 PUFAs (% of phospholipids) | ||||||
| Cord plasma DHA | 149 | 3.6 | 3.4 | 1.3 | 1.2–7.7 | — |
| Current plasma DHA | 151 | 2.4 | 2.2 | 1.0 | 0.6–5.5 | — |
| Cord plasma EPA | 149 | 0.4 | 0.3 | 0.4 | 0.0–2.3 | — |
| Current plasma EPA | 151 | 0.7 | 0.5 | 0.5 | 0.0–3.6 | — |
| Seafood contaminants | ||||||
| Cord plasma PCB 153 (μg/kg fat) | 150 | 122.2 | 99.5 | 92.5 | 9.7–653.6 | — |
| Current plasma PCB 153 (μg/kg fat) | 151 | 76.2 | 50.0 | 76.3 | 4.1–431.4 | — |
| Cord blood mercury (μg/L) | 151 | 21.0 | 15.6 | 17.6 | 1.0–99.3 | — |
| Current blood mercury (μg/L) | 151 | 4.8 | 2.8 | 5.5 | 0.1–34.1 | — |
| Other biological analyses (μg/dL) | ||||||
| Cord blood lead | 151 | 4.6 | 3.7 | 3.4 | 1.0–20.9 | — |
| Current blood lead | 151 | 2.5 | 1.8 | 2.2 | 0.4–12.8 | — |
| Cord plasma selenium | 136 | 348.8 | 292.5 | 193.8 | 157.9–1579.2 | — |
| Current plasma selenium | 151 | 199.3 | 173.7 | 107.8 | 67.9–947.5 | — |
| Child food frequency (no. of occasions) | ||||||
| Fish meals/wk | 120 | 1.5 | 1.1 | 1.5 | 0–7.3 | — |
| Marine mammal meals/wk | 122 | 0.8 | 0.2 | 1.5 | 0–8.8 | — |
| Behavioral performance on memory tasks | ||||||
| CRT discrimination accuracy (d′) | 154 | 1.4 | 1.4 | 0.9 | 0.01–3.6 | — |
| CRT RT New (ms) | 154 | 788.2 | 789.5 | 174.3 | 389.0–1251.0 | — |
| CRT RT Old (ms) | 154 | 815.6 | 817.0 | 183.7 | 345.0–1220.0 | — |
| WISC-IV Digit span forward | 154 | 9.1 | 9.0 | 2.4 | 3–16 | — |
| CVLT short-delay free recall | 152 | 8.1 | 8.0 | 2.4 | 3–13 | — |
| CVLT long-delay free recall | 152 | 8.5 | 8.0 | 2.5 | 1–14 | — |
| CVLT recognition discriminability (d′) | 152 | 92.5 | 95.6 | 8.9 | 53.3–100.0 | — |
PUFAs, polyunsaturated fatty acids; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PCB 153, polychlorinated biphenyl congener 153; CRT, continuous recognition memory task; RT, reaction time; WISC-IV, Wechsler Intelligence Scales for Children, 4th edition; CVLT, California Verbal Learning Test–Children's Version.
Associations among the nutrient and contaminant concentrations measured at birth and at the time of testing are presented in Table 2. As noted, cord DHA and EPA concentrations were strongly intercorrelated, as were current DHA and EPA concentrations. The 3 principal contaminants (ie, PCB 153, mercury, and lead) were moderately correlated with each other and with n−3 PUFA concentrations, presumably because all these substances are found at relatively high concentrations in traditional Inuit food.
TABLE 2.
Pearson's correlation coefficients among nutrients and contaminants1
| DHA |
EPA |
Selenium |
PCB 153 |
Mercury |
Lead |
|||||||
| Cord | Current | Cord | Current | Cord | Current | Cord | Current | Cord | Current | Cord | Current | |
| DHA | ||||||||||||
| Cord | 0.40** | 0.67** | 0.20* | 0.05 | 0.11 | 0.27** | 0.20* | 0.24** | 0.14† | 0.17* | 0.11 | |
| Current | 0.28** | 0.68** | 0.07 | 0.27** | 0.12 | 0.21* | 0.10 | 0.41** | 0.03 | 0.12 | ||
| EPA | ||||||||||||
| Cord | 0.16* | 0.16† | 0.14 | 0.32** | 0.36** | 0.32** | 0.28** | 0.27** | 0.09 | |||
| Current | 0.13 | 0.20* | 0.05 | 0.07 | 0.05 | 0.32** | 0.04 | 0.09 | ||||
| Selenium | ||||||||||||
| Cord | 0.31** | 0.30** | 0.19* | 0.44** | 0.22* | 0.17* | −0.02 | |||||
| Current | 0.26** | 0.42** | 0.23** | 0.63** | 0.06 | 0.24** | ||||||
| PCB 153 | ||||||||||||
| Cord | 0.47** | 0.45** | 0.25** | 0.28** | 0.05 | |||||||
| Current | 0.43** | 0.56** | 0.31** | 0.29** | ||||||||
| Mercury | ||||||||||||
| Cord | 0.46** | 0.34** | 0.11 | |||||||||
| Current | 0.22** | 0.29** | ||||||||||
| Lead | ||||||||||||
| Cord | 0.18* | |||||||||||
| Current | ||||||||||||
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PCB 153, polychlorinated biphenyl congener 153. **P < 0.01, *P < 0.05, †P < 0.10.
Behavioral results
Principal measures of task performance are presented in Table 1. Repeated-measures analysis of variance revealed that mean hit reaction times on the CRT were significantly longer for old than for new items (F[1,153] = 29.57, P < 0.001), although these 2 measures were highly intercorrelated (r = 0.94, P < 0.001). Correct identification rates for old and new items were moderately intercorrelated (r = 0.48, P < 0.001).
ERP results
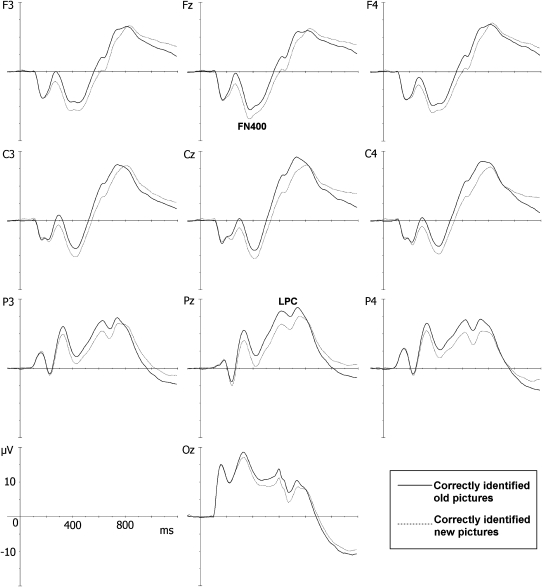
Average ERPs for correctly identified new and old items at 10 electrode locations are shown in Figure 2. These ERP response patterns were similar to those previously reported in children of these ages assessed on a continuous word-recognition task (47). FN400 was maximal at the midline frontal electrode (Fz), whereas the LPC was maximal at the midline parietal lead (Pz). FN400 amplitude (at Fz) was greater (more negative) for new than for old stimuli (F[1,153] = 49.02, P < 0.001), whereas the LPC amplitude (at Pz) was greater for old than for new stimuli (F[1,153] = 77.29, P < 0.001).
FIGURE 2.
Grand average event-related potentials (ERPs) for the continuous recognition task (n = 154). Dotted lines represent ERPs for correctly identified new pictures, and continuous lines represent ERPs for correctly identified old pictures. Frontocentral electrodes showed a negative deflection that occurred between 300 and 500 ms and reached a maximal amplitude at the Fz electrode, which is referred to as the FN400 component, and was followed by positive activity in the 500–800-ms interval and reached a maximal amplitude at the parietal electrodes, which is referred to as the late positive component (LPC). Both components showed more positive voltage for old than for new pictures, which is a phenomenon known as the FN400 and LPC old/new effects.
Associations of the ERP parameters elicited during the CRT with the child's behavioral performance on memory tasks are summarized in Table 3. A shorter FN400 latency to new stimuli was associated with a faster reaction time to both new and old stimuli on the CRT, better performance on the Digit span forward and better short-delay free recall on the CVLT. A shorter FN400 latency to old stimuli was also associated with a faster reaction time to new and old stimuli on the CRT as well as a better performance on both free-recall trials on the CVLT. Associations of FN400 amplitude to old stimuli with a poorer long-delay free recall and recognition discriminability on the CVLT fell short of conventional levels of statistical significance. The LPC amplitude to both new and old stimuli was associated with a better discrimination accuracy and shorter reaction time on the CRT, a longer auditory memory span on the Digit span forward, and better recall and recognition on the CVLT.
TABLE 3.
Pearson's correlation coefficients between event-related potential variables and memory performance1
| FN400 latency |
FN400 amplitude |
LPC amplitude |
|||||
| Condition | n | New | Old | New | Old | New | Old |
| CRT | |||||||
| Discrimination accuracy (d′) | 154 | −0.01 | −0.07 | −0.12 | −0.04 | 0.21** | 0.23** |
| Mean RT (new) | 154 | 0.23** | 0.32** | 0.08 | 0.09 | −0.19* | −0.25** |
| Mean RT (old) | 154 | 0.22** | 0.29** | 0.10 | 0.11 | −0.19* | −0.23** |
| WISC-IV | |||||||
| Digit span forward | 154 | −0.24** | −0.08 | 0.03 | −0.08 | 0.22** | 0.19* |
| CVLT | |||||||
| Short-delay free recall | 152 | −0.19* | −0.19* | 0.03 | 0.08 | 0.14† | 0.22** |
| Long-delay free recall | 152 | −0.11 | −0.20* | 0.10 | 0.14† | 0.23** | 0.22** |
| Recognition discriminability (d′) (log) | 152 | −0.13 | −0.15† | 0.05 | 0.15† | 0.24** | 0.30** |
LPC, late positive component; CRT, continuous recognition memory task; RT, reaction time; WISC-IV, Wechsler Intelligence Scales for Children, 4th edition; CVLT, California Verbal Learning Test–Children's Version. **P < 0.01, *P < 0.05, †P < 0.10.
Relation of DHA to memory
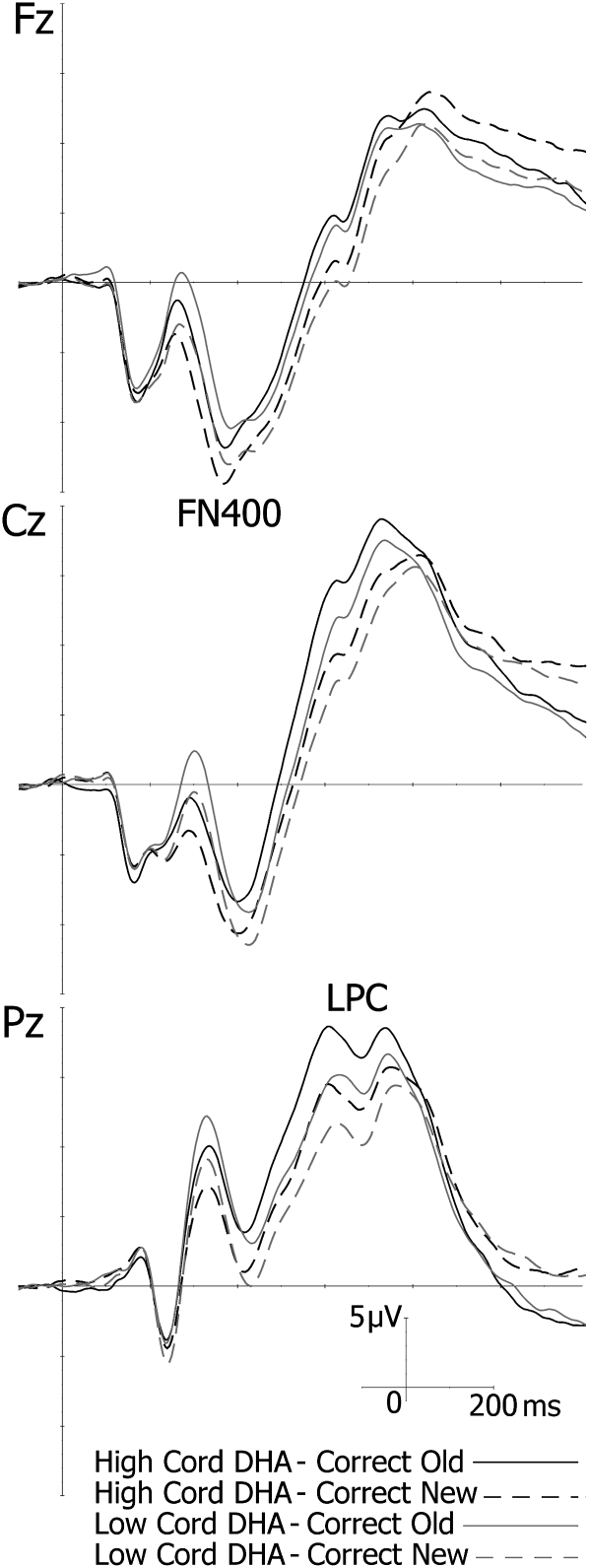
Results of the RM-MANOVA that compared children with high and low cord plasma DHA concentrations in terms of their ERP responses during the CRT are summarized in Table 4. The results relating to current DHA concentrations are presented in Table 5. Higher cord DHA concentrations were associated with a shorter latency of the FN400 component (F[1,136] = 23.23, P < 0.001) and a larger amplitude of the LPC (F[1,127] = 8.81, P < 0.01). This effect was clearly observable when ERP averages of the 2 cord DHA groups were compared (Figure 3). By contrast, a higher current DHA concentration was associated with a greater (ie, more negative) FN400 amplitude (F[1,129] = 4.25, P < 0.05). No DHA-by-condition interactions were observed. Because data regarding maternal binge drinking during pregnancy, which was missing for 31 children, was related to FN400 amplitude, the RM-MANOVA that related this endpoint to the current DHA concentration was rerun for the subset of children with alcohol exposure data to control for pregnancy binge drinking. In this analysis, the effect of the current DHA concentration on FN400 amplitude fell short of statistical significance (F[1,107] = 2.96, P = 0.09).
TABLE 4.
Repeated-measures multivariate ANOVA (RM-MANOVA) testing for effects of cord plasma docosahexaenoic acid (DHA) concentrations on event-related potential variables1
| Low cord DHA concentration (n = 75) |
High cord DHA concentration (n = 74) |
||||
| Component | New | Old | New | Old | P for DHA |
| FN400 latency (ms) | 410.6 ± 47.3 | 409.4 ± 42.4 | 377.4 ± 40.7 | 386.2 ± 40.7 | <0.001 |
| FN400 amplitude (μV) | −10.6 ± 6.9 | −7.9 ± 7.0 | −11.4 ± 6.1 | −8.9 ± 6.4 | 0.79 |
| LPC amplitude (μV) | 10.6 ± 6.5 | 13.5 ± 6.7 | 12.8 ± 7.1 | 16.4 ± 7.2 | 0.004 |
All values are means ± SDs for latencies and amplitudes for FN400 [300–500 ms, midline frontal electrode (Fz)] and the late positive component [LPC; 500-800 ms, midline parietal electrode (Pz)]. F-ratio P values are from RM-MANOVA with condition (old or new) as the within-subject factor and DHA group (split at the median) as the between-subjects factor. No condition × DHA interaction effect was significant (all P > 0.10). Covariates included in the RM-MANOVA models were as follows—for FN400 latency: child age, maternal age at delivery, breastfeeding duration, and cord mercury concentrations; for FN400 amplitude: child sex, socioeconomic status, tobacco during pregnancy, and cord and current polychlorinated biphenyl congener 153 (PCB 153) and cord lead concentrations; and for LPC amplitude: maternal education, maternal age at delivery, socioeconomic status, maternal Raven's score, tobacco during pregnancy, and current PCB 153, mercury, and lead concentrations.
TABLE 5.
Repeated-measures multivariate ANOVA (RM-MANOVA) testing for effects of current plasma docosahexaenoic acid (DHA) concentrations on event-related potential variables1
| Low current DHA concentration (n = 75) |
High current DHA concentration (n = 76) |
||||
| Component | New | Old | New | Old | P for DHA |
| FN400 latency (ms) | 399.7 ± 49.8 | 401.0 ± 45.6 | 387.5 ± 43.2 | 394.6 ± 40.9 | 0.11 |
| FN400 amplitude (μV) | −10.2 ± 6.2 | −7.7 ± 6.6 | −11.8 ± 6.7 | −9.2 ± 6.7 | 0.04 |
| LPC amplitude (μV) | 11.3 ± 7.0 | 14.6 ± 7.2 | 12.1 ± 6.7 | 15.4 ± 7.0 | 0.34 |
All values are means ± SDs for latencies and amplitudes for FN400 [300–500 ms, midline frontal electrode (Fz)] and the late positive component [LPC; 500–800 ms, midline parietal electrode (Pz)]. F-ratio P values are from RM-MANOVA with condition (old or new) as the within-subject factor and the DHA group (split at the median) as the between-subjects factor. No condition × DHA interaction effect was significant (all P > 0.20). Covariates included in the RM-MANOVA models were the same as listed in Table 4.
FIGURE 3.
Grand average event-related potentials (ERPs) for participants with low cord docosahexaenoic acid (DHA) concentrations (<3.42% of fatty acids; n = 75; gray lines) compared with high cord DHA concentrations (≥3.43% of fatty acids; n = 74; black lines). Dotted lines represent ERPs for correctly identified new pictures, and continuous lines represent ERPs for correctly identified old pictures. In the high-DHA group, the FN400 component (Fz) reached its peak earlier, and the late positive component (LPC; Pz) was larger.
Results from the regression analyses that tested for associations between DHA concentrations and the behavioral performance on neurobehavioral memory tasks are presented in Table 6. DHA concentrations in cord and current child plasma samples were not related to any of the CRT behavioral performance measures (all P > 0.20), but higher cord DHA concentrations were related to a more optimal performance on both the Digit span forward subtest (β = 0.35, P < 0.001) and the CVLT recognition memory measure (β = 0.18, P = 0.04). These effects were also observed when using dichotomous DHA measures (high compared with low) as predictors.
TABLE 6.
Associations between docosahexaenoic acid (DHA) concentrations and behavioral performance on memory tasks1
| Cord plasma DHA |
Current plasma DHA |
|||||
| n | r | Std β | n | r | Std β | |
| CRT | ||||||
| Discrimination accuracy (d′) | 149 | −0.04 | −0.02 | 151 | −0.05 | −0.04 |
| Mean RT (new) | 149 | −0.11 | −0.06 | 151 | −0.07 | −0.05 |
| Mean RT (old) | 149 | −0.11 | −0.09 | 151 | −0.10 | −0.07 |
| WISC-IV | ||||||
| Digit span forward | 149 | 0.26** | 0.35** | 151 | 0.12 | 0.15† |
| CVLT | ||||||
| Short-delay free recall | 148 | 0.10 | 0.12 | 149 | 0.13 | 0.09 |
| Long-delay free recall | 148 | 0.06 | 0.08 | 149 | 0.02 | −0.01 |
| Recognition discriminability (d′) (log) | 148 | 0.24** | 0.18* | 149 | 0.15† | 0.05 |
Std β, standardized regression coefficients; CRT, continuous recognition memory task; RT, reaction time; WISC-IV, Wechsler Intelligence Scales for Children, 4th edition; CVLT, California Verbal Learning Test–Children's Version. All values were calculated from multiple regression analyses. Covariates included in the regression models were as follows—for Discrimination accuracy (d′): parity and cord polychlorinated biphenyl congener 153 (PCB 153) and cord lead concentrations; for Mean RT (new): parity; for Mean RT (old): parity and cord PCB 153 concentrations; for Digit span forward: age at testing, maternal education, socioeconomic status, maternal Raven's score, tobacco during pregnancy, and current PCB 153 and cord mercury concentrations; for Short-delay free trial: child sex, maternal Raven's score, adoption status, and current PCB 153 concentrations; for Long delay free trial: child sex, maternal age, maternal Raven's score, and adoption status; and for Recognition discriminability: child sex, maternal age, parity, maternal education, socioeconomic status, maternal Raven's score, breastfeeding duration, tobacco during pregnancy, adoption status, and current PCB 153 concentrations. **P < 0.01, *P < 0.05, †P < 0.10.
Relation of seafood contaminants to memory
RM-MANOVA tests for effects of seafood contaminants on ERP components recorded during the CRT revealed significant interaction effects between the cord mercury group and stimulus condition (new compared with old) for both the FN400 (F[1,132] = 4.77, P = 0.03) and LPC (F[1,131] = 5.13, P = 0.03) amplitudes. Children with higher cord mercury concentrations had smaller (less negative) FN400 amplitudes to old items and smaller (less positive) LPC amplitudes to new items than did children with lower cord mercury concentrations. The inclusion of maternal binge drinking during pregnancy as a covariate in the F400 amplitude model did not alter the interaction of cord mercury by condition (F[1,110] = 3.82, P = 0.05). Current PCB 153 concentrations were also related to FN400 amplitudes; children with higher current PCB 153 concentrations had smaller (ie, less negative) FN400 amplitudes (F[1,132] = 4.39, P = 0.04). However, this effect was no longer significant after maternal binge drinking during pregnancy was controlled for (F[1,110] = 2.51, P = 0.12). Cord PCB 153 concentrations and current mercury-exposure groups did not relate to any ERP variable (all P > 0.20).
Multiple regression analyses that examined associations between seafood contaminants and the behavioral task performance during the CRT revealed that cord PCB 153 concentrations were associated with better recognition discrimination (β = 0.27, P = 0.001) and with a slower reaction time to old items (β = 0.17, P = 0.03). However, the latter association was no longer significant after maternal binge drinking during pregnancy was controlled for (β = 0.14, P = 0.14). Cord mercury and current mercury and PCB 153 concentrations were not related to any of the CRT behavioral performance measures (all P > 0.10). However, cord mercury and current PCB 153 concentrations were associated with a poorer Digit span performance (cord mercury concentrations: β = −0.20, P = 0.02; current PCB 153 concentrations: β = −0.18, P = 0.03) and the current PCB 153 body burden was associated with a better short-delay recall on the CVLT (β = 0.17, P = 0.03). These effects of contaminant exposures on the neuropsychological memory endpoints were also observed in ANCOVAs using dichotomous exposure measures as predictors.
Effect of contaminants on the relation of DHA to memory function
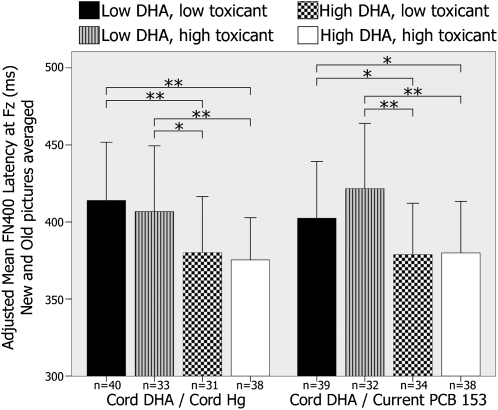
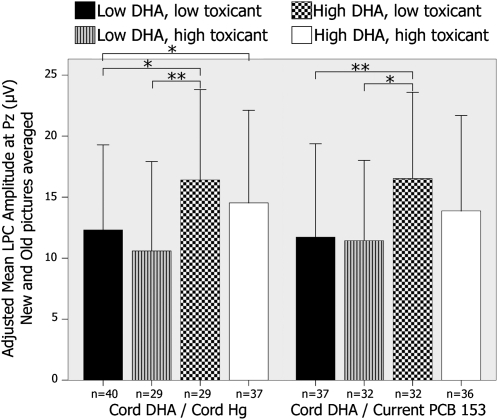
The degrees to which effects of cord DHA concentrations on FN400 latency and LPC amplitude were altered by prenatal exposure to mercury or current exposure to PCB 153 are examined in Figures 4 and 5. ANCOVA analyses that compared FN400 latency across 4 exposure groups revealed significant group differences (cord DHA and cord mercury: F[3,135] = 9.08, P < 0.001; cord DHA and current PCB 153: F[3,136] = 9.46, P < 0.001). Pairwise post hoc comparisons revealed that FN400 latency was significantly shorter in children with a higher prenatal DHA intake regardless of the concentration of cord mercury or current PCB 153. Similarly, ANCOVA analyses for the LPC amplitude revealed a significant difference across the 4 exposure groups (cord DHA and cord mercury: F[3,125] = 3.58, P = 0.02; cord DHA and current PCB 153: F[3,127] = 3.44, P = 0.02). Pairwise post hoc comparisons also showed that the effect of the cord DHA concentration on the LPC amplitude was significant regardless of the concentration of prenatal mercury exposure and that the LPC amplitude was larger in children with a higher prenatal DHA intake regardless of whether the current PCB 153 body burden was low or high.
FIGURE 4.
Mean (±SD) differences in FN400 latency at Fz averaged for new and old stimuli by exposure group. Exposure groups were created by using the median values of each exposure variable. Latencies were adjusted for child age at testing, maternal age at delivery, and breastfeeding duration. *,**Significant differences between groups in pairwise post hoc comparisons (ANCOVA): *P < 0.05, **P < 0.01. DHA, docosahexaenoic acid; Hg, mercury; PCB 153, polychlorinated biphenyl congener 153.
FIGURE 5.
Mean (±SD) differences in late positive component (LPC) amplitude at Pz averaged for new and old stimuli by exposure group. Exposure groups were created by using the median values of each exposure variable. Amplitudes were adjusted for maternal education, socioeconomic status, maternal Raven's score, tobacco during pregnancy, current blood lead concentrations, and maternal age at delivery. *,**Significant differences between groups in pairwise post hoc comparisons (ANCOVA): *P < 0.05, **P < 0.01. DHA, docosahexaenoic acid; Hg, mercury; PCB 153, polychlorinated biphenyl congener 153.
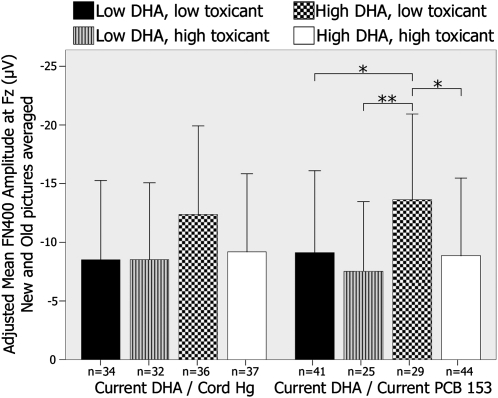
Moderating effects of contaminant exposure on the relation of the current DHA concentration to FN400 amplitude are examined in Figure 6. The ANCOVA analysis that examined the effects of the current DHA concentration on FN400 amplitude in relation to the cord mercury concentration revealed no significant differences across the 4 exposure groups (F[3,131] = 1.79, P = 0.15). The ANCOVA analysis for effects of the current DHA concentration on FN400 amplitude as a function of the current PCB 153 concentration revealed a significant difference across groups (F[3,131] = 3.07, P = 0.03). By contrast to the absence of a moderating effect of contaminants on the relation between the cord DHA concentration and FN400 latency and LPC amplitude, pairwise post hoc comparisons showed that the effect of the current DHA concentration on FN400 amplitude was moderated by the current PCB 153 concentration: children with high current DHA concentrations and low current PCB 153 concentrations had greater FN400 amplitudes than did children in the 3 other groups (all P < 0.05), which suggested that benefits related to the current DHA concentration are not seen in the presence of a high current PCB exposure.
FIGURE 6.
Mean (±SD) differences in FN400 amplitude at Fz averaged for new and old stimuli by exposure group. Exposure groups were created by using the median values of each exposure variable. Amplitudes were adjusted for child sex, socioeconomic status, tobacco during pregnancy and cord lead concentrations. *,**Significant differences between groups in pairwise post hoc comparisons (ANCOVA): *P < 0.05, **P < 0.01. DHA, docosahexaenoic acid; Hg, mercury; PCB 153, polychlorinated biphenyl congener 153.
DISCUSSION
This study examined associations of prenatal and childhood intakes of long-chain PUFAs and seafood contaminants with ERP response patterns during visual recognition memory processing in a sample of school-age children from an Inuit community where the consumption of fish and sea mammals is an important component of the daily diet. A higher prenatal DHA intake was associated with a shorter FN400 latency and larger LPC amplitude, and a higher childhood DHA intake was associated with a larger FN400 amplitude. By contrast, a higher prenatal mercury exposure was related to a smaller FN400 amplitude in the old condition and a smaller LPC amplitude in the new condition; a higher postnatal PCB exposure was related to a smaller FN400 amplitude. The effects of higher cord DHA concentrations on ERP variables were not moderated by the severity of exposure to seafood contaminants; however, The effects of current plasma DHA concentrations on FN400 amplitude were not seen in the presence of high current PCB exposure. DHA and mercury concentrations were not associated with differences in behavioral performances in this visual recognition memory task, which was designed to be easy enough to elicit a sufficient number of correct responses to permit a reliable assessment of the ERP response.
The study also examined the associations of prenatal and childhood intakes of long-chain PUFAs and seafood contaminants with performance on 2 well-established neuropsychological memory tasks. Higher cord plasma DHA concentrations were associated with better performances on the Digit span forward, which assessed immediate memory, and on the delayed recognition trial of the CVLT, which is a measure of episodic memory. Adverse effects on digit span performances were also shown for cord mercury and current PCB concentrations, which confirmed that the effects of these contaminant exposures on ERP response patterns in the behaviorally easier ERP tasks were also behaviorally seen on a more challenging neuropsychological assessment of memory function.
In line with dual-process models, the FN400 and LPC ERP components are thought to reflect 2 distinct retrieval processes underlying recognition memory, namely familiarity and recollection, respectively (48). In the current study, both ERP components were related to prenatal n−3 PUFA intakes. The effect of the cord plasma DHA concentration on FN400 latency suggested that a higher prenatal DHA intake results in faster judgment of familiarity of visual information, and the effect on LPC amplitude suggested an enhanced brain activity during the recollection of visual information in memory. Our finding that greater cord DHA concentrations were also associated with better behavioral performances on 2 neuropsychological memory tasks strengthened the assumption that these ERP effects reflect prenatal DHA-related enhancement of memory development. Beneficial effects of n−3 PUFA intake during the development on memory have previously been documented in rodents (49, 50) and human infants (9, 27, 51), but to our knowledge, the current study was the first to document benefits of prenatal DHA intake on memory function in school-age children. It has been suggested that the action of n−3 PUFAs on memory may be mediated by hippocampus-related mechanisms because experimental animal studies have shown that the hippocampus accumulates larger quantities of DHA than do other brain regions (49), and DHA promotes hippocampal neuronal growth and synaptic function (52–54). Thus, the data suggested that memory may constitute one of the principal targets of DHA action during development, and the failure to assess this cognitive function may account for the inconsistent results from earlier studies on the long-term benefits of prenatal n−3 PUFA intake in children (15, 16, 18, 19).
The magnitude of the association of the cord DHA concentrations with FN400 latency and LPC amplitude was substantially greater than the relation of the current DHA concentration to FN400 amplitude. Nevertheless, the latter finding was consistent with evidence from other studies that a higher DHA intake during childhood can enhance memory function. The FN400 amplitude finding suggested an association of the current DHA intake with increased brain activity during familiarity processing. Randomized controlled studies have reported associations between a higher DHA concentration in the child's blood at testing and better vocabulary at preschool age (55) and verbal recognition memory at school age (56). These findings suggested that the consumption of food rich in n−3 PUFAs may have a measurable beneficial effect on cognition even after the perinatal period. In a recent placebo-controlled functional magnetic resonance imaging study in school-age boys (57), 8 wk of supplementation with DHA increased the activation of the dorsolateral prefrontal cortex during a sustained attention task. Although a source-localization study would be required to identify the specific brain regions that generate the FN400 response, our finding of enhanced FN400, which is a frontally distributed ERP, in children with higher current DHA concentrations was consistent with these imaging data.
In the current study, higher cord mercury concentrations were associated with smaller FN400 amplitudes in processing old stimuli and smaller LPC amplitudes in processing new stimuli. On the basis of the relations shown between our ERP parameters and the behavioral performance on memory tasks, these results suggested that prenatal mercury exposure is associated with poorer memory performance. Data in the literature relating prenatal mercury exposure to poorer memory function in fish-eating populations are inconsistent. In the Faroe Islands, prenatal mercury exposure was related to impaired verbal learning and delayed figure reproduction at 7 y of age (24) but not at 14 y of age (58), and no effects were seen on memory in the Seychelles Islands (59–61). The effects reported in our study may have reflected the higher sensitivity of ERP measures of memory to detect mercury-related neurotoxic effects compared with that of some neurobehavioral learning tasks.
The finding of decreased FN400 amplitudes in children with higher current PCB body burdens contrasted with previous studies that reported adverse effects of prenatal, but not postnatal, PCB exposure on cognition (25, 62). A plausible explanation for this divergence is that the breastfeeding duration was typically much longer in this Inuit sample than the duration in other PCB cohort studies, which led to the transmission of much larger quantities of these contaminants to the child postnatally. Nevertheless, the effect on memory processing was consistent with findings from previous studies that linked prenatal PCB exposure to an impairment in visual recognition memory assessed during infancy (26, 63) and at preschool age (64). The absence of prenatal PCB effects in this study may have been due to differences in the mixtures of congeners to which the United States and Inuit populations were exposed. Compared with this Inuit sample, Michigan children were exposed to PCB mixtures with a larger proportion of potentially more toxic lower chlorinated congeners (31, 65). Our finding of decreased FN400 amplitude with higher PCB exposure suggested reduced frontal activity during information processing, which was consistent with the suggestion that PCBs may interfere more specifically with performance on tasks that tap frontal lobe function (23, 66, 67). More data are needed to test this hypothesis.
Our study sample also differed from those of previous cohort studies on DHA and/or contaminants in the high incidence of smoking during pregnancy in Inuit mothers. Eighty percent of the participating mothers reported smoking tobacco while pregnant, which was >3 times the proportion of smokers in young adult women from the general Canadian population (68). Prenatal tobacco exposure has been shown to be related to altered electrophysiologic responses to sensory stimuli in toddlers (69) and to poorer performances on cognitive and memory assessments during childhood (70). As noted in the footnotes to Tables 4 and 6, smoking during pregnancy was related to a smaller FN400 and LPC amplitude and to a poorer performance on the WISC-IV Digit span forward and CVLT recognition discriminability and was, therefore, statistically controlled in the models that tested for effects on these outcomes.
This study also provided new evidence that the FN400 and LPC components reflect memory-related processes on the basis of our analyses of the associations between their parameters and performance on standard neuropsychological memory tasks. The most consistent associations were with FN400 latency and LPC amplitude. Surprisingly, these correlations were only weakly influenced by the task condition (new compared with old), suggesting that future ERP studies that use continuous recognition paradigms should not limit their analyses to a contrasts of old compared with new effects.
In conclusion, to our knowledge, this study was the first to use ERP measures of recognition memory to assess the effects of n−3 PUFAs and seafood contaminants in school-age children. The results suggest that prenatal DHA intakes have important long-term beneficial effects for memory processing, and current DHA intakes can also be beneficial. Conversely, prenatal mercury and postnatal PCB exposure were both associated with reductions in ERP response amplitude measures, which indicated adverse effects on memory. The interpretation of these results was supported by our findings that these exposures were associated with performance on well-established neuropsychological memory tasks. Thus, the consumption of food rich in n−3 PUFAs during pregnancy and childhood should be encouraged to optimize cognitive function although, because of the contamination of seafood products by neurotoxic pollutants, caution should be taken when selecting dietary sources of n−3 PUFAs.
Acknowledgments
We are grateful to the Nunavik population and to all people involved in this study. We thank Renee Sun, Line Roy, Brenda Tuttle, and Alacie Pov for their contributions to data collection, Alissa Westerlund, Jocelyne Gagnon, and Neil Dodge for their work on data processing, and Célyne Bastien for her help with electrophysiologic recordings.
The authors' responsibilities were as follows—OB: wrote the manuscript, analyzed ERP data, and performed statistical analyses; MJB: implemented the ERP data collection in the field and performed preliminary analysis of ERP data; GM and SWJ: collaborated in the design and implementation of the study; DS-A: collaborated in implementing the ERP data collection in the field; PA: collaborated in the study design, particularly the plans for analysis of the biological data; ED: designed and supervised the Cord Blood Monitoring Program and collaborated in the design of this study; CAN: developed the CRT and trained and supervised MJB in the implementation of the ERP data collection in the field and the preliminary analysis of data; and JLJ: collaborated in the study design, planning of statistical analyses, and drafting of the manuscript. None of the authors declared a conflict of interest.
REFERENCES
- 1.Innis SM. Dietary omega-3 fatty acids and the developing brain. Brain Res 2008;1237:35–43 [DOI] [PubMed] [Google Scholar]
- 2.Birch EE, Garfield S, Castaneda Y, Hughbanks-Wheaton D, Uauy R, Hoffman D. Visual acuity and cognitive outcomes at 4 years of age in a double-blind, randomized trial of long-chain polyunsaturated fatty acid-supplemented infant formula. Early Hum Dev 2007;83:279–84 [DOI] [PubMed] [Google Scholar]
- 3.Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N., Jr Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins Leukot Essent Fatty Acids 2010;82:305–14 [DOI] [PubMed] [Google Scholar]
- 4.Judge MP, Harel O, Lammi-Keefe CJ. Maternal consumption of a docosahexaenoic acid-containing functional food during pregnancy: benefit for infant performance on problem-solving but not on recognition memory tasks at age 9 mo. Am J Clin Nutr 2007;85:1572–7 [DOI] [PubMed] [Google Scholar]
- 5.Judge MP, Harel O, Lammi-Keefe CJ. A docosahexaenoic acid-functional food during pregnancy benefits infant visual acuity at four but not six months of age. Lipids 2007;42:117–22 [DOI] [PubMed] [Google Scholar]
- 6.Malcolm CA, McCulloch DL, Montgomery C, Shepherd A, Weaver LT. Maternal docosahexaenoic acid supplementation during pregnancy and visual evoked potential development in term infants: a double blind, prospective, randomised trial. Arc Dis Child Fetal Neonatal Ed. 2003;88:F383–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlson SE, Werkman SH. A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids 1996;31:85–90 [DOI] [PubMed] [Google Scholar]
- 8.Clandinin MT, Van Aerde JE, Merkel KL, et al. Growth and development of preterm infants fed infant formulas containing docosahexaenoic acid and arachidonic acid. J Pediatr 2005;146:461–8 [DOI] [PubMed] [Google Scholar]
- 9.O'Connor DL, Hall R, Adamkin D, et al. Growth and development in preterm infants fed long-chain polyunsaturated fatty acids: a prospective, randomized controlled trial. Pediatrics 2001;108:359–71 [DOI] [PubMed] [Google Scholar]
- 10.Smithers LG, Gibson RA, McPhee A, Makrides M. Higher dose of docosahexaenoic acid in the neonatal period improves visual acuity of preterm infants: results of a randomized controlled trial. Am J Clin Nutr 2008;88:1049–56 [DOI] [PubMed] [Google Scholar]
- 11.Birch EE, Garfield S, Hoffman DR, Uauy R, Birch DG. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev Med Child Neurol 2000;42:174–81 [DOI] [PubMed] [Google Scholar]
- 12.Three randomized controlled trials of early long-chain polyunsaturated fatty acid supplementation on means-end problem solving in nine-month olds. Child Dev 2009;80:1376–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makrides M, Neumann M, Simmer K, Pater J, Gibson R. Are long-chain polyunsaturated fatty acids essential nutrients in infancy? Lancet 1995;345:1463–8 [DOI] [PubMed] [Google Scholar]
- 14.Morale SE, Hoffman DR, Castaneda YS, Wheaton DH, Burns RA, Birch EE. Duration of long-chain polyunsaturated fatty acids availability in the diet and visual acuity. Early Hum Dev 2005;81:197–203 [DOI] [PubMed] [Google Scholar]
- 15.Helland IB, Smith L, Saarem K, Saugstad OD, Drevon CA. Maternal supplementation with very-long-chain n−3 fatty acids during pregnancy and lactation augments children's IQ at 4 years of age. Pediatrics 2003;111:e39–44 [DOI] [PubMed] [Google Scholar]
- 16.Helland IB, Smith L, Blomén B, Saarem K, Saugstad OD, Drevon CA. Effect of supplementing pregnant and lactating mothers with n−3 very-long-chain fatty acids on children's IQ and body mass index at 7 years of age. Pediatrics 2008;122:e472–9 [DOI] [PubMed] [Google Scholar]
- 17.Bakker EC, Hornstra G, Blanco CE, Vles JSH. Relationship between long-chain polyunsaturated fatty acids at birth and motor function at 7 years of age. Eur J Clin Nutr 2009;63:499–504 [DOI] [PubMed] [Google Scholar]
- 18.Bakker EC, Ghys AJA, Kester ADM, et al. Long-chain polyunsaturated fatty acids at birth and cognitive function at 7 y of age. Eur J Clin Nutr 2003;57:89–95 [DOI] [PubMed] [Google Scholar]
- 19.Ghys A, Bakker E, Hornstra G, Van den Hout M. Red blood cell and plasma phospholipid arachidonic and docosahexaenoic acid levels at birth and cognitive development at 4 years of age. Early Hum Dev 2002;69:83–90 [DOI] [PubMed] [Google Scholar]
- 20.Su H-M. Mechanisms of n−3 fatty acid-mediated development and maintenance of learning memory performance. J Nutr Biochem 2010;21:364–73 [DOI] [PubMed] [Google Scholar]
- 21.National Academy of Sciences/National Research Council Seafood choices: balancing benefits and risks. Washington, DC: National Academy Press, 2006 [Google Scholar]
- 22.Hansen JC. Environmental contaminants and human health in the Arctic. Toxicol Lett 2000;112-113:119–25 [DOI] [PubMed] [Google Scholar]
- 23.Boucher O, Muckle G, Bastien C. Prenatal exposure to polychlorinated biphenyls: a neuropsychologic analysis. Environ Health Perspect 2009;117:7–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandjean P, Weihe P, White RF, et al. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol 1997;19:417–28 [DOI] [PubMed] [Google Scholar]
- 25.Jacobson JL, Jacobson SW. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med 1996;335:783–9 [DOI] [PubMed] [Google Scholar]
- 26.Jacobson SW, Fein GG, Jacobson JL, Schwartz PM, Dowler JK. The effect of intrauterine PCB exposure on visual recognition memory. Child Dev 1985;56:853–60 [PubMed] [Google Scholar]
- 27.Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: Evidence from the Inuit of Arctic Quebec. J Pediatr 2008;152:356–64 [DOI] [PubMed] [Google Scholar]
- 28.Saint-Amour D, Roy MS, Bastien C, et al. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology 2006;27:567–78 [DOI] [PubMed] [Google Scholar]
- 29.Jacques C, Levy E, Muckle G, et al. Long-term effects of prenatal omega-3 fatty acid intake on visual function in school-age children. J Pediatr 2011;158:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muckle G, Dewailly E, Ayotte P. Prenatal exposure of Canadian children to polychlorinated biphenyls and mercury. Can J Public Health 1998;89:S20–5 [PubMed] [Google Scholar]
- 31.Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Prenatal exposure of the northern Québec inuit infants to environmental contaminants. Environ Health Perspect 2001;109:1291–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jasper HA. The ten-twenty system of the international federation. Electroencephalogr Clin Neurophysiol 1958;10:371–5 [Google Scholar]
- 33.Gratton G, Coles MGH, Donchin E. A new method for off-line removal of ocular artefact. Electroencephalogr Clin Neurophysiol 1983;55:468–84 [DOI] [PubMed] [Google Scholar]
- 34.Curran T, Cleary AM. Using ERPs to dissociate recollection from familiarity in picture recognition. Brain Res Cogn Brain Res 2003;15:191–205 [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. Wechsler Intelligence Scales for Children. 4th ed. San Antonio, TX: The Psychological Corporation, 2003 [Google Scholar]
- 36.Delis DC, Kramer JH, Kaplan K, Ober BA. The California Verbal Learning Test for Children. San Antonio, TX: The Psychological Corporation, 1994 [Google Scholar]
- 37.Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyriod function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect 2009;117:1014–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E. Assessment of pre- and postnatal exposure to polychlorinated biphenyls: lessons from the Inuit cohort study. Environ Health Perspect 2003;111:1253–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollingshead AB. Four factor index of social status. New Haven, CT: Yale University Department of Sociology, 1975 [Google Scholar]
- 40.Raven JC, Court JH, Raven J. Manual for Raven's progressive matrices and vocabulary scales: standard progressive matrices. Oxford, United Kingdom: Psychologists Press, 1992 [Google Scholar]
- 41.Winer BJ. Statistical principles in experimental design. 2nd ed. New York: McGraw-Hill, 1971 [Google Scholar]
- 42.Lucas M, Dewailly E, Muckle G, et al. Gestational age and birth weight in relation to n−3 fatty acids among Inuit (Canada). Lipids 2004;39:617–26 [DOI] [PubMed] [Google Scholar]
- 43.Otto SJ, Houwelingen AC, Antal M, et al. Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. Eur J Clin Nutr 1997;51:232–42 [DOI] [PubMed] [Google Scholar]
- 44.Rhainds M, Levallois P, Dewailly E, Ayotte P. Lead, mercury, and organochlorine compound levels in cord blood in Québec, Canada. Arch Environ Health 1999;54:40–7 [DOI] [PubMed] [Google Scholar]
- 45.Longnecker MP, Wolff MS, Gladen BC, et al. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ Health Perspect 2003;111:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL. Determinants of polychlorinated biphenyls and methylmercury exposure in Inuit women of childbearing age. Environ Health Perspect 2001;109:957–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van Strien JW, Glimmerveen JC, Martens VEG, De Bruin EA. Age-related differences in brain activity during extended continuous word recognition in children. Neuroimage 2009;47:688–99 [DOI] [PubMed] [Google Scholar]
- 48.Rugg MD, Curran T. Event-related potentials and recognition memory. Trends Cogn Sci 2007;11:251–7 [DOI] [PubMed] [Google Scholar]
- 49.Chung W-L, Chen J-J, Su H-M. Fish oil supplementation of contral and (n−3) fatty acid-deficient male rats enhances reference and working memory performance and increases brain regional docosahexaenoic acid levels. J Nutr 2008;138:1165–71 [DOI] [PubMed] [Google Scholar]
- 50.Gamoh S, Hashimoto M, Sugioka K, et al. Chronic administration of doxosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience 1999;93:237–41 [DOI] [PubMed] [Google Scholar]
- 51.Henriksen C, Haugholt K, Lindgren M, et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008;121:1137–45 [DOI] [PubMed] [Google Scholar]
- 52.Calderon F, Kim H-Y. Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 2004;90:979–88 [DOI] [PubMed] [Google Scholar]
- 53.Cao D, Kevala K, Kim J, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem 2009;111:510–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida S, Yasuda A, Kawazato H, et al. Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of α-linolenate deficiency and a learning task. J Neurochem 1997;68:1261–8 [DOI] [PubMed] [Google Scholar]
- 55.Ryan AS, Nelson EB. Assessing the effect of docosahexaenoic acid on cognitive functions in healthy, preschool children: a randomized, placebo-controlled, double-blind study. Clin Pediatr (Phila) 2008;47:355–62 [DOI] [PubMed] [Google Scholar]
- 56.Dalton A, Wolmarans P, Witthuhn RC, Van Stuijvenberg ME, Swanevelder A, Smuts CM. A randomised control trial in school children showed improvement in cognitive function after consuming a bread spread, containing fish flour from a marine source. Prostaglandins Leukot Essent Fatty Acids 2009;80:143–9 [DOI] [PubMed] [Google Scholar]
- 57.McNamara RK, Able J, Jandacek R, et al. Docosahexaenoic acid supplementation increases prefrontal cortex activation during sustained attention in healthy boys: a placebo-controlled, dose-ranging, functional magnetic resonance imaging study. Am J Clin Nutr 2010;91:1060–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Debes F, Budtz-Jorgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioral function at age 14 years. Neurotoxicol Teratol 2006;28:363–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davidson PW, Sloane-Reeves J, Myers GJ, et al. Association between prenatal exposure to methylmercury and visuospatial ability at 10.7 years in the Seychelles Child Development Study. Neurotoxicology 2008;29:453–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davidson PW, Strain JJ, Myers GJ, et al. Neurodevelopmental effects of maternal nutritional status and exposure to methylmercury from eating fish during pregnancy. Neurotoxicology 2008;29:767–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Myers GJ, Marsh DO, Davidson PW, et al. Main neurodevelopmental study of Seychellois children following in utero exposure to methylmercury from a maternal fish diet: outcome at six months. Neurotoxicology 1995;16:653–64 [PubMed] [Google Scholar]
- 62.Jacobson JL, Jacobson SW. Prenatal exposure to polychlorinated biphenyls and attention at school age. J Pediatr 2003;143:780–8 [DOI] [PubMed] [Google Scholar]
- 63.Darvill T, Lonky E, Reihman J, Stewart P, Pagano J. Prenatal exposure to PCBs and infant performance on the Fagan Test of Infant Intelligence. Neurotoxicology 2000;21:1029–38 [PubMed] [Google Scholar]
- 64.Jacobson JL, Jacobson SW, Padgett RJ, Brumitt GA, Billings RL. Effects of prenatal PCB exposure on cognitive processing efficiency and sustained attention. Dev Psychol 1992;28:297–306 [Google Scholar]
- 65.Jacobson JL, Humphrey HEB, Jacobson SW, Schantz SL, Mullin MD, Welch R. Determinants of polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), and dichlorodiphenyl trichloroethane (DDT) in the sera of young children. Am J Public Health 1989;79:1401–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stewart P, Reihman J, Gump B, et al. Response inhibition at 8 and 9 1/2 years of age in children prenatally exposed to PCBs. Neurotoxicol Teratol 2005;27:771–80 [DOI] [PubMed] [Google Scholar]
- 67.Tilson HA, Jacobson JL, Rogan WJ. Polychlorinated biphenyls and the developing nervous system: cross-species comparisons. Neurotoxicol Teratol 1990;12:239–48 [DOI] [PubMed] [Google Scholar]
- 68.Statistics Canada Smoking and diabetes care: results from the CCHS cycle 3.1 (2005). Ottawa, Canada: Minister of Industry, 2006 [Google Scholar]
- 69.Scher MS, Richardson GA, Robles N, et al. Effects of prenatal substance exposure: altered maturation of visual evoked potentials. Pediatr Neurol 1998;18:236–43 [DOI] [PubMed] [Google Scholar]
- 70.Huizink AC, Mulder EJH. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev 2006;30:24–41 [DOI] [PubMed] [Google Scholar]