Abstract
The human Activator-Recruited Cofactor (ARC)/Mediator co-activator complex interacts with many transcriptional activators and facilitates recruitment of RNA polymerase II to promote target gene transcription. The MED25 (ARC92) subunit is a critical target of the potent Herpes simplex 1 viral transcriptional activator VP16. Here, we determine the solution structure of the MED25 VP16-binding domain (VBD), and define its binding site for the N-terminal portion of the VP16 transactivation domain (TADn). A hydrophobic furrow, formed by a β-barrel and two α-helices in MED25 VBD, interacts tightly with VP16 TADn. Mutations in this furrow prevent binding of VP16 TAD to MED25 VBD and interfere with the ability of over-expressed MED25 VBD to inhibit VP16-dependent transcriptional activation in vivo. This detailed molecular understanding of transactivation by the benchmark activator VP16 could provide important insights into viral and cellular gene activation mechanisms.
Introduction
Herpes simplex viruses (HSVs) usurp the host-cell transcriptional machinery upon infection to promote expression of viral genes1. Initial transcription of immediate-early (α) viral genes is activated by the tegument protein VP16 (also termed alpha-gene transactivating factor, α-TIF)1. VP16 possesses a potent transactivation domain (TAD) when fused to heterologous DNA-binding domains, and it has been suggested to be prototypic of “acidic” TADs2,3. The molecular mechanism of transcription activation mediated by the VP16 TAD has thus been extensively studied to provide insights into more general mechanisms of gene activation4.
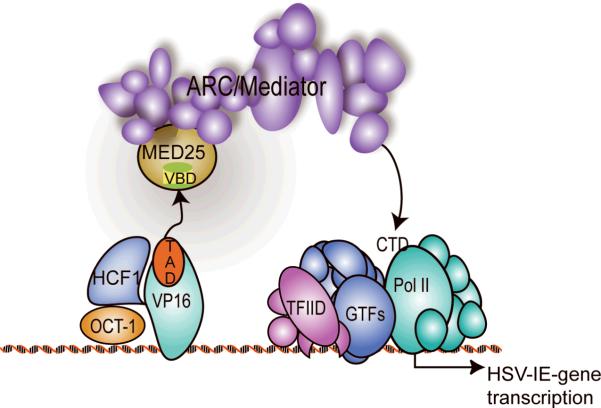
Previously, we and others identified a large multi-protein complex that is required for transcriptional activation by VP16, as well as for other cellular and viral transcriptional activators, including nuclear receptors, the cholesterol/lipid regulator SREBP, the inflammation and apoptosis regulator NF-κB, adenoviral E1A 13S, and the proto-oncoprotein p535,6. This human transcription co-activator complex, which we initially termed Activator-Recruited Cofactor (ARC), mediates activator-dependent recruitment of RNA polymerase II (Pol II) to target genes via binding to the C-terminal domain of the large Pol II subunit7 (Fig. 1). It is structurally and functionally related to the S. cerevisiae Mediator co-activator8, and we therefore refer to it as ARC/Mediator.
Figure 1.
VP16-activated transcription. VP16, OCT-1 and HCF-1 form a VP16-induced complex33. The VP16 transactivation domain (TAD) interacts with the MED25 VP16 binding domain (VBD) to recruit ARC/Mediator and activate transcription.
It is estimated that the ARC/Mediator co-activator complex is comprised of more than 30 subunits9. Growing experimental evidence suggests that many of these individual ARC/Mediator subunits can interact with transcriptional activators10. Previous studies by others and us indicated that the MED25 subunit (also known as ARC92 and ACID1) is a direct and functionally important target of the VP16 TAD11,12 (Fig. 1). Interestingly, residues 402–590 of MED25 display high sequence homology to a repeated sequence in a protein of unknown function, PTOV1, previously identified based on its overexpression in prostate tumors13 (Supplementary Fig. 1a). Moreover, deletion mutations showed that this MED25 region functioned as the VP16-binding domain (VBD), suggesting that this conserved sequence may represent a novel activator binding motif12 (Supplementary Fig. 1a). In our previous study, we also found that the amino-terminal portion of the VP16 TAD (TADn, Supplementary Fig. 1b) is critical for strong interaction with MED25, whereas the C-terminal portion only mediated weak binding to MED2512. On the other hand, the C-terminal domain of the VP16 TAD has been found to recruit the histone acetyltransferases CBP/p30014. In addition, the C-terminal domain has also been reported to interact with the p62/Tfb1 subunit of TFIIH15, with TAF916 and with TFIIA to stimulate assembly of a TFIIA-TFIID-promoter complex17. The full-length VP16 TAD has been further shown to interact with TFIIB18,19 and with PC420,21. The latter interaction was analyzed in detail by Jonker et al.22. Despite these advances, the precise molecular details of how VP16 activates transcription remain unclear.
Here, we determined the solution structure of the 19 kDa MED25 VBD (residues 391–553) to consist of a seven-stranded β-barrel flanked by three ⟨-helices. A hydrophobic groove formed by the ®-barrel and two of the three ⟨-helices serves as the VP16 TADn binding site. Point mutations targeting this groove prevent the full-length VP16 TAD from binding to MED25 VBD in vitro, and abrogate dominant negative inhibition of VP16 TAD-dependent transcriptional activation by soluble MED25 VBD in cell culture studies.
Results from this study may have important ramifications for both viral and cellular mechanisms of gene activation. First, the newly identified hydrophobic pocket on the surface of MED25 VBD could potentially serve as a novel target for small-molecule inhibitors that impinge on MED25-dependent transcriptional activity by viral transactivators of the Herpes family. These detailed structure-function studies will also guide future investigations elucidating the gene activation mechanism of putative cellular transcriptional activators involved in ARC/Mediator recruitment through interactions with MED25 and its structurally conserved activator-binding domain.
Results
Structure of MED25 VBD in solution
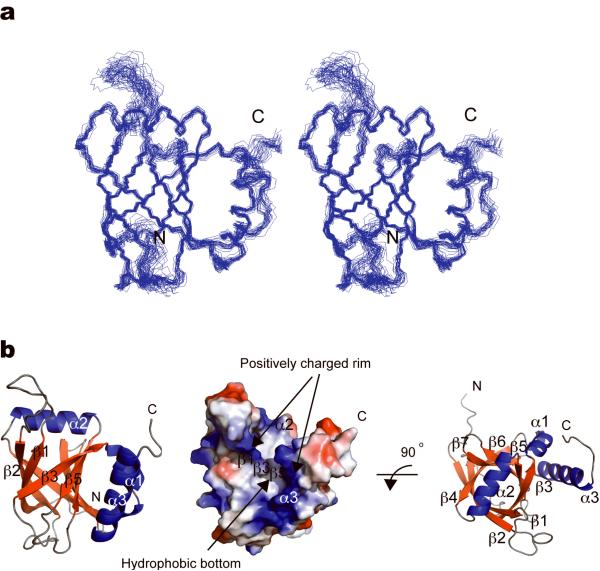
To obtain mechanistic insights into VP16 TAD-dependent transcriptional activation, we solved the three-dimensional solution structure of the MED25 VBD (amino acids 391–553, Supplementary Fig. 1a) by NMR spectroscopy. Table 1 lists the statistics of the structure calculations. A summary of a Ramachandran Plot generated by PROCHECK23 is presented in Supplementary Table 1. Supplementary Fig. 2 shows a 1H-15N-HSQC NMR spectrum with assigned peaks of the MED25 VBD. Procedures for sample preparation, data acquisition and structure calculation are described in the Methods section. The MED25 VBD exhibits a seven-stranded β-barrel flanked by three α-helices (Fig. 2a,b). The structure of the MED25 VBD contains large segments of high internal mobility that primarily localize to variable loops and to the unstructured N- and C-termini of the protein (Fig. 2a,b and Supplementary Fig. 3a). The interior of the β-barrel is tightly packed with aromatic and aliphatic side chains. The barrel is capped by helix α2 on one side and by two adjacent loops at the opposite end of the structure (Fig. 2b and Supplementary Fig. 3b). Helices α1 and α3 are located on one side of the barrel. The placement of strands β1, β3, β5, the long loop between Glu410 and Leu423, and the C-terminal α3-helix creates a deep furrow that is lined with positively charged residues on both sides and hydrophobic residues at its base (Fig. 2b). The shape and charge distribution of the furrow, as well as the presence of a central prominent hydrophobic pocket of a size suitable for accommodating a hydrophobic/aromatic side chain, together suggest a potential peptide interaction site.
Table 1.
NMR and refinement statistics for the MED25 VBD structure.
| MED25 VBD | |
|---|---|
| NMR distance and dihedral constraints | |
| Distance constraints | |
| Total NOE | 1755 |
| Intra-residue | 422 |
| Inter-residue | 1333 |
| Sequential (|i − j| =1) | 546 |
| Medium-range (|i − j| < 4) | 181 |
| Long-range (|i − j| > 5) | 606 |
| Intermolecular | n.a. |
| Hydrogen bonds | 90 |
| Total dihedral angle restraints | 208 |
| ϕ | 104 |
| ψ | 104 |
| Structure statistics | |
| Violations (mean and s.d.) | |
| Distance constraints (Å) | 0.046 ± 0.0193 |
| Dihedral angle constraints (°) | 0.88 ± 0.480 |
| Max. dihedral angle violation (°) | 2.90 |
| Max. distance constraint violation (Å) | 0.26 |
| Deviations from idealized geometry | |
| Bond lengths (Å) | 0.011 |
| Bond angles (°) | 1.17 |
| Impropers (°) | 1.46 |
| Average pairwise r.m.s. deviation** (Å) | |
| Heavy | 0.75 ± 0.08 |
| Backbone | 0.49 ± 0.07 |
Pairwise r.m.s. deviation was calculated among 25 refined structures. Values derived from the structured regions (Fig. 1b) of the protein.
Figure 2.
Solution structure of the MED25 VBD determined by NMR. (a) The 25 lowest energy structures are shown overlaid using the secondary structure elements in side-by-side stereoview. The bundles are displayed 2.4 inches apart. (b) Cartoon drawing of MED25 VBD; the seven β-strands forming the barrel and the three ⟨-helices are depicted in red and blue respectively. The long α3 helix docks on the barrel by making close contact with β5, β6 and α1. Helix α2 caps the barrel from one side. A color-coded electrostatic surface potential shows a negative patch surrounded by areas of positive potential with a hydrophobic furrow in the center (b, middle panel), highlighted by arrows.
15N T2 relaxation measurements showed a relatively uniform distribution of 15N T2 relaxation times of approximately 50 – 60 ms (Supplementary Fig. 3a). The N- and C-terminal residues of MED25 VBD and the residues forming the long loop between β1 and β2 exhibit significantly longer 15N T2 times, indicating higher local flexibility. Residues 500 – 508, constituting a loop between β5 and β6, display broad lines of amide resonances, which is most likely a result of local conformational exchange.
Interestingly, there are only two other human protein domains with considerable sequence homology to the MED25 VBD, both of which are present in PTOV1 (Fig. 1b), a protein of unknown function that is overexpressed in prostate cancer13. The high degree of sequence homology suggests that the two PTOV1 domains could indeed exhibit similar folding as the MED25 VBD, however, this will need to be experimentally confirmed.
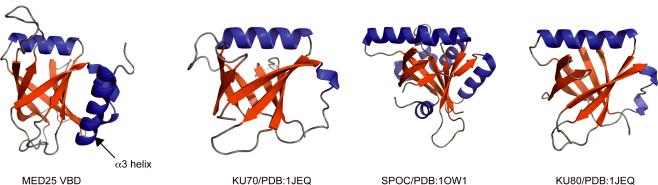
A DALI search24 identified three domains in the protein data bank that have folds similar to the MED25 VBD (Fig. 3 and Supplementary Fig. 4). However, they lack sequence homology to the MED25 VBD and exhibit distinct chain topologies. The Spen paralog and ortholog C-terminal (SPOC) domain (PDB ID 1OW125) is found in the Split ends family (SPEN) proteins, which regulate the expression of key transcriptional effectors in diverse signaling pathways. It was reported to mediate interactions with the silencing mediator for retinoid and thyroid receptors (SMRT) and the nuclear receptor co-repressor (NCoR) co-repressors, however, the precise function of this domain is unknown25. The other two structural homologs are found in the KU heterodimer (KU70-KU80) (PDB ID 1JEQ26), that is involved in double-stranded DNA repair26. Interestingly, all three structures lack the long C-terminal ⟨3 helix that together with β3 and β5 forms the hydrophobic pocket in MED25 VBD (Figs. 2 and 3). As our data discussed below indicate that the MED25 VBD hydrophobic pocket is critical for VP16 binding, this finding suggests that the structural homologues are unlikely to represent VP16 targets.
Figure 3.
MED25 VBD adopts a rare seven-strand β-barrel fold. Cartoon drawing of MED25 VBD accompanied by three structural homologues found using DALI35: Two β-barrel-domains from the KU70-KU80 complex (PDB 1JEQ26) and the SPOC (PDB 1OW125) domain of SPEN. The three homologous structures exhibit a different topology and lack the C-terminal helix present in MED25 VBD. The three MED25 VBD homologues are shown from left to right in decreasing degree of structural homology.
Molecular mechanism of MED25 VBD-VP16 TADn interaction
We tested two VP16 TAD constructs for binding to MED25 VBD (Supplementary Fig. 1b). A shorter, 42-residue fragment consisted of the N-terminal portion of the VP16 TAD (amino acids 411–452; termed here TADn), and a longer 80-residue fragment contained the full-length TAD (amino acids 411–490; termed here TAD) (Supplementary Fig. 1b). Both peptides displayed tight binding to MED25 VBD as determined by isothermal titration calorimetric (ITC) assays. The Kd of the VP16 TADn-MED25 VBD interaction was 1.6 μM (Supplementary Fig. 5a), whereas the full-length VP16 TAD binds to MED25 VBD with a Kd of approximately 50 nM (Supplementary Fig. 5b). The nanomolar affinity of the full-length VP16 TAD for the MED25 VBD is consistent with the notion that immobilized VP16 TAD can purify the ARC/Mediator complex from nuclear extracts in a single step27.
We mapped the binding site of VP16 TADn on MED25 VBD and the binding site of MED25 VBD on VP16 TADn by NMR spectroscopy (Fig. 4). 1H-15N- HSQC spectra of free VP16 TADn and TAD (Fig. 4a, blue signals and Supplementary Fig. 6a) display limited chemical shift dispersion, which is characteristic of unfolded polypeptide chains. In contrast to the free VP16 TADn and TAD, the 1H-15N-HSQC spectra of the VP16 TADn (Fig. 4a, red signals and Supplementary Fig. 7, red signals) and of the full-length TAD (Supplementary Fig. 7, black signals) in complex with the MED25 VBD displayed novel far-shifted NMR signals. The broad chemical shift dispersion of those signals indicated that the VP16 TAD adopts a partially folded conformation in the MED25 VBD-bound state. Moreover, a comparison of the spectra of the two bound peptides revealed a remarkable similarity in the far-shifted resonances, which suggested that the partially folded region was located in the TADn. (Fig. 4a, circled signals, and Supplementary Fig. 7 circled signals). The N-terminal VP16 TAD region was therefore identified as the primary MED25 VBD-binding portion. Consistent with this notion, a previous study showed that MED25-dependent transcriptional activation by the VP16 TAD required an intact TAD N-terminus12. Based on the above-mentioned results we employed the N-terminal VP16 TAD construct (TADn) for all further NMR experiments and the full-length VP16 TAD (TAD) for pull-down- and transcription activation-assays.
Figure 4.
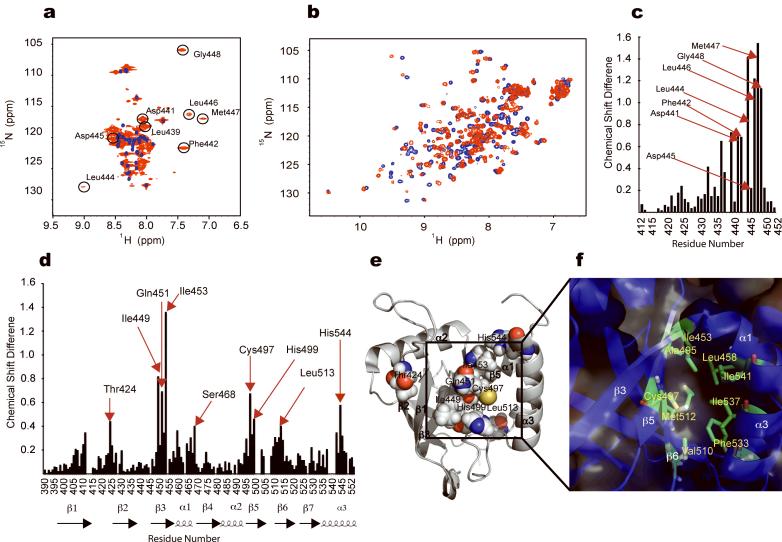
Interaction of VP16 TADn with MED25 VBD. (a) Overlay of 1H-15N-HSQC spectra of VP16 TADn alone (blue) and in the presence of 1.3 equivalents of unlabeled MED25 VBD (red). While the NMR signals of MED25 VBD in the bound state (b, red signals) still show reasonable line-width and intensity, the signals of the MED25-binding site of VP16 TADn show extensive chemical exchange line broadening and attenuation, in particular for far-shifted signals (circled peaks with assignment indicated). (b) Overlay of 1H-15N-HSQC spectra of MED25 VBD alone (blue) and in the presence of 1.3 equivalents of unlabeled VP16 TADn (red). (c) Changes in the chemical shifts of the amide proton and nitrogen atoms of VP16 TADn upon binding of MED25 VBD were plotted against the residue number. Amide resonances circled in (a) are highlighted by arrows. (d) Changes in the chemical shifts of the amide proton and nitrogen atoms of MED25 VBD upon binding of VP16 TADn were plotted against the residue number. Residues wherein amide signals experienced changes of the chemical shifts greater than 0.4 ppm are highlighted by arrows. (e) A cartoon representation of MED25 VBD shows the clustering of the residues experiencing chemical shifts changes greater than 0.4 ppm (drawn with space filling spheres) upon interaction with VP16 TADn in or near the hydrophobic furrow. The changes are clustered on β3, β5, β6 and α3. (f) Residues Ile453, Leu458, ALa495, Cys497, Val510, Met512, Phe533, Ile537, and Ile541 of MED25 VBD form a central hydrophobic pocket with in the large hydrophobic furrow. Several residues in or near this pocket showed the most pronounced chemical shift changes upon binding of VP16 TADn (Fig. 4e).
We obtained sequential backbone assignment of free VP16 TADn by means of standard triple resonance methods, while line broadening prevented us from obtaining continuous backbone resonances assignment of residues Ala434 – Gly448 of bound VP16 TADn. In addition to triple resonance backbone experiments we used Phenylalanine and Leucine amino acid-selective labeling and 15N-dispersed NOESY spectra to obtain a nearly complete assignment of bound TADn amide resonances. Only residue Ala438 and Asp443 of bound TADn could not be assigned. Residues D439, D441, F442, L444, D445, L446, M447, and G448 of bound VP16 TADn experience major chemical shift changes upon binding to MED25 VBD (circled peaks in Fig. 4a indicated with assignment and highlighted bars in Fig. 4c) and may constitute a core binding segment of alternating hydrophobic and negatively charged residues (Supplementary Fig. 1b shows a conserved region in the TADn between Asp437 and Gly448). In addition, Phe442 of the VP16 TADn was shown to be of critical importance for the interaction with the MED25 VBD, as a point mutation of Phe442 to Proline prevented binding of the TADn to the VBD11.
1H-15N-HSQC spectra of MED25 VBD showed substantial chemical shift changes upon addition of the VP16 TADn (Fig. 4b). The complex was in fast- to intermediate-exchange on the NMR time scale with regard to the dissociation kinetics, and shuttles among multiple bound conformations as discussed below. In order to locate the VP16 TADn-binding site on the MED25 VBD, we obtained greater than 90% of the backbone assignment of MED25 VBD bound to VP16 TADn and measured the magnitude of chemical shift changes of the 1H-15N HSQC cross peaks upon addition of the VP16 TADn peptide. The values are plotted versus the protein sequence in Fig. 4d. The most prominent chemical shift changes localized to a few discrete regions of the VBD that included residues T424, I449, Q451, I453, S468, C497, C499, L513, and H544. In a structural context, the strongest effects were observed for amino acids in β3 (Fig. 4d,e), and, to a lesser extent, β1, β5, and helices α1 and α3. Together, these structural elements form a patch of positively charged and hydrophobic residues that are likely to engage in charge-charge and hydrophobicity-complementary interactions with amino acids of the VP16 TADn. A hydrophobic pocket formed by MED25 VBD residues Gln451 and Ile453 of β3, Ala495 and Cys497 of β5, Phe533, Ile537 and Ile541 of α3, and Leu458 of α1 lie at the core of the VP16 binding site (Fig. 4f). Indeed, the strongest overall chemical shift changes upon binding of VP16 TADn were displayed by MED25 VBD residues that are situated close to or within this hydrophobic pocket (Fig. 4e,f).
Both proteins displayed exchange broadening of NMR resonances in the complex: Residues Leu449, Gln451, and Ile453 of the MED25 VBD showed line broadening upon binding to the VP16 TADn. Residues Arg538 to Ile541 of bound the MED25 VBD could not be assigned. Residues Val431 to Gly448 of the bound VP16 TADn exhibited extensive line broadening upon binding to MED25 VBD and Ala438 and Asp443 could not be assigned. These observations indicate that there are likely multiple bound conformations. We analyzed a similar situation previously in detail where we saw experimentally both phenomena, simple on/off binding and interaction with multiple bound states28.
In addition, we found evidence that the C-terminal portion of the VP16 TAD (TADc) interacts with a portion of MED25 VBD that is opposite on the barrel to the binding site of TADn. By comparing 1H-15N HSQC spectra of the MED25 VBD either bound to the TADn (Supplementary Fig. 8a, red signals) or bound to the full-length TAD (Supplementary Fig. 8a, black signals) one can monitor differences in the chemical shift of several amide resonances. Even without assignment of the MED25 VBD when bound to the TAD we could identify five distinct changes in amide chemical shifts and infer their assignment from the MED25 VBD bound to the TADn. We conclude that the observed changes are caused by the interaction of the C-terminal domain of VP16 TAD with MED25 VBD and mapped them to residues Gln456, Val471, Ile521, Gly524, and Leu525 of the VBD. Since Ile521, Gly524, Leu525 are located on β7 and Val471 is located on β4, the TADc binding site is distinct from the TADn binding site (Supplementary Fig. 8b). Gln456 is located on α1 and is possibly in contact with a hinge region of TAD. With the TADn interacting with β3 and β5 and the TADc binding to β4 and β7, the MED25 VBD is clamped by the VP16 TAD.
Point mutants impinge on MED25 VBD-VP16 TAD interaction
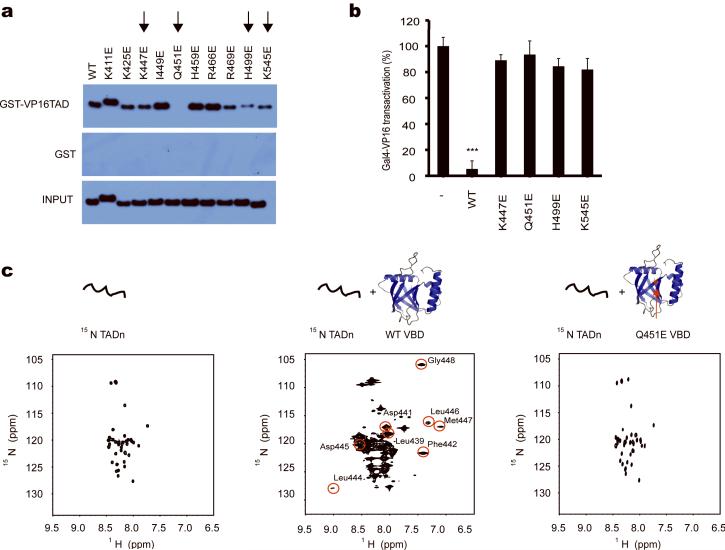
Guided by the experimentally observed chemical shift changes on the VBD, we designed ten MED25 VBD point mutants with amino acid substitutions in the VP16 TAD binding site and in areas nearby. Because both positively charged and hydrophobic interactions are thought to contribute to the MED25 VBD-VP16 TAD binding site, we aimed to disrupt the interaction by uniformly replacing all residues with glutamic acid. We screened all mutants in pull-down assays in which lysates from either wild-type or mutant MED25 VBD-expressing HEK293T cells were added to immobilized full-length VP16 TAD. Fractions of bound MED25 VBD protein were then probed by immunoblotting. As shown in Figure 5a, only the MED25 VBD Q451E mutant failed to bind to VP16 TAD in vitro, while several other mutants (e.g., K447E, H499E and K545E) exhibited considerably lower binding as compared to wild-type MED25 VBD. Subsequent NMR analyses confirmed that the Q451E mutant MED25 VBD indeed failed to interact with the VP16 TADn in solution, despite the fact that it remained properly folded (data not shown).
Figure 5.
Functional studies of mutant MED25 VBD. (a) VP16 full-length TAD-pull-down assays with MED25 VBD mutants. K447E, Q451E, H499E and K545E-mutated MED25 VBD exhibit weak or no binding to VP16 TAD (marked with an arrow). (b) While wild-type MED25 VBD acts in a dominant negative fashion to inhibit Gal4p-VP16 full-lenth TAD-dependent transcription, the K447E, Q451E, H499E and K545E-mutated MED25 VBD are incapable of inhibiting VP16-mediated transcription (***: p<0.01, t-test, one tailed, error bars represent s.d.). (c) The MED25 VBD Q451E mutation on β3 adjacent to the hydrophobic pocket disrupts binding of VP16 TADn to MED25 VBD. 1H-15N-HSQC spectra of free VP16 TADn (left panel), at 1:1.3 excess of wild-type MED25 VBD (middle panel) and with 1:1.5 excess of Q451E MED25 VBD (right panel) show that VP16 TADn only loosely binds, as seen by minor chemical shift changes, to mutant MED25 VBD without adopting a folded conformation. All far-shifted signals caused by the addition of wild-type MED25 VBD (middle panel, circled in red with assignment indicated) are missing when the mutant MED25 VBD is added to 15N-labeled VP16 TADn (right panel).
It has been shown previously that overexpressed, soluble MED25 VBD can prevent VP16 TAD-mediated transcriptional activation in a Gal4p/UAS transcription assay in human cells12. We therefore tested the four most attenuated binding mutants, K447E, Q451E, H499E, and K545E, for their ability to inhibit full-length VP16 TAD-mediated transcription in HEK293T cells. Indeed, as expected from the binding studies, all four MED25 VBD mutants were unable to interfere with Gal4p-VP16 TAD-activated transcription in a dominant negative manner, as compared to wild-type MED25 VBD (Fig. 5b). Mutant and wild-type MED25 VBD showed comparable level of expression when transfected into HEK293T cells (Supplementary Fig. 9).
NMR titrations of the unlabeled Q451E mutant MED25 VBD and 15N-labeled VP16 TADn displayed features of a remnant, very much reduced interaction. Although minor chemical shift changes were observed, all of the broadly dispersed resonances, characteristic of the partial folding transition of VP16, were absent (Fig. 5c, right panel in comparison to Fig. 5c, middle panel). We conclude that Q451E, sitting at the center of the hypothetical VP16 TADn binding site, greatly compromised the ability of MED25 VBD to interact with the viral peptide in vitro and in vivo.
Discussion
The ARC/Mediator co-activator complex is recruited by a number of viral and cellular transcription factors10. Previous studies by others and us showed that the Herpes simplex viral transactivator VP16 targets ARC/Mediator by interaction with the MED25 subunit, and deletion mutagenesis demonstrated that the MED25 VP16-binding domain is able to inhibit VP16-mediated transcriptional activation in vivo11,12. Our studies described here reveal the structure of the MED25 VBD and provide critical molecular details of the VP16 TAD – MED25 VBD interface. Our results also define an extended hydrophobic furrow on the MED25 VBD that is lined with positively charged residues, and present evidence that amino acids located in this furrow play a key role in VP16 TAD binding and VP16 TAD-dependent transcriptional activation. Indeed, our results show that a single MED25 VBD mutation (Q451E) located in a central hydrophobic pocket within the furrow prevents binding to the VP16 TAD in vitro and abolishes the dominant negative inhibition of MED25 VBD on VP16 TAD-dependent transcriptional activation in cellular contexts.
While several other entries in the protein data bank (i.e. the SPOC domain of SPEN and the two β-barrel structures of the KU70-KU80 heterodimer) exhibit superficial structural homology to the MED25 VBD (Fig. 3), they present different folding topologies and lack the long C-terminal helix 〈3. Since 〈3 together with β3 and β5 forms part of the hydrophobic VP16-binding pocket, these differences suggest that the MED25 VBD represents a structurally and functionally distinct domain and that these other proteins are unlikely targets of VP16 or other viral transactivators of the Herpes family.
Multiple sequence alignments indicate that residues in the hydrophobic pocket are well conserved among the N- and C-terminal domains of PTOV1 and MED25 VBD (Supplementary Fig. 1a). Although we have previously shown that the VP16 TAD does not associate with PTOV1 when expressed in cells12, our structural studies of MED25 VBD and its extensive conservation with the two PTOV1 domains suggest the possibility that PTOV1 could indeed represent a target for both viral and cellular transcription factors. Because PTOV1 was first identified as a putative prostate cancer oncogene13, it is tempting to speculate that PTOV1 (and the MED25 VBD-related domains) may play a role as a target for cellular activators in gene regulatory pathways contributing to prostate cancer proliferation.
The VP16 TADn peptide adopts a folded conformation upon binding to MED25 VBD, while retaining a certain degree of flexibility. We conclude from titration experiments that VP16 TADn samples two or more conformations in the bound state, consistent with an “induced fit” – type of interaction. This intrinsically unfolded structure of the un-liganded state of the VP16 TADn may allow a multitude of interactions with transcriptional targets. Indeed, while a single bound state may provide the tightest association, strong interaction with one partner might prevent binding to additional co-activator partners (e.g., CBP/p300), impeding efficient transactivation. Accordingly, as aforementioned, the VP16 TAD interacts with a number of targets in the basal transcription machinery, including TFIIB18,19, TFIIA17, the TFIID subunit TAFII3116, and the Tfb1 subunit of TFIIH15.
Interestingly, other viral activators of the Herpes family also employ MED25 and its VP16-binding domain as their transcription regulatory conduit. Indeed, both the Varicella-Zoster Virus IE62 protein and the Kaposi's sarcoma-associated herpesvirus LANA-1 protein target MED2529,30, and IE62 was shown to directly interact with the same region of MED25 as VP1631. Taken together with our findings presented here, as well as those of previous studies by others and us11,12, these results indicate that MED25 and the VP16-binding domain could serve a critical role in transactivation of viral genes in the Herpes family of viruses, and the molecular interface of viral activators and MED25 might thus provide a novel target for the development of anti-viral therapeutics.
Viruses commonly mimic, or hijack, cellular mechanisms for their maturation and propagation. It is therefore likely that cellular transactivators also target MED25 and the VP16-binding domain that we have structurally defined here for transcriptional regulation. In this regard, it is interesting to note that MED25 has been implicated in Charcot-Marie-Tooth neuropathy32, where it has been suggested to play an as yet poorly understood pathophysiological role in gene activation.
In conclusion, our study has revealed intricate structural and mechanistic details of the gene activation mechanism by the benchmark activator VP16 that might guide future investigations of transactivation mechanisms by both viral and cellular activators. These studies could also provide insights and tools to design novel therapeutic strategies that target the molecular underpinning of viral transactivation that is essential to propagation of Herpes family viruses.
Methods
Luciferase assay
For luciferase assays, approximately 5×105 HEK293T cells were plated into each well of 24-well plates and co-transfected with/or without 0.5 μg of MED25 VBD expression vector (wild-type or one of the 10 mutants), 100 ng of G4BE luciferase reporter, 5 ng of Renilla luciferase plasmid, and 2 ng of HA-Gal4-VP16 expression vector. Transfected cells were lysed after 24 hours and analyzed using the Dual Luciferase System (Promega).
Mutagenesis
In order to insert point mutations in our respective vectors, we employed the QuikChange Site-directed Mutagenesis kit from Stratagene, following the manufacturer's directions. Fully complementing primers (45-mer) containing nucleotide mutations within the middle of each primer were designed with an average of 18-mer flanking sequences. Primer sequences are provided upon request.
Protein Expression and Purification
The MED25 VBD (residues 390–553) was cloned into a pHis-Gb1-Parallel1 vector, a derivative of the pHis-Parallel136 using the EcoRI and KpnI cleavage sites. The construct was expressed in BL21(DE3) E. coli cells. The cells were grown to an OD600 of 0.8 and induced with 1 mM IPTG for 3–5 h at 37°C. Unlabeled proteins were grown in LB medium, labeled proteins in M9 containing 1 g 15N-NH4Cl, 2 g 13C glucose or 2 g 2H-13C-glucose and D2O for deuterated 13C-15N-labeled proteins. Cells were sonicated in 20 mM Tris-HCl pH 8.0, 300 mM NaCl, 10 mM Imidazole, 0.1 % (v/v) Triton X100, 1 mg ml−1 Lysozyme, 2 mM β-mercaptoethanol, 1 tablet of Roche Protease Inhibitor complete. The soluble fraction was obtained by centrifugation at 8,000 × g for 1h. The supernatant was applied to a Ni-NTA resin and washed with 10 volumes of 20 mM Tris-HCl pH 8.0, 300 mM NaCl, 10 mM Imidazole, 2 mM β-mercaptoethanol, and eluted with 5 volumes 20 mM Tris-HCl pH 8.0, 300 mM NaCl, 300 mM Imidazole, 2 mM β-mercaptoethanol. The eluted protein was cleaved with TEV-protease overnight at 4°C. The reaction mixture was diluted to roughly 50 mM NaCl and further purified by ion-exchange chromatography on a Resource S column (GE Healthcare) in 20 mM Na-Phosphate buffer pH 6.5, 3 mM DTT with a linear NaCl gradient from 50 to 500 mM NaCl. VP16 TADn (411–452) and VP16 TAD (411–490) were cloned into a pETM30 vector using the NpnI and KpnI restriction sites. BL21(DE3) E. coli cells were transformed with the above mentioned vector, grown to an OD600 of 0.8 and induced with 1 mM IPTG for 2–3 h at 37°C. Unlabeled proteins were grown in LB medium, labeled protein in M9 containing 1 g 15N-NH4Cl, 2 g 13C-glucose or 2 g 2H-13C-glucose and D2O for deuterated 13C-15N-labeled proteins. The MED25 VBD purification method with the subsequent TEV-cleavage step was also used for VP16 TADn and TAD. After TEV-cleavage, the protein solution was diluted to 1 mg ml−1 and subjected to a freeze-thaw cycle to precipitate GST. Precipitated GST was removed by centrifugation at 8,000 – g for 1 h. The remaining VP16 TADn/TAD peptide was concentrated and further purified by gel filtration on Superdex 75 (GE Healthcare) in 20 mM Tris-HCl pH 8.0, 150 mM NaCl, 3 mM DTT, 0.25 mM EDTA. Protein concentrations were determined at 280 nm. A S411W mutant of VP16 TADn was used to facilitate determination of protein concentrations at 280 nm. 15N Phenylalanine and 1-13C Leucine 15N (U) amino acid-selective labeled samples of TADn were prepared according to Takeuchi et al. and references therein37.
NMR Spectroscopy
All NMR samples were prepared in 20 mM sodium phosphate buffer pH 6.5, 150 mM NaCl, 3 mM DTT. When VP16 TADn was present, 0.25 mM EDTA was added to the solution to prevent proteolytic degradation. Spectra were recorded on Varian Inova 500 and 600 and on Bruker 600, 750 and 900 MHz spectrometers at 25°C. Resonance assignment was achieved by the use of standard multidimensional heteronuclear NMR-experiments38. NOEs were derived from 2D-1H-1H-NOESY, 3D 15N-dispersed NOESYHSQC, 3D 13C-dispersed NOESYHSQC and 4D HMQC-NOESY-HMQC experiments39. The mixing times were 60 ms for the 2D and 3D NOESYs and 150 ms for the 4D NOESY. The 4D-NOESY was recorded on a 1H-13C methyl-Ile,Leu,Val-labeled sample with a deuterated protein background40. Spectra were processed with NMRpipe41. The sparsely sampled 4D-NOESY was processed with MDD39 and subsequently with NMRpipe according to Hiller et al.39. Amino acid-selective labeled samples were recorded with HSQC spectra for 15N labeled Phenylalanine samples or with 1H-15N planes of HNCO spectrum38 for 1-13C Leucine 15N (U) labeled samples. NMR Spectra were plotted with the programs Sparky42 and CARA43.
Structure calculation
NOEs were picked and assigned manually in CARA43 or automatically derived from CYANA/CANDID44. Dihedral constraints were derived from Ca and Cb chemical shifts using the program TALOS+45. NOE intensities were integrated and converted into distance constraints using CYANA44. Hydrogen bond restraints for beta-sheets and helices were assigned when supported by NOEs and secondary structure prediction. 50 initial structures were created in a Simulated Annealing protocol using CYANA/CANDID44. The 25 structures with lowest energy were subsequently refined with CNS46 using explicit solvent in a MD simulation. Structures were visualized with PyMOL47. Sequence alignments were visualized with ESPRIPT34. PSVS48, CNS46 and PROCHECK-NMR23 were used for analysis of the structural ensemble and quality assessment of the structural data.
ITC
Protein samples were prepared in 20 mM sodium phosphate pH 6.5, 150 mM NaCl, 0.5 mM TCEP, 0.25 mM EDTA. A VP-ITC calorimeter (Microcal) was run at an equilibrium temperature of 25°C. The concentration of the protein in the well was roughly 10 times the estimated Kd and the concentration of the protein in the syringe was seven times the one in the well. Data were processed using Origin software.
GST-pulldown assays
GST and GST-VP16 TAD fusion protein were expressed in BL21(DE3) E. coli cells and purified by glutathione-sepharose (GE Healthcare) after multiple washes with 1 M and 0.5 M NaCl. For whole cell extracts (WCE), HEK293T cells were transfected with plasmid pcDNA4/TO (Invitrogen) harboring either wild-type or mutant Flag-MED25 VBD as per Mirus instructions. 18 hrs after transfection, cells were harvested with extraction buffer (20 mM Tris-HCl pH 8.0, 420 mM NaCl, 10 % (v/v) Glycerol, 0.5 % (v/v) NP-40, 0.1 mM NaCl, 1 mM PMSF, 1 mM DTT) for whole cell extract preparation. For pull-down experiments, WCEs were diluted to 100 mM NaCl HEG buffer (20 mM HEPES pH 7.9, 0.1 mM EDTA, 10 % (v/v) Glycerol) with 0.01 % (v/v) NP-40 to a final protein concentration of 2 μg per μl. 1 mg of WCEs were pre-cleared with glutathione-sepharose beads for 1 hr at 4°C and then were applied to 50 μl of 50 % slurry of either GST or GST-VP16 TAD beads and incubated at 4°C for 3 h. Beads were washed six times in 1 ml with 0.4 M KCl HEG buffer with 1 % (v/v) NP-40. Bound proteins were eluted with 0.3 % (w/v) sarkosyl.
Supplementary Material
ACKNOWLEDGMENTS
We thank M. Oberer for initial NMR-data collection, R. Rodriguez for critical advice on designing biochemical experiments, K. Edmonds for critical reading and python script writing, S. Hiller and V. Orekhov for advice and assistance with non-linear-sampling NMR data collection and processing, and H. Arthanari for advice on NMR data collection and manuscript editing. We are very grateful to M. Sattler and E. Vojnic of Technical University of Munich, Garching, Germany for critical discussion of unpublished data. We are also thankful for H.C-Seou's assistance with DNA cloning procedures and A. Koglin for help with CNS-calculations. A.G.M. was partially supported by a DAAD postdoctoral fellowship. P.S. was funded by the Human Frontier Science Program Organization long-term fellowship LT00686/2004-C. The work was supported by US National Institutes of Health grants CA127990 (G.W. and A.M.N.), GM47467 and EB002026 (G.W.), and GM071449 (A.M.N.).
Footnotes
Accession codes. The coordinates for the structure of MED25 VBD have been deposited in the Protein Data Bank with the accession code 2KY6. The chemical shifts were deposited under BMRB entry 17139.
References
- 1.Roizman B, Knipe D. Fundamental Virology Fourth Edition. 2001;33:1123–1183. [Google Scholar]
- 2.Triezenberg SJ, Kingsbury RC, McKnight SL. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988;2:718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]
- 3.Greaves R, O'Hare P. Separation of requirements for protein-DNA complex assembly from those for functional activity in the herpes simplex virus regulatory protein Vmw65. J Virol. 1989;63:1641–1650. doi: 10.1128/jvi.63.4.1641-1650.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghosh S, Toth C, Peterlin BM, Seto E. Synergistic activation of transcription by the mutant and wild-type minimal transcriptional activation domain of VP16. J Biol Chem. 1996;271:9911–9918. doi: 10.1074/jbc.271.17.9911. [DOI] [PubMed] [Google Scholar]
- 5.Naar AM, Lemon BD, Tjian R. Transcriptional coactivator complexes. Annu Rev Biochem. 2001;70:475–501. doi: 10.1146/annurev.biochem.70.1.475. doi:70/1/475 [pii] 10.1146/annurev.biochem.70.1.475. [DOI] [PubMed] [Google Scholar]
- 6.Boyer TG, Martin ME, Lees E, Ricciardi RP, Berk AJ. Mammalian Srb/Mediator complex is targeted by adenovirus E1A protein. Nature. 1999;399:276–279. doi: 10.1038/20466. doi:10.1038/20466. [DOI] [PubMed] [Google Scholar]
- 7.Naar AM, Taatjes DJ, Zhai W, Nogales E, Tjian R. Human CRSP interacts with RNA polymerase II CTD and adopts a specific CTD-bound conformation. Genes Dev. 2002;16:1339–1344. doi: 10.1101/gad.987602. doi:10.1101/gad.987602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornberg RD. Mediator and the mechanism of transcriptional activation. Trends Biochem Sci. 2005;30:235–239. doi: 10.1016/j.tibs.2005.03.011. doi:S0968-0004(05)00083-6 [pii] 10.1016/j.tibs.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Conaway RC, Sato S, Tomomori-Sato C, Yao T, Conaway JW. The mammalian Mediator complex and its role in transcriptional regulation. Trends Biochem Sci. 2005;30:250–255. doi: 10.1016/j.tibs.2005.03.002. doi:S0968-0004(05)00060-5 [pii] 10.1016/j.tibs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Malik S, Roeder RG. Dynamic regulation of pol II transcription by the mammalian Mediator complex. Trends Biochem Sci. 2005;30:256–263. doi: 10.1016/j.tibs.2005.03.009. doi:S0968-0004(05)00081-2 [pii] 10.1016/j.tibs.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Mittler G, et al. A novel docking site on Mediator is critical for activation by VP16 in mammalian cells. EMBO J. 2003;22:6494–6504. doi: 10.1093/emboj/cdg619. doi:10.1093/emboj/cdg619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang F, DeBeaumont R, Zhou S, Naar AM. The activator-recruited cofactor/Mediator coactivator subunit ARC92 is a functionally important target of the VP16 transcriptional activator. Proc Natl Acad Sci U S A. 2004;101:2339–2344. doi: 10.1073/pnas.0308676100. doi:101/8/2339 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedit P, et al. PTOV1, a novel protein overexpressed in prostate cancer containing a new class of protein homology blocks. Oncogene. 2001;20:1455–1464. doi: 10.1038/sj.onc.1204233. doi:10.1038/sj.onc.1204233. [DOI] [PubMed] [Google Scholar]
- 14.Ikeda K, Stuehler T, Meisterernst M. The H1 and H2 regions of the activation domain of herpes simplex virion protein 16 stimulate transcription through distinct molecular mechanisms. Genes Cells. 2002;7:49–58. doi: 10.1046/j.1356-9597.2001.00492.x. doi:492 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Langlois C, et al. NMR structure of the complex between the Tfb1 subunit of TFIIH and the activation domain of VP16: structural similarities between VP16 and p53. J Am Chem Soc. 2008;130:10596–10604. doi: 10.1021/ja800975h. doi:10.1021/ja800975h. [DOI] [PubMed] [Google Scholar]
- 16.Uesugi M, Nyanguile O, Lu H, Levine AJ, Verdine GL. Induced alpha helix in the VP16 activation domain upon binding to a human TAF. Science. 1997;277:1310–1313. doi: 10.1126/science.277.5330.1310. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi N, et al. DA-complex assembly activity required for VP16C transcriptional activation. Mol Cell Biol. 1998;18:4023–4031. doi: 10.1128/mcb.18.7.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayashi F, et al. Human general transcription factor TFIIB: conformational variability and interaction with VP16 activation domain. Biochemistry. 1998;37:7941–7951. doi: 10.1021/bi9801098. doi:10.1021/bi9801098 bi9801098 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Hall DB, Struhl K. The VP16 activation domain interacts with multiple transcriptional components as determined by protein-protein cross-linking in vivo. J Biol Chem. 2002;277:46043–46050. doi: 10.1074/jbc.M208911200. doi:10.1074/jbc.M208911200 M208911200 [pii] [DOI] [PubMed] [Google Scholar]
- 20.Kretzschmar M, Kaiser K, Lottspeich F, Meisterernst M. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell. 1994;78:525–534. doi: 10.1016/0092-8674(94)90429-4. doi:0092-8674(94)90429-4 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Ge H, Roeder RG. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994;78:513–523. doi: 10.1016/0092-8674(94)90428-6. doi:0092-8674(94)90428-6 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Jonker HR, Wechselberger RW, Boelens R, Folkers GE, Kaptein R. Structural properties of the promiscuous VP16 activation domain. Biochemistry. 2005;44:827–839. doi: 10.1021/bi0482912. doi:10.1021/bi0482912. [DOI] [PubMed] [Google Scholar]
- 23.Laskowski RA, Rullmannn JA, MacArthur MW, Kaptein R, Thornton JM. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 1996;8:477–486. doi: 10.1007/BF00228148. [DOI] [PubMed] [Google Scholar]
- 24.Holm L, Sander C. Protein structure comparison by alignment of distance matrices. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. doi:S0022-2836(83)71489-0 [pii] 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 25.Ariyoshi M, Schwabe JW. A conserved structural motif reveals the essential transcriptional repression function of Spen proteins and their role in developmental signaling. Genes Dev. 2003;17:1909–1920. doi: 10.1101/gad.266203. doi:10.1101/gad.266203 17/15/1909 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. doi:10.1038/35088000 35088000 [pii] [DOI] [PubMed] [Google Scholar]
- 27.Naar AM, et al. Composite co-activator ARC mediates chromatin-directed transcriptional activation. Nature. 1999;398:828–832. doi: 10.1038/19789. doi:10.1038/19789. [DOI] [PubMed] [Google Scholar]
- 28.Reibarkh M, Malia TJ, Wagner G. NMR distinction of single- and multiple-mode binding of small-molecule protein ligands. J Am Chem Soc. 2006;128:2160–2161. doi: 10.1021/ja055971z. doi:10.1021/ja055971z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang M, Hay J, Ruyechan WT. Varicella-zoster virus IE62 protein utilizes the human mediator complex in promoter activation. J Virol. 2008;82:12154–12163. doi: 10.1128/JVI.01693-08. doi:JVI.01693-08 [pii] 10.1128/JVI.01693-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roupelieva M, et al. Kaposi's sarcoma-associated herpesvirus Lana-1 is a major activator of the serum response element and mitogen-activated protein kinase pathways via interactions with the Mediator complex. J Gen Virol. 2010;91:1138–1149. doi: 10.1099/vir.0.017715-0. doi:vir.0.017715-0 [pii] 10.1099/vir.0.017715-0. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto S, Eletsky A, Szyperski T, Hay J, Ruyechan WT. Analysis of the varicella-zoster virus IE62 N-terminal acidic transactivating domain and its interaction with the human mediator complex. J Virol. 2009;83:6300–6305. doi: 10.1128/JVI.00054-09. doi:JVI.00054-09 [pii] 10.1128/JVI.00054-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leal A, et al. Identification of the variant Ala335Val of MED25 as responsible for CMT2B2: molecular data, functional studies of the SH3 recognition motif and correlation between wild-type MED25 and PMP22 RNA levels in CMT1A animal models. Neurogenetics. 2009;10:275–287. doi: 10.1007/s10048-009-0183-3. doi:10.1007/s10048-009-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wysocka J, Herr W. The herpes simplex virus VP16-induced complex: the makings of a regulatory switch. Trends Biochem Sci. 2003;28:294–304. doi: 10.1016/S0968-0004(03)00088-4. doi:S0968000403000884 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Gouet P, Courcelle E, Stuart DI, Metoz F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics. 1999;15:305–308. doi: 10.1093/bioinformatics/15.4.305. doi:btc028 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Holm L. Unification of protein families. Curr Opin Struct Biol. 1998;8:372–379. doi: 10.1016/s0959-440x(98)80072-9. doi:S0959-440X(98)80072-9 [pii] [DOI] [PubMed] [Google Scholar]
- 36.Harper SM, Neil LC, Gardner KH. Structural basis of a phototropin light switch. Science. 2003;301:1541–1544. doi: 10.1126/science.1086810. doi:10.1126/science.1086810 301/5639/1541 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi K, Ng E, Malia TJ, Wagner G. 1–13C amino acid selective labeling in a 2H15N background for NMR studies of large proteins. J Biomol NMR. 2007;38:89–98. doi: 10.1007/s10858-007-9152-z. doi:10.1007/s10858-007-9152-z. [DOI] [PubMed] [Google Scholar]
- 38.Ferentz AE, Wagner G. NMR spectroscopy: a multifaceted approach to macromolecular structure. Q Rev Biophys. 2000;33:29–65. doi: 10.1017/s0033583500003589. [DOI] [PubMed] [Google Scholar]
- 39.Hiller S, Ibraghimov I, Wagner G, Orekhov VY. Coupled decomposition of four-dimensional NOESY spectra. J Am Chem Soc. 2009;131:12970–12978. doi: 10.1021/ja902012x. doi:10.1021/ja902012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gardner KH, Kay LE. Production and incorporation of 15N, 13C, 2H (1H-1 methyl) isoleucine into proteins for multidimensional NMR studies. J. Am. Chem. Soc. 1997;119:7599–7600. [Google Scholar]
- 41.Delaglio F, et al. NMRPipe a Multidimensional Spectra Processing System Based on UNIX Pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 42.Sparky 3. San Fransisco: [Google Scholar]
- 43.Keller RLJ. The Computer Aided Resonance Assignment Tutorial. Cantina Verlag: 2004. [Google Scholar]
- 44.Herrmann T, Güntert P, Wüthrich K. Protein NMR structure determination with automated NOE-identification in the NOESY spectra using the new software ATNOS. J Biomol NMR. 2002;24:171–189. doi: 10.1023/a:1021614115432. doi:5111821 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Shen Y, Delaglio F, Cornilescu G, Bax A. TALOS+: a hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J Biomol NMR. 2009;44:213–223. doi: 10.1007/s10858-009-9333-z. doi:10.1007/s10858-009-9333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunger AT, et al. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 47.The PyMOL Molecular Graphics System. v.1.2r3pre Schrödinger, LLC; [Google Scholar]
- 48.Bhattacharya A, Tejero R, Montelione GT. Evaluating protein structures determined by structural genomics consortia. Proteins. 2007;66:778–795. doi: 10.1002/prot.21165. doi:10.1002/prot.21165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.