Abstract
Objective
Variation in expression of adhesion molecules plays a key role in regulating leukocyte behavior, but the contribution of fluid shear to these interactions cannot be ignored. Here we dissected the effects of each of these factors on leukocyte behavior in different venular regions.
Methods
Leukocyte behavior was quantified in blood perfused microvascular networks in anesthetized mouse cremaster muscle using intravital confocal microscopy. ICAM-1 expression and fluid shear rate were quantified using ICAM-1 fluorescent labeling, fluorescent particle tracking, and computational fluid dynamics.
Results
TNFα-induced an increase in ICAM-1 expression, and abolished the differences observed among control venules of different sizes. Consequently, leukocyte adhesion was increased to a similar level across all vessel sizes (5.1±0.46 leukocytes/100μm vs. 2.1±0.47 [control]), but remained significantly higher in venular convergences (7.8±0.4). Leukocyte transmigration occurred primarily in the smallest venules and venular convergences (23.9±5.1 and 31.9±2.7 leukocytes/10,000μm2 tissue, respectively). In venular convergences the two inlet vessels are predicted to create a region of low velocity, increasing leukocyte adhesion probability.
Conclusions
In straight regions of different sized venules the variability in ICAM-1 expression accounts for the differences in leukocyte behavior; in converging regions, fluid shear potentially has a greater effect on leukocyte-EC interactions.
Keywords: leukocyte rolling, leukocyte adhesion, ICAM-1, shear rate, vessel size, convergences, in-vivo, mouse
INTRODUCTION
Inflammation is a complex biological response of vascular tissue that provides protection against harmful stimuli, such as invasion of foreign bodies (e.g. splinters, dust, viruses and bacteria), as well as to initiate healing processes following physical tissue injury [5,11]. It is mainly characterized by the recruitment of leukocytes from the vasculature into regions of inflammation. The way that leukocytes interact with the endothelium varies greatly when one compares vessels of different origin or even further, when one focuses on individual vessels of the same type. For example, in individual venules, leukocyte rolling and adhesion occurs in some regions of the vascular wall, while other regions remain free of interacting leukocytes [8]. Furthermore, venular convergences support a higher number of interacting leukocytes than elsewhere [12], and the adhered leukocytes tend to be found preferentially near the EC junctions [29]. Finally, leukocyte transmigration occurs only at specific locations along the venular endothelial surface; prior to transmigration adhered leukocytes crawl on the vessel lumen until they encounter the location of specific “portals” which accommodate leukocyte migration across the vessel wall [20,29]. These data argue that some regions within the microvasculature exhibit a greater predisposition for supporting leukocyte interactions. They also raise the question as to the nature of the mechanisms responsible for these differences, and to what extent the regulating factors are a function of the location within the venular network.
One such factor is the surface expression of adhesion molecules. The profound effects of expression level and spatial distribution of adhesion molecules have been recognized, and enhanced leukocyte rolling, leukocyte adhesion and transmigration in-situ have all been observed in areas of higher expression levels of P-selectin [8], ICAM-1 [25,26] or PECAM-1 [16] respectively. The heterogeneous expression of these molecules also determines which cells will be recruited to which tissues during inflammation [3]. Likewise the expression of different adhesion molecules under similar shear conditions can have a different effect on leukocyte behavior [23], suggesting a key role for endothelial surface receptors in the regulation of leukocyte-EC interactions.
It has also been suggested that fluid shear forces affect leukocyte-EC interactions via direct or indirect mechanisms, although these effects are diverse, and vary with the experimental setup that was used. For example, the direct effect of fluid shear has been demonstrated by significantly decreased leukocyte delivery, hence the number of rolling and consequently the number of adhered cells under high hydrodynamic flow [13,27]. Likewise, fluid shear forces can have an anti-inflammatory effect, by attenuating the effects of pro-inflammatory cytokines such as TNFα on the endothelium [17]. On the other hand multiple studies have suggested that increased shear rate results in significant upregulation of adhesion molecules, such as ICAM-1 on EC surface [18,24,28], which in turn (indirectly) results in increased leukocyte adhesion and transmigration.
While the contributions of both adhesion molecules and fluid shear to the regulation of leukocyte-EC interactions have been extensively studied separately in different systems, only a few studies have addressed their mutual contributions and the interplay of biology and fluid mechanics on leukocyte behavior. For example, in different isolated cell systems it has been shown that neutrophil-facilitated melanoma extravasation [4] as well as leukocyte motility [23] required both microfluid mechanical events and endothelial surface receptors.
In the current work we show that it is not only one or the other factor, or even the interplay of molecular and/or hydrodynamic factors that account for differences in leukocyte-EC interactions; rather in different conditions as well as in different parts of the venular network, each of these factors has a predominant effect. We show that different leukocyte behavior is characteristic of not only specific vessel types (arterioles vs venules), but also vessel dimensions and different regions within the network. We provide evidence that in straight venular regions the variability in the expression of ICAM-1 could account for the differences in leukocyte behavior in different sized vessels. In venular convergences on the other hand, fluid shear potentially plays a more prominent role in regulating leukocyte-EC interactions.
MATERIALS and METHODS
Animal preparation
Experiments were performed on male wild type (WT) C57BL6J mice (Jackson Laboratories) between the age of 12–15 weeks old under control or inflammatory conditions. Inflammation was induced by local treatment with mouse recombinant TNFα (intrascrotal injection, 0.5 μg TNFα in 0.25ml saline, Sigma-Aldrich) 3 hours prior to the start of the surgical preparation [25]. Observations were made 4 – 5 hrs after the TNFα injection.
Anesthetized mice (sodium pentobarbital 65mg/kg i.p.) were prepared for intravital microscopy according to protocols approved by the Institutional Review Board of the University of Rochester as previously described [8]. Briefly, an endotracheal tube was inserted to insure a patent airway during the experiment, and the animal was kept warm by placing it on a warmer. Supplemental anesthetic was administered as needed throughout the experiment via a catheter inserted into the jugular vein. The right cremaster muscle was exteriorized, and gently pinned over a quartz pedestal for vizualisation by microscopy. During preparation and observation the tissue was continuously superfused with warmed physiological solution with the following composition: (in mM) NaCl, 131.9; KCL, 4.7; CaCl, 2.0; MgSO4, 1.2, NaHCO3, 18; pH 7.4 at 36°C, and equilibrated with gas containing 0% O2, 5% CO2 and 95% N2 to maintain tissue PO2 <15torr. Upon completion of the protocols, the animal was euthanised by anesthetic overdose.
Intravital microscopy
As described elsewhere [26] an Olympus BX61WI microscope with an Olympus PlanF1 immersion objective (20x, 0.65 NA) was used to acquire images. Brightfield images used to track leukocyte interactions with the vessel wall were acquired using a CCD camera (Dage MTI CD72). Fluorescence images were acquired by illuminating the tissue with a 50mW argon laser and imaging with a Nipkow disk confocal head (CSU 10, Yokogawa) and intensified CCD camera (XR Mega 10, Stanford Photonics): laser power and camera gain settings were unchanged throughout all the experiments. Images were recorded to a DVD recorder (SONY DVO100MD) for offline analysis. The spatial resolution in this imaging system is 0.5μm.
Shear rate estimation
In addition to the anesthetic catheter, a second catheter inserted into the same vein was used to inject fluorescently labeled beads (0.5 μm diameter, Fluorescebrite, Polysciences) in saline solution. After the completion of the preparation and the exteriorization of the cremaster muscle, the beads were injected in a bolus into the blood as needed. Newtonian wall shear rates were calculated as 8Vavg/D from the measured bead velocities and vessel diameter. Even though these flow-based estimates of shear rates can be significantly higher than those directly measured from the change of red blood velocity with the change in radial position [8] we utilized this approach for simplicity based on the demonstration that the relative relationships between shear rate and location within the microvasculature are not different with the two approaches [8].
Prediction of flow profile by simulation
A computational fluid dynamic (CFD) analysis was performed to predict how flow properties could be expected to vary in a model of an in vivo convergence. To create the CFD model, digitized images of the vessel were imported into ImageJ and salient features of the vessel were identified. The features were then used to generate a three-dimensional solid model of the vessel using SolidWorks™ (SolidWorks Corp., Concord, MA) modeling software. The solid model was then exported to GambitTM preprocessing software and FluentTM (ANSYS, Inc., Lebanon, NH) for CFD analysis. In Gambit, the boundary types specified for the vessel inlets and outlet were velocity inlets or outlets. In Fluent, the velocities specified as boundary conditions were in the range of the bulk flow velocities as previously measured for a similar geometry [8]. Both inlet velocities were equal. A Newtonian fluid with viscosity of 998.6 g·m−1 s−1 was modeled to mimic blood. The model convergence criterion was defined as 1e−06 and residuals were monitored to confirm that the model results were converged. A grid study was also performed to verify grid independence.
In situ immunofluorescence labeling of adhesion molecules
To immunofluorescently label ICAM-1 on the EC surface we utilized a previously developed approach to in vivo labeling and imaging of blood perfused microvessels [25,29]. Briefly, a main arteriole to the network of interest was cannulated using sharpened micropipettes upstream of the microvascular region targeted for observation. A separate glass occluding rod (modified micropipette) was used to occlude the main inflow arteriole to the tissue upstream of the cannulation site at the time of the antibody loading to facilitate complete perfusion of the downstream microvasculature with rat anti-mouse ICAM-1 (YN/1.7.4, eBioscience, 50 μg/ml) for 15 minutes, followed by goat anti-rat secondary fluorescent polyclonal antibody in saline (Alexa 488 anti-rat, Molecular Probes, 50 μg/ml) for 15 minutes. At the completion of the perfusion with the primary and secondary antibodies the cannulating and occluding micropipettes were removed and blood flow was re-established in the targeted microvascular region. Upon completion of all imaging protocols a third cannulation was used to perfuse the target vessels with fluorescent standard solution (0.05 mg/ml FITC-dextran in saline, 150 kDa M.W., Sigma-Aldrich). The measured intensity of ICAM-1 was then normalized to the intensity of FITC-dextran in the same region to account for differences in fluorescence produced by localized variability in the optical properties of the tissue. A representative venule filled with FITC-dextran is shown in Figure 4B, bottom panel. The specificity of the primary and secondary antibodies (using a relevant IgG-control followed by the secondary antibody, or alternatively the secondary antibody alone) as well as the linearity of fluorochrome in our system was confirmed as previously described [26].
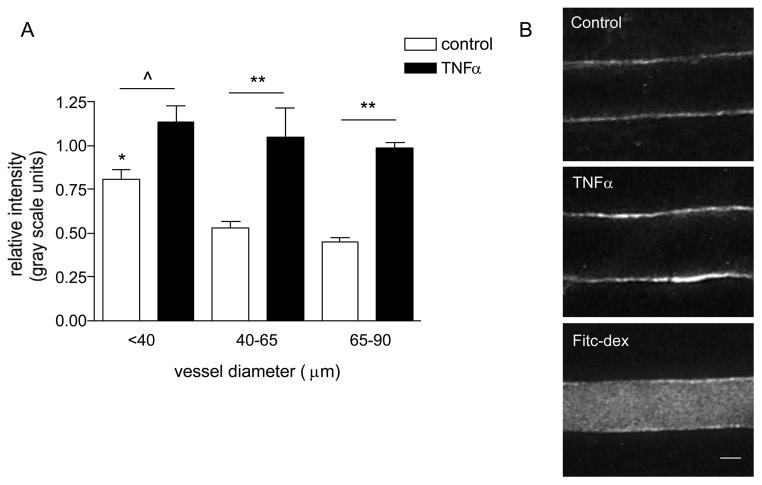
Figure 4. The expression of ICAM-1 varies in different sized venules.
(A) Mouse cremaster muscle venules were imunofluorescently labeled for ICAM-1 and the relative expression was quantified (as described in METHODS) under control and TNFα activated conditions. (B) Representative images of fluorescently labeled venular wall under control (upper panel) and TNFα activated conditions (enhanced ICAM-1 expression, middle panel), as well as an image of a venule which was internally perfused with FITC-dextran upon completion of data collection for normalization of intensity. The expression of ICAM-1 in small venules (< 40μm diameter) under control conditions was significantly higher than in larger vessels. Following TNFα treatment the expression of ICAM-1 significantly increased, but the difference among groups was abolished. * significantly different from other control groups (p<0.05), ^ significantly different from each other (p<0.05), ** significantly different from each other (p<0.01), n=8 venules for all groups.
Analyses
Venular convergences
A venular convergence was defined as region consisting of two converging vessels, specifically encompassing a region 40 μm length from the inner convergence point (the point where the two vessels meet) in each direction. A length of 40 μm was chosen as it is the average length of venular ECs in this tissue [25].
Leukocyte-EC interactions
All recorded images of intact blood perfused microvessels and leukocyte-EC interactions were analyzed using either Image J or NIH Image. Leukocyte-EC interactions were sampled in venules ranging from 25–90μm; where indicated, the data were binned into three groups according to vessel diameter (25–40μm, 40–65μm and 65–90μm). The diameter range for each of the groups was selected with respect to relative branch order and corresponded to third, second and first order of branching microvessels. Rolling leukocytes were defined as any leukocytes observed translating along the vessel wall in continuous contact with the endothelium that were moving slower than the hydrodynamic critical velocity as defined elsewhere [14]. Delivered leukocytes were defined as all leukocytes seen in the vicinity of the wall independently of whether they were rolling or carried in the free stream [9]. The number of rolling leukocytes on the vessel wall was calculated by counting leukocytes rolling past a line perpendicular to the vessel axis per 40 sec time interval. Firmly adhered leukocytes were defined as cells that remained stationary for ≥ 30 seconds. All leukocyte counts were performed within the same vessel depth irrespective of vessel diameter as previously described [26]. Leukocyte TEM was defined as the number of leukocytes in a defined tissue region adjacent to the venular wall. In venular convergences, perpendicular lines were projected at 40μm (the average EC length) distance from the inner converging point, and the average of this tissue area for all convergences (1545±21.6μm2) was used for comparative analysis of leukocyte TEM in the tissue adjacent to the straight venular regions. Vessel diameter in all straight venular regions was used as the width of the selected region (ROI) and the length was adjusted as needed to achieve an area of 1545μm2. The selected ROI for each of the straight venular regions was always downstream from the convergence, and was alternately located on opposite sides of the vessel wall (shown in Fig 3D). All TEM counts were normalized to 10,000μm2.
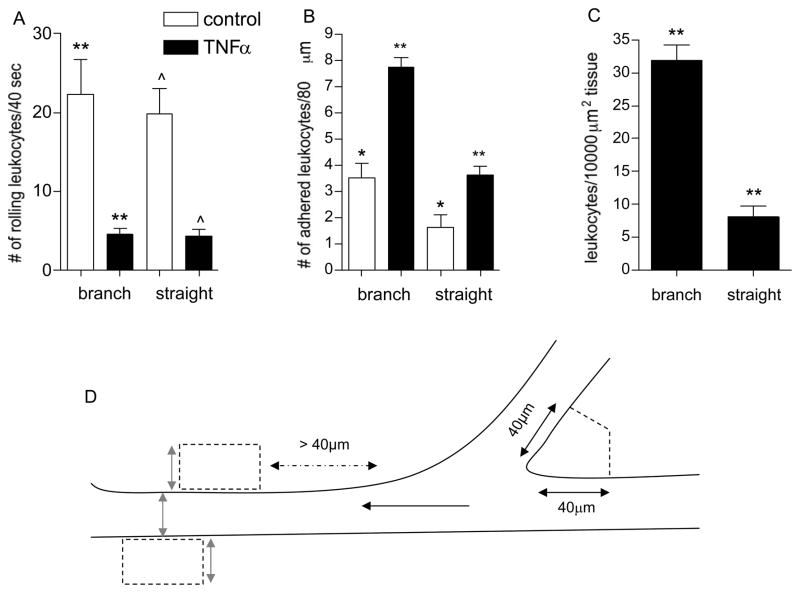
Figure 3. Regions of venular convergences support increased leukocyte adhesion and TEM.
The number of rolling (A), firmly adhered (B), and leukocytes that underwent TEM (C) was quantified in straight venular regions (vessels 30–80μm diameter) and compared to regions of venular convergences (as defined in METHODS) in the same vessels under control and TNFα activated conditions. (D) The cartoon demonstrates the tissue area adjacent to venular convergence and straight venular region that was used to quantify leukocyte TEM. In venular convergences perpendicular lines were projected 40 μm away from the inner converging point. An equal area was used to define tissue regions adjacent to the straight venular region, while keeping the width of the ROI equal to the vessel diameter (gray arrows). Black arrow indicates the direction of blood flow. Following TNFα treatment a significantly higher number of adhered leukocytes accumulated in venular convergences, compared to straight venular regions and consequently more TEM was observed in these regions. * significantly different from each other (p<0.05), **/^ significantly different from each other (p<0.01), n=8 venules, 4 mice.
ICAM-1 intensity
Fluorescence intensity levels, as an index of expression levels of ICAM-1, were analyzed using NIH-Image software as previously described [25]. Briefly, fluorescence was measured as gray scale units, where 0 = black and 255 = white, and was normalized and expressed as intensity relative to the intensity of the infused FITC-dextran solution. For all intensity measurements the laser power and camera gain settings were held constant, and the camera response was verified to be linear over the range used for these acquisitions.
Statistics
Statistical tests were performed using Graphpad Prism (v 4.0) to undertake t-tests, ANOVA, linear regression or correlation analyses as appropriate. Statistical significance was set at P < 0.05.
RESULTS
Leukocyte-EC interactions in different sized venules
Leukocyte-EC interactions vary greatly in vessels of different type and different origin, implying that leukocyte recruitment will be affected by the location in the vascular tree [26]. Here we asked whether these interactions might vary in vessels of different branch order (size) within the same microenvironment. We found that under control conditions the number of firmly adhered leukocytes in small venules was significantly higher than in larger vessels (3.2±0.27 vs 1.5±0.17 leukocytes/100μm vessel length, Fig 1B). The number of rolling leukocytes in the small vessels under similar conditions was significantly lower than in larger venules (11.2±0.85 leukocytes/40sec in venules 25–40 μm diameter vs. 19.2±1.2 leukocytes/40sec in venules 65–90μm diameter, Fig 1A). The lower number of rolling leukocytes in smaller venules compared to larger vessels is consistent with the expected leukocyte flux distribution through different venular branches in the intact microvascular network and further emphasizes the significance of the increased adhesion in these vessels. Leukocyte rolling velocities were not significantly different in all vessels sizes (Fig 1C), thus were not the cause for the lower number of rolling leukocytes.
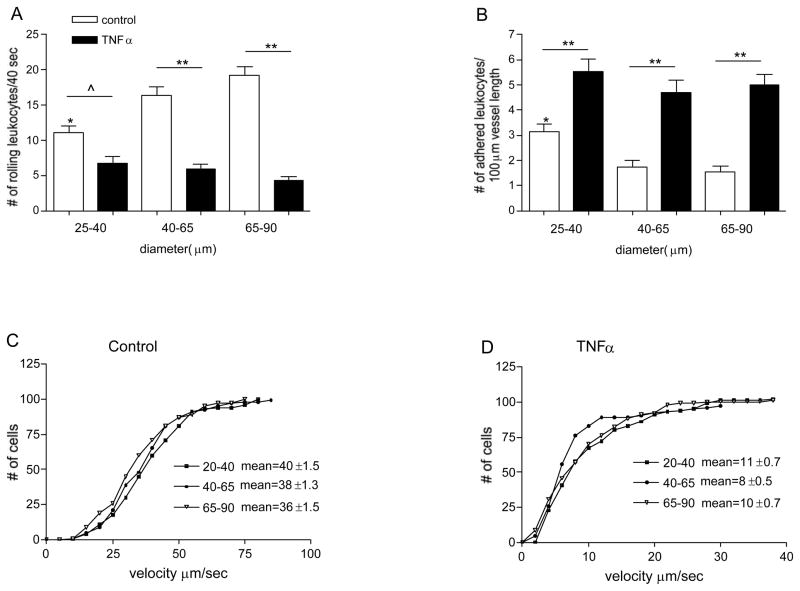
Figure 1. Leukocyte behavior is different in different sized venules.
The number of rolling (A) and firmly adhered leukocytes (B) was quantified in different sized, control and TNFα treated venules. Cumulative histograms show the velocity of rolling leukocytes in control (C) and TNFα treated venules (D). In small venules, most of the observed leukocytes were adhered rather than rolling; number of adhered leukocytes in these vessels is significantly higher and the number of rolling leukocytes is significantly lower than in the rest of the vessels. * significantly different from other control groups (p<0.05), ^ significantly different from each other (p<0.05), ** significantly different from each other (p<0.01), n=12–15 venules.
Interestingly, TNFα treatment, which evoked a significant increase in the average number of adhered leukocytes across all vessel sizes compared to control venules (5.1±0.46 vs. 2.1±0.47 leukocytes/100μm vessel length, Fig 1B), also abolished the differences in leukocyte rolling and adhesion in these vessels (Fig 1B). Since shear rate is not significantly different in TNFα activated venules [9], whereas the expression of adhesion molecules is increased [24], it is very likely that the latter are primarily responsible for the effect of TNFα on leukocyte behavior that we describe here.
Confirming our previous finding [26], the number of rolling leukocytes following TNFα treatment significantly decreased across all vessel sizes (5.7±0.69 vs 15.6±1.1 leukocytes/40sec, respectively, Fig 1A). The decrease in the number of rolling leukocytes in inflamed venules is due to both the significantly lower rolling velocities compared to control venules (Fig 1D) and the significantly higher number of adhered leukocytes along the vessel wall that results in a lower available area on the endothelial surface for rolling interactions to occur.
Leukocyte TEM was almost non-existent under control conditions (data not shown), but as expected was significantly increased in TNFα activated venules, primarily occurring in small venules (Fig 2). This suggests that some regions within the microvascular network are better equipped to accommodate leukocyte passage than others.
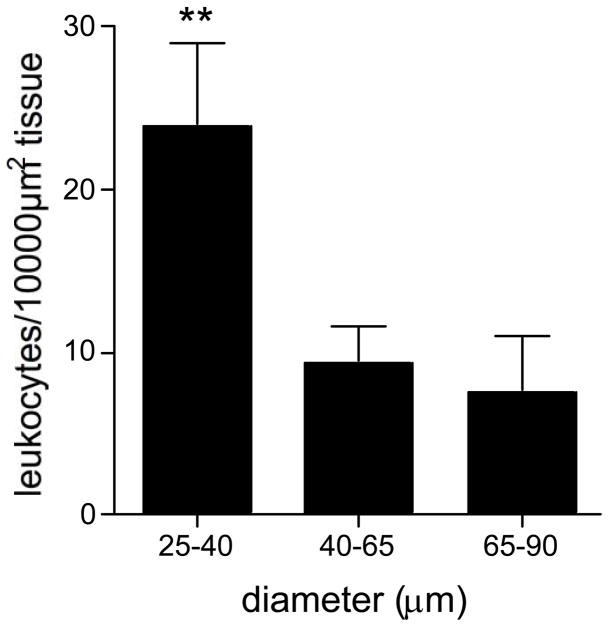
Figure 2. Leukocyte transmigration primarily occurs in small venules.
The number of leukocytes in the extravascular space adjacent to venules of different sizes was quantified and presented as leukocytes/10,000 μm2 tissue. TEM was significantly higher from small venules. ** significantly different from other groups (p<0.01), n=12 venules.
Leukocyte-EC interactions in straight vs converging venular regions
Another place where increased leukocyte-EC interactions have been observed is in venular convergences [12]. Thus we quantified leukocyte rolling, adhesion and TEM in these regions following TNFα treatment, and compared these to straight venular regions (vessels 30–80μm diameter). Under control conditions the number of adhered leukocytes in venular convergences was significantly higher compared to straight venular region (confirming our previous findings [12]). Following TNFα treatment, the number of adhered leukocytes increased significantly in both straight and converging regions, but remained significantly higher in venular convergences (7.8±0.4 vs. 3.6±0.3 leukocytes/80μm vessels length, respectively, p<0.001, Fig 3B). The number of adhered leukocytes in control and activated venular convergences was significantly higher than in straight venular regions despite the fact that leukocyte rolling fluxes in both regions were the same (4.5±0.8 vs. 4.3±0.9 leukocytes/40sec, Fig 3A). These data indicate that either the effect of TNFα on the expression of adhesion molecules in convergences is different from that in straight regions, or the factor leading to increased leukocyte adhesion is not the adhesion molecules, but possibly the fluid shear environment.
As expected form the higher number of adhered leukocytes, leukocyte TEM in venular convergences was significantly higher compared to straight venular regions (31.9±2.6 vs. 8.2±1.1 leukocytes/10,000μm2 tissue, respectively, p<0.001, Fig 3C).
Expression level of ICAM-1 in different sized vessels
It has been suggested the expression of adhesion molecules plays a more prominent role in regulating leukocyte-EC interactions than hemodynamic forces [14]. Thus we hypothesized that ICAM-1 expression patterns would account for the observed differences in leukocyte behavior in the different locations of the microvascular network. To test this, we used immunofluorescence labeling and confocal microscopy to quantify the relative expression of ICAM-1 in different venular regions.
As predicted from our earlier work [26], we found that the expression level of ICAM-1 in different sized venules closely paralleled the observed leukocyte behavior. Under control conditions the relative expression of ICAM-1 in smaller venules (25–40 μm diameter) was significantly (~1.6-fold) higher than in medium and larger vessels (40–90μm diameter, Fig 4), implying that this could account for the higher number of adhered leukocytes in these vessels as was demonstrated in Figure 1.
Following TNFα treatment the difference in expression of ICAM-1 across all vessel sizes was abolished, again consistent with the idea that the abrogation of the differences in leukocyte-EC interactions under these conditions was due to ICAM-1. Earlier we reported that in venules, TNFα evoked an overall ~2-fold increase in the expression level of ICAM-1 [25], however in the present study we found that this increase was not uniform, and varied significantly in different sized venules. In smaller venules, which had significantly higher baseline ICAM-1 expression, the increase was significant but relatively small (1.4-fold, Fig 4, p<0.05). In larger vessels (40–90μm diameter) which had a lower baseline ICAM-1 expression, a dramatic 2.2-fold increase was observed (Fig 4). This greater increase in the large venules resulted in there being no significant difference in ICAM-1 expression, and consequently no significant difference in leukocyte rolling and adhesion, among the three vessel groups, supporting the idea that adhesion molecules are key regulators of leukocyte-EC interactions.
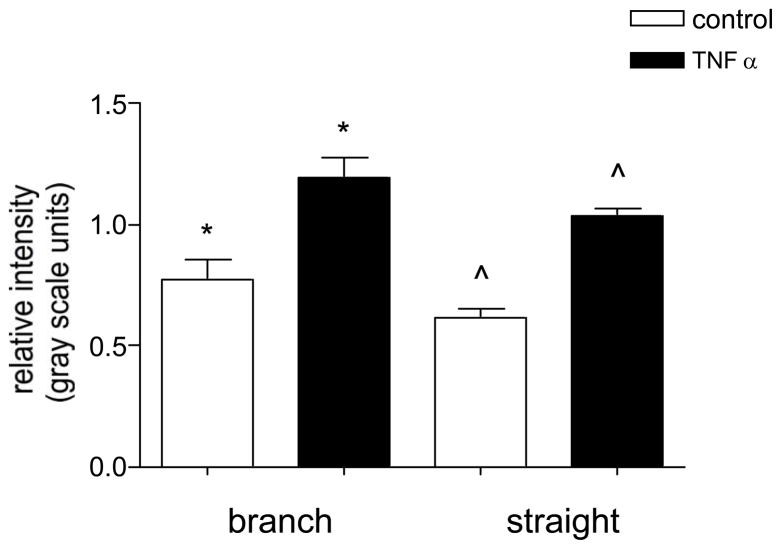
Expression level of ICAM-1 in straight vs converging venular regions
To test whether the expression of ICAM-1 in venular convergences could account for higher leukocyte adhesion in these regions, we compared the expression of ICAM-1 in venular convergences to that in straight venular regions (vessels 30–80μm diameter) in control and activated venules. We found that while the relative expression of ICAM-1 in TNFα activated venules significantly increased in both straight and converging regions, the levels remained not significantly different from each other (0.6±0.04 vs. 0.8±0.08 control, respectively and 1.0±0.03 vs. 1.2±0.08, TNFα, respectively, Fig 5). These results show that the effect of TNFα on ICAM-1 expression is similar in straight and converging regions, eliminating ICAM-1 expression from being the primary reason for the increased leukocyte adhesion in converging venules. Thus we concluded that the local shear environment, being an additional factor affecting leukocyte behavior, is a likely candidate to account for increased leukocyte adhesion in venular convergences.
Figure 5. The expression of ICAM-1 is not different in straight and converging venular regions.
Control and TNFα activated venules were imunofluorescently labeled for ICAM-1 and the relative expression was quantified in straight venular regions (vessels 30–80μm diameter) and compared to regions of venular convergences (as defined in METHODS) located upstream in the same vessels. The expression of ICAM-1 in both regions was not significantly different in control or TNFα activated venules. */^ significantly different from each other (p<0.05), n=8 vessels.
Increased leukocyte adhesion in small venules is independent of shear rate
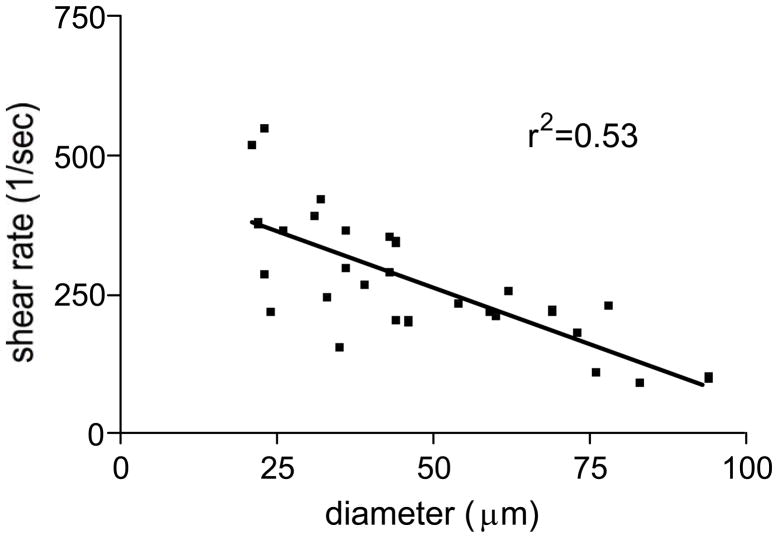
The close parallel between leukocyte behavior and the expression of ICAM-1 in different sized venules, together with previously published data [14], strongly suggests that adhesion molecules, rather than fluid shear are the primary regulations of leukocyte-EC interactions in these vessels. To confirm that leukocyte adhesion in straight venular regions was independent of fluid shear rate we measured the shear rate in venules of different sizes, after first quantifying leukocyte-EC interactions in these same vessels. The average value of shear rate across all venules ranging from 25–95μm diameter was 275±21.6 sec−1 (Fig 6). More importantly, and confirming our hypothesis, we found that in the venular network the shear rate linearly decreased with increasing vessel diameter, r2=0.53, thus could be predicted as a function of vessel size. The finding that more adhesion events were observed in smaller venules (as shown in Fig 1), where the shear rate is significantly higher than in larger vessels (as shown in Fig 6), suggests that in the range of shear rates measured in the straight regions of sampled venules (100–500 sec−1), leukocyte adhesion does not decrease as a functions of increased shear rate, as has been predicted from studies in isolated cell systems [13].
Figure 6. Shear rate in venules can be predicted by vessel size.
Shear rate in TNFα activated venules was assessed by tracking the displacement of fluorescently labeled beads (0.5μm) which were injected into the blood stream via a catheter inserted into the jugular vein. There was a significant correlation between vessel size and the prevailing shear rate in these vessels (p<0.05) linear correlation (r2=0.53).
Interestingly, studies in EC monolayers showed that ICAM-1 expression can be upregulated by flow [24]. This suggests that the higher expression of ICAM-1 that we observed in small venules under control conditions (as shown in Fig 4) could be a result of higher shear rates in these vessels, providing evidence for a possible, (indirect) effect of fluid shear on leukocyte-EC interactions via upregulation of ICAM-1 in-vivo.
Increased leukocyte adhesion in converging venules occurs in the stagnation regions or regions of low flow velocity
As previously discussed, the expression of ICAM-1 was not different in TNFα activated straight and converging venular regions (Fig 5), but the number of adhered leukocytes was significantly higher in these regions (Fig 3B). Likewise, we have previously showed that leukocyte-EC interactions under appropriate conditions can indeed be a function of shear rate [26]. Thus we hypothesized that in venular convergences, fluid shear rate has the capacity to have a greater impact on leukocyte-EC interactions.
Our initial question was whether there exist characteristically different shear fields (or velocity profiles) that would be consistent with our observation of preferential accumulation of leukocytes in these regions. Exploration of fluid shear field behavior through venular convergences in-vivo requires synthesis of fluid streamlines at very high temporal resolution from measuring velocity properties in a series of stacked confocal images. To simplify this analysis, we used CFD to predict the variations in flow velocities. Results from the CFD analysis indeed suggest that a region of low velocity is characteristically found at the junctional region between the two inlet vessels. Figure 7A depicts the predicted velocity vectors on a slice taken vertically through the center of a typical venular convergence. The region of low velocity (blue in the figure) is indicative of a stagnation region and suggests that there is an altered shear environment in this region. The results obtained from the simulation are thus consistent with the findings that leukocytes tend to accumulate in the regions of venular convergences, as the leukocytes entering the region of low velocity would have a higher tendency to remain in this region. Overall, the predicted presence of the stagnation region suggests that hydrodynamics have the capacity to play an important role in promoting the margination of leukocytes towards the vessel wall and increased leukocyte adhesion in convergence regions of the vascular network.
Figure 7. Two inlet venules create regions of low velocity which are indicative of stagnation region.
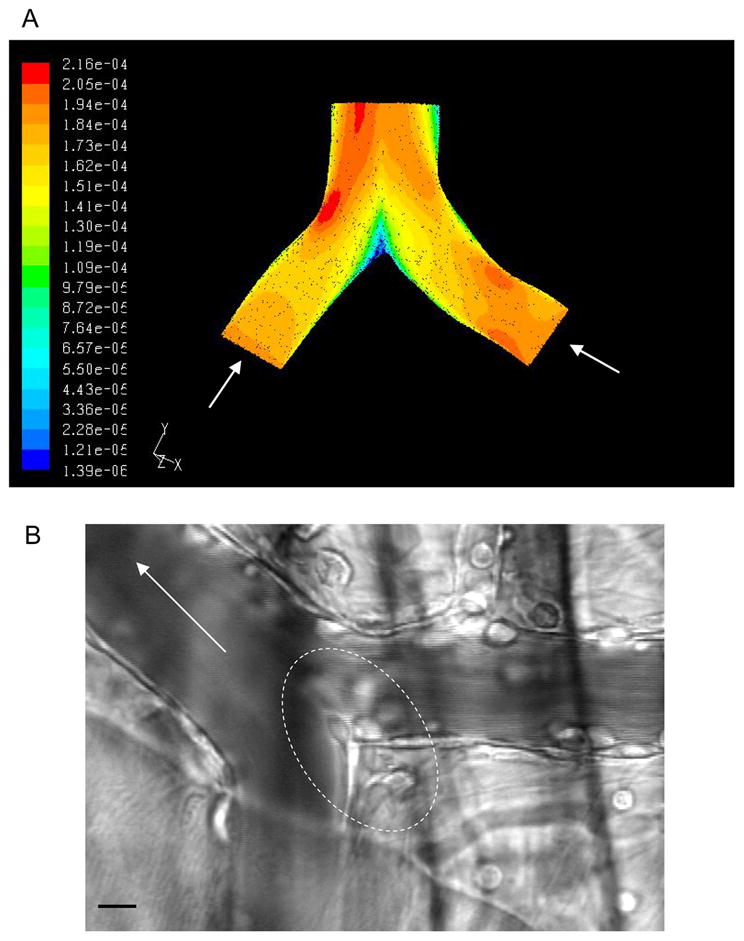
(A) A three-dimensional solid model of a venular convergence was generated (based on a live image obtained in vivo) using SolidWorks™ (SolidWorks Corp., Concord, MA) modeling software. This model was used to perform CFD analysis in order to predict variations in flow properties. The simulation results show that a region of low velocity can be found at the junctional region between the two inlet vessels, which could account for increased number of adhered transmigrating leukocytes in these regions. (B) Representative image of a venular convergence where enhanced leukocyte adhesion could be observed at the inner convergence points where the flow velocities are low as indicated by the simulation. The outlined regions is the region of enhanced leukocyte adhesion and leukocyte migration into the tissue. The bar is 10 micrometers. Arrows on both panels indicate flow direction.
DISCUSSION
In the current work we assessed the differences in leukocyte-EC interactions in different regions of the venular network, as well as the role of adhesion molecules such as ICAM-1 and the shear environment in regulating these interactions. Multiple members of the selectin and CAM families of adhesion molecules have been implicated in mediating the leukocyte recruitment cascade [10,31]. Similarly, secreted cytokines and chemokines play an important role in regulating recruitment, by either upregulating the levels of adhesion molecules [7,30] or acting as surface receptors that are capable of sustaining leukocyte interactions [6]. However, as ICAM-1 has been established as a key mediator of leukocyte adhesion, it was the main focus of the current work. We show that different leukocyte behavior is characteristic of not only specific vessel types (arterioles vs venules, as previously demonstrated [26]), but also vessel dimensions and different regions within the network. We provide evidence that in straight venular regions the variability in the expression of adhesion molecules such as ICAM-1 could account for the differences in leukocyte behavior in different sized venules. In venular convergences, on the other hand, fluid shear is likely to play a more prominent role in regulating leukocyte-EC interactions than it does in straight regions.
We and others have shown that adhesion molecules play a major role in the regulation of leukocyte-EC interactions in-vivo, but there is also evidence that changes in the balance between the expression levels of adhesion molecules and the shear rate might significantly affect these interactions. For example in arterioles, where the shear rate is characteristically higher (compared to venules, [15]), and the expression of adhesion molecules is significantly lower, shear rate indeed directly impacts leukocyte ability to firmly adhere as demonstrated by experimentally altering the local shear conditions [26]., Leukocyte adhesion in activated arterioles could be proportionately increased by gradually reducing the shear rate locally [26], suggesting that in these vessels, high fluid shear impairs the ability of leukocytes to firmly adhere. In venules, where the expression of adhesion molecules is significantly higher (compared to arterioles, [26]) and the shear rate is characteristically lower, higher shear rate supports leukocyte recruitment towards the wall but does not impact adhesion [9]. Thus, we conclude that in straight venular regions, ICAM-1 expression is the primary determinant of leukocyte adhesion; there may also be a possible indirect effect of shear rate on leukocyte adhesion in these regions, via the upregulation of ICAM-1. Our findings also suggest that in venular convergences low shear rates in combination with high expression of adhesion molecules (relatively to arterioles) could positively impact leukocyte accumulation.
In the current work we found that in different sized venules the expression patterns of ICAM-1closely paralleled the observed leukocyte behavior. In smaller venules under control conditions the expression of ICAM-1 was characteristically higher than in larger vessels, thus providing an explanation for the higher number of adhered leukocytes in these vessels (Fig 1).
TNFα treatment evoked an overall increase in the expression level of ICAM-1. Importantly, while the magnitude of that increase varied in different microvascular regions, ICAM-1 expression reached similar levels and was not different in all sampled venular regions (Fig 5). The differential increase in ICAM-1 expression in different sized venules to the same levels in all venular regions suggests that all ECs might have similar capacity to express ICAM-1, and that the expression might have been maximized following 500ng/mouse TNFα, consequently resulting in a high number of adhered leukocytes. That the expression of ICAM-1 in TNFα activated venular convergences was similar to straight regions was surprising to us, since the number of adhered leukocytes in these regions was significantly higher than in the straight venular regions. These data clearly indicate that another factor must be primarily responsible for the observed preferential recruitment of leukocytes in the regions of venular convergences. Thus, we explored the idea that possible perturbations in the blood flow in the regions of venular convergences will create preferred regions for leukocyte accumulation. By simulating the blood flow through a typical convergence and using CFD analysis we showed that the two inlet venules create regions of low velocity which are indicative of stagnation regions. Leukocytes entering these regions are more likely to marginate towards the vessel wall and stay there. Of course, as a next step, these findings should be confirmed in an in-vivo study. As has been previously mentioned, adhered leukocytes often crawl along the lumen of the blood vessel prior to TEM [29]. To date the direction of leukocyte crawling is assumed to be random. However, leukocyte crawling in the regions of venular convergences has not been studied. The possibility that shear will affect the directionality of leukocyte crawling, thus contributing to leukocyte accumulation in venular convergences merits investigation, but was beyond the scope of the present work.
Interestingly, our data show that leukocyte TEM in activated tissue is localized and primarily occurs in small venules and regions of venular convergences (Figs 2 and 3C). ICAM-1 is recruited to the sites of leukocyte TEM [2] and plays a crucial role in mediating TEM [1,19]. Thus one might assume that there is higher ICAM-1 expression in TNFα activated small venules and venular convergences, where increased leukocyte TEM was observed. In contrast, our findings show that there is no difference in the relative expression of ICAM-1 in these regions compared to other vascular regions, indicating that there is yet another factor that must contribute to higher number of transmigrating leukocytes in these regions. Our laboratory has previously demonstrated that ECs in larger venules have significantly larger area and a different shape, leading to decreased total junctional length/EC area in these vessels [25]; in like manner it is possible that EC junctional morphology varies between the straight and converging regions studied here. Since most leukocytes undergo transendothelial migration via junctional regions of the endothelium [22] we speculate that the effect of junctional morphology on leukocyte TEM will be substantial and should be addressed in future work. Similarly, expression patterns of ICAM-1 within individual ECs can be highly variable [25], suggesting another way in which more localized differences could result in preferred sites for leukocyte TEM. In the current work we assessed the average vessel wall expression of ICAM-1 in the regions of interest, thus it is possible that the more subtle local differences were not quantified. Less junctional length in larger venules (leading to a decreased number of available locations for leukocyte TEM) might contribute to the inefficient leukocyte extravasation that we documented in these vessels, and, as larger venules will have a thicker vessel wall and a denser layer of smooth muscle, this could also decrease leukocyte TEM.
An additional question that should be addressed in future work is how much the activation state of leukocytes themselves contributes to the observed results? It is possible that in microvessels of different branch orders, the leukocyte activation state will be different (e.g. the affinity state and the conformation of β2-integrins), resulting in different interactions with the vessel wall. It has been shown previously that β2-integrins in different affinity states are capable of mediating either leukocyte rolling or adhesion [21], implying further that if leukocyte activation state varies in different microvascular regions, this could by itself lead to local differences in leukocyte behavior.
Finally, in studies in isolated cell systems, the expression of ICAM-1 has been shown to increase significantly under high shear conditions [24,28], but thus far there is no evidence that this also occurs in vivo. Our work provides indirect evidence that this might also be the case in the intact blood perfused microvasculature. Our finding that ICAM-1 expression in small venules, which have characteristically higher fluid shear rates, is significantly higher than in larger vessels (Fig 6), suggests a possible indirect effect of flow on leukocyte interactions, by upregulating ICAM-1. Under pathological conditions such as atherosclerosis, which is characterized by vessel narrowing (thus with increased local blood velocity) the indirect effect of shear rate will have a crucial role in maintenance of the increased inflammatory state.
In summary, in the current work we show that in different regions of the microvascular network different factors are major regulators of leukocyte-EC interactions. We confirmed the previously suggested key role of adhesion molecules in regulating leukocyte interactions with the endothelium by demonstrating a striking correlation of leukocyte behavior and expression of ICAM-1 in the straight regions of different sized venules. However, in venular convergences, the disturbed flow and the variation in fluid velocity, but not the expression of ICAM-1, potentially play a primary role.
Acknowledgments
Sources of Support: The work was supported by NIH RO1 HL75186, NIH PO1 HL18208, and AHA 0615677T.
We thank Ms J.M. Kuebel for expert technical assistance and Hen Drori for thoughtful discussion.
References
- 1.Allingham MJ, van Buul JD, Burridge K. ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration. J Immunol. 2007;179:4053–64. doi: 10.4049/jimmunol.179.6.4053. [DOI] [PubMed] [Google Scholar]
- 2.Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. 2004;167:377–88. doi: 10.1083/jcb.200404129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cid MC, Cebrian M, Font C, Coll-Vinent B, Hernandez-Rodriguez J, Esparza J, Urbano-Marquez A, Grau JM. Cell adhesion molecules in the development of inflammatory infiltrates in giant cell arteritis: inflammation-induced angiogenesis as the preferential site of leukocyte-endothelial cell interactions. Arthritis Rheum. 2000;43:184–94. doi: 10.1002/1529-0131(200001)43:1<184::AID-ANR23>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 4.Dong C, Slattery MJ, Liang S, Peng HH. Melanoma cell extravasation under flow conditions is modulated by leukocytes and endogenously produced interleukin 8. Mol Cell Biomech. 2005;2:145–59. [PMC free article] [PubMed] [Google Scholar]
- 5.Feng D, Nagy JA, Pyne K, Dvorak HF, Dvorak AM. Neutrophils emigrate from venules by a transendothelial cell pathway in response to FMLP. J Exp Med. 1998;187:903–15. doi: 10.1084/jem.187.6.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 7.Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Semin Immunol. 2002;14:83–92. doi: 10.1006/smim.2001.0345. [DOI] [PubMed] [Google Scholar]
- 8.Kim MB, Sarelius IH. Distributions of wall shear stress in venular convergences of mouse cremaster muscle. Microcirculation. 2003;10:167–78. doi: 10.1038/sj.mn.7800182. [DOI] [PubMed] [Google Scholar]
- 9.Kim MB, Sarelius IH. Regulation of leukocyte recruitment by local wall shear rate and leukocyte delivery. Microcirculation. 2004;11:55–67. doi: 10.1080/10739680490266199. [DOI] [PubMed] [Google Scholar]
- 10.Kim MB, Sarelius IH. Role of shear forces and adhesion molecule distribution on P-selectin-mediated leukocyte rolling in postcapillary venules. Am J Physiol Heart Circ Physiol. 2004;287:H2705–11. doi: 10.1152/ajpheart.00448.2004. [DOI] [PubMed] [Google Scholar]
- 11.Kunkel EJ, Butcher EC. Plasma-cell homing. Nat Rev Immunol. 2003;3:822–9. doi: 10.1038/nri1203. [DOI] [PubMed] [Google Scholar]
- 12.Lamkin-Kennard KA, Chuang JY, Kim MB, Sarelius IH, King MR. The distribution of rolling neutrophils in venular convergences. Biorheology. 2005;42:363–83. [PubMed] [Google Scholar]
- 13.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–90. [PubMed] [Google Scholar]
- 14.Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. 1991;69:1034–41. doi: 10.1161/01.res.69.4.1034. [DOI] [PubMed] [Google Scholar]
- 15.Lipowsky HH, Kovalcheck S, Zweifach BW. The distribution of blood rheological parameters in the microvasculature of cat mesentery. Circ Res. 1978;43:738–49. doi: 10.1161/01.res.43.5.738. [DOI] [PubMed] [Google Scholar]
- 16.Mamdouh Z, Chen X, Pierini LM, Maxfield FR, Muller WA. Targeted recycling of PECAM from endothelial surface-connected compartments during diapedesis. Nature. 2003;421:748–53. doi: 10.1038/nature01300. [DOI] [PubMed] [Google Scholar]
- 17.Matharu NM, McGettrick HM, Salmon M, Kissane S, Vohra RK, Rainger GE, Nash GB. Inflammatory responses of endothelial cells experiencing reduction in flow after conditioning by shear stress. J Cell Physiol. 2008 doi: 10.1002/jcp.21457. [DOI] [PubMed] [Google Scholar]
- 18.Methe H, Balcells M, Alegret M, del C, Santacana M, Molins B, Hamik A, Jain MK, Edelman ER. Vascular bed origin dictates flow pattern regulation of endothelial adhesion molecule expression. Am J Physiol Heart Circ Physiol. 2007;292:H2167–75. doi: 10.1152/ajpheart.00403.2006. [DOI] [PubMed] [Google Scholar]
- 19.Millan J, Hewlett L, Glyn M, Toomre D, Clark P, Ridley AJ. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat Cell Biol. 2006;8:113–23. doi: 10.1038/ncb1356. [DOI] [PubMed] [Google Scholar]
- 20.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–75. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salas A, Shimaoka M, Phan U, Kim M, Springer TA. Transition from rolling to firm adhesion can be mimicked by extension of integrin alphaLbeta2 in an intermediate affinity state. J Biol Chem. 2006;281:10876–82. doi: 10.1074/jbc.M512472200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shaw SK, Bamba PS, Perkins BN, Luscinskas FW. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol. 2001;167:2323–30. doi: 10.4049/jimmunol.167.4.2323. [DOI] [PubMed] [Google Scholar]
- 23.Smith LA, Aranda-Espinoza H, Haun JB, Hammer DA. Interplay between shear stress and adhesion on neutrophil locomotion. Biophys J. 2007;92:632–40. doi: 10.1529/biophysj.105.079418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sultan S, Gosling M, Abu-Hayyeh S, Carey N, Powell JT. Flow-dependent increase of ICAM-1 on saphenous vein endothelium is sensitive to apamin. Am J Physiol Heart Circ Physiol. 2004;287:H22–8. doi: 10.1152/ajpheart.00880.2003. [DOI] [PubMed] [Google Scholar]
- 25.Sumagin R, Sarelius IH. TNF-alpha activation of arterioles and venules alters distribution and levels of ICAM-1 and affects leukocyte-endothelial cell interactions. Am J Physiol Heart Circ Physiol. 2006;291:H2116–25. doi: 10.1152/ajpheart.00248.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sumagin R, Sarelius IH. A role for ICAM-1 in maintenance of leukocyte-endothelial cell rolling interactions in inflamed arterioles. Am J Physiol Heart Circ Physiol. 2007;293:H2786–98. doi: 10.1152/ajpheart.00720.2007. [DOI] [PubMed] [Google Scholar]
- 27.Tegoulia VA, Cooper SL. Leukocyte adhesion on model surfaces under flow: effects of surface chemistry, protein adsorption, and shear rate. J Biomed Mater Res. 2000;50:291–301. doi: 10.1002/(sici)1097-4636(20000605)50:3<291::aid-jbm2>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Tsuboi H, Ando J, Korenaga R, Takada Y, Kamiya A. Flow stimulates ICAM-1 expression time and shear stress dependently in cultured human endothelial cells. Biochem Biophys Res Commun. 1995;206:988–96. doi: 10.1006/bbrc.1995.1140. [DOI] [PubMed] [Google Scholar]
- 29.Wojciechowski JC, Sarelius IH. Preferential binding of leukocytes to the endothelial junction region in venules in situ. Microcirculation. 2005;12:349–59. doi: 10.1080/10739680590934763. [DOI] [PubMed] [Google Scholar]
- 30.Wung BS, Ni CW, Wang DL. ICAM-1 induction by TNFalpha and IL-6 is mediated by distinct pathways via Rac in endothelial cells. J Biomed Sci. 2005;12:91–101. doi: 10.1007/s11373-004-8170-z. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–92. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]