Abstract
Under physiological conditions the gut associated lymphoid tissues not only prevent the induction of a local inflammatory immune response, but also induce systemic tolerance to fed antigens1,2. A notable counter-example is celiac disease, where genetically susceptible individuals expressing HLA-DQ2 or HLA-DQ8 molecules develop inflammatory T cell and antibody responses against dietary gluten, a protein present in wheat3. The mechanisms underlying this dysregulated mucosal immune response to a soluble antigen have not been identified. Retinoic acid, a metabolite of vitamin A, was shown to play a critical role in the induction of intestinal regulatory responses4–6. We found that in conjunction with IL-15, a cytokine greatly upregulated in the gut of celiac disease patients, retinoic acid rapidly activated dendritic cells to induce JNK phosphorylation and release the proinflammatory cytokines IL-12p70 and IL-23. As a result, in a stressed intestinal environment, retinoic acid acted as an adjuvant that promoted rather than prevented inflammatory cellular and humoral responses to fed antigen. Altogether, these findings unveil an unexpected role for retinoic acid and IL-15 in the abrogation of tolerance to dietary antigens.
Induction of regulatory intestinal responses to oral antigens prevents the subsequent development of systemic T helper type-1 (TH1) responses to those antigens, a phenomenon referred to as oral tolerance2. The difficulty of inducing TH1 immunity against soluble protein antigens in the highly regulatory environment of the gut has been a major limiting factor in the development of effective oral vaccines against invasive intracellular pathogens7. Mucosal tolerance has important fail-safe mechanisms that prevent the initiation of unnecessarily destructive inflammatory immune responses to harmless antigens contacted at mucosal surfaces. These mechanisms include induction of regulatory T cells (iTreg) expressing the transcription factor forkhead box P3 (Foxp3) and deletion of T cells specific to the ingested antigen1,2.
A notable exception is celiac disease (CD), where patients mount a TH1 immune response to dietary gluten3. Interestingly, interleukin-15 (IL-15), a cytokine induced upon NF-κB activation in multiple cell types8, is highly upregulated in the epithelium and the lamina propria (Lp) of CD patients9. Whereas IL-15 expressed by intestinal epithelial cells (IEC) was shown to license intraepithelial cytotoxic T lymphocytes (IEL) to become killer cells10, the potential impact of dysregulated IL-15 expression outside the intestinal epithelium on CD pathogenesis and, in particular, on T cell polarization has not been investigated.
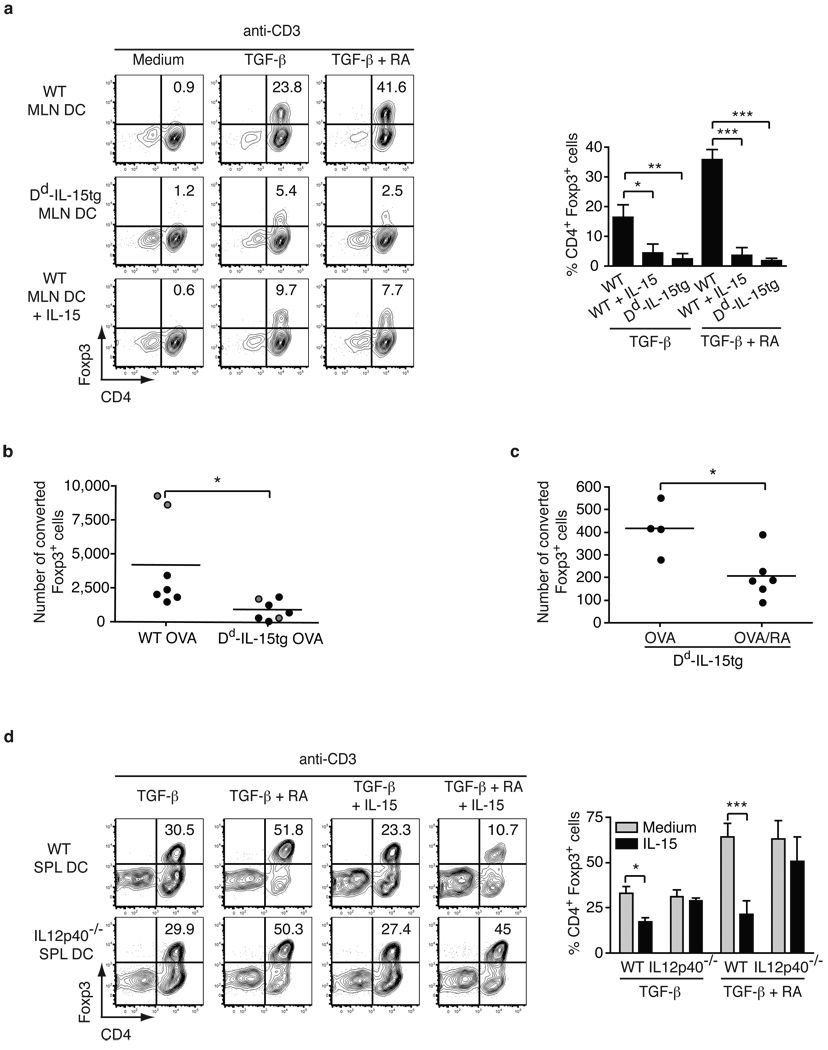
To determine whether the presence of IL-15 may impact intestinal homeostasis by promoting the development of inflammatory CD4+ T cell responses, we first examined its effects on the generation of iTreg. Foxp3+ iTreg are generated mainly in the gut-associated lymphoid tissue (GALT) during recognition of luminal antigens in the presence of retinoic acid (RA) and TGF-β1. Mesenteric lymph node (MLN) dendritic cells (DC) have tolerogenic functions which include the ability to drive de novo differentiation of iTreg4–6. Nevertheless, iTreg generation from unfractionated CD4+ T cells (Fig. 1a) or naïve CD44lo CD4+ T cells (Fig. S1a) was impaired in the presence of IL-15-stimulated MLN DC. Furthermore, IL-15 had no effect on iTreg differentiation in the presence of DC lacking the IL-2-IL-15Rβ/γc heterodimeric signaling receptor complex8 (Fig. S2a) and in DC-free systems (Fig. S1b and Fig. S5), demonstrating that IL-15 was acting at the level of DC and not T cells to block iTreg generation. To assess the relevance of our in vitro findings, we tested the response to fed chicken ovalbumin (OVA), a model antigen used in oral tolerance experiments, in Dd-IL-15 transgenic (tg) mice11 that over-express IL-15 in the Lp and MLN but not in the intestinal epithelium (Fig. S3). In agreement with our in vitro observations, the number of naïve OT-II RAG-1−/− CD4+ T cells converted into iTregs was significantly reduced in OVA fed Dd-IL-15tg mice as compared to WT mice (Fig. 1b). Intriguingly, RA further decreased the conversion of Treg cells in vivo in OVA-fed Dd-IL-15tg mice (Fig. 1c), suggesting that RA prevents rather than promotes iTreg differentiation in the presence of IL-15.
Figure 1. IL-15-activated DC in the presence of retinoic acid prevent induction of Foxp3+ regulatory T cells.
a, 105 CD4+ Foxp3− T cells were cultured with 4 × 104 MLN DC isolated from WT or Dd-IL-15tg mice with anti-CD3 alone or combined with IL-15, TGF-β and RA. The percentages of Foxp3+ cells are shown. Graph depicts pooled data ± s.e.m. (n=3). b, RAG1−/− OT-II CD45 congenic CD25− CD4+T cells were transferred into WT and Dd-IL-15tg mice that were fed OVA in drinking water for five days (black dots) or by gavage (grey dots). Treg cell conversion was assessed in the MLN by intracellular staining for Foxp3 and detected by flow cytometry. The absolute numbers of converted CD4+ Foxp3+ T cells are shown. Data are representative of two experiments performed independently. c, Ly5.2+ OT-II T cells were transferred into Ly5.1+ and Dd-IL-15tg-Ly5.1+ recipient mice that were fed OVA or OVA and RA five times during ten days. The absolute number of CD4+ Foxp3+ Ly5.2+ converted T cells in the MLN is shown as in a. The decrease in the number of converted iTreg was associated with a significant decrease in the number of detectable transferred T cells in Dd-IL-15tg mice (data not shown). This is likely due to the inability to detect inflammatory T cells that are more susceptible to cell death than Foxp3+ Tregs, which express anti-apoptotic factors. d, As in a, CD4+ Foxp3− T cells were cultured with SPL DC isolated from WT and IL-12p40−/− mice. The percentages of Foxp3+ cells are indicated. Graph depicts three pooled experiments ± s.e.m. *P<0.05, **P<0.01, ***P<0.001 (unpaired Student's t-test).
To determine the mechanisms by which IL-15-stimulated DC prevent iTreg and to further assess the role of RA, we used splenic (SPL) DC (Fig. S2b), which, unlike MLN, lack the ability to produce constitutively high levels of RA5,6. Having found that conditioned media obtained from IL-15-treated SPL DC decreased iTreg conversion (Fig. S2c), we analyzed the expression of cytokines in the supernatant of IL-15-treated SPL DC and found high levels of IL-12p70 and IL-23, but no IL-6 (Fig. S4a). In accordance with its ability to further block Treg generation in the presence of IL-15 (Fig. 1a), RA significantly amplified the release of all proinflammatory cytokines (Fig. S4a). As previously reported12, IL-12p70 significantly suppressed iTreg differentiation at concentrations found in IL-15-conditioned DC supernatant (Fig. S4b), and this effect was enhanced by IL-23 in a dose-dependent manner (Fig. S4b). The requirement for IL-12 in IL-15-mediated suppression of iTreg differentiation was demonstrated using IL-12p40-deficient DC (Fig. 1d and Fig. S4c) and neutralizing anti-IL-12p40 antibody (Fig. S4d).
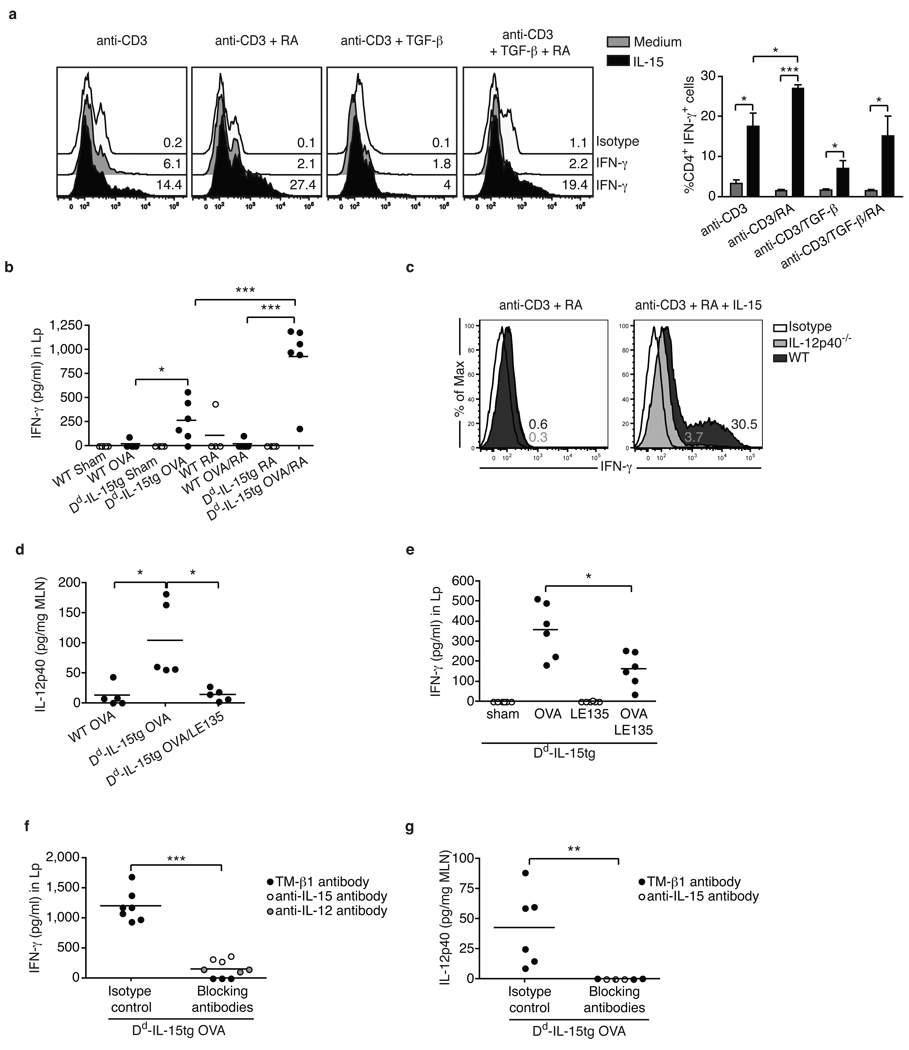
We next observed that IL-15-stimulated SPL DC (Fig. 2a) and conditioned media from IL-15-treated SPL DC (Fig. S6a) promoted in vitro differentiation of TH1 cells from CD4+ T cells under conditions that normally promote the generation of iTreg. This translated in vivo in the induction of TH1 responses in OVA fed Dd-IL-15tg mice (Fig. 2b and Fig. S6c). As expected, the ability of IL-15 to induce TH1 responses in vitro (Fig. 2c) and in vivo (Fig. 2f) was dependent on IL-12. In sharp contrast to previous studies suggesting that RA blocks the induction of inflammatory intestinal T cell responses6, RA promoted (Fig. 2a, Fig. 2b, Fig. S6a-c) and was also critical for TH1 polarization (Fig. 2d and Fig. 2e). In addition to its ability to promote IL-12 in DC, RA also acted at the level of T cells to amplify IL-12p70-mediated TH1 T cell differentiation (Fig. S5 and Fig. S6b). Surprisingly, RA also enhanced TH17 cell responses in vitro in the presence of IL-6 (Fig. S7a) and in vivo (Fig. S7b-d). Finally, the critical role of IL-15 in the induction of inflammatory T cell responses to dietary antigen was demonstrated by blocking IL-15 signaling in vivo using either a neutralizing anti-IL-15 or IL-15/IL-2Rβ antibody13 (Fig. 2f, Fig. 2g, Fig. S6d, Fig. S7e, and Fig. S7f). Altogether these observations suggest a sequential model (Fig. 4f) whereby IL-15 first acts in concert with RA to induce IL-12 and IL-23 in MLN DC (Fig. 2d and data not shown). Along with RA, these inflammatory mediators then operate at the level of T cells to promote TH1 cell differentiation and, when IL-6 is present14, TH17 cell differentiation. While our finding of the adjuvant effect of RA is unexpected within the field of mucosal immunity, it is consistent with its usage as a beneficial proinflammatory adjuvant in anti-tumor immunity15,16. Future studies using conditional RARα−/− mice will help further delineate the role of RA signaling in T cells and DC in the induction of inflammatory T cell responses to fed antigen.
Figure 2. Retinoic acid exerts an adjuvant effect on IL-15-mediated inflammatory T cell responses.
a, CD4+ T cells were cultured with WT SPL DC with the indicated cytokines. Representative histograms gated on CD4+ T cells show IFN-γ expression. The bar graph summarizes the percentage of IFN-γ-producing CD4+ T cells ± s.e.m. (n=5). b, Dd-IL-15tg and WT mice were fed PBS (sham), OVA, RA, or a mixture of OVA and RA. IFN-γ secretion by Lp cells re-stimulated for 24 h with OVA. The results are the means of triplicate samples obtained from two independent experiments. c, CD4+ T cells were cultured with SPL DC isolated from WT or IL-12p40−/− mice as described in a. Intracellular staining for IFN-γ of gated CD4 T cells is shown. Results are representative of two experiments. d, Levels of IL-12p40 in the MLN of WT and Dd-IL-15tg mice fed OVA, or a mixture of OVA and the RAR antagonist LE135. The results are the means of triplicate samples obtained from two independent experiments. Similar results were obtained for IL-12p70 and IL-23 (data not shown). e, IFN-γ secretion by Lp cells isolated from Dd-IL-15tg mice fed PBS (sham), OVA, and LE135. The results are the means of triplicate samples obtained from two independent experiments. f,g, Dd-IL-15tg mice were fed OVA and treated with blocking anti-IL-12p40, anti-IL-15, and TMβ-1 (anti-IL-2Rβ) or isotype control mAbs. The levels of IL-12p40 in the MLN (f) and IFN-γ in Lp cells re-stimulated overnight with OVA (g) were quantified. When anti-IL-15 and anti-IL-12 treatment experiments were performed in parallel, control mice received a mixture of corresponding isotype controls. Data represent two pooled experiments (n=6 mice per group) except for the anti-IL-12 treatment (n=3 individual mice). *P<0.05, ** P <0.01, *** P <0.001 (unpaired Student's t-test).
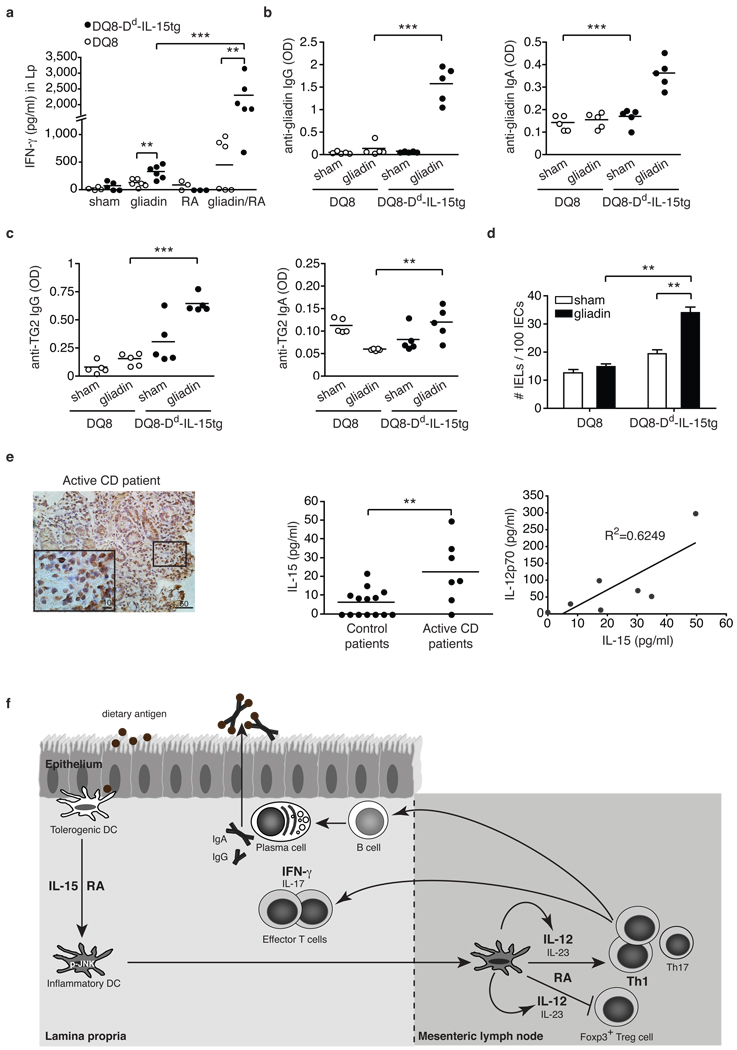
Figure 4. DQ8-Dd-IL-15tg mice fed gliadin mimic early stages of celiac disease reflecting dysregulation in the adaptive immune response to gluten.
a-d, DQ8 and DQ8-Dd-IL-15tg mice were fed gliadin every other day for ten days. a, IFN-γ secretion by Lp cells after overnight culture with gliadin. b, c, Anti-gliadin IgG, anti-gliadin IgA, anti-TG2 IgG and anti-TG2 IgA titers from serum collected fifteen days after feeding. d, Quantification of IEL among intraepithelial cells in small intestines fifteen days after the last feeding. e, IL-15 and IL-12 expression in the Lp of CD patients. Immunohistochemical stainings for IL-15 in gut tissue from an active celiac disease patient (left panel). Lp cells were harvested from biopsies obtained from control (n=14) or active CD patients (n=7) and assayed for levels of IL-15 and IL-12p70 by ELISA (middle and right panels). Equal concentration of total proteins was analyzed for each sample. **P<0.01, ***P<0.001 (unpaired Student's t-test). f, Proposed model for the co-adjuvant effects of RA and IL-15 in the intestinal mucosa. Under inflammatory conditions, the expression of the pro-inflammatory cytokine IL-15 is upregulated in the Lp of the small intestine. Through the synergistic action of IL-15 and RA, DC acquire the ability to release inflammatory cytokines, particularly IL-12 and IL-23. These inflammatory mediators then act in concert with RA to prevent the induction of Foxp3+ Treg cells and drive TH1 and potentially TH17 polarization. In turn, inflammatory T cells may provide help to B cells to produce specific IgG and IgA antibodies.
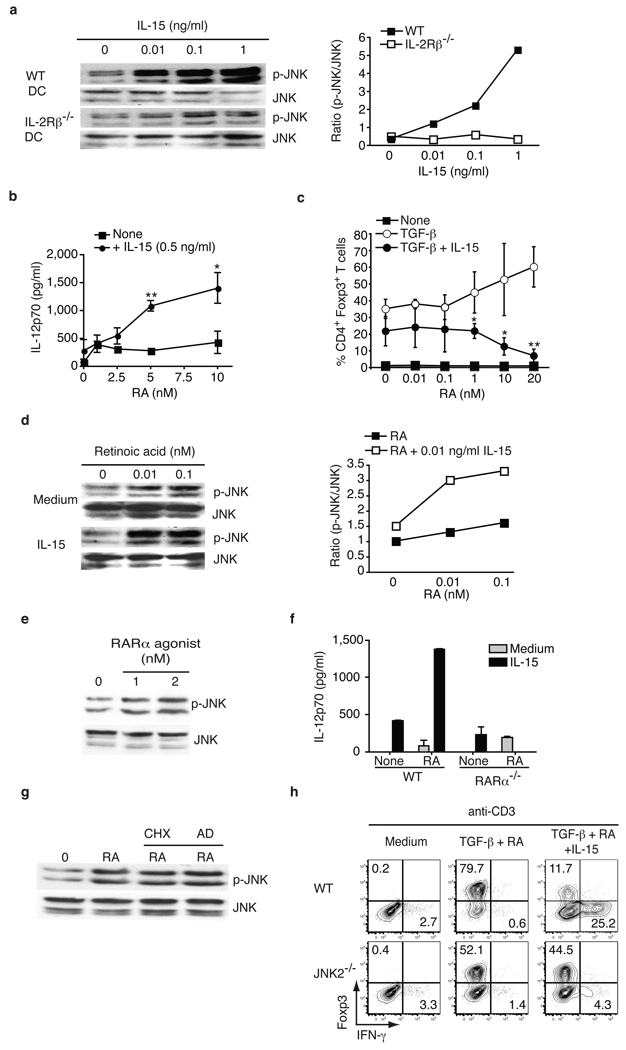
Based on our observations suggesting that IL-15 and RA act primarily at the level of DC to disrupt intestinal immune homeostasis, we investigated which signaling pathway was critical for their proinflammatory effects. We found that IL-15 rapidly induced JNK phosphorylation in a dose-dependent manner in DC (Fig. 3a). The ability of RA to synergize with IL-15 to promote IL-12p70 (Fig. 3b) and IL-23 (Fig. S8a) production by DC and significantly reduce iTreg differentiation (Fig. 3c) was paralleled by its ability to synergize with IL-15 to promote JNK phosphorylation (Fig. 3d). We then showed that RARα through which RA was shown to enhance conversion of iTreg17, was also mediating its proinflammatory effects. (Fig. 3e, Fig. S8b, Fig. 3f, and Fig. S8c). We cannot however definitively exclude the possibility that RA may signal in vivo via alternative RAR such as RARβ. In accordance with the rapid JNK phosphorylation kinetic (Fig. 3d and data not shown), RA-mediated JNK phosphorylation did not require de novo transcription and translation (Fig. 3g). Finally, using JNK2−/− SPL DC, we provided evidence that JNK plays a critical role in the co-adjuvant effects of IL-15 and RA (Fig. 3h and Fig. S8d). In contrast, extracellular signal-regulated kinase (ERK) and P38 MAPK were dispensable (Fig. S8e). Collectively, these results suggest that the adjuvant effects of RA are mediated via the RARα/JNK signaling pathway that may involve previously reported non-genomic signaling effects of RAR18.
Figure 3. Retinoic acid and IL-15 act in synergy to induce DC with proinflammatory properties in a JNK-dependent manner.
a, Concentration-dependent JNK phosphorylation in WT or IL-2Rβ−/− BMDC upon IL-15 stimulation analyzed by western blot (left panel) and quantified (right panel). b, IL-12p70 secretion after overnight culture of WT SPL DC with increasing doses of RA, with and without IL-15. Results are mean values ± s.e.m. (n=3). c, CD4+ Foxp3− T cells were cultured with WT SPL DC with anti-CD3 alone or combined with IL-15, TGF-β and increasing doses of RA. The percentages of Foxp3+ cells are shown. Graph depicts pooled data ± s.e.m (n=3). d, Concentration-dependent JNK phosphorylation in WT BMDC upon IL-15 (0.01 ng/ml) and increasing doses of RA stimulation by western blot (left panel) and quantified (right panel). e, Concentration-dependent JNK phosphorylation in WT BMDC upon stimulation with a RARα agonist (AM580). f, IL-12p70 secretion after overnight culture of WT and RARα−/− BMDC with IL-15 alone or combined with RA. Data are shown as means and s.e.m. (n=2). g, JNK phosphorylation in BMDC pretreated with cyclohexamide (CHX) or actinomycin D (AD) prior to stimulation with 0.1 nM RA. h, CD4+ Foxp3− T cells were cultured with SPL DC isolated from WT or JNK2−/− mice with the indicated cytokines. The percentages of Foxp3+ and IFN-γ+ among CD4+ T cells are indicated. Results are representative of two independent experiments. *P<0.05, ** P <0.01, (unpaired Student's t-test).
An intriguing aspect of CD pathogenesis is how an inflammatory TH1 response is induced against dietary gluten proteins. Due to the large number of proline residues they contain, gluten proteins are resistant to enzymatic degradation, leading to the generation of long peptides that are selectively presented in the gut by HLA-DQ2 or HLA-DQ8 molecules3. However, these observations alone fail to explain why 40% of the population expresses the CD-associated HLA-DQ2 and HLA-DQ8 molecules, yet induction of gluten-specific inflammatory T cell responses occurs in less than 2% of these individuals3. Our findings with the model antigen OVA (Fig. 2, Fig. S6, and Fig. S7) led us to hypothesize that dysregulated IL-15 expression in the Lp of CD patients9 (Fig. 4e) may support the development of TH1 immunity to dietary gluten.
To test this hypothesis, Dd-IL-15tg mice that have levels of IL-15 in the Lp comparable to those observed in the Lp of CD patients9 (compare Fig. S3 and Fig. 4e), were crossed onto humanized HLA-DQ8tg mice19 (DQ8-Dd-IL-15tg). As observed in CD patients20,21, anti-gluten CD4+ and CD8+ IFN-γ-producing T cells were induced in the MLN (Fig. S9a and Fig. S10) and Lp (Fig. 4a) of gliadin-fed DQ8-Dd-IL-15tg mice. Furthermore, this induction was dependent on the presence of RA (Fig. S11). The slight induction in IFN-γ producing T cells in DQ8tg mice fed gluten may be related to the reported innate effects of gluten3. In accordance with human studies22,23, IL-17-producing T cells were detected (Fig. S9b, S9c, S10c) only at very low frequency (compare Fig. S10b to Fig. S10c). As observed with OVA, feeding RA further enhanced inflammatory TH1 and TH17 responses to dietary gluten in the MLN (Fig. S9a and Fig. S9b) and the Lp (Fig. 4a and Fig. S9c). Patients with CD typically develop antibodies to gluten and to the enzyme tissue transglutaminase 2 (TG2) that binds and deamidates gluten in the intestine3,24. Strikingly, IgG and, to a lesser degree, IgA anti-gliadin and TG2 antibodies were significantly induced in DQ8-Dd-IL-15tg mice in the absence of exogenous adjuvant or systemic immunization as compared to DQ8tg mice (Fig. 4b and 4c). Finally, as seen in CD patients3, the presence of inflammatory anti-gluten T cell responses was associated with an increase in the number of intraepithelial lymphocytes (IEL) (Fig. 4d). However, no villous atrophy was observed and IEL failed to upregulate granzyme and activating NK receptors (data not shown). These findings are in agreement with the lack of IL-15 upregulation on IEC of Dd-IL-15tg mice (Fig. S3a), and this mirrors observations in humans that IEL require expression of IL-15 by IEC to become fully licensed killer cells3,10.
In further support of the hypothesis that IL-15 may disrupt tolerance to gluten in CD patients by inducing IL-12, we found that the levels of IL-15 and IL-12p70 were correlated (Fig. 4e). This discovery also gives a functional foundation to the identification of IL-12A as a genetic risk factor for CD by genome-wide association studies25. Taken together, the presence of IFN-γ-producing anti-gliadin T cells, anti-gliadin and anti-TG2 antibodies, and intraepithelial lymphocytosis in the absence of villous atrophy phenocopies an early stage of CD as defined in the modified Marsh 1 classification26, reflecting a selective dysregulation in the adaptive immune response to gluten. How adaptive anti-gluten immunity, epithelial distress and licensing of IEL to become killer cells are interrelated and contribute factors to the development of villous atrophy remains to be addressed.
From the perspective of mucosal immunity, our study reveals that in the presence of IL-15, RA has unforeseen co-adjuvant properties that induce TH1 immunity to fed antigens (Fig. 4f). It further suggests that under infectious conditions associated with induction of IL-15 and IL-6 in the intestinal mucosa, RA will also promote TH17 immunity. These observations caution against the use of vitamin A and RA for the treatment of autoimmunity and inflammatory intestinal disorders associated with high levels of IL-15. Indeed, a causal relationship between retinoids used for the treatment of acne and inflammatory bowel disease was suggested in a subset of patients27. Conversely, these findings provide an explanation as to why children suffering from vitamin A deficiency in developing countries28 respond less efficiently to oral vaccines than children from developed countries7,28, and also suggests that engineering mucosal vaccines that induce IL-15 may be beneficial due to their ability to induce concomitantly protective IgA antibodies and TH1 immunity.
More generally, our study supports the concept that there are no “unconditional” suppressive factors, and that integration of tissue and exogenous signals determine the class of the immune response, which ultimately needs to be tailored to the tissue and the antigen. In line with the idea that the same proinflammatory factors trigger different immunological outcomes depending on the tissue where they are induced, we found that the ability of IL-12 to inhibit iTreg induction was blocked by butyrate, a metabolite produced by commensal bacteria present in the colon but not in the small bowel (data not shown).
One final aspect of our study is that we may have in hand a long-awaited physiopathologically relevant murine model mimicking the early stages of CD. This model is unique in that development of inflammatory anti-gluten immunity develops without microbial adjuvant or systemic immunization in immunologically competent mice with a polyclonal T cell receptor repertoire. However, further studies are warranted to establish whether the anti-TG2 antibodies are gluten dependent. Especially relevant to CD is the identification of IL-15 as a causative factor driving the differentiation of anti-gluten CD4+ and CD8+ TH1 cells in the intestinal mucosa, resulting in the break of tolerance to gluten. Our observations may also explain why oral tolerance is disrupted in patients with inflammatory bowel disease29 who also have dysregulated IL-15 expression in the gut30. They finally suggest that inhibiting IL-15 signaling may constitute a therapeutic intervention to restore mucosal tolerance to luminal antigens.
Method Summary
All knock-out and transgenic mice used in these studies are on the C57BL/6 background. Dd-IL-15tg mice11 expressing IL-15 under the minimal MHC class I Dd promoter were used to perform the studies. To study the response to dietary gluten, Dd-IL-15tg mice were crossed to humanized HLA-DQ8 mice19 that had been backcrossed for twelve generations to MHC class II−/− C57BL/6 mice. To assess iTreg conversion in vivo, RAG−/− OT-II CD25− CD4+ T cells were adoptively transferred into congenic OVA-fed C57BL/6 and Dd-IL-15tg mice. For in vivo experiments mice were fed OVA, α-gliadin, or crude gliadin. Treg differentiation assays were performed using CD4+eGFP− T cells isolated from Foxp3eGFP reporter mice and MLN or SPL DC from IL-15tg or WT mice stimulated with IL-15, TGF-β and RA. To assess the role of IL-2Rβ, JNK2 or IL-12p40 on iTreg and TH1 cell differentiation, DC deficient in these different factors were purified from the corresponding knockout mice. To assess the role of RA, experiments were performed either in presence of the RAR antagonist (LE135), a RARα agonist, or with RARα −/− DC17. JNK phosphorylation was analyzed by western blot following DC stimulation with IL-15 and RA. Lp cells were isolated from intestinal biopsies of control and active CD patients to determine levels of IL-12 and IL-15 expression by ELISA.
Material and Methods
Mice
C57BL/6 WT, IL-12p40−/−, and IL-2Rβ−/− mice were purchased from Jackson Laboratory (Bar Harbor, ME). RAG−/−-OT-II mice were bred and housed in our animal facility. Foxp3eGFP reporter mice (Foxp3egfp) were previously described31. JNK2−/− mice were kindly provided by A. Lin (University of Chicago). Dd-IL-15tg11 were generously donated by Dr. Michael Caligiuri and crossed onto Ly5.1+ C57BL/6 mice or crossed onto HLA-DQ8 mice19 which were previously bred on a MHC Class II−/− C57BL/6 background. All mice were kept under specific pathogen free conditions at the animal facility of the University of Chicago. All mice were maintained on standard rodent chow, however DQ8 and DQ8-Dd-IL-15tg were maintained on gluten-free chow obtained from Research Diets Inc. (AIN-76A). All experiments were performed in accordance with the Institutional Biosafety Committee and the Institutional Care and Use Committee.
T cell and DC purification
For CD4+eGFP− T cell isolation, spleens and peripheral lymph nodes were mechanically disrupted through a 70 µm cell strainer. CD4+ cells were isolated by positive immunoselection using CD4-(L3T4) microbeads (Miltenyi Biotec). Purified CD4 T cells were sorted for GFP expression using BD FacsAria (BD Bioscience). In some experiments, purified CD4+ CD44loGFP− T cells were sorted using the FacsAria (BD Bioscience).
For DC isolation, MLN and spleen were digested with 400 units/ml collagenase type IV (Sigma-Aldrich, St. Louis, MO). Cells were filtered, resuspended in 22.5% Optiprep (Sigma-Aldrich), overlaid with HBS and centrifuged at 2000 rpm for 30 min. DC were then enriched from the interface by positive immunomagnetic selection using anti-CD11c-coated beads according to the manufacturer’s recommendations (Miltenyi Biotec). Purification yielded up to 90% CD11c+ cells.
In vitro Treg cell differentiation assay
For DC-free cultures, 2×105 CD4+eGFP− T cells were cultured for three days with 1µg/ml plate-bound anti-CD3ε(eBioscience, San Diego, CA) and 2 µg/ml anti-CD28 (eBioscience). For cultures containing DC, 1×105 CD4+eGFP− T cells and 4×104 purified SPL or MLN DC were cultured for three days with 1 µg/ml plate-bound anti-CD3ε (eBioscience). Specified recombinant cytokines (listed below) or neutralizing antibodies were added to the cultures.
Recombinant cytokines used were: TGF-β (2 µg/ml), IL-15 (20 ng/ml), IL-12p70 (250 pg/ml-2 ng/ml), IL-23 (250 pg/ml-2 ng/ml) (R&D). Neutralizing IL-12p40 antibody (1 µg/ml) (R&D). Retinoic acid (10 nM) (Sigma-Aldrich), Retinoic acid receptor antagonist, LE135, (1 µM ) (Tocris Bioscience, Ellisville, MO).
TH17 polarization
TH17 differentiation was performed as previously described32. Briefly, purified CD4+ T cells were stimulated with plate bound anti-CD3ε (1 µg/ml) and anti-CD28 (2 µg/ml) in the presence of 2 ng/ml TGF-β, 20 ng/ml IL-6 in the presence or absence of 10 nM RA and 20 ng/ml IL-15.
Bone marrow derived DC culture
Culture of BMDC were performed as previously described33. 105 BMDC from RARα−/− or WT mice were cultured overnight with IL-15 (20 ng/ml) and RA (10 nM) or IL-15 (20 ng/ml) and RA-receptor-α agonist AM580 (10 nM) (Tocris).
Antibodies and Flow cytometry
The following conjugated antibodies were purchased from eBioscience (San Diego): CD4 (GK1.5), CD11c (N418), CD8β (eBioH35-17.2), TCRβ (H57-597), CD45.1 (A20), CD45.2 (104), IFN-γ (XMG1.2), IL-17 (eBio17B7), Foxp3 (FJK-16a), and isotype controls. The following antibodies were purchased from BD Biosciences: CD44 (IM7), H-2Db (KH95), H-2Dd (34-2-12), H-2Kb (AF6-88.5), CD45 (30-F11), CD13 (R3-242), and isotype controls. Cells were permeabilized with the CytoFix/CytoPerm kit (BD Biosciences) for intra-cytoplasmic detection of IFN-γ and IL-17 mAb. Foxp3 fixation/permeabilization kit was used for intranuclear detection of Foxp3 (eBioscience). Flow cytometry analysis was performed with a FACsCanto (BD Biosciences).
Preparation of conditioned media and stimulation of T cell cultures
105 splenic CD11c+ cells were stimulated with 10 nM RA, 1 µM LE135 and/or 20 ng/ml rIL-15 and cultured for 24 h. 150 µl of the supernatants were added to 2×105 CD4+foxp3eGFP- T cells and differentiated on anti-CD3ε and anti-CD28 coated plates for 72 h as described above.
IEL and Lamina propria lymphocytes isolation
IEL34 and Lp35 were isolated as previously described using EDTA containing calcium-free media and collagenase VIII, respectively.
Cell signaling
Wild type or IL-2Rβ−/− DC were serum deprived overnight prior to stimulation with indicated doses of IL-15 or IL-15 and RA for 15 min. Preparation of cells and protein was performed as previously described33. Prior to stimulation with IL-15, BMDC were pre-incubated for 30 min with various concentrations of MAP kinase inhibitors specific for MEK1/2 (PD98059), JNK (SP600125) and P38 (SB203580). In some experiments BMDC were pre-incubated with cyclohexamide (10 µg/ml) (Sigma) or actinomycin D (10 µg/ml) (Sigma) for 15 min prior to stimulation with RA (0.1 ng/ml). Cells treated with DMSO were used as control for cells treated with MAP kinase inhibitors, cyclohexamide and actinomycin D.
T cell transfers
CD4+ CD25− T cells were purified from the spleen of RAG−/−-OT-II Ly5.1 or RAG−/−-OTII Ly5.2 mice. 5×105 cells were then transferred intravenously into naïve Ly5.1 or Ly5.2 C57BL/6 and Dd-IL-15tg mice. One day after transfer the mice received OVA dissolved in drinking water for five days or were administered 100 µg OVA every day for ten days by gavage as indicated in the figure legends. Mice were sacrificed one day after the last feeding and intranuclear levels of Foxp3 were evaluated by flow cytometry in the transferred T cells.
Antigen feeding and immunization
Mice were fed by intragastric gavage using an 18-gauge round-tipped needle (Kent Scientific, Torrington CT) with the following proteins: 100 µg ovalbumin (OVA) (Sigma Aldrich), 100 µg recombinant α-gliadin36 dissolved in water, or 20 mg crude gliadin (Sigma-Aldrich). In some experiments, mice were also fed with LE135 (1 µM) or RA (1 µM) resuspended in corn oil. Feeding occurred every other day for ten days. In some experiments, Dd-IL-15tg mice were injected i.p. with 100 µg of anti-IL-12p40 (R&D systems, clone C17.8, rat IgG2a) at the time of feedings, or with 20 µg of anti-mouse IL-15 antibody (M96, Amgen, mouse IgG2a) once a week before and during feeding, or 200 µg of a purified anti-mouse CD122 antibody (clone TM-β1, rat IgG2b) twice a week before and during feeding.
One day following the last feeding, Lp and MLN cells were isolated and re-stimulated for 24 h or 48 h, respectively with 50 µg/ml OVA or gliadin as noted in the figure legend. Supernatants were analyzed for cytokines by ELISA.
Detection of cytokines by ELISA
Cell supernatants were evaluated for IL-23 (R&D), IL-12p70 (BD Biosciences), IL-6, (BD Biosciences), IFN-γ (BD Biosciences) and IL-17 (R&D). Tissue pellets from epithelium, Lp and MLN were quantified for IL-15 (eBioscience).
Anti-TG2 and anti-gluten ELISA
Serum was harvested 15 days after mice received last gluten feeding. ELISA assays were performed as previously described37.
Patients and controls
Seven patients (age: 3–46 years) with active CD were investigated. Diagnosis of CD was based on the detection of anti-transglutaminase antibodies, the expression of HLA DQ2 or DQ8, villous atrophy, and clinical and histological response to gluten-free diet. Fourteen individuals (age: 1–55 years) undergoing endoscopies and biopsies for functional intestinal disorders of non-celiac origin were studied as controls. Lp cells were isolated from biopsies or surgical specimens as previously described23. All subjects gave written informed consent, and research was approved by the institutional review boards. Lp cells were centrifuged and resuspended in 500 ml PBS. Protein concentration was determined and total protein was analyzed for levels of IL-15 (eBioscience) and IL-12p70 (BD Biosciences).
Histology
Hematoxylin & Eosin staining was performed on 5µM 10% formalin-fixed paraffin embedded intestinal sections. Slides were analyzed under a Leica DM 2500 microscope with a HC PLAN APO 20×/0.7 NA and a HCX PL APO 100×/1.40-0.70 objectives.
Supplementary Material
Acknowledgements
We thank B. Sally, L.M. Sollid and M. Musch for critical reading of the manuscript. We thank C. Ciszewski, B. Uzunparmak, and N. Grandison for their help with the collection and analysis of human biopsies. We thank Mike Constantinides for technical assistance with mice breeding. We also thank the University of Chicago flow cytometry facility for technical assistance. Dd-IL-15tg mice were a generous gift from Dr. M. Caligiuri. RARα-deficient mice were kindly provided by Drs. P. Chambon and C. Benoist. This work was supported by the Digestive Disease Research Core Center at the University of Chicago (DK42086), R01 DK67180, and Crohn's and Colitis Foundation (for VA).
Abbreviations used
- CD
celiac disease
- DC
dendritic cells
- GALT
gut-associated lymphoid tissue
- IEC
intestinal epithelial cells
- IEL
intraepithelial cytotoxic T lymphocytes
- IL-15
interleukin-15
- iTreg
inducible regulatory T cells
- Lp
lamina propria
- MLN
mesenteric lymph node
- RA
retinoic acid
- RAR
retinoic-acid receptor
- SPL
splenic
- tg
transgenic
- TH
T helper
- WT
wild-type
Footnotes
Supplementary information is linked to online version of the paper at www.nature.com/nature
Author contributions
RWD and VA provided input into the conceptual development and execution of the studies, as well as preparation of the manuscript. FT, HFP, JAH, and WW provided outstanding technical assistance and input into data analyses. JAM and EVM helped with the analysis of the humanized HLA-DQ8 transgenic mice. DDK provided preparations of alpha-gliadin used in the feeding experiments, TAW provided TMβ-1 antibody, and YB helped with the realization of T cell transfer experiments and provided us with RARα-deficient bone-marrow. CS, SK, and SG followed patients with celiac disease and provided intestinal biopsies for cytokines analysis. YB, JAM, DDK, TAW participated in discussion and review of the manuscript. BJ conceived the idea, wrote the manuscript, and supervised all investigations.
Statistics
Statistical analyses were performed using unpaired Student t-test.
References
- 1.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jabri B, Sollid LM. Tissue-mediated control of immunopathology in coeliac disease. Nat Rev Immunol. 2009;9(12):858. doi: 10.1038/nri2670. [DOI] [PubMed] [Google Scholar]
- 4.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008 doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204(8):1757. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 7.Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11(4 Suppl):S45. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- 8.Tagaya Y, Bamford RN, DeFilippis AP, Waldmann TA. IL-15: a pleiotropic cytokine with diverse receptor/signaling pathways whose expression is controlled at multiple levels. Immunity. 1996;4(4):329. doi: 10.1016/s1074-7613(00)80246-0. [DOI] [PubMed] [Google Scholar]
- 9.Mention JJ, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125(3):730. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- 10.Meresse B, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21(3):357. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 11.Fehniger TA, et al. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol Dis. 2001;27(1):223. doi: 10.1006/bcmd.2001.0379. [DOI] [PubMed] [Google Scholar]
- 12.Caretto D, et al. Cutting edge: the Th1 response inhibits the generation of peripheral regulatory T cells. J Immunol. 2010;184(1):30. doi: 10.4049/jimmunol.0903412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokoyama S, et al. Antibody-mediated blockade of IL-15 reverses the autoimmune intestinal damage in transgenic mice that overexpress IL-15 in enterocytes. Proc Natl Acad Sci U S A. 2009;106(37):15849. doi: 10.1073/pnas.0908834106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 15.Gao Y, Camacho LH, Mehta K. Retinoic acid-induced CD38 antigen promotes leukemia cells attachment and interferon-gamma/interleukin-1beta-dependent apoptosis of endothelial cells: implications in the etiology of retinoic acid syndrome. Leuk Res. 2007;31(4):455. doi: 10.1016/j.leukres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Mohty M, et al. All-trans retinoic acid skews monocyte differentiation into interleukin-12-secreting dendritic-like cells. Br J Haematol. 2003;122(5):829. doi: 10.1046/j.1365-2141.2003.04489.x. [DOI] [PubMed] [Google Scholar]
- 17.Hill JA, et al. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29(5):758. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rochette-Egly C, Germain P. Dynamic and combinatorial control of gene expression by nuclear retinoic acid receptors (RARs) Nucl Recept Signal. 2009;7:e005. doi: 10.1621/nrs.07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Black KE, Murray JA, David CS. HLA-DQ determines the response to exogenous wheat proteins: a model of gluten sensitivity in transgenic knockout mice. J Immunol. 2002;169(10):5595. doi: 10.4049/jimmunol.169.10.5595. [DOI] [PubMed] [Google Scholar]
- 20.Mazzarella G, et al. Gliadin activates HLA class I-restricted CD8+ T cells in celiac disease intestinal mucosa and induces the enterocyte apoptosis. Gastroenterology. 2008;134(4):1017. doi: 10.1053/j.gastro.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nilsen EM, et al. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon gamma. Gut. 1995;37(6):766. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monteleone I, et al. Characterization of IL-17A-producing cells in celiac disease mucosa. J Immunol. 2010;184(4):2211. doi: 10.4049/jimmunol.0901919. [DOI] [PubMed] [Google Scholar]
- 23.Bodd M, et al. HLA-DQ2-restricted gluten-reactive T cells produce IL-21 but not IL-17 or IL-22. Mucosal Immunol. 2010;3(6):594. doi: 10.1038/mi.2010.36. [DOI] [PubMed] [Google Scholar]
- 24.Dieterich W, et al. Identification of tissue transglutaminase as the autoantigen of celiac disease [see comments] Nat Med. 1997;3(7):797. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 25.Hunt KA, et al. Newly identified genetic risk variants for celiac disease related to the immune response. Nat Genet. 2008;40(4):395. doi: 10.1038/ng.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue') Gastroenterology. 1992;102(1):330. [PubMed] [Google Scholar]
- 27.Reddy D, Siegel CA, Sands BE, Kane S. Possible association between isotretinoin and inflammatory bowel disease. Am J Gastroenterol. 2006;101(7):1569. doi: 10.1111/j.1572-0241.2006.00632.x. [DOI] [PubMed] [Google Scholar]
- 28.Stephensen CB, Livingston KA. Vitamin supplements and vaccines: maximize benefits, evaluate potential risks. Am J Clin Nutr. 2009;90(3):457. doi: 10.3945/ajcn.2009.28343. [DOI] [PubMed] [Google Scholar]
- 29.Kraus TA, et al. Failure to induce oral tolerance to a soluble protein in patients with inflammatory bowel disease. Gastroenterology. 2004;126(7):1771. doi: 10.1053/j.gastro.2004.03.076. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, et al. IL-15 is highly expressed in inflammatory bowel disease and regulates local T cell-dependent cytokine production. J Immunol. 2000;164(7):3608. doi: 10.4049/jimmunol.164.7.3608. [DOI] [PubMed] [Google Scholar]
- 31.Fontenot JD, et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24(2):179. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 33.Depaolo RW, et al. Toll-like receptor 6 drives differentiation of tolerogenic dendritic cells and contributes to LcrV-mediated plague pathogenesis. Cell Host Microbe. 2008;4(4):350. doi: 10.1016/j.chom.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park SH, et al. Selection and expansion of CD8alpha/alpha(1) T cell receptor alpha/beta(1) intestinal intraepithelial lymphocytes in the absence of both classical major histocompatibility complex class I and nonclassical CD1 molecules. J Exp Med. 1999;190(6):885. doi: 10.1084/jem.190.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lefrancois L, Lycke N. Isolation of mouse small intestinal intraepithelial lymphocytes, Peyer's patch, and lamina propria cells. Curr Protoc Immunol. 2001:19. doi: 10.1002/0471142735.im0319s17. Chapter 3, Unit 3. [DOI] [PubMed] [Google Scholar]
- 36.Bernardin JE, Kasarda DD, Mecham DK. Preparation and characterization of alpha-gliadin. J Biol Chem. 1967;242(3):445. [PubMed] [Google Scholar]
- 37.Marietta E, et al. A new model for dermatitis herpetiformis that uses HLA-DQ8 transgenic NOD mice. J Clin Invest. 2004;114(8):1090. doi: 10.1172/JCI21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.