Abstract
Microglia are critical cells in mediating the pathophysiology of neurodegenerative disorders such as HIV-associated neurocognitive disorders. We hypothesize that HIV-1 glycoprotein 120 (gp120) activates microglia by enhancing outward K+ currents, resulting in microglia secretion of neurotoxins and consequent neuronal dysfunction and death. To test this hypothesis, we studied the effects of gp120 on outward K+ current in cultured rat microglia. Application of gp120 enhanced outward K+ current in a dose-dependent manner, which was blocked by voltage-gated K+ (Kv) channel blockers. Western blot analysis revealed that gp120 produced an elevated expression of Kv channel proteins. Examination of activation and inactivation of outward K+ currents showed that gp120 shifted membrane potentials for activation and steady-state inactivation. The gp120-associated enhancement of outward K+ current was blocked by a CXCR4 receptor antagonist T140 or by a specific protein kinase A (PKA) inhibitor H89, suggesting the involvement of chemokine receptor CXCR4 and PKA in gp120-mediated enhancement of outward K+ current. Biological significance of gp120-induced enhancement of microglia outward K+ current was demonstrated by experimental results showing the neurotoxic activity of gp120-stimulated microglia, evaluated by TUNEL staining and MTT assay, was significantly attenuated by Kv channel blockers. Taken together, these results suggest that gp120 induces microglia neurotoxic activity by enhancing microglia outward K+ current and that microglia Kv channels may function as a potential target for the development of therapeutic strategies.
Keywords: Chemokine receptors, Voltage-gated K+ channel, Neuronal apoptosis, Neurodegeneration
Introduction
Microglia represent a population of resident immune cells in the brain. They are morphologically, immunophenotypically and functionally related to cells of the monocyte/macrophage lineage and play an important role as resident immunocompetent phagocytic cells in the pathogenesis of infectious, inflammatory and degenerative brain diseases. Upon challenging, microglia react by withdrawing their processes, becoming ameboid and macrophage-like and undergo dramatic phenotypic, immunochemical, and functional changes, collectively referred to as “activation”. The switch from resting to activation is characterized by an alteration of functional state. Resting microglia secrete neurotrophic factors, such as NGF, to support neuronal function and survival (Elkabes et al. 1998; Miwa et al. 1997). In contrast, the activated microglia produce reactive oxygen and nitrogen species and pro-inflammatory cytokines and chemokines with potential toxicity to neurons. Moreover, microglia express numerous chemokine receptors which are involved in cell migration and serve as co-receptors for HIV-1 infection. Indeed, microglia are the predominant resident CNS cell type productively infected by HIV-1 (Lipton and Gendelman 1995). Due to poor penetration of antiviral drugs through blood-brain barrier, resident microglia (and brain macrophages) constitute a cellular reservoir of HIV-1 in the brain and a source of potential neurotoxic products (Gendelman et al. 1997; Genis et al. 1992; Koenig et al. 1986). Studies have shown that HIV-1-infected and immune-activated microglia (and brain macrophages) release a number of soluble substances including, but not limited to, pro-inflammatory cytokines, chemokines, excitatory amino acids, nitric oxide and reactive oxygen species, as well as viral proteins, which can injure or kill neurons, contributing to the pathogenesis of HIV-1-associated neurocognitive disorders (HAND)(Garden 2002; Kaul et al. 2001; Kielian 2004). As such, it is imperative to identify potential target(s) for the development of therapeutic strategies to control microglia activation and/or suppress their subsequent neurotoxin production.
Increasing evidence indicates that voltage-gated K+ (Kv) channels play a pivotal role in the process of microglia activation (Farber and Kettenmann 2005; Walz and Bekar 2001). Non-activated microglia express little, if any, Kv channels whereas large outward K+ current has been observed in activated microglia (Farber and Kettenmann 2005). Exposure to a variety of activating stimuli produces a similar pattern of electrophysiological changes in microglia. For example, lipopolysaccharide (LPS), macrophage colony-stimulating factor or interferon-γ enhances outward K+ current in microglia (Eder et al. 1995; Fischer et al. 1995; Norenberg et al. 1994). These outward K+ currents, which share many properties with cloned Kv1.3 channels, including an activation threshold at about −40mV, strongly use-dependent inactivation and a high sensitivity to 4-aminopyridine (4-AP), agitoxin, margatoxin and charybdotoxin (Eder et al. 1995; Fordyce et al. 2005; Norenberg et al. 1994), are predominantly recorded in activated microglia (Kotecha and Schlichter 1999; Menteyne et al. 2009), suggesting a role for Kv channels in regulating microglia activation and cytotoxin production. It has also been shown that Kv channels expressed by activated microglia regulate their proliferation and migration (Kotecha and Schlichter 1999; Pannasch et al. 2006). Indeed, activated microglia injure neurons and blockade of microglia Kv channels inhibits microglia-induced neurotoxicity (Fordyce et al. 2005). These findings stimulate our hypothesis that HIV-1 infection activates microglia by enhancing outward K+ currents, resulting in microglia production of neurotoxins and consequent neuronal dysfunction and injury. In the present study, we tested our hypothesis by exploring the effects of HIV-1 glycoprotein 120 (gp120) on outward K+ current recorded in cultured rat microglia. Our results showed that gp120 enhanced microglia outward K+ current via CXCR4 and cAMP-dependent PKA signaling pathway, leading to microglia production of neurotoxins and resultant neuronal apoptosis.
Materials and Methods
Materials
HIV-1gp120 IIIB was purchased from Immunodiagnostics, Inc. (Woburn, MA). Aliquots of gp120 were kept as 100nM stock solution at −80°C. The stock solution was diluted to desired concentrations with artificial cerebrospinal fluid (ACSF) 2-5 min before test. T140 was kindly provided by Professor Nobutaka Fujii (Kyoto, Japan). All chemicals, unless otherwise specified, were from Sigma (St. Louis, MO).
Isolation and culture of microglia and cortical neurons
Microglia and cortical neuronal cultures were obtained from the cerebral cortices of 1–2d old or embryonic (E18) Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA). Briefly, the pups were anesthetized hypothermically and decapitated, and cerebral cortices were dissected out. The cortical tissues were enzymatically digested followed by a mechanical dissociation. The mixed primary cultures were grown on 75cm2 flasks in 30ml (106 cells/ml) DMEM supplemented with 10% FBS, 2mM glutamine, and 1% PEN/Strep (37°C, 5%CO2). After 7-10d in culture, microglial cells were harvested by gentle shaking, and then plated on uncoated 35mm plastic Petri dishes at a density of 0.5×106 cells per dish. Non-adhering cells were removed 30min after plating by changing the medium. The purity of resulting culture was judged by staining with OX-42 antibody (a marker for the microglia CR3/CD11b receptor). Cells were utilized for whole-cell recording 2 days after plating. In all cases, the culture medium was replaced with fresh ACSF in the experimental day. Experiments were conducted 1-2h after treatment with the reagents. Controls were performed in untreated and age-matched microglial cultures. For cortical neuronal cultures, the cells were plated in poly-D-lysine coated 24 well plates containing 1ml of medium, with a cell density of 1.0×105/ml. The cultures were maintained in neurobasal medium supplemented with 1% penicillin/streptomycin, B27(2%, v/v, Invitrogen, San Diego, CA), and L-glutamine (0.5mM) for at least 7-10 days before being used for experiments. All animal use procedures were strictly reviewed by the Institutional Animal Care and Use Committee (IACUC) of the University of Nebraska Medical Center (IACUC No. 00-062-07).
Electrophysiology
Whole-cell outward K+ currents were recorded from cultured microglia using an Axopatch 200B amplifier. After establishment of the whole-cell configuration, the cells were allowed to stabilize for 3-5min before recording. The recorded cells were voltage-clamped at −60mV and whole-cell outward K+ current was induced by voltage steps from the holding potential of −60mV to −40mV in the first step and then stepped to +60mV in increments of 10mV. The ACSF contained (in mM): 140 NaCl, 5 KCl, 2.0 CoCl2, 1 MgCl2, 10 D-glucose, 10 HEPES (pH 7.4 adjusted with NaOH, osmolarity: 310 mOsm). The recording electrodes were made from borosilcate glass capillaries and had resistance of 5–7.5MΩ when filled with an intracellular solution contained (in mM): 135 K-gluconate, 10 KCl, 1 CaCl2, 1 MgCl2, 10 EGTA, 0.5 Tris-GTP, 2 Mg-ATP,10 HEPES ( Adjusted pH to 7.3 with KOH, osmolarity: 300 mOsm). The seal resistance was 1–10GΩ. Junction potentials were corrected and the cell capacitance was compensated (~70%) in most cells. Current signals were filtered at 1kHz and digitized at 5kHz using a Digidata 1440A digitizer. The current and voltage traces were displayed and recorded in a Dell computer using pCLAMP 10 data acquisition/analysis system.
The activation was studied by measuring the peak K+ conductance (G) during a 700ms test pulse to various test potentials from a holding potential of −60mV. G was calculated from G = Ipeak/V, where Ipeak is the peak outward K+ current during the test potential (V). Data were normalized to maximum peak conductance (Gmax) and fitted to Boltzmann equation: G/Gmax= 1/[1+exp(V−V1/2)/k], where V1/2 is the voltage for half maximal activation, and k is the slope constant (mV). To study steady-state inactivation, cells were held at prepulse potentials ranging from −80 to +10mV for 60s and then subject to a +20mV test pulse for 200ms. Normalized steady-state currents were plotted versus prepulse potentials, and the curves were fitted by the Boltzmann function: I/Imax=1/[1+exp(Vpp−V1/2)/k], where Imax is the maximum current, V1/2 is the voltage for half maximal activation, and Vpp is the voltage of the prepulse potential.
Immunocytochemistry
Immunocytochemistry was performed to substantiate the capacity of gp120 enhancing expression of microglia Kv channel, particularly Kv1.3. Microglia were platted on poly-D-lysine coated coverslips at a density of 0.5×106 cells per well in 24 well plates. Twenty-four h later, microglia were activated by gp120 with or without tetraethylammonium (TEA, 5mM) or 4-AP (1mM). After another 24h, the microglia were washed in PBS 3 times, fixed with 4% paraformaldehyde (PFA) for 30min at room temperature, and after washing, blocked and permeabilized in PBS containing10% normal goat serum, 0.2% Triton X-100 and 0.1M glycine for 30min. Primary antibodies including rat polyclonal antibody Mac-1 (CD11b; 1/500; Serotec) and rabbit polyclonal antibody to Kv1.3 (Almonade Lab, Israel) were diluted in PBS with 10% goat serum and applied to coverslips for 1h. After washing in PBS (5min, 3 times) Alexa Fluor 488 and Alexa Fluor 594-conjugated secondary antibodies were added for 1 h. Coverslips were mounted on slides with ProLong Gold antifade reagent +DAPI (Molecular Probes), and images were taken with a 40× oil-immersion objective.
Microglia and neuronal co-culture and TUNEL Assay
TUNEL assay was performed using the in situ cell death detection kit, AP (Roche Applied Science, Indianapolis, IN). Rat microglia were seeded on transwell inserts (0.5×106 cells per well) in 24 well plates and left untreated or exposed to LPS (0.5μg/ml, as a positive control), gp120 or gp120 plus 4-AP/TEA/H89/T140. Twenty-four h later, microglia were washed and co-cultured with cortical neurons growing on poly-D-lysine coated coverslips at a density of 1.0×105 cells/well in 24-well plates for 24h. The cortical neurons were then washed in PBS (5 min, 3 times) and fixed with 4% PFA in PBS (pH 7.4) for 1 h at room temperature. After 3 times washing with PBS, neurons were permeabilized with 0.1% Triton X-100 in 0.1% sodium citrate for 2min on ice and then washed in PBS (5 min, 3 times). Then, the neurons were incubated with TUNEL reaction mixture that consists of terminal deoxynucleotide transferase and fluorescein-labeled nucleotides for incorporation into DNA strand breaks at 37°C. After a final wash in PBS (5 min, 3 times), coverslips were mounted in ProLong Gold antifade reagent with DAPI (Molecular Probes, Eugene, OR). Cells were visualized by Zeiss LSM 510 META NLO microscope in a 40× oil-immersion objective. TUNEL positive cells were counted and expressed as percentage of total number of cells counted.
MTT assay
The assay is based on the ability of active mitochondrial dehydrogenase to convert dissolved MTT to water-insoluble purple formazan crystals. Neurons washed in PBS (5min, 3 times) were incubated with fresh neuronal culture media containing MTT (500μg/ml) for 3h. At the end of incubation, the MTT solution was replaced with 500μl of dimethyl sulfoxide (DMSO) for cell lysis. The plate was shaken for 10min to solubilize the formazan crystals, and the optical density (OD) at 570nm was measured.
Statistical Analysis
Experimental data were expressed as mean±SEM. Statistical analyses were performed by ANOVA or Student t tests. A minimum p value of 0.05 was estimated as the significance level for all tests.
Results
1. Expression of Kv channels in microglia
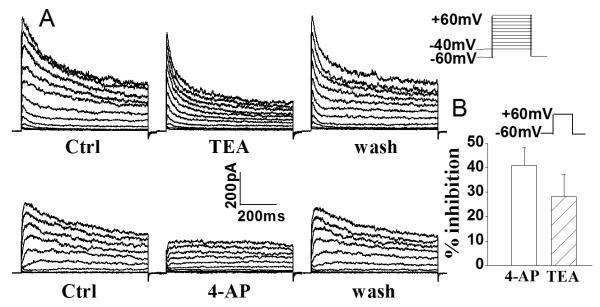
In seeking to determine whether Kv channels regulate microglia activation, we first examined Kv channel expression in rat microglia by recording the whole-cell outward K+ current induced by voltage steps (Fig. 1). In one group of microglia cultures (n=9), the average instantaneous outward K+ current (an A-type-like outward current) density was 79.8±6.3pA/pF and it was reduced to 46.7±5.5pA/pF when 4-AP was added to the bath. In another group of microglial cells (n=9), the average steady-state K+ current was 46.8±10.4pA/pF and it was reduced to 33.9±9.2pA/pF when 5mM TEA was introduced to the bath. Addition of 4-AP or TEA to the bath produced 41.5±7.2% or 27.6±8.9% reduction of outward K+ current, respectively (Fig. 1). To estimate K+ current density, the capacitance of microglial cells was determined and used to obtain an estimate of cell surface area. The average whole-cell capacitance was 12.4±3.0pF, with a range of 5–27.5pF (n=75).
Fig. 1.
Expression of outward K+ current in rat microglia. Panel A shows examples illustrating the voltage-dependent outward K+ current recorded in rat microglia and the partial blockade of outward K+ current by TEA (upper) and 4-AP (lower). Panel B depicts the average inhibition of whole-cell outward K+ current in microglia by 4-AP and TEA when measured at command voltage step of +60mV. Bars represent mean±SEM (the same in the following figures unless indicated). Voltage protocol employed to generate outward K+ current is shown above Panel B.
2. Enhancement of microglia outward K+ current by gp120
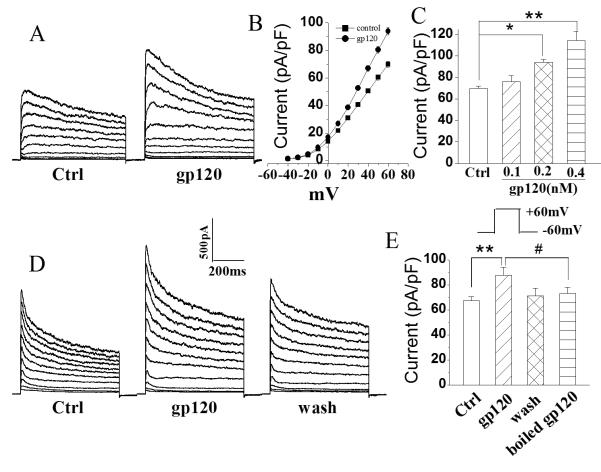
Following confirmation of Kv channel expression in microglia, we tested if gp120 could alter the outward K+ current in microglia. Incubation of microglia with gp120 for 1-2h enhanced whole cell outward K+ current in a dose-dependent manner (Fig. 2). When microglia were treated with gp120 at concentrations of 100, 200, and 400pM, the average instantaneous outward K+ current densities (pA/pF) were 76.1±5.7 (n=27), 94.0±2.6 (n=67) and 114.5±8.4 (n=11), respectively (Fig. 2). In comparison with the average outward K+ current density of 69.8±2.1pA/pF recorded in untreated (control) microglia (n=46), the differences were statistically significant (p<0.05), demonstrating an enhancement of whole cell outward K+ current by gp120 in cultured rat microglia. Incubation of microglia with heat (boiled)-inactivated gp120 (200pM) showed no significant effect on outward K+ current density with an average of 73.3±4.7pA/pF (Fig. 2, n=5), indicating a specific effect of gp120 on enhancing outward K+ current in microglia.
Fig. 2.
gp120 enhances microglia whole-cell outward K+ current in a dose-dependent manner. A. Typical outward K+ currents recorded from a control and a gp120-treated microglia as indicated. B. I-V curves showing gp120 increases outward K+ current. C. gp120 increases outward K+ current in a dose-dependent manner. The graph plots mean outward K+ current densities measured at +60 mV. D. Outward K+ current recorded in microglia before (Ctrl), during (gp120) and after (wash) bath application of gp120 (200pM). E. Average outward K+ current densities recorded in microglia cells as shown in D (n=5) and in another 5 microglia treated with heat (boiled)-inactivated gp120, illustrating gp120 specific enhancement of outward K+ current. *, p<0.05 vs Ctrl; **, p<0.01 vs Ctrl; #, p<0.05 vs boiled gp120.
3. Blockade of gp120-induced enhancement of outward K+ current by T140
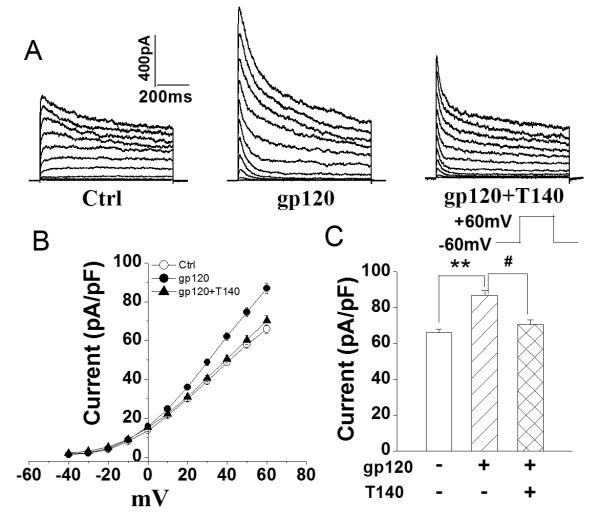
It is well-known that microglia express chemokine receptor CXCR4 (Albright et al. 1999; Lavi et al. 1997). To examine if the gp120-induced enhancement of outward K+ current was mediated through CXCR4 (a co-receptor for HIV-1 infection), we tested the effects of T140, a highly selective CXCR4 receptor antagonist, on gp120-induced enhancement of outward K+ current in another group of cultured microglia. While it per se had no significant effect on outward K+ current when added to the bath, T140 (50nM) significantly blocked gp120-induced enhancement of outward K+ current recorded in microglia. The average instantaneous K+ current densities without (control) and with addition of gp120 (200pM) to the bath solution were 65.9±2.2pA/pF (n=92) and 86.9±2.7pA/pF (n=75), respectively. In contrast, the current density was 70.6±2.5pA/pF (n=64) when both T140 and gp120 were added to the bath. In comparison with the K+ current recorded when gp120 was added alone, the difference was statistically significant (p<0.05), indicating that gp120 increases microglia outward K+ current via CXCR4 (Fig.3A, 3B).
Fig.3.
Blockade of gp120-induced enhancement of microglial outward K+ current by T140, a specific CXCR4 antagonist. A. Typical current traces recorded from control, gp120− and gp120+T140-treated microglia as indicated. B. I-V curves of peak outward K+ currents as shown in A. C. Bar graph showing the mean current densities (measured at +60mV) recorded in control, gp120− and gp120+T140-treated microglia. Note that gp120 enhanced outward K+ current and this enhancement was blocked by T140. ** p<0.01 vs ctrl; # p<0.05 vs gp120+T140.
4. Effect of gp120 on microglia Kv channel biophysical properties
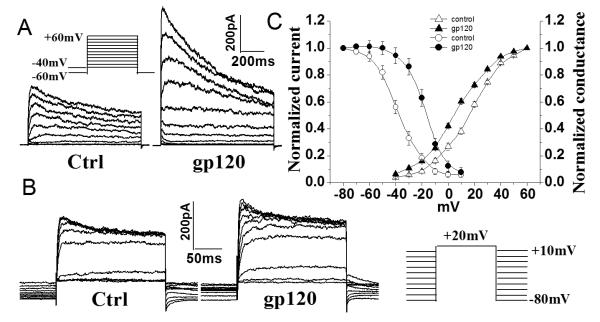
To determine if gp120 alters microglia Kv channel biophysical properties, we examined the influence of gp120 on the properties of microglia Kv channel activation and steady-state inactivation. Fig. 4A illustrates superimposed currents elicited by voltage steps applied in 10 mV increments from the holding potential −60 mV to +60mV. Outward K+ currents were first seen at −40mV in gp120-treated microglia and became larger at stronger depolarizing command voltages. Normalized outward K+ currents (Fig. 4A) were fitted to a Boltzmann equation: G/Gmax= 1/[1 +exp (V−V1/2)/k], from which the voltage for half-maximal activation (V1/2) and the slope factor (k) were calculated (Fig. 4C). Half-maximal activation occurred at 8.3±0.9mV and 12.9±0.9mV, with a slope factor, k, of 16.8±0.9mV and 15.2±0.8mV for gp120-treated and control microglia, respectively (Fig. 4C, n=21). The steady-state voltage dependence of inactivation of K+ currents was generated by varying the holding potential between −80 and +10mV in 10mV increments (Fig. 4B). After the holding potential was established for at least 1 min, cells were pulsed to a test potential of 20mV for 200ms. Steady-state inactivation began at approximately −70mV and was complete at ~0mV. Peak amplitudes of the evoked currents were measured, normalized and then plotted as a function of the holding potential (Fig. 4B, 4C). The region under the intersection of activation and inactivation illustrates a window of tonic channel activity between −35mV and 10mV with maximal activity at the intersection voltage of −10mV. Fitted with a Boltzmann function, half-maximal inactivation was at −15.4±0.7mV and −36.3± 0.8mV, with k=9.1±0.7mV and 10.1±0.7mV (n = 14) in gp120-treated and control microglia, respectively. In all cases, the voltage-dependent currents were highly K+ selective because the reversal potential was very close to the calculated Nernst potential (−85mV, data not shown) for K+ concentrations used in this study (see materials and methods).
Fig. 4.
Effects of gp120 on activation and inactivation of outward K+ currents. A. Activation of outward K+ current was induced using 700ms voltage steps from a holding potential of −60 mV to −40mV at the first step, and then step to +60mV in 10mV increments. To ensure complete recovery from inactivation, successive voltage steps were separated by 5 s. B. Steady-state inactivation was measured by varying the holding potential (−80 to +10mV) for 60s at each voltage, then applying a 200ms test pulse to +20mV. C. Average of activation and inactivation curves. Note that gp120 shifted activation and inactivation to more negative potential and more negative potential, respectively.
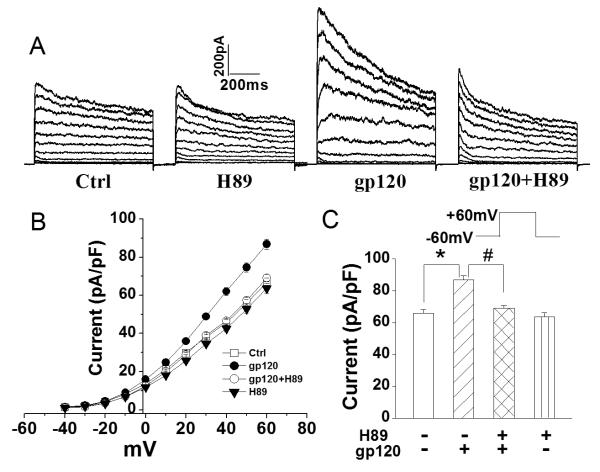
5. Involvement of PKA in gp120-induced increase of outward K+ current
Protein phosphorylation can profoundly influence ion channel activity. Accumulating evidence indicate that Kv channels can be regulated by c-AMP-dependent protein kinase (PKA) (Chung and Schlichter 1997; Fakler et al. 1994). We hypothesize that gp120 increases outward K+ current via CXCR4→PKA→Kv channel pathway. To test this hypothesis, we examined the effects of H89 (400nM) on gp120 (200pM)-induced enhancement of outward K+ current in rat microglia. When applied alone, H89 failed to inhibit outward K+ current (n=28). In contrast, when co-applied with gp120, H89 inhibited the gp120-associated increase of outward K+ current (Fig. 5). The average outward K+ current before (gp120) and after co-application of H89+gp120 were 86.2±2.7pA/pF (n=75) and 69.1±1.8pA/pF (n=42), respectively. The difference was statistically significant (p<0.05), suggesting the involvement of PKA in gp120-induced increase of outward K+ current.
Fig. 5.
Blockade of gp120-induced enhancement of outward K+ currents by H89, a specific inhibitor for PKA. A. Representative current traces recorded from a control cell (Ctrl) and cells treated respectively with H89 alone, gp120 alone and gp120+H89. The voltage protocol used to generate outward K+ current was the same as the one shown in Fig. 1. B. I–V relationship illustrating the outward K+ current densities as a function of voltage from the current traces shown in A. C. Summarized bar graphs showing average outward K+ current densities measured at a voltage step +60mV from microglia treated with H89 alone, gp120 alone and gp120+H89. Note a significant blockade of gp120 enhancement of outward K+ currents recorded in microglia. * p<0.05 vs. ctrl, # p<0.05 vs. gp120.
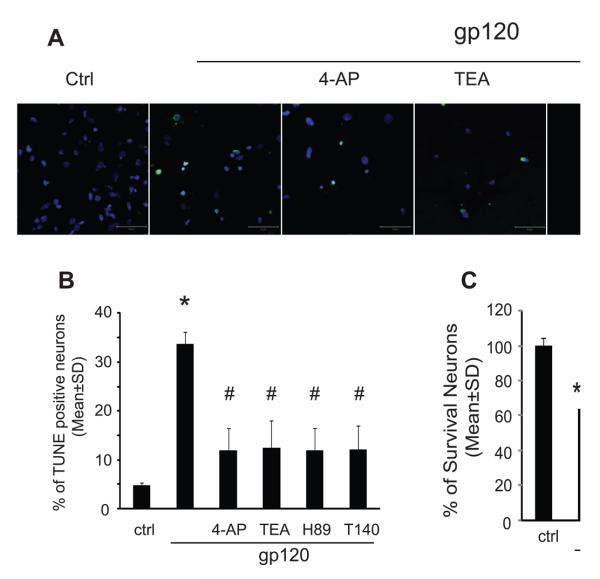
6. Blockade of microglia Kv channels inhibited microglia-induced neuronal injury
To examine biological significance of gp120-associated enhancement of outward K+ current, we studied neurotoxic activity of gp120-stimulated microglia and the protective effects of Kv channel blockers on microglia-induced neuronal injury. Rat microglia grown on the transwells were exposed to gp120 (200-500pM) with or without Kv channel blockers (4-AP, 1mM or TEA, 5mM) for 24 h. After washing, the rat microglia were co-cultured with rat cortical neurons for additional 24 h and results showed that gp120-stimulated microglia induced apoptosis in 33.2±3.9% of neurons examined (Fig. 6A, 6B). In comparison with the results of 4.7±1.7% obtained in neurons co-cultured with non-stimulated microglia (control), the difference is statistically significant (p<0.01), suggesting that gp120-stimulated microglia injure neurons. The injurious effect of microglia on neurons was confirmed by MTT assay (Fig. 6C). The neurotoxic effects of gp120-stimulated microglia were attenuated by Kv channel blockers (4-AP, TEA), CXCR4 receptor antagonist (T140) and PKA inhibitor (H89), respectively (Fig. 6). As a positive control, LPS (0.5μg/ml) was tested to stimulate microglia and the LPS-stimulated microglia, as anticipated, produced a significant neuronal apoptosis when co-cultured with neurons (data not shown).
Fig 6.
Gp120-activated microglia produced cytotoxicity on neurons. Panel A: apoptotic neurons were visualized by TUNEL staining at x400 original magnification (scale bar 50μm). Panel B: Neuronal apoptosis was analyzed by combined TUNEL/DAPI staining and percentage of apoptotic neurons was determined by TUNEL-positive cells to normalize the total number of DAPI-positive cells. Compared to control group, an increasing percentage of apoptotic neurons were notably observed in gp120 treated group (Control vs. gp120, 7.7% vs 33.77%). The apoptosis induced by gp120-stimulated microglia were attenuated by 4-AP, TEA, H89 and T140. Panel C: Neuronal viability was assessed by MTT assay. 4-AP, TEA, H89 and T140 significantly attenuated the neurotoxic activity of gp120-stimulated microglia. Values are expressed as mean±SD of triplicate cultures. The results are representative of three independent experiments performed in triplicate determinations. * p<0.05, ***p<0.001 vs Ctrl, # p<0.05, ## p<0.01 vs gp120
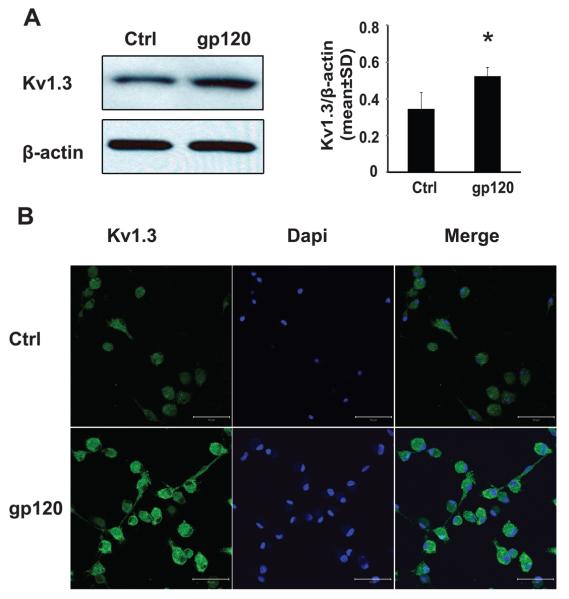
7. Effects of gp120 on microglia Kv channel expression
The voltage dependence of activation and inactivation of the outward K+ current recorded in rat microglia, plus its blockade by 4-AP and TEA, suggest a possible identity of Kv1.3 current, which is in agreement with the up-regulated expression levels of Kv1.3 mRNA observed by other investigators (Eder 1998; Norenberg et al. 1994; Schilling et al. 2000). To assess whether gp120 alters Kv1.3 protein expression, we examined the expression levels of Kv1.3 protein in microglia (2×106 cells/well, in 6 well plates) treated with or without gp120 for 24 h by western blot and immunocytochemistry. Our results showed that gp120 enhanced both the expression levels of Kv1.3 channel protein and immunofluorescent density of Kv1.3 staining in cultured microglia (Fig. 7). The increase of Kv1.3 channel expression following gp120 treatment may underlie gp120-associated enhancement of outward K+ current recorded in rat microglia.
Fig. 7.
gp120 enhanced expression levels of Kv1.3 in cultured microglia. Rat microglia (2×106 cells/well in 6 well plates or 0.5×106 cells/well in 24 well plates) were treated with or without gp120 (500pM) for 24h. The expression levels of Kv1.3 channel proteins were examined by Western blot and immunocytochemistry. Panel A: the representative scans showed Western blots for Kv1.3, and internal control β-actin (left). Each band density was normalized to its internal control and represents in bar graphs (right). Significant differences were detected in gp120 (1.5-fold increase) compared to non-treated microglia. *p<0.05 in comparison to control group were analyzed using Student's t-test. Panel B: Microglia were immunostained for expression of Kv1.3 (green). Images were visualized by fluorescent confocal microscopy at x400 original magnification (scale bar 50μm).
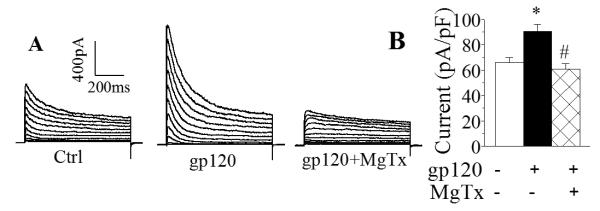
8. Specific Kv1.3 antagonist blocks gp120-induced increase of outward K+ current and neuronal apoptosis
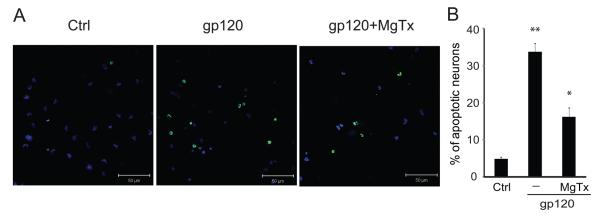
After demonstration of gp120 enhancement of Kv1.3 expression, we further verified if the newly formed channels were functional active and involved in gp120-associated increase of outward K+ current and neuronal apoptosis using a specific Kv1.3 blocker margatoxin (MgTx)(Knaus et al. 1995). In another group of experiments, incubation of microglia with gp120 produced a significant (p<0.01) enhancement of outward K+ current with an average current density of 90.4±5.7pA/pF (Fig. 8, n=12) when compared to the current density (65.7±4.3pA/pF, n=10) recorded in control (without gp120 treatment) microglia. Addition of MgTx (100nM) to the bath solution abolished gp120-induced increase of outward K+ current with an average of instantaneous current density of 60.5±4.5pA/pF (Fig. 8, n=8). In comparison with the current density recorded in microglia treated with gp120, the difference was statistically significant (p<0.01). The involvement of Kv1.3 in gp120-innduced neuronal injury was demonstrated by the results showing a significant attenuation of gp120-induced neuronal apoptosis by pretreatment of microglia with MgTx in microglia-neuronal co-culture system as shown in Fig. 9. These results indicate that the newly formed Kv1.3 channels were functional and involved in gp120-associated enhancement of outward K+ current in rat microglia.
Fig. 8.
Blockade of gp120 enhancement of outward K+ current by MgTx, a specific Kv1.3 blocker. A: an example showing blockade of gp120-induced enhancement of outward K+ current in rat microglia by MgTx. B: a summary bar graph illustrating MgTx significantly blocked gp120-induced enhancement of microglia outward K+ current. Instantaneous outward K+ current generated by a voltage step from −60mV to +60mV were measured and current densities were calculated. *, p<0.01 gp120−MgTx− vs gp120+MgTx−; #, p<0.01 gp120+MgTx− vs gp120+MgTx+.
Fig. 9.
Neurotoxic activity induced by gp120-stimulated microglia was blocked by MgTx in a microglia-neuronal co-culture system. A: Apoptotic neurons were assayed by TUNEL staining and visualized (green) via confocal microscopy at ×400 original magnifications. Scale bar equals 50μM. B: Quantification of apoptotic neurons was made by enumeration of TUNEL-positive cells and expressed as percentage of total number of DAPI-positive cells counted. Note that gp120-stimulated microglia produced a significant increase of apoptotic neurons and the blockade of microglia Kv1.3 channel by MgTx significantly reduced microglia-induced neuronal apoptosis.
Discussion
As the targets for HIV-1 infection and the producers of neurotoxins, microglia play an important role in the pathogenesis of HAND and other neurodegenerative disorders. It is widely accepted that the infected and immune-activated microglia secrete a variety of bioactive substances including viral protein gp120, resulting in neuronal dysfunction and death(Garden 2002; Glass and Wesselingh 2001). The mechanisms underlying microglia-associated neuropathogenesis are not fully understood. In the present study, we demonstrated that HIV-1 gp120 increased the levels of Kv1.3 expression and enhanced outward K+ current in cultured rat microglia via CXCR4 – PKA signaling pathways. The enhancement of outward K+ current was associated with microglia neurotoxicity as evidenced by experimental results showing the blockade of microglia Kv1.3 channels suppressed microglia-associated neurotoxicity in vitro.
In HIV-1-infected brain, gp120, shed from virions and/or secreted from HIV-1-infected microglia/macrophages, has the potential to diffuse and interact directly with surrounding and distant neural cells through activation of CXCR4 receptors(Bachis and Mocchetti 2004; Hesselgesser et al. 1998; Meucci et al. 1998), or by stimulation of uninfected microglia to release neurotoxins which act indirectly on local and distant neural cells, or both. Transgenic mice expressing gp120 manifest a spectrum of neuronal and glial changes resembling abnormalities in brains of HIV-1-infected humans and the severity of damage correlated positively with the brain levels of gp120 expression (Toggas et al. 1994). While the precise mechanisms on how gp120 stimulates microglia production of neurotoxins remains to be determined, the enhancement of outward K+ current by gp120 through CXCR4 may represent one of such potential mechanisms, as microglia-associated neurotoxicity was blocked either by a CXCR4 antagonist or by Kv channel blockers.
Microglia express a defined pattern of Kv channels, which is distinct from that of other glial cells and neurons. This pattern undergoes defined changes with microglia activation. It is believed that microglia Kv channels, albeit in a lower density than their excitable counterparts, play an important role in the switch from one functional state to another (Farber and Kettenmann 2005; Kotecha and Schlichter 1999). Patch clamp studies of microglia in cell culture and in tissue slices have demonstrated that outward rectifier Kv1.3 and Kv1.5 are the dominant Kv channels (Eder 1998; Kotecha and Schlichter 1999; Newell and Schlichter 2005; Pannasch et al. 2006; Schilling et al. 2000). Our results showed that elevated levels of Kv1.3 expression and enhanced outward K+ currents were detected in gp120-stimulated microglia and that the neuronal apoptosis induced by gp120-stimulated microglia was blocked by Kv channel blockers. These results suggest that gp120-induced elevation of Kv channel expression and enhancement of outward K+ current might trigger microglia-associated neurotoxicity. Whereas the functional roles of Kv 1.3 in microglia remain to be determined, evidence from this study and many others indicate that the newly formed Kv1.3 channels appear to be involved in microglia activation and subsequent production of neurotoxins (Eder 1998; Eder 2005; Fordyce et al. 2005; Kotecha and Schlichter 1999).
Ion channels are the targets of many intracellular signaling pathways including protein phosphorylation. These processes can modify channel activity and alter cellular electrophysiological properties. Thus, protein phosphorylation is an important physiological regulator of Kv channel function. Amino acid sequences of Kv1.3 clones from mouse, rat and human are very similar, with >98% homology between human and rat clones (Chandy 1995). All Kv1.3 clones contain several potential PKA and PKC phosphorylation sites, with one strong PKA site at the COOH terminus (Kennelly and Krebs 1991). Evidence that Kv1.3 channels can be regulated by PKA includes reports of enhancement of delayed rectifier K+ currents in vascular smooth muscle cells (Aiello et al. 1995), squid giant axon (Perozo et al. 1989) and human T lymphocytes (Chung and Schlichter 1997). Inhibition of serine/threonine protein phosphatases with okadaic acid increases Kv1.3 current and shifts the voltage dependence of activation and inactivation to more positive potentials. Inhibition of PKA by a specific PKA inhibitor decreases Kv1.3 current. These observations indicate that Kv1.3 activity can be regulated by serine/threonine phosphorylation. The results obtained in this study revealed that the gp120-induced enhancement of microglia outward K+ current was blocked by a specific PKA inhibitor H89, suggesting that activation of CXCR4 by gp120 in microglia causes cAMP-dependent PKA activation, resulting in Kv1.3 phosphorylation and consequent enhancement of channel activity.
Kv1.3 is predominantly expressed in immunocytes including microglia, lymphocytes, and macrophages and is important for immunocyte-mediated immune and inflammatory responses (Beeton et al. 2006; Chandy et al. 2004). Mounting evidence suggests that immune and inflammatory responses mediated by the activated microglia, the predominant resident CNS cell type productively infected by HIV-1(Lipton and Gendelman 1995), play a pivotal role in the pathogenesis of HAND (Dheen et al. 2007; Garden 2002; Glass and Wesselingh 2001). Thus, studies on identification of specific target(s) to regulate microglia activation and resultant production of neurotoxins are highly imperative. Our results, gp120-associated neurotoxicity was blocked by a specific Kv1.3 blocker MgTx, indicate that Kv1.3 expressed in microglia may function as a potential target for the development of therapeutic strategies for HAND and perhaps for other neurodegenerative disorders. We anticipate that blockade of Kv1.3 channels in microglia might attenuate microglia-associated neurotoxicity, resulting in the protection of neurons from HIV-associated challenge in the infected brain. It is worth pointing out that there may be side effects by systemic or intracranial administration of Kv1.3 blockers since small amounts of Kv1.3 are also expressed in several brain regions such as hippocampus, and pyriform cortex (Kues and Wunder 1992). However, such potential side effects might be minimal as animals deficient in Kv1.3 exhibited only a heightened sense of smell and distinct structure alterations in the olfactory bulb (Fadool et al. 2004). Thus, the potential for development of specific Kv1.3 blockers to suppress microglia-associated neurotoxicity is optimistic.
In summary, the experimental data provide in vitro evidence that HIV-1gp120 enhances outward K+ current in cultured rat microglia via CXCR4→cAMP-dependent PKA signaling pathway. Gp120 elevates the levels of Kv channel expression and alters Kv channel biophysical properties. Biological significance of gp120 enhancement of microglia outward K+ current was demonstrated by experimental results that blockade of microglia Kv channels by Kv channel blockers suppresses microglia-induced neurotoxicity. As such, the Kv channel expressed in microglia may function as a potential target in the development of therapeutic strategies for neurodegenerative disorders by which the activated resident microglia play a critical role in the pathogenesis.
Acknowledgements
The authors thank Mr. Bryan Katafiasz for reading of the manuscript. The authors extend special thanks to Ms. Julie Ditter, Ms. Robin Taylor and Ms. Johna Belling for their excellent administrative supports and to two anonymous reviewers for their critical criticisms and helpful comments. This work was supported by the NIH grants R01 NS041862-9 and 5 R01 NS063878.
References
- Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268:H926–34. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O'Connor MJ, Doms RW, Gonzalez-Scarano F. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73(1):205–13. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachis A, Mocchetti I. The chemokine receptor CXCR4 and not the N-methyl-D-aspartate receptor mediates gp120 neurotoxicity in cerebellar granule cells. J Neurosci Res. 2004;75(1):75–82. doi: 10.1002/jnr.10826. [DOI] [PubMed] [Google Scholar]
- Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci U S A. 2006;103(46):17414–9. doi: 10.1073/pnas.0605136103. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandy KG, Gutman GA. voltage-gated potassium channel genes. In: North RA, editor. Ligand and Voltage-gated Ion Channels. Bpca Raton; FL: CRC: 1995. pp. 1–71. [Google Scholar]
- Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25(5):280–9. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung I, Schlichter LC. Regulation of native Kv1.3 channels by cAMP-dependent protein phosphorylation. Am J Physiol. 1997;273:C622–33. doi: 10.1152/ajpcell.1997.273.2.C622. [DOI] [PubMed] [Google Scholar]
- Dheen ST, Kaur C, Ling EA. Microglial activation and its implications in the brain diseases. Curr Med Chem. 2007;14(11):1189–97. doi: 10.2174/092986707780597961. [DOI] [PubMed] [Google Scholar]
- Eder C. Ion channels in microglia (brain macrophages) Am J Physiol. 1998;275:C327–42. doi: 10.1152/ajpcell.1998.275.2.C327. [DOI] [PubMed] [Google Scholar]
- Eder C. Regulation of microglial behavior by ion channel activity. J Neurosci Res. 2005;81(3):314–21. doi: 10.1002/jnr.20476. [DOI] [PubMed] [Google Scholar]
- Eder C, Fischer HG, Hadding U, Heinemann U. Properties of voltage-gated currents of microglia developed using macrophage colony-stimulating factor. Pflugers Arch. 1995;430(4):526–33. doi: 10.1007/BF00373889. [DOI] [PubMed] [Google Scholar]
- Elkabes S, Peng L, Black IB. Lipopolysaccharide differentially regulates microglial trk receptor and neurotrophin expression. J Neurosci Res. 1998;54(1):117–22. doi: 10.1002/(SICI)1097-4547(19981001)54:1<117::AID-JNR12>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Fadool DA, Tucker K, Perkins R, Fasciani G, Thompson RN, Parsons AD, Overton JM, Koni PA, Flavell RA, Kaczmarek LK. Kv1.3 channel gene-targeted deletion produces “Super-Smeller Mice” with altered glomeruli, interacting scaffolding proteins, and biophysics. Neuron. 2004;41(3):389–404. doi: 10.1016/s0896-6273(03)00844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Brandle U, Glowatzki E, Zenner HP, Ruppersberg JP. Kir2.1 inward rectifier K+ channels are regulated independently by protein kinases and ATP hydrolysis. Neuron. 1994;13(6):1413–20. doi: 10.1016/0896-6273(94)90426-x. [DOI] [PubMed] [Google Scholar]
- Farber K, Kettenmann H. Physiology of microglial cells. Brain Res Brain Res Rev. 2005;48(2):133–43. doi: 10.1016/j.brainresrev.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fischer HG, Eder C, Hadding U, Heinemann U. Cytokine-dependent K+ channel profile of microglia at immunologically defined functional states. Neuroscience. 1995;64(1):183–91. doi: 10.1016/0306-4522(94)00398-o. [DOI] [PubMed] [Google Scholar]
- Fordyce CB, Jagasia R, Zhu X, Schlichter LC. Microglia Kv1.3 channels contribute to their ability to kill neurons. J Neurosci. 2005;25(31):7139–49. doi: 10.1523/JNEUROSCI.1251-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40(2):240–51. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Eiden L, Epstein L, Grant I, Lipton SA, McArthur JC, Pomerantz R, Price R, Swindells S. Neuropathogenesis of HIV-1 Dementia: A Panel Discussion. Chapman & Hall; New York: 1997. [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176(6):1703–18. doi: 10.1084/jem.176.6.1703. others. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL. Microglia in HIV-associated neurological diseases. Microsc Res Tech. 2001;54(2):95–105. doi: 10.1002/jemt.1124. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8(10):595–8. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410(6831):988–94. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kennelly PJ, Krebs EG. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991;266(24):15555–8. [PubMed] [Google Scholar]
- Kielian T. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front Biosci. 2004;9:732–50. doi: 10.2741/1266. [DOI] [PubMed] [Google Scholar]
- Knaus HG, Koch RO, Eberhart A, Kaczorowski GJ, Garcia ML, Slaughter RS. [125I]margatoxin, an extraordinarily high affinity ligand for voltage-gated potassium channels in mammalian brain. Biochemistry. 1995;34(41):13627–34. doi: 10.1021/bi00041a043. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233(4768):1089–93. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kotecha SA, Schlichter LC. A Kv1.5 to Kv1.3 switch in endogenous hippocampal microglia and a role in proliferation. J Neurosci. 1999;19(24):10680–93. doi: 10.1523/JNEUROSCI.19-24-10680.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kues WA, Wunder F. Heterogeneous Expression Patterns of Mammalian Potassium Channel Genes in Developing and Adult Rat Brain. Eur J Neurosci. 1992;4(12):1296–1308. doi: 10.1111/j.1460-9568.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, O'Connor M, Hoxie JA, Gonzalez-Scarano F. CXCR-4 (Fusin), a co-receptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151(4):1035–42. [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome [see comments] N Engl J Med. 1995;332(14):934–40. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Menteyne A, Levavasseur F, Audinat E, Avignone E. Predominant functional expression of Kv1.3 by activated microglia of the hippocampus after Status epilepticus. PLoS One. 2009;4(8):e6770. doi: 10.1371/journal.pone.0006770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci U S A. 1998;95(24):14500–5. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa T, Furukawa S, Nakajima K, Furukawa Y, Kohsaka S. Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. J Neurosci Res. 1997;50(6):1023–9. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1023::AID-JNR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Newell EW, Schlichter LC. Integration of K+ and Cl− currents regulate steady-state and dynamic membrane potentials in cultured rat microglia. J Physiol. 2005;567:869–90. doi: 10.1113/jphysiol.2005.092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norenberg W, Gebicke-Haerter PJ, Illes P. Voltage-dependent potassium channels in activated rat microglia. J Physiol. 1994;475(1):15–32. doi: 10.1113/jphysiol.1994.sp020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Farber K, Nolte C, Blonski M, Yan Chiu S, Messing A, Kettenmann H. The potassium channels Kv1.5 and Kv1.3 modulate distinct functions of microglia. Mol Cell Neurosci. 2006;33(4):401–11. doi: 10.1016/j.mcn.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Perozo E, Bezanilla F, Dipolo R. Modulation of K channels in dialyzed squid axons. ATP-mediated phosphorylation. J Gen Physiol. 1989;93(6):1195–218. doi: 10.1085/jgp.93.6.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling T, Quandt FN, Cherny VV, Zhou W, Heinemann U, Decoursey TE, Eder C. Upregulation of Kv1.3 K(+) channels in microglia deactivated by TGF-beta. Am J Physiol Cell Physiol. 2000;279(4):C1123–34. doi: 10.1152/ajpcell.2000.279.4.C1123. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367(6459):188–93. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Walz W, Bekar LK. Ion channels in cultured microglia. Microsc Res Tech. 2001;54(1):26–33. doi: 10.1002/jemt.1117. [DOI] [PubMed] [Google Scholar]