Abstract
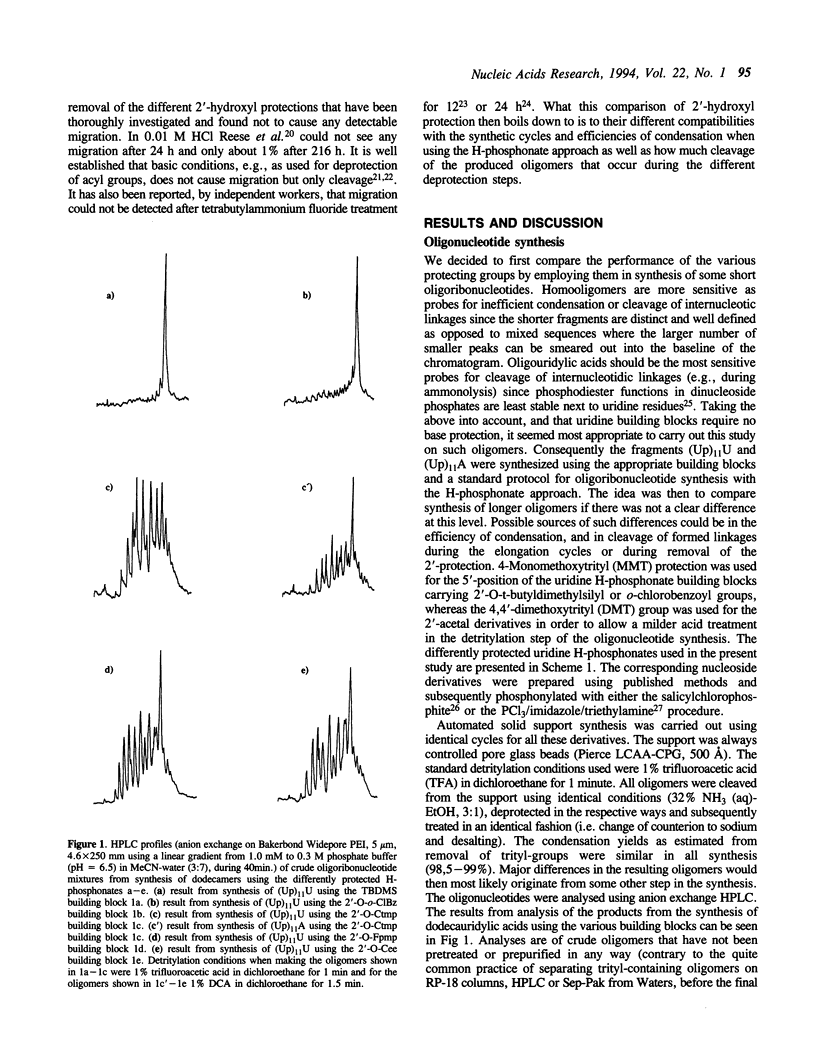
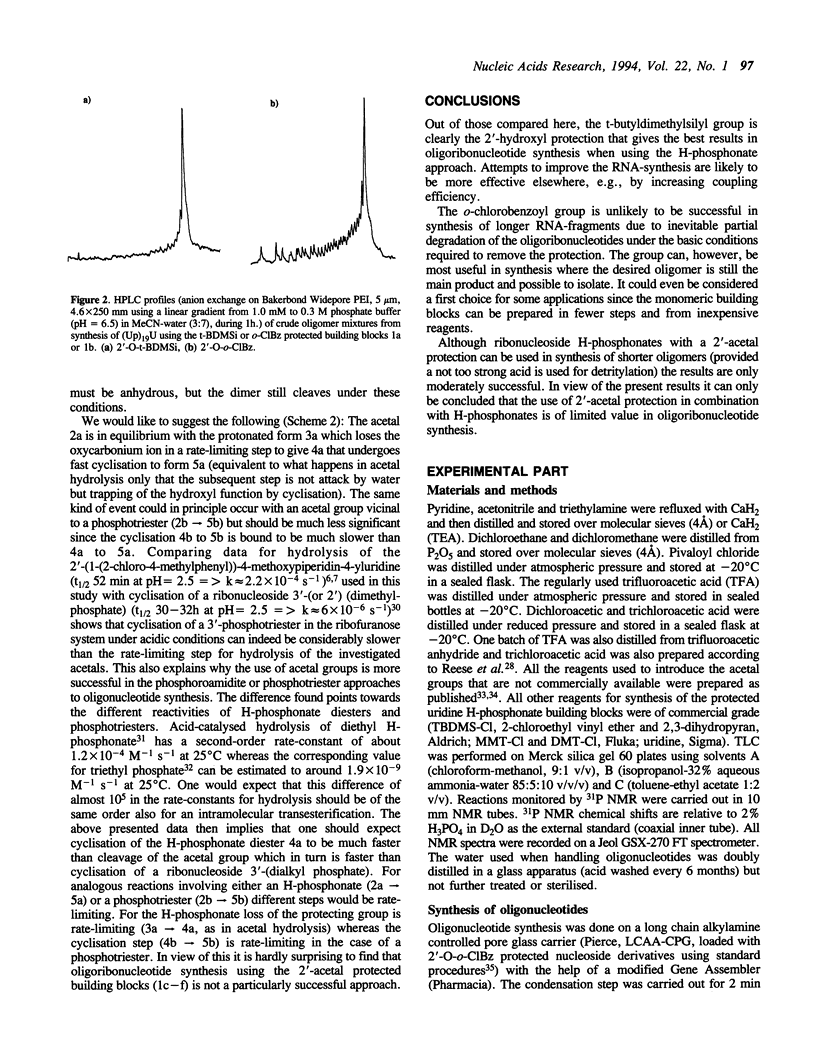
A number of different protecting groups were compared with respect to their usefulness for protection of 2'-hydroxyl functions during synthesis of oligoribonucleotides using the H-phosphonate approach. The comparison was between the t-butyldimethylsilyl (t-BDMSi), the o-chlorobenzoyl (o-CIBz), the tetrahydropyranyl (THP), the 1-(2-fluorophenyl)-4-methoxypiperidin-4-yl (Fpmp), the 1-(2-chloro-4-methylphenyl)-4-methoxypiperidin-4-yl (Ctmp), and the 1-(2-chloroethoxy)ethyl (Cee) protecting groups. All these groups were tested in synthesis of dodecamers, (Up)11U and (Up)11A, using 5'-O-(4-monomethoxytrityl) or (4,4'-dimethoxytrityl) uridine H-phosphonate building blocks carrying the respective 2'-protection. The performance of the t-BDMSi and o-CIBz derivatives were also compared in synthesis of (Up)19U. The most successful syntheses were clearly those where the t-butyldimethylsilyl group was used. The o-chlorobenzoyl group also gave satisfactory results but seems somewhat limited with respect to synthesis of longer oligomers. The results with all tested acetal derivatives (Fpmp, Ctmp, Cee, THP) were much less successful due to some accompanying cleavage of internucleotidic H-phosphonate functions during removal of 5'-O-protection (DMT).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beijer B., Sulston I., Sproat B. S., Rider P., Lamond A. I., Neuner P. Synthesis and applications of oligoribonucleotides with selected 2'-O-methylation using the 2'-O-[1-(2-fluorophenyl)-4-methoxypiperidin-4-yl] protecting group. Nucleic Acids Res. 1990 Sep 11;18(17):5143–5151. doi: 10.1093/nar/18.17.5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparutto D., Livache T., Bazin H., Duplaa A. M., Guy A., Khorlin A., Molko D., Roget A., Téoule R. Chemical synthesis of a biologically active natural tRNA with its minor bases. Nucleic Acids Res. 1992 Oct 11;20(19):5159–5166. doi: 10.1093/nar/20.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin B. E., Jarman M., Reese C. B. The synthesis of oligoribonucleotides. IV. Preparation of dinucleoside phosphates from 2',5'-protected ribonucleoside derivatives. Tetrahedron. 1968 Jan;24(2):639–662. doi: 10.1016/0040-4020(68)88015-9. [DOI] [PubMed] [Google Scholar]
- Ogilvie K. K., Usman N., Nicoghosian K., Cedergren R. J. Total chemical synthesis of a 77-nucleotide-long RNA sequence having methionine-acceptance activity. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5764–5768. doi: 10.1073/pnas.85.16.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RHODES D. N., LEA C. H. Composition of egg phospholipids. Nature. 1956 Jun 16;177(4520):1129–1130. doi: 10.1038/1771129a0. [DOI] [PubMed] [Google Scholar]
- Rozners E. Z., Rekis A. Kh, Kumpin'sh V. Kh, Bizdena E. O. Sintez oligoribonukleotidov N-fosfonatnym metodom s izpol'zovaniem shchelochnolabil'nykh 2'-o-zashchitnykh grupp. II. Nekotorye aspekty izpol'zovaniia 2'-o-benzoil'noi i anizoilnoi zashchitnykh grupp. Bioorg Khim. 1990 Nov;16(11):1531–1536. [PubMed] [Google Scholar]
- Sakatsume O., Ohtsuki M., Takaku H., Reese C. B. Solid phase synthesis of oligoribonucleotides using the 1-[(2-chloro-4-methyl)phenyl]-4-methoxypiperidin-4-yl (Ctmp) group for the protection of the 2'-hydroxy functions and the H-phosphonate approach. Nucleic Acids Res. 1989 May 25;17(10):3689–3697. doi: 10.1093/nar/17.10.3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawinski J., Strömberg R., Thelin M., Westman E. Studies on the t-butyldimethylsilyl group as 2'-O-protection in oligoribonucleotide synthesis via the H-phosphonate approach. Nucleic Acids Res. 1988 Oct 11;16(19):9285–9298. doi: 10.1093/nar/16.19.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tamatsukuri S., Ikehara M. Solid phase synthesis of oligoribonucleotides using the o-nitrobenzyl group for 2'-hydroxyl protection and H-phosphonate chemistry. Nucleic Acids Res. 1987 Sep 25;15(18):7235–7248. doi: 10.1093/nar/15.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ven'iaminova A. G., Gorn V. V., Zenkova M. A., Komarova N. I., Repkova M. N. Avtomaticheskii H-fosfonatnyi sintez oligoribonukleotidov s ispol'zovaniem 2'-O-tetragidropiranil'noi zashchitnoi gruppy. Bioorg Khim. 1990 Jul;16(7):941–950. [PubMed] [Google Scholar]
- Vinayak R., Anderson P., McCollum C., Hampel A. Chemical synthesis of RNA using fast oligonucleotide deprotection chemistry. Nucleic Acids Res. 1992 Mar 25;20(6):1265–1269. doi: 10.1093/nar/20.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]


