Abstract
The regenerative process of the perineurium and nerve function were examined using an in vivo model of perineurium resection in the rat sciatic nerve. Our hypothesis is that the regenerative process of the perineurium can be demonstrated by immunolabeling for tenascin-C and alpha smooth muscle actin after microsurgical resection of the perineurium in vivo. A total of 38 Lewis rats were used. Eight-week-old animals were assigned to one of two groups: the epi-perineurium removal group or the sham group. Under operative microscopy, the sciatic nerve was dissected from surrounding tissues at the thigh level from the ischial tuberosity to the fossa poplitea. The epi-perineurium was carefully removed by cutting circumferentially and stripping distally for 15 mm. For CatWalk® dynamic gait analysis, only right sciatic nerves underwent surgery; the left sciatic nerves were left intact. For pathological and electrophysiological tests, both the right and left sciatic nerves underwent surgery. Analysis of data was performed at each time interval with a two-group t-test. P < 0.05 was considered statistically significant. After resection of a 15-mm section of the epi-perineurium, immediate endoneurial swelling occurred in the outer portion and spread into the central portion. Although demyelination and axonal degeneration were found in the swollen area, remyelination and recovery of electrophysiological function were seen after regeneration of the perineurium. An immunohistological and electron microscopic study revealed that the perineurium regenerated via fusion of the residual interfascicular perineurium and endoneurial fibroblast-like cells of mesenchymal origin. CatWalk gait analysis showed not only motor paresis but also neuropathic pain during the early phases of this model.
Keywords: CatWalk, endoneurial fibroblast, endoneurial swelling, perineurium, regeneration, tenascin-C
Introduction
The perineurium represents a continuum with the pia-arachnoid in the central nervous system and surrounds nerve fascicles within the epineurium throughout the peripheral nervous system (Shanthaveerappa & Bourne, 1966). Cells comprising the perineurium are called perineurial cells; these cells have a polygonal morphology and are connected to each other by tight junctions (Pina-Oviedo & Ortiz-Hidalgo, 2008). The perineurial cell layers alternate with the extracellular matrix and basement membranes to form a distinctive lamellar structure. The function of the perineurium is to protect the nerve against mechanical stretching (Haftek, 1970) and to maintain the homeostasis of the nerve by acting as a diffusion barrier, called the blood–nerve barrier (Olsson & Kristens, 1973; Poduslo et al. 1994).
Bunge et al. (1989) conducted a lineage analysis of perineurial cells using beta-galactosidase as a cell marker and demonstrated that perineurial cells are of mesenchymal origin. Terho et al. (2002) observed perineurial regeneration after microsurgical perineurectomy and suggested that the fibroblast-like cells from the minifascicles may represent the preliminary stage of perineurial cells. However, little is known about the detailed behavior of perineurial cells or their precursors during perineurial regeneration in vivo.
Clinically, neurolysis can be classified into at least two procedures: external and internal neurolysis. The former refers to decompressing and freeing the nerve trunk from a constricting or distorting agent, and the latter refers to the exposure of fascicles by epineurotomy or epineurectomy and release of individual fascicles, if necessary. Because scar tissue is believed to constrict nerve fibers, thereby interfering with their function and regenerative capacity, neurolysis is, at least theoretically, a beneficial procedure (Brown, 1969, 1972).
In the peripheral nervous system, it is important for nerve trunks to be able to glide past surrounding tissue. Conjunctiva nervorum plays a key role in the gliding of a nerve trunk. This structure is attached to the surrounding tissue and the epifascicular epineurium and consists of several layers of loose connective tissue and capillaries. If the space that is normally filled with the gliding tissue becomes fibrotic, the nerve becomes completely immobile and can be mobilized again only with surgical procedures (Millesi et al. 1993).
There is little doubt about the value of external neurolysis; however, much controversy exists over the use of internal neurolysis. For example, some researchers have reported significant functional improvement after internal neurolysis in advanced cases of carpal tunnel syndrome (Curtis & Eversman, 1973; Sakurai & Miyasaka, 1986), whereas others strongly object to the procedure, insisting that both inevitable injury to the perineurium in the process of releasing fascicles and exaggerated scar formation following the procedure can interfere with treatment results (Phalen, 1981; Mackinnon et al. 1991). Therefore, such a procedure appears justifiable only when the preoperative intraneural fibrosis is more severe than the scarring induced by the surgical procedure (Rydevik et al. 1976).
Surgeons often have to deal with the epi-perineurium in peripheral nerve surgeries such as treating recurrent entrapment neuropathies with severe external and internal fibrosis (Sakurai & Miyasaka, 1986), performing interfascicular nerve graft or fascicular transfers (Oberlin et al. 1994; Sungpet et al. 2000), repairing nerves using end-to-side neurorrhaphy (Viterbo et al. 1994), or dealing with neuroma-in-continuity (Kato et al. 1998). However, taking the theoretical drawbacks of internal neurolysis into consideration, it is generally advisable to minimize intraneural dissection. There are several exceptions, such as the hourglass-like fascicular constriction seen in idiopathic anterior or posterior interosseous nerve palsy (Nagano et al. 1996) and nerve tumors such as Schwannoma and nonplexiform neurofibroma. These lesions usually require aggressive dissection of the interfascicular epineurium, which often causes temporary aggravation of nerve palsy after surgery (Donner et al. 1994; Russell, 2007), although favorable treatment outcomes have been reported. These positive results may indicate that the perineurium, the most important structural and functional barrier to nerve fibers, has a higher regeneration ability than generally thought.
Recently, several papers have reported constitutive expression of tenascin-C (TN-C) by perineurial cells (Nishimura et al. 2008; Hill, 2009). TN-C is an oligomeric glycoprotein of the extracellular matrix that has been found to be involved in processes such as wound healing, tumorigenesis, angiogenesis, and embryonic development (Jones & Jones, 2000; Chiquet-Ehrismann & Chiquet, 2003). Various domains of the tenascin molecule are associated with functions of the molecule including adhesion, anti-adhesion, and migration. Alternative mRNA splicing creates two major size variants identified as 220- and 320-kDa bands on Western blots (Erickson & Bourdon, 1989). In addition, PCR analysis demonstrated 22 human splice variants, suggesting the possibility of multiple functions of the molecule based on the particular splice variant. Although TN-C is diffusely expressed during neurogenesis in the peripheral nervous system, only the perineurium continues to express small splice variants after birth (Chiquet & Fambrough, 1984; Nishimura et al. 2008). Therefore, it is possible that small variant TN-C can be used to track perineurial cells during perineurial regeneration.
Myofibroblasts are a specialized type of fibroblast that share characteristics with smooth muscle cells expressing alpha smooth muscle actin (α-SMA) (Tamaoki et al. 2005). They play an important role in wound healing by synthesizing extracellular matrix components such as collagen type I and III; during normal wound healing, myofibroblasts disappear by apoptosis when epithelialization occurs (Gabbiani, 2003). Recent papers have demonstrated that myofibroblasts predominantly produce a large splice variant of TN-C (Tamaoki et al. 2005; Nishimura et al. 2008).
Our hypothesis is that the regenerative process of the perineurium can be demonstrated by immunolabeling for TN-C and α-SMA after microsurgical resection of the perineurium in vivo. The purpose of this study was to examine how the perineurium regenerates after resection of the epi-perineurium in the rat sciatic nerve and to examine the correlation between pathological and electrophysiological findings and clinical behavior in the model.
Experimental procedures
Animal model
All experimental protocols and animal maintenance procedures used in this study were approved by the Animal Ethics Research Committee of Nagoya University. A total of 38 Lewis rats (body weight approximately 250 g each) were used. Animals were anesthetized by intraperitoneal injection of 5% pentobarbital (Nembutal®, Dainippon Pharma Co., Ltd., Osaka, Japan). Under operative microscopy, the sciatic nerve was dissected from the surrounding tissues at the thigh level from the ischial tuberosity to the fossa poplitea. The epi-perineurium was carefully removed by cutting circumferentially and stripping distally for 15 mm, taking care to minimize damage to the axons (Fig. 1). After removal of the epi-perineurium, the muscle fascia and skin were closed with 5-0 Prolene® (Ethicon, Somerville, NJ, USA). For dynamic gait analysis, only the right sciatic nerves underwent surgery; the left sciatic nerves were left intact. For pathological and electrophysiological tests, both right and left sciatic nerves underwent surgery.
Fig. 1.
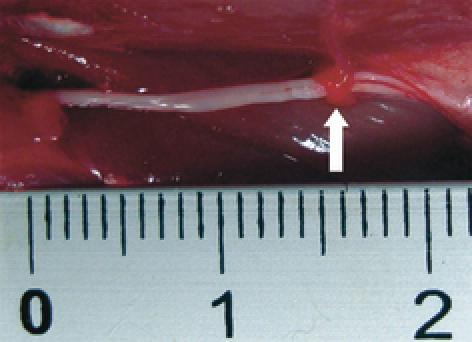
The epi-perineurium was stripped distally for 15 mm (white arrow) and removed microsurgically, taking care to minimize damage to the axons.
Experiment 1 for dynamic gait analysis
The CatWalk® (Noldus, Wageningen, Netherlands), a novel automated gait analysis system, was used for clinical examination. At the age of 8 weeks, 14 animals that had undergone surgery only on the right hind limbs were assigned to one of two groups: the epi-perineurium removal group (n = 9) and the sham group (n = 5). Examinations were performed before surgery and at 2, 7, 14, 21, 28, 35, and 42 days after surgery.
The CatWalk® gait analyses evaluate several dynamic and static gait parameters. Some of these parameters, including mean intensity of paw placement, stance duration, and swing duration of the hindpaw, have been associated with motor control and have also been linked to neuropathic pain (Vrinten & Hamers, 2003). However, most parameters may be regarded as strictly motor-related. By using this gait analysis, both motor function and neuropathic pain can be assessed after removal of the epi-perineurium.
Experiment 2 for electrophysiological, pathological, and wet muscle study
At the age of 8 weeks, 48 sciatic nerves of 24 rats were divided into two groups: the epi-perineurium removal group (n = 24) and the sham group (n = 24). At 2, 7, 20, and 42 days after surgery, electrophysiological, pathological, and wet muscle studies were performed.
Electrophysiological study
The compound muscle action potential (CMAP) of the tibialis anterior muscle was measured at room temperature (24 °C) under anesthesia with 25 mg kg−1 intraperitoneal pentobarbital injection (n = 6 from each group at days 2, 7, 20, and 42). Two stainless steel monopolar recording electrodes (H537A; Nihon Koden, Tokyo, Japan) were placed at the center of the belly of the anterior tibialis muscle after exposing the muscle. The sciatic nerve was carefully exposed and a bipolar stimulating electrode (UM2-5050; Nihon Koden) was placed around the nerve at the level of the sciatic notch. Electrical pulses (supramaximal; duration 100 ms; frequency 1 Hz; square wave) were applied with an isolator (SS-201J; Nihon Koden) connected to the electronic stimulator. CMAP latency was recorded to estimate electrophysiological function.
Pathologic study
After the electrophysiological study, nerves in each group were harvested and kept immersed in 4% paraformaldehyde overnight for histological and immunological evaluation. The specimens were embedded in paraffin and cut into 4-μm sections that were stained with hematoxylin and eosin (HE). Immunohistochemical studies were performed with monoclonal mouse anti-TN-C antibody clones 4F10TT (IBL, Gunma, Japan) and 4C8MS (IBL). 4C8MS specifically recognizes the alternative splicing sites, whereas 4F10TT reacts with constitutive sites of the TN-C molecules. Sections on slides were incubated with either rabbit polyclonal antibodies (1 μg mL−1), 4F10TT (2 μg mL−1), or 4C8MS (5 μg mL−1) overnight at 4 °C and subsequently with peroxidase-conjugated anti-mouse or anti-rabbit IgG Fab’ (1 : 500; MBL, Nagoya, Japan) for 1 h. After washing, diaminobenzidine/H2O2 solution was used to visualize antibody binding. The sections were then lightly counterstained with hematoxylin to facilitate orientation. The monoclonal antibody for TN-C (4F10TT) specifically recognizes the EGF-like domain of TN-C and therefore detects all TN-C isoforms. On the other hand, the monoclonal antibody for TN-C (4C8MS) specifically recognizes domain B in FNIII repeats in TN-C. Therefore, small variant TN-C is tracked by subtracting the immunolabeling of TN-C (4C8MS) from that of TN-C (4F10TT). Myofibroblasts were labeled by a direct immunoperoxidase method with anti-α-SMA antibody (M 0851; Dako Japan, Kyoto, Japan).
For electron microscopic evaluation, nerves in each group were harvested and immersed in 2% glutaraldehyde for 2 h. For postfixation, 1% osmic acid was used, and 24 h later, the nerve specimen was dehydrated and embedded. Transverse sections of the epi-perineurium were stained with toluidine blue and observed under a light microscope (BX60; Olympus, Tokyo, Japan). Ultrathin sections were double-stained with uranium and lead salt and observed under a transmission electron microscope (JEM-1400; JEOL, Tokyo, Japan).
To quantitatively evaluate the myelinated nerve fibers, digital images were obtained at 1000× magnification, and myelinated nerve fibers were counted directly on the toluidine blue-stained sections. Because the density of intact myelinated nerve fibers varied by location in the epi-perineurium group, i.e. in the center of the sciatic nerve or in the periphery, three samples were analyzed from each time point in each group and area. The approximate center and the most swollen peripheral areas of the sciatic nerve were chosen from the sample for analysis.
Semi-quantification of the pathological study
Semi-quantitative analysis of TN-C (4F10TT), TN-C (4C8MS), α-SMA, and toluidine blue-stained images was performed using the metamorph imaging analysis system (Version 7.5; Molecular Devices, Tokyo, Japan). Three samples from each group were analyzed from each time point. Sections were visualized, and the endoneurial TN-C (4F10TT and 4C8MS) and α-SMA expression in each sample was viewed using a low-power objective. Variation in the background staining was corrected prior to thresholding the image. A user-defined frame was then drawn around the outermost layer of the endoneurium. The total area within the endoneurium was assessed, and the percentage of the endoneurial area that represents the area thresholding that of TN-C (4F10TT and 4C8MS) and α-SMA stained per total area was calculated.
Analysis of the endoneurial swelling was performed by thresholding the toluidine blue-stained image per total area. The results were then expressed as a percentage of the endoneurial area and tested for significance with statcel qc software (OMS Publishing Inc., Saitama, Japan).
Wet muscle weight study
The tibialis anterior and gastrocnemius muscles from each animal were dissected free from the origin and insertion and weighed immediately (still wet) after euthanasia (n = 6 from each group at days 2, 7, 20, and 42). Tibialis anterior and gastrocnemius muscle weight data were calculated as percentages of the total body weight for each animal.
Statistical analysis
All data are presented as the means ± standard deviations (SD). Analysis of data was performed with statcel qc software at each time interval using a two-group t-test. P < 0.05 was considered statistically significant.
Results
Experiment 1 for dynamic gait analysis
All 14 animals recovered quickly from surgery. Although paresis of the right hind paw was observed in the group that underwent epi-perineurium removal, no other differences in animal behavior were observed between groups. Consistent with the overall good health of the animals, no significant differences between groups were observed in body weight. Weight gradually increased in both groups over the 42-day observation period, with no significant differences between groups. Further observation did not reveal any autotomy of the digits during the study.
CatWalk®: intensity of the right hind paws
Data analysis was performed with a threshold value of 40 arbitrary units (a.u.) with a possible range of 0–255 a.u. All pixels brighter than 40 were used. The mean intensity with which the paw was placed was computed over the whole stance period (Vrinten & Hamers, 2003). At days 2 and 7, the group that underwent epi-perineurium removal showed a significantly (P < 0.05) lower mean intensity of paw placement than the sham group (Fig. 2).
Fig. 2.
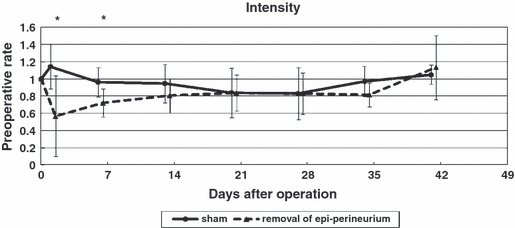
Mean intensity of paw placement. At days 2 and 7, the group that underwent epi-perineurium removal showed a significantly lower mean intensity of paw placement than the sham group (*P < 0.05).
CatWalk®: duration of stance and swing phase of the right hind paws
Two days after surgery, the time that the lesioned paw was in contact with the floor (stance phase) was reduced to 0.43 ± 0.39 of the preoperative value, with no significant differences between groups (Fig. 3A). Similarly, 2 days after surgery, the swing phase duration increased to 2.69 ± 1.25 relative to preoperative values, with no significant differences between groups (Fig. 3B).
Fig. 3.
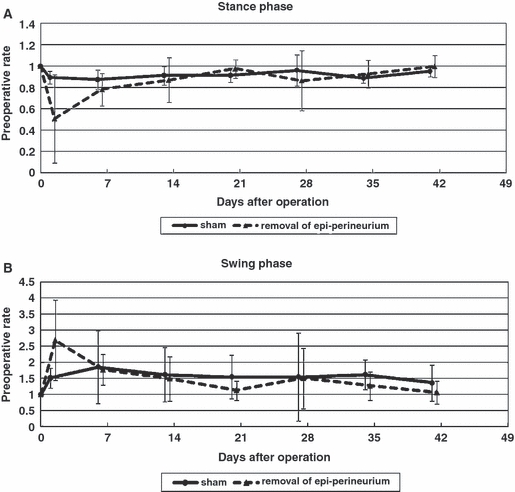
Duration of stance phase (A) and swing phase (B). Two days after surgery, the time that the lesioned paw was in contact with the floor (stance phase) was reduced to 0.43 ± 0.39 and the swing phase increased to 2.69 ± 1.25 of the preoperative value, with no significant differences between groups.
CatWalk®: individual paw parameters
Two days after surgery, there were significant (P < 0.05) differences between groups in terms of print area (Fig. 4A) and max area (Fig. 4B). In the following weeks, these parameters gradually increased in the group that underwent epi-perineurium removal, with no significant differences between groups.
Fig. 4.
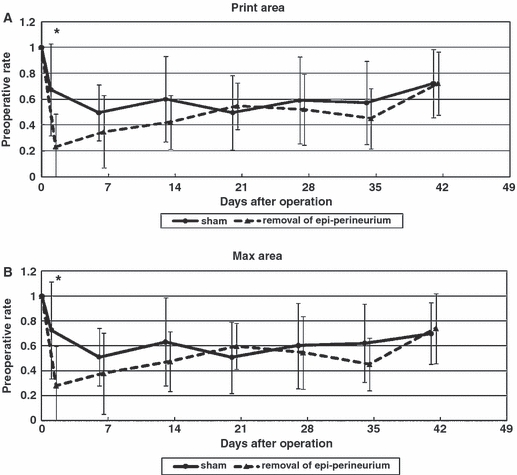
Individual paw parameters: print area (A), max area (B). Two days after surgery, there were significant (P < 0.05) differences between groups in terms of print area (A) and max area (B). In the following weeks, these parameters gradually increased in the group that underwent epi-perineurium removal, with no significant differences between groups (*P < 0.05).
Experiment 2 for electrophysiological, pathological, and wet muscle study
Electrophysiological study
CMAP latency of the anterior tibialis muscle was significantly (P < 0.05) longer for the epi-perineurium removal group than for the sham group 20 days after surgery (Fig. 5). At day 42, the nerve tended to recover in the epi-perineurium removal group.
Fig. 5.
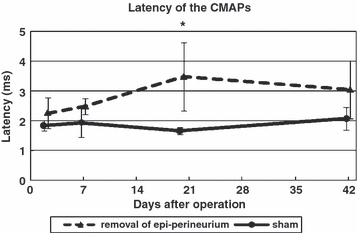
Time course of latency of CMAPs. The CMAP latency of the anterior tibialis muscle was significantly longer for the epi-perineurium removal group than for the sham group 20 days after surgery. At day 42, the nerve tended to recover in the epi-perineurium removal group (*P < 0.05).
Pathological study
In the sham group, the sciatic nerves looked normal both in toluidine blue-stained sections (Fig. 6A) and in electron microscopy (Fig. 6B). In addition, the expression patterns of TN-C and α-SMA were normal. TN-C (4F10TT) was expressed at the perineurium and around the vessel walls (Fig. 11A). In contrast, TN-C (4C8MS) and α-SMA were expressed only at the vessel walls (Figs 12A, 13A).
Fig. 6.
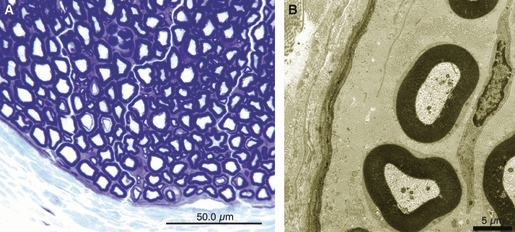
In the sham group, normal sciatic nerve was observed in sections stained for toluidine blue (bar = 50 μm) (A), electron microscopy (bar = 5 μm) (B).
Fig. 11.
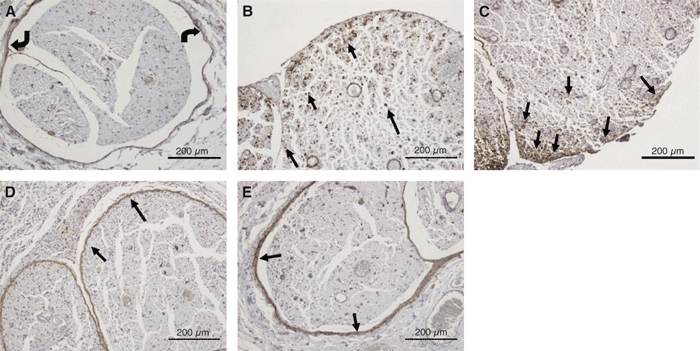
Immunohistochemical study of TN-C (4F10TT) expression in the sham group and the epi-perineurium removal group. In the sham group, TN-C (4F10TT) was expressed in the perineurium (curved arrows) (A). At 2 days after surgery, TN-C (4F10TT) was overexpressed in the nerve fascicle (black arrows), especially in the outer portion (B). At 7 days, TN-C (4F10TT) was overexpressed in the outer portion of the endoneurium (black arrows), particularly at the edge of the nerve fascicles (C). At 20 days, TN-C (4F10TT) was expressed in the periphery of the nerve fascicles with adhesion (black arrows), and was not expressed in the nerve fascicles (D). At 42 days, TN-C (4F10TT) surrounded the nerve fascicle (black arrows) and appeared to be an original perineurium (E) (bar = 200 μm).
Fig. 12.
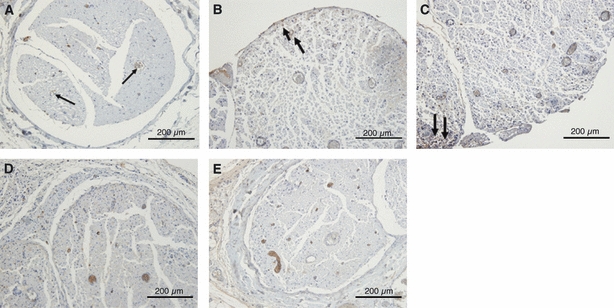
Immunohistochemical study of TN-C (4C8MS) expression in the sham group and the epi-perineurium removal group. In the sham group, TN-C (4C8MS) was expressed only at vessel walls (arrows). At 2 days after surgery, TN-C (4C8MS) was weakly expressed in the outer portion of the nerve (arrows) (B). At 7 days, TN-C (4C8MS) was weakly expressed at the edge of the nerve fascicles (arrow) (C). After 20 days, there were no appreciable differences in the expression pattern of TN-C (4C8MS) in terms of spatial distribution or intensity between the treated and sham groups (D,E).
Fig. 13.
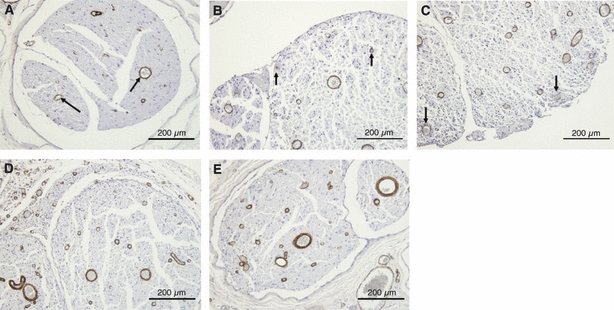
Immunohistochemical study of α-SMA expression in the sham group and the epi-perineurium removal group. In the sham group, α-SMA was expressed only at vessel walls (black arrows). At 2 days after surgery, α-SMA was expressed in the endoneurium, especially around vessels (arrows) (B). At 7 days, α-SMA was expressed at the edge of the nerve fascicles (arrows) (C). At 20 days, α-SMA was expressed in the endoneurium but not in the perineurium (D). There was a significant difference in the percentage area expressing α-SMA in the endoneurium removal group at days 2, 7 and 20 compared with the sham group. At 42 days, α-SMA was expressed only at the vascular wall, and the expression patterns matched those observed for the sham group (E). (bar = 200 μm).
Two days after removal of the epi-perineurium, marked endoneurial swelling in the outer portion of the nerve fascicles was observed in sections stained with toluidine blue (Fig. 7A). There was a significant difference in the percentage area stained with toluidine blue in the epi-perineurium removal group compared with the sham group (Table 1). In the central portion of the nerve, most of the myelinated nerve fibers remained intact (Fig. 7B); however, in the swollen outer portion, some demyelination and degeneration of nerve fibers was noted (Fig. 7C). Englobed myelinated nerves were observed by electron microscopic examination (Fig. 7D).
Fig. 7.
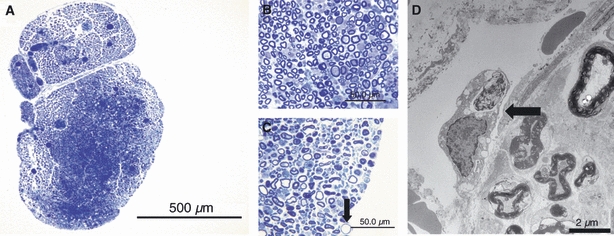
At 2 days after removal of the epi-perineurium, marked endoneurial swelling in the outer portion was observed in sections stained for toluidine blue (bar = 500 μm) (A). In the central portion of the nerve fascicle, most of the myelinated nerve fibers remained intact (bar = 50 μm) (B). However, in the swollen outer portion, degeneration of the nerve fibers was noted (arrow) (bar = 50 μm) (C). An englobed myelinated nerve (arrow) outside the endoneurium was observed by electron microscopic examination (bar = 2 μm) (D).
Table 1.
Comparison of the endoneurial toluidine blue-stained area
| Epi-perineurium removal group | |||||
|---|---|---|---|---|---|
| Group (n = 3) | Sham group | day 2 | day 7 | day 20 | day 42 |
| % area | 78.0 ± 0.04 | 55.3 ± 0.09* | 49.3 ± 0.02* | 39.3 ± 0.16* | 53.7 ± 0.25 |
Data are expressed as mean ± SD. Units are percentages. At days 2, 7, and 20 in the epi-perineurium removal group, the percentage area of toluidine blue staining in the endoneurium was significantly different from the sham group.
Significantly different from the sham group (P < 0.05).
TN-C (4F10TT) was overexpressed in the endoneurium, especially in the outer portion of the fascicles, and the difference was significant between groups (Fig. 11B, Table 2). TN-C (4C8MS) was expressed at low levels in the outer portion of the nerve (Fig. 12B, Table 3). α-SMA was expressed in the endoneurium, especially around vessels. There was a significant difference between the groups (Fig. 13B, Table 4).
Table 2.
Comparison of the endoneurial TN-C (4F10TT)-stained area
| Epi-perineurium removal group | |||||
|---|---|---|---|---|---|
| Group (n = 3) | Sham group | day 2 | day 7 | day 20 | day 42 |
| % area | 1.2 ± 0.00 | 12.2 ± 0.01** | 14.9 ± 0.07* | 3.0 ± 0.02 | 1.0 ± 0.00 |
Data are expressed as mean ± SD. Units are percentages. At days 2 and 7 in the epi-perineurium removal group, overexpression of TN-C (4F10TT) was significantly different from the sham group.
Significantly different from the sham group (P < 0.05).
Significantly different from the sham group (P < 0.01).
Table 3.
Comparison of the endoneurial TN-C (4C8MS)-stained area
| Epi-perineurium removal group | |||||
|---|---|---|---|---|---|
| Group (n = 3) | Sham group | day 2 | day 7 | day 20 | day 42 |
| % area | 0.5 ± 0.00 | 0.8 ± 0.00 | 1.2 ± 0.01 | 0.5 ± 0.00 | 0.3 ± 0.00 |
Data are expressed as mean ± SD. Units are percentages. Expression of TN-C (4C8MS) was increased at day 7 in the epi-perineurium removal group. However, the difference compared with the sham group was not significant.
Table 4.
Comparison of the endoneurial α-SMA-stained area
| Epi-perineurium removal group | |||||
|---|---|---|---|---|---|
| Group (n = 3) | Sham group | day 2 | day 7 | day 20 | day 42 |
| % area | 0.7 ± 0.00 | 1.5 ± 0.00* | 2.9 ± 0.01* | 1.4 ± 0.00* | 1.7 ± 0.01 |
Data are expressed as mean ± SD. Units are percentages. At days 2, 7, and 20, endoneurial expression of α-SMA was significantly increased.
Significantly different from the sham group (P < 0.05).
Seven days after removal of the epi-perineurium, the area of endoneurial swelling became larger (Fig. 8A). There was a significant difference in the percentage area stained with toluidine blue in the epi-perineurium removal group compared with the sham group (Table 1). Nerve fibers in the central portion of the nerve fascicles remained intact (Fig. 8B), but demyelinated and degenerated nerve fibers were noted in the outer portion of the endoneurium (Fig. 8C). Electron microscopy revealed englobed myelinated nerves and loss of collagen fibers in the extracellular matrix (Fig. 8D). TN-C (4F10TT) was overexpressed in the outer portion, particularly at the edge of the nerve fascicles (Fig. 11C). There was a significant difference in TN-C (4F10TT) expression in the epi-perineurium removal group compared with the sham group (Table 2). TN-C (4C8MS) was expressed at low levels at the edge of the nerve fascicles (Fig. 12C, Table 3). α-SMA was expressed in the endoneurium, especially around vessels (Fig. 13C). There was a significant difference in α-SMA expression in the epi-perineurium removal group compared with the sham group (Table 4). Therefore, myofibroblasts participated in regeneration of the perineurium.
Fig. 8.
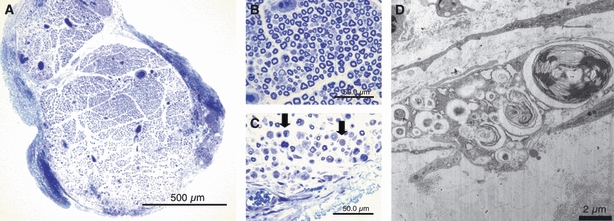
At 7 days after removal of the epi-perineurium, the area of endoneurial swelling became larger (bar = 500 μm) (A). The central portion of the endoneurium remained intact (bar = 50 μm) (B), but demyelinated and degenerated nerve fibers were noted in the outer portion of the endoneurium (arrows) (bar = 50 μm) (C). Electron microscopy revealed an englobed myelinated nerve and loss of collagen fibers in the extracellular matrix (bar = 2 μm) (D).
At 20 days after removal of the epi-perineurium, the area of endoneurial swelling spread further (Fig. 9A), and there was a significant difference between groups (Table 1). Demyelinated nerve fibers were noted in sections stained for toluidine blue in the outer portion (Fig. 9B) and near the central portion (Fig. 9C) of the endoneurium. Electron microscopy revealed that endoneurial fibroblast-like cells were assembling in the periphery of the nerve fascicles, forming a lamellar structure (Fig. 9D).
Fig. 9.
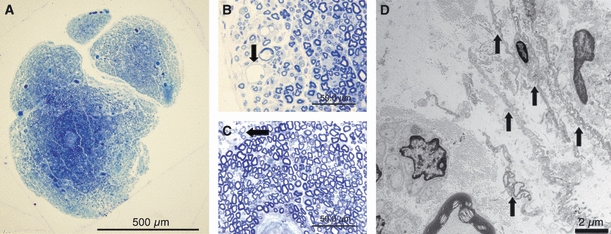
At 20 days after removal of the epi-perineurium, the area of endoneurial swelling spread further (bar = 500 μm) (A), and degenerated nerve fibers (arrow) were noted in toluidine blue-stained sections in the outer portion (bar = 50 μm) (B) and in the central portion (arrow) (bar = 50 μm) (C) of the endoneurium. However, regeneration of the perineurium by the laminar structure of endoneurial fibroblast-like cells (arrows) were observed by electron microscopic examination (bar = 2 μm) (D).
TN-C (4F10TT) was expressed in the periphery of the nerve fascicles with adhesion and was not expressed in the nerve fascicles (Fig. 11D, Table 2). There was no appreciable difference in the expression pattern of TN-C (4C8MS) in terms of spatial distribution or intensity between the operated and sham groups (Fig. 12D, Table 3). α-SMA was expressed in the endoneurium but not in the perineurium (Fig. 13D). There was a significant difference in the percentage area expressing α-SMA in the epi-perineurium removal group compared with the sham group (Table 4).
At 42 days after removal of the epi-perineurium, the difference in the outer and central portions of the nerve diminished, although the whole nerve was slightly swollen (Fig. 10A). There was no significant difference in the percentage area stained with toluidine blue between the operated and sham groups (Table 1). In the subperineurial area, re-myelinated nerve fibers were noted in sections stained with toluidine blue (Fig. 10B) and in electron microscopy (Fig. 10D). In addition, nerve fibers that had spread to the outside were also observed (Fig. 10C).
Fig. 10.
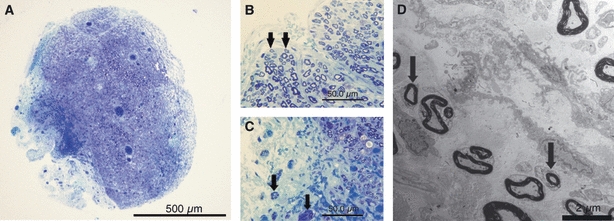
At 42 days after removal of the epi-perineurium, differences in the outer and central portions diminished (bar = 500 μm) (A), and the whole nerve was slightly swollen. In the subperineurial area, re-myelinated nerve fibers were noted in sections stained for toluidine blue (arrows) (bar = 50 μm) (B) and in electron microscopy (arrows) (bar = 2 μm) (D). However, nerve fibers that spread to the outside were also observed (arrows) (bar = 50 μm) (C).
TN-C (4F10TT) was expressed in the surrounding nerve fascicles and appeared to be an original perineurium (Fig. 11E). TN-C (4C8MS) and α-SMA were expressed only at the vascular wall, and the expression patterns matched those observed for the sham group (Figs 12E, 13E).
Figure 14 shows the number of myelinated nerve fibers in the center and the periphery of both groups. The number of myelinated nerve fibers decreased after resection of the epi-perineurium. There was a significant difference between the center and periphery in the epi-perineurium removal group at day 7. Recovery of the number of myelinated nerve fibers after epi-perineurium removal was observed at day 20.
Fig. 14.
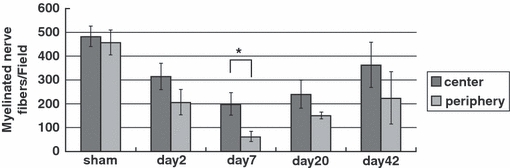
The number of myelinated nerve fibers in the center and periphery of both groups. The number of myelinated nerve fibers decreased after resection of the epi-perineurium. There was a significant difference between the center and periphery in the epi-perineurium removal group at day 7 (*P < 0.05). Recovery of the number of myelinated nerve fibers after epi-perineurium removal was observed after day 20.
Wet muscle study
Figure 15 shows the time course for wet muscle weights in the two groups. Percentages of wet muscle weights of the sum of tibialis anterior and gastrocnemius muscles to the total body weights in each group are shown. The epi-perineurium removal group showed a significantly (P < 0.05) lower muscle weight than the sham group at days 7 and 20 only. Recovery of muscle weight after epi-perineurium removal was observed at day 42.
Fig. 15.
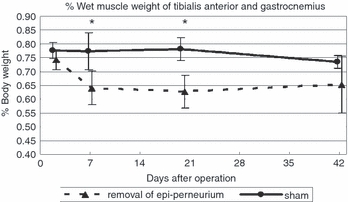
Time course of wet weight muscle changes in both groups. Percentages of wet muscle weights of the sum of tibialis anterior and gastrocnemius muscles to total body weights in each group are shown (*P < 0.05).
Discussion
A long-standing controversy about the origin of the perineurium was thought to have been settled by the work of Bunge et al. (1989). They used an in vitro method of cell marking with retroviral-mediated gene transfer and showed that the perineurium is derived from fibroblasts and not from Schwann cells. However, it is still not clear how the perineurium regenerates in vivo after removal. In this study, the regenerative process of the perineurium and nerve function were examined using an in vivo model of the perineurium of the rat sciatic nerve.
TN-C is a glycoprotein that is highly expressed during embryogenesis and transiently expressed during organogenesis; it is absent or reduced in developed organs, but reappears under pathological conditions caused by infection, inflammation, or during tumorigenesis (Chiquet-Ehrismann & Chiquet, 2003). Tenascins are synthesized by cells in connective tissue, and it is well known that fibroblasts produce TN-C (Chiquet-Ehrismann & Tucker, 2004). In the peripheral nervous system, although TN-C is diffusely expressed during neurogenesis, only the perineurium continues to express this molecule after birth (Chiquet & Fambrough, 1984). Five members of the tenascin glycoprotein family – X, Y, R, W, and C – have been identified. Of these, both TN-C and TN-R are involved in the early development of the nervous system. Once maturity has been achieved, levels are tightly regulated, particularly of TN-C, with altered expression patterns occurring as part of the regenerative response (Jones & Jones, 2000). Studies using rodent models of nerve injury appear to suggest that TN-C promotes recovery following nerve lesions, whereas TN-R may be inhibitory (Guntinas-Lichius et al. 2005). In this study, TN-C (4F10TT) was seen in the perineurium and vessel wall in the sham group. However, TN-C (4C8MS) was expressed only at the vessels in the sham group. At days 2 and 7, TN-C (4C8MS) was weakly expressed at the edge of the nerve fascicles. At days 20 and 42, TN-C (4C8MS) was expressed only at the vessels, similar to the pattern of expression in the sham group. In contrast, TN-C (4F10TT) was overexpressed in the outer portion of the nerve fascicle 2 days after removal of the epi-perineurium. At 7 days, it was overexpressed particularly at the edge of the nerve fascicles. This result indicates that TN-C (4F10TT)-positive endoneural fibroblast-like cells have the potential to become perineurial cells. At 20 days after removal of the epi-perineurium, expression of TN-C (4F10TT) was reduced in the endoneurium but was clearly positive around the nerve fascicles. In addition, the newly formed perineurium adhered to the fascicles. Furthermore, electron microscopy revealed regeneration of the perineurium by the lamellar formation of endoneurial fibroblasts-like cells at that time. At 42 days, TN-C (4F10TT) was expressed with a temperate gap between the endoneurium, similar to the expression pattern in the sham group. Therefore, the regenerative process was thought to be complete. These results show that perineurial cells or their precursors are present in the endoneurium and migrate to the outer side of the nerve to finally regenerate the damaged perineurium.
α-SMA was expressed at capillary vessels in both the sham group and the epi-perineurium removal group. Moreover, expression in the perineurium was not observed at any time point. Therefore, myofibroblasts do not migrate to the perineurium during the regeneration of the perineurium. However, the expression of α-SMA in the endoneurium was enhanced, especially around vessels at days 2, 7, and 20. Indirect participation of myofibroblasts during the regenerative process of the perineurium should be considered.
Marked endoneurial swelling in the outer portion was illustrated in sections stained with toluidine blue 2 days after removal of the epi-perineurium. In contrast, myelinated nerve fibers were maintained in the central portion of the endoneurium at that time. According to the time course, as the swollen area became larger, the number of demyelinated nerves increased. Consequently, latency of the CMAPs was extremely delayed in the epi-perineurium removal group at day 20. At day 42, although endoneurial swelling reached toward the central portion of the nerve, re-myelination was observed in the subperineurial endoneurium. Accordingly, recovery of electrophysiological function was observed. However, wet muscle weight recovery was delayed because some nerve fibers were not present inside the perineurium. It is possible that compartmentalization errors may occur during regeneration of the perineurium, resulting in a distance between the nerve lesion and the innervated muscle. Terho et al. (2002) reported an endoneurial response to microsurgically removed epi-perineurium of 4–5 mm in length. They showed that at the surgical area, the central portion (65% of the total area of the endoneurium) remained intact up to 5 weeks postoperatively, and the outer portion of the endoneurium (35% of the total area) reacted strongly and showed Wallerian degeneration (Terho et al. 2002). In our 15-mm resection model, two different lamellar structures consisting of the swollen outer portion and the central portion of the endoneurium were found. However, the ratio of the two components changed with time, and as the swollen outer portion became larger, the intact central portion became smaller. As electron microscopy revealed loss of collagen fibers in the extracellular matrix and the existence of endoneurial fibroblasts in various forms, endoneurial swelling may help endoneurial fibroblast-like cells migrate and regenerate in the perineurium.
In a previous report, animals did not show any paresis of the hindlimb, and no autotomy of the digits was observed clinically after a 4–5-mm section of the perineurium was microsurgically removed (Terho et al. 2002). In our study, a 15-mm section of epi-perineurium was removed. No autotomy of the digits was observed, but some paresis and reaction due to pain were found. Two days after resection of the epi-perineurium, the print area and max area decreased significantly with CatWalk® gait analysis. In the sciatic nerve resection model, these parameters were immediately and severely affected in the ipsilateral paw. The CatWalk® objectively detects dynamic and static gait impairments (Deumens et al. 2007).
A significant loss of intensity of the affected paw was observed 2 and 7 days after resection of the epi-perineurium. CatWalk® parameters such as intensity, swing phase, and stance phase show a high degree of correlation with mechanical withdrawal thresholds, as determined by von Frey filaments (Vrinten & Hamers, 2003; Gabriel et al. 2007). Using the acute inflammatory pain model, Gabriel et al. (2009) reported an absence of correlation between mechanical withdrawal threshold as assessed with the von Frey test and CatWalk® parameters of intensity and duty factor during the chronic phase. They concluded that the use of CatWalk®-based gait analysis as a pain test as well as the correlation of its data with the development of mechanical allodynia are highly dependent on the pain model used. Here, the presence of nerve damage may be pivotal (Gabriel et al. 2009). It is true that CatWalk® pain-related parameters such as intensity, duration of stance, and swing phase tend to recover as compared with the von Frey test. Accordingly, if CatWalk® pain-related parameters are significantly different than the sham group or preoperative value, the behavioral reactions can be considered to be due to pain. Therefore, resection of the epi-perineurium causes not only motor paresis but also neuropathic pain in the early phase.
Our method of removing a 15-mm section of the epi-perineurium caused short-interval motor paresis and neuropathic pain; however, with regeneration of the perineurium, the function of the nerves recovered within 6 weeks.
As this study was conducted in rats, differences between species may be significant regarding the regeneration process of the perineurium and nerve function. When considering the data in the realm of clinical practice, a careful justification is required.
Concluding remarks
The regenerative process of the perineurium and nerve function were evaluated by pathology, electrophysiology, a wet muscle study, and behaviorally using the CatWalk® gait analysis. After resection of a 15-mm section of the epi-perineurium, immediate endoneurial swelling occurred in the outer portion of the nerve and spread into the central portion of the nerve. Although demyelination and degeneration of the nerve fibers were found in the swollen area, remyelination and recovery of electrophysiological function were seen after regeneration of the perineurium. Immunohistological and electron microscopic studies revealed that the perineurium regenerated by fusion of the residual interfascicular perineurium and endoneurial fibroblast-like cells. CatWalk® gait analysis showed not only motor paresis but also neuropathic pain in the early phase of this model. For internal neurolysis, fasciculectomy may be an option for peripheral nerve surgery when care is taken during microsurgery to minimize damage to the axons.
Acknowledgments
None of the authors of this manuscript has received any type of support, benefits or funding from any commercial party related directly or indirectly to the subject of this article.
References
- Brown BA. Internal neurolysis in treatment of traumatic peripheral nerve lesions. Calif Med. 1969;110:460–462. [PMC free article] [PubMed] [Google Scholar]
- Brown BA. Internal neurolysis in traumatic peripheral-nerve lesions in continuity. Surg Clin North Am. 1972;52:1167–1175. doi: 10.1016/s0039-6109(16)39834-6. [DOI] [PubMed] [Google Scholar]
- Bunge MB, Wood PM, Tynan LB, et al. Perineurium originates from fibroblasts – demonstration in vitro with a retroviral marker. Science. 1989;243:229–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- Chiquet M, Fambrough DM. Chick myotendinous antigen. 1. A monoclonal-antibody as a marker for tendon and muscle morphogenesis. J Cell Biol. 1984;98:1926–1936. doi: 10.1083/jcb.98.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200:488–499. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Tucker RP. Connective tissues: signalling by tenascins. Int J Biochem Cell Biol. 2004;36:1085–1089. doi: 10.1016/j.biocel.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Curtis RM, Eversman WW. Internal neurolysis as an adjunct to treatment of carpal-tunnel syndrome. J Bone Joint Surg Am. 1973;55:733–740. [PubMed] [Google Scholar]
- Deumens R, Jaken RJP, Marcus MAE, et al. The CatWalk gait analysis in assessment of both dynamic and static gait changes after adult rat sciatic nerve resection. J Neurosci Methods. 2007;164:120–130. doi: 10.1016/j.jneumeth.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Donner TR, Voorhies RM, Kline DG. Neural sheath tumors of major nerves. J Neurosurg. 1994;81:362–373. doi: 10.3171/jns.1994.81.3.0362. [DOI] [PubMed] [Google Scholar]
- Erickson HP, Bourdon MA. Tenascin – an extracellular-matrix protein prominent in specialized embryonic-tissues and tumors. Annu Rev Cell Biol. 1989;5:71–92. doi: 10.1146/annurev.cb.05.110189.000443. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gabriel AF, Marcus MAE, Honig WMM, et al. The CatWalk method: a detailed analysis of behavioral changes after acute inflammatory pain in the rat. J Neurosci Methods. 2007;163:9–16. doi: 10.1016/j.jneumeth.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gabriel AF, Marcus MAE, Walenkamp GHIM, et al. The CatWalk method: assessment of mechanical allodynia in experimental chronic pain. Behav Brain Res. 2009;198:477–480. doi: 10.1016/j.bbr.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Guntinas-Lichius O, Angelov DN, Morellini F, et al. Opposite impacts of tenascin-C and tenascin-R deficiency in mice on the functional outcome of facial nerve repair. Eur J Neurosci. 2005;22:2171–2179. doi: 10.1111/j.1460-9568.2005.04424.x. [DOI] [PubMed] [Google Scholar]
- Haftek J. Stretch injury of peripheral nerve acute effects of stretching on rabbit nerve. J Bone Joint Surg Br. 1970;52:354–365. [PubMed] [Google Scholar]
- Hill R. Extracellular matrix remodelling in human diabetic neuropathy. J Anat. 2009;214:219–225. doi: 10.1111/j.1469-7580.2008.01026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones FS, Jones PL. The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Kato H, Minami A, Kobayashi M, et al. Functional results of low median and ulnar nerve repair with intraneural fascicular dissection and electrical fascicular orientation. J Hand Surg Am. 1998;23:471–482. doi: 10.1016/S0363-5023(05)80465-4. [DOI] [PubMed] [Google Scholar]
- Mackinnon SE, McCabe S, Murray JF, et al. Internal neurolysis fails to improve the results of primary carpal-tunnel decompression. J Hand Surg Am. 1991;16:211–218. doi: 10.1016/s0363-5023(10)80099-1. [DOI] [PubMed] [Google Scholar]
- Millesi H, Rath TH, Reihsner R, et al. Microsurgical neurolysis: its anatomical and physiological basis and its classification. Microsurgery. 1993;14:430–439. doi: 10.1002/micr.1920140703. [DOI] [PubMed] [Google Scholar]
- Nagano A, Shibata K, Tokimura H, et al. Spontaneous anterior interosseous nerve palsy with hourglass-like fascicular constriction within the main trunk of the median nerve. J Hand Surg Am. 1996;21:266–270. doi: 10.1016/S0363-5023(96)80114-6. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Hirata H, Tsujii M, et al. Pathomechanism of entrapment neuropathy in diabetic and nondiabetic rats reared in wire cages. Histol Histopathol. 2008;23:157–166. doi: 10.14670/HH-23.157. [DOI] [PubMed] [Google Scholar]
- Oberlin C, Beal D, Leechavengvongs S, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial-plexus – anatomical study and report of 4 cases. J Hand Surg Am. 1994;19:232–237. doi: 10.1016/0363-5023(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Olsson Y, Kristens K. Perineurium as a diffusion barrier to protein tracers following trauma to nerves. Acta Neuropathol. 1973;23:105–111. doi: 10.1007/BF00685764. [DOI] [PubMed] [Google Scholar]
- Phalen GS. The birth of a syndrome, or carpal-tunnel revisited. J Hand Surg Am. 1981;6:109–110. doi: 10.1016/s0363-5023(81)80163-3. [DOI] [PubMed] [Google Scholar]
- Pina-Oviedo S, Ortiz-Hidalgo C. The normal and neoplastic perineurium. Adv Anat Pathol. 2008;15:147–164. doi: 10.1097/PAP.0b013e31816f8519. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood nerve and blood-brain barriers. Proc Natl Acad Sci USA. 1994;91:5705–5709. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SM. Preserve the nerve: microsurgical resection of peripheral nerve sheath tumors. Neurosurgery. 2007;61:113–117. doi: 10.1227/01.neu.0000289724.89588.bc. [DOI] [PubMed] [Google Scholar]
- Rydevik B, Lundborg G, Nordborg C. Intraneural tissue-reactions induced by internal neurolysis – experimental-study on blood nerve barrier, connective tissues and nerve-fibers of rabbit tibial nerve. Scand J Plast Reconstr Surg Hand Surg. 1976;10:3–8. doi: 10.1080/02844317609169741. [DOI] [PubMed] [Google Scholar]
- Sakurai M, Miyasaka Y. Neural fibrosis and the effect of neurolysis. J Bone Joint Surg Br. 1986;68:483–488. doi: 10.1302/0301-620X.68B3.3015976. [DOI] [PubMed] [Google Scholar]
- Shanthaveerappa TR, Bourne GH. Perineural epithelium – a new concept of its role in integrity of peripheral nervous system. Science. 1966;154:1464–1467. doi: 10.1126/science.154.3755.1464. [DOI] [PubMed] [Google Scholar]
- Sungpet A, Suphachatwong C, Kawinwonggowit V, et al. Transfer of a single fascicle from the ulnar nerve to the biceps muscle after avulsions of upper roots of the brachial plexus. J Hand Surg Br. 2000;25:325–328. doi: 10.1054/jhsb.2000.0367. [DOI] [PubMed] [Google Scholar]
- Tamaoki M, Imanaka-Yoshida K, Yokoyama K, et al. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terho PM, Vuorinen VS, Roytta M. The endoneurial response to microsurgically removed epi- and perineurium. J Peripher Nerv Syst. 2002;7:155–162. doi: 10.1046/j.1529-8027.2002.02015.x. [DOI] [PubMed] [Google Scholar]
- Viterbo F, Trindade JC, Hoshino K, et al. Two end-to-side neurorrhaphies and nerve graft with removal of the epineural sheath – experimental study in rats. Br J Plast Surg. 1994;47:75–80. doi: 10.1016/0007-1226(94)90162-7. [DOI] [PubMed] [Google Scholar]
- Vrinten DH, Hamers FFT. ‘CatWalk’ automated quantitative gait analysis as a novel method to assess mechanical allodynia in the rat; a comparison with von Frey testing. Pain. 2003;102:203–209. doi: 10.1016/s0304-3959(02)00382-2. [DOI] [PubMed] [Google Scholar]


