Abstract
We analyzed renal cell cancer incidence patterns in the United States and reviewed recent epidemiologic evidence with regard to environmental and host genetic determinants of renal cell cancer risk. Renal cell cancer incidence rates continued to rise among all racial/ethnic groups in the United States, across all age groups, and for all tumor sizes, with the most rapid increases for localized stage disease and small tumors. Recent cohort studies confirmed the association of smoking, excess body weight, and hypertension with an elevated risk of renal cell cancer, and suggested that these factors can be modified to reduce the risk. There is increasing evidence for an inverse association between renal cell cancer risk and physical activity and moderate intake of alcohol. Occupational exposure to TCE has been positively associated with renal cell cancer risk in several recent studies, but its link with somatic mutations of the VHL gene has not been confirmed. Studies of genetic polymorphisms in relation to renal cell cancer risk have produced mixed results, but genome-wide association studies with larger sample size and a more comprehensive approach are underway. Few epidemiologic studies have evaluated risk factors by subtypes of renal cell cancer defined by somatic mutations and other tumor markers.
Keywords: renal cell cancer, incidence trends, cohort studies, smoking, obesity, hypertension, diet, occupation, genetic polymorphism, somatic mutation
Malignant tumors of the kidney account for about 4% of cancer incidence and 2% of cancer mortality in the United States, with over 54,000 new cases and 13,000 deaths having been estimated for 2008.1 More than 85% of kidney cancers arise in the renal parenchyma, with the remainder arising in the renal pelvis.2 Renal pelvis cancer is mostly of the transitional cell type and is not the focus of this review. Nearly all cancers originating in the renal parenchyma are adenocarcinomas (renal cell carcinoma), in which clear cell is the predominant subtype.3 The remainder is made up of the papillary type and other rare histologic types, such as collecting duct and chromophobe carcinomas.3,4 Distinct morphologic and genetic characteristics have also been described for renal cell carcinoma in familial cancer syndromes.5–7 Descriptive and analytic epidemiologic studies in general have not distinguished the subtypes of renal cell carcinoma, except for studies involving the von Hippel-Lindau (VHL) gene mutation that is seen primarily in the clear cell type.8 Since the clear cell type comprises 85% to 90% of renal cell carcinoma,3 the epidemiology of renal cell carcinoma is strongly influenced by that of the clear cell type.
DESCRIPTIVE EPIDEMIOLOGY
Incidence patterns and temporal trends
Incidence rates of renal cell carcinoma have been reported to be higher among men than women, and among African Americans than Caucasians in the United States.2,9,10 Herein, we used data from the U.S. Surveillance, Epidemiology, and End Results (SEER) program which has collected population-based cancer incidence and survival data among white and black residents of nine areas since 1973 (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco, Seattle, and Utah), an additional four areas since 1992 (Los Angeles, San Jose-Monterey, Rural Georgia, and Alaska Native Registry), and another four since 2000 (rest of California, Kentucky, Louisiana, and New Jersey.11–15 Data were available for the first time for Asian/Pacific Islanders, American Indian/Alaska Natives, and Hispanics since 1992. There were too few cases for analysis among American Indian/Alaska Natives, so recent data were based on the 12 SEER areas excluding Alaska. We found that incidence rates of renal cell cancer were highest among African Americans, whereas rates for whites and Hispanics were similar (Table 1). Asian/Pacific Islanders had about half the incidence rates of those of the other racial/ethnic groups. In general, the incidence rate in females was about half that of males, regardless of racial/ethnic origins. Compared to males, incidence rates among females were more comparable across racial/ethnic groups, except for the lower rates among Asian/Pacific Islanders. The substantially lower incidence rates among Asian Americans mirrored the international data showing lower incidence rates of kidney cancer in most Asian countries.16
Table 1.
Age-adjusted (2000 US standard) incidence rates of microscopically-confirmed renal cell carcinoma per 100,000 person-years by race/ethnicity and gender, SEER-12, 2002–2005.
| Male | Female | |
|---|---|---|
| African Americans | 17.0 | 7.5 |
| Whites | 14.3 | 7.2 |
| Asian/Pacific Islanders | 7.8 | 3.7 |
| Hispanics | 13.8 | 7.3 |
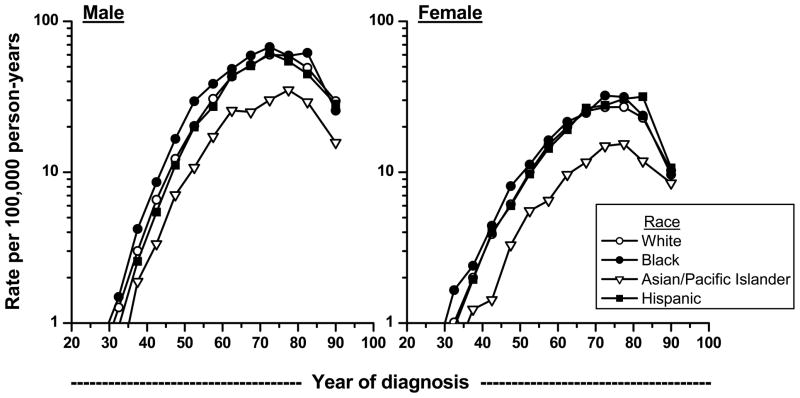
Incidence rates during 1992 to 2005 increased consistently with age, before plateauing around the age of 70 years (Figure 1). The drop in rates among the older age groups is likely an artifact due to less rigorous diagnostic workup. When we included cases without microscopic confirmation, the drop in rates for those over age 85 became less precipitous (data not shown). The higher rates for African Americans were seen across all age groups except the oldest in both men and women. Asian/Pacific Islanders of all ages had lower rates. It has been shown recently in 12 European centers that renal cell cancer patients aged 40 years or younger were more likely to have had symptoms and to be diagnosed at an early stage and with chromophobe and papillary cancers.3
Figure 1.
Age-specific incidence rates of microscopically-confirmed renal cell carcinoma by racial/ethnic group and sex, SEER-12 1992–2005.
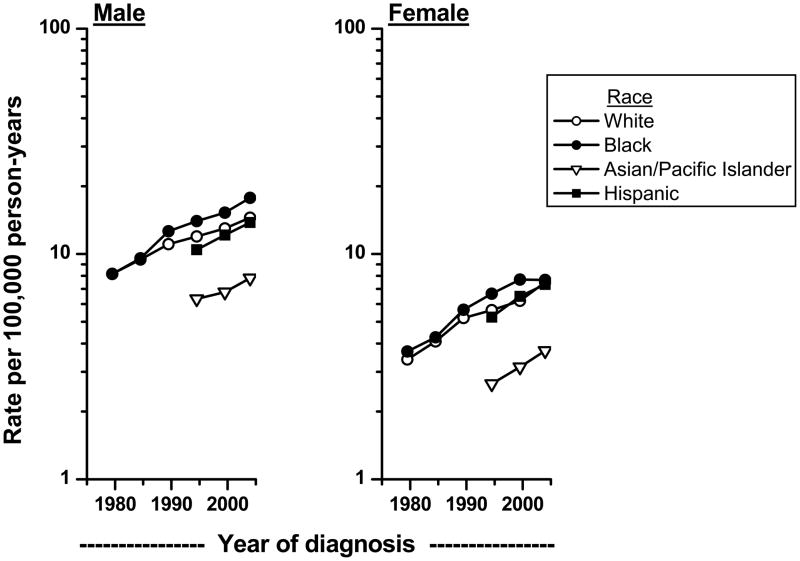
Increasing incidence rates of renal cell cancer have been previously reported in the U.S. and other countries.2,16–19 Recent analysis of the SEER data indicated that the increasing trends have continued into 2005 (Figure 2). The increases in incidence among African Americans have outpaced those of whites. The newly available data on Asian/Pacific Islanders and Hispanics showed that incidence rates in these groups also are increasing.
Figure 2.
Trends in age-adjusted (2000 US standard) microscopically-confirmed renal cell carcinoma incidence rates by sex among whites and blacks in SEER-9 during 1977–81 to 2002–05 and among Asian/Pacific Islanders and Hispanics in SEER-12 during 1992–96 to 2002–05.
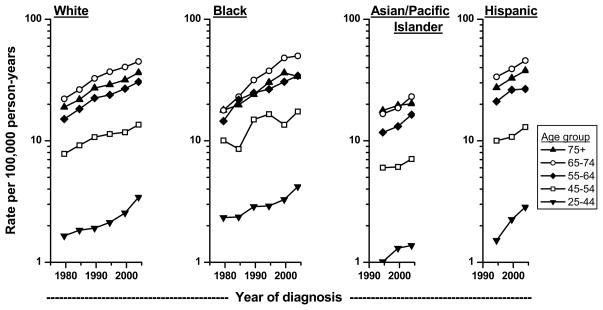
The upward trends of renal cell cancer occurred across all ages among all race/ethnic groups (Figure 3). The increases were remarkably comparable among the different age groups during the same time period, suggesting that factors contributing to the increasing incidence have affected the entire population rather than only certain subgroups over time. Of interest is the apparent more rapid increase in recent years among whites and blacks 25–44 years of age.
Figure 3.
Trends in age-adjusted (2000 US standard) microscopically-confirmed renal cell carcinoma incidence rates by age group among whites and blacks in SEER-9 during 1977–81 to 2002–05 and among Asian/Pacific Islanders and Hispanics in SEER-12 during 1992–96 to 2002–05.
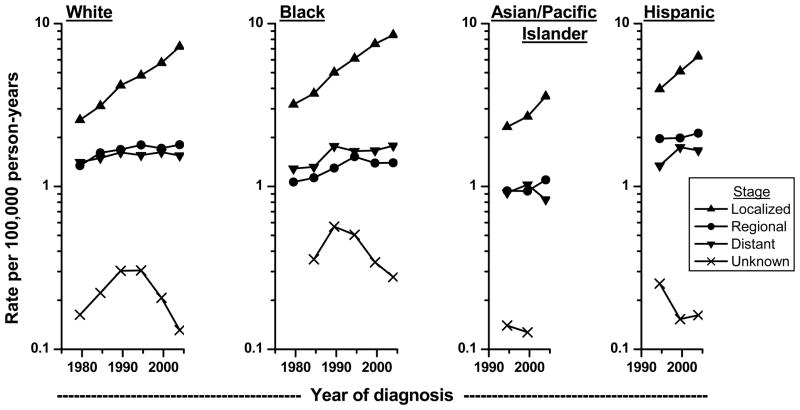
When examined by stage at diagnosis, most of the increases in incidence were seen in localized stage tumors (Figure 4). A small part of the increases by stage may be explained by the decline in tumors of unknown stage, possibly due to improvement in diagnostic workup over time. For whites and African Americans, the incidence trends for regional and distant tumors have leveled since the mid-1990s. For Asians and Hispanics, there is some suggestion of recent increase in regional tumors and decrease in distant tumors, but a longer time period is needed to definitively establish a trend pattern.
Figure 4.
Trends in age-adjusted (2000 US standard) microscopically-confirmed renal cell carcinoma incidence rates by stage of disease among whites and blacks in SEER-9 during 1977–81 to 2002–05 and among Asian/Pacific Islanders and Hispanics in SEER-12 during 1992–96 to 2002–05.
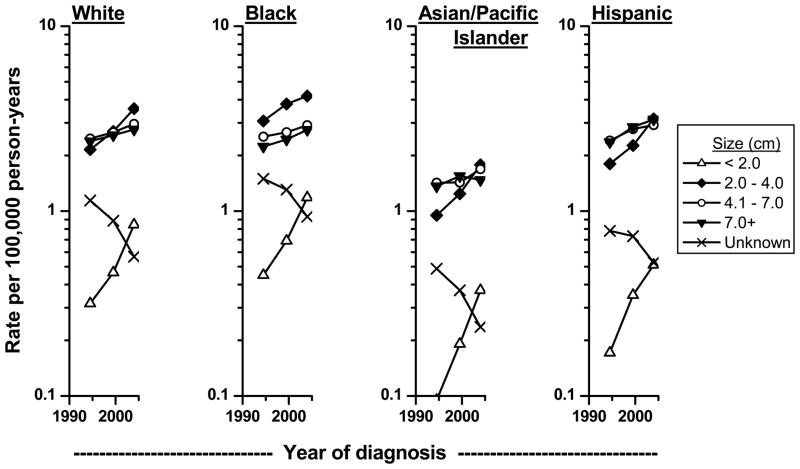
Tumor size data were available in SEER since 1988.12,13 Consistent with the trends by tumor stage, the most rapidly increasing trends are observed for the smallest tumors (< 2 cm), followed by tumors 2–4 cm in size (Figure 5). Of note, tumors of larger size also have increased over time. As with the stage findings, the decrease in tumors of unknown size may have contributed to the apparent increases in incidence rates for tumors of known size. These findings are consistent with previous observations that the average size of renal cell cancer at diagnosis has decreased over time.20,21 Even within Stage I tumors (defined as a tumor 7 cm or less that is confined to the kidney), the average size of renal cell carcinoma diagnosed in the U.S. has decreased over time.21 While some of the small tumors were likely diagnosed incidentally,22 it has been estimated that most of the preclinical renal carcinoma detected by CT screening among middle-aged Americans will progress to clinical symptoms and diagnosis in the absence of screening.23
Figure 5.
Trends in age-adjusted (2000 US standard) microscopically-confirmed renal cell carcinoma incidence rates by size of tumor among whites and blacks in SEER-9 during 1977–81 to 2002–05 and among Asian/Pacific Islanders and Hispanics in SEER-12 during 1992–96 to 2002–05.
In summary, we found that renal cell cancer incidence rates continued to rise among all racial/ethnic groups in the United States, across all age groups, and for all tumor sizes, with the most rapid increases for localized stage disease and small tumors. These findings imply that part of the rising rates may be related to improving detection and earlier diagnosis of cancers, but the increases in even large size cancers and young and middle-aged groups suggest that changes in the prevalence of risk factors may also be playing a role.
Stage at diagnosis and survival
With the more rapid increasing rate of localized tumors than tumors of other stages, an increasing proportion of renal cell carcinomas are diagnosed at the localized stage. During 2002–2005, the percentage of renal cell carcinoma diagnosed at the localized stage among men in the SEER-12 was 68% in African Americans, 64% in whites, 62% in Asian/Pacific Islanders, and 59% in Hispanics. The corresponding percentages of localized tumors diagnosed among females were 73%, 71%, 70%, and 64%, respectively.
The overall 5-year relative survival rate, which is the percentage of patients diagnosed with renal cell cancer who survived 5 years after diagnosis, adjusting for the expected survival experience of the general population, has improved over time (Table 2).14 Some of the improvement in the overall survival could be attributed to the increasing proportion of tumors diagnosed at an early stage. Among patients diagnosed during 1995–2004, the 5-year relative survival rates for patients with localized tumors ranged from 85.8% for African American men to 93.4% for white women, compared to a range of 55% to 64.2% for regional tumors and 11% or lower for patients with distant disease. Similarly, a recent study showed that the decrease in tumor size at diagnosis could be contributing in part to improved survival among more recently diagnosed patients.20 However, survival improved even after adjusting for tumor size, suggesting that there is advancement in the management of renal cell cancer patients over time.
Table 2.
Five-year relative survival rates (%) for patients with microscopically-confirmed renal cell carcinoma by race, gender, and time period, SEER-17, 1985–2004, with follow-up through 2005.
| White men | White women | African American men | African American women | |||||
|---|---|---|---|---|---|---|---|---|
| Stage at diagnosis | 1985–1994 | 1995–2004 | 1985–1994 | 1995–2004 | 1985–1994 | 1995–2004 | 1985–1994 | 1995–2004 |
| Total invasive | 64.3 | 70.6 | 66.1 | 74.6 | 58.2 | 66.6 | 63.3 | 70.1 |
| Localized | 90.5 | 92.0 | 89.3 | 93.4 | 81.4 | 85.8 | 87.2 | 88.1 |
| Regional | 62.1 | 64.2 | 60.6 | 63.8 | 54.8 | 55.0 | 50.4 | 55.3 |
| Distant | 9.6 | 10.0 | 8.5 | 8.3 | 7.9 | 7.2 | 4.3 | 11.3 |
| Unstaged | 47.2 | 56.0 | 41.4 | 44.3 | 26.9 | 35.3 | 46.2 | 41.3 |
The increase in 5-year relative survival rates is apparent in all racial and gender groups, although the improvement is largely confined to patients with localized and regional tumors (Table 2). Within each tumor stage and gender, African Americans generally had lower 5-year relative survival than white patients. The relatively poorer prognosis among African Americans has also been observed in previous.2,9,10
RISK FACTORS
Several risk factors have been well-established for renal cell carcinoma, including tobacco use, obesity, and hypertension, although the complexity of these associations and their mechanisms have yet to be elucidated. Other risk factors, such as dietary practices, physical activity, and occupational exposures, also have been implicated, but the evidence remains inconclusive. Most of the initial observations identifying these risk factors came from case-control studies, in which histories of exposures were obtained from renal cell cancer patients after their diagnosis and were compared to the exposure experience of the non-diseased population. Increasingly, results from large cohort studies are being published as a sufficient number of renal cell cancer patients are accumulated in these maturing cohorts. Since the exposure data and biological samples for molecular analyses in cohort studies are collected at baseline prior to cancer diagnosis, results from these studies are generally believed to be less prone to recall bias and are unaffected by the cancer and its treatment. To the extent possible, this review relies on recent results from cohort studies worldwide. Some results from case-control studies also are included when they are complementary to the cohort results.
Cigarette smoking
Cigarette smoking is considered a causal risk factor for renal cell cancer by both the International Agency for Research on Cancer and the U.S. Surgeon General.24,25 The association between cigarette smoking and renal cell cancer is relatively weak, but it tends to be stronger among men than women. A meta-analysis of 24 studies showed that compared to lifetime never smokers, ever smoking increased renal cell cancer risk by 54% among men and 22% among women.26 A clear dose-response pattern of risk was apparent, with risk doubling among men and increasing 58% among women who smoked more than a pack of cigarettes per day. Using a modeling analytic approach and data from a large cohort of smoking men in Finland, Lubin et al estimated that smoking fewer cigarettes per day for a longer number of years posted a greater renal cell cancer risk than smoking at a higher intensity for a shorter duration.27 Smoking cessation reduces the risk, but only among long-term quitters of ten or more years.26,28 There is also evidence to suggest that occasional smoking29 and passive exposure to tobacco smoke among nonsmokers30 may increase the risk of renal cell cancer. In addition to carcinogens in tobacco smoke,26 cigarette smoking is hypothesized to increase renal cell cancer risk through chronic tissue hypoxia due to smoking-related conditions such as chronic obstructive pulmonary disease and exposure to carbon monoxide.31
Obesity
Excess body weight is a well-established risk factor for renal cell carcinoma. It has been estimated that over 40% of renal cell cancers in the United States may be attributable to obesity and overweight.32 Recent cohort studies confirmed the association between obesity and renal cell cancer observed in previous case-control studies.33–44 A meta-analysis of data from prospective observational studies estimated that the risk of developing renal cell cancer increased 24% and 34% for men and women, respectively, for every 5 kg/m2 increase in body mass index (BMI).45
Limited cohort studies with measurements of waist and hip circumferences suggested that waist-to-hip ratio (WHR) is also a predictor of renal cell cancer risk, but it remains unclear whether BMI and WHR confer risk independent of each other.35,40,41,44 In a cohort of U.S. women, weight gain or loss of ten pounds or more has been shown to increase risk independent of BMI, particularly among individuals with frequent weight fluctuations of this magnitude.41 In another cohort study of Swedish men in which multiple measurements of height and weight were taken during a succession of health examinations, risk increased further with increasing BMI over time. Compared to men whose weight remained stable, the risk was doubled for men whose BMI had increased more than 14% over a six-year period.33 An independent effect of weight gain has been recently confirmed in a large cohort of U.S. men and women.44 This study further demonstrated that the increased risk was mostly associated with weight gain during early adulthood, whereas weight gain after midlife was unrelated to risk.
The pathophysiology underlying the association between excess weight and renal cell cancer risk is not fully understood. Several mechanisms have been proposed, including increased levels of insulin, insulin-like growth factor I (IGF-I) and sex steroids, production of cytokines and adipokines by adipose tissue, lipid peroxidation and oxidative stress, and increased glomerular filtration rate and renal plasma flow.32, 46–48 Chronic hypoxia, which may result from obesity and related sleep apnea, has also been hypothesized as a potential risk factor.31 Empirical data supporting these hypotheses are still limited, but active research is underway to address these issues.
Hypertension and Use of Antihypertensive Medications
The link between hypertension and renal cell cancer risk was first noted in case-control studies. Since certain type of renal cell cancer49 and cancer treatment50 itself can cause hypertension, data on hypertension collected after renal cell cancer diagnosis are more difficult to interpret. However, sufficient evidence from cohort studies have accumulated linking hypertension reported at baseline to subsequent renal cell cancer incidence.35,38,43,51 Cohort studies with blood pressure measurements taken at baseline clinic visits have generally shown increasing risk of renal cell cancer with increasing blood pressure.33, 52–54 Compared to individuals with normal blood pressure, those with the highest blood pressure (≥100 mmHg diastolic pressure or ≥160 mmHg systolic pressure) were found to have twofold or higher risks. Use of diuretics and other antihypertensive medications also has been associated with an elevated risk, but this association is likely confounded by a history of hypertension.35,38,43,51,54–56 In a comprehensive evaluation of various antihypertensive medications based on record-linkage of population-based databases in Denmark, excess risk of renal cell cancer was observed only during short-term follow-up, and risks were reduced to insignificant levels five or more years after the baseline.56 These findings suggest that use of antihypertensive medications is probably not a causal risk factor. Indeed, in a cohort of Swedish men with sequential blood pressure measurements during follow-up, the risk further increased among those whose blood pressure increased above the baseline level and reduced among those whose blood pressure declined over time.33 These data suggest that hypertension is a promoting factor in renal cell cancer development, and the risk can be modified with better control of blood pressure.
The association between hypertension and renal cell cancer risk has been shown to be independent of the effects of excess body weight and cigarette smoking.33,38,43,51,53,54 Individuals who are both obese and hypertensive have greater risk of developing renal cell cancer than those who have only one of these conditions.33,43,54 The biological mechanism underlying the observed association between hypertension and renal cell cancer risk has yet to be elucidated. Lipid peroxidation and formation of reactive oxygen species are found to be elevated in hypertensive individuals and are hypothesized to play a role in renal cell cancer development.57 The chronic renal hypoxia accompanying hypertension has also been hypothesized to increase renal cell cancer risk through up-regulation of hypoxia-inducible factors, which may be a key mediator of kidney oncogenesis.31,54,58,59 Hypoxia-associated hypertension has been shown to increase proximal tubular cell proliferation and glomerular hypertrophy in animal models.60
Other Preexisting Conditions and Medication Use
Increased incidence of renal cell cancer has been observed among patients with uremia undergoing hemodialysis, particularly among patients on long-term dialysis and those with acquired cystic kidney disease.61,62 Elevated renal cell cancer occurrence also has been reported among patients with end stage renal disease awaiting renal transplant as well as renal transplant patients.63,64 In renal transplant patients, subsequent renal cell cancer was more commonly diagnosed in the native kidney than the transplanted kidney.65,66
Survivors of certain childhood cancers and other cancers such as esophageal adenocarcinoma have been shown to have an increased risk of subsequent renal cell cancer.67,68 Likewise, renal cell cancer patients have been shown to have an elevated risk of a second primary cancer,69,70 including renal cell cancer in the contralateral kidney.71
Patients hospitalized for diabetes mellitus have been reported to have an excess risk of renal cell cancer.72,73 Recent studies have suggested that diabetes mellitus may not be an independent risk factor, since its association with renal cell cancer was rendered insignificant after adjusting for obesity and hypertension.35,43,52,74
The use of statins, a class of commonly prescribed cholesterol-lowering agents, has recently been associated with a reduced risk of renal cell carcinoma in a large case-control study nested within a large database of U.S. veterans.75 Statins have been shown to inhibit renal cancer cell growth in vitro and decrease the amount of pulmonary metastasis in animals administered oral dose of statins.76 Although statin use has been associated with reduced mortality rates and cancer risk in cohort studies,77,78 the specific link to a reduced risk of renal call cancer has yet to be confirmed in further epidemiologic investigations.
Use of aspirin and other non-steroidal anti-inflammatory drugs has not been consistently associated with renal cell cancer risk (reviewed in 79–81). An increased risk of renal cell cancer has been linked to use of phenacetin-containing analgesics and acetaminophen, a metabolite of phenacetin, although this association has not been confirmed in a recent cohort study.82
Reproductive and Hormonal Factors
Parity was first reported to be a risk factor for renal cell carcinoma in case-control studies and has been recently confirmed in a few cohort studies.83–85 Compared to nulliparous women, the risk of renal cell cancer increased 40 to 90 percent among women who had given birth.83–85 After controlling for age at first birth among parous women, a Swedish nationwide record-linkage study found a significant 15% increase in risk with each additional birth.83 Other reproductive-related factors, including the use of oral contraceptives and hormone replacement therapy, have not been consistently linked to risk.84,85 It is unclear whether gestational hypertension and associated renal stress or the large hormonal fluctuation during pregnancy is contributing to the elevated risk of renal cell cancer. Human renal cell cancer tissue has been shown to express steroid hormone receptors and luteinizing hormone releasing hormone receptors.86,87 In Eker rats, estrogen treatment has been shown to enhance the development of renal cell cancer while ovariectomy reduced neoplastic renal changes.88 There observations suggest that reproductive and hormonal factors may play a role in renal cell cancer development in susceptible individuals.
Physical Activity
Evidence linking physical activity to a reduction in renal cell cancer risk is accumulating. Data from most recent cohort studies suggest an inverse association between renal cell cancer risk and leisure time and/or occupational activity levels,36,43,52,89,90 although two large record-linkage studies from Sweden reported either no association91 or an increased risk with long-term employment in sedentary jobs only among men, but not among women.92 A dose-response trend of further reduction in risk with increasing levels of activity or energy expenditure was reported in some studies.43,89,90 The inverse trend was observed for current exercise, routine physical activity, recreational activity, or a composite of energy expenditure in a typical day. It has also been suggested that physical activity during adolescence may have a bearing on renal cell cancer risk later in life, but this observation needs confirmation in future studies.90 Physical activity may decrease renal cell cancer risk through a number of related pathways, including lowering body weight and blood pressure, improving insulin sensitivity, and reducing chronic inflammation and oxidative stress.57,93–95
Diet and Beverages
Diet rich in fruits and vegetables has been suggested to reduce renal cell cancer risk in some cohort studies,96–98 but not in others.35,99,100 Antioxidants such as vitamins A, C, and E, and carotenoids that are common in fruits and vegetables also have not been consistently linked to renal cell cancer risk.100,101 Of interest is a recent report from a cohort study of Swedish women that the risk of renal cell cancer was consistently reduced with increasing frequency of fatty fish consumption, but not with lean fish consumption.102 Fish oil is rich in docosahexaneoic acid and eicosapentaenoic acid that have been shown to reduce the invasive profile of renal cell cancer in vitro103 and suppress neovascular response in mice grafted with human kidney.104 Fatty fish are also a rich source of vitamin D3 which has been inversely associated with renal cell cancer risk and progression.105 These observations suggest that further investigations into the role of diet, particularly fatty fish consumption, on renal cell cancer risk and progression are warranted.
A report by the Swedish National Food Administration in April, 2002, indicating the detection of unexpectedly high levels of acrylamide in commonly consumed fried and baked foods generated substantial public health and media concerns with regard to the potential carcinogenic effects of acrylamide.106 Two earlier epidemiologic studies in Sweden reported generally no association between renal cell cancer risk and the daily amount of acrylamide consumption estimated from a Swedish food database. Consumption of food items with elevated acrylamide levels, including coffee, crisp breads and fried potatoes, also were not related to risk.107–109 In contrast, a recent cohort study from the Netherlands reported a moderate but statistically significant increase in renal cell cancer risk among individuals estimated to have the highest quintile of acrylamide intake when compared to those in the lowest quintile of intake.110 A consistent dose-response pattern of increasing risk with higher intake was not observed, however. Since acrylamide has been shown to be mutagenic and carcinogenic in animal models and to induce the formation of hemoglobin adducts in humans,111,112 further assessment of cancer risk in epidemiologic studies is prudent.
Alcohol consumption has been inversely associated with renal cell cancer in a number of recent cohort studies,35,113–115 although the association is not statistically significant or is observed only in subgroups of subjects with certain age or BMI level in some studies.113,115 The inverse association was found for all types of alcoholic drinks, including beer, wine, and liquor. In a pooled analysis of 12 prospective studies, renal cell cancer risk decreased with increasing amount of alcohol consumption, with a significant 28% reduction in risk among those who drank ≥15 g/day, equivalent to slightly more than one alcoholic drink per day.116 In contrast, total fluid intake from all beverages, including coffee, tea, milk, juice, soda, and water, has not been consistently linked to risk.115 A potential mechanism by which moderate consumption of alcohol may reduce renal cell cancer risk is through improvement in insulin sensitivity,117,118 thus lowering the risk of type 2 diabetes, production of insulin-like growth factor-I, and subsequent risk of renal cell cancer.
Occupation and Environment
Although generally not considered an occupational disease, renal cell carcinoma has been linked to some occupational exposures. In particular, trichloroethylene (TCE), a chlorinated solvent commonly used as a degreaser in metal industries and as a general solvent in other industries, has been extensively studied, and evidence with regard to its renal toxicity and carcinogenicity reviewed.119–122 Research on the health effects of TCE exposure has intensified since the publication of a scientific article in 1999123 linking heavy cumulative TCE exposure to somatic mutations in the von Hippel Lindau (VHL) gene of exposed renal cell cancer patients, but not in tumors of unexposed patients. Most of the recent case-control121,124 and cohort studies involving TCE-exposed workers125–127 have reported results suggestive of an association with renal cell carcinoma, although the findings did not reach statistical significance in some studies.127 In contrast, no association was reported in a small cohort study of TCE-exposed workers in Denmark128 and another retrospective cohort mortality study of workers exposed to chlorinated organic solvents in Taiwan.129
One of the most challenging methodologic issues for epidemiologic studies of TCE and cancer risk is the assessment of historic exposure to TCE. A recent case-control study conducted in the Arve valley of France, an area with high TCE exposure from local industries and the availability of atmospheric TCE measurements and patient monitoring data, applied substantial efforts in exposure assessment using a semi-quantitative approach.130 The assessment was blinded to the case-control status. This study reported a 64% increase in risk with ever exposure to TCE, with the risk more than doubled and reaching statistical significance for individuals exposed to high cumulative dose of TCE.121 The risk increased further when both cumulative and peak exposures were considered, suggesting additional effects with high intensity exposure. Positive results from this and other recent epidemiologic studies, along with experimental studies providing plausible biological mechanisms and modes of action (reviewed in119,122,131), add to the evidence for a possible role of TCE in renal cell cancer development. However, given the methodologic challenges and the lack of association in some studies, further investigations with improved methodology are warranted before causal conclusions can be drawn.
An increased risk of renal cell carcinoma has also been linked to other industrial exposures, including chromium compounds, cadmium, lead, copper sulfate, solvents, benzene, vinyl chloride, asbestos, pesticides, and herbicides.132–136 Employment in certain occupations also has been associated with renal cell cancer risk, such as printers, aircraft mechanics, farmers, railroad workers, metal workers, mechanics and repairers, workers employed in vitamins A and E synthesis, and service station employees.132,136–138 However, none of these occupations or exposures has been conclusively related to risk in epidemiologic studies.
Exposure to low levels of arsenic in drinking water has not been associated with renal cell cancer risk.139–141 Nitrate in public water supplies also has been investigated recently, but no association with renal cell cancer risk was found.142 Radons in drinking water from drilled wells, with uranium-238 being the major naturally occurring radionuclides contributing to the radiation dose, were not associated kidney cancer risk in another study.143 Following the Chernobyl accident in Ukraine in 1986, renal cell cancer patients were found to have increased proliferative histopathological changes in kidney tissues adjacent to their cancer when compared to a series of unexposed but otherwise comparable renal cell cancer patients in Spain.144,145 These changes, including proliferating cell nuclear antigen, K-ras expression, proliferative atypical nephropathy, tubular epithelial nuclear atypia and carcinoma in situ, increased with duration of radiation exposure and with increased level of chronic persistent exposure to low-dose ionizing radiation among residents of the cesium-137 contaminated areas.
GENETIC SUSCEPTIBILITY AND ENVIRONMENT
Inherited renal cell carcinoma has been identified in a number of familial cancer syndromes. A number of inherited high penetrant mutations have been discovered, including the VHL gene in patients with clear cell tumors,146 the MET proto-oncogene in patients with papillary tumors,147 the fumarate hydratease (FH) gene in patients with papillary tumors and accompanying leiomyomatosis and uterine fibroids,148 and a gene associated with the Birt-Hogg-Dube (BHD) syndrome.149 Furthermore, the importance of polymorphic genetic variants and epigenetic changes in the development of sporadic renal cell cancer is increasingly recognized. The discovery of these low-penetrant genetic variants and their interactions with environmental exposures is the subject of intense current epidemiologic research.
Host susceptibility
Patients diagnosed with a sporadic renal cell carcinoma were more likely to report having a family history of kidney cancer in first-degree relatives than the control population in case-control studies.150–152 An analysis based on record-linkage in Sweden showed that kidney cancer risk increased 50% with an affected parent and to over fourfold if a sibling was affected with kidney cancer.153 In an Icelandic study based on linked genealogic data and nationwide cancer registry data, patients diagnosed with sporadic renal cell cancer over a 45-year period were found to be more related to each other than were members of a randomly selected control group with similar distributions in age, sex, and number of ancestors.154 The excess risk of renal cell cancer was observed not only in first-degree relatives, but also in second- and third-degree relatives, suggesting a possible role of inherited low-penetrant genetic variants that may increase the susceptibility to sporadic renal cell cancer.
Investigations of single nucleotide polymorphisms (SNPs) in relation to cancer risk have flourished in recent years. Renal cell cancer studies published to date have been based on the pathway approach, identifying genes involved in biological pathways that are relevant for renal cell cancer, such as genes involved in the metabolism of tobacco smoke and other xenobiotics, DNA repair, cell cycle control and apoptosis, angiogenesis, oxidative stress, immune function, obesity and hormone metabolism, and hypertension and renal function. These studies tend to be relatively small in size, and generally did not attempt comprehensive selection of the genes in a specific pathway nor complete coverage of the selected genes. Larger studies with a more comprehensive approach, such as genome-wide association studies, are underway. SNPs that have been examined in relation to the risk of renal cell cancer and its predisposing conditions are summarized in many databases available on the internet, such as the HuGE Navigator (http://www.hugenavigator.net).155
Polymorphism in genes encoding the glutathione S-transferase (GST) superfamily of inducible enzymes is probably the most studied genetic variants in relation to renal cell cancer risk to date.156–163 GST enzymes catalyze conjugation of electrophilic substrates with glutathione, usually resulting in detoxification of reactive intermediates.164 Within this family of genes, GSTM1 and GSTT1 are most frequently studied in relation to renal cell cancer, and are active in the detoxification of polycyclic aromatic hydrocarbons in tobacco smoke and halogenated solvents. Studies of the main effects of GSTM1 and GSTT1 have not produced consistent association with renal cell cancer,157–162 although the largest study with 925 cases and 1247 control subjects found no association.160 When examined with environmental exposures, several studies reported differences in risk in subgroups defined by genotype status. However, inconsistent findings were reported for association with smoking158,160 and TCE exposure156,161 when stratified by GSTM1 or GSTT1 genotype. With regard to pesticide exposure, two studies have shown greater magnitude of elevated risk among individuals carrying the active GSTM1 or GSTT1 genotype.159,163 Given the small numbers of exposed individuals and the lack of information on specific pesticides in these studies, further investigations are needed to clarify these associations.
Polymorphisms in genes encoding other Phase I (activation) and Phase II (detoxification) metabolic enzymes and their interaction with environmental exposures on renal cell cancer risk have also been examined, including GSTP1, NAT2, CYP1A1, CYP1B1, CYP2E1, CYP2D6, and NQ01.157,158,160–162,165–167 Of the few studies that have examined GSTP1, no main effect of the gene was observed, but some suggested interactions with other genes to influence cancer risk.157,158,160,161 Elevated risk of renal cell carcinoma was reported among individuals with the slow acetylator genotypes of the N-acetyltransferase 2 (NAT2) gene,166 but this was not confirmed in other studies.157,161 Differential effect by NAT2 acetylation status was reported for cigarette smoking,166 but not for TCE exposure.161 Associations with other metabolic genes have been reported, but have not been replicated, and are therefore not discussed in this review.
The role of vitamin D receptor (VDR) gene polymorphism has been examined in relation to renal cell cancer risk.168–170 The kidney is a major vitamin D regulating organ and vitamin D receptor levels were found to be lower in renal cell cancer tissue than autologous normal kidney tissue.171 Vitamin D has been shown to suppress renal cancer cell growth and angiogenesis in experimental studies.172 An elevated risk of renal cell carcinoma has been associated with VDR variants, including the TT genotype of the TaqI polymorphism168 and the AA genotype of the ApaI polymorphism,169 but a recent large study reported no association with the TaqI, BsmI, or FokI.170 This study, however, suggested that the association with VDR variants may differ by age and a family history of cancer.170 Given the suggestive findings and the plausibility of biological mechanism, a comprehensive examination of the VDR gene in relation to renal cell cancer risk is warranted.
Polymorphisms in genes encoding the DNA repair enzymes have also been examined in relation to renal cell cancer risk, including the xeroderma pigmentosum complementation groups C (XPC), D (XPD) and G (XPG), X-ray repair cross-complementing groups 1 (XRCC1) and 3 (XRCC3), and mismatch repair genes (hMLH1 and hMSH2).173–175 A significant excess risk was associated with the AA genotype of the XRCC1 Arg399Gln polymorphism.173 An association with polymorphisms in the hMLH1 and hMSH2 genes were also suggested.175 No association was reported for the other DNA repair gene variants.
Genes involved in the folate (one-carbon) metabolism pathway have been studied in relation to risk of a number of cancers and recently have been linked to risk of renal cell carcinoma.176,177 Further, interaction with vegetable intake appeared to enhance the associations of the gene variants with renal cell cancer.177 Polymorphism in genes involved in cell cycle control, including the checkpoint kinase 2 (CHEK2) and cyclin D1 (CCND1) genes, has also been associated with kidney cancer risk.178,179 Genes involved in the immune function pathways, including IL-6, IL-10, TNF-α, IFN-γ, TGF-β, and CTLA-4, have been evaluated in a few small studies with suggestive results.180–183 Other studies have examined polymorphism in genes involved in steroid hormone metabolism,184 the hypoxia inducible factor-1α gene (HIF1A),185 and the matrix metalloproteinase (MMP) genes encoding a family of extracellular matrix-degrading enzymes.186,187 The findings from these studies are tantalizing and can serve to guide the next phase of research design for a more thorough evaluation of renal cell cancer risk. However, given the mixed results, the lack of replication data, and the limitations of most studies to date, no firm conclusion can be drawn with regard to the role of genetic polymorphism in renal cell cancer risk. Furthermore, few studies have attempted to evaluate interaction of these genes with relevant environmental exposure in relation to renal cell cancer risk.
Renal cell cancer patients were found to have significantly shorter telomere length in DNA from peripheral blood lymphocytes than control subjects.188 An interaction between cigarette smoking and telomere length was also suggested, with the highest risk among smokers with short telomere length compared to non-smokers with long telomere length. Further research showed that the elevated renal cell cancer risk with telomere shortening was seen in both CD4+ T cells and CD8+ T cells, as well as the overall peripheral blood lymhocytes. A significant inverse trend of increasing risk with decreasing telomere length was also observed.189 These findings suggest that germline chromosomal instability as a result of telomere dysfunction is related to renal cell cancer risk. Confirmation of these findings is needed, preferably in cohort studies with prediagnostic and pretreatment peripheral blood DNA.
Tumor markers
Somatic mutations in sporadic renal cell cancers have been well documented, most notably VHL alterations in clear cell tumors and MET mutations in a small proportion of papillary tumors.4 It has been shown recently that the VHL gene is altered in as high as 91% of clear cell cancers through mutation or promoter hypermethylation.190 A specific somatic mutation pattern with a hot spot at codon 81 was previously observed in renal cell cancer patients who experienced high cumulative occupational exposure to TCE, but not in unexposed patients.123 This finding was not confirmed in a recent series of patients with high TCE exposure in France.191 Somatic VHL mutation has also been evaluated in conjunction with other environmental and lifestyle exposures in renal cell cancer risk in the Netherlands Cohort Study.51,101,192 The association with cigarette smoking and intake of carotenoids and vitamins were comparable for renal cell cancer patients with and without somatic VHL mutations.101,192 However, a history of hypertension was associated with renal cell cancer risk only among those with VHL mutated tumors, whereas an association with diuretic use was observed only among patients without somatic VHL mutation.51 In an exploratory study of a small series of renal cell cancer patients in Sweden, somatic VHL mutations were inversely associated with consumption of vegetables and citrus fruits while exposure to welding fumes increased the risk of multiple VHL mutations.193 Despite the limited epidemiologic data, laboratory studies have linked tobacco carcinogens to VHL mutations in renal tumors of Wistar rats194 and deletions in chromosome 3p in peripheral blood lymphocytes of renal cell cancer patients,195 suggesting the possibility of a targeted pathway through VHL and perhaps other genes on chromosome 3p for tobacco-related renal cell cancer development.
Evaluation of somatic VHL mutations in epidemiologic studies is complicated by technical difficulties, including variations in the quality of DNA from archived tissue blocks. Most previous studies have reported VHL mutations in about 55% to 70% of renal cell cancers,196–198 well below the 91% with VHL alterations (82% with mutation and 8% with promoter hypermethylation) reported in a recent series of clear cell renal cancers with DNA from fresh frozen tissues.190 These observations suggest that misclassification of somatic VHL mutations could be substantial in some studies, rendering the results difficult to interpret.
Numerous other genetic and epigenetic alternations in renal cell cancer have been reported, but few have been evaluated in light of specific environmental and lifestyle risk factors for renal cell cancer. Promoter hypermethylation of the tumor suppressor genes on chromosome 9p21 (INK4A and ARF) was observed in some renal cell cancers among patients residing in radiation-contaminated areas following the Chernobyl accident.199 In another small series of renal cell cancer patients, somatic mutations in the p53 tumor suppressor gene and the Ras oncogene were not related to gasoline exposure, a postulated renal cancer risk factor, but the majority of the mutations were seen in smokers.200 Increased age and adiposity, risk factors for renal cell cancer, were shown to enhance promoter hypermethylation of the RASSF1A tumor suppressor gene in normal kidney tissue.201 Molecular changes in renal tissues other than VHL mutation have also been studied in relation to TCE and its metabolite in experimental,202,203 but data in human populations are still limited.
Recently, high throughput methods for genome-wide screening of DNA copy number alterations and loss of heterozygosity have been developed and tested in small series of renal cell cancers.204–206 If proven reliable and feasible, these powerful methods hold promise for novel discoveries in large scale investigations of gene-environment and gene-gene interactions.
CONCLUSIONS
Renal cell cancer incidence has continued to rise among all race/ethnic groups in the United States through 2005, and the increases were mostly due to localized disease and tumors of small size. Improved screening and incidental diagnosis may have contributed to these increases, but it has been estimated that most of the preclinical small tumors would become clinically evident eventually. The rising prevalences of obesity and hypertension likely have contributed to the upward cancer trends. Results from recent cohort studies, however, suggested that the obesity- and hypertension-related renal cell cancer risk may be tempered by effective hypertension control and reduced weight fluctuations. There is also increasing epidemiologic evidence that physical activity and moderate alcohol intake may reduce risk. Several recent investigations have confirmed an association between renal cell cancer risk and occupational exposure to TCE, although the link between high cumulative TCE exposure and somatic VHL mutation remains inconclusive. Epidemiologic investigations have increasingly incorporated analysis of germline and somatic mutations and assessed their interactions with environmental exposures in cancer risk. Polymorphisms in genes involved in a variety of pathways, including genes encoding metabolic enzymes, vitamin D receptor, DNA repair, and immune function, have been examined in relation to renal cell cancer risk, but the results have been mixed. Telomere shortening in peripheral blood DNA also has been linked to elevated risk in a dose-response manner, but it is unclear whether this association is modified by any environmental exposures. Somatic mutations of VHL and other genes have been observed in subtypes of renal cell cancer, but their link to environmental exposures has yet to be clarified. The recent rapid advancement in high-throughput laboratory technology, such as the genome-wide scanning, will allow for more thorough evaluation of molecular changes. In conjunction with detailed environmental exposure data in large epidemiologic studies, these technological innovations hold promise for novel discoveries in the etiology and prognosis of renal cell cancer.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health. The authors express their appreciation to the dedicated staff of NCI’s Surveillance, Epidemiology, and End Results Program and cancer registries contributing data to the SEER program. We thank Mr. John Lahey of IMS, Inc., and Mr. David Check of the Division of Cancer Epidemiology and Genetics for data tabulation and figure development.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chow WH, Devesa SS, Warren JL, Fraumeni JF., Jr Rising incidence of renal cell cancer in the United States. JAMA. 1999;281:1628–1631. doi: 10.1001/jama.281.17.1628. [DOI] [PubMed] [Google Scholar]
- 3.Verhoest G, Veillard D, Guillé F, et al. Relationship between age at diagnosis and clinicopathologic features of renal cell carcinoma. Eur Urol. 2007;51:1298–1305. doi: 10.1016/j.eururo.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 4.Zambrano NR, Lubensky IA, Merino MJ, et al. Histopathology and molecular genetics of renal tumors toward unification of a classification system. J Urol. 1999;162:1246–1258. [PubMed] [Google Scholar]
- 5.Zbar B, Lerman M. Inherited carcinomas of the kidney. Adv Cancer Res. 1998;75:163–201. doi: 10.1016/s0065-230x(08)60742-3. [DOI] [PubMed] [Google Scholar]
- 6.Pavlovich CP, Walther MM, Eyler RA, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–1552. doi: 10.1097/00000478-200212000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Merino MJ, Torres-Cabala C, Pinto P, Linehan WM. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31:1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 8.Linehan WM, Lerman MI, Zbar B. Identification of the von Hippel-Lindau (VHL) gene. JAMA. 1995;273:564–570. [PubMed] [Google Scholar]
- 9.Vaishampayan UN, Do H, Hussain M, Schwartz K. Racial disparity in incidence patterns and outcome of kidney cancer. Urol. 2003;62:1012–1017. doi: 10.1016/j.urology.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Stafford HS, Saltzstein SL, Shimasaki S, et al. Racial/ethnic and gender disparities in renal cell carcinoma incidence and survival. J Urol. 2008;179:1704–1708. doi: 10.1016/j.juro.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, Mariotto A, Miller BA, Feuer EJ, Altekruse SF, Lewis DR, Clegg L, Eisner MP, Reichman M, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2005. National Cancer Institute; Bethesda, MD: 2008. http://seer.cancer.gov/csr/1975_2005/, based on November 2007 SEER data submission, posted to the SEER web site. [Google Scholar]
- 12.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use, Nov 2007 Sub (1973–2005) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2005 Counties. ( www.seer.cancer.gov) released April 2008, based on the November 2007 submission. [Google Scholar]
- 13.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 13 Regs Limited-Use, Nov 2007 Sub (1992–2005) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2005 Counties. ( www.seer.cancer.gov) released April 2008, based on the November 2007 submission. [Google Scholar]
- 14.Surveillance, Epidemiology, and End Results (SEER) Program. National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; SEER*Stat Database: Incidence - SEER 17 Regs Limited-Use + Hurricane Katrina Impacted Louisiana Cases, Nov 2007 Sub (1973–2005 varying) -Linked To County Attributes - Total U.S., 1969–2005 Counties. ( www.seer.cancer.gov) released April 2008, based on the November 2007 submission. [Google Scholar]
- 15.Surveillance Research Program, National Cancer Institute SEER*Stat software. ( www.seer.cancer.gov/seerstat) version 6.4.4.
- 16.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973–1992. Eur J Cancer Prev. 2002;11:171–178. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Katz DL, Zheng T, Holford TR, Flannery J. Time trends in the incidence of renal carcinoma: analysis of Connecticut Tumor Registry data, 1935–1989. Int J Cancer. 1994;58:57–63. doi: 10.1002/ijc.2910580111. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Semenciw R, Morrison H, et al. Kidney cancer in Canada: the rapidly increasing incidence of adenocarcinoma in adults and seniors. Can J Public Health. 1997;88:99–104. doi: 10.1007/BF03403870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg T, Martel S. Cancer trends from 1972–1991 for registered Indians living on Manitoba reserves. Int J Circumpolar Health. 1998;57 (Suppl 1):391–398. [PubMed] [Google Scholar]
- 20.Nguyen MM, Gill IS, Ellison LM. The evolving presentation of renal carcinoma in the United States: trends from the Surveillance, Epidemiology, and End Results program. J Urol. 2006;176:2397–2400. doi: 10.1016/j.juro.2006.07.144. [DOI] [PubMed] [Google Scholar]
- 21.Cooperberg MR, Mallin K, Ritchey J, et al. Decreasing size at diagnosis of stage I renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. J Urol. 2008;179:2131–2135. doi: 10.1016/j.juro.2008.01.097. [DOI] [PubMed] [Google Scholar]
- 22.Pankhurst T, Howie AJ, Adu D, et al. Incidental neoplasms in renal biopsies. Nephrol Dial Transplant. 2006;21:64–69. doi: 10.1093/ndt/gfi149. [DOI] [PubMed] [Google Scholar]
- 23.Fenton JJ, Weiss NS. Screening computed tomography: Will it result in overdiagnosis of renal carcinoma? Cancer. 2004;100:986–990. doi: 10.1002/cncr.20055. [DOI] [PubMed] [Google Scholar]
- 24.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiologic evidence. Lung Cancer. 2004;45 (Suppl 2):53–59. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- 26.Hunt JD, van der Hel OL, McMillan GP, et al. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114:101–108. doi: 10.1002/ijc.20618. [DOI] [PubMed] [Google Scholar]
- 27.Lubin JH, Virtamo J, Weinstein SJ, Albanes D. Cigarette smoking and cancer: Intensity patterns in the alpha-tocopherol, beta-carotene cancer prevention study in Finnish men. Am J Epidemiol. 2008;167:970–975. doi: 10.1093/aje/kwm392. [DOI] [PubMed] [Google Scholar]
- 28.Parker AS, Cerhan JR, Janney CA, et al. Smoking cessation and renal cell carcinoma. Ann Epidemiol. 2003;13:245–251. doi: 10.1016/s1047-2797(02)00271-5. [DOI] [PubMed] [Google Scholar]
- 29.Bjerregaard BK, Raaschou-Nielsen O, Sørensen M, et al. The effect of occasional smoking on smoking-related cancers: in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2006;17:1305–1309. doi: 10.1007/s10552-006-0068-9. [DOI] [PubMed] [Google Scholar]
- 30.Hu J, Ugnat AM. Active and passive smoking and risk of renal cell carcinoma in Canada. Eur J Cancer. 2005;41:770–778. doi: 10.1016/j.ejca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Sharifi N, Farrar WL. Perturbations in hypoxia detection: A shared link between hereditary and sporadic tumor formation? Med Hypotheses. 2006;66:732–735. doi: 10.1016/j.mehy.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Rev. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 33.Chow WH, Gridley G, Fraumeni JF, Jr, Järvholm B. Obesity, hypertension, and the risk of kidney cancer in men. New Engl J Med. 2000;343:1305–1311. doi: 10.1056/NEJM200011023431804. [DOI] [PubMed] [Google Scholar]
- 34.Calle EE, Rodriguez C, Walker-Thurmond K, et al. Overweight, obesity, and mortality from cancer in a prospectively studied cohort cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 35.Nicodemus KK, Sweeney C, Folsom AR. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer. 2004;108:115–121. doi: 10.1002/ijc.11532. [DOI] [PubMed] [Google Scholar]
- 36.Van Dijk BA, Schouten LJ, Kiemeney LA, et al. Relation of height, body mass, energy intake, and physical activity to risk of renal cell carcinoma: results from the Netherlands Cohort Study. Am J Epidemiol. 2004;160:1159–1167. doi: 10.1093/aje/kwh344. [DOI] [PubMed] [Google Scholar]
- 37.Bjørge T, Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 2004;160:1168–1176. doi: 10.1093/aje/. [DOI] [PubMed] [Google Scholar]
- 38.Flaherty KT, Fuchs CS, Colditz GA, et al. A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States) Cancer Causes Control. 2005;16:1099–1106. doi: 10.1007/s10552-005-0349-8. [DOI] [PubMed] [Google Scholar]
- 39.Oh SW, Yoon YS, Shin SA. Effects of excess weight on cancer incidences depending on cancer sites and histologic findings among men: Korea National Health Insurance Corporation Study. J Clin Oncol. 2005;23:4742–4754. doi: 10.1200/JCO.2005.11.726. [DOI] [PubMed] [Google Scholar]
- 40.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2006;118:728–738. doi: 10.1002/ijc.21398. [DOI] [PubMed] [Google Scholar]
- 41.Luo J, Margolis KL, Adami HO, et al. Body size, weight cycling, and risk of renal cell carcinoma among postmenopausal women: The Women’s Health Initiative (United States) Am J Epidemiol. 2007;166:752–759. doi: 10.1093/aje/kwm137. [DOI] [PubMed] [Google Scholar]
- 42.Reeves GK, Pirie K, Beral V, et al. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335:1134–1144. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setiawan VW, Stram DO, Nomura AMY, et al. Risk factors for renal cell cancer: The Multiethnic Cohort. Am J Epidemiol. 2007;166:932–940. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 44.Adams KF, Leitzmann MF, Albanes D, et al. Body size and renal cell cancer incidence in a large US cohort study. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn122. EPub June 12, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 46.Gago-Dominguez M, Castelao JE. Lipid peroxidation and renal cell carcinoma: further supportive evidence and new mechanistic insights. Free Radic Biol Med. 2006;40:721–733. doi: 10.1016/j.freeradbiomed.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Spyridopoulos TN, Petridou ET, Skalkidou A, et al. Low adiponectin levels are associated with renal cell carcinoma: A case-control study. Int J Cancer. 2007;120:1573–1578. doi: 10.1002/ijc.22526. [DOI] [PubMed] [Google Scholar]
- 48.Renehan AG, Roberts DL, Dive C. Obesity and cancer: pathophysiological and biological mechanisms. Arch Physiol Biochem. 2008;114:71–83. doi: 10.1080/13813450801954303. [DOI] [PubMed] [Google Scholar]
- 49.Steffens J, Bock R, Braedel HU, et al. Renin-producing renal cell carcinomas – clinical and experimental investigations on a special form of renal hypertension. Urol Res. 1992;20:111–115. doi: 10.1007/BF00296521. [DOI] [PubMed] [Google Scholar]
- 50.Wu S, Chen JJ, Kudelka A, et al. Incidence and risk of hypertension with sorafenib in patients with cancer: a systematic review and meta-analysis. Lancet Oncol. 2008;9:117–123. doi: 10.1016/S1470-2045(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 51.Schouten LJ, van Dijk BAC, Oosterwijk E, et al. Hypertension, antihypertensives and mutations in the Von Hippel-Lindau gene in renal cell carcinoma: results from the Netherlands Cohort Study. J Hypertension. 2005;23:1997–2004. doi: 10.1097/01.hjh.0000186023.74245.48. [DOI] [PubMed] [Google Scholar]
- 52.Choi MY, Jee SH, Sull JW, Nam CM. The effect of hypertension on the risk for kidney cancer in Korean men. Kidney Int. 2005;67:647–652. doi: 10.1111/j.1523-1755.2005.67137.x. [DOI] [PubMed] [Google Scholar]
- 53.Vatten LJ, Trichopoulos D, Holmen J, Nilsen TIL. Blood pressure and renal cancer risk: the HUNT Study in Norway. Brit J Cancer. 2007;97:112–114. doi: 10.1038/sj.bjc.6603823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weikert S, Boeing H, Pischon T, et al. Blood pressure and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am J Epidemiol. 2008;167:438–446. doi: 10.1093/aje/kwm321. [DOI] [PubMed] [Google Scholar]
- 55.Friis S, Sørensen HT, Mellemkjær L, et al. Angiotensin-converting enzyme inhibitors and the risk of cancer. Cancer. 2001;92:2462–2470. doi: 10.1002/1097-0142(20011101)92:9<2462::aid-cncr1596>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 56.Fryzek JP, Poulsen AH, Johnsen SP, et al. A cohort study of antihypertensive treatments and risk of renal cell cancer. Brit J Cancer. 2005;92:1302–1306. doi: 10.1038/sj.bjc.6602490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gago-Dominguez M, Castelao JE, Yuan JM, et al. Lipid peroxidation: a novel and unifying concept of the etiology of renal cell carcinoma (United States) Cancer Causes Control. 2002;13:287–293. doi: 10.1023/a:1015044518505. [DOI] [PubMed] [Google Scholar]
- 58.Kaelin WG., Jr The von Hippel-Lindau gene, kidney cancer, and oxygen sensing. J Am Soc Nephrol. 2003;14:2703–2711. doi: 10.1097/01.asn.0000092803.69761.41. [DOI] [PubMed] [Google Scholar]
- 59.Koshiji M, Kageyama Y, Pete EA, et al. HIF-1α induces cell cycle arrest by functionally counteracting Myc. EMBO J. 2004;23:1949–1956. doi: 10.1038/sj.emboj.7600196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mazzali M, Jefferson A, Ni Z, et al. Microvascular and tubulointerstitial injury associated with chronic hypoxia-induced hypertension. Kidney Int. 2003;63:2088–2093. doi: 10.1046/j.1523-1755.2003.00011.x. [DOI] [PubMed] [Google Scholar]
- 61.Peces R, Martinez-Ara J, Miguel JL, et al. Renal cell carcinoma co-existent with other renal disease: clinico-pathological features in pre-dialysis patients and those receiving dialysis or renal transplantation. Nephrol Dial Transplant. 2004;19:2789–2796. doi: 10.1093/ndt/gfh458. [DOI] [PubMed] [Google Scholar]
- 62.Kojima Y, Takahara S, Miyake O, et al. Renal cell carcinoma in dialysis patients: A single center experience. Int J Urol. 2006;13:1045–1048. doi: 10.1111/j.1442-2042.2006.01498.x. [DOI] [PubMed] [Google Scholar]
- 63.Farivar-Mohseni H, Perlmutter AE, Wilson S, et al. Renal cell carcinoma and end stage renal disease. J Urol. 2006;175:2018–2021. doi: 10.1016/S0022-5347(06)00340-5. [DOI] [PubMed] [Google Scholar]
- 64.Schwarz A, Vatandasiar S, Merkel S, Haller H. Renal cell carcinoma in transplant recipients with acquired cystic kidney disease. Clin J Am Soc Nephrol. 2007;2:750–756. doi: 10.2215/CJN.03661106. [DOI] [PubMed] [Google Scholar]
- 65.Neuzillet Y, Lay F, Luccioni A, et al. De novo renal cell carcinoma of native kidney in renal transplant recipients. Cancer. 2005;103:251–257. doi: 10.1002/cncr.20745. [DOI] [PubMed] [Google Scholar]
- 66.Ianhez LE, Lucon M, Nahas WC, et al. Renal cell carcinoma in renal transplant patients. Urol. 2007;69:462–464. doi: 10.1016/j.urology.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 67.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24:476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 68.Das A, Thomas S, Zablotska LB, et al. Association of esophageal adenocarcinoma with other subsequent primary cancers. J Clin Gastroenterol. 2006;40:405–411. doi: 10.1097/00004836-200605000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Thompson RH, Leibovich BC, Cheville JC, et al. Second primary malignancies associated with renal cell carcinoma histological subtypes. J Urol. 2006;176:900–903. doi: 10.1016/j.juro.2006.04.080. [DOI] [PubMed] [Google Scholar]
- 70.Beisland C, Talleraas O, Bakke A, Norstein J. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int. 2006;97:698–702. doi: 10.1111/j.1464-410X.2006.06004.x. [DOI] [PubMed] [Google Scholar]
- 71.Bani-Hani AH, Leibovich BC, Lohse CM, et al. Associations with contralateral recurrence following nephrectomy for renal cell carcinoma using a cohort of 2,352 patients. J Urol. 2005;173:391–394. doi: 10.1097/01.ju.0000148951.71353.8b. [DOI] [PubMed] [Google Scholar]
- 72.Wideroff L, Gridley G, Mellemkjær L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–1365. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 73.Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999;42:107–112. doi: 10.1007/s001250051122. [DOI] [PubMed] [Google Scholar]
- 74.Zucchetto A, Dal Maso L, Tavani A, et al. History of treated hypertension and diabetes mellitus and risk of renal cell cancer. Ann Onc. 2007;18:596–600. doi: 10.1093/annonc/mdl438. [DOI] [PubMed] [Google Scholar]
- 75.Khurana V, Caldito G, Ankem M. Statins might reduce risk of renal cell carcinoma in humans: case-control study of 500,000 veterans. Urol. 2008;71:118–122. doi: 10.1016/j.urology.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 76.Horiguchi A, Sumitomo M, Asakuma J, et al. 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin Cancer Res. 2004;10:8648–8655. doi: 10.1158/1078-0432.CCR-04-1568. [DOI] [PubMed] [Google Scholar]
- 77.Friis S, Poulsen AH, Johnsen SP, et al. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114:643–647. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 78.Mehta JL, Bursac Z, Hauer-Jensen M, et al. Comparison of mortality rates in statin users versus nonstatin users in a United States veteran population. Am J Cardiol. 2006;98:923–928. doi: 10.1016/j.amjcard.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 79.Baron JA. Epidemiology of non-steroidal anti-inflammatory drugs and cancer. Prog Exp Tumor Res. 2003;37:1–24. doi: 10.1159/000071364. [DOI] [PubMed] [Google Scholar]
- 80.Harris RE, Beebe-Donk J, Doss H, Burr Doss D. Aspirin, Ibuprofen, and other non-steroidal anti-inflammatory drugs in cancer prevention: a critical review of non-selective COX-2 blockade (review) Oncol Rep. 2005;13:559–583. [PubMed] [Google Scholar]
- 81.Bosetti C, Gallus S, La Vecchia C. Aspirin and cancer risk: an updated quantitative review to 2005. Cancer Causes Control. 2006;17:871–888. doi: 10.1007/s10552-006-0033-7. [DOI] [PubMed] [Google Scholar]
- 82.Friis S, Nielsen GL, Mellemkjaer L, et al. Cancer risk in persons receiving prescriptions for paracetamol: a Danish cohort study. Int J Cancer. 2002;97:96–101. doi: 10.1002/ijc.1581. [DOI] [PubMed] [Google Scholar]
- 83.Lambe M, Lindblad P, Wuu J, et al. Pregnancy and risk of renal cell cancer: a population-based study in Sweden. Br J Cancer. 2002;86:1425–1429. doi: 10.1038/sj.bjc.6600263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kabat GC, Silvera SA, Miller AB, Rohan TE. A cohort study of reproductive and hormonal factors and renal cell cancer risk in women. Br J Cancer. 2007;96:845–849. doi: 10.1038/sj.bjc.6603629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Molokwu JC, Prizment AE, Folsom AR. Reproductive characteristics and risk of kidney cancer: Iowa Women’s Health Study. Maturitas. 2007;58:156–163. doi: 10.1016/j.maturitas.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 86.Langner C, Ratschek M, Rehak P, et al. Steroid hormone receptor expression in renal cell carcinoma: an immunohistochemical analysis of 182 tumors. J Urol. 2004;171:611–614. doi: 10.1097/01.ju.0000108040.14303.c2. [DOI] [PubMed] [Google Scholar]
- 87.Keller G, Schally AV, Gaiser T, et al. Receptors for luteinizing hormone releasing hormone expressed on human renal cell carcinomas can be used for targeted chemotherapy with cytotoxic luteinizing hormone releasing hormone analogues. Clin Cancer Res. 2005;11:5549–5557. doi: 10.1158/1078-0432.CCR-04-2464. [DOI] [PubMed] [Google Scholar]
- 88.Wolf DC, Goldsworthy TL, Donner EM, et al. Estrogen treatment enhances hereditary renal tumor development in Eker rats. Carcinogenesis. 1998;19:2043–2047. doi: 10.1093/carcin/19.11.2043. [DOI] [PubMed] [Google Scholar]
- 89.Mahabir S, Leitzmann MF, Pietinen P, et al. Physical activity and renal cell cancer risk in a cohort of male smokers. Int J Cancer. 2004;108:600–605. doi: 10.1002/ijc.11580. [DOI] [PubMed] [Google Scholar]
- 90.Moore SC, Chow WH, Schatzkin A, et al. Physical activity during adulthood and adolescence in relation to renal cell cancer. Am J Epidemiol. 2008 doi: 10.1093/aje/kwn102. EPub May 8, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bergström A, Terry P, Lindblad P, et al. Physical activity and risk of renal cell cancer. Int J Cancer. 2001;92:155–157. [PubMed] [Google Scholar]
- 92.Bergström A, Moradi T, Lindblad P, et al. Occupational physical activity and renal cell cancer: a nationwide cohort study in Sweden. Int J Cancer. 1999;83:186–191. doi: 10.1002/(sici)1097-0215(19991008)83:2<186::aid-ijc7>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 93.Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290:1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 94.Stewart KJ, Bacher AC, Turner KL, et al. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. 2005;165:756–62. doi: 10.1001/archinte.165.7.756. [DOI] [PubMed] [Google Scholar]
- 95.Mora S, Cook N, Buring JE, et al. Physical activity and reduced risk of cardiovascular events: potential mediating mechanisms. Circulation. 2007;116:2110–2118. doi: 10.1161/CIRCULATIONAHA.107.729939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fraser GE, Phillips RL, Beeson WL. Hypertension, antihypertensive medications and risk of renal carcinoma in California Seventh-Day Adventists. Int J Epidemiol. 1990;19:832–838. doi: 10.1093/ije/19.4.832. [DOI] [PubMed] [Google Scholar]
- 97.Rashidkhani B, Lindblad P, Wolk A. Fruits, vegetables and risk of renal cell carcinoma: a prospective study of Swedish women. Int J Cancer. 2005;113:451–455. doi: 10.1002/ijc.20577. [DOI] [PubMed] [Google Scholar]
- 98.Lee JE, Giovannucci E, Smith-Warner SA, et al. Intakes of fruits, vegetables, vitamins A, C, and E, and carotenoids and risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2445–2452. doi: 10.1158/1055-9965.EPI-06-0553. [DOI] [PubMed] [Google Scholar]
- 99.Van Dijk BA, Schouten LJ, Kiemeney LA, et al. Vegetable and fruit consumption and risk of renal cell carcinoma: results from the Netherlands cohort study. Int J Cancer. 2005;117:648–654. doi: 10.1002/ijc.21203. [DOI] [PubMed] [Google Scholar]
- 100.Weikert S, Boeing H, Pischon T, et al. Fruits and vegetables and renal cell carcinoma: Findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2006;118:3133–3139. doi: 10.1002/ijc.21765. [DOI] [PubMed] [Google Scholar]
- 101.Van Dijk BAC, Schouten LJ, Oosterwijk E, et al. Carotenoid and vitamin intake, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Cancer Causes Control. 2008;19:125–134. doi: 10.1007/s10552-007-9078-5. [DOI] [PubMed] [Google Scholar]
- 102.Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296:171–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- 103.McCabe AJ, Wallace JM, Gilmore WS, et al. Docosahexaenoic acid reduces in vitro invasion of renal cell carcinoma by elevated levels of tissue inhibitor of metalloproteinase-1. J Nutr Biochem. 2005;16:17–22. doi: 10.1016/j.jnutbio.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Skopinska-Rozewska E, Krotkiewski M, Sommer E, et al. Inhibitory effect of shark liver oil on cutaneous angiogenesis induced in Balb/c mice by syngeneic sarcoma L-1, human urinary bladder and human kidney tumour cells. Oncol Rep. 1999;6:1341–1344. doi: 10.3892/or.6.6.1341. [DOI] [PubMed] [Google Scholar]
- 105.Fujioka T, Suzuki Y, Okamoto T, et al. Prevention of renal cell carcinoma by active vitamin D3. World J Surg. 2000;24:1205–1210. doi: 10.1007/s002680010206. [DOI] [PubMed] [Google Scholar]
- 106.Törnqvist M. Acrylamide in food: the discovery and its implications: a historical perspective. Adv Exp Med Biol. 2005;561:1–19. doi: 10.1007/0-387-24980-X_1. [DOI] [PubMed] [Google Scholar]
- 107.Mucci LA, Dickman PW, Steineck G, et al. Dietary acrylamide and cancer of the large bowel, kidney, and bladder: absence of an association in a population-based study in Sweden. Br J Cancer. 2003;88:84–89. doi: 10.1038/sj.bjc.6600726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mucci LA, Dickman PW, Steineck G, et al. Dietary acrylamide and cancer risk: additional data on coffee. Br J Cancer. 2003;89:735–736. [Google Scholar]
- 109.Mucci LA, Lindblad P, Steineck G, Adami HO. Dietary acrylamide and risk of renal cell cancer. Int J Cancer. 2004;109:774–776. doi: 10.1002/ijc.20011. [DOI] [PubMed] [Google Scholar]
- 110.Hogervorst JG, Schouten LJ, Konings EJ, et al. Dietary acrylamide intake and the risk of renal cell, bladder, and prostate cancer. Am J Clin Nutr. 2008;87:1428–1438. doi: 10.1093/ajcn/87.5.1428. [DOI] [PubMed] [Google Scholar]
- 111.Besaratinia A, Pfeifer GP. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis. 2007;28:519–528. doi: 10.1093/carcin/bgm006. [DOI] [PubMed] [Google Scholar]
- 112.Wirfält E, Paulsson B, Törnqvist M, et al. Association between estimated acrylamide intakes, and hemoglobin AA adducts in a sample from the Malmö Diet and Cancer Cohort. Eur J Clin Nutr. 2008;62:314–323. doi: 10.1038/sj.ejcn.1602704. [DOI] [PubMed] [Google Scholar]
- 113.Rashidkhani B, Ǻkesson A, Lindblad P, Wolk A. Alcohol consumption and risk of renal cell carcinoma: A prospective study of Swedish women. Int J Cancer. 2005;117:848–853. doi: 10.1002/ijc.21231. [DOI] [PubMed] [Google Scholar]
- 114.Mahabir S, Leitzmann MF, Virtanen MJ, et al. Prospective study of alcohol drinking and renal cell cancer risk in a cohort of Finnish male smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:170–175. [PubMed] [Google Scholar]
- 115.Lee JE, Giovannucci E, Smith-Warner SA, et al. Total fluid intake and use of individual beverages and risk of renal cell cancer in two large cohorts. Cancer Epidemiol Biomarkers Prev. 2006;15:1204–1211. doi: 10.1158/1055-9965.EPI-05-0889. [DOI] [PubMed] [Google Scholar]
- 116.Lee JE, Hunter DJ, Spiegelman D, et al. Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst. 2007;99:801–810. doi: 10.1093/jnci/djk181. [DOI] [PubMed] [Google Scholar]
- 117.Lazarus R, Sparrow D, Weiss ST. Alcohol intake and insulin levels. The Normative Aging Study. Am J Epidemiol. 1997;145:909–916. doi: 10.1093/oxfordjournals.aje.a009050. [DOI] [PubMed] [Google Scholar]; Br J Cancer. 2007;96:845–849. doi: 10.1038/sj.bjc.6603629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davies MJ, Baer DJ, Judd JT, et al. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287:2559–2562. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]
- 119.Harth V, Brüning T, Bolt HM. Renal carcinogenicity of trichloroethylene: update, mode of action, and fundamentals for occupational standard setting. Rev Environ Health. 2005;20:103–118. [PubMed] [Google Scholar]
- 120.Scott CS, Chiu WA. Trichloroethylene cancer epidemiology: a consideration of select issues. Environ Health Perspect. 2006;114:1471–8. doi: 10.1289/ehp.8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Charbotel B, Fevotte J, Hours M, et al. Case-control study on renal cell cancer and occupational exposure to trichloroethylene. Part II: Epidemiological aspects. Ann Occup Hyg. 2006;50:777–787. doi: 10.1093/annhyg/mel039. [DOI] [PubMed] [Google Scholar]
- 122.Lock EA, Reed CJ. Trichloroethylene: Mechanisms of renal toxicity and renal cancer and relevance to risk assessment. Toxicol Sci. 2006;91:313–331. doi: 10.1093/toxsci/kfj107. [DOI] [PubMed] [Google Scholar]
- 123.Brauch H, Weirich G, Hornauer MA, et al. Trichloroethylene exposure and sporadic somatic mutations in patients with renal cell carcinoma. J Natl Cancer Inst. 1999;91:854–861. doi: 10.1093/jnci/91.10.854. [DOI] [PubMed] [Google Scholar]
- 124.Brüning T, Pesch B, Wiesenhütter B, et al. Renal cell cancer risk and occupational exposure to trichloroethylene: results of a consecutive case-control study in Arnsberg, Germany. Am J Ind Med. 2003;43:274–285. doi: 10.1002/ajim.10185. [DOI] [PubMed] [Google Scholar]
- 125.Raaschou-Nielsen O, Hansen J, McLaughlin JK, et al. Cancer risk among workers at Danish Companies using trichloroethylene: a cohort study. Am J Epidemiol. 2003;158:1182–1192. doi: 10.1093/aje/kwg282. [DOI] [PubMed] [Google Scholar]
- 126.Zhao Y, Krishnadasan A, Kennedy N, et al. Estimated effects of solvents and mineral oils on cancer incidence and mortality in a cohort of aerospace workers. Am J Ind Med. 2005;48:249–58. doi: 10.1002/ajim.20216. [DOI] [PubMed] [Google Scholar]
- 127.Boice JD, Jr, Marano DE, Cohen SS, et al. Mortality among Rocketdyne workers who tested rocket engines, 1948–1999. J Occup Environ Med. 2006;48:1070–1092. doi: 10.1097/01.jom.0000240661.33413.b5. [DOI] [PubMed] [Google Scholar]
- 128.Hansen J, Raaschou-Nielsen O, Christensen JM, et al. Cancer incidence among Danish workers exposed to trichloroethylene. J Occup Environ Med. 2001;43:133–139. doi: 10.1097/00043764-200102000-00012. [DOI] [PubMed] [Google Scholar]
- 129.Chang YM, Tai CF, Yang SC, et al. A cohort mortality study of workers exposed to chlorinated organic solvents in Taiwan. Ann Epidemiol. 2003;13:652–660. doi: 10.1016/S1047-2797(03)00038-3. [DOI] [PubMed] [Google Scholar]
- 130.Fevotte J, Charbotel B, Muller-Beaute P, et al. Case-control study on renal cell cancer and occupational exposure to trichloroethylene. Part I: Exposure assessment. Ann Occup Hyg. 2006;50:765–775. doi: 10.1093/annhyg/mel040. [DOI] [PubMed] [Google Scholar]
- 131.Chiu WA, Caldwell JC, Keshava N, Scott CS. Key scientific issues in the health risk assessment of trichloroethylene. Environ Health Perspect. 2006;114:1445–1449. doi: 10.1289/ehp.8690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parent ME, Hua Y, Siemiatycki J. Occupational risk factors for renal cell carcinoma in Montreal. Am J Ind Med. 2000;38:609–618. doi: 10.1002/1097-0274(200012)38:6<609::aid-ajim1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 133.Pesch B, Haerting J, Ranft U, et al. Occupational risk factors for renal cell carcinoma: agent-specific results from a case-control study in Germany. Int J Epidemiol. 2000;29:1014–1024. doi: 10.1093/ije/29.6.1014. [DOI] [PubMed] [Google Scholar]
- 134.Buzio L, Tondel M, De Palma G, et al. Occupational risk factors for renal cell cancer. An Italian case-control study. Med Lav. 2002;93:303–309. [PubMed] [Google Scholar]
- 135.Hu J, Mao Y, White K. Renal cell carcinoma and occupational exposure to chemicals in Canada. Occup Med. 2002;52:157–164. doi: 10.1093/occmed/52.3.157. [DOI] [PubMed] [Google Scholar]
- 136.Mattioli S, Truffelli D, Baldasseroni A, et al. Occupational risk factors for renal cell cancer: a case-control study in Northern Italy. J Occup Environ Med. 2002;44:1028–1036. doi: 10.1097/00043764-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 137.Richard S, Carrette MN, Béroud C, et al. High incidence of renal tumours in vitamins A and E synthesis workers: a new cause of occupational cancer? [Letter] Int J Cancer. 2004;108:942–944. doi: 10.1002/ijc.11637. [DOI] [PubMed] [Google Scholar]
- 138.Zhang Y, Cantor KP, Lynch CF, Zheng T. A population-based case-control study of occupation and renal cell carcinoma risk in Iowa. J Occup Environ Med. 2004;46:235–240. doi: 10.1097/01.jom.0000116805.62079.d6. [DOI] [PubMed] [Google Scholar]
- 139.Guo HR, Chiang HS, Hu H, et al. Arsenic in drinking water and incidence of urinary cancers. Epidemiology. 1997;8:545–550. doi: 10.1097/00001648-199709000-00012. [DOI] [PubMed] [Google Scholar]
- 140.Kurttio P, Pukkala E, Kahelin H, et al. Arsenic concentrations in well water and risk of bladder and kidney cancer in Finland. Environ Health Perspect. 1999;107:705–710. doi: 10.1289/ehp.99107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baastrup R, Sørensen M, Baistrøm T, et al. Arsenic in drinking-water and risk for cancer in Denmark. Environ Health Perspect. 2008;116:231–237. doi: 10.1289/ehp.10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ward MH, Rusiecki JA, Lynch CF, Cantor KP. Nitrate in public water supplies and the risk of renal cell carcinoma. Cancer Causes Control. 2007;18:1141–1151. doi: 10.1007/s10552-007-9053-1. [DOI] [PubMed] [Google Scholar]
- 143.Kurttio P, Salonen L, Ilus T, et al. Well water radioactivity and risk of cancers of the urinary organs. Environ Res. 2006;102:333–338. doi: 10.1016/j.envres.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 144.Romanenko A, Morell-Quadreny L, Nepomnyaschy V, et al. Pathology and proliferative activity of renal-cell carcinomas (RCCS) and renal oncocytomas in patients with different radiation exposure after the Chernobyl accident in Ukraine. Int J Cancer. 2000;87:880–883. doi: 10.1002/1097-0215(20000915)87:6<880::aid-ijc19>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 145.Romanenko A, Morell-Quadreny L, Nepomnyaschy V, et al. Radiation sclerosing proliferative atypical nephropathy of peritumoral tissue of renal-cell carcinomas after the Chernobyl accident in Ukraine. Virchows Arch. 2001;438:146–153. doi: 10.1007/s004280000334. [DOI] [PubMed] [Google Scholar]
- 146.Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 147.Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet. 1997;16:68–73. doi: 10.1038/ng0597-68. [DOI] [PubMed] [Google Scholar]
- 148.Tomlinson IP, Alam NA, Rowan AJ, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat Genet. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 149.Nickerson ML, Warren MB, Toro JR, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. doi: 10.1016/s1535-6108(02)00104-6. [DOI] [PubMed] [Google Scholar]
- 150.Negri E, Foschi R, Talamini R, et al. Family history of cancer and the risk of renal cell cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2441–2444. doi: 10.1158/1055-9965.EPI-06-0382. [DOI] [PubMed] [Google Scholar]
- 151.Hung RJ, Moore L, Boffetta P, et al. Family history and the risk of kidney cancer: a multicenter case-control study in Central Europe. Cancer Epidemiol Biomarkers Prev. 2007;16:1287–1290. doi: 10.1158/1055-9965.EPI-06-0963. [DOI] [PubMed] [Google Scholar]
- 152.Randi G, Pelucchi C, Negri E, et al. Family history of urogenital cancers in patients with bladder, renal cell and prostate cancers. Int J Cancer. 2007;121:2748–2752. doi: 10.1002/ijc.23037. [DOI] [PubMed] [Google Scholar]
- 153.Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer. 2001;92:144–150. [PubMed] [Google Scholar]
- 154.Gudbiartsson T, Jónasdóttir T, Thoroddsen Á, et al. A population-based familial aggregation analysis indicates genetic contribution in a majority of renal cell carcinomas. Int J Cancer. 2002;100:476–479. doi: 10.1002/ijc.10513. [DOI] [PubMed] [Google Scholar]
- 155.Yu W, Gwinn M, Clyne M, et al. A navigator for human genome epidemiology. Nature Genetics. 2008;40:124–125. doi: 10.1038/ng0208-124. [DOI] [PubMed] [Google Scholar]
- 156.Brüning T, Lammert M, Kempkes M, et al. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch Toxicol. 1997;71:596–599. doi: 10.1007/s002040050432. [DOI] [PubMed] [Google Scholar]
- 157.Longuemaux S, Deloménie C, Gallou C, et al. Candidate genetic modifiers of individual susceptibility to renal cell carcinoma: a study of polymorphic human xenobiotic-metabolizing enzymes. Cancer Res. 1999;59:2903–2908. [PubMed] [Google Scholar]
- 158.Sweeney C, Farrow DC, Schwartz SM, et al. Glutathione S-transferase M1, T1, and P1 polymorphisms as risk factors for renal cell carcinoma: a case-control study. Cancer Epidemiol Biomarkers Prev. 2000;9:449–454. [PubMed] [Google Scholar]
- 159.Buzio L, De Palma G, Mozzoni P, et al. Glutathione S-transferases M1-1 and T1-1 as risk modifiers for renal cell cancer associated with occupational exposure to chemicals. Occup Environ Med. 2003;60:789–793. doi: 10.1136/oem.60.10.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moore LE, Brennan P, Karami S, et al. Glutathione S-transferase polymorphisms, cruciferous vegetable intake and cancer risk in the Central and Eastern European Kidney Cancer Study. Carcinogenesis. 2007;28:1960–1964. doi: 10.1093/carcin/bgm151. [DOI] [PubMed] [Google Scholar]
- 161.Wiesenhütter B, Selinski S, Golka K, et al. Re-assessment of the influence of polymorphisms of phase-II metabolic enzymes on renal cell cancer risk of trichloroethylene-exposed workers. Int Arch Occup Environ Health. 2007;81:247–251. doi: 10.1007/s00420-007-0200-5. [DOI] [PubMed] [Google Scholar]
- 162.Smits KM, Schouten LJ, van Dijk BAC, et al. Polymorphisms in genes related to activation or detoxification of carcinogens might interact with smoking to increase renal cancer risk: results from The Netherlands Cohort Study on diet and cancer. World J Urol. 2008;26:103–110. doi: 10.1007/s00345-007-0220-5. [DOI] [PubMed] [Google Scholar]
- 163.Karami S, Boffetta P, Rothman N, et al. Renal cell carcinoma, occupational pesticide exposure, and modification by glutathione S-transferase polymorphisms. Carcinogenesis. 2008 June 19; doi: 10.1093/carcin/bgn153. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30:445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 165.Farker K, Lehmann MH, Kästner R, et al. CYP2E1 genotyping in renal cell/urothelial cancer patients in comparison with control populations. Int J Clin Pharmacol Ther. 1998;36:463–468. [PubMed] [Google Scholar]
- 166.Semenza JC, Ziogas A, Largent J, et al. Gene-environment interactions in renal cell carcinoma. Am J Epidemiol. 2001;153:851–859. doi: 10.1093/aje/153.9.851. [DOI] [PubMed] [Google Scholar]
- 167.Sasaki M, Tanaka Y, Okino ST, et al. Polymorphisms of the CYP1B1 gene as risk factors for human renal cell cancer. Clin Cancer Res. 2004;10:2015–2019. doi: 10.1158/1078-0432.ccr-03-0166. [DOI] [PubMed] [Google Scholar]
- 168.Ikuyama T, Hamasaki T, Inatomi H, et al. Association of vitamin D receptor gene polymorphism with renal cell carcinoma in Japanese. Endocrine J. 2002;49:433–438. doi: 10.1507/endocrj.49.433. [DOI] [PubMed] [Google Scholar]
- 169.Obara W, Suzuki Y, Kato K, et al. Vitamin D receptor gene polymorphisms are associated with increased risk and progression of renal cell carcinoma in a Japanese population. Int J Urol. 2007;14:483–487. doi: 10.1111/j.1442-2042.2007.01771.x. [DOI] [PubMed] [Google Scholar]
- 170.Karami S, Brennan P, Hung RJ, et al. Vitamin D receptor polymorphisms and renal cancer risk in Central and Eastern Europe. J Toxicol Environ Health. 2008;71 (part A):367–372. doi: 10.1080/15287390701798685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Trydal T, Bakke A, Aksnes L, Aarskog D. 1,25-dihydroxyvitamin D3 receptor measurement in primary renal cell carcinomas and autologous normal kidney tissue. Cancer Res. 1988;48:2458–2461. [PubMed] [Google Scholar]
- 172.Fujioka T, Hasegawa M, Ishikura K, et al. Inhibition of tumor growth and angiogenesis by vitamin D3 agents in murine renal cell carcinoma. J Urol. 1998;160:247–251. [PubMed] [Google Scholar]
- 173.Hirata H, Hinoda Y, Matsuyama H, et al. Polymorphisms of DNA repair genes are associated with renal cell carcinoma. Biochem Biophys Res Communications. 2006;342:1058–1062. doi: 10.1016/j.bbrc.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 174.Sakano S, Hinoda Y, Okayama N, et al. The association of DNA repair gene polymorphisms with the development and progression of renal cell carcinoma. Ann Oncol. 2007;18:1817–1827. doi: 10.1093/annonc/mdm337. [DOI] [PubMed] [Google Scholar]
- 175.Rubio-Del-Campo A, Salinas-Sánchez AS, Sánchez-Sánchez F, et al. Implications of mismatch repair genes hMLH1 and hMSH2 in patients with sporadic renal cell carcinoma. BJU Int. 2008 doi: 10.1111/j.1464-410X.2008.07581.x. Epub. [DOI] [PubMed] [Google Scholar]
- 176.Heijmans BT, Boer JM, Suchiman HE, et al. A common variant of the methylenetetrahydrofolate reductase gene (1p36) is associated with an increased risk of cancer. Cancer Res. 2003;63:1249–1253. [PubMed] [Google Scholar]
- 177.Moore LE, Hung R, Karami S, et al. Folate metabolism genes, vegetable intake and renal cancer risk in central Europe. Int J Cancer. 2008;122:1710–1715. doi: 10.1002/ijc.23318. [DOI] [PubMed] [Google Scholar]
- 178.Yu J, Habuchi T, Tsuchiya N, et al. Association of the cyclin D1 gene G870A polymorphism with susceptibility to sporadic renal cell carcinoma. J Urol. 2004;172:2410–2413. doi: 10.1097/01.ju.0000138156.24384.16. [DOI] [PubMed] [Google Scholar]
- 179.Brennan P, McKay J, Moore L, et al. Uncommon CHEK2 mis-sense variant and reduced risk of tobacco-related cancers: case-control study. Human Molecular Genetics. 2007;16:1794–1801. doi: 10.1093/hmg/ddm127. [DOI] [PubMed] [Google Scholar]
- 180.Chen T, Jackson C, Costello B, et al. An intronic variant of the TGFBR1 gene is associated with carcinomas of the kidney and bladder. Int J Cancer. 2004;112:420–425. doi: 10.1002/ijc.20419. [DOI] [PubMed] [Google Scholar]
- 181.Havranek E, Howell WM, Fussell HM, et al. An interleukin-10 promotor polymorphism may influence tumor development in renal cell carcinoma. J Urol. 2005;173:709–712. doi: 10.1097/01.ju.0000152493.86001.91. [DOI] [PubMed] [Google Scholar]
- 182.Baştürk B, Yavaşçaoğlu İ, Vuruşkan H, et al. Cytokine gene polymorphisms as potential risk and protective factors in renal cell carcinoma. Cytokine. 2005;30:41–45. doi: 10.1016/j.cyto.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 183.Cozar JM, Romero JM, Aptsiauri N, et al. High incidence of CTLA-4 AA (CT60) polymorphism in renal cell cancer. Human Immunol. 2007;68:698–704. doi: 10.1016/j.humimm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 184.Tanaka Y, Hirata H, Chen Z, et al. Polymorphisms of catechol-O-methyltransferase in men with renal cell cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:92–97. doi: 10.1158/1055-9965.EPI-06-0605. [DOI] [PubMed] [Google Scholar]
- 185.Ollerenshaw M, Page T, Hammonds J, Demaine A. Polymorphisms in the hypoxia inducible factor-1α gene (HIF1A) are associated with the renal cell carcinoma phenotype. Cancer Genetics Cytogenetics. 2004;153:122–126. doi: 10.1016/j.cancergencyto.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 186.Hirata H, Okayama N, Naito K, et al. Association of a haplotype of matrix metalloproteinase (MMP)-1 and MMP-3 polymorphisms with renal cell carcinoma. Carcinogenesis. 2004;25:2379–2384. doi: 10.1093/carcin/bgh254. [DOI] [PubMed] [Google Scholar]
- 187.Awakura Y, Ito N, Nakamura E, et al. Matrix metalloproteinase-9 polymorphisms and renal cell carcinoma in a Japanese population. Cancer Lett. 2006;241:59–63. doi: 10.1016/j.canlet.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 188.Wu X, Amos CI, Zhu Y, et al. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- 189.Shao L, Wood CG, Zhang D, et al. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol. 2007;178:1492–1496. doi: 10.1016/j.juro.2007.05.112. [DOI] [PubMed] [Google Scholar]
- 190.Nickerson ML, Jaeger E, Durocher JA, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. doi: 10.1158/1078-0432.CCR-07-4921. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Charbotel B, Gad S, Caïola D, et al. Trichloroethylene exposure and somatic mutations of the VHL gene in patients with renal cell carcinoma. J Occup Med Toxicol. 2007;2:13–19. doi: 10.1186/1745-6673-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Van Dijk BAC, Schouten LJ, Oosterwijk E, et al. Cigarette smoking, von Hippel-Lindau gene mutations and sporadic renal cell carcinoma. Br J Cancer. 2006;95:374–377. doi: 10.1038/sj.bjc.6603281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Hemminki K, Jiang Y, Ma X, et al. Molecular epidemiology of VHL gene mutations in renal cell carcinoma patients: relation to dietary and other factors. Carcinogenesis. 2002;23:809–815. doi: 10.1093/carcin/23.5.809. [DOI] [PubMed] [Google Scholar]
- 194.Shiao YH, Rice JM, Anderson LM, et al. von Hippel-Lindau gene mutations in N-nitroso-dimethylamine-induced rat renal epithelial tumors. J Natl Cancer Inst. 1998;90:1720–1723. doi: 10.1093/jnci/90.22.1720. [DOI] [PubMed] [Google Scholar]
- 195.Zhu Y, Horikawa Y, Yang H, et al. BPDE induced lymphocytic chromosome 3p deletions may predict renal cell carcinoma risk. J Urol. 2008;179:2416–2421. doi: 10.1016/j.juro.2008.01.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Gnarra JR, Tory K, Weng Y, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 197.Van Houwelingen KP, van Dijk BA, Hulsbergen-van de Kaa CA, et al. Prevalence of von Hippel-Lindau gene mutations in sporadic renal cell carcinoma: results from The Netherlands cohort study. BMC Cancer. 2005;5:57–67. doi: 10.1186/1471-2407-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Banks RE, Tirukonda P, Taylor C, et al. Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene alterations and relationship with clinical variables in sporadic renal cancer. Cancer Res. 2006;66:2000–2011. doi: 10.1158/0008-5472.CAN-05-3074. [DOI] [PubMed] [Google Scholar]
- 199.Romanenko A, Morell-Quadreny L, Lopez-Guerrero JA, et al. The INK4a/ARF locus: role in cell cycle control for renal cell epithelial tumor growth after the Chernobyl accident. Virchows Arch. 2004;445:298–304. doi: 10.1007/s00428-004-1056-7. [DOI] [PubMed] [Google Scholar]
- 200.Roth S, Partanen T, Suitiala T, et al. Molecular analysis of occupational cancer: infrequent p53 and ras mutations in renal-cell cancer in workers exposed to gasoline. Int J Cancer. 1997;73:492–496. doi: 10.1002/(sici)1097-0215(19971114)73:4<492::aid-ijc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 201.Peters I, Vaske B, Albrecht K, et al. Adiposity and age are statistically related to enhanced RASSF1A tumor suppressor gene promoter methylation in normal autopsy kidney tissue. Cancer Epidemiol Biomarkers Prev. 2007;16:2526–2532. doi: 10.1158/1055-9965.EPI-07-0203. [DOI] [PubMed] [Google Scholar]
- 202.Mally A, Walker CL, Everitt JI, et al. Analysis of renal cell transformation following exposure to trichloroethene in vivo and its metabolite S-(dichlorovinyl)-L-cysteine in vitro. Toxicology. 2006;224:108–118. doi: 10.1016/j.tox.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 203.Lock EA, Barth J, Argraves SW, Schnellmann RG. Changes in gene expression in human renal proximal tubule cells exposed to low concentrations of S-(1,2-dichlorovinyl)-L-cysteine, a metabolite of trichloroethylene. Toxicol Appl Pharmacol. 2006;216:319–330. doi: 10.1016/j.taap.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 204.Yoshimoto T, Matsuura K, Kaman S, et al. High-resolution analysis of DNA copy number alterations and gene expression in renal clear cell carcinoma. J Path. 2007;213:392–401. doi: 10.1002/path.2239. [DOI] [PubMed] [Google Scholar]
- 205.Cifola I, Spinelli R, Beltrame L, et al. Genome-wide screening of copy number alterations and LOH events in renal cell carcinomas and integration with gene expression profile. Molecular Cancer. 2008;7:6–17. doi: 10.1186/1476-4598-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Monzon FA, Hagerkord JM, Lyons-Weiler MA, et al. Whole genome SNP arrays as a potential diagnostic tool for the detection of characteristic chromosomal aberrations in renal epithelial tumors. Modern Path. 2008;21:599–608. doi: 10.1038/modpathol.2008.20. [DOI] [PubMed] [Google Scholar]