Abstract
Receptor editing is the process of ongoing antibody gene rearrangement in a lymphocyte that already has a functional antigen receptor. The expression of a functional antigen receptor will normally terminate further rearrangement (allelic exclusion). However, lymphocytes with autoreactive receptors have a chance at escaping negative regulation by “editing” the specificities of their receptors with additional antibody gene rearrangements. Nemazee points out, “receptor editing separates receptor selection from cellular selection.”1 As such, editing complicates the Clonal Selection Hypothesis, because edited cells are not simply endowed for life with a single, invariant antigen receptor.2 For example, an edited B cell changes the specificity of its B cell receptor (BCR), and if the initial immunoglobulin gene is not inactivated during the editing process, allelic exclusion is violated, and the B cell can exhibit two specificities. Here we will describe the discovery of editing, the pathways of receptor editing at the heavy (H) and light (L) chain loci, and current evidence regarding how and where editing happens and what effects it has on the antibody repertoire.
Keywords: receptor editing, antibody, autoimmunity, B cell, V(D)J recombination
Scope of review and a word about editing nomenclature
This review focuses on receptor editing and receptor revision in normal and autoimmune B cells of mouse and man. Table 1 provides a glossary of terminology. Terminology that is defined in Table 1 is given in italics the first time it is used. Table 2 summarizes different methods that have been used to analyze editing.
Table 1.
Editing and Antibody Glossary
| Term | Definition |
|---|---|
| allelic exclusion | The phenomenon that B cells usually express a single kind of antibody H chain and L chain; exclusion is typically enforced at the genetic level—with only one allele being productively rearranged |
| allelic inclusion | The presence of more than one productively rearranged H or L chain allele in a single B cell; inclusion when it occurs, usually involves the L chain and may result in the expression of more than one BCR per B cell; competition for H chain pairing or intracellular sequestration limit phenotypic inclusion |
| BCR | B cell receptor; membrane bound antibody expressed on the surface of B cells |
| CDR3 | Third complementarity determining region or hypervariable portion of an antibody peptide chain. The H chain CDR3 is encoded by the 3′ end of the VH, the entire D segment and the 5′ end of the JH; the L chain CDR3 is encoded by the 3′ end of the VL and the 5′ end of the JL |
| cryptic heptamer | 7 nucleotide sequence recognized and cleaved by RAG in rearrangements that do not necessarily obey the 12/23 rule; a cryptic heptamer is present in the Jκ-Cκ intron that participates in iRS rearrangement; cryptic heptamers are found in the 3′ ends of most murine and human VH genes and can serve as substrates for VH replacement |
| H chain | Antibody heavy chain |
| isotypic exclusion | The phenomenon that B cells usually express a single L chain isotype (either κ or λ, not both) see above |
| L chain | Antibody light chain |
| leapfrogging | The process of ongoing Vκ-Jκ rearrangement in which an existing Vκ-Jκ rearrangement is replaced by an upstream Vκ rearranging of to a downstream Jκ gene segment; leapfrogging can also occur in human λ rearrangements by rearrangement of an upstream Vλ to a downstream JC cassette and in the H chain locus by secondary DJH rearrangement. |
| Primary rearrangement | Initial rearrangement attempt at heavy or light chain loci |
| RAG | The recombinase activating genes, rag1 and rag2, encode enzymes that are part of the multi-protein complex that carries out V(D)J recombination |
| receptor editing | The process of ongoing gene rearrangement in a B cell that already has a productive heavy or light chain gene rearrangement; successful editing converts the specificity of a self-reactive antibody into a non-self-reactive antibody. |
| receptor revision | Term used in some papers to describe secondary rearrangement that occurs in mature (typically antigen-experienced) B cells |
| RS rearrangement | Cκ deletion by rearrangement of the RS (recombining sequence, a non-coding gene segment downstream of Cκ; known as the κ deleting element in humans) in B cells that have typically already endured multiple κ light chain gene rearrangements; there are two kinds of RS rearrangement, iRS (involving the cryptic heptamer in the Jκ-Cκ intron) or Vκ-RS. |
| RSS (recombination signal sequence) | A conserved heptamer and nonamer separated by a non- conserved spacers of approximately 12 or 23 nucleotides; RSSs are bound and cleaved by RAG, the recombinase that carries out V(D)J recombination |
| Secondary rearrangement | One or more rearrangements that occurs after primary rearrangement |
| V(D)J recombination | Site-directed somatic DNA recombination between variable (V), diversity (D, heavy chain only) and joining (J) gene segments that generates a diverse repertoire of antigen receptors in B cells and T cells; V(D)J recombination is also referred to as antibody gene rearrangement. |
| VH replacement | The process of H chain rearrangement on an allele that already contains a H chain rearrangement; VH replacement occurs by invasion of a cryptic heptamer (see above); the resulting rearrangement product consists of the new VH and most of the CDR3 (including the DH and JH gene segments) from the previous H chain rearrangement |
Table 2.
Methods for the Study of Receptor Editing
| Method Description |
|---|
| cell culture systems. Bone marrow stromal pre-B cell cultures in which IL-7 is withdrawn will undergo sequential rearrangement of antibody L chains. |
| excision circles. Closed loops of DNA containing earlier Vκ-Jκ rearrangements can be detected in B cells that have undergone only a few cell divisions since L chain rearrangement. Sequence analysis can be used to evaluate editing precursors; see also reciprocal products |
| heavy chain allotype. Allelic differences in H chain constant regions can be used to generate antibodies that recognize H chains of particular mouse strains. These serological markers can be used to evaluate allelic inclusion and editing of H chains in mice that are heterozygous for two different H chain allotypes. |
| human Cκ heterozygous mice. This site-directed transgenic provides an allelic marker for the murine κ locus. |
| hybridomas and other transformed B cells. These monoclonal cell lines facilitate genetic analysis of reciprocal products from inversions and RS deleted alleles. |
| Ig transgenic and site-directed transgenic mouse models (see Table 3) |
| Jκ skewing. An increased usage of distal Jκ segments in highly edited B cell populations arises as a consequence of leapfrogging rearrangement at the κ L chain locus. |
| L chain isotype. Kappa (κ) and lambda (λ) L chains are the two L chain isotypes. Since the λ locus usually rearranges after the κ locus, B cells with more λ rearrangements have undergone more rounds of L chain gene rearrangement and may represent an edited population. |
| ligation-mediated PCR of DNA breaks. A technique that detects double strandedDNA breaks that are present transiently during the process of immunoglobulin gene rearrangement. |
| RAG transcript abundance or RAG reporter mice. RAG mRNA or protein expression in a particular B cell population or subset is consistent with ongoing immunoglobulin gene rearrangement. |
| reciprocal products. Vκ-Jκ rearrangements that occur by chromosomal DNA inversion create novel segments of DNA that may contain previous Vκ-Jκ rearrangements. This facilitates the analysis of editing precursors. See also excision circles. |
| RS deletion frequency. An increased frequency of RS rearrangement in B cells indicates more rounds of L chain gene rearrangement. |
| Sequence analysis. Ig gene sequence can identify VH replacement footprints. Single cell PCR amplification and sequencing of immunoglobulin genes can detect multiple productive rearrangements in a single cell. |
Germline antibody genes undergo primary somatic gene rearrangement (also known as V(D)J recombination) in order to generate the antibody repertoire. This first attempt may be productive (resulting in a functional H chain, H+, or functional L chain, L+) or non-productive (H− or L−). Secondary rearrangement refers to one or more rearrangements that occur after primary rearrangement. Included amongst secondary rearrangements are those that rescue B cells with non-productive primary rearrangements and those that alter BCR specificity. Secondary rearrangements can occur on the same or a different chromosome from the primary rearrangement. Rearrangements that alter the specificity of the BCR to avoid autoreactivity are referred to as receptor editing and usually occur early during B cell development, typically in the bone marrow. Secondary rearrangements that occur in mature B cells are referred to as receptor revision and their effects on autoreactivity are controversial.
The potential advantages and disadvantages of receptor editing
At the outset one might ask, why edit? The major biological justification for the existence of receptor editing is likely to be the elimination of self-reactive B cells and the prevention of autoimmune manifestations. In mice expressing the apoptosis inhibitory genes bcl-2 and bcl-xL, editing of self-reactive B cells that escape deletion is an important mechanism to prevent autoimmunity 3, 4. By giving B cells with autoreactive receptors a second chance to survive, editing potentially increases the efficiency of primary B cell generation. Editing most often involves the L chain, and B cells undergoing L chain rearrangement have nearly always completed H chain rearrangement, pre-BCR selection and expansion (Fig. 1). In other words, a fair amount of energy has already been invested in creating B cells with a “functional” H chain. Once the H chain is fixed, the L chain can then be edited to modify BCR specificity. Furthermore, editing may represent a diversification mechanism for the immune system, i.e. a way to create additional specificities. The ability to undergo multiple rearrangement attempts may allow for a more thorough sampling of the germline repertoire through rearrangement. This is particularly apt for L chain rearrangements, which can potentially occur multiple times per κ allele by leapfrogging and, failing success at κ, move on to λ. Recently, defects in receptor editing have been documented in autoimmune-prone strains of mice and in humans with lupus and type 1 diabetes.5, 6 If editing can correct autoreactivity, will it be possible to manipulate the extent of recombination to increase the stringency of B cell selection in the setting of autoimmune disease? Will it be possible to use assays of antibody gene rearrangement to predict who will develop autoimmunity in the future or who will have a type of disease that requires a particular form of B cell targeted therapy?
Figure 1. B cell development and potential tolerance checkpoints.
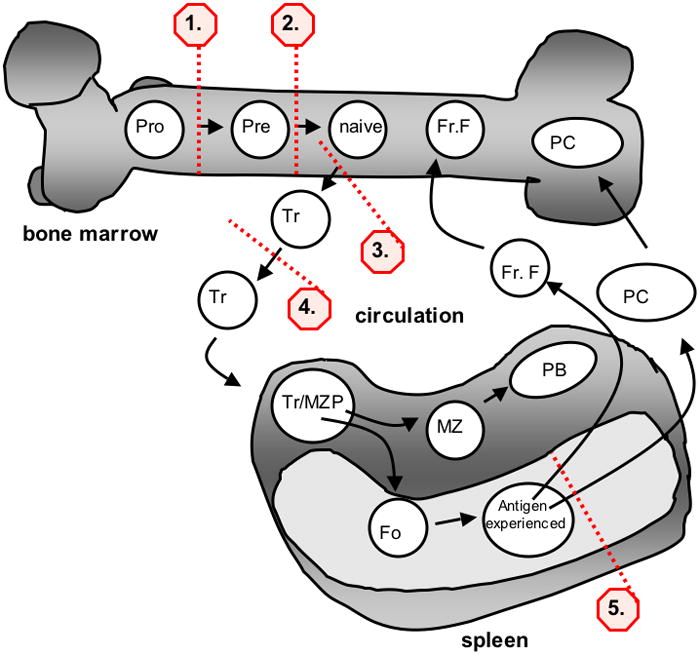
Black arrows denote stages of B cell development in the bone marrow, circulation and spleen. For illustrative purposes, development is shown as a single, linear process as follows: pro-B (where H chain rearrangement occurs, corresponds to Hardy Fr. B-C, as described in 196), pre-B (where L chain gene rearrangement occurs, corresponds to Hardy Fr. D), naive (or newly formed IgM+ B cells; Hardy Fr. E), Tr (transitional B cells), Tr/MZP (transitional/marginal zone precursor), MZ (marginal zone), Fo (follicular), PB (plasmablast, short-lived antibody forming cells), Antigen-experienced (B cells which have received T cell help and are undergoing somatic mutation and differentiation into memory cells and long-lived plasma cells), Fr. F (Hardy fraction F, mature B cells that recirculate to the bone marrow), PC (plasma cells). Numbered red dashed lines correspond to the potential tolerance checkpoints: 1. At the pro-B to pre-B cell transition H chains undergo selection and may undergo additional rounds of rearrangement if the expressed H chain is autoreactive; 2. At the pre-B to naive-B cell transition antibody specificity is tested and B cells with autoreactive BCR may revert to surface immunoglobulin negative status (Hardy Fr. D) due to leapfrogging or RS deletion, as they undergo additional rounds of L chain rearrangement; 3–4. IgM expressing newly formed B cells and transitional cells may undergo additional rounds of rearrangement before (editing) or after (revision) they leave the bone marrow; 5. receptor revision may occur in more mature B cells.
On the other hand, editing and receptor revision may have undesirable consequences. Editing may not always correct autoreactivity. In the case of VH replacement, the retention of much of the CDR3 may preserve some of the autoreactivity. In addition, the VH replaced antibody will usually have a longer CDR3, which in itself may predispose to autoreactivity or polyreactivity.7 Likewise, L chain editing, while more frequent than H chain editing and more likely to occur at a time when BCR specificity is being tested, does not necessarily result in the correction of autoreactivity. As the antibody H chain often contributes in a dominant fashion to autoantibody specificity8 and much of the normal primary antibody repertoire is autoreactive,9 switching an antibody’s L chain may not fully abrogate autoreactivity. For example, B cells with autoreactive H chains that are paired with editor L chains are sometimes still polyreactive.10, 11 Even if the editor L chain successfully alters autoantibody specificity, the editing process does not always delete the initially produced autoreactive L chain. Such allelically or isotypically included B cells may in fact bypass the naive/transitional tolerance checkpoint, if the non-autoreactive L chain can out-compete the autoreactive L chain either for H chain pairing or surface expression (Fig. 1). In the periphery, however, the expressed L chain may be inactivated, perhaps by somatic mutation, and the autoreactive L chain can be re-expressed, now unopposed by the inactivated editor L chain.12 It is even possible that an editing event may occur in peripheral mature B cells, and may produce de novo an autoreactive specificity. Importantly, the creation of an autoreactive B cell in the periphery may be particularly problematic, as many of the primary B cell tolerance checkpoints have been bypassed (Fig. 1). In addition to concerns about autoreactivity, a correlation between high levels of editing and chromosome translocations has been described.13 Rearrangement generates double-stranded DNA breaks. When these breaks occur in B cells that are undergoing cell division and have relaxed cell cycle checkpoint controls and/or defective DNA repair enzymes, there is an increased risk of chromosomal translocations and oncogenic transformation. In this connection, it has been proposed that mantle cell lymphomas may arise in autoimmune-prone B cells that are excluded from the germinal center.14
Origins of receptor editing: allelic exclusion
The concept of receptor editing originated from studies that analyzed allelic exclusion of antibodies. In this section we will describe these studies and link them to the discovery of receptor editing. In these early studies, immunophenotyping showed that nearly all B cells in an F1 animal having two different heavy chain allotypes expressed only a single allotype on the surface of individual B cells. In rabbits, mice, chickens and humans, the proportion of B cells that expressed both alleles was less than one percent, and it was not clear if the few “double-expressers” found were truly allelically included or represented antibodies that happened to cross-react with the other allotype.15–21 The possibility that some allelically included B cells appeared to have only one BCR on the surface but actually expressed more than one H chain inside the cell was later ruled out in mice and rabbits using intracellular staining.22, 23 L chains also showed isotypic exclusion: B cells typically expressed either κ or λ L chains, but not both (reviewed in 22). Southern blotting and PCR studies demonstrated that the major reason for allelic exclusion was not phenotypic, but genetic: B cells typically harbored only one productively rearranged H chain allele (VDJ+). The other allele was either non-productively (VDJ−) or incompletely rearranged (DJ). The later findings for L chains turned out to be more complex (discussed further below). These early findings gave rise to the notion of “allelic” or “haplotype” exclusion at the level of antibody gene rearrangement: once a productive rearrangement was made, the other allele was somehow inhibited from undergoing additional rearrangements. Two models were proposed to account for exclusion, the stochastic model and the deterministic model.
According to the stochastic model, rearrangements occur randomly, are relatively infrequent and are usually unsuccessful, so that most B cells wind up being allelically excluded by chance24. Initiation of rearrangement depends upon the probability of rearrangement at a given locus. The temporal ordering of rearrangement can be accounted for (in part) by having higher rearrangement probabilities at the H chain locus than the L chain loci and a higher probability of rearrangement at κ than at λ. If the probabilities for H and L chain rearrangement are not extremely different, one would predict that rearrangements would occasionally go “out of order.” Consistent with this prediction, there are examples of murine and human B cells in which L chain rearrangement precedes H chain rearrangement.25, 26
On the other hand, approximately one third of mouse κ+ B cells have germline κ alleles and this, along with the high κ:λ ratio, is difficult to accommodate in a model with synchronous parallel rearrangement of multiple loci.24 One way to increase the frequency of germline alleles is to invoke a short time window of opportunity for rearrangement and limit the amount of recombinase or locus-accessibility to recombination, such that a very low fraction of alleles is undergoing rearrangement at a given time. 27–29 In this model, rearrangement is like playing Russian roulette: at each spin of the wheel, the cell has a chance of either rearranging or dying. If there is a high chance of dying with each spin (a high crash factor) and there are relatively few spins, cells that initially undergo productive rearrangement are the most likely to survive. Such cells would often have a productive rearrangement on one κ allele and no other L chain rearrangements. However, this model still does not readily accommodate B cells with λ rearrangements, which usually have exhaustively rearranged κ alleles (see below).
The low probability of rearrangement at an immunoglobulin locus is enhanced by a high proportion of dysfunctional rearrangements.24 If rearrangements are random, two thirds of them are out of frame to begin with. Consistent with this prediction, approximately two thirds of H chain rearrangements are out-of-frame in pro-B cells, prior to pre-BCR expression.30 Furthermore, not all of the in-frame rearrangements are functional: there can be termination codons and pseudogenes. Finally, even if the rearrangements are productive, the H chain may not pair well with the L chain.
Alternatively, the deterministic model does not leave allelic exclusion to chance, but rather invokes ordered initiation of recombination and active feedback inhibition once a productive rearrangement is made 31, 32. A series of epigenetic mechanisms appear to control H and L chain locus accessibility and dictate which allele is rearranged first. These mechanisms include replication timing, DNA methylation, histone modification, nucleosome positioning and heterochromatization (reviewed in 33). According to the deterministic model, the sequential rearrangement of H and L chain loci during the pro-B and pre-B cell stages of B cell development is influenced not only by regulated accessibility to recombination machinery, but by active feedback inhibition of rearrangement in between H chain and L chain rearrangement. The deterministic model posits a kinetic argument to account for low frequencies of RAG-accessibility or low levels of RAG expression within single developing B cells: at any given instant, the fraction of B cells undergoing active rearrangement is low, but most B cells eventually undergo rearrangement at multiple loci.34 Once the H chain protein is complexed to an invariant surrogate light chain, λ5/VpreB, the resultant pre-BCR is expressed on the cell surface and the rearrangement machinery is turned off. 35, 36, 37 Thus, the prevention of signaling from the pre-BCR by deletion of Igα or Igβ leads to the loss of H chain allelic inclusion. 38, 39 Also consistent with the deterministic model is the down-regulation of rag transcripts in developing B cells after successful H chain rearrangement and their up-regulation before L chain rearrangement. 40
Isotypic exclusion of L chains is also consistent with a regulated model of rearrangement. According to the regulated model, isotypic exclusion is explained by two properties of L chain rearrangement. The first property is that κ and λ rearrange at different times during B cell development. 41, 42, 43 The second is that λ expressing B cells often have both κ alleles deleted. Based primarily on the analysis of transformed cell lines in mouse and man, it was shown that κ nearly always rearranged first, followed by locus deletion (termed RS rearrangement in mice, and κ deleting element in humans: see below) followed finally by λ 44–49 All of these findings are accommodated by a model in which H chain and L chain rearrangements occur in a regulated and ordered manner.
Fundamental to either theory of allelic exclusion is that B cells with more than one productive antibody rearrangement are uncommon, either by chance or by regulated rearrangement. Yet, over time, examples of secondary rearrangement in B cells with productive antibody rearrangements began to accumulate. Primary and secondary rearrangements at the allele level are depicted schematically for H chains in Fig. 2a and for L chains in Fig. 2b.
Figure 2. Schematics of heavy and light chain allele configurations during primary and editing rearrangements.

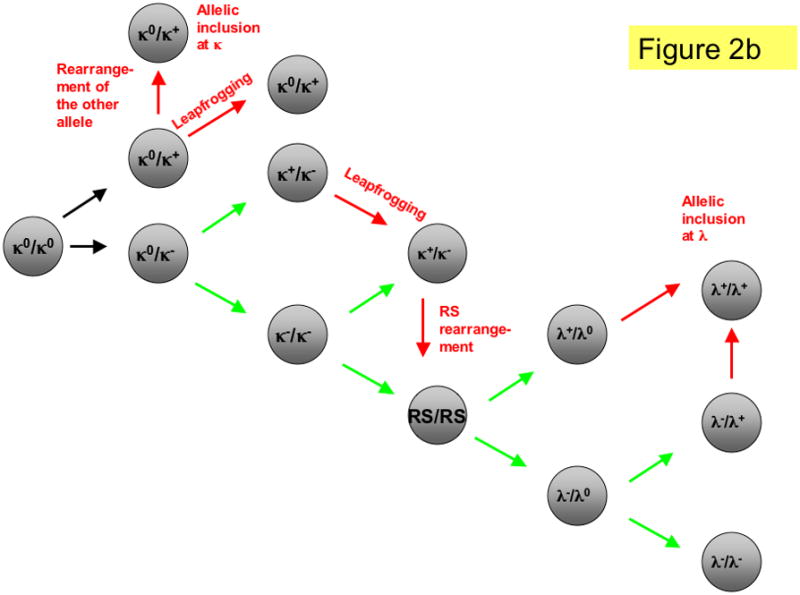
a. Heavy chain rearrangement in mouse and man. At the pro-B cell stage, B cells undergo D to J rearrangement on both alleles (DJ/DJ). This is followed by V to DJ rearrangement on one or both alleles. The allele genotypes are: DJ, DJs (secondary DJH rearranged allele; also included here but not shown are alleles that have undergone D-D fusion), H+ (productive VDJ rearrangement), H− (non-productive VDJ rearrangement), VHR (VH replacement). Black arrows indicate primary rearrangement attempts. Green arrows indicate secondary rearrangements in B cells with one or more prior non-productive rearrangements and red arrows indicate editing rearrangements in B cells with one or more prior productive rearrangements. b. Light chain rearrangements in mouse. B cells initially have both κ and λ loci in the germline configuration (κ0/κ0, λ0/λ0). Most B cells rearrange κ first (VκJκ) then undergo RS rearrangement and, if needed, progress to λ rearrangement. Arrows indicate rearrangement attempts using the same color scheme as in panel a. Shown is only one of many possible orderings of L chain rearrangement. To save space, only the loci that are undergoing rearrangement are shown. κ+ (productive κ light chain gene rearrangement), κ− (non-productive κ rearrangement), RS (RS deletion or inversion by either V-RS or iRS rearrangement), λ+ (productive lambda light chain rearrangement), λ−(non-productive lambda rearrangement).
Secondary heavy chain rearrangements
The H chain locus can undergo a leapfrogging type of rearrangement called secondary DJH rearrangement. During secondary DJH rearrangement, a DH gene upstream of the existing DJH rearrangement recombines with a JH gene downstream of the DJH rearrangement, replacing the preceding DJH joint (Fig. 3a) by a leapfrogging deletional rearrangement (Fig. 3b). In a library of neonatal VHDJH rearrangements from the autoimmune MRL and the non-autoimmune parental C3H strains, the control C3H mice often rearranged DQ52 to JH1. (DQ52-JH1 is by definition a primary rearrangement since these are the DH and JH gene segments that are closest to each other on the chromosome.) In contrast, DQ52-JH1 rearrangements were rare in the SLE-prone MRL strain.50 The MRL mice preferentially used upstream D genes and the downstream JH4 gene, potentially arising by secondary DJH rearrangement.50 In humans, rearrangements to the most DH-distal JH gene segment, JH6, appear to be counter-selected during early B cell development 9. As JH6 is considerably longer than the other JH gene segments, it likely contributes to CDR3 elongation and potentially to antibody multireactivity.
Figure 3. Pathways of heavy chain rearrangement and editing.
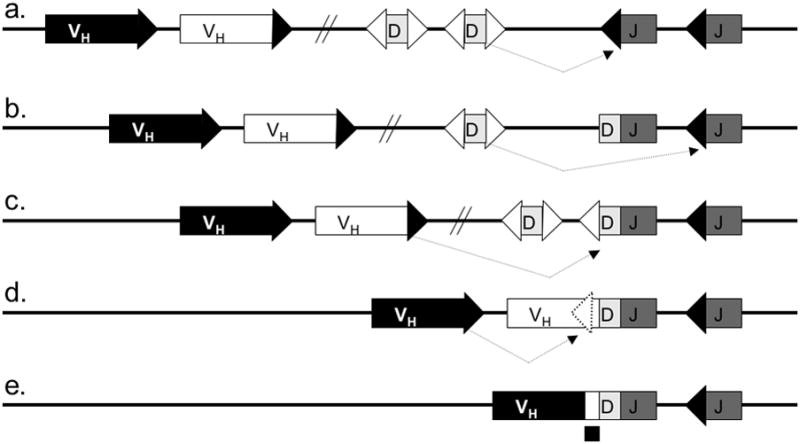
A simplified murine heavy chain locus with two H chain variable region genes (VH), two diversity segments (D H) and two joining gene segments (JH) is shown. Five heavy chain allele configurations are shown: a. Germline heavy chain locus undergoing D to J rearrangement; b. H chain locus with a primary DJH rearrangement undergoing secondary DJH rearrangement; c. DJ rearranged locus undergoing V to DJH rearrangement; d. locus with a completed VDJ rearrangement undergoing VH replacement; e. locus with a completed VH replacement. Dashed arrows indicate the rearrangement pathways. Boxes denote gene segments, lines introns, white triangles recombination signal sequences with 12-nucleotide spacers, black triangles recombination signal sequences with 23-nucleotide spacers, dashed triangle cryptic recombination signal sequence, and diagonal parallel lines indicate large gaps in the intervening sequence (the intervening sequence is much longer than can be shown herein). The footprint of the replaced VH is marked by a black square under the VH replaced allele in panel e.
Other types of secondary rearrangements at the H chain locus violate the 12/23 rule. According to the 12/23 rule, rearrangement preferentially occurs between RSSs (recombination signal sequences) having dissimilar spacer lengths.51 For example, in the mouse H chain locus, the DH segment RSSs have 12-nucleotide spacers (12-RSS), whereas the VH and JH segments have 23-RSSs.52 Thus DHs recombine with JH and VH but VH cannot directly recombine with JH (Figs. 3a–3c). Because all of the upstream DH segments are deleted when a VH rearranges to DJH, editing of the H chain VH by leapfrogging appears to be impossible.53 But in fact rearrangements that violate the 12/23 rule do occur at both the H chain and the κ loci, and include DH-DH, VH replacement, Jκ-Jκ, Vκ-Vκ, Vκ-iRS and other combinations.54–56
In a DH-DH fusion, the recombination process joins a 5′ DH segment to a preceding DH-JH rearrangement rather than to a 3′ JH gene. Although DH-DH fusions violate the 12/23 rule, they may do so because heptamer-like sequences within DH coding sequences make the DH-RSSs look more like 23-RSSs than 12-RSSs.57 DH-DH fusions occur more frequently in murine lupus than in non-autoimmune strains of mice.50, 58 Approximately 10–20% of human antibody sequences have highly elongated CDR3s, some of which appear to contain DH-DH fusions.57, 59, 60 The significance of DH-DH fusions in human antibody sequences is unclear.
Another mechanism of secondary rearrangement at the H chain locus, called VH replacement, provides a means of editing a fully rearranged (VDJ) H chain.61, 62 During VH replacement (Figs. 3d, 3e), the conventional 23-RSS of an upstream murine VH undergoes RAG-dependent deletional rearrangement with the cryptic RSS of an existing downstream VH gene. The downstream VH gene is part of an existing VDJ rearrangement on the same allele.63 In mice, the cryptic RSS usually resembles a 12-RSS, having a conserved nonamer 12 nt upstream of the heptamer, and is found in over 80% of VHs.64 This rearrangement results in replacement of all but the very 3′ end of the previously rearranged VH with a new VH. As a result, a “footprint” containing the 3′ portion of the CDR3 sequence from the original rearrangement is retained in the new rearrangement. VH replacement was initially discovered in transformed cell lines, but later also observed in H chain knock-in mice.61, 62, 64, 65 Footprints of VH replacement have been found in human VH gene sequences.7
Secondary DH-JH rearrangements, DH-DH fusions and VH replacement can contribute to elongation of the CDR3 and promote autoreactivity. Whereas most murine antibodies use the D gene reading frame 1 (RF1) which codes for a tyrosine-glycine rich amino acid sequence, a DH-DH fusion fails to preserve the preferred reading frame, often resulting in RF3 usage. RF3 sequences often contain an arginine codon66. VH replacement tends to increase the CDR3 length because of the retention of the “footprint” at the 3′ end of the original VH. The 3′ ends of VH gene segments usually contain an arginine residue. Together, the longer CDR3 and the additional arginine residue contribute to autoreactivity. Arginine residues are positively charged and increase antibody binding to DNA.8
Longer H chain CDR3s with altered of amino acid composition are features of the antibody repertoire in strains such as MRL/MpJ and MRL/lpr.67–69 Similar observations were made in another mouse model of lupus that carries the NZM2410-derived interval Sle3/5 on the C57BL/6 background.70 In comparison with the non-autoimmune B6 strain, B6.Sle3/5 H chains exhibited longer CDR3 sequences with frequent arginine residues, greater usage of the RF3 reading frame, DH-DH fusions, and skewed DH and JH usage 70. In addition, mature B cells from B6.Sle3/5 mice showed evidence of re-expression of the rag genes with presence of VH-DH excision circles, suggesting that VH replacement rearrangements took place in the periphery of lupus-prone mice 70.
Secondary light chain rearrangements
Secondary rearrangement can also occur at the κ locus. Rearrangements at the κ locus can occur by deletion, in which case the chromosomal DNA between the recombining gene segments is excised and released as an episome (Fig. 4a and Table 2). Alternatively, κ rearrangement can occur by inversion, in which case the chromosomal DNA between the recombining gene segments is retained on the chromosome in the form of a reciprocal product (Fig. 4b). 55, 71 Productive Vκ-Jκ rearrangements have been recovered from both excision circles 72 and reciprocal products.73, 74 Single cell PCR studies showed that the frequency of κ+/κ+ in individual B cells is approximately 10%. 75, 76 Lambda L chain rearrangements in murine and human B cells were also frequently allelically included. 75, 77, 78 The potential for allelic inclusion of B cells that undergo multiple λ rearrangements is illustrated in Fig. 5 and discussed further below. Taken together, these findings are compatible with feedback inhibition of rearrangement only if the mechanism is frequently faulty.
Figure 4. Pathways of kappa light chain rearrangement and editing.
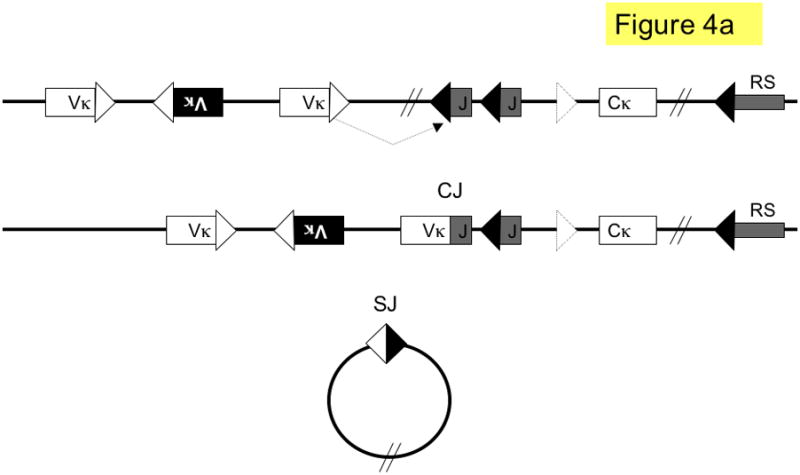
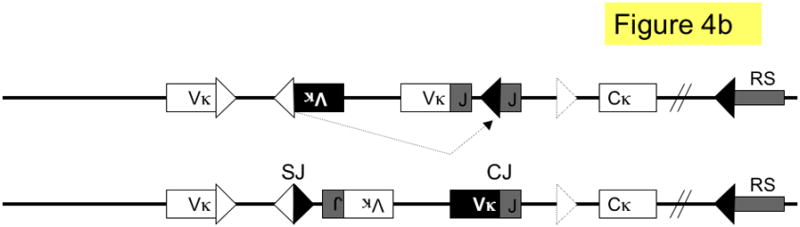
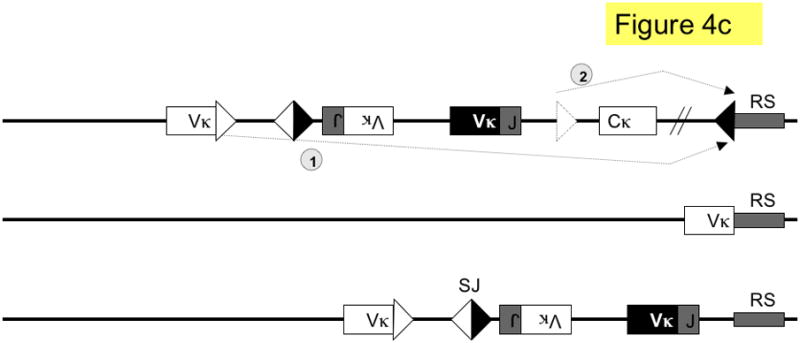
A simplified κ locus with three variable region gene segments (Vκ), two joining segments (J), one constant region (Cκ) and the recombining sequence (RS) is shown. Three different kinds of κ rearrangement are shown, which could have occurred sequentially: a. deletional κ rearrangement, producing a coding joint (CJ) and a signal joint (SJ); the piece of DNA containing the SJ is released as an episome that is not replicated with successive cell divisions. b. inversional secondary rearrangement of a Vκ gene present in reverse orientation; leapfrogging over an existing Vκ-Jκ rearrangement here produces an SJ that is retained on the chromosome in the reciprocal product; c. κ locus silencing by two pathways of RS rearrangement on a locus with at two prior Vκ-Jκ rearrangements; arrows are labeled to illustrate the two pathways: pathway 1. Vκ-RS deletional rearrangement results in excision of Cκ and pathway 2. iRS rearrangement results in excision of Cκ but retention of both preceding Vκ-Jκ rearrangements. Dashed arrows denote rearrangements, boxes represent gene segments, lines introns, white triangles recombination signal sequences with 12-nucleotide spacers, black triangles recombination signal sequences with 23-nucleotide spacers, dashed triangle J-C intron heptamer, diagonal parallel lines indicate large gaps in the intervening sequence. RS = recombining sequence.
Figure 5. Pathways of lambda light chain rearrangement and editing.
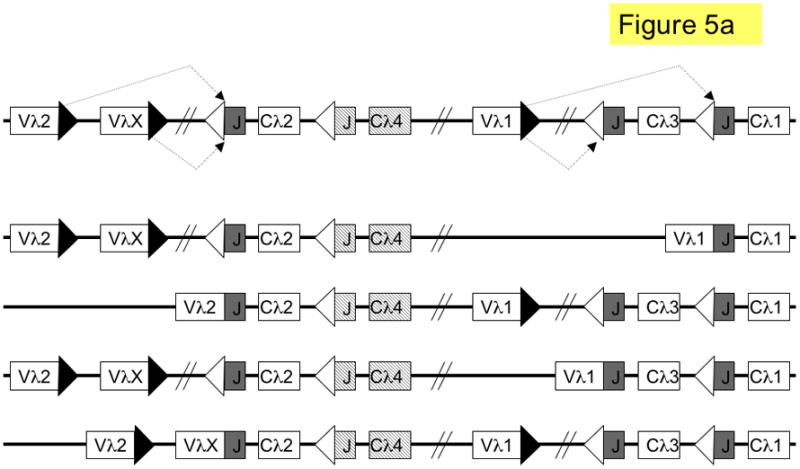
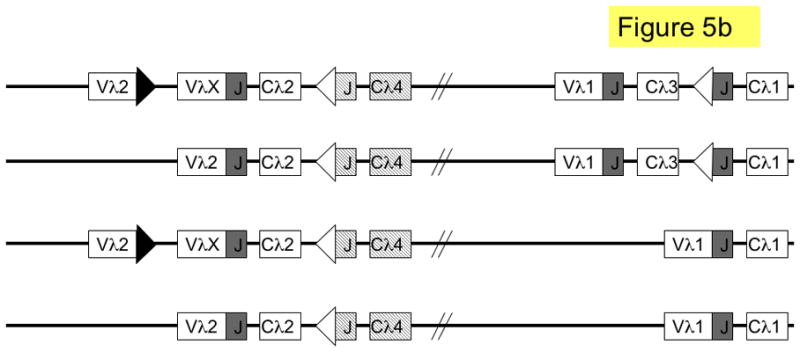
The murine lambda locus is shown. a. primary λ rearrangements (one rearrangement per allele); shown from top to bottom are: germline (unrearranged) locus; Vλ1 to JCλ1 rearrangement (λ1); Vλ2 to JCλ2 rearrangement (λ2); Vλ1 to JCλ3 rearrangement (λ3) and VλX to JCλ2 rearrangement (λX). b. secondary L rearrangements (two rearrangements per allele); shown from top to bottom are: λX and λ3; λ2 and λ3; λX andλ1; λ2 and λ1. Boxes denote gene segments, the JCλ4 is represented by dashed boxes to indicate that it is a pseudogene; lines denote introns, white triangles recombination signal sequences with 12-nucleotide spacers, black triangles recombination signal sequences with 23-nucleotide spacers and diagonal parallel lines indicate large gaps in the intervening sequence. SJ = signal joint.
Mathematical modeling suggests that normal mature murine B cells have rearranged their L chain loci 2.5 times, on average.79 However, there are unresolved issues with respect to the frequency of secondary rearrangement. For example, why is the κ/λ ratio lower and RS rearrangement frequency higher in bone marrow pre-B cells than in mature splenic B cells?6, 27, 42 Most κ rearrangements in normal splenic murine B cells involve Jκ1 or Jκ2, suggesting that they are primary rather than secondary rearrangements. Are highly edited B cells counter-selected or sequestered somewhere?
Additional evidence for ongoing rearrangement emerged from the analysis of endogenous antibody gene rearrangements in immunoglobulin transgenic mice. When B cells were analyzed in detail from immunoglobulin transgenic mice, not all of the endogenous antibody genes were found to be in the germline configuration, as would have been predicted by the feedback inhibition model.80–85 Mice bearing autoantibody transgenes had even higher levels of allelic inclusion.83, 86, 87 Taken together, these findings suggested that there might be an important physiological process underlying “failed” allelic exclusion. Further studies showed that ongoing rearrangement might occur to edit the specificity of an autoreactive B cell receptor.
Receptor Editing in Mice with Conventional Autoantibody Transgenes
The initial descriptions of receptor editing were based on studies of two conventional autoantibody transgenic mice with normal (non-autoimmune) genetic backgrounds.83, 86, 87 One of the transgenes, called 3–83, consisted of an antibody H chain and L chain pair that recognized particular MHC class II alleles (H-2Kk or b but not H-2Kd). Therefore, the fates of the transgenic B cells could be compared in autoreactive and non-autoreactive situations (this is sometimes referred to as a facultative autoantigen).88 The second autoantibody transgene, 3H9, consisted of a H chain that had been cloned from a hybridoma panel derived from an autoimmune MRL/lpr mouse.89 The 3H9 H chain, when paired with most endogenous L chains, recognized DNA, a constitutive autoantigen associated with systemic autoimmunity.
In the presence of the ubiquitously expressed autoantigen H-2Kk, some 3–83 transgene-expressing B cells are deleted in the bone marrow, but others inactivate their transgene and upregulate the recombinase pathway in order to produce new endogenous rearrangements that do not bear the autoreactive transgene specificity. Tiegs and Nemazee demonstrated that 3–83 tg, H-2Kk mice exhibited: (1) Increased rag 1 and rag2 gene expression in the bone marrow, including in the B cells that expressed the transgenic H chain; (2) elevated levels of excision circles indicative of endogenous L chain rearrangement and (3) increased endogenous L chain expression. These features were not found in 3–83 tg mice that did not express the autoantigen (i.e., H-2Kd mice) or in mice that expressed the H-2Kk autoantigen only in the liver. The authors did not explain how the transgenic Ig chains were inactivated, although some cells were shown to express both the transgenic L chain and an endogenous L chain (see below). They postulated that in the physiologic situation, an ‘autoreactive’ L chain could be removed and replaced by another L chain by a process of secondary recombination. In part, this process should be reflected by an increased frequency of λ chain usage.90
The analysis of 3H9 H chain only transgenic mice provided evidence that endogenous L chains could edit the specificity of anti-DNA antibodies by leapfrogging rearrangement.83, 87 Because there are numerous Vκ and Jκ gene segments, Vκ gene segments upstream of an existing Vκ-Jκ rearrangement can rearrange to downstream Jκ segments. This form of secondary rearrangement, also referred to as “leapfrogging”, is particularly efficient for editing, because it not only generates a new L chain, but also inactivates the preceding rearrangement (Fig 4b). A prediction of editing by leapfrogging is that editor κ L chains should exhibit a skewing towards the use of more distal Jκ segments (e.g., use Jκ4 or 5 more frequently than Jκ1 or Jκ2). The 3H9 H chain produces anti-DNA antibodies with most L chains. Thus, in 3H9 H chain transgenic mice, most initial L chain rearrangements (rearranged to Jκ1 or Jκ2) would require editing.91 Consistent with the prediction of increased editing, a higher fraction than expected of hybridomas from 3H9 mice had Vκ-Jκ rearrangements that used distal Jκ gene segments.87 Distal Jκ skewing was even more pronounced in 3H9 transgenic mice that lacked one κ allele, in which κ chain rearrangement can only occur on the single remaining κ allele.78 However these studies did not analyze the L chains that were edited to determine if they were autoreactive. They also did not rule out the possibility that particular Jκ segments were selected in combination with particular Vκs, which could cause non-random Jκ skewing for reasons other than increased editing.92
Editing can also occur by rearrangement on a second κ allele or at λ. In this case, the first L chain rearrangement is not necessarily erased, and allelic inclusion may result. If both L chains are functional, they may compete for pairing to the same H chain in the same B cell, creating up to 3 different BCRs for the dimeric IgMs. Indirect evidence for editing by L chain competition was described in mice that had both the 3H9 H chain and the Vκ4 L chain transgene (creating the 3H9/Vκ4 antibody which binds to dsDNA).83 In order to avoid autoreactivity, 3H9/Vκ4 transgenic animals produce B cells with endogenous L chain rearrangements. These B cells fail to bind dsDNA or express the 3H9/Vκ4 antibody on the cell surface. The failure to express the 3H9/Vκ4 antibody is not due to loss of either transgene. Furthermore Vκ4 L chain only transgenic mice express the L chain transgene and exclude most endogenous L chain rearrangements. Taken together, these results suggest that the endogenous L chain out-competes the transgenic Vκ4 L chain for binding to the 3H9 H chain or the 3H9/Vκ4 antibody is retained inside the cell. The latter mechanism can occur with some autoantibodies, particularly those having specificity for the Golgi apparatus.93
If multiple κ rearrangements fail to yield a suitable L chain, the κ locus undergoes rearrangement to a non-coding gene called RS in mice (RS is referred to as the κ deleting element in humans).46, 47 RS rearrangement results in either deletion or inversion of Cκ, consequently inactivating κ L chain expression on that allele. In cell lines with λ rearrangements, one or usually both κ alleles are deleted, whereas in κ-expressing B cells the fraction of RS rearrangements is approximately 15%.6, 44, 45, 94, 95 There are two different kinds of RS rearrangements, rearrangement involving the cryptic heptamer in the J-Cκ intron to RS (iRS-RS) or rearrangement involving a Vκ gene segment to RS (Vκ-RS; Fig. 4c). In the mouse, the major pathway of κ locus deletion is Vκ-RS, whereas in man it is iRS-RS.49, 95 When λ+ B cells and B cell hybridomas from wild type mice were analyzed for Vκ-Jκ rearrangements that were on the same allele (in cis) with iRS-RS deletions, 47% were found to be in-frame.95 Thus, RS rearrangement is a means of enforcing isotypic exclusion in B cells that have gone on to λ rearrangement. RS knock-out mice exhibit reduced frequencies of λ+ B cells but, surprisingly, do not have substantially increased isotypic inclusion.96 Although gene targeting studies of the murine κ locus indicate that RS rearrangement is not required for the production of λ B cells, the RS knock-out mouse phenotype nevertheless suggests that RS rearrangement promotes λ B cell development.96–98 The reduced frequency of λ B cells in RS deficient mice may reduce the observed level of isotypic inclusion.
Editing at the λ locus can occur by leapfrogging in man, due to the presence of multiple JC cassettes. There are different λ alleles in the human population that vary in the number of JCλ cassettes and, consequently, individuals may vary in the extent of editing.99 In the mouse, the λ locus is simpler. It consists of two V-JC clusters that are separated by over 190 kb of genomic DNA (Fig. 5a).100 Each murine λ cluster can only undergo one functional rearrangement, and rearrangements between clusters are very infrequent 101, excluding the use of leapfrogging as a major editing pathway. There is also no known λ deleting element. Thus, unlike in the κ locus, which has both leapfrogging and deletion mechanisms, editing of murine λ L chains is more likely to result in inclusion (Fig. 5b).
As discussed above, receptor editing was originally described in two mouse models that expressed an autoreactive transgenic immunoglobulin receptor.83, 86, 87 A pre-rearranged immunoglobulin transgene that affects efficient allelic exclusion of endogenous immunoglobulin loci provides a convenient means of tracking the effects of receptor editing. In most studies, the transgene results in autoreactive immunoglobulin. Thus, the role of receptor editing in the induction of self-tolerance can be assessed in the context of a normal mouse strain. Conversely, the potential mechanisms for failure of self-tolerance that lead to autoimmunity can be investigated in the context of an autoimmune mouse strain. Most of the interest has focused on anti-DNA autoantibodies, particularly in lupus-prone mouse strains. However, other specificities have also been studied and are summarized in Table 3.
Table 3.
Antibody Transgenic and Knock-in Mouse Models Used to Study Receptor Editing
| Transgene target | Autoimmune background | reference(s) | comments |
|---|---|---|---|
| laminin | No | 184 | |
| kappa | No | 129 | |
| H-2K | No | 3, 86, 90, 102, 109, 111, 131, 163, 164, 168, 171–173, 181, 185 | |
| H-2K | Yes | 5* 148* 166 | *Non-physiologic “editing” of a conventional transgene |
| HEL | No | 4, 76, 108, 162, 174, 186, 187 | |
| HEL | Yes | 144* | *Non-physiologic “editing” of a conventional transgene |
| αGal | No | 188 | |
| NP | No | 120 | |
| DNA | No | 6, 11, 64, 78, 83, 87, 92, 103, 104, 106, 110, 115, 124, 149, 167, 189–191 | *Non-physiologic “editing” of a conventional transgene |
| DNA | Yes | 114, 134, 135, 141–143, 151, 192, 193 | |
| GPI | No | 112 | |
| Phosphatidylserine | No | 125 | |
| Phosphatidylcholine | No | 170 | |
| Myelin Oligodendocyte Glycoprotein | No | 194* | *Non-physiologic “editing” of a conventional transgene |
| α3(IV)NC1 | No | 195* | *Non-physiologic “editing” of a conventional transgene |
| None | No | 104 ** 113 *** | **VκJκ knock-in transgene; ***Vλ1Jλ knock-in transgene |
| other | Various | 147 | mouse model with all antibody loci substituted with their human equivalents, and autoimmunity induced by drug treatment |
Editing of antibodies on a non-autoimmune background
In this section we will concentrate mainly on transgenic models that are antibody knock-ins (site-directed antibody transgenes). We will first discuss the insights regarding receptor editing that have come from normal mouse strains expressing a transgene, and then contrast what occurs in various autoimmune models in the next section.
The importance of autoantibody knock-in mice in relation to receptor editing should be emphasized. A conventional transgene will be integrated randomly in the genome, so it will not be subject to any of the normal mechanisms of receptor editing.102 (In some cases the transgene can undergo deletion by intrachromosomal recombination, but this happens infrequently.103) Although the term ‘receptor editing’ was originally applied to the substitution of a conventional transgene with an endogenous immunoglobulin gene in order to bypass autoreactivity, this is not necessarily a physiological process in many ways.83, 86, 87 To address this concern, a knock-in transgene is inserted in the immunoglobulin locus so that it mimics an endogenous productive rearrangement.104 A variation of this approach involves the creation of a mouse by transfer of a whole nucleus from a mature B cell.105 Therefore, the knock-in antibody can be excised or invaded by rearrangement of upstream V genes. In the κ locus, the molecular events are no different from what would be predicted to happen in the absence of a pre-rearranged transgene. Thus, the mechanisms of receptor editing of a knock-in may be considered to be physiologic. For the H chain knock-in, the interpretation is somewhat more complicated, since the knock-in locus retains D regions upstream of the rearranged H chain, which is not physiologic.64, 65
Editing appears to be critical for the survival of functional B cells that initially express autoreactive BCRs, as is the case with constitutive antoantigen models, such as anti-DNA. If editing is blocked in mice that are rag deficient and have H chain/L chain knock-ins directed against dsDNA, B cells are deleted in the bone marrow by apoptosis,106 while similar transgenics with a specificity for ssDNA produce B cell anergy.107 In either case, little serum Ig is found.106 Editing may be essential for the maintenance of a robust and functional B cell repertoire. This is illustrated, for example, in 3H9 knock-in mice in which most antigen-specific hybridomas isolated after KLH immunization do not express the 3H9 H chain.64
Studies in Vκ knock-in mice with a human κ constant region on one κ allele and a mouse Vκ knock-in on the other allele permitted the degree of editing to be estimated at 25%.108 Furthermore, editing was associated with a prolongation of the pre-BII stages of B-cell ontogeny in the bone marrow.108, 109 In the presence of a transgene that promotes survival (bcl-2), the degree of editing is increased, perhaps because of further extension of the relevant stage of B cell maturation in the bone marrow.3 In some cases, the edited κ allele is not inactivated, which can lead to L chain allelic inclusion. The presence of a pre-rearranged transgene changes the kinetics of B-cell ontogeny in the bone marrow, and this will influence the degree and timing of secondary rearrangements.
The potential for the edited gene to remain functional is particularly great when the initial autoreactive gene is a Vλ, which cannot be deleted by normal mechanisms (as discussed above).11, 78, 110 Such cases of allelic inclusion have been described in which the edited autoreactive allele is silenced to some degree,105 possibly in part through intracellular sequestration.93 It is possible that such a ‘cryptic’ autoreactive immunoglobulin chain may later be expressed, particularly in the context of a developing autoimmune diasthesis.111, 112 The occurrence of allelic inclusion correlates with a prolongation of the pre-B cell stage, which allows for a secondary rearrangement to occur even if the pre-rearranged knock-in is not inactivated.113 However, in other models of systemic autoimmunity, allelic inclusion is found to be quite rare.114
When the repertoire is skewed strongly towards autoreactivity, then editing can play a major role in maintaining tolerance. The VH56R transgene was created by introducing an additional arginine residue at position 56 of CDR2 of the 3H9 H chain transgene.8 This modification increases binding to DNA so much that only a few L chains can pair with VH56R to produce a non-autoreactive antibody. Accordingly, VH56R- knock-in mice bred onto the B6 or BALB/c background have a B cell repertoire with evidence of extensive receptor editing.64, 115 Nearly all B cells that express the VH56R knock-in use one of the few L chains that are sufficiently acidic to reduce DNA binding (V κ38c, Vκ20, Vκ21D, Vκ12 or Vλx; these L chains are referred to as “editors”).115 The presence of extensive L chain editing in the 56R mouse is also demonstrated by high levels of the RS rearrangement.6 Because RS rearrangements are non-functional, this finding suggests that 56R mice have a higher level of editing, rather than simply selecting for L chain editors. In other B cells, the VH56R knock-in is inactivated and an endogenous VH gene is expressed, usually from the other H chain allele.64, 92, 116 In some cases, the VH56R knock-in can be edited by invasion of upstream VH and/or DH elements, referred to as VH replacement (see Fig. 3d–3e) or DH invasion, respectively.
The developmental timing, frequency and relevance of VH replacement as an editing mechanism are controversial. Most evidence suggests that VH replacement occurs early during B cell development, prior to the expression of a BCR. This evidence includes the presence of junctional modifications consistent with N-additions in VH replaced rearrangements on the transgenic allele in VH knock-in mice, suggesting that VH replacement occurred during early B cell development, when the enzyme TdT is active.64, 65, 116 Furthermore, the lack of H chain rearrangement products cloned by LM-PCR from dsDNA breaks beyond the pro-B cell stage suggests that H chain rearrangements are occurring in pro-B cell stage and not later.117, 118 These findings call into question how VH replacement is linked to BCR selection if replacement is confined to the pro-B cell stage, which precedes L chain gene rearrangement. One possibility is that VH replacement is important for pre-BCR rather than BCR selection. Recent studies linking abnormalities in pre-BCR selection to autoreactivity (for example the production of autoreactive H chains in mice lacking the λ5 surrogate L chain) could point to a potential role for VH replacement in altering the autoantibody repertoire.119 Alternatively, perhaps VH replacement can occur in peripheral B cells, as has been documented in the quasi-monoclonal (QM) mouse (described further below) and the B6.Sle3/5 mouse.120, 70
Another uncertainty with VH replacement is its frequency in the normal B cell repertoire. In autoreactive H chain knock-in mice, the vast majority of B cells that have edited the H chain knock-in do so via D-invasion followed by conventional rearrangement on either the targeted or the endogenous H chain allele.65, 116, 64 Attempts to find evidence of VH replacement in non-transgenic mice have met with limited success. art of the difficulty lies in establishing that there was a preceding VDJ rearrangement. As discussed above, one identifier is a duplication of the 3′ VH sequence, just downstream from the cryptic heptamer known as a footprint. However, extensive junctional modifications sometimes obscure the footprint. When TdT deficient mice, which lack N-additions, were used to look for footprints, very few if any unequivocal footprints were identified.121 In humans, the frequency of VH replacement may be increased in inflammatory lesions from patients with autoimmune disease (reviewed in reference 122). It is intriguing that antibody sequences cloned from human cell lines can exhibit extraordinarily long CDR3 sequences, some of which appear to arise from sequential VH replacement.123
In regulating the extent to which receptor editing is used to maintain tolerance, the strength of recognition of an autoantigen may be important. Thus, a H/L double knock-in mouse with a BCR that binds dsDNA poorly (i.e., 3H9/Vκ8R) maintains the knock-in and anergizes its B cells; while a H/L double knock-in mouse with a BCR that binds dsDNA well (i.e., 3H9/Vκ4R) undergoes extensive editing to rescue B cells that would otherwise be deleted in the bone marrow.124 Although this finding might imply that editing is at least in part an artifact of using a somatically mutated (and thus high affinity) heavy chain knock-in, similar levels of editing have been found using a knock-in from 3H9 by reversion to its germline sequence, which turns out to bind phosphatidylserine.125 On the other hand, a BCR that recognizes a cell surface antigen (H-2K) can be better induced to editing in vitro by a low avidity interaction.126 In several in vitro systems, the editing process is induced in bone marrow B cells by BCR cross-linking, while such signaling suppresses editing in splenic B cells.90, 126 In the bone marrow, membrane antigens with a wide variety of affinities can induce editing, although this may be due to the great enhancement of avidity secondary to multivalent binding.127 Parallel results were obtained in the HEL/anti-HEL system. In anti-HEL transgenic mice, the presence of the strongly crosslinking autoantigen mHEL largely causes massive deletion of B cells in the bone marrow. If an additional survival signal is present as a bcl-xL transgene, then receptor editing is seen as well.4 If the HEL antigen is present in soluble form (sHEL), then substantial editing is seen, but this can be diminished in the presence of a cross-reactive ‘autoantigen’ transgene, e.g. duck egg lysozyme (DEL).128
A unique transgenic model utilizes a membrane-bound anti-κ construct (“κ-macroself antigen”), which makes all κ chains operationally autoimmune, but maintains the diversity of the κ repertoire and the normal process of B-cell ontogeny.129 Igκ expression is completely suppressed in this model, and all B cells and serum antibodies are λ+. Since normally the κ loci rearrange before λ, and the great majority of murine B cells are κ+, it would be predicted that most λ+ cells in this model would have first expressed κ L chains, and then edited them in response to self-recognition of the κ-macroself antigen. In fact, bone marrow B cells showed substantially increased levels of rag1 and rag2 gene expression, consistent with a prolonged window of L chain rearrangement, perhaps accompanied by a developmental arrest at the bone marrow pre-B cell stage.108, 113 Splenic and bone marrow B cells showed increased RS recombination sequences in their DNA, which is indicative of inactivation of the κ allele by the editing process. Although the authors did not examine individual B cells to determine the actual frequency of κ chain editing in λ+ cells, they argue that their evidence suggests that nearly all B cells in their model follow this route.129
Secondary rearrangements in a non-autoimmune mouse may not always be demonstrably related to autoreactivity. The QM mouse has genetically inactivated κ alleles, one non-functional VH allele, and a site-directed VH transgene (17.2.25) that binds to nitrophenyl (not known to be an autoantigen) in combination with λ L chains.130 VH replacement is seen in only a small fraction of B cells, yet VH replaced B cells appear to account for the majority of the serum Ig.120, 130 Thus, it is unclear if VH replacement in this mouse model is being driven by autoreactivity. It could not be ruled out that 17.2.25 might be autoreactive with at least one Vλ gene. Alternatively, the greater H chain diversity afforded by VH replacement allows for enrichment of VH replaced B cells amongst antigen-responsive B cells. A similar conclusion was reached in the 3–83 Ig+ model.131 In B6 mice with the 56R transgene, editing of the site-directed transgene VH appeared to occur before the formation of a functional BCR, although this does not rule out the possibility that VH replacement is relevant for selection of H chains at the pre-BCR checkpoint (see above).116 In other instances, low levels of cell surface expression of the BCR lead to editing, even in the absence of autoreactivity. BCR downregulation by contact with autoantigen may thus be part of the mechanism of the maintenance of the recombination machinery and the consequent facilitation of editing.132 In another system, however, increased expression of a H chain knock-in led to increased use of receptor editing to maintain tolerance.133
Editing of antibodies on an autoimmune background
Since receptor editing appears to be an important normal mechanism to purge the B-cell repertoire of autoreactivity, it might be expected that editing would be faulty in the context of a mouse strain that shows lupus-like autoreactivity. In fact, the investigation of antibody transgenic autoimmune mice has led to a number of curious findings, which do not yet give a clear picture of the role of receptor editing in the failure of tolerance.
VH76R is a variant of the 3H9 H chain transgene that has two additional arginine residues, at positions 56 and 76.8 VH76R produces an autoantibody with every L chain. Mice with a VH76R knock-in show a marked reduction in the number of B cells in the bone marrow, and those that do proceed to the periphery are largely Vκ38c+.134 This occurs equally on a normal genetic background (BALB/c) and an autoimmune one (MRL/lpr). The normal mouse maintains tolerance to DNA, while the MRL/lpr.76R knock-in mouse exhibits accelerated development of autoimmunity, in comparison with non-tg MRL/lpr.134 The IgG anti-dsDNA antibodies appeared to be mainly made by the H76R sd-tg in combination with an unusual L chain gene, Vκ23.134 It is of particular interest in this model that the Vκ23 gene accumulates far fewer somatic mutations than the VH76R knock-in, suggesting that the L chain gene may have been activated by a second round of receptor editing that occurred after the H chain began to be mutated, that is, presumably after AID induction and class switching.
The MRL/lpr.56R strain also exhibits accelerated production of anti-dsDNA autoantibodies, compared to non-transgenic MRL/lpr. Vκ23 is only one of several κ genes found in IgG anti-dsDNA hybridomas from MRL/lpr.56R. In addition, most of these clones do not utilize the 56R knock-in.135 Thus, the loss of tolerance in this model appears to be associated with abnormally extensive receptor editing or altered selection of the H chain edited B cells. In the MRL/lpr.3H9.Vκ8 double knock-in, some of the anti-dsDNA autoantibodies appear to arise as a consequence of L chain revision, since the transgenic L chain can show inactivation by the (peripheral process of) somatic hypermutation and the endogenous L chains expressed by these B cells have fewer somatic mutations.136 In NZBxNZW strains bearing the knock-in anti-DNA H chain D42H, and one of three different site-directed κ chains, the transgenic κ chain that induced the most editing in normal background (C57BL/6xBALB/c, ref.137) behaved similarly on the autoimmune genetic background. This L chain knock-in also produced the highest frequency of high affinity anti-DNA hybridomas that expressed a particular endogenous Vκ gene, VκRF.138 Thus, a high level of editing appeared to favor the loss of tolerance on an autoimmune background; however, with only three different Vκ transgenes that were studied, this association could be coincidental or could reflect altered B cell selection stringency on the autoreactive background.
The cGVH lupus model provides further insights into the role of receptor editing in the formation of anti-dsDNA autoantibodies. B6 mice injected with coisogenic bm12 I-A incompatible CD4+ T cells develop a spectrum of autoantibodies and immunopathology characteristic of spontaneous lupus.139 If the B6 mice bear the knock- in anti-DNA VH genes 3H9 or 56R, the induction of anti-DNA is somewhat enhanced.140, 141 Based on hybridoma analysis, IgM anti-DNA antibodies from these models were usually found to use the 3H9 or 56R trangenes.10, 141 In the B6.56R H chain knock-in model, several of the H chain knock-in expressing B cells also expressed editor L chains that incompletely vetoed DNA reactivity and were polyreactive, binding to DNA, phosphatidylserine and myelin basic protein.10 On the other hand, about half of the IgG anti-DNA hybridomas from 3H9 cGVH and all the IgG hybridomas (irrespective of DNA binding) from 56R cGVH did not use the knock-in VH gene. Furthermore, the 3H9 anti-DNA hybridomas that retained the knock-in H chain showed increased evidence of extensive L chain editing.140 In the 56R cGVH, most anti-DNA hybridomas used VH genes from the endogenous allele, particularly two J558 genes with residues that enhanced the positive charge of CDR2. IgG anti-DNA antibodies in the serum of these 56R cGVH mice also showed a substantial contribution from the endogenous allele, based on IgG2a allotype analysis. Although this unexpected skewing away from the 56R H chain could be the result of selective pressures on the minority population of H chain edited B cells that emerge from the bone marrow, it is not obvious how this selection could operate to disfavor the transgene (but see below), and why its effects would be largely confined to IgG and not IgM. It is possible that the transgene was revised in the periphery, either because it was inactivated by hypermutation, or because the cGVH re-induced the rearrangement machinery. If so, TdT must have also been up-regulated, as the IgG anti-DNA autoantibodies using an endogenous VH gene showed evidence of N addition. An alternative interpretation of the data is that the 56R tg bearing cells are indeed disfavored because they have already been extensively edited, or because they still have some DNA recognition which causes them to be anergized. Consistent with the latter alternative, most B cells expressing the IgM allotype of the transgenic allele in B6.56R mice have low levels of IgM.92, 93, 116
Several other models suggest that receptor revision may occur in the periphery during the loss of tolerance to lupus autoantigens. CD22−/− mice with an anti-DNA H chain knock-in and a Vκ knock-in spontaneously produce anti-DNA autoantibodies that mainly express an endogenous Vκ gene (VκRF). Interestingly, expression of VκRF, as well as expression of rag2 and the presence of κ-locus double stranded DNA breaks, are found in peripheral splenic B cells, but not in immature B cells in the bone marrow.142 VκRF expression was also seen in CD22 wt mice with the same antibody knock-ins. No mice in this study showed glomerular disease, so it is not certain that the anti-DNA antibodies produced are representative of lupus autoantibodies. As discussed above, the issue of receptor revision remains controversial, and L chain and H chain genes may function differently in this regard.
On the other hand, several models find evidence of decreased receptor editing of a transgenic BCR in the context of an autoimmune background. The Sle2z locus (from NZW) suppresses the degree of editing of both the H chain and the L chain in B6.Sle2z.56R mice, and it is conceivable but unproven that the deficiency of receptor editing is responsible for the failure of tolerance to DNA.143 The Sle1z also appeared to suppress editing of in the sHEL/anti-HEL model, although this was not tested on an antibody knock-in.144 Similarly, the cGVH model of autoimmunity suppressed receptor editing in the bone marrow in the same double transgenic system.145 MRL/lpr mice expressing the 56R anti-DNA H chain have altered L chain usage, suggestive of altered editing or selection or both.5, 135 MRL/lpr mice have lower frequencies of RS rearrangement (a non-productive L chain gene rearrangement product), suggestive of reduced L chain editing.5, 6 Lamoureux et al. also showed that upon engagement of the B cell receptor in bone marrow cultures, MRL/lpr B cells exhibited lower expression of rag and underwent fewer κ rearrangements, further supporting the conclusion that receptor editing is decreased on the MRL/lpr background 5.
The Sle1 interval, derived from the NZM2410 mouse strain, contains several genes that predispose to lupus.146 The identification of Ly108 as one of the Sle1 genes suggests that differences in receptor editing directly contribute to lupus pathogenesis.144 Ly108 belongs to the SLAM family of costimulatory molecules with at least two isoforms, Ly108.2 a normal allele and Ly108.1 associated with lupus. Following B cell receptor engagement, the expression of Ly108.2, but not that of Ly108.1, promotes calcium flux and rag re-expression. These data therefore suggest that the autoimmune Ly108.1 allele within the Sle1 interval plays a role in lupus by decreasing the ability of self-reactive B cells to edit their receptors.144 An interesting variant on the concept that editing defects are associated with autoimmunity is the effect of hydralazine, an anti-hypertensive known to induce a lupus-like syndrome in humans. Hydralazine appears to suppress in vitro editing in response to BCR ligation of bone marrow B cells from a mouse made transgenic for all the human immunoglobulin loci.147
On the other hand, the NOD autoimmune mouse showed evidence for normal L chain editing in the presence of two conventional autoreactive transgenes, while measures of editing in this strain in the absence of a transgene showed a deficiency.6, 148 B6.56R H chain knock-in mice lose tolerance to DNA, while BALB/c.56R mice do not, but this does not appear to be due to differences in receptor editing.149 However, the anti-DNA response in the former strain does not appear to be associated with glomerular pathology, nor is it T-cell dependent.150 Thus, as in the case of the CD22−/− mice discussed above, it may not represent lupus-associated autoimmunity. Central editing was normal in another anti-DNA transgenic model on the NZB×NZW background.151
As with the murine studies, investigations of human B cells have shown that a large part of the repertoire is self-reactive and that editing is a critical mechanism in preventing the emergence of autoreactive specificities.9, 152 Also as with mice, human studies resulted in significant contradictions about the role of editing in autoimmune patients.
Studies of receptor editing in humans
In human lupus, an early study noted that anti-DNA antibodies used mostly downstream Vκ genes that were proximal to the Jκ cluster.153 In addition, these lupus Vκ genes were often rearranged with upstream Jκ genes.153 The authors interpreted this observation as suggesting that mostly primary rearrangements had taken place in anti-DNA B cells and that a failure to edit these self-reactive rearrangements was a contributor to disease. Alternatively, the bias in Vκ and Jκ usage may have been a consequence of the positive selection of certain specificities during the autoimmune process. In a different study of lupus patients, Suzuki et al. reported that the A30 Vκ germline gene, in association with Jκ2, encodes a nephritogenic anti-DNA antibody.154 While normal B cells edit the A30-Jκ2 rearrangement, probably by an inversion mechanism, this rearrangement is expressed in B cells from patients with lupus nephritis. Once again, these authors interpreted the results as evidence for a failure of receptor editing rather than a consequence of selection. Panigrahi et al. observed that RS rearrangement frequency was reduced in patients with SLE and type 1 diabetes compared to normal control subjects.6 As RS rearrangements are non-functional, this study is not confounded by selection and indicates that the extent of secondary L chain rearrangement is reduced. It is striking that patients with two different autoimmune diseases in which B cells play prominent pathogenic roles both have lower levels of L chain rearrangement. These findings could indicate a primary defect in receptor editing or a defect that is secondary to a more global process, such as accelerated bone marrow B cell maturation.
Conversely, some studies have reported evidence for increased editing in lupus. Dorner et al. examined the κ repertoire of lupus patients and observed biased utilization of Vκ and Jκ gene segments among productive rearrangements.155 Of particular interest was the increased frequency of Jκ5, a downstream Jκ gene, suggesting increased κ leapfrogging. In addition, Jκ5-containing rearrangements contained fewer somatic mutations than other rearranged genes, suggesting that they might be the product of receptor revision. More recently, it has been proposed that aberrant production of IL-6 in lupus patients is responsible for the increased and prolonged expression of RAG proteins.156
Suggestions of increased receptor editing also come from the study of human rheumatoid arthritis patients. Meffre et al. have isolated unconventional B cells that express both a standard L chain and a surrogate light chain.157 These B cells can contain long H chain CDR3s, are often self-reactive and display evidence of having been edited and they accumulate within the joints of rheumatoid patients.157 Consistent with this observation, the same group of investigators reported that approximately 8% of the cloned VH-D-JH sequences from these rheumatoid arthritis joints had undergone VH replacement.158
Regulation of Receptor Editing
The genetic control of receptor editing has been studied in several transgenic models. Pre-B cells expressing IκB show evidence of receptor editing, consistent with a role for NFκB, although the precise mechanism remains to be elucidated.159–161 PLCγ2-deficiency suppresses editing in the HEL/anti-HEL system.162 PLCγ2 is present in higher quantities in immature B cells, and shows increased phosphorylation in response to BCR crosslinking, which may explain the induction of rag2 in this population.163, 164 This signaling pathway may also pass through the Foxo1 transcription factor.165 In fact, rag2 may be induced in immature (but not mature) B cells by Ca++ mobilization alone.163 However, other data suggest that PLCγ2 transmits the signals necessary to down-regulate rag and thus terminates receptor editing.166 SLP65 deficient mice also show impaired receptor editing,167 as do E2A+/− mice.168 Interestingly, the OcaB transcriptional co-activator may impact the editing of some Vκ genes, but not others.169 The requirement of RAG1 for the production of dsDNA breaks at signal ends, consistent with ongoing editing, was demonstrated in H50G (anti-NP) traditional H chain transgenic mice.117 Immature B cells can be induced to edit by BCR crosslinking, while transitional B cells cannot, perhaps because of an altered signaling pathway through PLCγ2.166, 170
The investigations of editing in normal mice have been extended in some cases to identify mechanisms that suppress editing, and thus potentially lead to autoimmune disease. The TLR9 agonist CpG suppressed rag2 gene expression in the bone marrow of 3–83 H-2b/d mice.171 Heterozygous deficiency at the rag1 locus appeared to hinder editing in the κ-macroself model.
The process of editing can be modeled in vitro, facilitating studies of the underlying mechanisms. Immature B cells in bone marrow stromal cell cultures grown in the presence of IL-7 will undergo maturation when IL-7 is withdrawn. If antigen or anti-BCR antibody is present, then B cell development is blocked and secondary rearrangements can occur.172 If development proceeds beyond the IgMlo stage to the IgMhi stage, then BCR crosslinking induces apoptosis rather than receptor editing.173 Thus, a limited time interval in ontogeny may be available for an autoreactive B cell to be rescued by editing. The length of this time interval may be determined by type of autoantigen that is being bound; for example, in the sHEL/anti-HEL system, even immature BM B cells can be induced to up-regulate rag and rearrange endogenous κ chains.174 Alternatively or in addition, the level of basal signaling of the BCR dictates whether immature B cells are positively selected or up-regulate rag expression and undergo receptor editing. Consistent with a basal signaling model, Cre-mediated deletion of a floxed H chain in IL-7 bone marrow stromal cell cultures led to rag up-regulation, as did BCR signaling blockade via a PI3 kinase inhibitor.166, 175 It appears that insufficient BCR signaling reprograms B cells to not only undergo receptor editing but to adopt the transcriptional programme of an earlier developmental stage (neoteny).175, 176
An area of ongoing controversy is whether receptor editing can be reinduced in vivo (receptor revision). In several systems, rag transcript levels are increased in spleens or lymph nodes after immunization, particularly in the germinal centers.177–181 Double stranded DNA breaks and receptor revision of κ knock-in alleles can be found in germinal center (GC) B cells.181 Receptor revision is inhibited by crosslinking the BCR. Double strand DNA breaks have likewise been found in splenic B cells in QM transgenic mice.120 In other systems, transgenic spleen cells can be induced to show receptor revision, if they are cultured with LPS and IL-4.181 Some authors argue that in the course of immunization immature B cells from the bone marrow are exported in larger numbers, and that accounts for the signs of receptor revision, rather than a re-induction of the editing machinery.182 Interestingly, in a 56RVκ8 double transgenic model, high levels of rag and the presence of dsDNA breaks in the κ locus were detected disproportionately in T3 transitional cells in the spleen, suggestive of receptor revision.118, 183 On the other hand, in the same model secondary rearrangements in the H chain were confined to the bone marrow.118 This issue awaits a more definitive resolution.
Conclusion
Seventeen years after its discovery, receptor editing stands as a critical event during B cell development. There is now little doubt that it is a major mechanism in preventing the emergence of self-reactive specificities and maintaining normal tolerance. Other aspects of receptor editing function still remain to be clarified. For instance, is an increase in receptor diversity one of the purposes of receptor editing? The elegance of conventional B cell diversification is that the process starts with antigen-independent broad repertoire creation (V(D)J rearrangement) followed by fine-tuning (somatic mutations) after antigen encounter. It seems unlikely that receptor editing, which usually affects specificity drastically, would be involved in antigen-driven responses. In addition, an antigen-specific role would increase the risk of creating autoimmune B cells in the periphery where tolerance checkpoints are probably less stringent than in the bone marrow. An important question nevertheless remains whether the purpose of editing rearrangements in the bone marrow is to diversify the primary B cell repertoire. In this case, the impetus for editing would not necessarily depend upon the specificity of the original rearrangement, but would be a quasi-stochastic process in many B cells. Numerous studies have suggested that primary rearrangements are often biased towards gene segments with preferred chromosomal locations and editing these initial rearrangements could result in a more comprehensive use of the germline repertoire.
One of the major difficulties in studying receptor editing is that secondary rearrangements, which often occur via deletion, do not leave traces of the editing precursors on the chromosome. Thus, the demonstration of editing is often indirect, relying upon clues such as biased gene segment usage, rag expression or the presence of dsDNA breaks. Many essential insights into the process of receptor editing have thus come from the use of immunoglobulin transgenic and knock-in mice. Nevertheless, such transgenic models are not fully physiological. The B cell repertoire is obviously restricted, even if only a L chain or only a H chain transgene are used in combination with endogenous H or L chains, respectively. Furthermore, the presence of the pre-rearranged transgene bypasses some of the normal steps of B-cell ontogeny in the bone marrow, resulting in abnormally accelerated B cell development. Therefore, it will be important to extend any mechanistic conclusions gleaned from transgenic mouse models to non-transgenic mice, both in normal individuals and in the context of autoimmunity. In addition, the transgenic approach obviously cannot be translated directly to human studies.
A critical question is whether alterations in receptor editing play a role in the pathogenesis of autoimmune diseases. It is remarkable that, in both animal and human studies, diametrically opposed conclusions have been reached by different investigators. A basic question such as, “Is receptor editing increased or decreased during lupus?” requires further study. Obviously, differences in patient populations and experimental systems may be responsible for some of these discrepancies. For instance, animal studies suggest that H and L editing are differentially regulated during autoimmunity with increased rearrangements usually described at the H chain locus while decreased editing is often seen at the L chain level. In addition, the Janus nature of editing renders both increased and decreased editing compatible with the pathogenesis of the disease. Whereas a decline in editing may not efficiently eliminate self-reactive antibodies, a greater rate of secondary rearrangements could generate autoantibodies in an uncontrolled manner. It is therefore conceivable that opposing abnormalities in editing co-exist at various stages of the autoimmune process. Decreased editing in the bone marrow during B cell development could result in a lack of negative selection of autoreactive specificities while increased editing in the periphery could diversify the repertoire towards autoantibody production.
Acknowledgments
The authors are grateful to Dr. Debora Sekiguchi for helpful comments on the manuscript. ELP is supported by the NIH (R01 DE017590, N01-AI-50024, R56-AI090842, U01 DK-070430) and the Pennsylvania Department of Health. RAE was supported by the NIH (R01-AR34156, and R01-AI036206), the Lupus Research Institute, the Alliance for Lupus Research, the American Autoimmune Related Diseases Association, the Arthritis Foundation and the Lupus Foundation of South Jersey. MM was supported by NIH grant ES-12646.
Contributor Information
Eline T. Luning Prak, Email: luning@mail.med.upenn.edu.
Marc Monestier, Email: marcm@temple.edu.
Robert A. Eisenberg, Email: raemd@mail.med.upenn.edu.
References
- 1.Nemazee D. Receptor editing in lymphocyte development and central tolerance. Nat Rev Immunol. 2006;6:728–40. doi: 10.1038/nri1939. [DOI] [PubMed] [Google Scholar]
- 2.Cohn M, et al. Reflections on the clonal-selection theory. Nat Rev Immunol. 2007;7:823–30. doi: 10.1038/nri2177. [DOI] [PubMed] [Google Scholar]
- 3.Lang J, et al. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J Exp Med. 1997;186:1513–22. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang W, et al. Self-reactive B lymphocytes overexpressing Bcl-xL escape negative selection and are tolerized by clonal anergy and receptor editing. Immunity. 1998;9:35–45. doi: 10.1016/s1074-7613(00)80586-5. [DOI] [PubMed] [Google Scholar]
- 5.Lamoureux JL, et al. Reduced receptor editing in lupus-prone MRL/lpr mice. J Exp Med. 2007;204:2853–64. doi: 10.1084/jem.20071268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panigrahi AK, et al. RS rearrangement frequency as a marker of receptor editing in lupus and type 1 diabetes. J Exp Med. 2008;205:2985–94. doi: 10.1084/jem.20082053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Burrows PD, Cooper MD. The molecular basis and biological significance of VH replacement. Immunol Rev. 2004;197:231–42. doi: 10.1111/j.0105-2896.2004.0107.x. [DOI] [PubMed] [Google Scholar]
- 8.Radic MZ, et al. Residues that mediate DNA binding of autoimmune antibodies. J Immunol. 1993;150:4966–77. [PubMed] [Google Scholar]
- 9.Wardemann H, et al. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 10.Witsch EJ, et al. Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction. J Exp Med. 2006;203:1761–72. doi: 10.1084/jem.20060075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle CM, et al. Consequences of receptor editing at the lambda locus: multireactivity and light chain secretion. Proc Natl Acad Sci U S A. 2006;103:11264–9. doi: 10.1073/pnas.0604053103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181–8. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JH, et al. Mechanisms promoting translocations in editing and switching peripheral B cells. Nature. 2009;460:231–6. doi: 10.1038/nature08159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Munoz R, et al. Autoimmunity and lymphoma: is mantle cell lymphoma a mistake of the receptor editing mechanism? Leuk Res. 2009;33:1437–9. doi: 10.1016/j.leukres.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Hanly WC, Knight KL. Cellular distribution of rabbit IgA allotypes. J Immunol. 1975;115:590–4. [PubMed] [Google Scholar]
- 16.Jones PP, Cebra JJ, Herzenberg LA. Restriction of gene expression in B lymphocytes and their progeny. I. Commitment to immunoglobulin allotype. J Exp Med. 1974;139:581–99. doi: 10.1084/jem.139.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pernis B, et al. Cellular localization of immunoglobulins with different allotypic specificities in rabbit lymphoid tissues. J Exp Med. 1965;122:853–76. doi: 10.1084/jem.122.5.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cebra JJ, Colberg JE, Dray S. Rabbit lymphoid cells differentiated with respect to alpha-, gamma-, and mu- heavy polypeptide chains and to allotypic markers Aa1 and Aa2. J Exp Med. 1966;123:547–58. doi: 10.1084/jem.123.3.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warner NL, et al. Allotypes of mouse IgM immunoglobulin. Nature. 1977;265:447–9. doi: 10.1038/265447a0. [DOI] [PubMed] [Google Scholar]
- 20.Litwin SD. Allelic and class exclusion of membrane-associated immunoglobulins of human lymphocytes. J Immunol. 1972;108:1129–31. [PubMed] [Google Scholar]
- 21.Ivanyi J, Hudson L. Allelic exclusion of M1 (IgM) allotype on the surface of chicken B cells. Immunology. 1978;35:941–5. [PMC free article] [PubMed] [Google Scholar]
- 22.Hayward AR, et al. Pre-B and B cells in rabbits. Ontogeny and allelic exclusion of kappa light chain genes. J Exp Med. 1978;148:1367–77. doi: 10.1084/jem.148.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreto V, Cumano A. Frequency and characterization of phenotypic Ig heavy chain allelically included IgM-expressing B cells in mice. J Immunol. 2000;164:893–9. doi: 10.4049/jimmunol.164.2.893. [DOI] [PubMed] [Google Scholar]
- 24.Coleclough C, et al. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 1981;290:372–8. doi: 10.1038/290372a0. [DOI] [PubMed] [Google Scholar]
- 25.Kubagawa H, et al. Light-chain gene expression before heavy-chain gene rearrangement in pre-B cells transformed by Epstein-Barr virus. Proc Natl Acad Sci U S A. 1989;86:2356–60. doi: 10.1073/pnas.86.7.2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novobrantseva TI, et al. Rearrangement and expression of immunoglobulin light chain genes can precede heavy chain expression during normal B cell development in mice. J Exp Med. 1999;189:75–88. doi: 10.1084/jem.189.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn M, Langman RE. The protection: the unit of humoral immunity selected by evolution. Immunol Rev. 1990;115:11–147. doi: 10.1111/j.1600-065x.1990.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 28.Louzoun Y, et al. Analysis of B cell receptor production and rearrangement. Part I. Light chain rearrangement. Semin Immunol. 2002;14:169–90. doi: 10.1016/s1044-5323(02)00041-6. discussion 221–22. [DOI] [PubMed] [Google Scholar]
- 29.Liang HE, et al. Variegated transcriptional activation of the immunoglobulin kappa locus in pre-b cells contributes to the allelic exclusion of light-chain expression. Cell. 2004;118:19–29. doi: 10.1016/j.cell.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 30.Chukwuocha RU, Nadel B, Feeney AJ. Analysis of homology-directed recombination in VDJ junctions from cytoplasmic Ig- pre-B cells of newborn mice. J Immunol. 1995;154:1246–55. [PubMed] [Google Scholar]
- 31.Alt FW, et al. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 1980;21:1–12. doi: 10.1016/0092-8674(80)90109-9. [DOI] [PubMed] [Google Scholar]
- 32.Alt FW, et al. Ordered rearrangement of immunoglobulin heavy chain variable region segments. Embo J. 1984;3:1209–19. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergman Y, Cedar H. A stepwise epigenetic process controls immunoglobulin allelic exclusion. Nat Rev Immunol. 2004;4:753–61. doi: 10.1038/nri1458. [DOI] [PubMed] [Google Scholar]
- 34.Mostoslavsky R, Alt FW, Rajewsky K. The lingering enigma of the allelic exclusion mechanism. Cell. 2004;118:539–44. doi: 10.1016/j.cell.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 35.Martensson IL, et al. The pre-B cell receptor and its role in proliferation and Ig heavy chain allelic exclusion. Semin Immunol. 2002;14:335–42. doi: 10.1016/s1044-5323(02)00066-0. [DOI] [PubMed] [Google Scholar]
- 36.ten Boekel E, Melchers F, Rolink AG. Precursor B cells showing H chain allelic inclusion display allelic exclusion at the level of pre-B cell receptor surface expression. Immunity. 1998;8:199–207. doi: 10.1016/s1074-7613(00)80472-0. [DOI] [PubMed] [Google Scholar]
- 37.Loffert D, et al. Surrogate light chain expression is required to establish immunoglobulin heavy chain allelic exclusion during early B cell development. Immunity. 1996;4:133–44. doi: 10.1016/s1074-7613(00)80678-0. [DOI] [PubMed] [Google Scholar]
- 38.Papavasiliou F, et al. The role of Ig beta in precursor B cell transition and allelic exclusion. Science. 1995;268:408–11. doi: 10.1126/science.7716544. [DOI] [PubMed] [Google Scholar]
- 39.Papavasiliou F, et al. The cytoplasmic domains of immunoglobulin (Ig) alpha and Ig beta can independently induce the precursor B cell transition and allelic exclusion. J Exp Med. 1995;182:1389–94. doi: 10.1084/jem.182.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grawunder U, et al. Down-regulation of RAG1 and RAG2 gene expression in preB cells after functional immunoglobulin heavy chain rearrangement. Immunity. 1995;3:601–8. doi: 10.1016/1074-7613(95)90131-0. [DOI] [PubMed] [Google Scholar]
- 41.Arakawa H, Shimizu T, Takeda S. Re-evaluation of the probabilities for productive arrangements on the kappa and lambda loci. Int Immunol. 1996;8:91–9. doi: 10.1093/intimm/8.1.91. [DOI] [PubMed] [Google Scholar]
- 42.Lindsley RC, et al. Generation of peripheral B cells occurs via two spatially and temporally distinct pathways. Blood. 2007;109:2521–8. doi: 10.1182/blood-2006-04-018085. [DOI] [PubMed] [Google Scholar]
- 43.Takemori T, Rajewsky K. Lambda chain expression at different stages of ontogeny in C57BL/6, BALB/c and SJL mice. Eur J Immunol. 1981;11:618–25. doi: 10.1002/eji.1830110806. [DOI] [PubMed] [Google Scholar]
- 44.Korsmeyer SJ, et al. Normal human B cells display ordered light chain gene rearrangements and deletions. J Exp Med. 1982;156:975–85. doi: 10.1084/jem.156.4.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hieter PA, et al. Human immunoglobulin kappa light-chain genes are deleted or rearranged in lambda-producing B cells. Nature. 1981;290:368–72. doi: 10.1038/290368a0. [DOI] [PubMed] [Google Scholar]
- 46.Siminovitch KA, et al. The human kappa deleting element and the mouse recombining segment share DNA sequence homology. Nucleic Acids Res. 1987;15:2699–705. doi: 10.1093/nar/15.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moore MW, et al. Deletions of kappa chain constant region genes in mouse lambda chain-producing B cells involve intrachromosomal DNA recombinations similar to V-J joining. Proc Natl Acad Sci U S A. 1985;82:6211–5. doi: 10.1073/pnas.82.18.6211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langerak AW, et al. Unraveling the consecutive recombination events in the human IGK locus. J Immunol. 2004;173:3878–88. doi: 10.4049/jimmunol.173.6.3878. [DOI] [PubMed] [Google Scholar]
- 49.Brauninger A, et al. Regulation of immunoglobulin light chain gene rearrangements during early B cell development in the human. Eur J Immunol. 2001;31:3631–7. doi: 10.1002/1521-4141(200112)31:12<3631::aid-immu3631>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 50.Klonowski KD, Primiano LL, Monestier M. Atypical VH-D-JH rearrangements in newborn autoimmune MRL mice. J Immunol. 1999;162:1566–72. [PubMed] [Google Scholar]
- 51.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–81. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 52.Sakano H, et al. Identification and nucleotide sequence of a diversity DNA segment (D) of immunoglobulin heavy-chain genes. Nature. 1981;290:562–5. doi: 10.1038/290562a0. [DOI] [PubMed] [Google Scholar]
- 53.Brodeur PH, et al. The organization of the mouse Igh-V locus. Dispersion, interspersion, and the evolution of VH gene family clusters. J Exp Med. 1988;168:2261–78. doi: 10.1084/jem.168.6.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seidman JG, Leder P. A mutant immunoglobulin light chain is formed by aberrant DNA- and RNA-splicing events. Nature. 1980;286:779–83. doi: 10.1038/286779a0. [DOI] [PubMed] [Google Scholar]
- 55.Lewis S, et al. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982;30:807–16. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- 56.Vinocur JM, et al. Violations of the 12/23 rule at the mouse immunoglobulin kappa locus, including V kappa-V kappa rearrangement. Mol Immunol. 2009;46:2183–9. doi: 10.1016/j.molimm.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meek KD, Hasemann CA, Capra JD. Novel rearrangements at the immunoglobulin D locus. Inversions and fusions add to IgH somatic diversity. J Exp Med. 1989;170:39–57. doi: 10.1084/jem.170.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Klonowski KD, Monestier M. Heavy chain revision in MRL mice: a potential mechanism for the development of autoreactive B cell precursors. J Immunol. 2000;165:4487–93. doi: 10.4049/jimmunol.165.8.4487. [DOI] [PubMed] [Google Scholar]
- 59.Sanz I. Multiple mechanisms participate in the generation of diversity of human H chain CDR3 regions. J Immunol. 1991;147:1720–9. [PubMed] [Google Scholar]
- 60.Collins AM, et al. Partitioning of rearranged Ig genes by mutation analysis demonstrates D-D fusion and V gene replacement in the expressed human repertoire. J Immunol. 2004;172:340–8. doi: 10.4049/jimmunol.172.1.340. [DOI] [PubMed] [Google Scholar]
- 61.Reth M, et al. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. Nature. 1986;322:840–2. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- 62.Kleinfield R, et al. Recombination between an expressed immunoglobulin heavy-chain gene and a germline variable gene segment in a Ly 1+ B-cell lymphoma. Nature. 1986;322:843–6. doi: 10.1038/322843a0. [DOI] [PubMed] [Google Scholar]
- 63.Usuda S, et al. Immunoglobulin V gene replacement is caused by the intramolecular DNA deletion mechanism. Embo J. 1992;11:611–8. doi: 10.1002/j.1460-2075.1992.tb05093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen C, et al. Immunoglobulin heavy chain gene replacement: a mechanism of receptor editing. Immunity. 1995;3:747–55. doi: 10.1016/1074-7613(95)90064-0. [DOI] [PubMed] [Google Scholar]
- 65.Taki S, Schwenk F, Rajewsky K. Rearrangement of upstream DH and VH genes to a rearranged immunoglobulin variable region gene inserted into the DQ52-JH region of the immunoglobulin heavy chain locus. Eur J Immunol. 1995;25:1888–96. doi: 10.1002/eji.1830250715. [DOI] [PubMed] [Google Scholar]
- 66.Raaphorst FM, et al. Molecular mechanisms governing reading frame choice of immunoglobulin diversity genes. Immunol Today. 1997;18:37–43. doi: 10.1016/s0167-5699(97)80013-8. [DOI] [PubMed] [Google Scholar]
- 67.Zemlin M, et al. Adult lupus-prone MRL/MpJ2+ mice express a primary antibody repertoire that differs in CDR-H3 length distribution and hydrophobicity from that expressed in the C3H parental strain. Mol Immunol. 2005;42:789–98. doi: 10.1016/j.molimm.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 68.Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. doi: 10.1146/annurev.iy.12.040194.002415. [DOI] [PubMed] [Google Scholar]
- 69.Monestier M. Autoantibodies to nucleosomes and histone-DNA complexes. Methods. 1997;11:36–43. doi: 10.1006/meth.1996.0385. [DOI] [PubMed] [Google Scholar]
- 70.Wakui M, et al. Genetic dissection of lupus pathogenesis: Sle3/5 impacts IgH CDR3 sequences, somatic mutations, and receptor editing. J Immunol. 2004;173:7368–76. doi: 10.4049/jimmunol.173.12.7368. [DOI] [PubMed] [Google Scholar]
- 71.Shapiro MA, Weigert M. How immunoglobulin V kappa genes rearrange. J Immunol. 1987;139:3834–9. [PubMed] [Google Scholar]
- 72.Harada K, Yamagishi H. Lack of feedback inhibition of V kappa gene rearrangement by productively rearranged alleles. J Exp Med. 1991;173:409–15. doi: 10.1084/jem.173.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feddersen RM, Van Ness BG. Double recombination of a single immunoglobulin k-chain allele: implications for the mechanism of rearrangement. Proc Natl Acad Sci (USA) 1985;82:4793–4797. doi: 10.1073/pnas.82.14.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feddersen RM, Van Ness BG. Corrective recombination of mouse immunoglobulin kappa alleles in Abelson murine leukemia virus-transformed pre-B cells. Mol Cell Biol. 1990;10:569–76. doi: 10.1128/mcb.10.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamagami T, et al. Frequencies of multiple IgL chain gene rearrangements in single normal or kappaL chain-deficient B lineage cells. Immunity. 1999;11:317–27. doi: 10.1016/s1074-7613(00)80107-7. [DOI] [PubMed] [Google Scholar]
- 76.Casellas R, et al. Igkappa allelic inclusion is a consequence of receptor editing. J Exp Med. 2007;204:153–60. doi: 10.1084/jem.20061918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van der Burg M, et al. Ordered recombination of immunoglobulin light chain genes occurs at the IGK locus but seems less strict at the IGL locus. Blood. 2001;97:1001–8. doi: 10.1182/blood.v97.4.1001. [DOI] [PubMed] [Google Scholar]
- 78.Prak EL, et al. Light chain editing in kappa-deficient animals: a potential mechanism of B cell tolerance. J Exp Med. 1994;180:1805–15. doi: 10.1084/jem.180.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nemazee D. Theoretical limits to massive receptor editing in immature B cells. Curr Top Microbiol Immunol. 1998;229:163–71. doi: 10.1007/978-3-642-71984-4_12. [DOI] [PubMed] [Google Scholar]
- 80.Ritchie KA, Brinster RL, Storb U. Allelic exclusion and control of endogenous immunoglobulin gene rearrangement in kappa transgenic mice. Nature. 1984;312:517–20. doi: 10.1038/312517a0. [DOI] [PubMed] [Google Scholar]
- 81.Rusconi S, Kohler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. Nature. 1985;314:330–4. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- 82.Carmack CE, et al. Influence of a V kappa 8 L chain transgene on endogenous rearrangements and the immune response to the HA(Sb) determinant on influenza virus. J Immunol. 1991;147:2024–33. [PubMed] [Google Scholar]
- 83.Gay D, et al. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993;177:999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Imanishi-Kari T, et al. Endogenous Ig production in mu transgenic mice. I. Allelic exclusion at the level of expression. J Immunol. 1993;150:3311–26. [PubMed] [Google Scholar]
- 85.Nussenzweig MC, et al. Allelic exclusion in transgenic mice that express the membrane form of immunoglobulin mu. Science. 1987;236:816–9. doi: 10.1126/science.3107126. [DOI] [PubMed] [Google Scholar]
- 86.Tiegs SL, Russell DM, Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993;177:1009–20. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Radic MZ, et al. B lymphocytes may escape tolerance by revising their antigen receptors. J Exp Med. 1993;177:1165–73. doi: 10.1084/jem.177.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nemazee DA, Burki K. Clonal deletion of B lymphocytes in a transgenic mouse bearing anti-MHC class I antibody genes. Nature. 1989;337:562–6. doi: 10.1038/337562a0. [DOI] [PubMed] [Google Scholar]
- 89.Shlomchik M, et al. Anti-DNA antibodies from autoimmune mice arise by clonal expansion and somatic mutation. J Exp Med. 1990;171:265–92. doi: 10.1084/jem.171.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hertz M, Nemazee D. BCR ligation induces receptor editing in IgM+IgD- bone marrow B cells in vitro. Immunity. 1997;6:429–36. doi: 10.1016/s1074-7613(00)80286-1. [DOI] [PubMed] [Google Scholar]
- 91.Ibrahim SM, et al. Light chain contribution to specificity in anti-DNA antibodies. J Immunol. 1995;155:3223–33. [PubMed] [Google Scholar]
- 92.Sekiguchi DR, et al. Development and selection of edited B cells in B6.56R mice. J Immunol. 2006;176:6879–87. doi: 10.4049/jimmunol.176.11.6879. [DOI] [PubMed] [Google Scholar]
- 93.Khan SN, et al. Editing and escape from editing in anti-DNA B cells. Proc Natl Acad Sci U S A. 2008;105:3861–6. doi: 10.1073/pnas.0800025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dunda O, Corcos D. Recombining sequence recombination in normal kappa-chain-expressing B cells. J Immunol. 1997;159:4362–6. [PubMed] [Google Scholar]
- 95.Retter MW, Nemazee D. Receptor editing occurs frequently during normal B cell development. J Exp Med. 1998;188:1231–8. doi: 10.1084/jem.188.7.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vela JL, et al. Rearrangement of mouse immunoglobulin kappa deleting element recombining sequence promotes immune tolerance and lambda B cell production. Immunity. 2008;28:161–70. doi: 10.1016/j.immuni.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zou YR, Takeda S, Rajewsky K. Gene targeting in the Ig kappa locus: efficient generation of lambda chain-expressing B cells, independent of gene rearrangements in Ig kappa. Embo J. 1993;12:811–20. doi: 10.1002/j.1460-2075.1993.tb05721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen J, et al. B cell development in mice that lack one or both immunoglobulin kappa light chain genes. Embo J. 1993;12:821–30. doi: 10.1002/j.1460-2075.1993.tb05722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lefranc MP, et al. IMGT, the international ImMunoGeneTics database. Nucleic Acids Res. 1999;27:209–12. doi: 10.1093/nar/27.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Storb U, et al. Physical linkage of mouse lambda genes by pulsed-field gel electrophoresis suggests that the rearrangement process favors proximate target sequences. Mol Cell Biol. 1989;9:711–8. doi: 10.1128/mcb.9.2.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sanchez P, Nadel B, Cazenave PA. V lambda-J lambda rearrangements are restricted within a V-J-C recombination unit in the mouse. Eur J Immunol. 1991;21:907–11. doi: 10.1002/eji.1830210408. [DOI] [PubMed] [Google Scholar]
- 102.Caucheteux SM, et al. Tolerance induction to self-MHC antigens in fetal and neonatal mouse B cells. Int Immunol. 2008;20:11–20. doi: 10.1093/intimm/dxm116. [DOI] [PubMed] [Google Scholar]
- 103.Chen C, et al. Deletion and editing of B cells that express antibodies to DNA. J Immunol. 1994;152:1970–82. [PubMed] [Google Scholar]
- 104.Prak EL, Weigert M. Light chain replacement: a new model for antibody gene rearrangement. J Exp Med. 1995;182:541–8. doi: 10.1084/jem.182.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gerdes T, Wabl M. Autoreactivity and allelic inclusion in a B cell nuclear transfer mouse. Nat Immunol. 2004;5:1282–7. doi: 10.1038/ni1133. [DOI] [PubMed] [Google Scholar]
- 106.Xu H, et al. Regulation of anti-DNA B cells in recombination-activating gene-deficient mice. J Exp Med. 1998;188:1247–54. doi: 10.1084/jem.188.7.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Erikson J, et al. Expression of anti-DNA immunoglobulin transgenes in non-autoimmune mice. Nature. 1991;349:331–4. doi: 10.1038/349331a0. [DOI] [PubMed] [Google Scholar]
- 108.Casellas R, et al. Contribution of receptor editing to the antibody repertoire. Science. 2001;291:1541–4. doi: 10.1126/science.1056600. [DOI] [PubMed] [Google Scholar]
- 109.Pelanda R, et al. Receptor editing in a transgenic mouse model: site, efficiency, and role in B cell tolerance and antibody diversification. Immunity. 1997;7:765–75. doi: 10.1016/s1074-7613(00)80395-7. [DOI] [PubMed] [Google Scholar]
- 110.Li Y, Louzoun Y, Weigert M. Editing anti-DNA B cells by Vlambdax. J Exp Med. 2004;199:337–46. doi: 10.1084/jem.20031712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liu S, et al. Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens. J Immunol. 2005;175:5067–76. doi: 10.4049/jimmunol.175.8.5067. [DOI] [PubMed] [Google Scholar]
- 112.Huang H, et al. Induction of tolerance in arthritogenic B cells with receptors of differing affinity for self-antigen. Proc Natl Acad Sci U S A. 2006;103:3734–9. doi: 10.1073/pnas.0600214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oberdoerffer P, Novobrantseva TI, Rajewsky K. Expression of a targeted lambda 1 light chain gene is developmentally regulated and independent of Ig kappa rearrangements. J Exp Med. 2003;197:1165–72. doi: 10.1084/jem.20030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Makdasi E, et al. Autoreactive anti-DNA transgenic B cells in lupus-prone New Zealand black/New Zealand white mice show near perfect L chain allelic exclusion. J Immunol. 2009;182:6143–8. doi: 10.4049/jimmunol.0803610. [DOI] [PubMed] [Google Scholar]
- 115.Li H, et al. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–57. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- 116.Yunk L, et al. Antibodies in a heavy chain knock-in mouse exhibit characteristics of early heavy chain rearrangement. J Immunol. 2009;183:452–61. doi: 10.4049/jimmunol.0804060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Davila M, et al. Multiple, conserved cryptic recombination signals in VH gene segments: detection of cleavage products only in pro B cells. J Exp Med. 2007;204:3195–208. doi: 10.1084/jem.20071224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nakajima PB, et al. Two distinct populations of H chain-edited B cells show differential surrogate L chain dependence. J Immunol. 2009;182:3583–96. doi: 10.4049/jimmunol.0802533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Keenan RA, et al. Censoring of autoreactive B cell development by the pre-B cell receptor. Science. 2008;321:696–9. doi: 10.1126/science.1157533. [DOI] [PubMed] [Google Scholar]
- 120.Bertrand FE, Golub R, Wu GE. V(H) gene replacement occurs in the spleen and bone marrow of non-autoimmune quasi-monoclonal mice. Eur J Immunol. 1998;28:3362–70. doi: 10.1002/(SICI)1521-4141(199810)28:10<3362::AID-IMMU3362>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 121.Watson LC, et al. Paucity of V-D-D-J rearrangements and VH replacement events in lupus prone and nonautoimmune TdT−/− and TdT+/+ mice. J Immunol. 2006;177:1120–8. doi: 10.4049/jimmunol.177.2.1120. [DOI] [PubMed] [Google Scholar]
- 122.Liu Y, et al. Potential contribution of VH gene replacement in immunity and disease. Ann N Y Acad Sci. 2005;1062:175–81. doi: 10.1196/annals.1358.020. [DOI] [PubMed] [Google Scholar]
- 123.Zhang Z, et al. Contribution of Vh gene replacement to the primary B cell repertoire. Immunity. 2003;19:21–31. doi: 10.1016/s1074-7613(03)00170-5. [DOI] [PubMed] [Google Scholar]
- 124.Chen C, Prak EL, Weigert M. Editing disease-associated autoantibodies. Immunity. 1997;6:97–105. doi: 10.1016/s1074-7613(00)80673-1. [DOI] [PubMed] [Google Scholar]
- 125.Li H, et al. Regulation of anti-phosphatidylserine antibodies. Immunity. 2003;18:185–92. doi: 10.1016/s1074-7613(03)00026-8. [DOI] [PubMed] [Google Scholar]
- 126.Hertz M, et al. V(D)J recombinase induction in splenic B lymphocytes is inhibited by antigen-receptor signalling. Nature. 1998;394:292–5. doi: 10.1038/28419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lang J, et al. B cells are exquisitely sensitive to central tolerance and receptor editing induced by ultralow affinity, membrane-bound antigen. J Exp Med. 1996;184:1685–97. doi: 10.1084/jem.184.5.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tze LE, et al. Ig light chain receptor editing in anergic B cells. J Immunol. 2000;165:6796–802. doi: 10.4049/jimmunol.165.12.6796. [DOI] [PubMed] [Google Scholar]
- 129.Ait-Azzouzene D, et al. An immunoglobulin C kappa-reactive single chain antibody fusion protein induces tolerance through receptor editing in a normal polyclonal immune system. J Exp Med. 2005;201:817–28. doi: 10.1084/jem.20041854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cascalho M, et al. A quasi-monoclonal mouse. Science. 1996;272:1649–52. doi: 10.1126/science.272.5268.1649. [DOI] [PubMed] [Google Scholar]
- 131.Braun U, Rajewsky K, Pelanda R. Different sensitivity to receptor editing of B cells from mice hemizygous or homozygous for targeted Ig transgenes. Proc Natl Acad Sci U S A. 2000;97:7429–34. doi: 10.1073/pnas.050578497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kouskoff V, et al. B cell receptor expression level determines the fate of developing B lymphocytes: receptor editing versus selection. Proc Natl Acad Sci U S A. 2000;97:7435–9. doi: 10.1073/pnas.130182597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu X, Shen S, Manser T. Influence of B cell antigen receptor expression level on pathways of B cell tolerance induction. J Immunol. 2009;182:398–407. doi: 10.4049/jimmunol.182.1.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen C, et al. Selection of anti-double-stranded DNA B cells in autoimmune MRL-lpr/lpr mice. J Immunol. 2006;176:5183–90. doi: 10.4049/jimmunol.176.9.5183. [DOI] [PubMed] [Google Scholar]
- 135.Li Y, et al. Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern. J Exp Med. 2002;196:1543–52. doi: 10.1084/jem.20021560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brard F, et al. Somatic mutation and light chain rearrangement generate autoimmunity in anti-single-stranded DNA transgenic MRL/lpr mice. J Exp Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yachimovich N, et al. The efficiency of B cell receptor (BCR) editing is dependent on BCR light chain rearrangement status. Eur J Immunol. 2002;32:1164–74. doi: 10.1002/1521-4141(200204)32:4<1164::AID-IMMU1164>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 138.Yachimovich-Cohen N, et al. Autoimmune NZB/NZW F1 mice utilize B cell receptor editing for generating high-affinity anti-dsDNA autoantibodies from low-affinity precursors. Eur J Immunol. 2003;33:2469–78. doi: 10.1002/eji.200324025. [DOI] [PubMed] [Google Scholar]
- 139.Eisenberg R. The chronic graft-versus-host model of systemic autoimmunity. Curr Dir Autoimmun. 2003;6:228–44. doi: 10.1159/000066864. [DOI] [PubMed] [Google Scholar]
- 140.Sekiguchi DR, et al. Chronic graft-versus-host in Ig knockin transgenic mice abrogates B cell tolerance in anti-double-stranded DNA B cells. J Immunol. 2002;168:4142–53. doi: 10.4049/jimmunol.168.8.4142. [DOI] [PubMed] [Google Scholar]
- 141.Sekiguchi DR, Eisenberg RA, Weigert M. Secondary heavy chain rearrangement: a mechanism for generating anti-double-stranded DNA B cells. J Exp Med. 2003;197:27–39. doi: 10.1084/jem.20020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yarkoni Y, et al. Peripheral B cell receptor editing may promote the production of high-affinity autoantibodies in CD22-deficient mice. Eur J Immunol. 2006;36:2755–67. doi: 10.1002/eji.200636190. [DOI] [PubMed] [Google Scholar]
- 143.Liu Y, et al. Lupus susceptibility genes may breach tolerance to DNA by impairing receptor editing of nuclear antigen-reactive B cells. J Immunol. 2007;179:1340–52. doi: 10.4049/jimmunol.179.2.1340. [DOI] [PubMed] [Google Scholar]
- 144.Kumar KR, et al. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–9. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 145.Feuerstein N, et al. Chronic graft-versus-host reaction is associated with a decrease in Ig light chain receptor editing in bone marrow self-reactive B cells. Eur J Immunol. 2004;34:1361–70. doi: 10.1002/eji.200324743. [DOI] [PubMed] [Google Scholar]
- 146.Morel L, et al. The major murine systemic lupus erythematosus susceptibility locus, Sle1, is a cluster of functionally related genes. Proc Natl Acad Sci U S A. 2001;98:1787–92. doi: 10.1073/pnas.031336098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mazari L, Ouarzane M, Zouali M. Subversion of B lymphocyte tolerance by hydralazine, a potential mechanism for drug-induced lupus. Proc Natl Acad Sci U S A. 2007;104:6317–22. doi: 10.1073/pnas.0610434104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Silveira PA, et al. B cell selection defects underlie the development of diabetogenic APCs in nonobese diabetic mice. J Immunol. 2004;172:5086–94. doi: 10.4049/jimmunol.172.8.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Witsch EJ, Bettelheim E. Allelic and isotypic light chain inclusion in peripheral B cells from anti-DNA antibody transgenic C57BL/6 and BALB/c mice. J Immunol. 2008;180:3708–18. doi: 10.4049/jimmunol.180.6.3708. [DOI] [PubMed] [Google Scholar]
- 150.Tsao PY, et al. T cell-independent spontaneous loss of tolerance by anti-double-stranded DNA B cells in C57BL/6 mice. J Immunol. 2008;181:7770–7. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- 151.Wellmann U, Werner A, Winkler TH. Altered selection processes of B lymphocytes in autoimmune NZB/W mice, despite intact central tolerance against DNA. Eur J Immunol. 2001;31:2800–10. doi: 10.1002/1521-4141(200109)31:9<2800::aid-immu2800>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 152.Meffre E, et al. Surrogate light chain expressing human peripheral B cells produce self-reactive antibodies. J Exp Med. 2004;199:145–50. doi: 10.1084/jem.20031550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bensimon C, Chastagner P, Zouali M. Human lupus anti-DNA autoantibodies undergo essentially primary V kappa gene rearrangements. Embo J. 1994;13:2951–62. doi: 10.1002/j.1460-2075.1994.tb06593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Suzuki N, et al. Characterization of a germline Vk gene encoding cationic anti-DNA antibody and role of receptor editing for development of the autoantibody in patients with systemic lupus erythematosus. J Clin Invest. 1996;98:1843–50. doi: 10.1172/JCI118985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dorner T, et al. Immunoglobulin kappa chain receptor editing in systemic lupus erythematosus. J Clin Invest. 1998;102:688–94. doi: 10.1172/JCI3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hillion S, et al. Interleukin-6 is responsible for aberrant B-cell receptor-mediated regulation of RAG expression in systemic lupus erythematosus. Immunology. 2007;122:371–80. doi: 10.1111/j.1365-2567.2007.02649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Meffre E, et al. Circulating human B cells that express surrogate light chains and edited receptors. Nat Immunol. 2000;1:207–13. doi: 10.1038/79739. [DOI] [PubMed] [Google Scholar]
- 158.Itoh K, et al. Immunoglobulin heavy chain variable region gene replacement As a mechanism for receptor revision in rheumatoid arthritis synovial tissue B lymphocytes. J Exp Med. 2000;192:1151–64. doi: 10.1084/jem.192.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Derudder E, et al. Development of immunoglobulin lambda-chain-positive B cells, but not editing of immunoglobulin kappa-chain, depends on NF-kappaB signals. Nat Immunol. 2009;10:647–54. doi: 10.1038/ni.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cadera EJ, et al. NF-kappaB activity marks cells engaged in receptor editing. J Exp Med. 2009;206:1803–16. doi: 10.1084/jem.20082815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bai L, et al. Phospholipase Cgamma2 contributes to light-chain gene activation and receptor editing. Mol Cell Biol. 2007;27:5957–67. doi: 10.1128/MCB.02273-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Benschop RJ, et al. Distinct signal thresholds for the unique antigen receptor-linked gene expression programs in mature and immature B cells. J Exp Med. 1999;190:749–56. doi: 10.1084/jem.190.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Benschop RJ, et al. Unique signaling properties of B cell antigen receptor in mature and immature B cells: implications for tolerance and activation. J Immunol. 2001;167:4172–9. doi: 10.4049/jimmunol.167.8.4172. [DOI] [PubMed] [Google Scholar]
- 165.Amin RH, Schlissel MS. Foxo1 directly regulates the transcription of recombination-activating genes during B cell development. Nat Immunol. 2008;9:613–22. doi: 10.1038/ni.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Verkoczy L, et al. Basal B cell receptor-directed phosphatidylinositol 3-kinase signaling turns off RAGs and promotes B cell-positive selection. J Immunol. 2007;178:6332–41. doi: 10.4049/jimmunol.178.10.6332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hayashi K, et al. Impaired receptor editing in the primary B cell repertoire of BASH-deficient mice. J Immunol. 2004;173:5980–8. doi: 10.4049/jimmunol.173.10.5980. [DOI] [PubMed] [Google Scholar]
- 168.Quong MW, et al. Receptor editing and marginal zone B cell development are regulated by the helix-loop-helix protein, E2A. J Exp Med. 2004;199:1101–12. doi: 10.1084/jem.20031180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Casellas R, et al. OcaB is required for normal transcription and V(D)J recombination of a subset of immunoglobulin kappa genes. Cell. 2002;110:575–85. doi: 10.1016/s0092-8674(02)00911-x. [DOI] [PubMed] [Google Scholar]
- 170.Wang H, et al. Transitional B cells lose their ability to receptor edit but retain their potential for positive and negative selection. J Immunol. 2007;179:7544–52. doi: 10.4049/jimmunol.179.11.7544. [DOI] [PubMed] [Google Scholar]
- 171.Azulay-Debby H, Edry E, Melamed D. CpG DNA stimulates autoreactive immature B cells in the bone marrow. Eur J Immunol. 2007;37:1463–75. doi: 10.1002/eji.200636878. [DOI] [PubMed] [Google Scholar]
- 172.Melamed D, et al. A functional B cell receptor transgene allows efficient IL-7-independent maturation of B cell precursors. J Immunol. 1997;159:1233–9. [PubMed] [Google Scholar]
- 173.Melamed D, et al. Developmental regulation of B lymphocyte immune tolerance compartmentalizes clonal selection from receptor selection. Cell. 1998;92:173–82. doi: 10.1016/s0092-8674(00)80912-5. [DOI] [PubMed] [Google Scholar]
- 174.Tze LE, Hippen KL, Behrens TW. Late immature B cells (IgMhighIgDneg) undergo a light chain receptor editing response to soluble self-antigen. J Immunol. 2003;171:678–82. doi: 10.4049/jimmunol.171.2.678. [DOI] [PubMed] [Google Scholar]
- 175.Tze LE, et al. Basal immunoglobulin signaling actively maintains developmental stage in immature B cells. PLoS Biol. 2005;3:e82. doi: 10.1371/journal.pbio.0030082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schram BR, et al. B cell receptor basal signaling regulates antigen-induced Ig light chain rearrangements. J Immunol. 2008;180:4728–41. doi: 10.4049/jimmunol.180.7.4728. [DOI] [PubMed] [Google Scholar]
- 177.Hikida M, et al. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–4. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 178.Hikida M, et al. Characterization of B cells expressing recombination activating genes in germinal centers of immunized mouse lymph nodes. J Immunol. 1997;158:2509–12. [PubMed] [Google Scholar]
- 179.Han S, et al. Neoteny in lymphocytes: Rag1 and Rag2 expression in germinal center B cells. Science. 1996;274:2094–7. doi: 10.1126/science.274.5295.2094. [DOI] [PubMed] [Google Scholar]
- 180.Han S, et al. V(D)J recombinase activity in a subset of germinal center B lymphocytes. Science. 1997;278:301–5. doi: 10.1126/science.278.5336.301. [DOI] [PubMed] [Google Scholar]
- 181.Papavasiliou F, et al. V(D)J recombination in mature B cells: a mechanism for altering antibody responses. Science. 1997;278:298–301. doi: 10.1126/science.278.5336.298. [DOI] [PubMed] [Google Scholar]
- 182.Nagaoka H, et al. Immunization and infection change the number of recombination activating gene (RAG)-expressing B cells in the periphery by altering immature lymphocyte production. J Exp Med. 2000;191:2113–20. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kiefer K, et al. Antigen receptor editing in anti-DNA transitional B cells deficient for surface IgM. J Immunol. 2008;180:6094–106. doi: 10.4049/jimmunol.180.9.6094. [DOI] [PubMed] [Google Scholar]
- 184.Brady GF, et al. Kappa editing rescues autoreactive B cells destined for deletion in mice transgenic for a dual specific anti-laminin Ig. J Immunol. 2004;172:5313–21. doi: 10.4049/jimmunol.172.9.5313. [DOI] [PubMed] [Google Scholar]
- 185.Shivtiel S, et al. Impaired light chain allelic exclusion and lack of positive selection in immature B cells expressing incompetent receptor deficient of CD19. J Immunol. 2002;168:5596–604. doi: 10.4049/jimmunol.168.11.5596. [DOI] [PubMed] [Google Scholar]
- 186.Hippen KL, et al. In vivo assessment of the relative contributions of deletion, anergy, and editing to B cell self-tolerance. J Immunol. 2005;175:909–16. doi: 10.4049/jimmunol.175.2.909. [DOI] [PubMed] [Google Scholar]
- 187.Pogue SL, Goodnow CC. Gene dose-dependent maturation and receptor editing of B cells expressing immunoglobulin (Ig)G1 or IgM/IgG1 tail antigen receptors. J Exp Med. 2000;191:1031–44. doi: 10.1084/jem.191.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Benatuil L, et al. Ig knock-in mice producing anti-carbohydrate antibodies: breakthrough of B cells producing low affinity anti-self antibodies. J Immunol. 2008;180:3839–48. doi: 10.4049/jimmunol.180.6.3839. [DOI] [PubMed] [Google Scholar]
- 189.Heltemes-Harris L, Liu X, Manser T. Progressive surface B cell antigen receptor down-regulation accompanies efficient development of antinuclear antigen B cells to mature, follicular phenotype. J Immunol. 2004;172:823–33. doi: 10.4049/jimmunol.172.2.823. [DOI] [PubMed] [Google Scholar]
- 190.Liu Y, Li L, Mohan C. The role of rearrangement at the second Ig heavy chain locus in maintaining B cell tolerance to DNA. J Immunol. 2008;180:7721–7. doi: 10.4049/jimmunol.180.11.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Pewzner-Jung Y, et al. B cell deletion, anergy, and receptor editing in “knock in” mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J Immunol. 1998;161:4634–45. [PubMed] [Google Scholar]
- 192.Friedmann D, et al. Production of high affinity autoantibodies in autoimmune New Zealand Black/New Zealand white F1 mice targeted with an anti-DNA heavy chain. J Immunol. 1999;162:4406–16. [PubMed] [Google Scholar]
- 193.Tarasenko T, Kole HK, Bolland S. A lupus-suppressor BALB/c locus restricts IgG2 autoantibodies without altering intrinsic B cell-tolerance mechanisms. J Immunol. 2008;180:3807–14. doi: 10.4049/jimmunol.180.6.3807. [DOI] [PubMed] [Google Scholar]
- 194.Litzenburger T, et al. Development of myelin oligodendrocyte glycoprotein autoreactive transgenic B lymphocytes: receptor editing in vivo after encounter of a self-antigen distinct from myelin oligodendrocyte glycoprotein. J Immunol. 2000;165:5360–6. doi: 10.4049/jimmunol.165.9.5360. [DOI] [PubMed] [Google Scholar]
- 195.Zhang Y, et al. Central tolerance regulates B cells reactive with Goodpasture antigen alpha3(IV)NC1 collagen. J Immunol. 2008;181:6092–100. doi: 10.4049/jimmunol.181.9.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hardy RR, et al. Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J Exp Med. 1991;173:1213–25. doi: 10.1084/jem.173.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]


