Abstract
Synthetic retinoid fenretinide is one of the most promising clinically tested retinoids. Previously, we have shown that fenretinide induces apoptosis of Huh7 cells, but HepG2 cells are relatively resistant to fenretinide-induced apoptosis. The current study examines the interactive role of fenretinide and histone deacetylase inhibitors (HDACi) in inducing apoptosis of human hepatocellular carcinoma (HCC) cells and the underlying mechanism. Trichostatin A (TSA) and scriptaid can either enhance fenretinide-induced apoptosis in the fenretinide sensitive HCC cells (Huh7 and Hep3B) or sensitize the fenretinide resistant cells (HepG2) to become sensitive to the apoptotic effect of fenretinide in a cancer-cell specific manner. The sensitivity of cells to fenretinide-induced apoptosis was neither associated with ROS production nor anti-oxidant gene expression. However, the level of RARβ and Nur77 was important for inducing apoptosis. Upon fenretinide and HDACi treatment, the expression of RARβ and Nur77 were induced and co-localized in the cytosol. The induction of Nur77 protein level, but not the mRNA level, was RARβ-dependent. In addition, RARβ interacted with Nur77. Nur77 was essential for fenretinide- and HDACi-induced apoptosis of Huh7 cells. Induction of the expression, the interaction, and the nuclear export of RARβ and Nur77 mediate fenretinide and HDACi induced apoptosis. Our findings suggest that targeting Nur77 and RARβ simultaneously provides an effective way to induce HCC cell death.
Keywords: translocation, nuclear receptor, retinoid, TSA, scriptaid
Introduction
Fenretinide inhibited the carcinogenic process and induced apoptosis in hematological malignancy and head and neck, breast, bladder and oral cancer.1 Fenretinide used in chemotherapy of cancer was more effective than natural retinoids in clinical trials.2 In addition, recent studies have shown that fenretinide can also prevent high-fat diet-induced hepatic steatosis as well as reduce insulin resistance, ameliorate CCl4-induced liver fibrosis and inhibit angiogenesis.3–5 Thus, it seems that fenretinide has a broad spectrum of effects in regulating liver function and disease process. The objectives of the current study are to improve the efficacy of fenretinide and to understand the underlying mechanism.
Histone modification is one of the epigenetic hallmarks of the cancer cell.6–8 Imbalance of deacetylase activity is involved in cancer onset and progression. Thus, histone modification is becoming a therapeutic target for cancer.9–11 Histone deacetylase inhibitors (HDACi) have shown great anti-tumor activity by inducing apoptosis, differentiation, and/or cell-cycle arrest in cancer cells. The combination effects of HDACi and retinoids have been indicated in in vitro, animal, and clinical trial studies such as acute promyelocytic leukemia and neuroblastoma.12 HDACi suberoylanilide hydroxamic acid synergizes with zoledronic acid to induce apoptosis of prostate cancer cell lines LNCap and PC-3.13 The combination effect of HDACi and all-trans-retinoic acid was demonstrated in neuroblastoma cells and a xenograft model of neuroblastoma.14 The combination therapies using HDACi and retinoids hold enormous promise for the treatment of hematological and solid tumors. However, the underlying mechanism is not clear.
The current study tested the combination effect of fenretinide with two HDACi: TSA and scriptaid, which has relatively lower toxicity in comparison with TSA.15 Our data showed, for the first time, that HDACi could sensitize the fenretinide-resistant HCC cells to the apoptotic effect of fenretinide. In addition, the apoptotic effect is cancer cell specific and is mediated via induction and interaction as well as nuclear export of Nur77 and RARβ. Our findings suggest that targeting Nur77 and RARβ simultaneously provides an effective way to induce HCC cell death.
Materials and Methods
Cell cultures and treatment
Huh7 cells were maintained in Dulbecco’s Modification of Eagle’s Medium; HepG2 and Hep3B cells in Minimum Essential Medium. Primary human hepatocytes were generously provided by XenoTech, LLC (Lenexa, KS, USA) as a gift, and cultured according to our previous publication.16 Fresh medium containing corresponding compounds was provided every 24 hrs.
Cell death assay
Cell viability and caspase 3/7 activity were determined by CellTiter-Glo® Luminescent Cell Viability assay and Caspase-Glo® 3/7 kit, respectively (Promega, Madison, WI).
Total RNA preparation and quantitative real-time PCR
Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) and real-time PCR was performed according to our previous publication.17 Primers used are described in the Supporting Materials and Methods.
Western blotting and confocal microscopy
Preparation of subcellular fraction and performance of western blotting as well as confocal microscopy were described previously.17
siRNA transfection
Scramble siRNA and pre-designed siRNA specific for human Nur77 and RARβ genes were purchased from Ambion (Austin, TX), which were described previously.17
Double immunostaining (Nur77 and cleaved caspase 3 as well as Nur77 and RARβ)
Forty-eight hrs after the siRNA transfection, cells were treated by indicated chemicals followed by immunofluorescence staining and confocal microscopy, which were described previously.17
Co-immunoprecipitation
The interaction between RARβ and Nur77 was assessed by using Immunoprecipitation Kit-Dynabeads® Protein G (Invitrogen Carlsbad, CA) followed by western blot. In brief, IgG and anti-RARβ or -Nur77 antibody was immobilized on Dynabeads® protein G. Dynabeads-antibody complex was isolated using magnet. Then, proteins (300 µg) extracted from treated cell were incubated with Dynabeads-antibody complex overnight at 4°C. The Dynabeads-antibody-antigen complex was isolated by magnet. After extensive washing, the complex was denatured by NuPAGE® LDS Sample Buffer/NuPAGE® Reducing Agent mix followed by isolation of protein using magnet and western blot using anti-RARβ or -Nur77 antibody.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using Student's t-test or one-way ANOVA. Significance was defined by p < 0.05.
Results
HDACi enhanced fenretinide-induced apoptosis in human HCC cells, but not in primary human hepatocytes
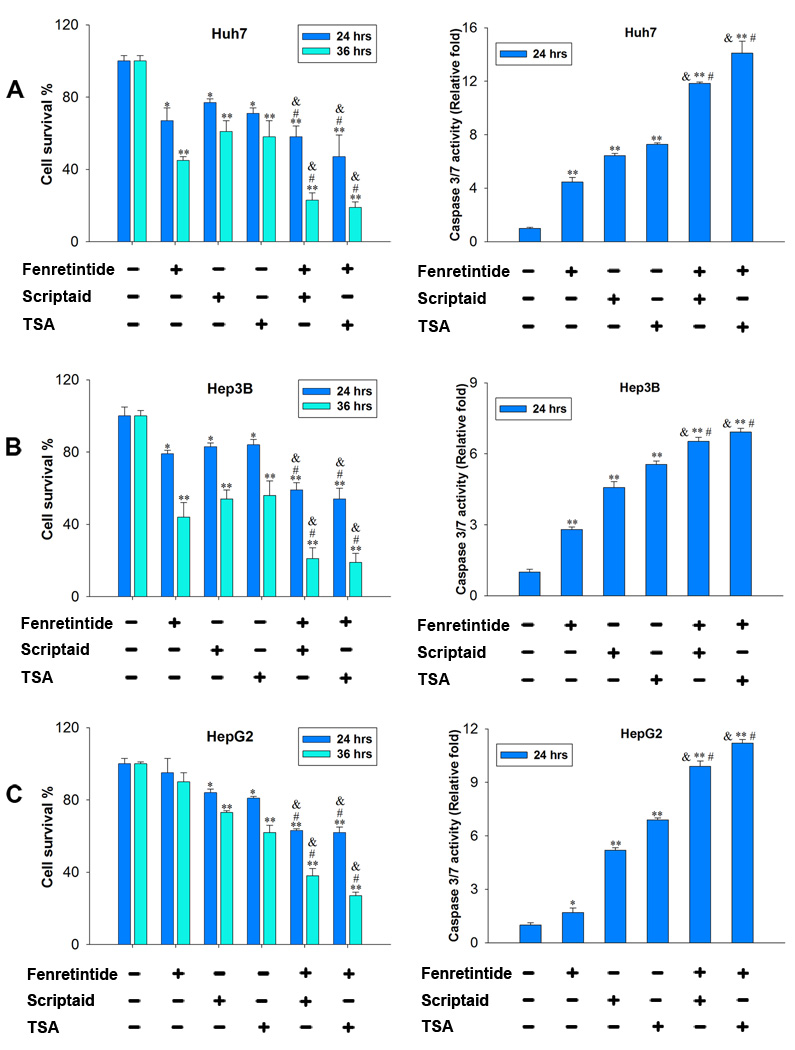
To study the effect of fenretinide and HDACi in inducing the death of HCC cells, Huh7, Hep3B and HepG2 cells were treated with fenretinide (10 µM), TSA (1 µM), scriptaid (10 µM), and fenretinide plus HDACi. Apoptosis was evaluated by cell survival and caspase 3/7 activity. In Huh7 and Hep3B cells, reduction of viability was observed in all the treatment groups. Both TSA and scriptaid were able to enhance fenretinide-induced cell death (Figure 1A, 1B). In comparison with Huh7 and Hep3B cells, HepG2 cells were relatively resistant to fenretinide, but TSA and scriptaid could induce HepG2 cell death. In addition, the cell death effect was enhanced when the combination treatments were used, and the effect was time-dependent (Figure 1C). More than 60% of HepG2 cells died after 36 hrs combination treatments. Consistent findings were noted when caspase 3/7 activity was monitored in three HCC cell lines (Figure 1A–C). The effect of these compounds was tested in freshly isolated primary human hepatocytes, which had not gone through any passage. Hepatocytes derived from three individuals were tested. Neither single nor combination treatments induced the death of primary human hepatocytes (Supplementary Figure 1).
Figure 1.
HDACi enhanced fenretinide-induced apoptosis of human HCC cells. Huh7 (A), Hep3B (B) and HepG2 (C) cells were treated with fenretinide (10 µM), scriptaid (10 µM), TSA (1 µM), and combination of fenretinide plus HDACi for indicated time. Controls were treated with DMSO. Cell survival and caspase 3/7 activity were determined by CellTiter-Glo® Luminescent Cell Viability Assay and Caspase-Glo® 3/7 kit (Promega), respectively. Data were expressed as mean ± SD from three independent experiments, *=p<0.05, **=p<0.01 vs. DMSO; &=p<0.05, vs. fenretinide; #=p<0.05 vs. TSA or scriptaid.
Fenretinide and HDACi-induced apoptosis did not correlate with the level of ROS generation and anti-oxidant gene expression in HCC cells
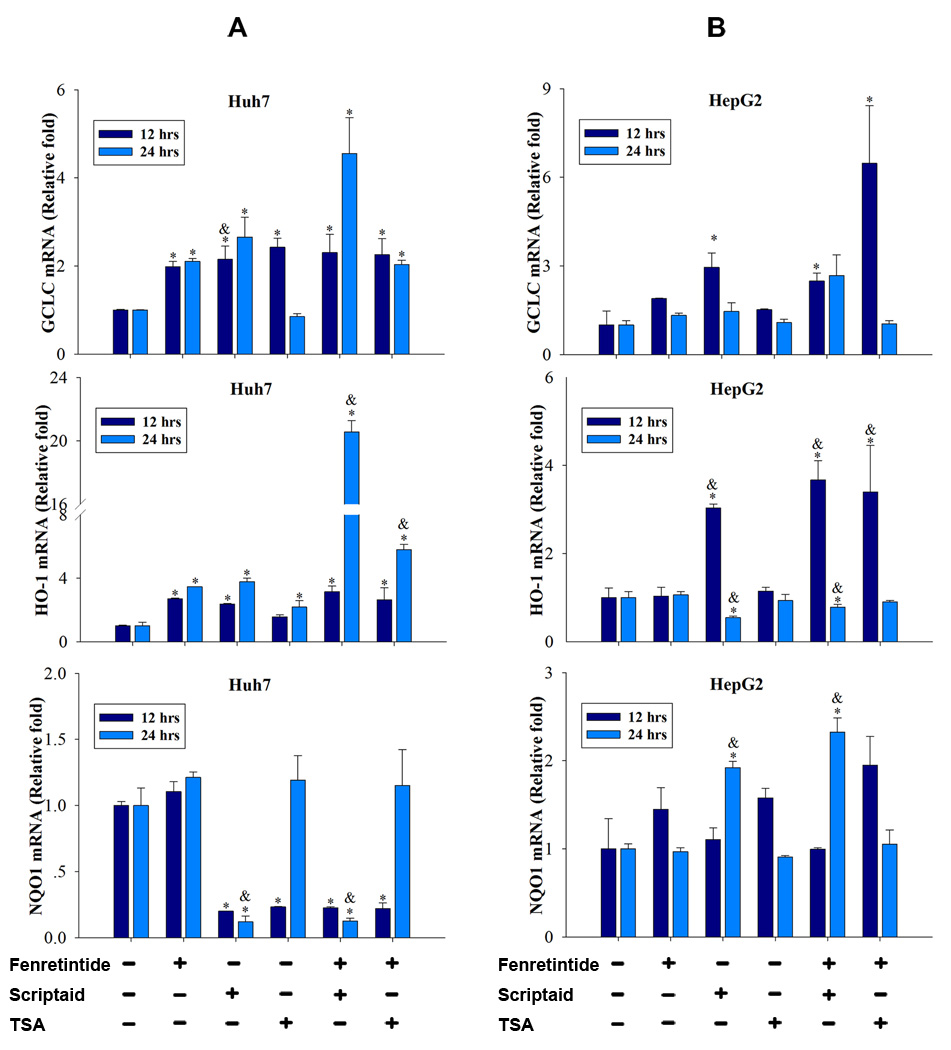
ROS generation has been considered the key mechanism accounting for fenretinide-induced apoptosis in cancer cells.1 To study whether ROS generation was associated with the enhanced apoptosis induced by the combination treatment, Huh7 and HepG2 cells were treated with fenretinide, TSA, scriptaid, and fenretinide plus HDACi for 6 hrs. Mitochondrial ROS was evaluated by flow cytometry using MitoSOX™ Red mitochondrial superoxide indicator (Invitrogen Carlsbad, CA). In Huh7 cells, the mitochondrial ROS generation was modestly increased by fenretinide, TSA, and fenretinide plus HDACi (Supplementary Figure 2A). However, the levels of induction were not correlated with the degree of cell death. In HepG2 cells, increased production of ROS was not detected in any of the treatment groups (Supplementary Figure 2B). It has been shown that RA inhibits Nrf2 and the expression of its target antioxidative genes. Nrf2-silenced cells are vulnerable to all-trans RA-induced mitochondrial toxicity and apoptosis.18 Thus, we studied the possibility that fenretinide-induced apoptosis may be a result of a compromise in Nrf2 signaling. The expression of three Nrf2 target genes (GCLC, HO-1, and NQO1) was studied. There was no consistent pattern in terms of the expression of those genes in either cell line (Figure 2A, 2B). The expression levels were not correlated with the level of cell death. Thus, ROS generation and anti-stress gene expression do not seem to play a key role in fenretinide/HDACi-induced apoptosis of HCC cells.
Figure 2.
Fenretinide and HDACi-induced apoptosis did not correlate with the expression level of the anti-oxidant genes in HCC cells. Huh7 (A) and HepG2 (B) cells were treated with fenretinide (10 µM), scriptaid (10 µM), TSA (1 µM), and combination of both for 12 and 24 hrs followed by RNA extraction to study the expression level of Nrf2 target genes. The results were generated from three independent experiments. *=p<0.05 vs. DMSO, &=p<0.05, vs. fenretinide.
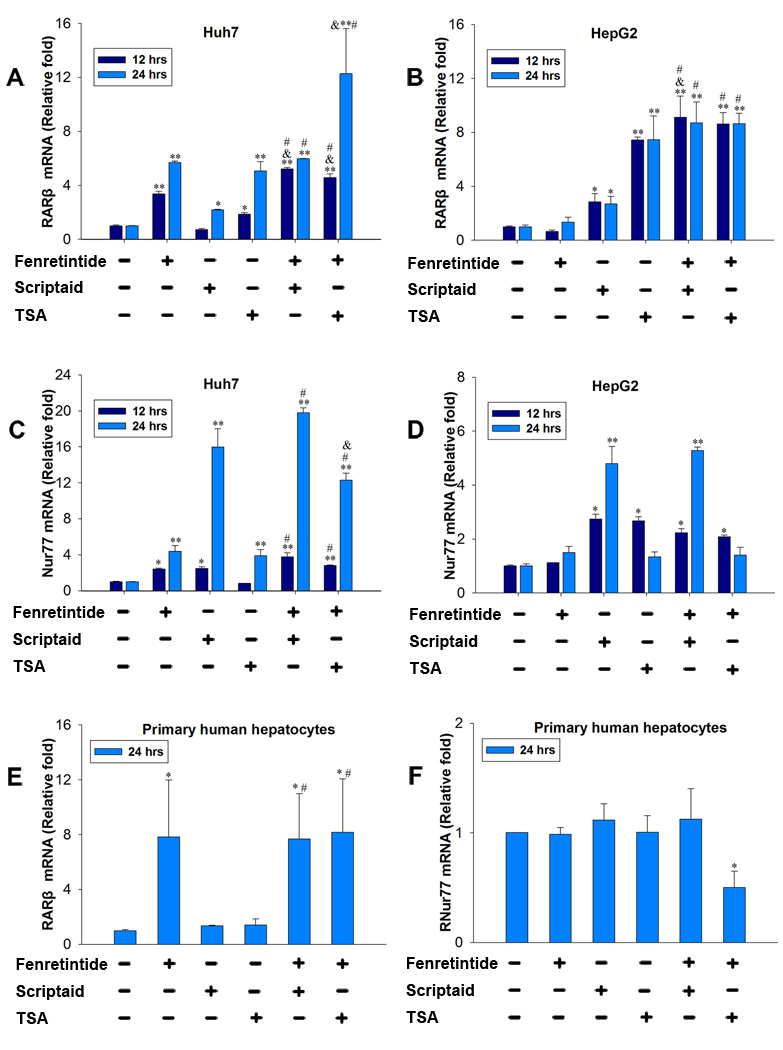
The effect of fenretinide and/or HDACi in regulating the expression of RARβ and Nur77 mRNA levels in HCC cells and primary human hepatocytes
To assess the effect of fenretinide and HDACi on RARβ and Nur77 expression, HCC cells and primary human hepatocytes were treated with fenretinide (10 µM), TSA (1 µM), scriptaid (10 µM), and fenretinide plus HDACi for 12 and 24 hrs. RARβ and Nur77 mRNA level was quantified. Fenretinide, scriptaid (24 hrs), and TSA induced the mRNA level of RARβ, and the levels of RARβ mRNA were further induced by combination treatments in Huh7 cells (Figure 3A). In HepG2 cells, fenretinide had no effect in inducing RARβ mRNA level. In contrast to fenretinide, HDACi increased RARβ mRNA levels. Similar to what occurred in Huh7 cells, the RARβ mRNA levels were further increased by combination treatments (Figure 3B). Nur77 mRNA levels in Huh7 cells were increased in fenretinide, scriptaid, TSA (24 hrs), and combination treatment groups (Figure 3C). Fenretinide had no effect in inducing Nur77 mRNA level in HepG2 cells. However, HDACi (12 hrs) did. Combination treatments did not further induce the Nur77 mRNA level (Figure 3D). In primary human hepatocytes, fenretinide, but not HDACi, increased RARβ mRNA level (Figure 3E). Combination treatments did not further increase the expression level. Since apoptosis did not occur in primary human hepatocytes, the induction of RARβ was not sufficient for fenretinide to induce apoptosis. Neither fenretinide nor HDACi changed Nur77 mRNA level in primary human hepatocytes. Fenretinide plus TSA significantly reduced the mRNA level of Nur77 in primary human hepatocytes (Figure 3F).
Figure 3.
The effect of fenretinide and/or HDACi in regulating the expression of RARβ and Nur77 mRNA levels in HCC cells and primary human hepatocytes. Huh7 and HepG2 cells were treated as described in Figure legend 1 for 12 and 24 hrs. Hepatocytes were obtained from three individuals and were treated for 24 hrs. RARβ and Nur77 mRNA levels were measured by RT-PCR. The results generated from cell lines were done by three independent experiments. *=p<0.05, **=p<0.01 vs. DMSO; &=p<0.05, vs. fenretinide; #=p<0.05 vs. TSA or Scriptaid.
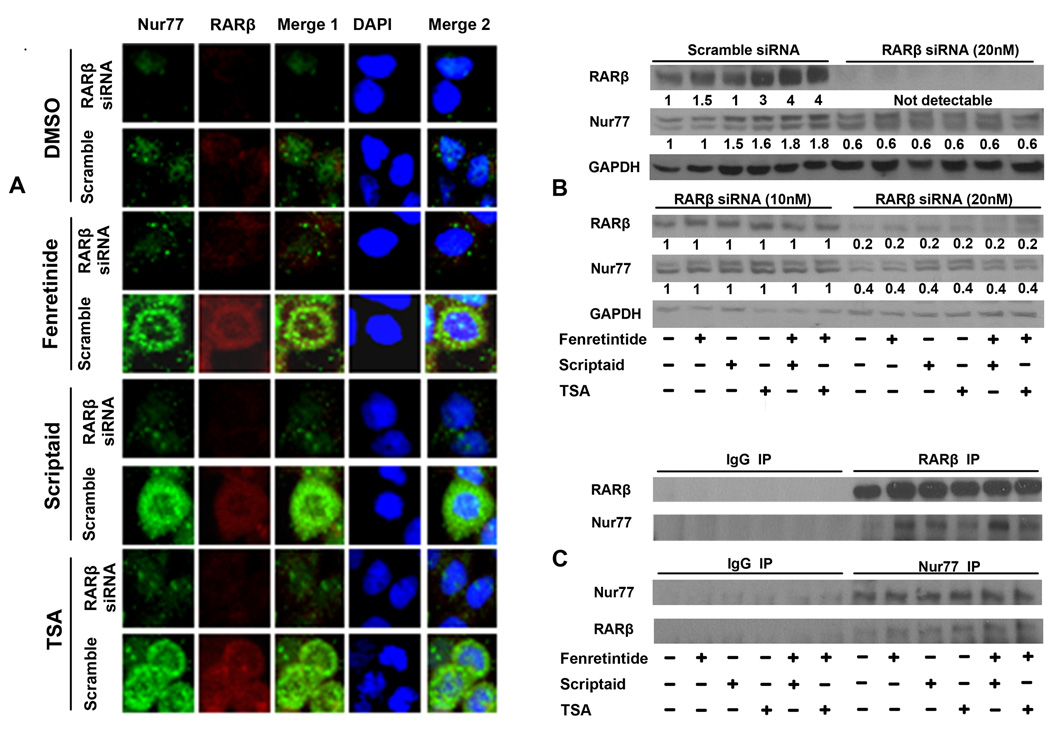
The interaction between Nur77 and RARβ
Our previous publication also shows that fenretinide-induced apoptosis of Huh7 cell is RARβ and Nur77 dependent.17, 19 Thus, it is important to determine whether these two nuclear receptors interact with each other or whether they represent two independent pathways. We studied the effect of RARβ on Nur77 expression. Huh7 cells were transfected with RARβ or scramble siRNA for 48 hrs followed by fenretinide or HDACi treatment to study the expression of Nur77 by immunostaining and western blot. The anti-RARβ antibody used was specific. We have used this antibody to show increased recruitment of RARβ to the RARβ and Cyp26a1 promoters.19 The data showed that fenretinide and HDACi induced the expression level of Nur77 and RARβ. Surprisingly, confocal microscopy showed cytoplasmic localization of Nur77 and RARβ in fenretinide and HDACi treated Huh7 cells. RARβ siRNA blocked fenretinide- and HDACi-induced RARβ expression. Interestingly, Nur77 also became undetectable after RARβ expression was reduced (Figure 4A). Thus, the expression of Nur77 was dependent on the presence of RARβ. Western blot confirmed the findings. We also studied 12-hr time point because the majority of Huh7 cells died after 24 hrs combination treatments. Using 30 µg of protein, RARβ could be detected in control DMSO treated cells by western blot. Combination treatments substantially induced RARβ and Nur77 protein level in scramble siRNA-transfected cells. RARβ siRNA transfection substantially reduced RARβ protein in all treatment groups, which was accompanied by decreased Nur77 protein level (Figure 4B). In addition, Nur77 level was no longer regulated by fenretinide and HDACi treatments in RARβ siRNA-transfected cells. In an independent experiment, when two doses of RARβ siRNA were used side-by-side (10 and 20 nM), a dose-dependent decrease of RARβ as well as Nur77 was noted (Figure 4B). Consistently, Nur77 level was no longer regulated by fenretinide and HDACi after RARβ expression was reduced. Thus, the Nur77 protein level was influenced by the presence of RARβ. We also tested the hypothesis that RARβ can regulate the level of Nur77 mRNA although the data generated using primary human hepatocytes, in which RARβ induction was not accompanied by an increased level of Nur77 mRNA (Figure 3F), did not support this scenario. Our data indicated that knockdown in the expression of RARβ was not accompanied with reduction of Nur77 mRNA level (data not shown). Thus, RARβ has an impact on Nur77 protein level, but not mRNA level.
Figure 4.
The interaction between Nur77 and RARβ. Huh7 cells were transfected with either scramble or RARβ siRNA (20 nM) for 48 hrs. Then, cells were treated with indicated chemicals for an additional 24 hrs (A) followed by immunostaining with anti-Nur77 antibody and goat anti-mouse IgG Alexa Fluor® 488 second antibody (green) and with anti-RARβ antibody followed by Texas Red-conjugated secondary antibody. The staining was viewed using confocal microscopy (A). In an independent experiment, Huh7 cells were transfected with either scramble or RARβ siRNA (10 or 20 nM) for 48 hrs and treated with indicated chemicals for an additional 12 hrs. Then, the treated cells were subjected to protein extraction and western blot to study the expression of RARβ and Nur77. Numbers indicate the relative level of the protein level normalized by the level of GAPDH (B). Proteins extracted from 12 hrs treated cells were immunoprecipitated by anti-RARβ (upper panel) or anti-Nur77 antibody (lower panel) or pre-immune IgG followed by western blot using anti-Nur77 (upper panel) or anti-RARβ (lower panel) antibody (C).
The potential interaction between Nur77 and RARβ was studied by immunoprecipitation followed by western blot. Figure 4C showed that RARβ could interact with endogenous Nur77. These data indicate that RARβ and Nur77 directly or indirectly interact with each other.
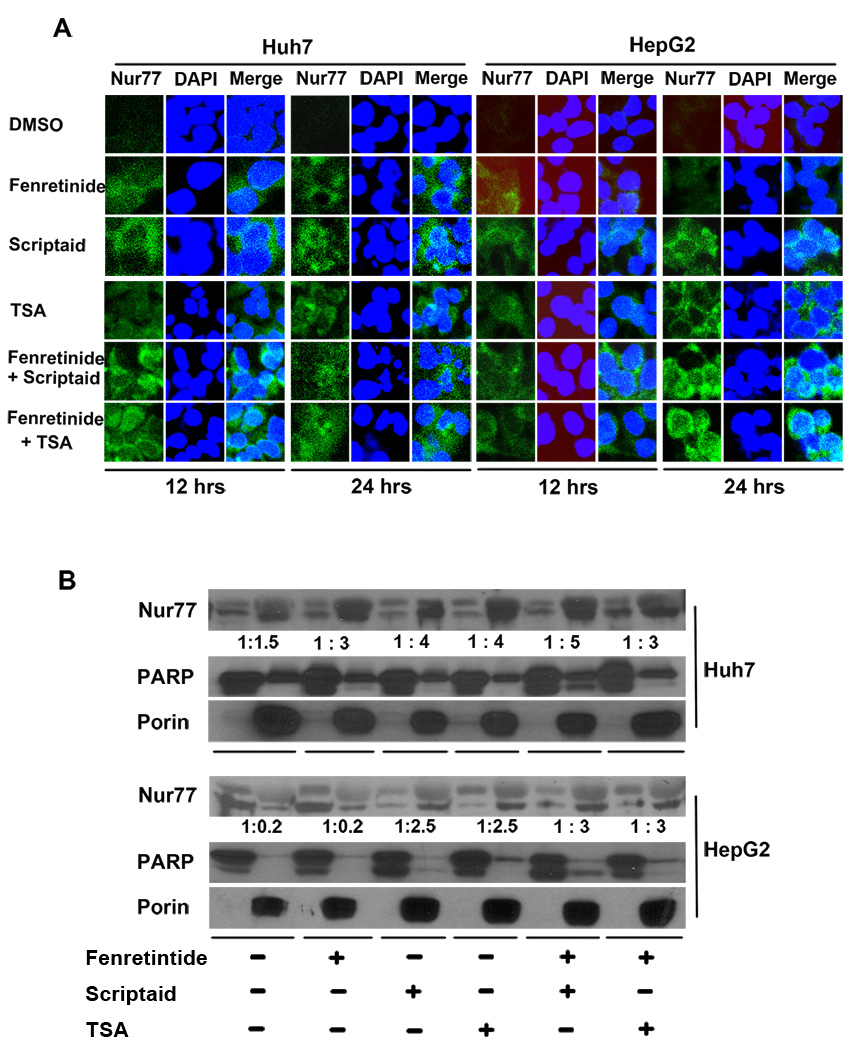
HDACi and/or fenretinide induced cytoplasmic Nur77 in HCC cells
The biological function of Nur77 is dependent on its intracellular localization. Nur77 induces apoptosis by binding to mitochondrial Bcl-2.20 Our previous publication shows that induction and intracellular localization of Nur77 dictate fenretinide-induced apoptosis of HCC cells.17 Thus, we studied whether HDACi can regulate intracellular location of Nur77 as well. When fenretinide alone was used, the cytoplasmic staining of Nur77 was apparent after 24 hrs treatment. When combination treatments were used, the induced Nur77 level was much higher than that by single treatments within 12 hrs. Combination treatment of Huh7 cells using fenretinide plus HDACi for 24 hrs induced cell death as revealed by condensed and fragmented DAPI staining of the nucleus. The expression pattern of Nur77 in those cells was diffused and the level was reduced (Figure 5A). In HepG2 cells, fenretinide modestly induced Nur77, which was found in both the nucleus and cytosol (Figure 5A). HDACi and HDACi plus fenretinide induced cytoplasmic Nur77 expression in a time-dependent manner.
Figure 5.
HDACi and/or fenretinide induced cytoplasmic Nur77 expression in HCC cells. (A) HCC cells were treated as described in Figure legend 1. Immunofluorescence staining was performed using anti-Nur77 antibody and nuclear counterstaining with DAPI and viewed by confocal microscopy. (B) Nuclear (Nu) and Mitochondria (Mit) enriched fractions were isolated from treated cells. Proteins were fractionated followed by western blot using antibodies specific to Nur77, PARP, and Porin. Numbers indicate the ratios of nuclear protein vs. mitochondria protein.
To determine the subcellular localization of Nur77 in response to the treatments, nuclear- and mitochondria-enriched fractions were isolated. Porin and Poly (ADP-ribose) polymerase (PARP) were used as mitochondrial and nuclear markers, respectively. In Huh7 cells, Nur77 protein was predominantly found in the mitochondria-enriched fraction (Figure 5B). In the fenretinide-resistant HepG2 cells, Nur77 was mainly expressed in the nuclear-enriched fraction of DMSO and fenretinide treated cells. However, HDACi and combination treatments changed the intracellular location of Nur77, which was mainly expressed in mitochondria-enriched fractions. Thus, the mitochondrial localization of Nur77 was positively associated with the sensitivity of the cell to the apoptotic effect of HDACi and/or fenretinide (Figure 5B).
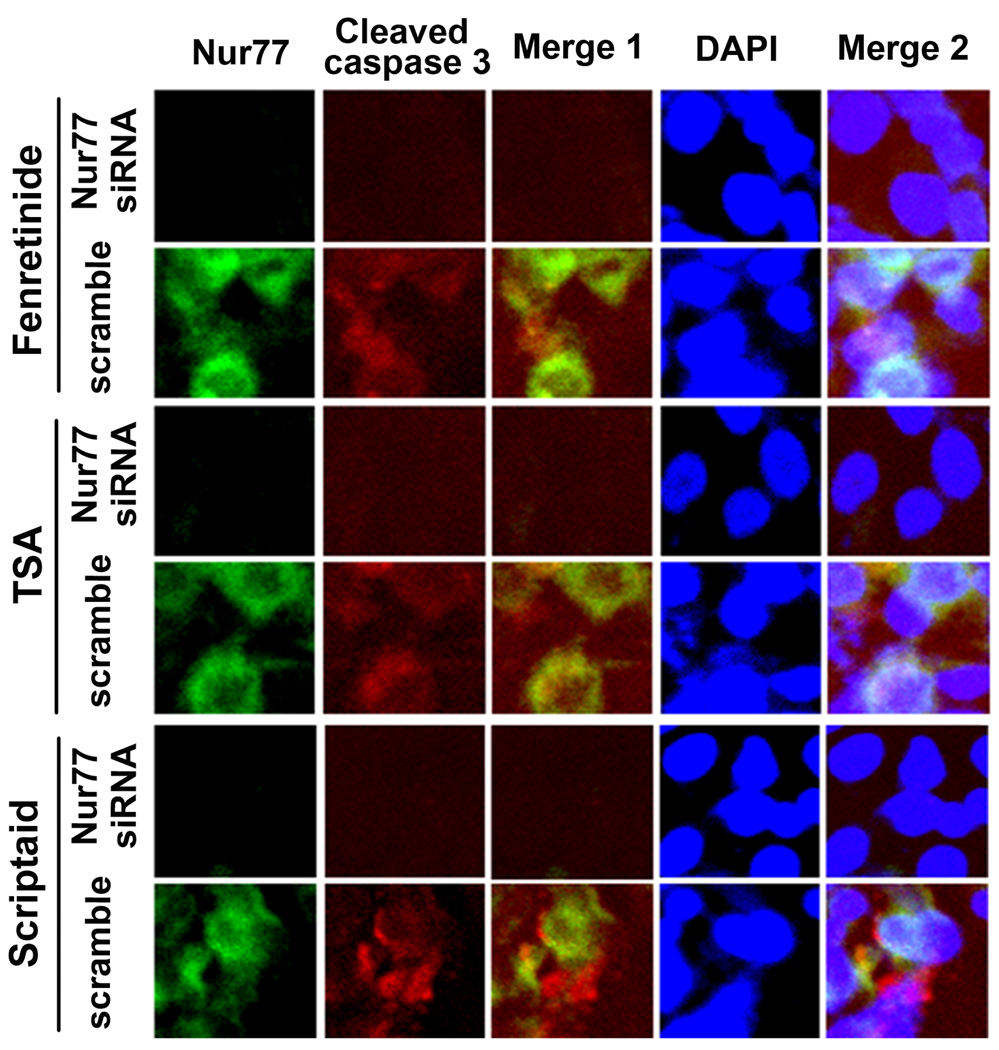
Nur77 was essential for fenretinide- and HDACi-induced apoptosis of Huh7 cells
In order to determine whether Nur77 was required for fenretinide- and HDACi-induced apoptosis, the expression of Nur77 was knocked down by siRNA. Double immunofluorescence staining (Nur77 and cleaved caspase 3) was performed to evaluate the role of Nur77 on fenretinide and HDACi-induced apoptosis. Fenretinide and HDACi induced the expression of cytoplasmic Nur77, which co-existed with the induction of cleaved caspase 3 in scramble siRNA-transfected Huh7 cells. Nur77 was not detected in Nur77 siRNA transfected Huh7 cells, in which cleaved caspase 3 was also undetectable after fenretinide or HDACi treatment (Figure 6). These findings indicate that Nur77 is the common mechanism for fenretinide and HDACi to induce apoptosis of HCC cells. Taken together, our data showed that induction, interaction, and nuclear export of RARβ and Nur77 mediate fenretinide and HDACi induced apoptosis of HCC cells. The presented data are summarized in figure 7.
Figure 6.
Nur77 was essential for fenretinide- and HDACi-induced apoptosis of Huh7 cells. Huh7 cells were transfected with either scramble or Nur77 siRNA (50 nM) for 48 hrs and treated with fenretinide or HDACi for an additional 24 hrs. Then, cells were immunostained with anti-Nur77 antibody and FITC-conjugated goat anti-rabbit IgG (green) followed by staining with anti-cleaved caspase 3 antibody and sheep anti-goat IgG-Texas Red. The staining was viewed under confocal microscope. A representative result from three independent experiments is shown.
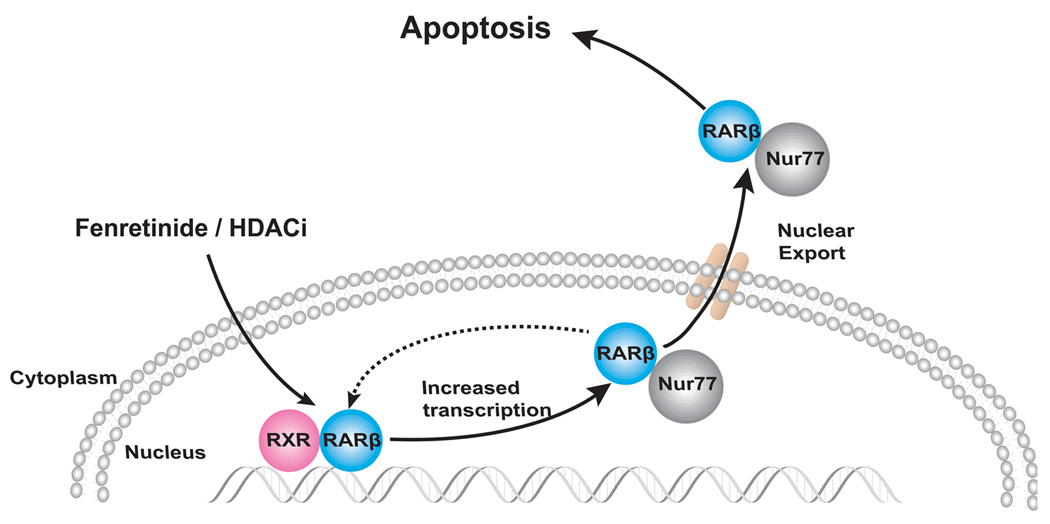
Figure 7.
Fenretinide can mediate via RARβ to increase the expression level of RARβ.19 The fenretinide-induced RARβ directly or indirectly interacts with Nur77. Such interaction stabilizes Nur77 protein level. The RARβ/Nur77 protein complex is exported to the cytosol and most likely targets mitochondria to induce apoptosis.
Discussion
Among the retinoic acid receptors, RARβ is a tumor suppressor gene. Its expression is frequently suppressed in lung cancer cell lines, 21, 22 head and neck, 23 and breast 24 as well as liver cancer cell lines.25 The expression of RARβ can be regulated transcriptionally by RAR and epigenetically by DNA methylation and histone acetylation.19, 26–28 However, our unpublished data indicated that azacitidine, which causes DNA demethylation, was not able to enhance fenretinide-induced apoptosis in HCC cells (data not shown). It has been shown that HDACi plus 13-cis retinoic acid restores retinoid sensitivity by reverting RARβ epigenetic silencing in human melanoma cell lines.29 In addition, HDACi and acyclic retinoid cooperatively induce the expression of RARβ and cause apoptosis.30 Furthermore, the anticancer effect of HDACi operates at least in part through retinoic acid signaling.31 These data along with ours shown in the current study indicate that RARβ is the common target for HDACi and fenretinide. Thus, they enhance each other’s effect in inducing liver cancer cell death. However, it remains unclear how RARβ induces apoptosis. For the first time, we show that RARβ may interact with Nur77 to cause apoptosis.
Nur77 displays opposing effects on cell death and survival.32 The translocation of Nur77 from nucleus to mitochondria confers its apoptotic effect.33 Nur77 targets mitochondria through its interaction with mitochondrial Bcl-2, which unmasks its hidden BH-3 domain. This conformational change converts Bcl-2 into a pro-apoptotic molecule.34 Nur77 interacts with nuclear receptors such as small heterodimeric partner (SHP) in HepG2 cells. This interaction not only blocks Nur77-mediated transcription, but also causes resistance of HepG2 cells to interferon γ and anti-Fas antibody-induced apoptosis.35 Interestingly, we showed that Nur77 interacts with RARβ and that the expression of Nur77 is dependent upon the presence of RARβ. Knockdown experiments suggested that Nur77 and RARβ interaction might stabilize Nur77 and sustain its expression level. RARβ may also serve a chaperone and carry Nur77 for nuclear export. Thus, it is possible that SHP and RARβ compete with each other for binding to Nur77 in HepG2 cells. The induction of RARβ by fenretinide and HDACi favors the interaction between RARβ and Nur77, and thus leads to apoptosis. This may also explain why the level of RARβ can be used to determine the efficacy of retinoids and the prognosis of cancer.36 Additional experimentation is needed to prove this.
The expression of Nur77 is regulated transcriptionally by AP-1.37 It is important to determine the mechanism by which fenretinide induces Nur77 expression. We have previously shown that fenretinide regulates RARβ-mediated gene transcription. 19 Data generated using primary human hepatocytes do not support the idea that RARβ directly regulates Nur77 gene expression. In addition, putative RARβ binding sites have not been found in the Nur77 gene. It is important to note that non-genomic action of retinoids is known 36 and this possibility cannot be excluded. Since scriptaid and TSA had a differential effect in inducing Nur77, multiple mechanisms at transcriptional and post-transcriptional levels might be involved. Based on the data presented in the current study, the increased expression of Nur77 could be due to histone acetylation of RARβ, which increased the expression level of RARβ and therefore the stabilization of Nur77 protein.
Mitochondrial oxidative stress is an early event of apoptosis.38 Several studies have shown that ROS production plays a key role in HDACi- as well as fenretinide-induced apoptosis.11, 39 However, neither ROS production nor the expression of anti-oxidant genes correlated with fenretinide and HDACi-induced apoptosis in HCC cells in the current study. Thus, other mechanisms such as RARβ/Nur77-mediated cell death may play a more significant role.
Supplementary Material
Acknowledgement
The authors thank XenoTech, LLC (Lenexa, KS, USA) for generous gift of primary human hepatocytes. The authors thank Ms. Zoe Raglow for editing the manuscript and Dr. Yun Zhong for preparation of the figures.
Grant Support: This work was supported by NIH grants (CA 53596 and P20RR021940 Molecular Biology Core) and the National Natural Science Foundation of China (No.81001109).
Abbreviations
- HDACi
histone deacetylase inhibitors
- TSA
trichostatin A
- ROS
Reactive oxygen species
- PARP
Poly (ADP-ribose) polymerase
- DAPI
4', 6-diamidino-2-phenylindole
- HCC
human hepatocellular carcinoma
Footnotes
Financial Disclosures: No conflicts of interest exist.
Authors’ Contribution:
Hui Yang – experiment design, data acquisition, analysis, and interpretation, manuscript preparation
Qi Zhan – ROS data acquisition and material support.
Yu-Jui Yvonne Wan – generate idea and hypothesis, experiment design, analysis and interpretation of data, manuscript preparation, and obtain research funding.
References
- 1.Hail N, Jr, Kim HJ, Lotan R. Mechanisms of fenretinide-induced apoptosis. Apoptosis. 2006;11:1677–1694. doi: 10.1007/s10495-006-9289-3. [DOI] [PubMed] [Google Scholar]
- 2.Lippman SM, Lee JJ, Martin JW, El-Naggar AK, Xu X, Shin DM, Thomas M, et al. Fenretinide activity in retinoid-resistant oral leukoplakia. Clin Cancer Res. 2006;12:3109–3114. doi: 10.1158/1078-0432.CCR-05-2636. [DOI] [PubMed] [Google Scholar]
- 3.Qian J, Zhang JS, Wang XQ, Ji JL, Mei S. Fenretinide stimulates the apoptosis of hepatic stellate cells and ameliorates hepatic fibrosis in mice. Hepatol Res. 2009;39:1229–1247. doi: 10.1111/j.1872-034X.2009.00562.x. [DOI] [PubMed] [Google Scholar]
- 4.Preitner F, Mody N, Graham TE, Peroni OD, Kahn BB. Long-term Fenretinide treatment prevents high-fat diet-induced obesity, insulin resistance, and hepatic steatosis. Am J Physiol Endocrinol Metab. 2009;297:E1420–E1429. doi: 10.1152/ajpendo.00362.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sogno I, Vene R, Ferrari N, De Censi A, Imperatori A, Noonan DM, Tosetti F, et al. Angioprevention with fenretinide: targeting angiogenesis in prevention and therapeutic strategies. Crit Rev Oncol Hematol. 2010;75:2–14. doi: 10.1016/j.critrevonc.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Hadnagy A, Beaulieu R, Balicki D. Histone tail modifications and noncanonical functions of histones: perspectives in cancer epigenetics. Mol Cancer Ther. 2008;7:740–748. doi: 10.1158/1535-7163.MCT-07-2284. [DOI] [PubMed] [Google Scholar]
- 7.Jones PA, Baylin SB. The epigenomics of cancer. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 9.Smith KT, Workman JL. Histone deacetylase inhibitors: anticancer compounds. Int J Biochem Cell Biol. 2009;41:21–25. doi: 10.1016/j.biocel.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Carey N, La Thangue NB. Histone deacetylase inhibitors: gathering pace. Curr Opin Pharmacol. 2006;6:369–375. doi: 10.1016/j.coph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Carew JS, Giles FJ, Nawrocki ST. Histone deacetylase inhibitors: mechanisms of cell death and promise in combination cancer therapy. Cancer Lett. 2008;269:7–17. doi: 10.1016/j.canlet.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Bots M, Johnstone RW. Rational combinations using HDAC inhibitors. Clin Cancer Res. 2009;15:3970–3977. doi: 10.1158/1078-0432.CCR-08-2786. [DOI] [PubMed] [Google Scholar]
- 13.Sonnemann J, Bumbul B, Beck JF. Synergistic activity of the histone deacetylase inhibitor suberoylanilide hydroxamic acid and the bisphosphonate zoledronic acid against prostate cancer cells in vitro. Mol Cancer Ther. 2007;6:2976–2984. doi: 10.1158/1535-7163.MCT-07-0221. [DOI] [PubMed] [Google Scholar]
- 14.Coffey DC, Kutko MC, Glick RD, Butler LM, Heller G, Rifkind RA, Marks PA, et al. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer Res. 2001;61:3591–3594. [PubMed] [Google Scholar]
- 15.Su GH, Sohn TA, Ryu B, Kern SE. A novel histone deacetylase inhibitor identified by high-throughput transcriptional screening of a compound library. Cancer Res. 2000;60:3137–3142. [PubMed] [Google Scholar]
- 16.Yang H, Nie Y, Li Y, Wan YJ. Histone modification-mediated CYP2E1 gene expression and apoptosis of HepG2 cells. Exp Biol Med (Maywood) 2010;235:32–39. doi: 10.1258/ebm.2009.009252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang H, Bushue N, Bu P, Yvonne Wan YJ. Induction and intracellular localization of Nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem Pharmacol. 2010;79:948–954. doi: 10.1016/j.bcp.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan KP, Kosuge K, Yang M, Ito S. NRF2 as a determinant of cellular resistance in retinoic acid cytotoxicity. Free Radic Biol Med. 2008;45:1663–1673. doi: 10.1016/j.freeradbiomed.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Bu P, Wan YJ. Fenretinide-induced apoptosis of Huh-7 hepatocellular carcinoma is retinoic acid receptor beta dependent. BMC Cancer. 2007;7:236. doi: 10.1186/1471-2407-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang XK. Targeting Nur77 translocation. Expert Opin Ther Targets. 2007;11:69–79. doi: 10.1517/14728222.11.1.69. [DOI] [PubMed] [Google Scholar]
- 21.Houle B, Leduc F, Bradley WE. Implication of RARB in epidermoid (Squamous) lung cancer. Genes Chromosomes Cancer. 1991;3:358–366. doi: 10.1002/gcc.2870030506. [DOI] [PubMed] [Google Scholar]
- 22.Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, et al. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst. 1999;91:1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 23.Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54:3580–3587. [PubMed] [Google Scholar]
- 24.Widschwendter M, Berger J, Daxenbichler G, Muller-Holzner E, Widschwendter A, Mayr A, Marth C, et al. Loss of retinoic acid receptor beta expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Res. 1997;57:4158–4161. [PubMed] [Google Scholar]
- 25.Li C, Wan YJ. Differentiation and antiproliferation effects of retinoic acid receptor beta in hepatoma cells. Cancer Lett. 1998;124:205–211. doi: 10.1016/s0304-3835(97)00475-8. [DOI] [PubMed] [Google Scholar]
- 26.De los Santos M, Zambrano A, Sanchez-Pacheco A, Aranda A. Histone deacetylase inhibitors regulate retinoic acid receptor beta expression in neuroblastoma cells by both transcriptional and posttranscriptional mechanisms. Mol Endocrinol. 2007;21:2416–2426. doi: 10.1210/me.2007-0151. [DOI] [PubMed] [Google Scholar]
- 27.Youssef EM, Lotan D, Issa JP, Wakasa K, Fan YH, Mao L, Hassan K, et al. Hypermethylation of the retinoic acid receptor-beta(2) gene in head and neck carcinogenesis. Clin Cancer Res. 2004;10:1733–1742. doi: 10.1158/1078-0432.ccr-0989-3. [DOI] [PubMed] [Google Scholar]
- 28.Cras A, Darsin-Bettinger D, Balitrand N, Cassinat B, Soulie A, Toubert ME, Delva L, et al. Epigenetic patterns of the retinoic acid receptor beta2 promoter in retinoic acid-resistant thyroid cancer cells. Oncogene. 2007;26:4018–4024. doi: 10.1038/sj.onc.1210178. [DOI] [PubMed] [Google Scholar]
- 29.Kato Y, Salumbides BC, Wang XF, Qian DZ, Williams S, Wei Y, Sanni TB, et al. Antitumor effect of the histone deacetylase inhibitor LAQ824 in combination with 13-cis-retinoic acid in human malignant melanoma. Mol Cancer Ther. 2007;6:70–81. doi: 10.1158/1535-7163.MCT-06-0125. [DOI] [PubMed] [Google Scholar]
- 30.Tatebe H, Shimizu M, Shirakami Y, Sakai H, Yasuda Y, Tsurumi H, Moriwaki H. Acyclic retinoid synergises with valproic acid to inhibit growth in human hepatocellular carcinoma cells. Cancer Lett. 2009;285:210–217. doi: 10.1016/j.canlet.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 31.Epping MT, Meijer LA, Bos JL, Bernards R. UNC45A confers resistance to histone deacetylase inhibitors and retinoic acid. Mol Cancer Res. 2009;7:1861–1870. doi: 10.1158/1541-7786.MCR-09-0187. [DOI] [PubMed] [Google Scholar]
- 32.Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25:4725–4743. doi: 10.1038/sj.onc.1209601. [DOI] [PubMed] [Google Scholar]
- 33.Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000;289:1159–1164. doi: 10.1126/science.289.5482.1159. [DOI] [PubMed] [Google Scholar]
- 34.Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, et al. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004;116:527–540. doi: 10.1016/s0092-8674(04)00162-x. [DOI] [PubMed] [Google Scholar]
- 35.Yeo MG, Yoo YG, Choi HS, Pak YK, Lee MO. Negative cross-talk between Nur77 and small heterodimer partner and its role in apoptotic cell death of hepatoma cells. Mol Endocrinol. 2005;19:950–963. doi: 10.1210/me.2004-0209. [DOI] [PubMed] [Google Scholar]
- 36.Bushue N, Wan YJ. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010 doi: 10.1016/j.addr.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Chen X, Zachar V, Chang C, Ebbesen P. Transcriptional activation of human TR3/nur77 gene expression by human T-lymphotropic virus type I Tax protein through two AP-1-like elements. J Gen Virol. 1999;80(Pt 12):3073–3081. doi: 10.1099/0022-1317-80-12-3073. [DOI] [PubMed] [Google Scholar]
- 38.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–418. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 39.Kadara H, Tahara E, Kim HJ, Lotan D, Myers J, Lotan R. Involvement of Rac in fenretinide-induced apoptosis. Cancer Res. 2008;68:4416–4423. doi: 10.1158/0008-5472.CAN-08-0031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.