Abstract
Excessive exposure of the skin to solar ultraviolet (UV) radiation is one of the major factors for the development of skin cancers, including nonmelanoma. For the last several centuries the consumption of dietary phytochemicals has been linked to numerous health benefits including the photoprotection of the skin. Green tea has been consumed as a popular beverage world-wide and skin photoprotection by green tea polyphenols (GTPs) has been widely investigated. In this article, we have discussed the recent investigations and mechanistic studies which define the potential efficacy of GTPs on the prevention of non-melanoma skin cancer. UV-induced DNA damage, particularly the formation of cyclobutane pyrimidine dimers, has been implicated in immunosuppression and initiation of skin cancer. Topical application or oral administration of green tea through drinking water of mice prevents UVB-induced skin tumor development, and this prevention is mediated, at least in part, through rapid repair of DNA. The DNA repair by GTPs is mediated through the induction of interleukin (IL)-12 which has been shown to have DNA repair ability. The new mechanistic investigations support and explain the anti-photocarcinogenic activity, in particular anti-non-melanoma skin cancer, of green tea and explain the benefits of green tea for human health.
Keywords: Chemoprevention, contact hypersensitivity, cyclobutane pyrimidine dimers, green tea polyphenols, nucleotide excision repair, non-melanoma skin cancer, ultraviolet radiation
Introduction
Skin cancer, including melanoma and non-melanoma, represents a major public health problem as the incidence of skin cancer is equivalent to the incidence of cancers in all other organs combined [1]. It is also a major burden on the health care system as it has been estimated that the cost of treating non-melanoma and melanoma skin cancers in the United States is in billions dollar annually (www.cancer.org/statistics). The chronic exposure of the skin to solar ultraviolet (UV) radiation is a major etiologic factor for initiation of skin cancers. The depletion of the ozone layer that allows more solar UV radiation to reach at the surface of the Earth, together with continuing increase in life expectancy and changing dietary habits and lifestyle appear to be contributing factors for the increasing risk of cutaneous malignancies. Effective chemopreventive and chemotherapeutic agents and strategies to address this issue and disease are urgently needed. One such strategy includes the use of dietary phytochemicals, and it is becoming popular as a means to protect skin diseases including the risk of skin cancer.
Naturally occurring phytochemicals, specifically phenolics, are widely distributed in plant foods, including fruits, vegetables, seeds, nuts, flowers and bark. Important dietary sources of polyphenols are grape seeds, grape skin, tea, apples, red wine, onions and cacao, etc. [2]. These polyphenols possess beneficial health effects of dietary sources. Among the extensive studied polyphenols, the green tea is better known for its health benefits in many organs as well as in general health. In this review article, we particularly describe and discuss the prevention of non-melanoma skin cancer by green tea polyphenols with particular emphasis on their DNA repair abilities in UV exposed skin.
Green tea polyphenols
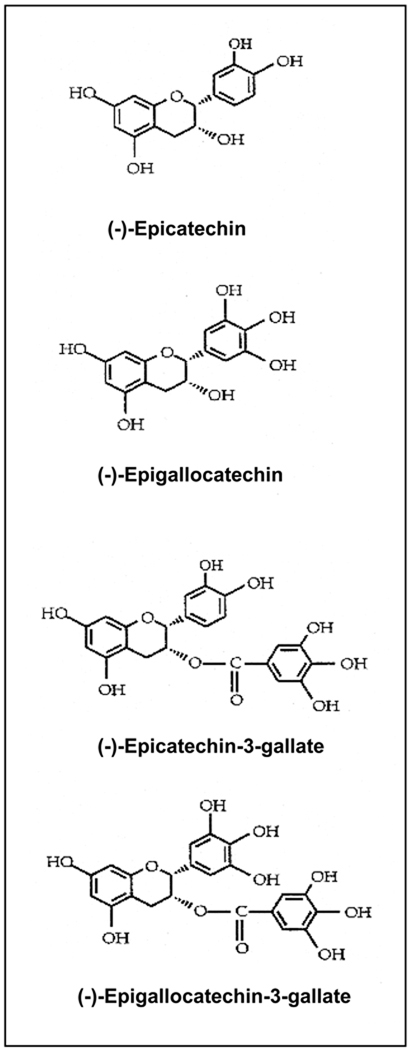
Tea has been consumed as a popular beverage world-wide for the last thousands of years because of its health benefits and pleasant aroma. Beverage grade commercial tea is manufactured from the leaves of the plant Camellia sinensis and is commercially available mainly in three forms: green, black and oolong tea [2–4]. Of the total commercial tea production worldwide, about 78% is consumed in the form of black tea, mainly in western countries and some Asian countries, and 20% is consumed in the form of green tea. Green tea is largely consumed in some Asian countries like, Japan, China, Korea, parts of India and a few countries in North Africa and the Middle East [3, 4]. The consumption of green tea is now increasing in western countries including the United States because of increasingly new investigations on its health benefits and anti-carcinogenic activities in various organs. Approximately 2% of tea is consumed in the form of oolong tea, which is a partially fermented tea product, and largely consumed in some parts of South-eastern China [3, 4]. The characteristic aroma and health benefits of tea are associated with the presence of catechin/epicatechin molecules and their derivatives, which are commonly called “polyphenols”. The major polyphenols present in green tea are: (–)-epicatechin, (–)-epigallocatechin, (–)-epicatechin-3-gallate and (–)-epigallocatechin-3-gallate (EGCG), and are shown in Figure 1.
Figure 1.
Chemical structures of major epicatechins present in green tea. Epicatechins are also known as “polyphenols”.
Studies have shown that polyphenols possess anti-oxidant, anti-inflammatory and anti-carcinogenic activities in several in vitro and in vivo systems [5, 6]. Of these major polyphenols, EGCG is the major component and the most effective molecule. EGCG has been extensively studied in several disease models including its beneficial effects against the harmful effects of solar ultraviolet radiation. The polyphenols present in black tea are less well-defined and known as thearubigens and theaflavins. Investigations revealed that thearubigins and theaflavins are also anti-carcinogenic in nature. The in vitro cell culture and in vivo animal studies indicate that the polyphenols present in green tea (abbreviated as GTPs: a mixture of green tea epicatechins or polyphenols) are better chemopreventive agents than those present in black tea.
Skin
The skin is the largest organ of the body and comprising a surface area of approximately 1.5–2.0 m2 which protects the internal organs of the body against the detrimental effects of environmental and xenobiotic agents. Thus skin provides a protective covering at this crucial interface between inside and outside; and therefore is a physical barrier between the external environment and internal tissues. Morphologically, skin is a composite of a variety of cell types and organellar bodies, each of which has a particular function, and is made up of several different layers, each with particular properties. The major layers include the epidermis and the dermis. The major cell type of the epidermis is the keratinocytes. Keratinocytes comprise >90% of the cells of the epidermal layer. Other cell types are: Langerhans cells, melanocytes and γδ T cells. The dermal components of the skin, including dermal fibroblast, microvasculature endothelial cells, dermal dendritic cells, mast cells, and resident perivascular T-cells, also participate in the cutaneous functions. These epidermal and dermal components have evolved into a dynamic network of interacting cells capable of sensing a variety of perturbations including trauma, ultraviolet irradiation, toxic environmental chemicals, and pathogenic microorganisms in the cutaneous environment. Among many environmental and xenobiotic factors, the exposure of the skin to solar UV radiation is the key factor in the initiation of various skin disorders, such as wrinkling, hypopigmentation and hyperpigmentation and skin cancer [7–9]. Statistically, the average annual UV dose that an average American typically receive in a year is about 2,500–3,300 mJ/cm2. An average female is exposed to an average 2,200 mJ/cm2 and males 2,800 mJ/cm2 each year with an additional exposure of about 800 mJ/cm2 of solar UVB radiation during a conservative vacation period [10, 11].
UV radiation
Sunlight is a major source of UV radiation. Solar UV spectrum is divided into three major components based on their wavelengths and biological effects [5, 7]: (a) long-wave UVA (320–400 nm), (b) mid-wave UVB (290–320 nm), and (c) short-wave UVC (200–290 nm). The UV spectrum which reaches on the surface of the Earth consists of approximately 5% UVB and 95% UVA. Most of the UVC fraction of solar UV spectrum is blocked by the Earth’s stratospheric ozone layer. Solar UV radiation constitutes approximately 5% of the electromagnetic spectrum that reaches the Earth’s surface. UVA comprises the largest spectrum of solar UV radiation (≈95%). UVA radiation can penetrate deeper into the epidermis and dermis of the skin. Extensive exposure of the skin to UVA can lead to benign tumor formation [12, 13]. Exposure of the skin to UVA induces the generation of singlet oxygen and hydroxyl free radicals, which can cause damage to cellular macromolecules, like proteins, lipids and DNA [14]. UVA-induced oxidative stress can enhance the process of photoaging in the form of skin sagging rather than wrinkling [15] and also can suppress some immunological functions [16]. UVB radiations are mutagenic and carcinogenic in nature and responsible for a variety of skin diseases. UVB radiation can penetrate inside epidermis of the skin and can induce oxidative stress, immunosuppression, DNA damage, premature aging of the skin and skin cancers including the melanoma and nonmelanoma [6, 7]. UVC radiation have enormous energy and mutagenic in nature, and therefore comparatively more harmful than UVA or UVB radiation.
Multistage model of UV-carcinogenesis
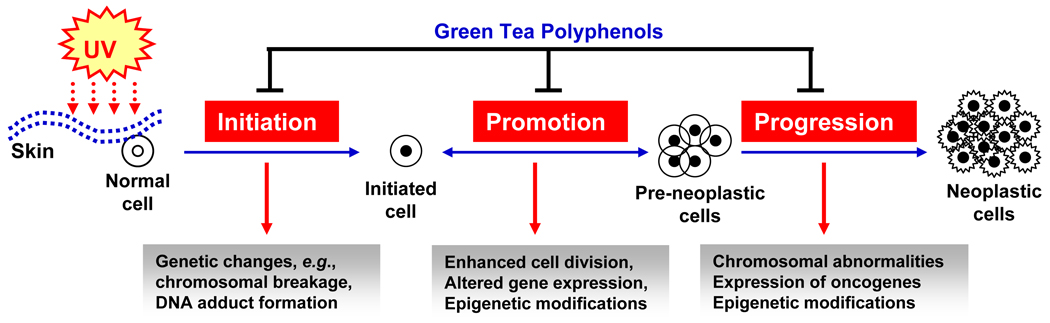
UV radiation, particularly UVB, can act as a tumor initiator [17], tumor promoter [18] and co-carcinogen [19, 20]. The process of UV-carcinogenesis can be divided into three distinct stages: initiation, promotion and progression (Figure 2). The tumor initiation stage is an irreversible process associated with the genotoxic damage of cellular DNA upon exposure to endogenous or exogenous carcinogens, such as UV radiation. In contrast, tumor promotion is a long-lasting reversible process characterized by clonal expansion of initiated cells to form a benign tumor with rapidly proliferating potential. This stage requires repeated multiple exposures of the UV radiation or any other tumor promoting agent, to give rise to a benign tumor. Tumor progression involves the transformation of the benign tumor into an invasive and malignant tumor. Both UVA and UVB irradiation can act as a complete carcinogen. UVA is a relatively weak complete carcinogen than UVB because of its weak activity as an initiating agent.
Figure 2.
Schematic representation of UV radiation-induced multi-stage skin carcinogenesis, showing initiation, promotion and progression stages. In the initiation stage genetic changes occurs in the cells. At the promotion stage, additional UV irradiation leads to the clonal expansion of initiated cells. Tumor progression stage involves the transformation of the benign tumor into an invasive and potentially malignant tumor. Green tea polyphenols can reverse, inhibit or slow down the process of skin carcinogenesis at one or at all the stages of carcinogenesis.
Skin cancer is mainly divided into two major types of cancers, melanoma and nonmelanoma. Melanoma develops from melanocytes, a kind of skin cells, which contain a pigment called melanin. Depending on the type of cells, nonmelanoma skin cancers are further classified as, squamous cell and basal cell carcinomas. Nonmelanoma skin cancers rarely metastasize while melanoma cancer cells can metastasize to other parts of the body, and may lead to death. Most of the mortality occurs due to melanoma because of its ability to metastasize to other organs. The incidence of melanoma has increased dramatically in the past few decades in the United States [21, 22], and is increasing rapidly in children [23]. Nonmelanoma skin cancers can metastasize but much less often than melanoma, therefore mortality is rare. Protection from melanoma and nonmelanoma skin cancers requires protection from the exposure to solar UV radiation.
Phytochemicals and skin cancer
Since ancient time, natural products, herbs and spices have been used for preventing several diseases, including cancer. Since early in the history of medicine, an association between diet and disease has persisted. Almost 25 centuries ago, Hippocrates, the father of modern medicine, proclaimed “Let food be thy medicine and medicine be thy food”. Approximately 35 years ago, the National Cancer Institute initiated a Diet and Cancer Program to provide researchers with the resources needed to better elucidate the role of dietary phytochemicals in cancer prevention. The phytochemicals possessing anti-inflammatory, immunomodulatory and anti-oxidant properties are among the most promising group of natural products that can be exploited as ideal chemopreventive agents for a variety of skin disorders in general. Recent advances in our understanding at the cellular and molecular levels of carcinogenesis have led to the development of promising strategies for the chemoprevention of cancers. Chemoprevention is a means of cancer control by the use of specific natural or synthetic chemical substances which can suppress, retard or reverse the process of carcinogenesis, and thus offers a realistic strategy for controlling the risk of skin diseases. Studies have shown the chemopreventive potential of several phytochemicals, such as green tea polyphenols, silymarin, retinoids, and grape seed proanthocyanidins, etc. against UV radiation-induced adverse effects, which include inflammation, oxidative stress, DNA damage and photocarcinogenesis [4, 5, 24, 25]. Here, I will summarize and discuss the recent developments in the area of anti-photocarcinogenic potential of green tea polyphenols with emphasis on prevention of UV-carcinogenesis through rapid repair of damaged DNA.
Green tea inhibits non-melanoma skin cancer
Following standard photocarcinogenesis protocols using SKH-1 hairless mice, it has been found that oral administration of GTPs in drinking water of mice resulted in significant protection against the development of non-melanoma skin cancer in terms of tumor incidence (percentage of mice with tumors), tumor multiplicity and tumor size compared to non-GTPs-treated UVB-irradiated mice [26]. A water extract of green tea leaves, which primarily contained a mixture of polyphenolic ingredients, when given as the sole source of drinking water to mice afforded protection against UVB radiation-induced skin tumorigenesis [27], and also promoted partial regression of established skin papillomas in mice [28]. Long-term oral administration or topical application of GTPs or EGCG did not show signs of visible toxicity. Our laboratory developed a hydrophilic ointment based formulation for the topical treatment of green tea polyphenols [29]. Treatment of SKH-1 hairless mice with the topical formulation of EGCG resulted in exceptionally high protection against photocarcinogenesis in terms of tumor incidence, tumor multiplicity and tumor size [29]. These results indicated that the use of EGCG with topical formulation might increase the penetration or absorption capacity of EGCG inside the skin layers and the presence of higher concentration may be responsible for the higher photoprotection. The photoprotective effects of GTPs or EGCG by topical treatment were greater than oral administration which may be due to presence of higher content of these polyphenols in topical application [30]. Record and Dreosti [31] have shown that green tea can protect against both UVA and UVB radiation-induced skin cancer in mice. Experimental studies conducted in in vitro and in vivo models indicate that GTPs or EGCG prevent photocarcinogenesis following several mechanisms which involve multiple molecular targets [5, 6].
Prevention of non-melanoma skin cancer by GTPs/EGCG requires interleukin (IL)-12
UVB-induced immunosuppression has been implicated in non-melanoma skin cancer caused by UV irradiation, and topical treatment of EGCG inhibits UVB-induced immunosuppression in mice through the induction of IL-12, an immunoregulatory cytokine. It was hypothesized that prevention of non-melanoma skin cancer by EGCG may be associated with the induction of IL-12 in mice. Topical application of EGCG resulted in a significant reduction in UVB-induced skin tumor development compared to non-EGCG treated wild-type mice. However, the treatment of IL-12 KO mice with EGCG did not inhibit tumor development in these mice [32]. The analysis of tumor data indicates that mice deficient in IL-12 are at greater risk of UVB-induced carcinogenesis, and suggest that the chemopreventive effect of EGCG against skin cancer is mediated through the induction of IL-12. Identical information were also observed when IL-12 KO and their wild-type counterparts were given GTPs in drinking water of mice and subjected to UV-induced non-melanoma skin cancer protocol [33], suggesting that IL-12 is required for the anti-non-melanoma skin cancer effect of EGCG or GTPs.
GTPs inhibit UVB-induced immunosuppression
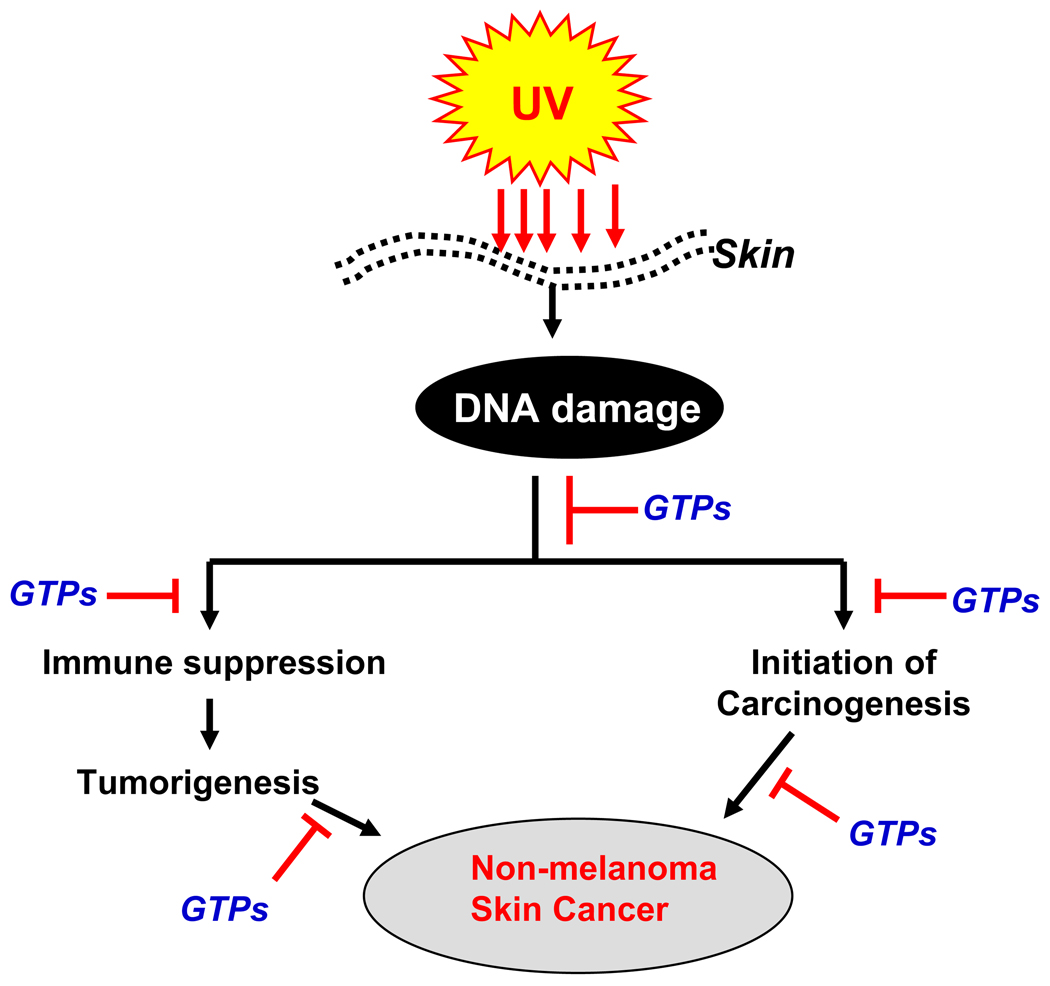
The immunosuppressive effects of solar UVB radiation are well established, most clearly by the effects of UVB radiation in inhibiting contact hypersensitivity (CHS) response, which is a prototypic T-cell-mediated immune response [34]. Some of the adverse effects of solar UV radiation on human health, including exacerbation of infectious diseases and initiation of skin cancer, are mediated at least in part by the ability of UV radiation to induce immunosuppression [35–37]. Ample clinical and experimental evidence suggests that UV-induced immunosuppression is a risk factor for skin cancer in mice and probably in humans as well [38, 39]. Chronically immunosuppressed patients living in regions of intense sun exposure experience an exceptionally high rate of skin cancer [40]. Therefore, the inhibition of UV-induced immunosuppression can be considered as a potential strategy for managing skin cancer (summarized in Figure 3). In that context the effect of GTPs was examined on UVB-induced immunosuppression in C3H/HeN mice. Topical application of the mouse skin with GTPs before UVB exposure resulted in significant protection against local and systemic suppression of CHS response [41]. Among the four major epicatechin derivatives present in GTPs, EGCG was found to be the most effective polyphenol affording protection against UVB-induced suppression of CHS. In this study, topical treatment of GTPs or its individual epicatechin derivatives also resulted in inhibition of UV-induced inflammation, which suggests an association between the induction of inflammation and suppression of CHS after UV irradiation of skin [41].
Figure 3.
Schematic diagram depicting the chemopreventive mechanism of UV radiation-induced non-melanoma skin cancer by green tea polyphenols (GTPs). GTPs inhibit UVB-induced immunosuppression and initiation of skin cancer through rapid repair of UVB-induced DNA damage in the skin.
UV-induced DNA damage: A molecular trigger for immunosuppression and non-melanoma skin cancer/photocarcinogenesis
Exposure of the skin to UV radiation resulted in DNA damage or DNA photoproducts which initiates an important cascade of signaling pathways. The DNA photoproducts are altered DNA structures that activate a cascade of responses, beginning with the initiation of cell cycle arrest and activation of DNA repair mechanisms. The biologically harmful effects associated with UV irradiation are largely the result of errors in DNA repair, which can lead to oncogenic mutations [reviewed in 42]. UV-induced DNA damage in the form of thymine dimers or cyclobutane pyrimidine dimers (CPD) is a molecular trigger for the induction of immunosuppression and initiation of photocarcinogenesis [43, 44]. Studies have revealed that exposure of the skin to UV radiation results in the formation of CPDs in skin cells [45]. The formation of CPDs occurs immediately after the interaction of photons with the DNA molecule.
GTPs rapidly repair UVB-induced DNA damage in skin cells
Topical treatment of mouse skin with GTPs significantly inhibited UVB-induced DNA damage as assessed using a 32P-postlabelling technique [46]. Similarly, topical treatment of human skin with GTPs prior to UV exposure resulted in a dose-dependent repair of CPDs [47]. Camouse et al. [48] found that topical application of green tea or white tea extracts provided human skin protection from solar-simulated ultraviolet light. These tea extracts were shown to provide protection against the detrimental effects of UV light on cutaneous immunity. The investigators also concluded that these protective effects were not due to direct UV absorption or sunscreen effects. Studies on the effects of green tea polyphenols on the DNA repair kinetics and repair mechanisms of UV-induced CPDs have been carried out using in vitro cell culture and in vivo animal models as well as in human skin. Studies showed that topical treatment of skin with EGCG does not prevent UVB-induced CPDs formation immediately after UVB irradiation, which indicated that EGCG does not have a significant sunscreen effect. However; in skin samples obtained at 24 h or 48 h after UVB exposure, the numbers of CPD-positive cells were significantly reduced (or repaired) in the EGCG-treated mouse skin compared to the control group of mice which were not treated with EGCG [49]. Studies of the DNA repair mechanisms suggested that the rapid repair of UV-induced CPDs by EGCG was mediated through stimulation of IL-12 on application of the EGCG onto the mouse skin [49]. IL-12 is a 70-kDa heterodimeric protein composed of two disulfide-bonded chains, the p40 and p35 subunits [50, 51]. IL-12 has been shown to possess potent antitumor activity in a wide variety of murine tumor models [52–54], and has the capacity to induce DNA repair [55–57] and this concept was verified by testing the effect of EGCG on UV-induced CPD formation in IL-12 knockout mice. EGCG does not remove or repair UV-induced CPDs in the skin of IL-12 knockout mice but repaired in the skin of their wild-type counterparts, further confirming the role of IL-12 in rapid repair of DNA damage by this polyphenol [49]. Studies on the effects of oral administration of GTPs in the drinking water of mice on UVB-induced DNA damage also were carried out and it was found that UV-induced DNA damage in the form of CPDs was resolved rapidly in the GTPs-treated mice compared to non-GTPs-treated mice [58]. Schwarz et al. [59] observed that treatment of normal human keratinocytes and “human skin equivalent” with GTPs reduced UVB-induced DNA damage in the form of CPDs and that this effect was mediated through the stimulation of IL-12 production. These investigations suggest that the difference in the GTPs-associated DNA repair capacity between IL-12 knockout mice and their wild-type counterparts may be due to the absence of IL-12 in the IL-12 knockout mice. Wei et al. [60] have shown that water extract of green tea scavenges H2O2 and inhibits UV-induced oxidative DNA damage in an in vitro system. Zhao et al. [61] demonstrated that application of green tea extract to Epiderm, a reconstituted human skin equivalent, also inhibited psoralen-UVA-induced formation of 8-methoxypsoralen-DNA adducts. Clifford et al. [62] have shown that EGCG or drinking green tea protects human cellular DNA from UV and visible radiation-induced DNA damage, and also protect DNA damage in human peripheral blood cells after tea ingestion. These observations demonstrate the potential chemopreventive effects of green tea polyphenols against UVB-induced DNA damage.
It also has been recognized that UV-induced DNA damage is an important molecular trigger for the migration of antigen presenting cells (i.e., epidermal Langerhans cells) from the skin to the draining lymph nodes. DNA damage in antigen presenting cells impairs their capacity to present Ag, which in turn results in a lack of sensitization [53]. CPD-containing antigen presenting cells have been found in the draining lymph nodes of UV-exposed mice [54]. These antigen presenting cells were identified to be of epidermal origin and exhibited an impaired Ag presentation capacity. Since the treatment of EGCG induces IL-12 in mice [63], and IL-12 has the capacity to induce DNA repair [51, 56, 57], the effect of EGCG on the migration of CPD-positive cells from the UV-exposed skin to the draining lymph nodes was studied [64]. Immunohistochemical analysis of CPD-positive cells in lymph nodes after 36 h of UV irradiation revealed that the numbers of CPD-positive cells in the lymph nodes of the UV-exposed IL-12 KO mice being approximately 4-fold higher than in the lymph nodes of their wild-type counterparts. The lower numbers of CPD-positive cells in the lymph nodes of UV-exposed wild-type mice compared to IL-12 KO mice may be attributable to the presence of endogenous IL-12 in the wild-type mice at levels that are capable of partial repair of the damaged DNA in the migrating cells. Treatment with EGCG resulted in a significant reduction in the numbers of CPD-positive cells in the lymph nodes of UV-exposed wild-type mice compared to UV-exposed wild-type mice that did not receive EGCG. In contrast, there was no significant difference in the number of CPD-positive cells in the lymph nodes between EGCG-treated and non-EGCG-treated UV-exposed IL-12 KO mice. This observation supports the evidence that the reduction in the numbers of CPD-positive cells in the lymph nodes of wild-type mice after EGCG treatment may be due to EGCG-induced IL-12-mediated repair of CPD-positive cells.
GTPs repair UVB-induced DNA damage through enhancement of nuclear excision repair (NER) genes
It has been shown that application of DNA repair enzymes that reduce the numbers of CPD-positive cells prevents UV-induced immunosuppression and incidence of skin cancer [44, 65]. It was found that administration of GTPs in drinking water of mice has the ability to prevent UVB-induced immunosuppression in xeroderma pigmentosum complementation group A-positive (XPA+/+) mice but not in XPA-deficient (XPA−/−) mice, which are devoid of NER genes and that is necessary for the repair of UV-induced DNA damage in mammalian cells. Therefore, further studies were conducted to examine whether the GTPs-mediated repair of UV-induced DNA damage requires NER gene. For this purpose, XPA+/+ and XPA−/− mice were exposed to UVB with and without the treatment of mice with GTPs in drinking water, and mice were sacrificed 72 h later. In skin samples obtained from XPA−/− mice, no significant difference in the staining pattern of CPDs was observed whether or not they were treated with GTPs. In contrast, in UVB-exposed skin samples obtained from XPA+/+ mice, the numbers of CPD+ cells were significantly lower in the GTPs-treated mice than those mice which were not treated with GTPs [66]. Further studies were conducted to identify the DNA repair mechanism by GTPs in UVB-exposed cells. To determine whether the NER mechanism is required for the EGCG-enhanced repair of UVB-induced DNA damage, NER-deficient fibroblasts from a person suffering from xeroderma pigmentosum complementation group A (XPA) and NER-proficient fibroblasts from a healthy person (XPA-proficient) were exposed to UVB with or without prior treatment with EGCG. The data analysis of CPD-positive cells revealed that the numbers of CPD-positive cells were significantly lower in EGCG-treated group at 24 h after UVB exposure in the XPA-proficient cells compared to non-EGCG-treated cells. However, EGCG did not significantly remove or repair UVB-induced CPDs in NER-deficient cells [66]. This in vitro observation indicated that EGCG-induced DNA repair is mediated through a functional NER mechanism.
GTPs prevent UVB-induced immunosuppression in NER-proficient mice but not in NER-deficient mice
As the enhanced repair of UVB-induced DNA damage in the form of CPDs by GTPs may be associated with the inhibition of UVB-induced immunosuppression in mice, it was determined whether GTPs prevent UVB-induced immunosuppression in XPA-deficient (XPA−/−) mice which do not have the ability to repair UVB-induced DNA damage because of absence of functional NER genes [66]. For this purpose, XPA−/− and their wild-type counterparts (XPA+/+) were subjected to CHS protocol or experiment with and without the treatment of GTPs in drinking water of mice. Following the CHS protocol, it was observed that in the absence of treatment with GTPs, the CHS response was significantly lower in XPA−/− mice that were UVB-irradiated than those XPA−/− mice that were not UVB-irradiated, indicating the immunosuppressive effect of UVB radiation in XPA−/− mice. The group of mice that were treated with GTPs in drinking water also exhibited a significant UVB-induced suppression of CHS response which was similar to non-GTPs-treated UVB-exposed mice. It suggests that administration of GTPs did not prevent UVB-induced suppression of CHS response to 2,4-dinitrofluorobenzene, a contact sensitizer, in XPA−/− mice. In contrast, the administration of GTPs to the wild-type counterparts significantly induces contact sensitization reaction and ear swelling response to 2,4-dinitrofluorobenzene and was significantly higher than those mice which were not given GTPs in drinking water and exposed to UVB radiation [66]. The change in ear skin thickness in XPA−/− mice in response to 2,4-dinitrofluorobenzene sensitization in GTPs +UVB was also compared to the change in ear skin thickness in XPA+/+ mice in response to GTPs +UVB. The increase in ear skin thickness after sensitization to 2,4-dinitrofluorobenzene was greater in the XPA+/+ mice treated with GTPs +UVB as compared to increase in ear skin thickness after sensitization to contact sensitizer in XPA−/− mice treated with GTPs +UVB. These observations suggest that prevention of UVB-induced immunosuppression by GTPs requires NER genes, which have a role in repair of UVB-induced DNA damage.
Repair of UVB-induced DNA damage by GTPs leads to suppression of inflammation
UVB irradiation resulted in inflammation in the skin, and there is increasing evidence that chronic inflammation promotes the development of skin cancers [Reviewed in 9, 14]. Both UV-induced inflammatory responses and UV-induced skin tumor development are causally related to UV-induced DNA damage. Therefore, it was of interest to explore the effects of green tea polyphenols on DNA repair and their relationship with inflammatory responses in the skin. It is well known that CPDs are formed immediately after the exposure of the skin to UV radiation, and inflammation develops at later stages. Following UV exposure, UV-induced DNA damage in the form of CPDs was repaired or removed more rapidly in the skin of mice that had been treated either with topical application of EGCG or administration of GTPs in drinking water of mice. Subsequently, the levels of UVB-induced inflammation were lowered in the treated mice than the non-treated mice. The levels of inflammation in the mouse skin were assessed through analysis of biomarkers of inflammation, such as cyclooxygenase-2 (COX-2) expression, PGE2 production and the levels of pro-inflammatory cytokines. In contrast, this effect of EGCG or GTPs was not observed in IL-12-deficient mice [58, 64]. This may be due to the fact that the treatment of mice with EGCG or GTPs was not able to repair UV-induced DNA damage significantly in the absence of IL-12 [64, 66]. This information supports the concept that UV-induced CPDs and inflammatory responses are causally related with the increased risk of photocarcinogenesis. This in vivo experimental evidence indicates that the prevention of UVB-induced skin cancer by GTPs or EGCG is mediated through inhibition of UVB-induced inflammation, which in turn is mediated, at least in part, through rapid repair of damaged DNA. The outcome of the studies therefore suggests that regular consumption of green tea or green tea polyphenols may be considered as an effective strategy for the prevention of inflammation-associated skin diseases including UV irradiation-caused skin tumor development.
Inhibition of UVB-induced immunosuppression by GTPs is mediated through enhancement of NER genes
NER is the main mechanism of repair in mammalian cells for the removal of UV radiation-induced DNA damage. Since the treatment of GTPs enhances the removal or repair of UVB-induced DNA damage, it was of interest to examine whether the removal or repair of UV-induced CPDs by GTPs is mediated via induction of NER genes. Subsequent analysis of data reveals that treatment of mice with GTPs in drinking water of mice increases the levels of some NER genes (e.g., XPA, XPC and RPA1) in UVB-exposed skin sites compared to non-GTPs-fed mice and that may have contributed to the rapid repair of damaged DNA in mouse skin [66]. The role of NER was further confirmed by assessing the effect of GTPs on UVB-induced immunosuppression in XPA−/− mice and data were compared with the XPA+/+ mice. Treatment of mice with GTPs in drinking water prevents UVB-induced suppression of contact hypersensitivity response in XPA+/+ mice but do not prevent in XPA−/− mice further support the observations that inhibition of UVB-induced immunosuppression by GTPs require functional NER genes. This observation was important as the treatment of GTPs do not remove or repair UVB-induced DNA damage in XPA−/− (NER-deficient) mice but repair in XPA+/+ (NER-proficient or wild-type) mice which were exposed to UVB. These observations were further verified by using NER-deficient cells from XPA-patients and NER-proficient cells from healthy persons. Cells derived from patients suffering from xeroderma pigmentosum either lack or have reduced DNA repair capacity due to genetic mutations in several components of the NER. The XPA complementation type represents the most severe phenotype, because the XPA gene is the most crucial component in the repair process and, thus, cells lacking the XPA gene are completely deficient in NER [67, 68]. Following these experiments, GTPs were able to remove UV-induced CPDs in NER-proficient cells but was not able to remove or repair in NER-deficient human fibroblast cells. These observations indicate that repair of UV-induced DNA damage by GTPs is mediated through the NER mechanism or GTPs-induced DNA repair requires functional NER. These findings have important implications for the chemopreventive mechanism of skin cancer protection by GTPs, and identify a new mechanism by which GTPs prevent UV-induced immunosuppression.
Together, the studies conducted with green tea polyphenols indicate that the prevention of UV radiation-induced immunosuppression and subsequently the prevention of non-melanoma skin cancer by GTPs either through topical application or in drinking water of mice are mediated through rapid repair of UVB-induced DNA damage, as summarized in Figure 3. As UV-induced DNA damage and immunosuppression play an important role in nonmelanoma skin cancer, it is tempting to suggest that drinking green tea should be further investigated as a chemopreventive agent for the prevention of skin cancers in humans, and its possible use in future practice of medicine.
Bioavailability and metabolism of green tea polyphenols
The bioavailability and metabolism of phytochemicals may influence their effectiveness. Small molecules, like catechin monomers in green tea, can be easily absorbed through the gut barrier, whereas the large molecular weight polyphenols, such as (-)-epigallocatechin-3-gallate or polyphenols from black tea, are poorly absorbed. Once absorbed, polyphenols are conjugated to glucuronide, sulfate and methyl groups in the gut mucosa and inner tissues. Non-conjugated polyphenols are virtually not found in plasma. As, the upper layer of the skin is stratum corneum, which is hydrophobic in nature, some vehicle are required for effective penetration of the molecules inside the skin during topical application or treatment. Therefore, successful delivery of plant polyphenols requires cream-based, organic solvent-based or lipid soluble topical formulations that can enhance the penetration of the polyphenols, and that will result in more efficient skin photoprotection. Mittal et al [29] used hydrophilic cream-based topical formulation of EGCG for the protection of UVB-induced skin carcinogenesis in mice. This hydrophilic cream-based formulation provides moisturizing environment for a longer period of time to EGCG for its penetration inside the skin in addition to other ingredients which enhance the penetration ability of phytochemicals into the skin layers.
Translation of animal studies to human system
The photoprotective effects of polyphenols on UVB-induced DNA damage contribute to the prevention of non-melanoma skin cancer and act to abrogate the various biochemical processes induced or mediated by solar UV radiation. Based on the epidemiological evidences and in vitro and in vivo laboratory studies, it is suggested that routine consumption or topical treatment of skin with green tea polyphenols may provide efficient protection against the harmful effects of solar ultraviolet radiation in humans. Based on the information obtained in animal models, it can be suggested that the regular consumption of 5–6 cups of green tea (1 g green tea leaves/150 ml water =1 cup) per day by humans may provide the same level of photoprotective effect in human system as was observed in animal models. However, the magnitude of photoprotective effect in terms of UVB-induced immunosuppression and DNA repair by green tea may differ person to person based on the differences in race, and intensity, exposure time and frequency of UV irradiation. For appropriate conversion of chemopreventive agent doses from animal studies to human system, the body surface area normalization method has been prescribed [69]. The human equivalent dose of any chemopreventive agent can be calculated using the following formula:
Further, the use of polyphenols in combination with sunscreens or skin care lotions may provide an effective strategy for mitigating the effects of UV radiation that will lead to the prevention of the skin from different skin diseases caused by excessive sun exposures including the risk of non-melanoma skin cancer in humans.
Acknowledgments
The work reported from Dr. Katiyar’s laboratory was supported by the funds from National Institutes of Health (CA104428, CA140832, AT002536) and Veteran Affairs Merit Review Award. The content of this article does not necessarily reflect the views or policies of the funding agencies. Grateful thanks to all of the past and present members of my research group for their outstanding contributions.
Abbreviations
- CHS
contact hypersensitivity
- CPD
cyclobutane pyrimidine dimer
- IL
interleukin
- GTPs
green tea polyphenols
- EGCG
(-)-epigallocatechin-3-gallate
- NER
nucleotide excision repair
- XPA
xeroderma pigmentosum complementation group A
- UV
ultraviolet
References
- 1.Housman TS, Feldman SR, Williford PM, Fleischer AB, Jr, Goldman ND, Acostamadiedo JM, Chen GJ. J. Am. Acad. Dermatol. 2003;48:425–429. doi: 10.1067/mjd.2003.186. [DOI] [PubMed] [Google Scholar]
- 2.Nichols JA, Katiyar SK. Arch. Dermatol. Res. 2010;302:71–83. doi: 10.1007/s00403-009-1001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hara Y. Green Tea, Health Benefits and Applications. New York: Marcel Dekker; 2001. [Google Scholar]
- 4.Katiyar SK, Elmets CA. Int. J. Oncol. 2001;18:1307–1313. doi: 10.3892/ijo.18.6.1307. [DOI] [PubMed] [Google Scholar]
- 5.Baliga MS, Katiyar SK. Photochem. Photobiol. Sci. 2006;5:243–253. doi: 10.1039/b505311k. [DOI] [PubMed] [Google Scholar]
- 6.Katiyar S, Elmets CA, Katiyar SK. J. Nutr. Biochem. 2007;18:287–296. doi: 10.1016/j.jnutbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 7.deGruijl FR, van der Leun JC. Health Phys. 1994;67:319–359. doi: 10.1097/00004032-199410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Ichihashi M, Ueda M, Budiyanto A, Bito T, Oka M, Fukunaga M, Tsuru K, Horikawa T. Toxicology. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 9.Mukhtar H, Elmets CA. Photochem. Photobiol. 1996;63:355–447. doi: 10.1111/j.1751-1097.1996.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 10.Godar DE, Wengraitis SP, Shreffler J, Sliney DH. Photochem. Photobiol. 2001;73:621–629. doi: 10.1562/0031-8655(2001)073<0621:udoa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Godar DE. Photochem. Photobiol. 2001;74:787–793. doi: 10.1562/0031-8655(2001)074<0787:UDOACA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 12.Bachelor MA, Bowden GT. Semin. Cancer Biol. 2004;14:131–138. doi: 10.1016/j.semcancer.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. J. Am. Acad. Dermatol. 2001;44:837–846. doi: 10.1067/mjd.2001.114594. [DOI] [PubMed] [Google Scholar]
- 14.DiGiovanni J. Pharmacol. Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 15.Krutmann J. Eur. J. Dermatol. 2001;11:170–171. [PubMed] [Google Scholar]
- 16.Ullrich SE. Hum. Exp. Toxicol. 1995;14:89–91. doi: 10.1177/096032719501400118. [DOI] [PubMed] [Google Scholar]
- 17.Kligman LH, Akin FJ, Kligman AM. J. Am. Acad. Dermatol. 1980;3:30–35. doi: 10.1016/s0190-9622(80)80221-0. [DOI] [PubMed] [Google Scholar]
- 18.Katiyar SK, Korman NJ, Mukhtar H, Agarwal R. J. Natl. Cancer Inst. 1997;89:556–566. doi: 10.1093/jnci/89.8.556. [DOI] [PubMed] [Google Scholar]
- 19.Donawho CK, Kripke ML. Cancer Res. 1991;51:4176–4181. [PubMed] [Google Scholar]
- 20.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 21.American Cancer Society. Cancer facts and figures. 2007 http://www.cancer.org/.
- 22.Maddodi N, Setaluri V. Photochem. Photobiol. 2008;84:528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 23.Strouse JJ, Fears TR, Tucker MA, Wayne AS. J. Clin. Oncol. 2005;23:4735–4741. doi: 10.1200/JCO.2005.02.899. [DOI] [PubMed] [Google Scholar]
- 24.Surh Y-J. Mutat. Res. 1999;428:305–327. doi: 10.1016/s1383-5742(99)00057-5. [DOI] [PubMed] [Google Scholar]
- 25.Pinnell SR. J. Am. Acad. Dermatol. 2003;48:1–19. doi: 10.1067/mjd.2003.16. [DOI] [PubMed] [Google Scholar]
- 26.Mantena SK, Meeran SM, Elmets CA, Katiyar SK. J. Nutr. 2005;135:2871–2877. doi: 10.1093/jn/135.12.2871. [DOI] [PubMed] [Google Scholar]
- 27.Wang ZY, Huang MT, Ferraro T, Wong CQ, Lou YR, Reuhl K, Iatropoulos M, Yang CS, Conney AH. Cancer Res. 1992;52:1162–1170. [PubMed] [Google Scholar]
- 28.Wang ZY, Huang MT, Ho CT, Chang R, Ma W, Ferraro T, Reuhl KR, Yang CS, Conney AH. Cancer Res. 1992;52:6657–6665. [PubMed] [Google Scholar]
- 29.Mittal A, Piyathilake C, Hara Y, Katiyar SK. Neoplasia. 2003;5:555–565. doi: 10.1016/s1476-5586(03)80039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vayalil PK, Elmets CA, Katiyar SK. Carcinogenesis. 2003;24:927–936. doi: 10.1093/carcin/bgg025. [DOI] [PubMed] [Google Scholar]
- 31.Record IR, Dreosti IE. Nutr. Cancer. 1998;32:71–75. doi: 10.1080/01635589809514721. [DOI] [PubMed] [Google Scholar]
- 32.Meeran SM, Mantena SK, Elmets CA, Katiyar SK. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 33.Meeran SM, Akhtar S, Katiyar SK. J. Invest. Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Gruijl FR, Sterenborg HJ, Forbes PD, Davies RE, Cole C, Kelfkens G, van Weelden H, Slaper H, van der Leun JC. Cancer Res. 1993;53:53–60. [PubMed] [Google Scholar]
- 35.Toews GB, Bergstresser PR, Streilein JW, Sullivan S. J. Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 36.Cooper KD, Oberhelman L, Hamilton TA, Baadsgaard O, Terhune M, LeVee G, Anderson T, Koren H. Proc. Natl. Acad. Sci. U S A. 1992;89:8497–8501. doi: 10.1073/pnas.89.18.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman RS, Cooper KD, De Fabo EC, Frederick JE, Gelatt KN, Hammond SP, Hersey P, Koren HS, Ley RD, Noonan F. Photochem. Photobiol. 1995;61:223–247. doi: 10.1111/j.1751-1097.1995.tb03966.x. [DOI] [PubMed] [Google Scholar]
- 38.Yoshikawa T, Rae V, Bruins-Slot W, vand-den-Berg JW, Taylor JR, Streilein JW. J. Invest. Dermatol. 1990;95:530–536. doi: 10.1111/1523-1747.ep12504877. [DOI] [PubMed] [Google Scholar]
- 39.Meunier L, Raison-Peyron N, Meynadier J. Rev. Med. Int. 1998;19:247–254. doi: 10.1016/S0248-8663(97)89326-5. [DOI] [PubMed] [Google Scholar]
- 40.Katiyar SK. Cancer Letts. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katiyar SK, Elmets CA, Agarwal R, Mukhtar H. Photochem. Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- 42.Timares L, Katiyar SK, Elmets CA. Photochem. Photobiol. 2008;84:422–436. doi: 10.1111/j.1751-1097.2007.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kripke ML, Cox PA, Alas LG, Yarosh DB. Proc. Natl. Acad. Sci. USA. 1992;89:7516–7520. doi: 10.1073/pnas.89.16.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yarosh D, Alas LG, Yee V, Oberyszyn A, Kibitel JT, Mitchell D, Rosenstein R, Spinowitz A, Citron M. Cancer Res. 1992;52:4227–4231. [PubMed] [Google Scholar]
- 45.Katiyar SK, Matsui MS, Mukhtar H. Photochem. Photobiol. 2000;72:788–793. doi: 10.1562/0031-8655(2000)072<0788:koulic>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 46.Chatterjee ML, Agarwal R, Mukhtar H. Biochem. Biophys. Res. Comm. 1996;229:590–595. doi: 10.1006/bbrc.1996.1848. [DOI] [PubMed] [Google Scholar]
- 47.Katiyar SK, Perez A, Mukhtar H. Clinical Cancer Res. 2000;6:3864–3869. [PubMed] [Google Scholar]
- 48.Camouse MM, Domingo DS, Swain FR, Conrad EP, Matsui MS, Maes D, Declercq L, Cooper KD, Stevens SR, Baron ED. Exp. Dermatol. 2009;18:522–526. doi: 10.1111/j.1600-0625.2008.00818.x. [DOI] [PubMed] [Google Scholar]
- 49.Meeran SM, Mantena SK, Elmets CA, Katiyar SK. Cancer Res. 2006;66:5512–5520. doi: 10.1158/0008-5472.CAN-06-0218. [DOI] [PubMed] [Google Scholar]
- 50.Wolf SF, Temple PA, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick RM. J. Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 51.Katiyar SK. Toxicol. Appl. Pharmacol. 2007;224:220–227. doi: 10.1016/j.taap.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colombo MP, Vagliani M, Spreafico F, Parenza M, Chiodoni C, Melani C, Stoppacciaro A. Cancer Res. 1996;56:2531–2534. [PubMed] [Google Scholar]
- 53.Brunda MJ, Luistro L, Warrier RR, Wright RB, Hubbard BR, Murphy M, Wolf SF, Gately MK. J. Exp. Med. 1993;178:1223–1230. doi: 10.1084/jem.178.4.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robertson MJ, Ritz J. Oncologist. 1996;1:88–97. [PubMed] [Google Scholar]
- 55.Meeran SM, Mantena SK, Meleth S, Katiyar SK. Mol. Cancer Ther. 2006;5:825–832. doi: 10.1158/1535-7163.MCT-06-0003. [DOI] [PubMed] [Google Scholar]
- 56.Schwarz A, Maeda A, Kernebeck K, van Steeg H, Beissert S, Schwarz T. J. Exp. Med. 2005;201:173–179. doi: 10.1084/jem.20041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz A, Stander S, Berneburg M, Böhm M, Kulms D, van Steeg H, Grosse-Heitmeyer K, Krutmann J, Schwarz T. Nat. Cell Biol. 2002;4:26–31. doi: 10.1038/ncb717. [DOI] [PubMed] [Google Scholar]
- 58.Meeran SM, Akhtar S, Katiyar SK. J. Invest. Dermatol. 2009;129:1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwarz A, Maeda A, Gan D, Mammone T, Matsui MS, Schwarz T. Photochem. Photobiol. 2008;84:350–355. doi: 10.1111/j.1751-1097.2007.00265.x. [DOI] [PubMed] [Google Scholar]
- 60.Wei H, Ca Q, Rahn R, Zhang X, Wang Y, Lebwohl M. Biochemistry. 1998;37:6485–6490. doi: 10.1021/bi972702f. [DOI] [PubMed] [Google Scholar]
- 61.Zhao JF, Zhang YJ, Jin XH, Athar M, Santella RM, Bickers DR, Wang ZY. J. Invest. Dermatol. 1999;113:1070–1075. doi: 10.1046/j.1523-1747.1999.00806.x. [DOI] [PubMed] [Google Scholar]
- 62.Morley N, Clifford T, Salter L, Campbell S, Gould D, Curnow A. Photodermatol. Photoimmunol. Photomed. 2005;21:15–22. doi: 10.1111/j.1600-0781.2005.00119.x. [DOI] [PubMed] [Google Scholar]
- 63.Katiyar SK, Challa A, McCormick TS, Cooper KD, Mukhtar H. Carcinogenesis. 1999;20:2117–2124. doi: 10.1093/carcin/20.11.2117. [DOI] [PubMed] [Google Scholar]
- 64.Meeran SM, Mantena SK, Katiyar SK. Clinical Cancer Res. 2006;12:2272–2280. doi: 10.1158/1078-0432.CCR-05-2672. [DOI] [PubMed] [Google Scholar]
- 65.Yarosh D, Klein J, O'Connor A, Hawk J, Rafal E, Wolf P. Lancet. 2001;357:926–929. doi: 10.1016/s0140-6736(00)04214-8. [DOI] [PubMed] [Google Scholar]
- 66.Katiyar SK, Vaid M, van Steeg H, Meeran SM. Cancer Prev. Res. 2010;3:179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carreau M, Eveno E, Quilliet X, Chevalier-Lagente O, Benoit A, Tanganelli B, Stefanini M, Vermeulen W, Hoeijmakers JH, Sarasin A. Carcinogenesis. 1995;16:1003–1009. doi: 10.1093/carcin/16.5.1003. [DOI] [PubMed] [Google Scholar]
- 68.Muotri AR, Marchetto MC, Zerbini LF, Libermann TA, Ventura AM, Sarasin A, Menck CF. Hum. Gene Ther. 2002;13:1833–1844. doi: 10.1089/104303402760372936. [DOI] [PubMed] [Google Scholar]
- 69.Reagan-Shaw S, Nihal M, Ahmad N. FASEB J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]