A pro-apoptotic function of activated PKCδ may be mediated by several downstream nuclear regulators involved in apoptotic cell death. Vaccinia-related kinase 1 (VRK1) is a new nuclear target of PKCδ in the regulation of apoptotic cell death induced by DNA damage.
Abstract
Vaccinia-related kinase 1 (VRK1) is a novel serine/threonine kinase that plays an important role in cell proliferation. However, little is known about the upstream regulators of VRK1 activity. Here we provide evidence for a role of protein kinase Cδ (PKCδ) in the regulation of murine VRK1. We show that PKCδ interacts with VRK1, phosphorylates the Ser-355 residue in the putative regulatory region, and negatively regulates its kinase activity in vitro. Intriguingly, PKCδ-induced cell death was facilitated by phosphorylation of VRK1 when cells were exposed to a DNA-damaging agent. In addition, p53 played a critical role in the regulation of DNA damage–induced cell death accompanied by PKCδ-mediated modulation of VRK1. In p53-deficient cells, PKCδ-mediated phosphorylation of VRK1 had no effect on cell viability. However, cells overexpressing p53 exhibited significant reduction of cell viability when cotransfected with both VRK1 and PKCδ. Taken together, these results indicate that PKCδ regulates phosphorylation and down-regulation of VRK1, thereby contributing to cell cycle arrest and apoptotic cell death in a p53-dependent manner.
INTRODUCTION
Vaccinia-related kinase 1 (VRK1), a novel family of mammalian serine/threonine protein kinases, was initially identified by its homology to the catalytic domain of the vaccinia virus B1R kinase, which is essential for viral DNA replication (Rempel et al., 1990; Nezu et al., 1997; Nichols and Traktman, 2004; Klerkx et al., 2009). VRK1 shows ubiquitous expression in embryonic and adult tissues. This gene is especially highly expressed in proliferating tissues, such as fetal liver, testis, and thymus and in several cancer cell lines (Nezu et al., 1997; Santos et al., 2006). A previous study demonstrated that VRK1 is a nuclear kinase that stabilizes the intracellular protein levels of p53 by a posttranscriptional modification and thus affects p53-dependent transcription (Vega et al., 2004). Moreover, VRK1 phosphorylates and associates with other transcription factors, such as ATF2 or c-Jun, resulting in an incremental increase in transcriptional activity (Sevilla et al., 2004a, 2004b). In addition, it has been shown that VRK1 may modulate the association of Barrier to Autointegration Factor (BAF) with nuclear components and thus play a role in maintaining appropriate nuclear architecture (Nichols et al., 2006; Gorjánácz et al., 2007). According to recent reports, VRK1 phosphorylates histone H3 on Thr-3 and Ser-10 required for mitotic chromatin condensation (Kang et al., 2007). Additionally, VRK1 mediates cyclin D1 expression through the phosphorylation of cAMP response element-binding protein (CREB) (Kang et al., 2008). Mouse VRK1 is composed of three differentially spliced isoforms: full-length, lacking exon 12 (mVRK1Δ), or lacking exons 12 and 13 (mVRK1ΔΔ) (Nichols and Traktman, 2004). The mVRK1ΔΔ isoform is identical to human VRK1 in length and alignment of carboxy termini of the sequences (Nichols and Traktman, 2004). However, little is known about the cellular functions and the upstream regulatory elements of these murine kinases.
Protein kinase C (PKC), a family of serine/threonine protein kinases, is activated by diverse stimuli and participates in cellular processes such as growth, differentiation, and apoptosis (Hug and Sarre, 1993; Griner and Kazanietz, 2007). PKC is composed of three subclasses consisting of the classical (α, βI, βII, and γ), novel (δ, ε, η, and θ), and atypical (ζ and ι/λ) PKC isoforms. In addition, two other members, PKCμ and PKCν, exhibit unique characteristics (Johannes et al., 1994). Activation of PKC is usually associated with membrane translocation, and prolonged cellular exposure to PKC activators can cause its degradation or down-regulation (Lu et al., 1998). PKC can also be activated by cleavage to generate an active catalytic fragment freed from inhibition by the regulatory domain (Emoto et al., 1995; Smith et al., 2000; Basu et al., 2002).
PKCδ has been shown to regulate the mitochondrial-dependent pathway of apoptosis, and a selective inhibitor of PKCδ or a dominant-negative mutant of PKCδ attenuates apoptotic cell death induced by several stimuli, including glutamate, H2O2, phorbol ester, UV, taxol, and etoposide (Konishi et al., 1997; Denning et al., 1998; Majumder et al., 2000; Matassa et al., 2001; Choi et al., 2006). In addition, smooth muscle cells derived from PKCδ knockout mice are defective in mitochondrial-dependent apoptosis, leading to a diminished cell death response to H2O2, UV, TNF-α, and IL-1β (Leitges et al., 2001). A previous study demonstrated that an apoptotic stimulus results in proteolytic activation of PKCδ, which is inhibited by treatment with caspase inhibitors and transfection with anti-apoptotic proteins, such as Bcl-2 or Bcl-xL (Emoto et al., 1995). This observation is in agreement with previous results demonstrating that overexpression of the catalytic fragment of PKCδ, but not full-length PKCδ or a kinase-inactive fragment, was sufficient to induce apoptotic cell death (Ghayur et al., 1996; Bharti et al., 1998). Thus, PKCδ activity seems to have an important role in the regulation of apoptosis in a cell type–specific and context-specific manner. However, the mechanism by which activation of PKCδ drives cell death remains largely unknown (Yoshida, 2007).
In this study, we show that PKCδ plays a role as an upstream regulator of VRK1 in the nucleus by physically interacting with VRK1, thereby inducing its phosphorylation and down-regulation of its kinase activity. We also demonstrate that PKCδ-mediated VRK1 phosphorylation and p53 act synergistically to arrest the cell cycle and trigger cell death when DNA damage occurs.
RESULTS
VRK1 associates with PKCδ in cells
To identify whether PKCδ is an upstream regulatory element of VRK1, we first examined the binding between PKCδ and VRK1. mVRK1 fused with Flag epitope tag at its N terminus (Flag-VRK1) was transiently transfected into CHO-K1 cells along with PKCδ. Whole-cell lysates were subjected to immunoprecipitation with anti–Flag antibody. As seen in Figure 1A, PKCδ was coimmunoprecipitated with VRK1. Proteolytic activation of PKCδ by caspases results in the generation of PKCδ catalytic fragment (PKCδ CF), which is constitutively active (Ghayur et al., 1996; Koriyama et al., 1999). To assess the interaction of VRK1 with PKCδ CF, cells were transfected with Flag-VRK1 and PKCδ CF, followed by immunoprecipitation with anti–Flag antibody. Interestingly, VRK1 was also coimmunoprecipitated with the PKCδ CF (Supplemental Figure 1). To investigate the endogenous interaction between PKCδ and VRK1, we performed coimmunoprecipitation assay before and after exposing the cells to DNA damage. We could detect weak endogenous interactions between PKCδ and VRK1 only under DNA-damaging conditions, suggesting that they could associate each other in the conditions of cell death (Figure 1B). These data also indicate that the endogenous interaction between PKCδ and VRK1 occurs transiently for enzymatic reaction.
FIGURE 1:
VRK1 interacts with PKCδ in the nucleus. (A) Whole-cell extracts from CHO-K1 transfected with either a combination of pFlag-VRK1 and and pcDNA3-PKCδ were immunoprecipitated with anti–Flag antibody, and the precipitates, as well as the input (40 μg), were immunoblotted with antibodies to PKCδ or Flag. (B) Whole-cell extracts from HT22 cells treated with dimethyl sulfoxide or etoposide (50 μM) for 24 h were immunoprecipitated with anti–PKCδ antibody, and the precipitates were immunoblotted with antibodies to PKCδ or VRK1. (C) CHO-K1 cells were transiently transfected with a plasmid encoding GFP-PKCδ FL, GFP-PKCδ CF, or RFP-VRK1. Cells were also transfected with empty vector as controls. Nuclei were stained with Hoechst, and the localization of PKCδ and VRK1 was observed by fluorescence microscopy. (D) Cells transiently transfected with a plasmid encoding HA-PKCδ FL, PKCδ CF, or Flag-VRK1 were subjected to subcellular fractionation. Cytosol (Cyto) and nuclear (Nu) fractions were analyzed by immunoblot with antibodies to PKCδ, Flag, or lamin B.
To determine whether VRK1 and PKCδ localize in the same subcellular compartment, we used a series of green fluorescent protein (GFP)-tagged full-length and catalytic fragments of PKCδ, and red fluorescent protein (RFP)-tagged VRK1. As shown in Figure 1C, the cytoplasmic localization of GFP-PKCδ was more prominent than the nuclear portion, whereas GFP-PKCδ CF was predominantly distributed in the nucleus and to a lesser extent in the cytoplasm. In contrast, RFP-VRK1 was mainly localized in the nucleus (Figure 1C). To confirm this result, we used subcellular fractionation of the cytoplasm and nucleus. As expected, Flag-VRK1 was identified in the nuclear fractions, whereas HA-PKCδ and PKCδ CF were recovered from both the cytoplasmic and nuclear fractions (Figure 1D). These results suggest that nuclear-localized PKCδ and PKCδ CF can associate with VRK1.
PKCδ phosphorylates VRK1 on Ser-355 in vitro
Because PKCδ CF can phosphorylate several proteins in the nucleus (Bharti et al., 1998; Ren et al., 2002), we examined the enzymatic activity of PKCδ in nuclear lysates from full-length PKCδ- and PKCδ CF-transfected cells using an in vitro kinase assay. As shown in Figure 2A, PKCδ CF became more activated than full-length PKCδ in the nucleus. In addition, because PKCδ CF was mainly localized in the nucleus, as was VRK1 (Figure 1), thereafter we only used PKCδ CF to test whether PKC is implicated in the posttranslational modification of VRK1. To determine whether VRK1 is a substrate for PKCδ, PKCδ immunoprecipitates were incubated with recombinant glutathione S-transferase (GST)-VRK1 fusion proteins. Preimmune serum (PIS) was used as a negative control. Because VRK1 has an autophosphorylating activity (Lopez-Borges and Lazo, 2000), phosphorylation of VRK1 was detected in an in vitro kinase assay using PIS immunoprecipitates (Figure 2B). On the other hand, phosphorylation of VRK1 was more dramatically increased by PKCδ CF immunoprecipitates than by PIS (Figure 2B), and its phosphorylation by PKCδ CF was confirmed using the VRK1 inactive mutant (K179E) (Figure 2C), indicating that VRK1 was phosphorylated by PKCδ in vitro.
FIGURE 2:
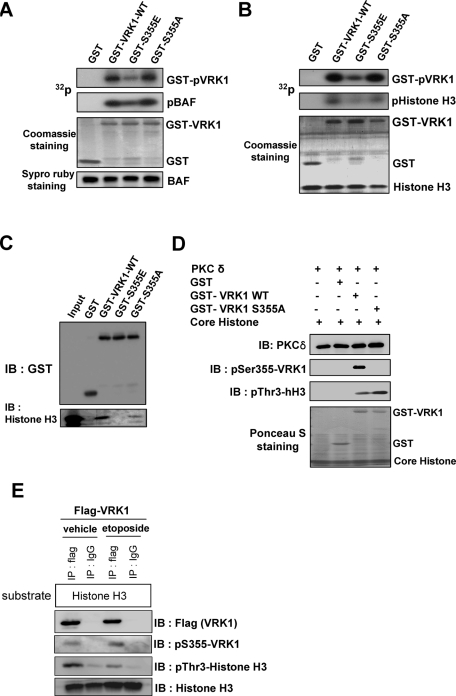
PKCδ phosphorylates VRK1. (A) CHO-K1 cells were transfected with a plasmid encoding HA-PKCδ FL or PKCδ CF. After 24 h, the nuclear fraction was subjected to immunoprecipitation with anti-PKCδ. In vitro immune complex kinase assays were performed using histone H1 as a substrate. (B, C) Whole-cell extracts from CHO-K1 transfected with PKCδ CF were immunoprecipitated with anti–PKCδ antibody followed by in vitro immune complex kinase assay using GST-VRK1 (B) or its inactive mutant GST-VRK1 K179E (C) as substrates. The left panel shows Coomassie blue staining, and the right panel shows the incorporation of radioactivity. (D) A schematic representation of deletion mutants generated in the VRK1-GST fusion constructs used in subsequent experiments. (E, F) Whole-cell extracts from CHO-K1 transfected with PKCδ CF were immunoprecipitated with anti–PKCδ antibody, followed by in vitro immune complex kinase assay using GST-VRK1 full-length, deletion mutants (VRK1-N130, -M131–280, and –ΔN280) (E) or single or double amino acid substituted mutants (S355A, T390A, and S355A/T390A) (F) as substrates. The lower panel shows Coomassie blue staining, and the upper panel shows the incorporation of radioactivity. (G) Recombinant PKCδ was incubated with GST, GST-VRK1 wild type, or GST-VRK1 S355A mutant. The samples were analyzed by immunoblotting with anti–PKCδ or anti–phospho-VRK1(Ser-355) antibody, respectively.
To confirm the phosphorylation of VRK1 by PKCδ, we further constructed GST-VRK1 fusion proteins carrying segments from the C or N terminus, or the internal segment of VRK1 (schematically depicted in Figure 2D). Because the N-terminal portion (N130) and the internal segment (M131–280) bear an ATP-binding domain and motifs for kinase activity, respectively, autophosphorylation of GST-VRK1 mutant proteins was not detected (Figure 2E). When these mutant proteins were incubated with PKCδ immunoprecipitates, only the GST-ΔN280 mutant was phosphorylated (Figure 2E), confirming that the C-terminal region of VRK1 is phosphorylated by PKCδ in vitro.
Additionally, bioinformatics tools predicted that the C-terminal region of VRK1 might be phosphorylated by PKCδ. The NetworKIN database (http://networkin.info) provides improved information for the prediction of cellular kinase–substrate relations (Linding et al., 2007). This database predicted that PKCδ would phosphorylate the C-terminal motif of VRK1 with the highest probability. Therefore we focused on the C-terminal portion that might have regulatory roles through specific interaction with other proteins (Lopez-Borges and Lazo, 2000). We tested the phosphorylation of mutant forms of GST-VRK1 in which Ser-355 or Thr-390 residues located in the C-terminal region were substituted with alanine residues. As shown in Figure 2F, wild-type VRK1 and the T390A mutant were more highly phosphorylated by PKCδ than by PIS. However, the S355A mutant showed no additional phosphorylation by PKCδ compared with PIS (Figure 2F). In addition, recombinant PKCδ was incubated with GST, GST-VRK1 wild type, or GST-VRK1 mutant in which Ser-355 is replaced by alanine. Kinase-active recombinant PKCδ actively phosphorylated GST-VRK1 wild type, but not GST (Figure 2G). In particular, the GST-VRK1 S355A mutant showed no reactivity with anti–pSer-355 antibody. These results suggest that Ser-355 of VRK1 is a target for PKCδ, at least in vitro.
PKCδ negatively regulates VRK1 kinase activity in vitro
To elucidate whether the phosphorylation of VRK1 by PKCδ affects its kinase activity, we constructed a phosphomimetic mutant, in which Ser-355 is substituted with glutamic acid (S355E). We used two typical substrates of VRK1, BAF and histone H3, to simulate the effect of PKCδ-mediated Ser-355 phosphorylation of VRK1 in vitro (Nichols et al., 2006; Kang et al., 2007). As expected, GST-VRK1 actively phosphorylated BAF in vitro. GST-VRK1 S355A mutant also phosphorylated BAF to the same extent as wild-type VRK1 because this mutation has no effect on its kinase activity (Figure 3A). Interestingly, the GST-VRK1 S355E phosphomimetic mutant showed not only decreased autophosphorylation, but also lower kinase activity on BAF compared with the activity of wild type. Additionally, this phosphomimetic mutant also displayed decreased kinase activity on histone H3 (Figure 3B). These results suggest that the phosphorylation of VRK1 by PKCδ leads to negative regulation of its kinase activity.
FIGURE 3:
PKCδ negatively regulates VRK1 kinase activity in vitro. (A, B) VRK1 kinase assay was performed using recombinant GST, GST-VRK1, GST-VRK1 S355E, or GST-VRK1 S355A. BAF (A) and histone H3 (B) were used as substrates. (C) GST pull-down assays were performed using core histone proteins with GST or GST-VRK1 wild type, GST-VRK1 S355E, or GST-VRK1 S355A, followed by immunoblotting with anti–histone H3 antibody. (D) In vitro phosphorylation assay was carried out with recombinant PKCδ and GST, GST-VRK1, or GST-VRK1 S355A in the presence of core histone as a substrate. Phosphorylations of VRK1 and core histone were analyzed by immunoblotting with anti–phospho-VRK1 (Ser-355) and anti–histone H3 (Thr-3) antibodies, respectively. (E) HEK 293T cells were transfected with plasmids encoding Flag-VRK1. After 24 h, whole-cell lysates were subjected to immunoprecipitation with anti-Flag or control IgG. In vitro immune complex kinase assays were performed using histone H3 as a substrate. Phosphorylations of VRK1 and histone H3 were analyzed by immunoblotting with anti–phospho-VRK1 (Ser-355) and anti–histone H3 (Thr-3) antibodies, respectively.
To determine whether the phosphorylation of Ser-355 could modulate VRK1 activity, we tested the association between VRK1 and its substrate, histone H3. GST pull-down assays showed that VRK1 wild type binds more efficiently to histone H3 than VRK1 S355E mutant (Figure 3C). Moreover, we examined whether PKCδ downregulates VRK1 kinase activity on Thr-3 of histone H3 in vitro. When VRK1 was phosphorylated on Ser-355 by PKCδ, its kinase activity on Thr-3 of histone H3 was diminished (Figure 3C).
To investigate the regulation of VRK1 activity in cells, we performed immunocomplex protein kinase assays before and after exposing the cells to DNA damage. HEK 293T cells were transfected with Flag-VRK1. Cell lysates were immunoprecipitated with anti-Flag or control immunoglobulin G (IgG), and histone H3 was used as a substrate to detect VRK1 kinase activity. Immunoblot analysis showed that the phosphorylation of VRK1 on Ser-355 was increased when cells were exposed to etoposide, whereas histone H3 phosphorylation on Thr-3 was decreased (Figure 3E). Taken together, these data suggest that PKCδ is involved in the negative regulation of VRK1 through phosphorylation.
PKCδ-induced cell death is potentiated by VRK1
To find the physiological condition in which VRK1 is phosphorylated by PKCδ, we tested the effect of VRK1 in PKCδ-induced cell death. First, we tested whether VRK1 affects cell viability. Wild-type and S355A mutant VRK1 showed little effect on CHO-K1 cell proliferation. However, the phosphomimetic mutant S355E showed an inhibitory effect on cell viability (Figure 4A). This result suggests that phosphorylated VRK1 by PKCδ might be involved in the cell death pathway.
FIGURE 4.
VRK1 potentiates PKCδ-induced cell death. (A) CHO-K1 cells were transfected with mock vector, Flag-VRK1, Flag-VRK1 S355A, or Flag-VRK1 S355E. After 24 h, cell viability was measured by MTT assay, and whole-cell extracts were analyzed by immunoblotting with antibodies to Flag or GAPDH. (B, C) CHO-K1 cells were transfected with PKCδ CF alone, PKCδ CF with Flag-VRK1, or PKCδ CF with Flag-VRK1 S355A. After 24 h, cell viability was measured by MTT assay (B), and cells were viewed using fluorescence microscopy (C). Nuclei were stained with Hoechst. (D, E) C6 cells were transfected with PKCδ CF alone, PKCδ CF with Flag-VRK1, or PKCδ CF with Flag-VRK1 S355A. After 24 h, cell viability was measured by MTT assay (D), and whole-cell extracts were analyzed by immunoblotting with antibodies to cleaved caspase 3 or GAPDH (E). Results are mean ± SEM value from three separate experiments. **, P < 0.01. (F) CHO-K1 cells were transiently transfected with a plasmid encoding dsRed-VRK1 wild type or its S355A mutant. Nuclei were stained with Hoechst, and the nuclear morphology was observed by using fluorescence microscope. (G) CHO-K1 cells were transfected with PKCδ CF alone, PKCδ CF with Flag-VRK1, or PKCδ CF with Flag-VRK1 S355A. After 24 h, cells were fixed with 70% ethanol and stained with propidium iodide. Sub-G1 population was analyzed by flow cytometry.
As mentioned, VRK1 mainly resides in the nucleus because it has a nuclear localization signal. A previous study demonstrated that overexpression of PKCδ CF results in nuclear localization and regulates an essential nuclear event that is required for activation of the apoptotic pathway (DeVries et al., 2002). Therefore we reasoned that PKCδ might interact with VRK1 in the nucleus in certain conditions, such as apoptotic cell death. We thus examined whether overexpression of PKCδ CF induces cell death in CHO-K1 cells. On the basis of the MTT assay, we found that more than 30% of the cells underwent cell death (Figure 4B). As we previously showed that PKCδ contributes to regulation of VRK1 activity in vitro, we next investigated whether VRK1 is involved in PKCδ CF–induced cell death. Interestingly, PKCδ CF–induced cell death was synergistically increased when cells were transfected with VRK1 (Figure 4, B and C), and more dramatic results were obtained from the MTT assay and cleaved caspase-3 in transfected C6 glioma cells (Figure 4, D and E), suggesting that VRK1 participated in the potentiation of PKCδ CF–induced cell death. On the other hand, the VRK1-S355A mutant itself did not affect cell viability but completely rescued the effect of wild-type VRK1 on PKCδ-induced cell death (Figure 4, B and D). These results suggest that negative regulation of VRK1 activity plays an important role in PKCδ-induced cell death. Moreover, VRK1 S355A mutant retained its kinase activity and abrogated PKCδ-induced cell death. This result also supports the notion that the VRK1 S355A mutant might have a dominant negative effect against VRK1 in cell death conditions. In addition, both VRK1-WT and VRK1-S355A mutant are localized in the nucleus. As shown in Figure 4F, ds-Red VRK1-S355A mutant is located in the nucleus like wild-type VRK1.
We additionally performed the flow cytometric analysis to verify the role of VRK1 in PKCδ-induced apoptosis. As shown in Figure 4G, apoptotic cell population (sub-G1) was increased when cells were cotransfected with PKCδ CF and VRK1-WT. However, apoptotic cell death was diminished when cells were cotransfected with PKCδ CF and VRK1-S355A mutant. This result is correlated with MTT and active caspase-3 assay.
PKCδ phosphorylates VRK1 in DNA damage-induced cell death
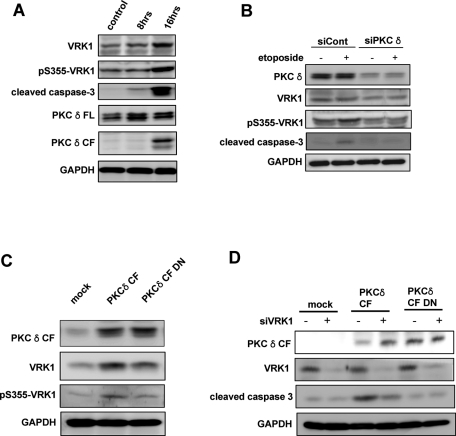
To clarify the association between PKCδ and VRK1 in the apoptotic pathway, we checked whether endogenous VRK1 is phosphorylated on Ser-355 by PKCδ in DNA damage–induced cell death. HT22 cells were treated with etoposide to induce DNA damage and apoptosis. As expected, apoptotic cell death signaling was evident when the increase in cleaved caspase-3 and PKCδ catalytic fragment were measured during the incubation period (Figure 5A). Interestingly, the level of pSer355-VRK1 was elevated as apoptotic cell death progressed, even though the level of VRK1 was also augmented by etoposide treatment. In HEK293T cells, the level of human VRK1 was also increased in etoposide-induced cell death (Supplemental Figure 2). Etoposide induces G2/M arrest in a variety of cell lines (Wei et al., 2010). We have previously reported that VRK1 expression is regulated in a cell cycle–dependent manner (Kang et al. 2007). The expression level of VRK1 reached the highest point in G2/M phase. That might be the reason why the expression of VRK1 is increased by etoposide.
FIGURE 5:
PKCδ is involved in phosphorylation of VRK1 on Ser-355 in response to DNA damage. (A) HT22 cells were treated with etoposide (50 μM) for the indicated times. Cell lysates were subjected to immunoblot with specified antibodies. (B) HT22 cells transfected with control scramble siRNA or PKCδ siRNA were left untreated or treated by etoposide. After 24 h, cell lysates were subjected to immunoblot with specified antibodies. (C) HT22 cells were transfected with enhanced green fluorescent protein (EGFP), EGFP-PKCδ CF, or EGFP-PKCδ CF DN. After 24 h, cell lysates were subjected to immunoblot with specified antibodies. (D) HT22 cells transfected with control scramble siRNA or VRK1 siRNA were cotransfected with EGFP, EGFP-PKCδ CF, or EGFP-PKCδ. After 24 h, cell lysates were subjected to immunoblot with specified antibodies.
When PKCδ was depleted in cells, the phosphorylation on S355 of VRK1 was diminished as well as the apoptotic cell death in response to etoposide (Figure 5B). In addition, expression of PKCδ CF dominant negative mutant relieved the etoposide-induced phosphorylation of VRK1 on Ser-355 (Figure 5C). Collectively, these data indicate that the PKCδ catalytic fragment phosphorylates VRK1 in the nucleus during apoptotic cell death.
To further verify the role of VRK1 in PKCδ-mediated cell death, we knocked down VRK1 by introducing VRK1 small interfering (si)RNAs. As shown in Figure 5D, knocking down of VRK1 was associated with the attenuation of apoptotic cell death induced by PKCδ CF. This result also supports the role of VRK1 in conjunction with PKCδ in apoptotic cell death.
Phosphorylation of VRK1 by PKCδ is required for the p53-dependent cell death pathway
A recent study demonstrated that VRK1 might function as a switch controlling the proteins that interact with p53 and thus modifying p53 stability and activity during cell proliferation (Vega et al., 2004). We therefore examined whether p53 was involved in our system. For this purpose, we first examined the effect of etoposide on cellular p53 levels. In agreement with previous studies (Blass et al., 2002; Jinag et al., 2006), etoposide elicited accumulation of the p53 protein in the nucleus and nuclear translocation of the catalytic fragment of PKCδ (Figure 6, A and B). Next we examined changes in the levels of endogenous p53 protein in DNA damage–induced cell death. A slight increment in levels of p53 protein was detected in C6 glioma cells transfected with wild-type VRK1 (Figure 6, C and D), suggesting that p53 might be involved in VRK1-induced cell death.
FIGURE 6:
Involvement of p53 in VRK1-mediated cell death induced by PKCδ. (A) C6 cells treated with etoposide (50 μM) for 12 h were subjected to subcellular fractionation. Cytosol (Cyto) and nuclear (Nu) fractions were analyzed by immunoblot with antibodies to PKCδ, p53, or Lamin. (B) C6 cells were treated with etoposide (50 μM) for 12 h, and the accumulation of p53 in the nucleus was assessed using immunofluorescence staining. (C) C6 cells overexpressing mock vector, Flag-VRK1, or Flag-VRK1 S355A were treated with or without etoposide (50 μM) for 8 h. Cell viability was measured by MTT assay. (D) Whole-cell extracts were analyzed by immunoblot with antibodies to p53, cleaved caspase 3, or GAPDH. **, P < 0.01.
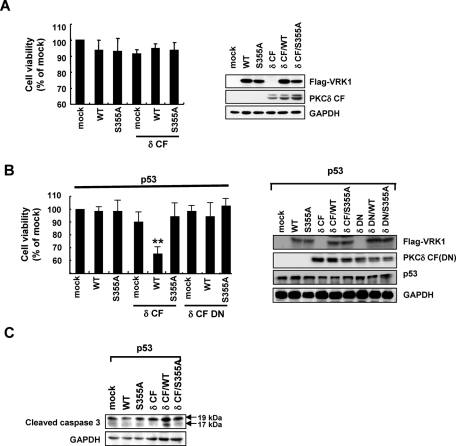
To further investigate the effect of p53 on PKCδ-mediated VRK1 modulation, we used p53-deficient H1299 cells. Cells were transfected with plasmids expressing wild-type VRK1 or its S355A mutant, with or without PKCδ CF plasmid, and the MTT assay was performed. Interestingly, the effect of VRK1 and PKCδ on cell viability was not observed in p53 knockout cells (Figure 7A). On the other hand, p53 overexpressing H1299 cells exhibited significant reduction of cell viability when the cells were cotransfected with both VRK1 and PKCδ CF (Figure 7B). The role of p53 was confirmed by its ability to increase the level of cleaved caspase-3 in cells cotransfected with VRK1 and PKCδ (Figure 7C).
FIGURE 7:
p53 is involved in VRK-1-mediated cell death induced by PKCδ. (A) H1299 (p53 null) cells were transfected with a plasmid encoding wild-type VRK1 or its S355A mutant either alone or together with PKCδ. Cells were also transfected with empty vector or PKCδ alone as controls. After 24 h, cell viability was measured by MTT assay, and whole-cell extracts were analyzed by immunoblot with antibodies to Flag, PKCδ, or GAPDH. (B, C) H1299 (p53 null) cells were cotransfected with a plasmid encoding wild-type VRK1 or its S355A mutant either alone or together with PKCδ or PKCδ dominant negative in the presence of p53. After 24 h, cell viability was measured by MTT assay, and whole-cell extracts were analyzed by immunoblotting with antibodies to Flag, PKCδ, p53 (B), or cleaved caspase 3 (C). Results are mean ± SEM value from three separate experiments. **, P < 0.01 value compared with p53 alone.
Actually, overexpression of PKCδ CF showed little effect on apoptosis in p53-deficient H1299 cells (Figure 7A). However, HCT116 (p53 −/−) and HL60 (p53 −/−) cells are sensitive to PKCδ CF–induced apoptosis even though they do not express p53 (Supplemental Figure 3, A and C). Notably, apoptotic cell death by PKCδ and VRK1 is also dramatically potentiated in the presence of wild-type p53 both in HCT116 (p53 −/−) and HL60 (p53 −/−) cells (Supplemental Figure 3, B and D). Taken together, these results suggest that p53 plays an important role in the regulation of cell death accompanied by PKCδ-mediated phosphorylation of VRK1.
DISCUSSION
In the present study, we suggest that PKCδ plays an important role as an upstream regulator of VRK1 in the nucleus and contributes to the acceleration of apoptotic cell death following the DNA damage response. This study also provides evidence that phosphorylation of VRK1 on Ser-355 by PKCδ is required for the induction of the p53-dependent cell death pathway.
The distinct pattern of subcellular localization of PKCδ in response to various stimuli may determine the effects of PKCδ on cell apoptosis. For example, when cells overexpressing PKCδ were treated with phorbol ester, PKCδ translocated to the mitochondria, resulting in loss of mitochondrial membrane potential and release of cytochrome c (Li et al., 1999; Majumder et al., 2000). In addition, PKCδ translocated to the nucleus in response to etoposide, followed by elevation of caspase-3 activity in C6 glioma cells (Blass et al., 2002). Translocation of PKCδ to specific cellular compartments may lead to the phosphorylation of specific substrates and to the association of PKCδ with distinct proteins present in these locations (Brondie and Blumberg, 2003). Until now, a number of downstream targets of PKCδ have been described in different cellular systems. Some of these PKCδ substrates are nuclear proteins that participate in cell apoptosis. One of these proteins is the DNA-dependent protein kinase (DNA-PK), an enzyme essential for the repair of double-stranded DNA breaks that is inhibited by PKCδ-dependent phosphorylation (Bharti et al., 1998). Rad9, a key component of the genotoxin-activated checkpoint signaling complex, also binds to anti–apoptotic Bcl-2 family proteins and mediates apoptotic responses to DNA damage when phosphorylated by PKCδ (Yoshida et al., 2003). In addition, it has been reported that lamin B and p73β are nuclear substrates of PKCδ involved in apoptotic cell death (Cross et al., 2000; Ren et al., 2002). Here we show that both full-length PKCδ and its catalytic fragment associated with VRK1 (Figure 1A), causing VRK1 phosphorylation on Ser-355 residue (Figure 2) and negatively regulating its kinase activity (Figure 3) in vitro.
We propose that VRK1 is one of the downstream targets of PKCδ in the nucleus that might contribute to PKCδ-induced apoptotic cell death. Under physiological conditions, the majority of VRK1 is localized in the nucleus, with some present in the perinuclear area (Vega et al., 2004), in agreement with our present results (Figure 1). In contrast, PKCδ is predominantly distributed in the cytoplasm, showing a punctate, perinuclear distribution (DeVries et al., 2002). However, we found that overexpressed full-length PKCδ and its catalytic fragment were also distributed in the nucleus (Figure 1, B and C). We showed that PKCδ-induced cell death is potentiated by wild-type VRK1, but not the S355A mutant, suggesting that phosphorylation of VRK1 by PKCδ plays a critical role in cell cycle arrest and cell death (Figure 4B).
VRK1 leads to p53 accumulation and transcriptional activity (Vega et al., 2004). Moreover, a recent study indicated that PKCδ functions downstream of the p53 response in DNA damage–induced apoptosis in vivo and in vitro (Humphries et al., 2006). Thus, we focused on the possible role of VRK1 in the PKCδ-induced apoptotic cell death following the DNA damage response. Surprisingly, the effect of VRK1 and PKCδ on cell death was not apparent in p53-depleted H1299 cells (Figure 7A). Furthermore H1299 cells overexpressing p53 exhibited significant reduction of cell viability when the cells were cotransfected with both VRK1 and PKCδ (Figure 7B). The effect of p53 on cell death was confirmed by its ability to increase the level of cleaved caspase-3 in cells cotransfected with VRK1 and PKCδ (Figure 7C), demonstrating that p53 plays a critical role in the regulation of cell death concomitant with PKCδ-mediated VRK1 phosphorylation.
The mammalian VRK family and the related vaccinia virus B1R kinase are classified as a distinct branch of the casein kinase-1 group, and their biological roles are less well known compared with other kinases in the kinome (Gross and Anderson, 1998). A recent study demonstrated that VRK1 might play a role in enabling DNA replication. This study showed that expression of VRK1 from the temperature-sensitive mutant B1 vaccinia virus genome restored viral DNA replication, whereas a catalytically inactive mutant of VRK1 was not able to rescue the virus (Boyle and Traktman, 2004). In addition, VRK1 was also implicated in cell cycle progression. Suppression of VRK1 expression by specific small interfering RNA (siRNA) leads to a retardation of cell division and proliferation (Vega et al., 2004), and ablation of the Caenorhabditis elegans VRK homolog through the use of siRNA-mediated depletion resulted in early embryonic lethality due to a problem in cell cycle progression (www.wormbase.org). In addition, VRK1 phosphorylates histone H3 on Thr-3 and Ser-10, resulting in chromatin condensation and cell division (Kang et al., 2007). These recent findings collectively support the notion that VRK1 is a positive regulator for cell proliferation and cell cycle progression. Therefore it is conceivable that the kinase activity of VRK1 needs to be down-regulated in conditions where cells are undergoing cell cycle arrest and death. This study suggests that VRK1 is negatively regulated by PKCδ in DNA damage–induced cell death conditions for cell cycle arrest and activation of apoptotic pathways. It is probable that Ser-355 phosphorylated VRK1 associates with another nuclear target to regulate p53-dependent cell death pathway, even though its kinase activity is down-regulated.
Thus we propose a dual model of VRK1 function in cell proliferation and death (Figure 8). In cell proliferation conditions, VRK1 is mainly localized in the nucleus and phosphorylates several nuclear targets essential for cell cycle progression and division, such as CREB, histone H3, and BAF. On the contrary, in cell death conditions, VRK1 is phosphorylated by PKCδ and contributes to cell cycle arrest and apoptosis.
FIGURE 8:
Hypothetical model: dual signaling pathways of VRK1. In cell proliferation conditions, VRK1 is mainly localized in the nucleus and phosphorylates several nuclear targets essential for cell cycle progression and division, such as CREB, histone H3, and BAF. On the contrary, in cell death conditions, VRK1 is phosphorylated by PKCδ and contributes to cell cycle arrest and apoptosis.
Taken together, it is concluded that the active form of PKCδ is translocated into the nucleus, where PKCδ associates with VRK1, leading to down-regulation of its kinase activity and thus accelerating cell cycle arrest and activation of apoptotic pathways via a p53-dependent mechanism.
MATERIALS AND METHODS
Reagents and antibodies
Histone H1 and etoposide were purchased from Calbiochem (La Jolla, CA). [γ-32P]ATP was from NEN Life Science Products (Boston, MA). Recombinant PKCδ was from Upstate Biotechnology (Lake Placid, NY). The anti–Flag antibody was purchased from Sigma (St. Louis, MO). Polyclonal antibodies against PKCδ and lamin B and monoclonal antibody (mAb) against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were from Santa Cruz Biotechnology (Santa Cruz, CA), and mAb against p53 and polyclonal antibody against cleaved caspase-3 were from Cell Signaling Technology (Beverly, MA).
Plasmids
We obtained 1191 base pairs ORF of mVRK1 from mouse liver cDNA, which is splice variant lacking exon 12 and 13 of mVRK1. Amplification of cDNA was performed with pfu polymerase (Solgent, Daejon, Republic of Korea) and a primer pair specific for the VRK1 coding region (forward 5′-AAAGATCTAATGCCCCGTGTAAAAGCAGC-3′ and reverse 5′-AATCTAGATTACTTCTGGGCTTTCTTTC-3′). The amplified DNA fragment was digested with BglII and XbaI and cloned into pFlag-CMV-1 (Sigma). This construct was used as a template to produce phosphorylation mutant constructs and kinase inactive mutant. VRK1 mutations S355A, T390A, and K179E were generated using complementary oligonucleotides containing the mutation desired, and also cloned into pFlag-CMV-1. All mutations were confirmed by DNA sequencing. VRK1 and mutants fused to RFP were cloned into the XhoI and BamHI sites of pDsRed1-C1 (Clontech, Mountain View, CA). Mouse PKCδ cloned into pHACE vector was generously provided by Y. S. Lee (Lee et al., 2002). The constitutive active form of PKCδ (amino acids 325–674, CAT) was prepared by subcloning the PCR-amplified fragment into pcDNA3.1. Wild-type PKCδ and PKCδ-CAT fused to GFP were cloned into the XhoI and BamHI sites of pEGFP-C1 (Clontech).
Preparation of recombinant VRK1 proteins
Complete VRK1 coding region, the deletion mutants, and the phosphorylation mutants were cloned into pGEX-4T-1 (Pharmacia Biotech, Uppsala, Sweden). The deletion mutant constructs including the amino acid fragments 1-130 (N130), 131-280 (131–280), and 281-396 (ΔN280) were generated by PCR-based amplification. The resulting plasmids were transformed into Escherichia coli BL21(DE3) to produce GST tag-VRK1 fusion proteins after treating with 0.1 M isopropyl-1-thio-β-d-galactopyranoside for 24 h at 16°C. Bacteria were lysed in phosphate-buffered saline (PBS) containing 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM Na3VO4. The GST fusion proteins were then purified using glutathione-sepharose resin (Amersham Biosciences, Little Chalfont, UK) and eluted from the beads with reduced glutathione according to the manufacturer’s recommendations. The mutant constructs were confirmed by DNA sequencing.
Preparation of anti–mouse VRK1 antibody
Mouse VRK1 antisera were generated in rabbit using recombinant mouse VRK1 (accession no. NM 011705.3) as immunogen. Approximately 1 mg of recombinant mouse VRK1 was used to immunize rabbit with complete Freund’s adjuvant through subcutaneous injection. After 2 wk of first immunization, the rabbit was boosted once again using incomplete adjuvant. Then, the rabbit was boosted once more with only recombinant protein after 2 wk of second immunization. Rabbit serum was collected and then subjected to HiTrap Protein G column (GE Healthcare, Uppsala, Sweden) for affinity purification. Phosphorylated Ser-355 of mouse VRK1 was raised against peptides VKTRPApSKK.
Cell culture and transfection
CHO-K1 cells were maintained in DMEM/F12 containing 10% bovine calf serum and antibiotics in a humidified 5% CO2 incubator at 37°C. H1299 (human lung cancer cell line, p53−/−) was grown in RPMI 1640 containing 10% fetal calf serum (FCS), glutamine, HEPES, and antibiotics in a humidified 5% CO2 incubator at 37°C. C6 glioma cells were cultured in DMEM containing 10% FCS and antibiotics in a humidified 5% CO2 incubator at 37°C. CHO-K1 cells were transfected by the electroporation method with the plasmid indicated in the specific experiments. After incubation for 24 h, the transfected cells were treated as indicated for analysis. H1299 and C6 cells were transfected with the plasmid indicated in the specific experiments by using Metafectene reagent (Biontex, Munich, Germany). After incubation for 24 h, the transfected cells were treated as indicated for analysis.
Cytoplasmic and nuclear fractionation
Cells were resuspended in hypotonic buffer (10 mM HEPES, 10 mM KCl, 1.5 mM MgCl2, 1 mM DTT, 0.2 mM PMSF, and 0.5% Nonidet P-40) and incubated at 4°C for 30 min. Samples were agitated every 10 min and then centrifuged at 3500 rpm for 4 min to collect the cytoplasmic fraction. To isolate nuclei, pellets were resuspended, incubated in nuclear extraction buffer (20 mM HEPES, 450 mM NaCl, 1.5 mM MgCl2, 1 mM DTT, and 0.2 mM PMSF) for 20 min, and then repeated freezing and thawing five times. The nuclear suspension was centrifuged at 15,000 rpm for 20 min to obtain the nuclear fraction.
Immunoblotting and immunoprecipitation
Immunoblot analysis was performed as we previously described (Choi et al., 2006). For immunoprecipitation, cell lysates were prepared in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 2.5 mM sodium pyrophosphate, 1 mM β-glycerol phosphate, 10 μg/ml pepstatin A, 10 μg/ml leupeptin, 1 mM PMSF, 10 μg/ml aprotinin, 10 mM NaF, and 1 mM Na3VO4). Equal amounts of proteins were immunoprecipitated using anti–PKCδ and anti–Flag antibodies and collected with protein A-Sepharose beads (Santa Cruz Biotechnology) at 4°C for 16 h. The immunoprecipitate was then washed four times in cold lysis buffer and subjected to Western blot analysis and PKCδ kinase assay.
PKCδ kinase assay
The activity of PKCδ was determined by immune complex kinase assay as previously described (Choi et al., 2006). Briefly, the anti–PKCδ immunoprecipitate was washed in a kinase reaction buffer (25 mM Tris-HCl, pH 7.5, 5 mM β-glycerol phosphate, 2 mM DTT, 0.1 mM sodium orthovanadate, and 10 mM MgCl2). The kinase assay was carried out in a total volume of 30 μl of a kinase reaction buffer containing 50 μM ATP, 1 μCi of [γ-32P]ATP, and affinity-purified GST fusion proteins or 200 μg/ml histone H1 used as substrates for 30 min incubation at 30°C. The phosphorylated proteins were resolved on 10% SDS-polyacrylamide gels followed by autoradiography.
VRK1 protein kinase assay
The kinase activity was performed as previously described (Vega et al., 2004). Briefly, aliquots of GST fusion proteins were resuspended in kinase reaction buffer (20 mM Tris-HCl, pH 7.5, 5 mM MgCl2, 0.5 mM DTT, and 150 mM KCl) and incubated for 30 min at 30°C with 1 μCi of [γ-32P]ATP and 1 μg myelin basic protein used as substrate. Substrate phosphorylation was detected by autoradiography.
Cell viability assay
Cell viability was assessed by measuring their ability to metabolize 3-(4,5-dimethyldiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) as we described in detail elsewhere (Choi et al., 2006).
siRNA experiments
siRNA duplexes targeting VRK1 and PKCδ were purchased from Dharmacon (Lafayette, CO). Transfection of siRNA duplexes was carried out using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) or Neon transfection system (Invitrogen).
Immunofluorescence staining and immunocytochemical analysis
Cells were grown on coated glass coverslips. Then, 24 h posttransfection, cells were fixed with 4% paraformaldehyde (PFA) in PBS for 30 min at 4°C and then washed in PBS. Slides were mounted and visualized by fluorescence microscopy (Axioplan2; Zeiss, Oberkochen, Germany). For immunocytochemistry, etoposide-treated C6 cells were fixed with 4% PFA and then incubated with blocking solution (2.5% bovine serum albumin and 2.5% equine serum in PBS) for 1 h at room temperature. The samples were incubated overnight at 4°C with monoclonal anti–p53 antibody, followed by incubation with FITC-conjugated anti–mouse IgG. Slides were mounted and visualized by fluorescence microscopy. Postacquisition processing of images was performed with Adobe Photoshop 7.0 software.
Statistical analysis
All traces and immunoblots presented are representative of three separate experiments. All quantitative data are presented as means ± SEM of a minimum of three experiments. Comparisons between two groups were analyzed via t test, and values of P < 0.05 were considered to be significant.
Supplementary Material
Acknowledgments
This work was supported by the National Research Foundation of Korea (No. 20090063547, 20090081464, and WCU program R31–2008–000–10105–0). This work was also supported by the Brain Korea 21 program and the Regional Core Research Program/Anti-aging and Well-being Research Center of the Korean Ministry of Education, Science and Technology.
Abbreviations used:
- CF
catalytic fragment
- DN
dominant negative
- EGFP
enhanced green fluorescent protein
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IgG
immunoglobulin G
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PKCδ
protein kinase Cδ
- siRNA
small interfering RNA
- VRK1
vaccinia-related kinase 1
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-08-0717) on February 23, 2011.
REFERENCES
- Basu A, Lu D, Sun B, Moor AN, Akkaraju GR, Huang J. Proteolytic activation of protein kinase C-ε by caspase-mediated processing and transduction of antiapoptotic signals. J Biol Chem. 2002;277:41850–41856. doi: 10.1074/jbc.M205997200. [DOI] [PubMed] [Google Scholar]
- Bharti A, et al. Inactivation of DNA-dependent protein kinase by protein kinase Cδ: implication for apoptosis. Mol Cell Biol. 1998;1:6719–6728. doi: 10.1128/mcb.18.11.6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass M, Krefeld I, Kazimirsky G, Blumberg PM, Brodie C. Tyrosine phosphorylation of protein kinase Cδ is essential for its apoptotic effect in response to etoposide. Mol Cell Biol. 2002;22:182–195. doi: 10.1128/MCB.22.1.182-195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle KA, Traktman P. Members of a novel family of mammalian protein kinases complement the DNA-negative phenotype of a vaccinia virus ts mutant defective in the B1 kinase. J Virol. 2004;78:1992–2005. doi: 10.1128/JVI.78.4.1992-2005.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brondie C, Blumberg PM. Regulation of cell apoptosis by protein kinase Cδ. Apoptosis. 2003;8:72–91. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- Choi BH, Hur EM, Lee JH, Jun DJ, Kim KT. Protein kinase Cδ-mediated proteosomal degradation of MAP kinase phosphatase-1 contributes to glutamate-induced neuronal cell death. J Cell Sci. 2006;119:1329–1340. doi: 10.1242/jcs.02837. [DOI] [PubMed] [Google Scholar]
- Cross T, Griffiths G, Deacon E, Sallis R, Gough M, Watters D, Lord JM. PKC-δ is an apoptotic lamin kinase. Oncogene. 2000;19:2331–2337. doi: 10.1038/sj.onc.1203555. [DOI] [PubMed] [Google Scholar]
- Denning MF, Wang T, Nickoloff BJ, Wrone-Smith T. Protein kinase C delta is activated by caspase-dependent proteolysis during ultraviolet radiation-induced apoptosis of human keratinocytes. J Biol Chem. 1998;273:29995–30002. doi: 10.1074/jbc.273.45.29995. [DOI] [PubMed] [Google Scholar]
- DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCδ is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emoto Y, et al. Proteolytic activation of protein kinase C delta by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghayur BT, et al. Proteolytic activation of protein kinase Cδ by an ICE/CED 3-like protease induced characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorjánácz M, Klerkx EP, Galy V, Santarella R, López-Iglesias C, Askjaer P, Mattaj IW. Caenorhabditis elegans BAF-1 and its kinase VRK-1 participate directly in postmitotic nuclear envelope assembly. EMBO J. 2007;26:132–143. doi: 10.1038/sj.emboj.7601470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- Gross SD, Anderson RA. Casein kinase I: spatial organization and positioning of a multifunctional protein kinase family. Cell Signal. 1998;10:699–711. doi: 10.1016/s0898-6568(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Hug H, Sarre TF. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase Cδ null mouse in vivo. J Biol Chem. 2006;281:9728–9737. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- Jinag P, Du W, Heese K, Wu M. The bad guy cooperates with a good cop p53: Bad is transcriptionally up-regulated by p53 and forms Bad/p53 complex at the mitochondria to induce apoptosis. Mol Cell Biol. 2006;26:9071–9082. doi: 10.1128/MCB.01025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes FJ, Prestle J, Eis S, Oberhagemann P, Pfizenmaier K. PKCu is a novel, atypical member of the protein kinase C family. J Biol Chem. 1994;269:6140–6148. [PubMed] [Google Scholar]
- Kang TH, Park DY, Choi YH, Kim KJ, Yoon HS, Kim KT. Mitotic histone phosphorylation by vaccinia-related kinase 1 in mammalian cells. Mol Cell Biol. 2007;27:8533–8546. doi: 10.1128/MCB.00018-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang TH, Park DY, Kim W, Kim KT. VRK1 phosphorylates CREB and mediates CCND1 expression. J Cell Sci. 2008;121:3035–3041. doi: 10.1242/jcs.026757. [DOI] [PubMed] [Google Scholar]
- Klerkx EP, Lazo PA, Askjaer P. Emerging biological functions of the vaccinia-related kinase (VRK) family. Histol Histopathol. 2009;24:749–759. doi: 10.14670/HH-24.749. [DOI] [PubMed] [Google Scholar]
- Konishi H, Takaka M, Takemura Y, Matsuzaki H, Ono Y, Kikkawa U, Nishizuka Y. Activation of protein kinase C by tyrosine phosphorylation in response to H2O2. Proc Natl Acad Sci USA. 1997;94:11233–11237. doi: 10.1073/pnas.94.21.11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koriyama H, Kouchi Z, Umede T, Saido TC, Momoi T, Ishiura S, Suzuki K. Proteolytic activation of protein kinase C δ and ε by caspase-3 in U937 cells during chemotherapeutic agent-induced apoptosis. Cell Signal. 1999;11:831–838. doi: 10.1016/s0898-6568(99)00055-8. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Soh JW, Dean NM, Cho CK, Kim TH, Lee SJ, Lee YS. Protein kinase C delta overexpression enhances radiation sensitivity via extracellular regulated protein kinase 1/2 activation, abolishing the radiation-induced G2-M arrest. Cell Growth Differ. 2002;13:237–246. [PubMed] [Google Scholar]
- Leitges M, Mayr M, Braun U, Mayr U, Li C, Pfister G, Ghaffari-Tabrizi N, Baier G, Hu Y, Xu Q. Exacerbated vein graft arteriosclerosis in protein kinase C delta-null mice. J Clin Invest. 2001;108:1505–1512. doi: 10.1172/JCI12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH. Protein kinase Cδ targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic kerainocytes when overexpressed by an adenoviral vector. Mol Cell Biol. 1999;19:8547–8558. doi: 10.1128/mcb.19.12.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linding R, et al. Systematic discovery of in vivo phosphorylation networks. Cell. 2007;129:1415–1426. doi: 10.1016/j.cell.2007.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Borges S, Lazo PA. The human vaccinia-related kinase 1 (VRK1) phosphorylates threonine-18 within the mdm-2 binding site of the p53 tumour suppressor protein. Oncogene. 2000;19:3656–3664. doi: 10.1038/sj.onc.1203709. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu D, Hornia D, Devonish W, Pagano M, Foster DA. Activation of protein kinase C triggers its ubiquitination and degradation. Mol Cell Biol. 1998;18:839–845. doi: 10.1128/mcb.18.2.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder PK, Pandey P, Sun X, Cheng K, Datta R, Saxena S, Kharbanda S, Kufe D. Mitochondrial translocation of protein kinase C delta in phorbol ester-induced cytochrome c release and apoptosis. J Biol Chem. 2000;275:21793–21796. doi: 10.1074/jbc.C000048200. [DOI] [PubMed] [Google Scholar]
- Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKC delta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276:29719–29728. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- Nezu JI, Oku A, Jones MH, Shimane M. Identification of two novel human putative serine/threonine kinases, VRK1 and VRK2, with structural similarity to vaccinia virus B1R kinase. Genomics. 1997;45:327–331. doi: 10.1006/geno.1997.4938. [DOI] [PubMed] [Google Scholar]
- Nichols RJ, Traktman P. Characterization of three paralogous members of the mammalian vaccinia related kinase family. J Biol Chem. 2004;279:7934–7946. doi: 10.1074/jbc.M310813200. [DOI] [PubMed] [Google Scholar]
- Nichols RJ, Wiebe MS, Traktman P. The vaccinia-related kinases phosphorylate the N terminus of BAF, regulating its interaction with DNA and its retention in the nucleus. Mol Biol Cell. 2006;17:2451–2464. doi: 10.1091/mbc.E05-12-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel RE, Anderson MK, Evans E, Traktman P. Temperature-sensitive vaccinia virus mutants identify a gene with an essential role in viral replication. J Virol. 1990;64:574–583. doi: 10.1128/jvi.64.2.574-583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Datta R, Shioya H, Li Y, Oki E, Biedermann V, Bharti A, Kufe D. p73β is regulated by protein kinase Cδ catalytic fragment generated in the apoptotic response to DNA damage. J Biol Chem. 2002;277:33758–33765. doi: 10.1074/jbc.M110667200. [DOI] [PubMed] [Google Scholar]
- Santos CR, et al. VRK1 signaling pathway in the context of the proliferation phenotype in head and neck squamous cell carcinoma. Mol Cancer Res. 2006;4:177–185. doi: 10.1158/1541-7786.MCR-05-0212. [DOI] [PubMed] [Google Scholar]
- Sevilla A, Santos CR, Barcia R, Vega FM, Lazo PA. c-Jun phosphorylation by the human vaccinia-related kinase 1 (VRK1) and its cooperation with the N-terminal kinase of c-Jun (JNK) Oncogene. 2004a;23:8950–8958. doi: 10.1038/sj.onc.1208015. [DOI] [PubMed] [Google Scholar]
- Sevilla A, Santos CR, Vega FM, Lazo PA. Human vaccinia-related kinase 1 (VRK1) activates the ATF2 transcriptional activity by novel phosphorylation on Thr-73 and Ser-62 and cooperates with JNK. J Biol Chem. 2004b;279:27458–27465. doi: 10.1074/jbc.M401009200. [DOI] [PubMed] [Google Scholar]
- Smith L, Chen L, Reyland ME, DeVries TA, Talanian RV, Omura S, Simth B. Activation of atypical protein kinase C ζ by caspase processing and degradation by the ubiquitin-proteasome system. J Biol Chem. 2000;275:40620–40627. doi: 10.1074/jbc.M908517199. [DOI] [PubMed] [Google Scholar]
- Vega FM, Sevilla A, Lazo PA. p53 stabilization and accumulation induced by human vaccinia-related kinase 1. Mol Cell Biol. 2004;24:10366–10380. doi: 10.1128/MCB.24.23.10366-10380.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Xie Y, Tao L, Tang D. Both ERK1 and ERK2 kinases promote G2/M arrest in etoposide-treated MCF7 cells by facilitating ATM activation. Cell Signal. 2010;22:1783–1789. doi: 10.1016/j.cellsig.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Yoshida K. PKC delta signaling: mechanisms of DNA damage response and apoptosis. Cell Signal. 2007;19:892–901. doi: 10.1016/j.cellsig.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Wang HG, Miki Y, Kufe D. Protein kinase Cδ is responsible for constitutive and DNA damage-induced phosphorylation of Rad9. EMBO J. 2003;22:1431–1441. doi: 10.1093/emboj/cdg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.