Abstract
Background
Hypotension and reduced cerebral blood flow secondary to brachiocephalic occlusion (BCO) stimulate various homeostatic physiological and endocrine responses. Our previous studies have also suggested a role of estradiol in augmenting the fetal stress response to BCO. Objectives: We tested the hypothesis that gonadotropins and/or prolactin (PRL) are upregulated in fetal pituitary in response to fetal stress and play a role in the response to BCO-induced stress.
Methods
We performed 3 studies: one in which we measured ovine fetal pituitary PRL, luteinizing hormone (LH), and follicle-stimulating hormone (FSH) mRNA throughout the latter half of gestation in order to better understand the ontogenetic changes upon which dynamic responses are superimposed; one in which we measured these mRNA abundances in response to BCO and/or estrogen treatment, and one in which we measured plasma LH responses to BCO in chronically catheterized late-gestation fetal sheep.
Results
PRL gene expression is increased dramatically in the last 20% of gestation. LH and FSH mRNAs were unchanged except for a transient dip in the expression of LH in the last few days before the normal time of spontaneous parturition. Chronic treatment with estradiol decreased LH and FSH mRNA, but increased PRL mRNA abundance after BCO. In contrast, BCO alone increases the abundance of LH, but not FSH or PRL mRNA in fetal pituitary. Plasma LH concentrations were not increased in response to BCO.
Conclusions
We conclude that the late-gestation fetal sheep responds to hypotensive stress with increases in LH mRNA but not LH secretion. LH, FSH and PRL changes are therefore unlikely to contribute to the fetal response to cerebral hypoperfusion.
Key Words: Estradiol, Fetal stress, Late-gestation fetal sheep
Introduction
Fetuses respond to stress with responses that are homeostatic [1, 2]. For example, we and others have demonstrated robust hypothalamic-pituitary-adrenal (HPA) axis activation in response to hypotension [3, 4], hypoxia [5, 6], hypercapnia [7], and acidemia [8]. While much is known about HPA axis responses to stress in the fetus, little is known about the responsiveness of other hormonal systems and their interaction in the response to fetal stress. Of particular interest to us is the influence of fetal stress on hormonal systems potentially controlling estrogen biosynthesis in the fetus. Estrogen influences fetal neuroendocrine responsiveness to stress [2, 9]; changes in fetal plasma estradiol [10] and estradiol-3-sulfate concentrations [11] stimulate fetal HPA axis activity.
We have suspected that pituitary hormones other than ACTH might respond to fetal stressors. This possibility seems feasible to us in light of the apparent dissociation of ACTH and cortisol responses to cerebral hypoperfusion [12], combined with the possibility that pituitary hormones other than ACTH or pro-opiomelanocortin (POMC) might alter adrenal and placental steroidogenesis [13, 14]. We speculated that there might be involvement of the fetal hypothalamic-pituitary-gonadal axis in fetal stress responses because we had previously demonstrated that cerebral hypoperfusion resulted in upregulation of estrogen receptor α in the fetal pituitary and medullary brainstem [15]. We hypothesized that gonadotropins and/or prolactin (PRL) are upregulated in fetal pituitary in response to hypoperfusion stress and that the synthesis of these hormones in both unstressed and stressed fetuses is modulated by estrogen. The present study is a test of this hypothesis.
Materials and Methods
Experimental Protocols
Study 1: Ontogeny of Pituitary Hormone mRNA Expression. We analyzed pituitary mRNA previously isolated and converted to cDNA as a part of a previously published study of genes integral to the function of the HPA axis [16]. We collected pituitaries from fetal sheep at 80, 100, 120, 130, and 145 days of gestation, and at 1 and 7 days postnatally (n = 4–5/group). The animals used in this study were not instrumented or chronically catheterized, so tissues were collected from unstressed and otherwise untreated animals. The details of the tissue collection have been previously described [16].
Study 2. We studied 16 fetal sheep that were 124–128 days of gestation at the time of study. Blood pressure, blood gas, plasma hormone data, and gene/protein expression data for prostaglandin endoperoxide synthase and nitric oxide synthase isoforms in these experiments have been published elsewhere [17, 18]. The fetuses were chronically catheterized with vascular catheters in femoral and lingual arteries and an extravascular occluder (8 mm diameter; In Vivo Metric, Ukiah, Calif., USA) was placed around the brachiocephalic artery, as previously described [17, 18]. One half of the fetuses were treated with subcutaneous pellets that released approximately 0.25 mg estradiol per day and increased fetal plasma estradiol concentration to 103 ± 12 pg/ml. The other half of the fetuses received placebo pellets, and plasma estradiol concentrations of 62 ± 6 pg/ml were measured in this group [17]. Experiments were performed after at least 5 days of recovery from surgery and while the animals were conscious. Each fetus was subjected to a 10-min period of brachiocephalic occlusion (BCO), a manipulation that lowers cerebral perfusion pressure and flow but is not severe enough to cause any histologically identifiable tissue damage [19]. Before, during, and after the BCO, femoral and lingual arterial pressures and amniotic fluid pressures were measured using disposable transducers, National Instruments (Austin, Tex., USA) analog-to-digital conversion board and LabView software (National Instruments). Changes in lingual arterial pressure were used to confirm the efficacy of the BCO. One hour after the onset of BCO (50 min after the termination of BCO), fetuses were humanely euthanized and tissues were rapidly dissected and snap-frozen as previously described [17].
Study 3: Plasma Luteinizing Hormone Responses to BCO. In a separate group of 12 fetuses, we measured the fetal plasma luteinizing hormone (LH) responses to BCO. These fetuses were surgically prepared as described in study 2, and were 124–136 days of gestation on the day of study. BCO was performed as described in study 2. Fetal blood samples were drawn for hormone (5 ml each) and blood gas (1 ml each) analysis. Blood for hormone analysis was centrifuged at 3,000 g for separation of plasma from erythrocytes; the plasma was divided into two equal aliquots and frozen at −20°C until analysis. Plasma LH concentrations were measured using a commercial immunoradiometric assay from DSL Labs (catalog number DSL-4600; Webster, Tex., USA). Blood gases were analyzed using a Radiometer ABL77 blood gas analyzer (Radiometer Corp., Copenhagen, Denmark). Throughout each experiment, fetal arterial blood and amniotic fluid pressures were measured as described for study 2. Blood pressure and blood gases from these experiments have been reported previously [20].
mRNA Analysis
mRNA was isolated using Trizol (Life Technologies, Invitrogen, Carlsbad, Calif., USA) according to the manufacturer's instructions. Total mRNA in each sample (4 μg) was converted to cDNA with a High Capacity cDNA Archive kit using the methodology recommended by the kit manufacturer (Applied Biosystems, Foster City, Calif., USA). The newly synthesized cDNA, stored at −20°C, was used for assay of mRNA for ovine LH, ovine follicle-stimulating hormone (FSH), and ovine PRL by real-time polymerase chain reaction. Real-time polymerase chain reactions were run using AmpliTaq Gold DNA Polymerase (Applied Biosystems) and primers and probes (Applied Biosystems) were specifically designed using Primer Express software (Applied Biosystems). Probes were labeled with 6-carboxyfluoresceine (6-FAM) or VIC® in the 5′ position and carboxytetramethyl rhodamine (TAMRA) in the 3′ position. Sequences of primers and probes have been reported previously [21]. In each sample, 18S ribosomal RNA was also measured using real-time reverse transcriptase polymerase chain reaction methodology, with probes, primers, and reagents purchased from Applied Biosystems. All mRNA abundances for genes of interest were normalized to the abundance of 18S rRNA, using the relative cycle threshold (ΔCt) method. All reactions were performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems), using 100 ng of cDNA, 100 nM primers, and 200 nM probes.
Reactions were amplified using the following conditions: 48°C for 30 min and 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 1 min. For all assays, we ran appropriate ‘no reverse transcriptase’ (reaction using mRNA alone as the substrate) and ‘no template’ (reaction using no cDNA) controls.
Statistical Analyses
In study 1, mRNA abundances were analyzed statistically using two-way analysis of variance (ANOVA) of the measured values of ΔCt [17]. In study 2, mRNA abundances were analyzed using two-way ANOVA. In study 3, variables were analyzed using one-way ANOVA. The criterion for acceptance of statistical significance for all analyses was p < 0.05. Post hoc analysis of the data was performed using the Bonferroni test. For graphical representation, fold changes in mRNA abundance from the mean abundance in the 80-day group (study 1) or in the ‘control/control’ group (placebo pellet, no BCO in study 2) were calculated as 2−ΔΔCt[22].
Results
Ontogeny of Gonadotropin and PRL Expression
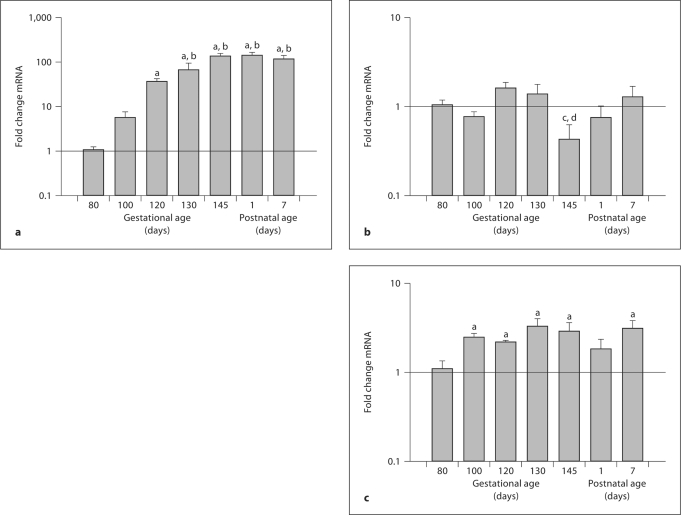
The responses to BCO and estradiol were studied at approximately 85% gestation (term in fetal sheep is approximately 147 days of gestation). PRL mRNA abundance was dramatically increased in late gestation, significantly increased by 120 days of gestation (compared to 80 days of gestation) and by 130 days of gestation and throughout the first week of postnatal life, compared to 100 days of gestation (fig. 1a). LH mRNA abundance was relatively constant, except for a statistically significant and transient decrease in the term (145 days of gestation) fetuses (fig. 1b). FSH mRNA abundance was increased in fetuses at 100, 120, 130, and 145 days of gestation and in lambs at 7 days postnatally compared to fetuses at 80 days of gestation (fig. 1c).
Fig. 1.
Ontogeny of mRNA abundances of pituitary PRL (a), LH (b), and FSH (c) in fetal sheep throughout the latter half of gestation (80, 100, 120, 130, and 145 days of gestation) and in lambs (1 and 7 days postnatally). Data are reported as mean values of relative expression levels ± SEM at each developmental age (n = 4–5/ group). Superscripts ‘a’, ‘b’, ‘c’, and ‘d’ represent statistically significant difference compared to 80, 100, 120, and 130 days, respectively.
Gonadotropin and PRL Responses to BCO and Estradiol
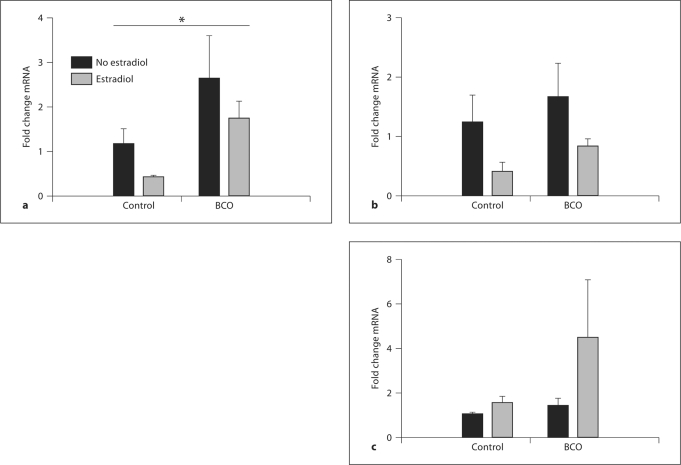
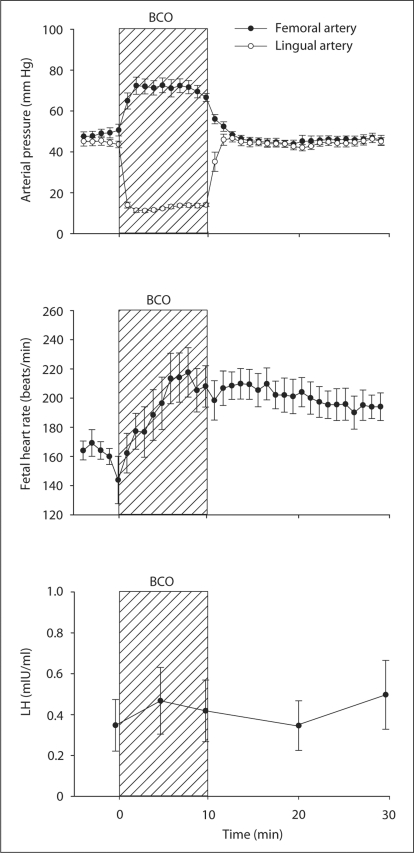
LH mRNA was significantly increased by BCO (p < 0.005 by two-way ANOVA), but not significantly altered by estradiol treatment (fig. 2). The effect of BCO on LH mRNA abundance was also revealed by significant differences (p < 0.05 by Bonferroni test) between BCO-treated and BCO-untreated groups in both placebo and estradiol-treated fetuses. There were no statistically significant changes in either FSH or PRL mRNA resulting from either BCO or estradiol treatment (fig. 2). In a separate experiment (study 3), we tested the hypothesis that plasma concentrations of LH are increased in response to BCO. We measured plasma LH concentrations before, during, and after BCO (fig. 3). Hemodynamics are shown in figure 3 for comparison, although values of these variables have been reported as a part of a larger study [20]. BCO decreased lingual arterial pressure (downstream from the occlusion) and stimulated reflex increases in femoral arterial blood pressure and heart rate. Although these fetuses responded to the BCO with endocrine responses typical of responses to stress (increased POMC, ACTH, and cortisol plasma concentrations as previously reported), plasma LH concentrations were low and not significantly increased by the BCO (fig. 3).
Fig. 2.
Relative mRNA abundance of ovine LH β-subunit (a), ovine FSH β-subunit (b), and ovine PRL (c) in fetal pituitaries. The fetuses had or had not been subjected to BCO, and either had or had not been treated with estradiol. Data are reported as mean values 8 SEM of relative abundances. The asterisk represents a statistically significant effect of BCO (p < 0.005 by ANOVA).
Fig. 3.
Hemodynamic and plasma LH responses to BCO in 11 fetal sheep. Data are reported as mean values ± SEM.
Discussion
The results of the present study reveal that hypotension stimulates pituitary expression of LH mRNA without an accompanying increase in fetal plasma LH concentrations. The results are consistent with the conclusion that plasma LH, FSH, and PRL do not respond to acute fetal stress in late gestation, using BCO as a model of acute stress.
Prolactin
Our studies demonstrate an increase in PRL as the fetus matures. In the adult, PRL is stimulated by estrogen [23]. For example, PRL is dramatically increased in pregnancy [24]; this increase is likely dependent upon stimulation of PRL with estrogen. In the present study, we measured an increase in PRL mRNA in response to combined estradiol and BCO. It is possible that a stronger stimulus to PRL is required to increase mRNA abundance, that milder stimuli could not increase expression above the already elevated expression levels in late gestation (fig. 3). In adult human and animal subjects, PRL secretion is increased in response to stress [25, 26]. However, this does not appear to be true in the case of hypotension stress in the ovine fetus. In a study by Drummond et al. [27], hemorrhage did not alter ovine fetal plasma PRL concentrations. Reports of PRL responsiveness to stress at delivery are inconsistent [28, 29]. It is an interesting possibility that PRL might stimulate adrenal steroidogenesis. Freemark and colleagues [30, 31, 32] have demonstrated that PRL receptors are expressed in the adrenal cortex of nonhuman primates and of rodents. Albrecht, Pepe, and collaborators [13, 33] have reported that, in the baboon, PRL stimulates fetal dehydroepiandrosterone secretion. It is possible, therefore, that in estrogen-treated fetuses PRL secretion in response to BCO contributes to the stimulation of adrenocortical cortisol secretion. Nevertheless, at present there is no evidence that PRL stimulates adrenocortical secretion in this species.
Gonadotropins and PRL
We know of no other studies demonstrating that LH mRNA is increased in response to either hypotension or cerebral hypoperfusion in the fetus, and we know of no studies in which LH responses to cardiovascular stimuli have been tested. Although novel in the fetus, the concept that LH secretion is modified by stress is not, by itself, new. For example, plasma LH concentrations in rats are increased after exposure to ether vapors [34]. Stress is perhaps more often associated with suppression of LH secretion, as reviewed by Breen and Karsch [35]. Although the LH mRNA abundance was significantly increased after BCO, the plasma concentrations were not (fig. 3). Consistent with reports by Grumbach and colleagues [36, 37], the circulating concentration of LH in fetal blood is low in late gestation, perhaps the result of negative feedback inhibition by circulating placental steroids. The data suggest that the increase in LH mRNA is not translated efficiently into protein and/or secreted into blood. This impaired translation is consistent with the reported dissociation of mRNA and secreted protein in response to inhibition of LH secretion by estrogen [38]. One interesting possibility is that, because of the common developmental origin of corticotropes and gonadotropes, and because of the similarity of the LH response to the POMC response to BCO [Wood and Keller-Wood, unpubl. obs.], it is possible that some of the POMC, LH, and perhaps FSH mRNA in the fetal pituitary might reside in pituitary cells that are immature and that do not actively secrete either peptide [39]. If this were true, the POMC peptides secreted in response to BCO would presumably originate in more fully differentiated cells. In support of this notion is the report by Bell et al. [40] that not all corticotropes express prohormone convertase 1: in other words, not all corticotropes are capable of secreting ACTH.
Estradiol alone decreased LH and FSH mRNA. The increase in LH mRNA in response to BCO was not attenuated by estradiol, suggesting that the negative feedback inhibition of LH is on basal secretion only or, alternatively, that the feedback-sensitive pathways within the fetal brain are not involved in the stimulation of LH responses to BCO. Because fetal plasma estrogen concentrations increase in term fetuses [41], negative feedback inhibition of LH might also explain the statistically significant decrease in LH mRNA in fetal sheep at 145 days of gestation compared to younger and older animals. The feedback effects of the sex steroids on LH and FSH are complex, comprised of both positive and negative feedback influences [42].
Overall, the results of the present study reveal that, in response to relatively severe hypotension, pituitary LH mRNA abundance is increased but that the increase in mRNA is not accompanied by an increase in plasma concentrations of LH. These data are consistent with our overall thesis that increased estrogen action in the fetus in late gestation stimulates and coordinates an augmentation of the fetal response to stress.
Acknowledgements
This work was supported by NIH grants HD42035, HD33053, and DK62080 (to M.K.-W.). We thank Ms. Xiaoyang (Lisa) Fang for the performance of real-time RT-PCR and Dr. Yun-Ju He for performance of hormone assays. Finally, we thank Drs. Damian Giroux, Christine Schlaerth, and Jason Gersting for their contributions to various aspects of these experiments.
References
- 1.Toubas PL, Silverman NH, Heymann MA, Rudolph AM. Cardiovascular effects of acute hemorrhage in fetal lambs. Am J Physiol. 1981;240:H45–H48. doi: 10.1152/ajpheart.1981.240.1.H45. [DOI] [PubMed] [Google Scholar]
- 2.Wood CE. Estrogen/hypothalamus-pituitary-adrenal axis interactions in the fetus: the interplay between placenta and fetal brain. J Soc Gynecol Investig. 2005;12:67–76. doi: 10.1016/j.jsgi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Wood CE, Chen HG, Bell ME. Role of vagosympathetic fibers in the control of adrenocorticotropic hormone, vasopressin, and renin responses to hemorrhage in fetal sheep. Circ Res. 1989;64:515–523. doi: 10.1161/01.res.64.3.515. [DOI] [PubMed] [Google Scholar]
- 4.Tong H, Lakhdir F, Wood CE. Endogenous prostanoids modulate the ACTH and AVP responses to hypotension in late-gestation fetal sheep. Am J Physiol. 1998;275:R735–R741. doi: 10.1152/ajpregu.1998.275.3.R735. [DOI] [PubMed] [Google Scholar]
- 5.Gardner DS, Jamall E, Fletcher AJ, Fowden AL, Giussani DA. Adrenocortical responsiveness is blunted in twin relative to singleton ovine fetuses. J Physiol. 2004;557:1021–1032. doi: 10.1113/jphysiol.2004.061796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giussani DA, McGarrigle HH, Moore PJ, Bennet L, Spencer JA, Hanson MA. Carotid sinus nerve section and the increase in plasma cortisol during acute hypoxia in fetal sheep. J Physiol Lond. 1994;477:75–80. doi: 10.1113/jphysiol.1994.sp020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HG, Wood CE. The adrenocorticotropic hormone and arginine vasopressin responses to hypercapnia in fetal and maternal sheep. Am J Physiol. 1993;264:R324–R330. doi: 10.1152/ajpregu.1993.264.2.R324. [DOI] [PubMed] [Google Scholar]
- 8.Cudd TA, Wood CE. Does thromboxane mediate the fetal ACTH response to acidemia? Am J Physiol. 1996;270:R594–R598. doi: 10.1152/ajpregu.1996.270.3.R594. [DOI] [PubMed] [Google Scholar]
- 9.Purinton SC, Wood CE. Oestrogen augments the fetal ovine hypothalamus- pituitary-adrenal axis in response to hypotension. J Physiol. 2002;544:919–929. doi: 10.1113/jphysiol.2002.025635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saoud CJ, Wood CE. Modulation of ovine fetal adrenocorticotropin secretion by androstenedione and 17beta-estradiol. Am J Physiol. 1997;272:R1128–R1134. doi: 10.1152/ajpregu.1997.272.4.R1128. [DOI] [PubMed] [Google Scholar]
- 11.Wood CE, Gridley KE, Keller-Wood M. Biological activity of 17betaestradiol-3-sulfate in ovine fetal plasma and uptake in fetal brain. Endocrinology. 2003;144:599–604. doi: 10.1210/en.2002-220764. [DOI] [PubMed] [Google Scholar]
- 12.Wood CE, Powers MJ, Keller-Wood M. Blockade of PGHS-2 inhibits the hypothalamus-pituitary-adrenal axis response to cerebral hypoperfusion in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1813–R1819. doi: 10.1152/ajpregu.90917.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pepe GJ, Waddell BJ, Albrecht ED. The effects of adrenocorticotropin and prolactin on adrenal dehydroepiandrosterone secretion in the baboon fetus. Endocrinology. 1988;122:646–650. doi: 10.1210/endo-122-2-646. [DOI] [PubMed] [Google Scholar]
- 14.Alevizaki M, Saltiki K, Mantzou E, Anastasiou E, Huhtaniemi I. The adrenal gland may be a target of LH action in postmenopausal women. Eur J Endocrinol. 2006;154:875–881. doi: 10.1530/eje.1.02165. [DOI] [PubMed] [Google Scholar]
- 15.Wood CE. Cerebral hypoperfusion increases estrogen receptor abundance in the ovine fetal brain and pituitary. Neuroendocrinology. 2008;87:216–222. doi: 10.1159/000112844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller-Wood M, Powers MJ, Gersting JA, Ali N, Wood CE. Genomic analysis of neuroendocrine development of fetal brain-pituitary-adrenal axis in late gestation. Physiol Genomics. 2006;24:218–224. doi: 10.1152/physiolgenomics.00176.2005. [DOI] [PubMed] [Google Scholar]
- 17.Wood CE, Giroux D. Central nervous system prostaglandin endoperoxide synthase-1 and -2 responses to oestradiol and cerebral hypoperfusion in late-gestation fetal sheep. J Physiol. 2003;549:573–581. doi: 10.1113/jphysiol.2002.038398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood CE, Giroux D. Expression of nitric oxide synthase isoforms in the ovine fetal brain: alteration by hormonal and hemodynamic stimuli. J Soc Gynecol Investig. 2006;13:329–337. doi: 10.1016/j.jsgi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Tong H, Wood CE. Indomethacin attenuates the cerebral blood flow response to hypotension in late-gestation fetal sheep. Am J Physiol. 1999;277:R1268–R1273. doi: 10.1152/ajpregu.1999.277.5.R1268. [DOI] [PubMed] [Google Scholar]
- 20.Powers MJ, Wood CE. Ketamine inhibits fetal ACTH responses to cerebral hypoperfusion. Am J Physiol Regul Integr Comp Physiol. 2007;292:1542–1549. doi: 10.1152/ajpregu.00300.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaub CE, Keller-Wood M, Wood CE. Blockade of estrogen receptors decreases CNS and pituitary prostaglandin synthase expression in fetal sheep. Neuroendocrinology. 2008;87:121–128. doi: 10.1159/000109664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Shupnik MA, Baxter LA, French LR, Gorski J. In vivo effects of estrogen on ovine pituitaries: prolactin and growth hormone biosynthesis and messenger ribonucleic acid translation. Endocrinology. 1979;104:729–735. doi: 10.1210/endo-104-3-729. [DOI] [PubMed] [Google Scholar]
- 24.Rigg LA, Lein A, Yen SS. Pattern of increase in circulating prolactin levels during human gestation. Am J Obstet Gynecol. 1977;129:454–456. doi: 10.1016/0002-9378(77)90594-4. [DOI] [PubMed] [Google Scholar]
- 25.Blackwell RE, Guillemin R. Hypothalamic control of adenohypophysial secretions. Annu Rev Physiol. 1973;35:357–390. doi: 10.1146/annurev.ph.35.030173.002041. [DOI] [PubMed] [Google Scholar]
- 26.Neill JD. Effect of ‘stress’ on serum prolactin and luteinizing hormone levels during the estrous cycle of the rat. Endocrinology. 1970;87:1192–1197. doi: 10.1210/endo-87-6-1192. [DOI] [PubMed] [Google Scholar]
- 27.Drummond WH, Rudolph AM, Keil LC, Gluckman PD, Macdonald AA, Heymann MA. Arginine vasopressin and prolactin after hemorrhage in the fetal lamb. Am J Physiol. 1980;238:E214–E219. doi: 10.1152/ajpendo.1980.238.3.E214. [DOI] [PubMed] [Google Scholar]
- 28.Ramin SM, Porter JC, Gilstrap LC, Rosenfeld CR. Stress hormones and acid-base status of human fetuses at delivery. J Clin Endocrinol Metab. 1991;73:182–186. doi: 10.1210/jcem-73-1-182. [DOI] [PubMed] [Google Scholar]
- 29.Vogl SE, Worda C, Egarter C, Bieglmayer C, Szekeres T, Huber J, Husslein P. Mode of delivery is associated with maternal and fetal endocrine stress response. BJOG. 2006;113:441–445. doi: 10.1111/j.1471-0528.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 30.Freemark M. Ontogenesis of prolactin receptors in the human fetus: roles in fetal development. Biochem Soc Trans. 2001;29:38–41. doi: 10.1042/0300-5127:0290038. [DOI] [PubMed] [Google Scholar]
- 31.Freemark M, Driscoll P, Maaskant R, Petryk A, Kelly PA. Ontogenesis of prolactin receptors in the human fetus in early gestation. Implications for tissue differentiation and development. J Clin Invest. 1997;99:1107–1117. doi: 10.1172/JCI119239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royster M, Driscoll P, Kelly PA, Freemark M. The prolactin receptor in the fetal rat: cellular localization of messenger ribonucleic acid, immunoreactive protein, and ligand-binding activity and induction of expression in late gestation. Endocrinology. 1995;136:3892–3900. doi: 10.1210/endo.136.9.7649097. [DOI] [PubMed] [Google Scholar]
- 33.Walker ML, Pepe GJ, Albrecht ED. Regulation of baboon fetal adrenal androgen formation by pituitary peptides at mid- and late gestation. Endocrinology. 1988;122:546–551. doi: 10.1210/endo-122-2-546. [DOI] [PubMed] [Google Scholar]
- 34.Euker JS, Meites J, Riegle GD. Effects of acute stress on serum LH and prolactin in intact, castrate and dexamethasone-treated male rats. Endocrinology. 1975;96:85–92. doi: 10.1210/endo-96-1-85. [DOI] [PubMed] [Google Scholar]
- 35.Breen KM, Karsch FJ. New insights regarding glucocorticoids, stress and gonadotropin suppression. Front Neuroendocrinol. 2006;27:233–245. doi: 10.1016/j.yfrne.2006.03.335. [DOI] [PubMed] [Google Scholar]
- 36.Gluckman PD, Marti-Henneberg C, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. 14. The effect of 17-beta estradiol infusion on fetal plasma gonadotropins and prolactin and the maturation of sex steroid-dependent negative feedback. Endocrinology. 1983;112:1618–1623. doi: 10.1210/endo-112-5-1618. [DOI] [PubMed] [Google Scholar]
- 37.Albers N, Bettendorf M, Herrman H, Kaplan SL, Grumbach MM. Hormone ontogeny in the ovine fetus. 27. Pulsatile and copulsatile secretion of luteinizing hormone, follicle-stimulating hormone, growth hormone, and prolactin in late gestation: a new method for analysis of copulsatility. Endocrinology. 1993;132:701–709. doi: 10.1210/endo.132.2.8425489. [DOI] [PubMed] [Google Scholar]
- 38.Mercer JE, Phillips DJ, Clarke IJ. Short-term regulation of gonadotropin subunit mRNA levels by estrogen: studies in the hypothalamo-pituitary intact and hypothalamo-pituitary disconnected ewe. J Neuroendocrinol. 1993;5:591–596. doi: 10.1111/j.1365-2826.1993.tb00526.x. [DOI] [PubMed] [Google Scholar]
- 39.Antolovich GC, Perry RA, Trahair JF, Silver M, Robinson PJ. The development of corticotrophs in the fetal sheep pars distalis: the effect of adrenalectomy or cortisol infusion. Endocrinology. 1989;124:1333–1339. doi: 10.1210/endo-124-3-1333. [DOI] [PubMed] [Google Scholar]
- 40.Bell ME, Myers TR, Myers DA. Expression of proopiomelanocortin and prohormone convertase-1 and -2 in the late gestation fetal sheep pituitary. Endocrinology. 1998;139:5135–5143. doi: 10.1210/endo.139.12.6374. [DOI] [PubMed] [Google Scholar]
- 41.Yu HK, Cabalum T, Jansen CA, Buster JE, Nathanielsz PW. Androstenedione, testosterone, and estradiol concentrations in fetal and maternal plasma in late pregnancy in the sheep. Endocrinology. 1983;113:2216–2220. doi: 10.1210/endo-113-6-2216. [DOI] [PubMed] [Google Scholar]
- 42.Fink G. Oestrogen and progesterone interactions in the control of gonadotrophin and prolactin secretion. J Steroid Biochem. 1988;30:169. doi: 10.1016/0022-4731(88)90090-8. [DOI] [PubMed] [Google Scholar]