Abstract
Alcohol abuse is a major global health problem, but there is still much uncertainty about the mechanisms of action. So far, the effects of ethanol on ion channels in the plasma membrane have received the most attention. We have now investigated actions on intracellular calcium channels in pancreatic acinar cells. Our aim was to discover the mechanism by which alcohol influences calcium homeostasis and thereby understand how alcohol can trigger premature intracellular trypsinogen activation, which is the initiating step for alcohol-induced pancreatitis. We used intact or two-photon permeabilized acinar cells isolated from wild-type mice or mice in which inositol trisphosphate receptors of type 2 or types 2 and 3 were knocked out. In permeabilized pancreatic acinar cells even a relatively low ethanol concentration elicited calcium release from intracellular stores and intracellular trypsinogen activation. The calcium sensor calmodulin (at a normal intracellular concentration) markedly reduced ethanol-induced calcium release and trypsinogen activation in permeabilized cells, effects prevented by the calmodulin inhibitor peptide. A calmodulin activator virtually abolished the modest ethanol effects in intact cells. Both ethanol-elicited calcium liberation and trypsinogen activation were significantly reduced in cells from type 2 inositol trisphosphate receptor knockout mice. More profound reductions were seen in cells from double inositol trisphosphate receptor (types 2 and 3) knockout mice. The inositol trisphosphate receptors, required for normal pancreatic stimulus–secretion coupling, are also responsible for the toxic ethanol action. Calmodulin protects by reducing calcium release sensitivity.
Alcohol abuse is a major global health problem, but there is still much uncertainty about the primary mechanisms of alcohol action. It is now clear that general biophysical lipid effects cannot explain relevant alcohol actions, but that alcohol influences the open state probability of specific ion channels including receptors for NMDA, GABA, and acetylcholine (ACh) (1). Alcohol could also have effects on intracellular ion channels, but this has not so far been investigated. The pancreatic acinar cell is a classical preparation for studying intracellular ion channels (2, 3) and we have therefore investigated alcohol actions on intracellular calcium (Ca2+) channels in pancreatic acinar cells.
Excessive alcohol intake is one of the major causes of acute pancreatitis, a potentially fatal human disease in which the pancreas digests itself and its surroundings (4). Repeated episodes of acute pancreatitis can lead to chronic pancreatitis, which markedly increases the risk of pancreatic cancer (4). Autodigestion is initiated by trypsin activation inside the pancreatic acinar cells, which is mediated by the generation of excessive intracellular Ca2+ signals elicited by unphysiologically high concentrations of ACh or cholecystokinin, by long-chain fatty acids (FAs) or fatty acid ethyl esters (FAEEs), or by bile acids (4, 5). Although there is no doubt about the crucial role of malfunctioning acinar cells in the initiation of acute pancreatitis, important pathological changes in duct cell function can also be influential (6, 7).
Despite the well established correlation between alcohol intake and development of acute pancreatitis (4), the effect of ethanol on isolated pancreatic acinar cells is very variable. In a previous study, we found that ethanol even in very high concentrations (>100 mM) mostly had only minor effects on Ca2+ homeostasis (8). The work of Laposata and Lange (9) indicated that the toxic effect of alcohol was largely due to production of nonoxidative metabolites, namely FAEEs, and later work has highlighted the damaging effects of these substances on pancreatic acinar cells (8, 10, 11). Recent work has shown that palmitoleic acid ethyl ester (POAEE) releases Ca2+ from both the endoplasmic reticulum (ER) and an acid store in the apical part of pancreatic acinar cells and indicates that it is the release from the acid store, predominantly through IP3 receptors (IP3Rs) of types 2 and 3, that is principally responsible for the intracellular activation of trypsinogen (12). Long-chain fatty acids, for example palmitoleic acid, can induce a more slowly developing Ca2+ release also causing intracellular trypsinogen activation (10, 13, 14), which to a large extent depends on inhibition of mitochondrial function and subsequent reduction in the capacity to remove excess Ca2+ from the cytosol through ATP-dependent Ca2+ pumps (10, 15).
The evidence implicating FAEEs as important mediators of the crucial intracellular trypsinogen activation initiating alcohol-related pancreatitis does not exclude other effects of alcohol (16, 17). In view of the variable acute effects of ethanol itself on isolated pancreatic acinar cells (8) we have carried out a detailed study of the action of alcohol, using two-photon permeabilized cells, which have turned out to be useful preparations for studies of Ca2+ homeostasis (12, 18, 19).
In two-photon permeabilized acinar cells, ethanol—in a concentration as low as 10 mM—consistently evoked release of Ca2+ from intracellular stores and induced trypsinogen activation. An important part of this Ca2+ release occurred from an acid bafilomycin-sensitive store in the apical part of the cells and—as shown in IP3R knockout and double-knockout experiments—this was principally mediated by IP3Rs of types 2 and 3. The reason for the difference between the effects of alcohol on intact and permeabilized acinar cells was explored. Addition of the Ca2+ sensor calmodulin (CaM) to the external solution—which had access to the intracellular (cytosolic) compartment through the hole in the plasma membrane created by two-photon laser light—markedly reduced the ability of ethanol to release Ca2+ from intracellular stores and also markedly inhibited trypsinogen activation. A CaM concentration of 2.5 μM, which corresponds roughly to the normal intracellular CaM concentration in intact acinar cells (20), abolished the Ca2+-releasing effect of 10 mM ethanol as well as the trypsinogen activation and markedly reduced the effects of 100 mM ethanol. The effects of CaM in the permeabilized cells were abolished by addition of CaM inhibitor peptide and the relatively small effects of ethanol on intact acinar cells were virtually abolished by the CaM activator CALP-3. We conclude that the IP3 receptors, required for normal pancreatic stimulus–secretion coupling, are also responsible for the toxic ethanol action and that calmodulin exerts a protective effect by reducing Ca2+ release sensitivity.
Results
Ethanol Releases Ca2+ from Intracellular Stores in Permeabilized Cells.
To study the role of intracellular Ca2+ stores in ethanol-induced Ca2+ signal generation, we used a low-Kd Ca2+ indicator (Fluo-5N AM) to measure [Ca2+] changes inside the stores ([Ca2+]store) in two-photon permeabilized pancreatic acinar cells (12, 18, 19). Ethanol (100 mM) evoked a marked reduction in [Ca2+]store, similar to that previously shown to occur in response to palmitoleic acid ethyl ester (12) (Fig. S1 A and C). The permeabilized cells responded with marked Ca2+ release to much lower ethanol concentrations than were required to elicit Ca2+ release in intact cells (8). Inhibition of IP3Rs with 2-aminoethoxydiphenilborinate (2-APB) or heparin markedly reduced the responses to alcohol as did inhibition of ryanodine receptors (RyRs) with Ruthenium Red (RR). Combined inhibition of both receptors reduced Ca2+ release more effectively than separate applications of the inhibitors, but still did not abolish the ethanol response (Fig. S1 A and B).
Ethanol-Induced Ca2+ Release and Trypsin Activity Are Calmodulin Dependent.
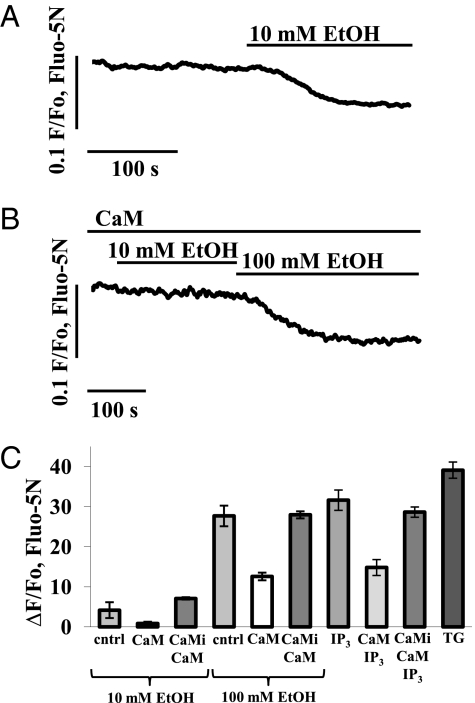
CaM is an important intracellular Ca2+ sensor, influencing many cellular events (21). CaM is a relatively small protein, which can be dialyzed into cells via a patch clamp pipette (whole-cell configuration) (20), and is therefore likely to be washed out of two-photon permeabilized cells. We tested the hypothesis that the difference between the modest effects of ethanol in intact cells and the substantial effects in two-photon permeabilized cells might be due to lack of CaM in the permeabilized preparation. In these experiments we used a CaM concentration of 2.5 μM, because this is the estimated normal cytoplasmic CaM level in pancreatic acinar cells (20). Fig. 1A shows a typical Ca2+ release response to 10 mM ethanol in a permeabilized cell (n = 8). In the presence of CaM, the same concentration of ethanol failed to induce any Ca2+ release (Fig. 1 B and C; n = 9; P < 0.006). However, after pretreatment of permeabilized cells with CaM in the presence of CaM inhibitor peptide (20 μM), responses to 10 mM ethanol were restored (Fig. 1C; n = 8; P > 0.07). At the higher ethanol concentration of 100 mM, Ca2+ release was observed in the presence of CaM, but it was markedly reduced (Fig. 1 B and C; n = 7; P < 0.01) compared with the release obtained without CaM (Fig. 1C; n = 10). Responses to 100 mM ethanol were restored by addition of 20 μM CaM inhibitor peptide (Fig. 1C; n = 8; P > 0.1). We also tested the effect of CaM on the Ca2+ release from internal stores evoked by IP3. CaM inhibited markedly the IP3-induced Ca2+ release (Fig. 1C; n = 5; P < 0.0008). The CaM inhibitor peptide (20 μM) fully restored the responses to 10 μM IP3 (Fig. 1C; n = 5; P < 0.2).
Fig. 1.
Ethanol-induced Ca2+ release from all stores is reduced by adding CaM to the solution in which the permeabilized cells are suspended. (A) Normal (control) reduction in [Ca2+]store in response to stimulation with 10 mM ethanol. (B) In the presence of CaM (2.5 μM), the same ethanol concentration (10 mM) fails to induce any change in [Ca2+]store (compare with Fig. 1A). A higher concentration of ethanol (100 mM) induces small Ca2+ release. (C) Quantitative summary of results concerning CaM inhibition of ethanol-induced Ca2+ release. Ethanol responses were restored in the presence of a mixture of CaM (2.5 μM) and CaM inhibitory peptide (20 μM). Also included are data showing that Ca2+ release elicited by IP3 (10 μM) was reduced by CaM and that CaM inhibitory peptide (20 μM) restored the responses. For comparison, the amplitude of the response to thapsigargin (10 μM) is included. Error bars indicate SEM.
Responses Evoked by Ethanol in Intact Cells.
As previously reported (15), the effects of ethanol on intact pancreatic acinar cells are very modest. Using an ethanol concentration of 100 mM, which is within the range of ethanol levels that have been measured in plasma from individuals caught driving under the influence of alcohol (22), more than half of the Fura-2–loaded cells (56%, 10 of 18) developed only a tiny sustained elevation of the cytosolic Ca2+ concentration ([Ca2+]i) above the initial basal level (average amplitude of elevation: 31 ± 6 nM, n = 18). The other 8 cells (44%) did not respond at all.
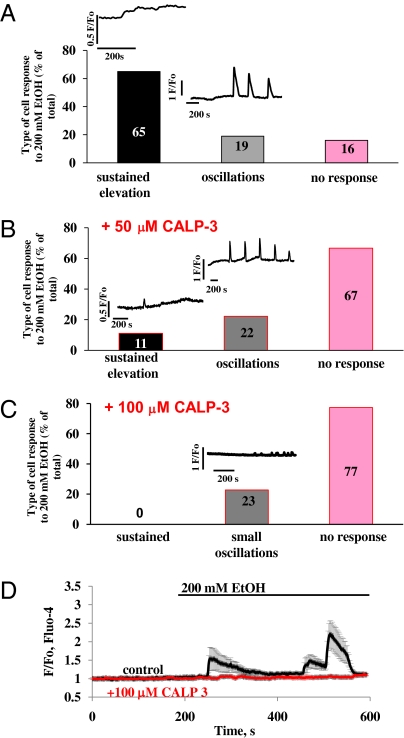
In Fluo-4–loaded cells, we tested the effects of 200 mM ethanol (Fig. 2). The majority of cells (65%, 28 cells of 43) again responded with a small, sustained increase in [Ca2+]i (Fig. 2A), whereas a smaller population (16%, 7 of 43 cells) did not show any response. However, we observed a unique phenomenon of delayed oscillatory [Ca2+]i elevations in 19% (8 of 43) of the examined cells. In these cases the ethanol response started with the usual tiny rise in [Ca2+]i, but thereafter (200–400 s) a set of robust [Ca2+]i spikes could be observed (Fig. 2A). The elevations lasted ∼50–75 s and repeated with reducing amplitude within ∼100 s. Although ethanol-induced Ca2+ oscillations have not previously been reported, the principal result of our new experiments on intact cells, namely the very small or absent responses to even a relatively high ethanol concentration, is in agreement with our earlier report (8).
Fig. 2.
In intact cells, ethanol (200 mM EtOH) mostly evokes small sustained elevations in [Ca2+]I and some broad oscillations and in some cases fails to evoke any response. The calmodulin activator CALP-3 effectively blocks ethanol-induced calcium responses. (A) Percentage of cells giving small sustained [Ca2+]i elevation responses, broad oscillations, or no response to stimulation with ethanol. (B) Percentage of cells giving small sustained elevation responses, short oscillations, or no response to stimulation with ethanol in the presence of 50 μM CALP-3. (C) Percentage of cells giving very small oscillations or no response to stimulation with ethanol in presence of 100 μM CALP-3. (D) Average responses to ethanol in the presence (red) and the absence (black) of CALP-3 (100 μM). Error bars indicate SEM.
As already mentioned, the CaM inhibitor peptide restored ethanol-induced Ca2+ release in permeabilized cells, which had been inhibited by CaM (Fig. 1C), and we therefore decided to test the potential effect of a cell-permeable CaM activator (CALP-3) on the sensitivity of intact cells to ethanol. The CaM activator almost abolished the ethanol-induced [Ca2+]i elevation (Fig. 2B). The percentage of cells responding with a small sustained [Ca2+]i elevation to ethanol was reduced from 65% (Fig. 2A) to 11% (Fig. 2B, n = 18) by CALP-3 (50 μM). Interestingly, 100 μM CALP-3 completely blocked sustained responses to ethanol (Fig. 2C, n = 22), but 23% of the cells still responded with oscillations, similarly to the situation at 50 μM or without CALP-3 (Fig. 2 A–C). However, the amplitude of the ethanol-induced Ca2+ oscillations in the presence of 100 μM CALP-3 was very dramatically reduced (to 0.12 + 0.05 ΔF/F0, Fig. 2C) compared with those seen at 50 μM CALP-3 (Fig. 2B) or without the CaM activator (Fig. 2A). The averaged responses to 200 mM ethanol are shown in Fig. 2D in the presence (red trace, n = 18) and the absence (black trace, n = 10) of CALP-3 (100 μM).
Involvement of Acidic Stores in Ca2+ Release Induced by Ethanol.
The principal compartment from which Ca2+ can be liberated in pancreatic acinar cells is the ER (3). However, it has become clear in recent years that intracellular Ca2+ pools in acid organelles also play important roles in intracellular Ca2+ homeostasis (3, 23–25) and particularly do so in pancreatic acinar cells (12, 18, 19, 26, 27). Secretory (zymogen) granules (ZG) constitute a major part of the acid pool and contain large amounts of Ca2+ (3). They also contain large amounts of Zn2+ (28). [In the case of Zn2+ it is known that the ZG membrane is provided with a specific Zn2+ transporter (ZnT2) (28), whereas the Ca2+ uptake mechanism into ZGs still remains obscure (3)].
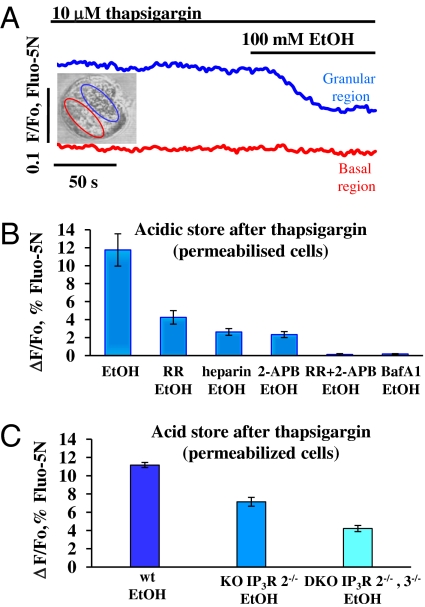
To test the possible involvement of the acid store in the ethanol-elicited Ca2+ release, we depleted the ER stores by arresting the ER Ca2+ pumps using the specific inhibitor thapsigargin (29) and tested the effect of ethanol. As previously documented, thapsigargin (10 μM) causes a marked reduction of [Ca2+]store that can be observed in all parts of the cells (12, 18, 19, 30). After thapsigargin reduced [Ca2+]store to a lower stable level, ethanol (100 mM) reduced [Ca2+]store in the granular part of the cell, but had no effect in the basal region (Fig. 3A, n = 8). Next, we investigated the nature of the response to ethanol in the granular region. To test whether the ethanol-sensitive apical Ca2+ response depends on the store being acid, we reduced the transmembrane H+ gradient by inhibiting the vacuolar type H+ ATPase activity with bafilomycin A1 (100 nM) (12, 18, 31). Preincubation with bafilomycin A1 did not affect the Ca2+ depletion of ER stores induced by thapsigargin [see figure 2 in Gerasimenko et al. (19)], but abolished the subsequent response to ethanol (Fig. 3B and Fig. S2A; n = 5; P < 0.0001). The ethanol-induced Ca2+ release from the acidic stores was markedly reduced by the IP3R inhibitors heparin (Fig. 3B; n = 6; P < 0.004) or 2-APB (Fig. 3B; n = 5; P < 0.004). The RyR inhibitor RR also inhibited Ca2+ release, but was less effective than the IP3R inhibitors (Fig. 3B; n = 5; P < 0.004). Combined inhibition of IP3Rs and RyRs (2-APB and RR) abolished Ca2+ release from the acidic stores (Fig. 3B; n = 5; P < 0.003).
Fig. 3.
Ethanol (100 mM) elicits Ca2+ release from thapsigargin-insensitive calcium stores (permeabilized cells). (A) After depletion of ER stores with thapsigargin, ethanol induces Ca2+ release from the granular area. Fluorescence traces show [Ca2+]store changes in a single permeabilized cell. Ethanol evokes marked reduction in [Ca2+]store in the granular region (blue trace), but not in the basal region (red trace). The transmitted light picture shows the cell and the two regions of interest. (B) Quantification of [Ca2+]acid store reduction evoked by ethanol after preincubation with thapsigargin in the absence and the presence of various inhibitors used in experiments of the type shown in Fig. S2A. Preincubation with bafilomycin A1 (100 nM Baf A1: 30 min) or with a mixture of 2-APB and RR (100 μM and 10 μM, respectively) blocked, whereas heparin (250 μg/mL), 2-APB (100 μM), or ruthenium red (RR) (10 μM) reduced to varying extents the ethanol-induced Ca2+ release. (C) Ethanol-induced Ca2+ release from thapsigargin-insensitive stores is severely reduced in permeabilized cells isolated from IP3R2−/− knockout and even more in IP3R2−/−, 3−/− double-knockout mice (quantitative summary of experiments shown in Fig. S2B).
We also tested the importance of RyRs in experiments in which IP3Rs were inhibited by subtype-specific antibodies (12). In the presence of antibodies to IP3Rs of types 1 and 2, RR caused further inhibition of ethanol-induced Ca2+ release from the acidic stores. In the combined presence of antibodies to IP3Rs of types 2 and 3 as well as RR, there was hardly any ethanol-induced Ca2+ release (Fig. S3).
Ethanol-Evoked Ca2+ Release Is Greatly Reduced by Knockouts of IP3Rs of Types 2 and 3.
Using pharmacological tools, it would appear that ethanol-induced Ca2+ release is highly dependent on functional IP3Rs (Fig. 3B and Fig. S1 A and B). However, the most conclusive and direct approach would be to compare the results from mice in which specific types of IP3Rs have been knocked out with those from the appropriate wild-type controls (12, 32). The ethanol-induced Ca2+ release from the acidic store in acinar cells from IP3R2−/− mice was markedly reduced (Fig. 3C and Fig. S2B; n = 9; P < 0.0001) compared with that in wild-type controls (Fig. 3C; n = 5), but a stronger reduction in the ethanol-elicited Ca2+ release from the acid store was observed in acinar cells isolated from mice in which both types 2 and 3 IP3Rs had been knocked out (Fig. 3C; n = 18). The Ca2+ release response from the double-KO (IP3R2−/−, IP3R3−/−) mice was significantly smaller (P < 0.00004) than that from the single-KO (IP3R2−/−) mice.
Ethanol Induces Trypsinogen Activation.
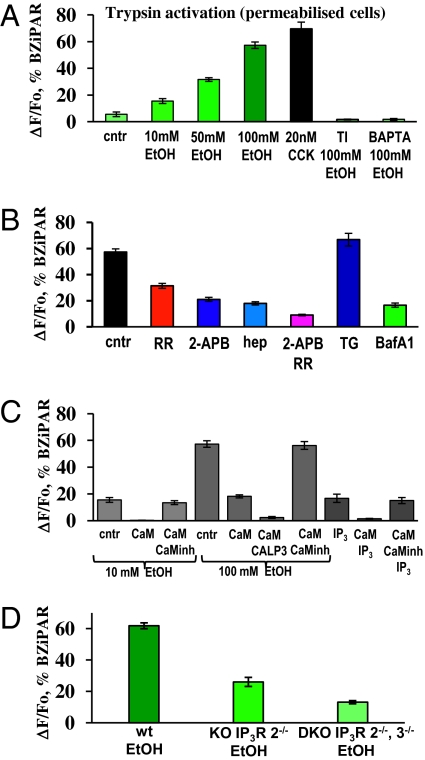
We monitored the time course of ethanol-induced trypsin activity in permeabilized cells by using a probe (BZiPAR) that becomes fluorescent when trypsin cleaves the two oligopeptide side chains (33). Control cells, not exposed to ethanol, have only a low-level background activity of trypsin (Fig. 4A; n = 7) probably due to a small proportion of cells damaged during isolation. A low dose of ethanol (10 mM) induced a small but clearly observable increase in trypsin activity (Fig. 4A; n = 8; P < 0.001). At a concentration of 50 mM, ethanol induced a more substantial activation of trypsin (Fig. 4A; n = 5; P < 0.0001) and at 100 mM a much higher degree of activation (Fig. 4A; n = 10; P < 0.0001). Soybean trypsin inhibitor (TI), at a concentration of 0.01%, completely blocked 100 mM ethanol-induced trypsin activity (Fig. 4A, n = 6; P < 0.0001 compared with response to 100 mM ethanol). To check the Ca2+ dependence of trypsin activation we performed experiments in high Ca2+ buffer conditions, where the bath—and therefore the cytosolic—[Ca2+] was clamped close to the physiological level (10−7 M) by a mixture of 10 mM BAPTA and 2 mM CaCl2. Under this condition, application of 100 mM ethanol failed to induce trypsin activity (Fig. 4A; n = 6; P < 0.0001 compared with control). Hyperstimulation with the Ca2+ mobilizing hormone cholecystokinin (CCK) using a concentration of 20 nM (the physiological concentration range is 1–20 pM) induced strong activation of trypsin (Fig. 4A; n = 5; P < 0.0001 compared with control) in agreement with previous data (34).
Fig. 4.
Ethanol-induced trypsin activity in permeabilized cells. (A) Concentration dependence of ethanol-evoked increase in intracellular trypsin activity. The results of control (no ethanol) experiments and the effects of trypsin inhibitor (TI), CCK, and clamping the external and therefore the cytosolic [Ca2+] at the normal resting level (Ca2+-BAPTA) are also shown. (B) Inhibition of ethanol-induced trypsin activity by 2-APB, heparin, Ruthenium Red, and a mixture of 2-APB and RR, as well as responses after preincubation with thapsigargin or Bafilomycin A1. Representative traces are shown in Fig. S4 A and B. (C) Ethanol-induced trypsin activity is dramatically reduced by CaM (2.5 μM) and practically blocked by calmodulin activator CALP-3. In the presence of CaM (2.5 μM), 10 mM ethanol fails to induce trypsin activity, but at 100 mM induces small activation. A combination of CALP-3 (100 μM) and CaM (2.5 μM) practically blocked trypsin activation by 100 mM ethanol. IP3-induced trypsin activation was also inhibited by CaM. Responses were restored in the presence of 20 μM CaM inhibitory peptide. Representative traces are shown in Fig. S4 C–E. (D) Ethanol elicits reduced trypsin activity in permeabilized cells isolated from IP3R2−/− mice compared with wild-type control mice and the response is even more severely reduced in cells isolated from IP3R2−/−, IP3R3−/− mice (quantitative summary of results of the type shown in Fig. S2C).
Ethanol-Induced Trypsinogen Activation Is Highly Dependent on Ca2+ Release from Acidic Stores.
Inhibition of IP3Rs with 100 μM 2-APB substantially reduced ethanol-induced trypsin activity (Fig. 4B; n = 5; P < 0.0001). Heparin also inhibited ethanol-induced trypsin activation to a similar degree (Fig. 4B; n = 5; P < 0.0001). Inhibition of RyRs with RR (10 μM) also reduced trypsin activity, but to a lesser extent (Fig. 4B and Fig. S4A; n = 7; P < 0.0001). Inhibition of both IP3Rs (with 100 μM of 2-APB) and RyRs (with 10 μM of RR) reduced activation to a very low level (Fig. 4B and Fig. S4A; n = 6; P < 0.0001). To explore the relative importance of the two different Ca2+ stores in trypsinogen activation we used thapsigargin and bafilomycin A1 (Fig. 4B and Fig. S4B). We emptied the ER store of Ca2+ slowly by initially using a thapsigargin concentration of 1 nM and subsequently increasing it to 10 μM (12); thereafter ethanol (100 mM) still elicited substantial trypsin activity (Fig. 4B and Fig. S4B; n = 6; P > 0.07), quantitatively similar to that seen in cells that had not been poisoned with thapsigargin. Preincubation of cells with 100 nM bafilomycin A1 for 30 min slowly emptied the acidic Ca2+ store (12); thereafter the ethanol-induced trypsin activity was very markedly reduced and much lower than in control cells (Fig. 4B and Fig. S4B; n = 7; P < 0.0001), indicating that Ca2+ release from the acidic store is particularly important for trypsinogen activation, possibly due to the previously shown cathepsin dependence of activation (12, 35). The neutralization of the intragranular pH is unlikely to have inhibited trypsinogen activation directly. A shift in pH in the neutral direction would be expected to promote trypsinogen activation, because the two predominant forms of trypsinogen—known as PRSS1 and PRSS2—autoactivate with a pH maximum ∼7 (36).
Calmodulin Inhibits Ethanol-Induced Trypsinogen Activation.
As for the ethanol-induced intracellular Ca2+ release (Fig. 2A), the ethanol-induced trypsin activity was also inhibited by CaM (Fig. 4C and Fig. S4 C and D). The activation by 10 mM ethanol was abolished (Fig. S4D; n = 6; P < 0.0001), whereas in parallel controls without CaM (Fig. 4C and Fig. S4C; n = 8) or with CaM plus the CaM inhibitor peptide (Fig. 4C; n = 5), 10 mM ethanol elicited a clear increase in trypsin activity (Fig. 4C). The trypsin activity induced by 100 mM ethanol was markedly reduced by CaM (Fig. 4C and Fig. S4D; n = 6; P < 0.0001) compared with parallel controls without CaM (Fig. 4C; n = 10) or in the presence of CaM and the CaM inhibitor peptide (Fig. 4C; n = 5).
The CaM activator CALP-3 together with CaM practically blocked trypsin activity induced by 100 mM ethanol (Fig. 4C and Fig. S4E; n = 10; P < 0.0001 compared with responses in the presence of CaM).
IP3 (10 μM) induced trypsin activity of a magnitude similar to that elicited by 10 mM ethanol (Fig. 4C; n = 7). Calmodulin blocked IP3-elicited trypsin activation whereas the CaM inhibitory peptide restored the response to 10 μM IP3 (Fig. 4C; n = 7; P > 0.3). IP3 (10 μM) evokes a smaller degree of trypsin activation than 100 mM ethanol (Fig. 4C), which may seem surprising in view of its strong effect on Ca2+ release from all of the stores (Fig. 1C). However, it is the release from the acid store that is important for trypsin activation (Fig. 3) and IP3 is not such a powerful releaser of Ca2+ from the acid store (figure S4 in ref. 12).
Ethanol-Elicited Trypsinogen Activation Depends on Functional IP3Rs of Types 2 and 3: Knockout of Types 2 and 3 IP3Rs.
Ethanol-elicited trypsin activity was measured in permeabilized pancreatic acinar cells from wild-type, IP3R2−/−, and IP3R2−/−, 3−/− mice (Fig. 4D and Fig. S2C). The ethanol-elicited trypsin activation was markedly reduced in the experiments on acinar cells from IP3R2−/− mice (Fig. 4D; n = 6; P < 0.0001) compared with controls (Fig. 4D; n = 5) and even more reduced in experiments on cells from the double-KO (IP3R2−/−, 3−/−) mice (Fig. 4D; n = 14). The ethanol-elicited trypsin activity was significantly lower in the double-KO experiments compared with the single KOs (P < 0.00004) (Fig. 4D).
Discussion
Our results show that ethanol, in concentrations (10–100 mM) that are pathophysiologically relevant (1), can generate substantial release of Ca2+ from intracellular stores, leading to intracellular protease activation. The effect of ethanol is largely due to Ca2+ release from acid stores mediated by IP3Rs and this process is CaM sensitive.
IP3Rs are the crucial molecules responsible for physiological agonist-elicited intracellular Ca2+ release and therefore also for normal stimulus–secretion coupling (3, 25, 32, 37, 38). Our data now demonstrate the crucial role of types 2 and 3 IP3Rs for the pathophysiology of alcohol-related pancreatitis. Although ethanol can release Ca2+ through these channels from both the ER and an acid granular store, our results indicate that the release from the acid store is particularly important for trypsinogen activation. This result is consistent with previous data on the effects of fatty acid ethyl esters and bile acids (12, 19).
The inhibitory effects of CaM on ethanol-induced Ca2+ release and trypsinogen activation can explain the apparent discrepancy between the weak effects of alcohol on intact pancreatic acinar cells and the strong effects on permeabilized cells, from which we expect CaM to have been—at least partially—washed out. The effect of CaM could be due to inhibition of IP3R opening, which has been reported in some systems (39–41), although there is currently no agreement about the mechanism of action of CaM on IP3Rs (42). Irrespective of the mechanism of action, the protective effect of CaM on the granular store is of considerable interest, particularly in view of data showing that the physiological local Ca2+ spikes in the granular region elicited by hormonal stimulation recruit CaM from the basolateral cytoplasm to the granular region (20). This recruitment of CaM occurring during physiological stimulation (20) may in view of our data be regarded as a functionally important process evolved to protect the granular region from excessive Ca2+ liberation that could lead to the potentially dangerous intragranular trypsinogen activation (43, 44) due to the ion exchange concept by Quesada and Verdugo (5, 45). The results obtained by Craske et al. (20) showed that supramaximal hormone stimulation, leading to a sustained [Ca2+]i elevation, produces only a single transient phase of CaM recruitment to the granular pole, whereas each physiologically occurring repetitive Ca2+ spike elicits movement of CaM from the basolateral part of the cell to the granular pole. Thus, repetitive Ca2+ spiking (3) seems a safer option than generation of a sustained [Ca2+]i increase. The marked inhibition of ethanol-induced Ca2+ release and trypsin activation by a calmodulin activator indicate potential therapeutic benefits for treatment of pancreatitis.
Recent work on mast cells demonstrated the potential benefit of reducing excessive Ca2+ signal generation for combating nasal polyposis and mast cell-dependent allergies (46, 47). Our data emphasize the potential therapeutic benefit of reducing excessive intracellular Ca2+ signal generation in the treatment of pancreatitis. In our study, knockout of the type 2 IP3R alone caused a significant reduction in alcohol-elicited intracellular Ca2+ release as well as trypsinogen activation, whereas in a previous study it was shown that deletion of the type 2 IP3R alone had no effect on acetylcholine-induced Ca2+ release (32). Thus, development of subtype-specific IP3R inhibitors could have therapeutic benefit.
Materials and Methods
Our methods, for assessing the [Ca2+] concentration in the cytosol or intracellular stores and intracellular trypsinogen activation in intact or two-photon permeabilized pancreatic acinar cells from normal WT mice or from mice in which IP3Rs of type 2—or both types 2 and 3—were knocked out, have been described in previous publications (12, 18). Details of the methods and reagents used are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the RIKEN Brain Science Institute-Olympus Collaboration Centre for technical assistance with confocal image acquisition. O.H.P. is a Medical Research Council Professor (G19/22). This work was supported by Grant G0700167 from The Medical Research Council (United Kingdom). G.L. and P.F. are Wellcome Trust Prize PhD students. K.M. was supported by a grant from the Japanese Science and Technology Agency Calcium Oscillation Project and a grant from the Japanese Society for Promotion of Science. M.W.S. was supported by a Japanese Society for Promotion of Science Postdoctoral Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016534108/-/DCSupplemental.
References
- 1.Spanagel R. Alcoholism: A systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 2.Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 3.Petersen OH, Tepikin AV. Polarized calcium signaling in exocrine gland cells. Annu Rev Physiol. 2008;70:273–299. doi: 10.1146/annurev.physiol.70.113006.100618. [DOI] [PubMed] [Google Scholar]
- 4.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: Bench to the bedside. Gastroenterology. 2007;132:1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Petersen OH, Sutton R. Ca2+ signalling and pancreatitis: effects of alcohol, bile and coffee. Trends Pharmacol Sci. 2006;27:113–120. doi: 10.1016/j.tips.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Venglovecz V, et al. Effects of bile acids on pancreatic ductal bicarbonate secretion in guinea pig. Gut. 2008;57:1102–1112. doi: 10.1136/gut.2007.134361. [DOI] [PubMed] [Google Scholar]
- 7.Lee MG, Muallem S. Pancreatitis: The neglected duct. Gut. 2008;57:1037–1039. doi: 10.1136/gut.2008.150961. [DOI] [PubMed] [Google Scholar]
- 8.Criddle DN, et al. Ethanol toxicity in pancreatic acinar cells: Mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA. 2004;101:10738–10743. doi: 10.1073/pnas.0403431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laposata EA, Lange LG. Presence of nonoxidative ethanol metabolism in human organs commonly damaged by ethanol abuse. Science. 1986;231:497–499. doi: 10.1126/science.3941913. [DOI] [PubMed] [Google Scholar]
- 10.Criddle DN, et al. Fatty acid ethyl esters cause pancreatic calcium toxicity via inositol trisphosphate receptors and loss of ATP synthesis. Gastroenterology. 2006;130:781–793. doi: 10.1053/j.gastro.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Werner J, et al. Pancreatic injury in rats induced by fatty acid ethyl ester, a nonoxidative metabolite of alcohol. Gastroenterology. 1997;113:286–294. doi: 10.1016/s0016-5085(97)70106-9. [DOI] [PubMed] [Google Scholar]
- 12.Gerasimenko JV, et al. Pancreatic protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc Natl Acad Sci USA. 2009;106:10758–10763. doi: 10.1073/pnas.0904818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang F, et al. The role of free fatty acids, pancreatic lipase and Ca+ signalling in injury of isolated acinar cells and pancreatitis model in lipoprotein lipase-deficient mice. Acta Physiol (Oxf) 2009;195:13–28. doi: 10.1111/j.1748-1716.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58:422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 15.Petersen OH, et al. Fatty acids, alcohol and fatty acid ethyl esters: Toxic Ca2+ signal generation and pancreatitis. Cell Calcium. 2009;45:634–642. doi: 10.1016/j.ceca.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Cosen-Binker LI, Binker MG, Wang CC, Hong W, Gaisano HY. VAMP8 is the v-SNARE that mediates basolateral exocytosis in a mouse model of alcoholic pancreatitis. J Clin Invest. 2008;118:2535–2551. doi: 10.1172/JCI34672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaisano HY, Gorelick FS. New insights into the mechanisms of pancreatitis. Gastroenterology. 2009;136:2040–2044. doi: 10.1053/j.gastro.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Gerasimenko JV, Sherwood M, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP, cADPR and IP3 all release Ca2+ from the endoplasmic reticulum and an acidic store in the secretory granule area. J Cell Sci. 2006;119:226–238. doi: 10.1242/jcs.02721. [DOI] [PubMed] [Google Scholar]
- 19.Gerasimenko JV, et al. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem. 2006;281:40154–40163. doi: 10.1074/jbc.M606402200. [DOI] [PubMed] [Google Scholar]
- 20.Craske M, et al. Hormone-induced secretory and nuclear translocation of calmodulin: Oscillations of calmodulin concentration with the nucleus as an integrator. Proc Natl Acad Sci USA. 1999;96:4426–4431. doi: 10.1073/pnas.96.8.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: From activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- 22.Jones AW. The drunkest drinking driver in Sweden: Blood alcohol concentration 0.545% w/v. J Stud Alcohol. 1999;60:400–406. doi: 10.15288/jsa.1999.60.400. [DOI] [PubMed] [Google Scholar]
- 23.Gerasimenko JV, Tepikin AV, Petersen OH, Gerasimenko OV. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr Biol. 1998;8:1335–1338. doi: 10.1016/s0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 24.Churchill GC, et al. NAADP mobilizes Ca2+ from reserve granules, lysosome related organelles, in sea urchin eggs. Cell. 2002;27:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 25.Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 26.Gerasimenko OV, Gerasimenko JV, Belan PV, Petersen OH. Inositol trisphosphate and cyclic ADP-ribose-mediated release of Ca2+ from single isolated pancreatic zymogen granules. Cell. 1996;84:473–480. doi: 10.1016/s0092-8674(00)81292-1. [DOI] [PubMed] [Google Scholar]
- 27.Yamasaki M, et al. Organelle selection determines agonist-specific Ca2+ signals in pancreatic acinar and beta cells. J Biol Chem. 2004;279:7234–7240. doi: 10.1074/jbc.M311088200. [DOI] [PubMed] [Google Scholar]
- 28.Guo L, et al. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc Natl Acad Sci USA. 2010;107:2818–2823. doi: 10.1073/pnas.0914941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thastrup O, Cullen PJ, Drøbak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerasimenko JV, et al. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol. 2003;163:271–282. doi: 10.1083/jcb.200306134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forgac M. Vacuolar ATPases: Rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol. 2007;8:917–929. doi: 10.1038/nrm2272. [DOI] [PubMed] [Google Scholar]
- 32.Futatsugi A, et al. IP3 receptor types 2 and 3 mediate exocrine secretion underlying energy metabolism. Science. 2005;309:2232–2234. doi: 10.1126/science.1114110. [DOI] [PubMed] [Google Scholar]
- 33.Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raraty M, et al. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA. 2000;97:13126–13131. doi: 10.1073/pnas.97.24.13126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Halangk W, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nemoda Z, Sahin-Tóth M. Chymotrypsin C (caldecrin) stimulates autoactivation of human cationic trypsinogen. J Biol Chem. 2006;281:11879–11886. doi: 10.1074/jbc.M600124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vanderheyden V, et al. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim Biophys Acta. 2009;1793:959–970. doi: 10.1016/j.bbamcr.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mikoshiba K. IP3 receptor/Ca2+ channel: From discovery to new signaling concepts. J Neurochem. 2007;102:1426–1446. doi: 10.1111/j.1471-4159.2007.04825.x. [DOI] [PubMed] [Google Scholar]
- 39.Michikawa T, et al. Calmodulin mediates calcium-dependent inactivation of the cerebellar type 1 inositol 1,4,5-trisphosphate receptor. Neuron. 1999;23:799–808. doi: 10.1016/s0896-6273(01)80037-4. [DOI] [PubMed] [Google Scholar]
- 40.Adkins CE, et al. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1, -2 and -3 inositol trisphosphate receptors. Biochem J. 2000;345:357–363. [PMC free article] [PubMed] [Google Scholar]
- 41.Kasri NN, et al. The N-terminal Ca2+-independent calmodulin-binding site on the inositol 1,4,5-trisphosphate receptor is responsible for calmodulin inhibition, even though this inhibition requires Ca2+ Mol Pharmacol. 2004;66:276–284. doi: 10.1124/mol.66.2.276. [DOI] [PubMed] [Google Scholar]
- 42.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87:593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sherwood MW, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104:5674–5679. doi: 10.1073/pnas.0700951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murphy JA, et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology. 2008;135:632–641. doi: 10.1053/j.gastro.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 45.Quesada I, Chin WC, Verdugo P. ATP-independent luminal oscillations and release of Ca2+ and H+ from mast cell secretory granules: Implications for signal transduction. Biophys J. 2003;85:963–970. doi: 10.1016/S0006-3495(03)74535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Di Capite J, Parekh AB. CRAC channels and Ca2+ signaling in mast cells. Immunol Rev. 2009;231:45–58. doi: 10.1111/j.1600-065X.2009.00808.x. [DOI] [PubMed] [Google Scholar]
- 47.Di Capite J, Nelson C, Bates G, Parekh AB. Targeting Ca2+ release-activated Ca2+ channel channels and leukotriene receptors provides a novel combination strategy for treating nasal polyposis. J Allergy Clin Immunol. 2009;124:1014–1021. doi: 10.1016/j.jaci.2009.08.030. e1–e3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.