Abstract
Conventional nicotinic acetylcholine receptor (nAChR) agonists, such as acetylcholine, act at an extracellular “orthosteric” binding site located at the interface between two adjacent subunits. Here, we present evidence of potent activation of α7 nAChRs via an allosteric transmembrane site. Previous studies have identified a series of nAChR-positive allosteric modulators (PAMs) that lack agonist activity but are able to potentiate responses to orthosteric agonists, such as acetylcholine. It has been shown, for example, that TQS acts as a conventional α7 nAChR PAM. In contrast, we have found that a compound with close chemical similarity to TQS (4BP-TQS) is a potent allosteric agonist of α7 nAChRs. Whereas the α7 nAChR antagonist metyllycaconitine acts competitively with conventional nicotinic agonists, metyllycaconitine is a noncompetitive antagonist of 4BP-TQS. Mutation of an amino acid (M253L), located in a transmembrane cavity that has been proposed as being the binding site for PAMs, completely blocks agonist activation by 4BP-TQS. In contrast, this mutation had no significant effect on agonist activation by acetylcholine. Conversely, mutation of an amino acid located within the known orthosteric binding site (W148F) has a profound effect on agonist potency of acetylcholine (resulting in a shift of ∼200-fold in the acetylcholine dose-response curve), but had little effect on the agonist dose-response curve for 4BP-TQS. Computer docking studies with an α7 homology model provides evidence that both TQS and 4BP-TQS bind within an intrasubunit transmembrane cavity. Taken together, these findings provide evidence that agonist activation of nAChRs can occur via an allosteric transmembrane site.
Keywords: allosteric potentiation, neurotransmitter receptor
Nicotinic acetylcholine receptors (nAChRs) are pentameric neurotransmitter-gated ion channels and are members of the superfamily of Cys-loop receptors that includes receptors for 5-hydroxytryptamine (5-HT), GABA, and glycine (1). Nicotinic receptors are, themselves, a diverse family of receptors (2, 3). In vertebrates, 17 nAChR subunits (α1–α10, β1–β4, γ, δ, and ε) have been identified and can coassemble to generate a wide variety of nAChR subtypes with distinct biophysical and pharmacological properties (3). For example, important nAChR subtypes expressed in the brain include heteromeric receptors (such as those containing α4 and β2 subunits) and homomeric receptors (such as those containing five copies of the α7 subunit). Nicotinic receptors have been implicated in a number of neurological and psychiatric disorders, including Alzheimer's disease, Parkinson disease, epilepsy, and schizophrenia (4–6). As a consequence, nAChRs are viewed as being important targets for therapeutic drug discovery (7, 8).
3D structural information is available for individual nAChR subunit domains (9) and for intact nAChRs (10). In addition, high-resolution structural information has been obtained from acetylcholine binding proteins, which share close sequence similarity to the nAChR extracellular region (11). Such data have helped to confirm that conventional agonists (such as acetylcholine) and competitive antagonists [such as methyllycaconitine (MLA)] bind to an extracellular agonist-binding domain (the “orthosteric” binding site) located at the interface between adjacent subunits (12, 13) and distinct from the transmembrane ion-channel pore. Each of the five subunits in a nAChR contains four α-helical transmembrane domains (TM1–4), with the pore of the channel being lined by the second of these transmembrane domains.
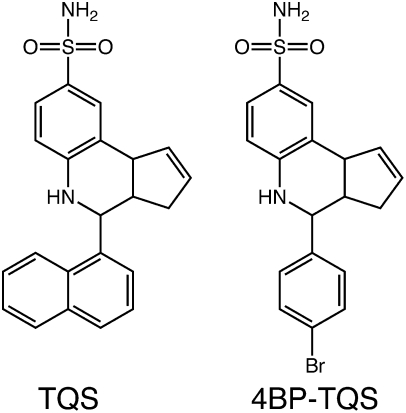
In addition to extensive studies aimed at characterizing nAChR-selective agonists and antagonists, considerable interest has been generated by the identification of a diverse group of allosteric modulators of nAChRs (14–16). These modulators include both positive allosteric modulators (PAMs) and noncompetitive antagonists (also referred to as negative allosteric modulators). Studies of α7-selective PAMs have identified two classes (type I and type II). Although both types potentiate agonist-evoked responses, they differ in their effects on receptor desensitization. Type I PAMs increase peak agonist-evoked responses but have little or no effect on the rate of desensitization of α7 nAChRs (17, 18), whereas type II PAMs also cause a dramatic reduction in desensitization (19, 20). Here, we have examined the properties of 4-(1-napthyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (TQS), which is a type II PAM, as has been described previously (20). In addition, we report studies conducted with 4-(4-bromophenyl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinoline-8-sulfonamide (4BP-TQS), a compound that is similar in chemical structure to TQS (Fig. 1), and which we have found to have potent, but atypical, agonist activity on α7 nAChRs.
Fig. 1.
Chemical structure of TQS and 4BP-TQS.
In previous studies (21) we have identified an intrasubunit cavity, located between the four transmembrane α-helices, as the allosteric binding site for both type I and type II PAMs acting on α7 nAChRs (21, 22). Here, we provide evidence that ligand binding at this transmembrane allosteric site is able to activate nAChRs in the absence of agonist binding at the orthosteric site. We conclude that ligand-induced channel activation can be driven by both orthosteric and allosteric agonists, presumably by affecting rate constants for transitions between resting and open states.
Results
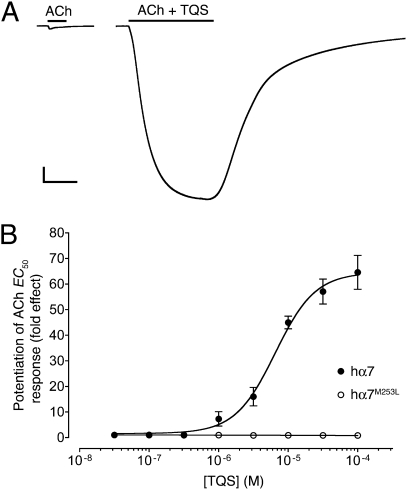
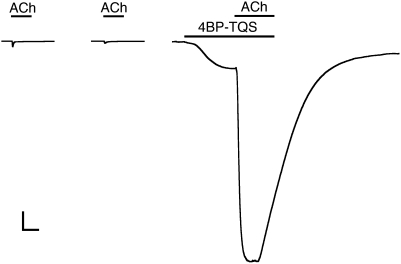
Agonist activation of α7 nAChRs by acetylcholine caused rapidly desensitizing responses (Figs. 2A, 3, and 4A), a finding that is consistent with previous studies (23). In contrast, TQS, an α7-selective type II PAM (20), displayed no agonist activity on α7 nAChRs but caused dramatic potentiation of agonist-evoked responses, together with a dramatic reduction in the rate of desensitization (Fig. 2A). These findings are also in agreement with previous independent studies (20). Dose-response data determined from a range of concentrations of TQS revealed that it potentiated responses to a submaximal concentration of acetylcholine (100 μM, close to the EC50 for ACh), with an EC50 value of 6.2 ± 0.6 μM and a Hill coefficient of 1.5 ± 0.1 (n = 3) (Fig. 2B). It has also been reported previously that type II PAMs can cause reactivation of α7 nAChRs from a desensitized state (21). We have confirmed that, as was shown previously for the type II PAM PNU-120596 (21), coapplication of TQS with acetylcholine causes reactivation of desensitized α7 nAChRs (Fig. 3).
Fig. 2.
Positive allosteric modulation of α7 nAChRs by TQS, examined by two-electrode voltage-clamp recording in Xenopus oocytes. (A) Representative recordings are shown illustrating responses to the application of acetylcholine (100 μM) (Left) and of acetylcholine (100 μM) coapplied with TQS (100 μM) (Right). The duration of agonist applications are indicated by a horizontal line. (Scale bars: vertical, 1 μA; horizontal, 5 s.) (B) Dose-response data are presented for a range of concentrations of TQS (0.03–100 μM) on responses evoked by a submaximal (EC50) concentration of acetylcholine. Data were obtained with either wild-type α7 nAChRs (●) or α7 nAChRs containing the M253L mutation (○). Data are means ± SEM of at least three independent experiments, each from different oocytes.
Fig. 3.
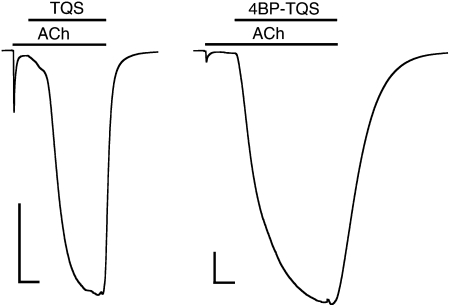
TQS and 4BP-TQS facilitate recovery of α7 nAChRs from desensitization. Prolonged exposure of α7 nAChRs to a high concentration of acetylcholine (100 μM) results in receptor activation, followed by rapid desensitization. In the continued presence of acetylcholine (100 μM), coapplication of either TQS (10 μM) (Left) or 4BP-TQS (10 μM) (Right) results in reactivation of desensitized receptors. Applications of acetylcholine and allosteric modulators (TSQ or 4BP-TQS) are indicated by horizontal lines. (Scale bars: vertical, 0.5 μA; horizontal, 10 s.)
Fig. 4.
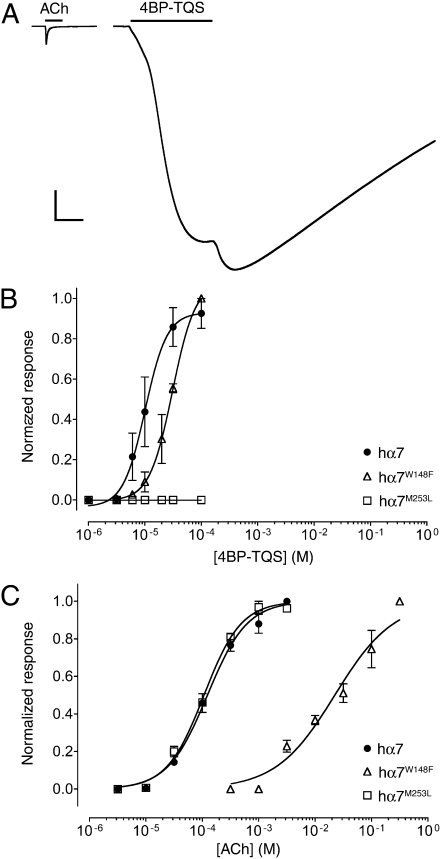
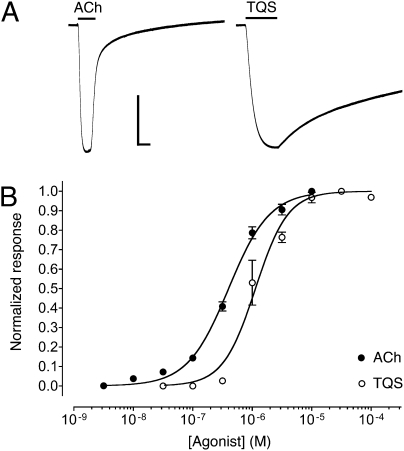
Agonist activation of α7 nAChRs by acetylcholine and 4BP-TQS, examined by two-electrode voltage-clamp recordings in Xenopus oocytes. (A) Representative recordings are shown illustrating responses to the application of acetylcholine (3 mM) (Left) and of 4BP-TQS (60 μM) (Right). The duration of agonist applications are indicated by a horizontal line. (Scale bars: vertical, 1 μA; horizontal, 5 s.) Dose-response data are presented for a range of concentrations of 4BP-TQS (B) or acetylcholine (C), with wild-type α7 nAChRs (●) or with α7 nAChRs containing either the W148F mutation (△) or the M253L mutation (□). Data are means ± SEM of at least three independent experiments, each from different oocytes.
Despite the absence of agonist activity of TQS on α7 nAChRs, even at the highest concentrations tested (0.1 mM), a compound with very close chemical similarity to TQS (4BP-TQS) (Fig. 1) was found to act as a potent agonist of α7 nAChRs (Fig. 4A). However, in contrast to agonist activation by acetylcholine, responses evoked by 4BP-TQS displayed no evidence of desensitization (Fig. 4A). Responses to 4BP-TQS had a relatively slow onset and were slow to reach a plateau (Fig. 4A). The differences in kinetics and in the extent of receptor desensitization suggest that acetylcholine and 4BP-TQS activate α7 nAChRs through different mechanisms. A further difference between agonist activation by the two compounds is that the agonist dose-response curve observed with 4BP-TQS is significantly steeper (nH = 2.3 ± 0.4) (Fig. 4B) than that observed with acetylcholine (nH = 1.3 ± 0.2) (Fig. 4C). Additionally, responses generated by a maximal concentration of 4BP-TQS were substantially larger (45 ± eightfold) than maximal acetylcholine responses recorded from the same oocyte. Half-maximal agonist activation of α7 nAChRs occurred with lower concentrations of 4BP-TQS (EC50 = 17 ± 3 μM) than with acetylcholine (EC50 = 128 ± 12 μM) (Fig. 4 B and C). At high concentrations (above ∼30 μM) an increase in the response was observed after agonist application ceased (Fig. 4A), suggesting that 4BP-TQS also has some receptor/channel blocking activity at high concentrations. As shown above for TQS (Fig. 3), exposure of desensitized α7 nAChRs to 4BP-TQS also resulted in recovery of receptors from the desensitized state (Fig. 3).
In previous studies, aimed at characterizing the binding site of α7-selective PAMs such as PNU-120596, we exploited a series of chimeras containing domains from the α7 nAChR subunit and the 5-HT3A subunit (21). The rationale behind these experiments was that PNU-120596 acted as a potentiator of α7 nAChRs but had no effect on 5-HT3 receptors. We have examined the influence of 4BP-TQS on 5-HT3A and detected no evidence of agonist activity. Similarly, when 4BP-TQS was tested on an α7 subunit chimera (α74TM-5HT3A) containing the transmembrane region from 5-HT3A (24), no agonist activity was observed. This finding suggests that agonist activation of α7 nAChRs, like allosteric modulation by compounds such as PNU-120596, is dependent on the α7 transmembrane region.
We have shown previously that mutations located within a proposed intrasubunit transmembrane cavity of α7 nAChRs reduce levels of potentiation by both type I and type II PAMs (21, 22). For example, a single-point mutation (M253L) located in the second transmembrane domain (TM2), reduced levels of potentiation by PNU-120596 to about 10% of that observed with wild-type α7 nAChRs (21). Here, the influence of the M253L mutation was examined with regard to potentiation by TQS and was found to have an even more dramatic effect, causing a complete loss of potentiation (Fig. 2B), even at the highest concentrations of TQS tested (0.1 mM). The M253L mutation had a similarly dramatic effect on the agonist activity of 4BP-TQS, causing a complete loss of drug-evoked responses, even at concentrations of 4BP-TQS that produced maximal agonist activity on wild-type α7 nAChRs (0.1 mM) (Fig. 4B). In contrast, the M253L mutation had no significant effect on agonist activity of acetylcholine (Fig. 4C). These findings provide further support for the hypothesis that acetylcholine and 4BP-TQS activate α7 nAChRs through different mechanisms of action.
The binding site for orthosteric agonists, such as acetylcholine, is well established and mutations located at this site in α7 nAChRs have been shown to cause a substantial rightward shift in the dose-response curve for acetylcholine (25). Here, we examined the influence of one such mutation (W148F), located close to the known binding site of acetylcholine. Comparison of wild-type and mutant (W148F) α7 nAChRs revealed that the W148F mutation caused a large (188-fold) rightward shift in the acetylcholine dose-response curve (Fig. 4C). The same mutation caused a much smaller rightward shift (∼twofold) in the 4BP-TQS dose-response curve that was not significantly different from wild-type (Fig. 4B). Therefore, we have found that a mutation located close to the known orthosteric binding site (W148F) causes a greater reduction in agonist potency of acetylcholine, whereas a mutation close to the proposed transmembrane binding site of allosteric modulators (M253L) has a selective effect on agonist activity of 4BP-TQS.
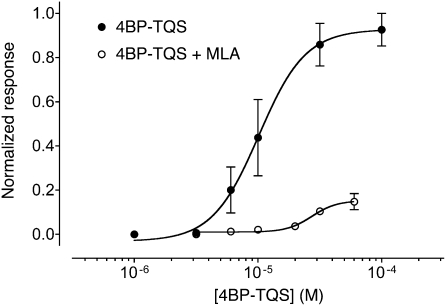
MLA is a potent reversible competitive antagonist of α7 nAChRs. As has been demonstrated previously, MLA causes a surmountable block of acetylcholine-evoked responses (22, 26). Here, MLA was found to act as a reversible antagonist of responses evoked by 4BP-TQS (Fig. 5 and Fig. S1A). However, in contrast to its effects on acetylcholine (22, 26), antagonism of 4BP-TQS responses by MLA was not surmountable (Fig. 5), a feature that is characteristic of noncompetitive antagonism. The reversible nature of antagonism by MLA (Fig. S1A) indicates that it is reasonable to attribute the nonsurmountable block of 4BP-TQS responses to noncompetitive binding, rather than irreversible binding. Antagonism of 4BP-TQS responses was also observed with α-bungarotoxin. However, in contrast to MLA, antagonism by α-bungarotoxin was not readily reversible (Fig. S1B). Typically, antagonism by α-bungarotoxin required more than 6 min to achieve complete block and responses did not recover substantially, even after washing for 15 min (Fig. S1B). The differential effect of MLA (noncompetitive, rather than competitive antagonism) provides strong evidence that 4BP-TQS binds at a site that is distinct from the conventional orthosteric binding site and supports the mutagenesis studies above, showing that 4BP-TQS causes agonist activation of α7 nAChRs via a distinct site.
Fig. 5.
MLA is a noncompetitive antagonist of 4BP-TQS. Dose-response data are presented for a range of concentrations of acetylcholine acting on α7 nAChRs either in the absence (●) or presence (○) of MLA (5 nM). In all cases, MLA was preapplied for 15 s and then coapplied with 4BP-TQS. Data are means ± SEM of at least three independent experiments, each from different oocytes.
An interesting question is whether, in addition to its action as an allosteric agonist, 4BP-TQS is also able to act as a potentiator of acetylcholine responses. To examine this question, acetylcholine was coapplied with a submaximal concentration of 4BP-TQS (10 μM). After preapplying 4BP-TQS, coapplication of 25 μM acetylcholine (equivalent to an EC10 concentration in the absence of 4BP-TQS) resulted in a dramatically potentiated response to acetylcholine (Fig. 6). Indeed, the magnitude of the secondary response (i.e., that caused by the coapplication of an EC10 concentration of acetylcholine) was 542 ± 32-fold (n = 3) larger than the response elicited by an EC10 concentration of acetylcholine applied in the absence of 4BP-TQS (Fig. 6). Furthermore, this potentiated response to acetylcholine was 41 ± 5-fold larger than that of a maximal concentration of acetylcholine applied in the absence of 4BP-TQS (Fig. 6). In addition, the potentiated acetylcholine responses showed no evidence of desensitization, a feature that is characteristic of type II PAMs, such as TQS. Similar experiments were performed with α7 nAChRs containing the M253L mutation and revealed that this single-point mutation abolished completely both the agonist and potentiating effects of 4BP-TQS (Fig. S2).
Fig. 6.
4BP-TQS acts as potentiator of acetylcholine responses. Representative traces showing agonist responses on α7 nAChRs to a maximal concentration (3 mM) (Left) and an EC10 concentration (25 μM) (Center) of acetylcholine. Also shown (Right) is a response to 4BP-TQS (10 μM). After the response to 4BP-TQS had reached a plateau, acetylcholine (25 μM) was coapplied with 4BP-TQS (10 μM), resulting in a secondary response of much greater magnitude (542 ± 32; n = 3) than was observed when the same concentration of acetylcholine was applied alone (Center).
Mutations of amino acid L247 (such as L247T) in α7 nAChRs have been found to have particularly dramatic effects on the functional properties of this receptor (27, 28). The effects of the L247T mutation include increased potency of agonists such as acetylcholine, reduced levels of desensitization (27), and an increase in spontaneous openings (29). Indeed, the desensitization profile of acetylcholine responses on α7 receptors containing the L247T mutation resembles that observed in wild-type α7 receptors in the presence of type II PAMs, such as PNU-120596 and TQS (19, 20). In addition, the L247 mutation causes some competitive antagonists to act as agonists (28). Because of these interesting and diverse effects, we examined the influence of the L247T mutation on α7 PAMs. Interestingly, we find that the L247T mutation converts TQS, an α7-selective type II PAM, into an agonist (Fig. 7). Higher concentrations of TQS were required to obtain half-maximal activation of α7L247T nAChRs (EC50 = 1.2 ± 0.3 μM) than with acetylcholine (EC50 = 0.4 ± 0.04 μM). However, whereas the allosteric agonist 4BP-TQS generated much larger maximal responses than acetylcholine in wild-type α7 nAChRs (45-fold) (Fig. 4A), maximal responses to TQS on α7L247T nAChRs were not significantly larger than the maximal responses to acetylcholine (Fig. 7A).
Fig. 7.
The α7 nAChR transmembrane mutation L247T converts TQS from a PAM into an agonist. (A) Representative recordings are shown illustrating responses to the application of acetylcholine (3 μM) (Left) and of TQS (100 μM) (Right). The duration of agonist applications are indicated by a horizontal line. (Scale bars: vertical, 0.5 μA; horizontal, 5 s.) (B) Dose-response data are presented for a range of concentrations of acetylcholine (●) and TQS (○) acting on α7 nAChRs containing the transmembrane L247T mutation. Data are means ± SEM of at least three independent experiments, each from different oocytes.
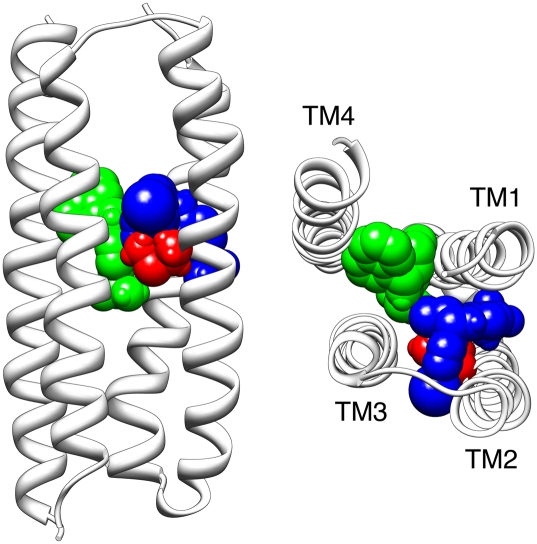
Previously, computer-docking simulations were conducted with an α7 nAChR homology model and provided support for the proposal that α7 nAChR PAMs, such as PNU-120596, act via a transmembrane binding site (21). Using the same approach, we have obtained evidence that both TQS and 4BP-TQS bind favorably at a similar transmembrane location on the α7 nAChR. The most favorable (lowest energy) docked conformation of TQS was in a position broadly similar to that identified previously for PNU-120596 (21), within a presumed intrasubunit cavity located between the four α-helical transmembrane domains (Fig. 8). The lowest energy docked conformation of 4BP-TQS was also within the transmembrane intrasubunit cavity but was located closer toward the inner face of the M2 helix and was in very close proximity (within 3Å) of M253, the amino acid which, when mutated, caused a dramatic loss of agonist activity by 4BP-TQS.
Fig. 8.
Computer-docking simulation performed with a homology model of the α7 transmembrane domain (21). The backbone of the four transmembrane α-helices (TM1–TM4) are shown in gray. The side chain of M253 is in red (with atoms represented as spheres) the lowest energy (highest predicted binding affinity) docked position of 4BP-TQS is illustrated in blue. For comparison, the lowest energy docked position of TQS is illustrated in green. The model is shown from a side-on view (Left) and as viewed from above, looking down from the extracellular face of the lipid membrane (Right).
Discussion
Ligand-gated ion channels such as nAChRs are complex multisubunit allosteric proteins, containing a variety of binding sites for pharmacologically active compounds (13, 30). In the present study, we have presented evidence demonstrating that 4BP-TQS acts as an agonist of α7 nAChRs by binding to an allosteric transmembrane site. Several lines of experimental evidence support the hypothesis that 4BP-TQS and conventional agonists such as acetylcholine exert their agonist effects on α7 nAChRs through different mechanisms. The most obvious difference between agonist activation by acetylcholine and 4BP-TQS is that activation by acetylcholine causes rapid desensitization of α7 nAChRs, whereas no desensitization was observed when receptors were activated with 4BP-TQS (Fig. 4A). Indeed, the nondesensitizing responses to 4BP-TQS resemble responses with acetylcholine in the presence of allosteric potentiators, such as TQS (20). In addition, responses to acetylcholine had a much faster onset that those evoked by 4BP-TQS (Fig. 4A). At high concentrations of 4BP-TQS (above ∼30 μM), an increase in the response was observed after agonist application ceased (Fig. 4A). This finding would suggest that 4BP-TQS also has some receptor/channel-blocking activity, a feature that is also observed with high concentrations of conventional agonists, such as acetylcholine (31).
The agonist dose-response curve observed with 4BP-TQS is significantly steeper (nH = 2.3 ± 0.4) (Fig. 4B) than that observed with acetylcholine (nH = 1.3 ± 0.2) (Fig. 4C). This finding may reflect a greater degree of cooperativity associated with agonist activation by 4BP-TQS, although the difference may be, at least in part, because of an underestimation of the Hill coefficient with acetylcholine caused by the high level of desensitization that occurs with high agonist concentrations (32, 33).
Data obtained from mutated α7 nAChRs provide support for the conclusion that acetylcholine and 4BP-TQS cause receptor activation through different binding sites. A mutation located in the nAChR transmembrane domain (M253L) causes a complete loss of agonist activity by 4BP-TQS but has no significant effect on acetylcholine. In contrast, a mutation known to be located close to the conventional agonist-binding site (W148F) had a significantly greater effect on agonist potency of acetylcholine than 4BP-TQS. Mutations do not necessarily have to lie in close proximity to an agonist binding site to have dramatic effects on agonist potency, but these data support the conclusion that acetylcholine and 4BP-TQS act through different mechanisms. It is well established that W148F is located close to the acetylcholine binding site and computer modeling data, presented in this study, supports the conclusion that M253L is located close to the binding site for 4BP-TQS.
Perhaps the most direct evidence that acetylcholine and 4BP-TQS bind to distinct sites comes from studies with the α7-selective antagonist MLA. MLA is a competitive antagonist of acetylcholine acting on α7 nAChRs (22, 26), but acts as a noncompetitive antagonist of 4BP-TQS. When acting as an antagonist of acetylcholine, MLA binds competitively at the conventional (orthosteric) binding site. We assume that the nonsurmountable antagonism of 4BP-TQS responses by MLA are also a result of MLA binding to its extracellular (orthosteric) site. In binding to this site, MLA may stabilize the closed state of the receptor or may inducing a conformational change that affects binding of 4BP-TQS at a distinct site. We have described this as noncompetitive antagonism, although MLA could also be considered to be acting as an inverse agonist. In support of the latter possibility are previous studies demonstrating that MLA can block spontaneous openings in mutant α7 nAChRs (29). Taken together, these findings provide strong evidence that acetylcholine and 4BP-TQS cause agonist activation of α7 nAChRs by binding to distinct (orthosteric and allosteric) sites. The site-directed mutagenesis data support the hypothesis that 4BP-TQS acts as an agonist by binding to the same allosteric site that is responsible for potentiation by PAMs, such as LY-2087101, PNU-120596, and TQS. It has been proposed previously that α7 PAMs, such as LY-2087101 and PNU-120596, bind to an intrasubunit transmembrane cavity (21). The likelihood that 4BP-TQS acts as an agonist by binding at a similar location is supported by computer docking simulations conducted with an α7 nAChR homology model (Fig. 8). It is likely that this intrasubunit transmembrane cavity corresponds to a conserved allosteric modulatory site among Cys-loop receptors. There is evidence, for example, that GABAA and glycine receptor allosteric modulators, such as neurosteroids and volatile anesthetics, interact in this region (34–36). Furthermore, recent photoaffinity labeling studies with purified Torpedo nAChR has identified interactions of photoaffinity ligands, including anesthetics, within the transmembrane region (37, 38).
There is clear evidence that nAChRs undergo spontaneous openings in the absence of agonists (39) and that the frequency of such events can be increased through multiple mechanisms. For example, increased levels of spontaneous opening of nAChRs have been reported in several mutated nAChRs (29, 40, 41) and as a result of the omission of subunits in heterologously expressed muscle nAChRs (42). In the same way, it is plausible that stabilization of the open conformation of a nAChR might occur as a result of ligands binding to multiple distinct sites. Here we have shown that in α7 nAChRs containing the L247T mutation, a receptor known to have a higher frequency of spontaneous openings (29), the allosteric potentiator TQS acquires the ability to act as an agonist, just as 4BP-TQS has agonist activity on wild-type α7 nAChRs. By reference to previously described models for allosteric proteins (30, 43), the agonist effects of 4BP-TQS and TQS can be explained in terms of their influence on rate constants for transitions between resting and open states of the receptor.
Conventional agonists, PAMs, and allosteric agonists, provide a range of possible avenues for therapeutic drug discovery. Conventional agonists of α7 nAChRs have been extensively studied in preclinical models of neuropsychiatric disorders (8), although few have progressed into clinical studies. Studies of α7-selective PAMs have been reported in animal models (19, 44, 45) but there is currently no evidence as to the potential therapeutic usefulness of allosteric modulators or allosteric agonists acting on α7 nAChRs. Theoretically, it is possible that a type II PAM might have therapeutic benefits in situations where stronger agonist responses were desirable. A nondesensitizing allosteric agonist, on the other hand, might be particularly beneficial under conditions of severe loss of endogenous acetylcholine, for example in advanced Alzheimer's disease, where potentiation might be less effective. A potential concern is that excessive receptor activation might lead to excitotoxicity because of excessive calcium influx. It is of interest, therefore, that a recent study has shown that α7-selective type II PAMs do not exert cytotoxic effects, at least in the cell types that were examined (46).
Agonist activation by ligands interacting with an allosteric site (sometimes referred to as “ago-allosteric” ligands) has been described previously with respect to G protein-coupled receptors (47, 48). There have also been reports demonstrating that allosteric potentiators of ligand-gated ion channels can also have direct agonist activity. Such effects have been described, for example, for the barbiturate anesthetic pentobarbital acting on GABAA receptors, albeit at fairly high concentrations (49). Here we have identified a compound that is capable of potent agonist activation of α7 nAChRs. Significantly, however, 4BP-TQS is a more potent and efficacious agonist of α7 nAChRs than is acetylcholine (8-fold lower EC50 and 45-fold larger maximal response). In addition, we have obtained evidence that this activation occurs via interaction with a transmembrane site. It is possible that this allosteric agonist binding site on nAChRs may provide a novel avenue for pharmaceutical drug discovery.
Materials and Methods
Materials.
All chemicals were obtained from Sigma, with the exception of TQS and 4BP-TQS (Fig. 1), which were obtained from Chembridge Corporation, and MLA, which was obtained from Tocris Bioscience. Several plasmid constructs used in this study have been described previously. These constructs include plasmids containing human α7 cDNA in pSP64GL (50), mouse 5-HT3A in pRK5 (51), and an α7/5-HT3A subunit chimera (α74TM-5HT3A) in pZeoSV2 (24).
Site-Directed Mutagenesis and cRNA Synthesis.
Site-directed mutagenesis was performed on human nAChR α7 subunit cDNA in plasmid pSP64GL (50) using the QuikChange mutagenesis kit (Stratagene) and verified by nucleotide sequencing. Plasmid pSP64GL containing wild-type or mutated human α7 cDNA was linearized with BamHI and purified with QIAQuik PCR purification kit (Qiagen). In vitro synthesis of cRNA was performed using Message Machine SP6 transcription kit (Ambion). For consistency with previous studies, the numbering of amino acids altered by site-directed mutagenesis is based on the predicted signal sequence cleavage site in the mature α7 protein (see figure 2 in ref. 23).
Xenopus Oocyte Electrophysiology.
Xenopus laevis oocytes were isolated and defolliculated, as described previously (52). Heterologous expression was achieved by injection of either cRNA (6–12 ng) into oocyte cytoplasm in the case of wild-type and mutated α7, or plasmid cDNA constructs (10–30 ng) into oocyte nuclei in the case of 5-HT3A and α7/5-HT3A subunit chimeras. Oocytes were injected in a volume of 32.2 nL using a Drummond variable volume microinjector. Two electrode voltage-clamp recordings were performed essentially as described previously (52).
Computer Docking Simulations.
Computational molecular docking was performed with AutoDock 4 (53) using a homology model of the human α7 nAChR transmembrane region (54), as described previously (21). To avoid bias, a blind-docking approach was used in which no assumptions were made concerning where within the transmembrane region 4BP-TQS and TQS might be expected to bind. Flexibility of rotatable bonds in 4BP-TQS and TQS was permitted during the docking simulation.
Supplementary Material
Acknowledgments
We thank Jeppe Kejser Christensen and Antonio Nardi for advice during the early stages of this project. J.K.G. and G.T.Y. were supported by Biotechnology and Biological Science Research Council Collaborative Awards in Science and Engineering PhD studentships that were co-funded by the Biotechnology and Biological Science Research Council and Eli Lilly; M.S. was supported by an Erasmus Bilateral Agreement between University College London and the University of Helsinki, Finland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017975108/-/DCSupplemental.
References
- 1.Lester HA, Dibas MI, Dahan DS, Leite JF, Dougherty DA. Cys-loop receptors: New twists and turns. Trends Neurosci. 2004;27:329–336. doi: 10.1016/j.tins.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Le Novère N, Changeux J-P. Molecular evolution of the nicotinic acetylcholine receptor: An example of multigene family in excitable cells. J Mol Evol. 1995;40:155–172. doi: 10.1007/BF00167110. [DOI] [PubMed] [Google Scholar]
- 3.Millar NS, Gotti C. Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology. 2009;56:237–246. doi: 10.1016/j.neuropharm.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Changeux J-P, Taly A. Nicotinic receptors, allosteric proteins and medicine. Trends Mol Med. 2008;14:93–102. doi: 10.1016/j.molmed.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Weiland S, Bertrand D, Leonard S. Neuronal nicotinic acetylcholine receptors: From the gene to the disease. Behav Brain Res. 2000;113:43–56. doi: 10.1016/s0166-4328(00)00199-6. [DOI] [PubMed] [Google Scholar]
- 6.Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jensen AA, Frølund B, Liljefors T, Krogsgaard-Larsen P. Neuronal nicotinic acetylcholine receptors: structural revelations, target identifications, and therapeutic inspirations. J Med Chem. 2005;48:4705–4745. doi: 10.1021/jm040219e. [DOI] [PubMed] [Google Scholar]
- 8.Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: A perspective on two decades of drug discovery research. Biochem Pharmacol. 2007;74:1092–1101. doi: 10.1016/j.bcp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Dellisanti CD, Yao Y, Stroud JC, Wang Z-Z, Chen L. Crystal structure of the extracellular domain of nAChR α1 bound to α-bungarotoxin at 1.94 Å resolution. Nat Neurosci. 2007;10:953–962. doi: 10.1038/nn1942. [DOI] [PubMed] [Google Scholar]
- 10.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4Å resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 11.Brejc K, et al. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 12.Sine SM, Engel AG. Recent advances in Cys-loop receptor structure and function. Nature. 2006;440:448–455. doi: 10.1038/nature04708. [DOI] [PubMed] [Google Scholar]
- 13.Taly A, Corringer P-J, Guedin D, Lestage P, Changeux J-P. Nicotinic receptors: Allosteric transitions and therapeutic targets in the nervous system. Nat Rev Drug Discov. 2009;8:733–750. doi: 10.1038/nrd2927. [DOI] [PubMed] [Google Scholar]
- 14.Bertrand D, Gopalakrishnan M. Allosteric modulation of nicotinic acetylcholine receptors. Biochem Pharmacol. 2007;74:1155–1163. doi: 10.1016/j.bcp.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Moaddel R, Jozwiak K, Wainer IW. Allosteric modifiers of neuronal nicotinic acetylcholine receptors: new methods, new opportunities. Med Res Rev. 2007;27:723–753. doi: 10.1002/med.20091. [DOI] [PubMed] [Google Scholar]
- 16.Faghih R, Gopalakrishnan M, Briggs CA. Allosteric modulators of the α7 nicotinic acetylcholine receptor. J Med Chem. 2008;51:701–712. doi: 10.1021/jm070256g. [DOI] [PubMed] [Google Scholar]
- 17.Krause RM, et al. Ivermectin: A positive allosteric effector of the α7 neuronal nicotinic acetylcholine receptor. Mol Pharmacol. 1998;53:283–294. doi: 10.1124/mol.53.2.283. [DOI] [PubMed] [Google Scholar]
- 18.Broad LM, et al. Identification and pharmacological profile of a new class of selective nicotinic acetylcholine receptor potentiators. J Pharmacol Exp Ther. 2006;318:1108–1117. doi: 10.1124/jpet.106.104505. [DOI] [PubMed] [Google Scholar]
- 19.Hurst RS, et al. A novel positive allosteric modulator of the α7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J Neurosci. 2005;25:4396–4405. doi: 10.1523/JNEUROSCI.5269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grønlien JH, et al. Distinct profiles of α7 nAChR positive allosteric modulation revealed by structurally diverse chemotypes. Mol Pharmacol. 2007;72:715–724. doi: 10.1124/mol.107.035410. [DOI] [PubMed] [Google Scholar]
- 21.Young GT, Zwart R, Walker AS, Sher E, Millar NS. Potentiation of α7 nicotinic acetylcholine receptors via an allosteric transmembrane site. Proc Natl Acad Sci USA. 2008;105:14686–14691. doi: 10.1073/pnas.0804372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins T, Millar NS. Nicotinic acetylcholine receptor transmembrane mutations convert ivermectin from a positive to a negative allosteric modulator. Mol Pharmacol. 2010;78:198–204. doi: 10.1124/mol.110.064295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couturier S, et al. A neuronal nicotinic acetylcholine receptor subunit (α7) is developmentally regulated and forms a homo-oligomeric channel blocked by α-BTX. Neuron. 1990;5:847–856. doi: 10.1016/0896-6273(90)90344-f. [DOI] [PubMed] [Google Scholar]
- 24.Gee VJ, Kracun S, Cooper ST, Gibb AJ, Millar NS. Identification of domains influencing assembly and ion channel properties in α7 nicotinic receptor and 5-HT3 receptor subunit chimaeras. Br J Pharmacol. 2007;152:501–512. doi: 10.1038/sj.bjp.0707429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galzi JL, et al. Functional significance of aromatic amino acids from three peptide loops of the α7 neuronal nicotinic receptor site investigated by site-directed mutagenesis. FEBS Lett. 1991;294:198–202. doi: 10.1016/0014-5793(91)80668-s. [DOI] [PubMed] [Google Scholar]
- 26.Drasdo A, Caulfield M, Bertrand D, Bertrand S, Wonnacott S. Methyl lycaconitine: A novel nicotinic antagonist. Mol Cell Neurosci. 1992;3:237–243. doi: 10.1016/1044-7431(92)90043-2. [DOI] [PubMed] [Google Scholar]
- 27.Revah F, et al. Mutations in the channel domain alter desensitization of a neuronal nicotinic receptor. Nature. 1991;353:846–849. doi: 10.1038/353846a0. [DOI] [PubMed] [Google Scholar]
- 28.Bertrand D, et al. Unconventional pharmacology of a neuronal nicotinic receptor mutated in the channel domain. Proc Natl Acad Sci USA. 1992;89:1261–1265. doi: 10.1073/pnas.89.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand S, et al. Paradoxical allosteric effects of competitive inhibitors on neuronal α7 nicotinic receptor mutants. Neuroreport. 1997;8:3591–3596. doi: 10.1097/00001756-199711100-00034. [DOI] [PubMed] [Google Scholar]
- 30.Monod J, Wyman J, Changeux J-P. On the nature of allosteric transitions: A plausible model. J Mol Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 31.Liu Q, Yu K-W, Chang Y-C, Lukas RJ, Wu J. Agonist-induced hump current production in heterologously-expressed human α4β2-nicotinic acetylcholine receptors. Acta Pharmacol Sin. 2008;29:305–319. doi: 10.1111/j.1745-7254.2008.00760.x. [DOI] [PubMed] [Google Scholar]
- 32.Gerzanich V, Anand R, Lindstrom J. Homomers of α8 and α7 subunits of nicotinic receptors exhibit similar channel but contrasting binding site properties. Mol Pharmacol. 1994;45:212–220. [PubMed] [Google Scholar]
- 33.Papke RL, Thinschmidt JS. The correction of alpha7 nicotinic acetylcholine receptor concentration-response relationships in Xenopus oocytes. Neurosci Lett. 1998;256:163–166. doi: 10.1016/s0304-3940(98)00786-1. [DOI] [PubMed] [Google Scholar]
- 34.Mihic SJ, et al. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 35.Ye Q, et al. Enhancement of glycine receptor function by ethanol is inversely correlated with molecular volume at position α267. J Biol Chem. 1998;273:3314–3319. doi: 10.1074/jbc.273.6.3314. [DOI] [PubMed] [Google Scholar]
- 36.Hosie AM, Wilkins ME, da Silva HMA, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 37.Ziebell MR, Nirthanan S, Husain SS, Miller KW, Cohen JB. Identification of binding sites in the nicotinic acetylcholine receptor for [3H]azietomidate, a photoactivatable general anesthetic. J Biol Chem. 2004;279:17640–17649. doi: 10.1074/jbc.M313886200. [DOI] [PubMed] [Google Scholar]
- 38.Garcia G, 3rd, et al. [3H]Benzophenone photolabeling identifies state-dependent changes in nicotinic acetylcholine receptor structure. Biochemistry. 2007;46:10296–10307. doi: 10.1021/bi7008163. [DOI] [PubMed] [Google Scholar]
- 39.Jackson MB. Spontaneous openings of the acetylcholine receptor channel. Proc Natl Acad Sci USA. 1984;81:3901–3904. doi: 10.1073/pnas.81.12.3901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohno K, et al. Congenital myasthenic syndrome caused by prolonged acetylcholine receptor channel openings due to a mutation in the M2 domain of the ε subunit. Proc Natl Acad Sci USA. 1995;92:758–762. doi: 10.1073/pnas.92.3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosman C, Auerbach A. Kinetic, mechanistic, and structural aspects of unliganded gating of acetylcholine receptor channels: A single-channel study of second transmembrane segment 12′ mutants. J Gen Physiol. 2000;115:621–635. doi: 10.1085/jgp.115.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jackson MB, et al. Spontaneous and agonist-induced openings of an acetylcholine receptor channel composed of bovine muscle α-, β- and δ-subunits. Pflugers Arch. 1990;417:129–135. doi: 10.1007/BF00370689. [DOI] [PubMed] [Google Scholar]
- 43.Changeux J-P, Edelstein SJ. Nicotinic Acetylcholine Receptors from Molecular Biology to Cognition. New York: Odile Jacob; 2005. [Google Scholar]
- 44.Ng HJ, et al. Nootropic α7 nicotinic receptor allosteric modulator derived from GABAA receptor modulators. Proc Natl Acad Sci USA. 2007;104:8059–8064. doi: 10.1073/pnas.0701321104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Timmermann DB, et al. An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J Pharmacol Exp Ther. 2007;323:294–307. doi: 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- 46.Hu M, Gopalakrishnan M, Li J. Positive allosteric modulation of α7 neuronal nicotinic acetylcholine receptors: lack of cytotoxicity in PC12 cells and rat primary cortical neurons. Br J Pharmacol. 2009;158:1857–1864. doi: 10.1111/j.1476-5381.2009.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Langmead CJ, Christopoulos A. Allosteric agonists of 7TM receptors: Expanding the pharmacological toolbox. Trends Pharmacol Sci. 2006;27:475–481. doi: 10.1016/j.tips.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz TW, Holst B. Allosteric enhancers, allosteric agonists and ago-allosteric modulators: Where do they bind and how do they act? Trends Pharmacol Sci. 2007;28:366–373. doi: 10.1016/j.tips.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 49.Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. doi: 10.1038/366565a0. [DOI] [PubMed] [Google Scholar]
- 50.Broadbent S, et al. Incorporation of the β3 subunit has a dominant-negative effect on the function of recombinant central-type neuronal nicotinic receptors. Mol Pharmacol. 2006;70:1350–1357. doi: 10.1124/mol.106.026682. [DOI] [PubMed] [Google Scholar]
- 51.Harkness PC, Millar NS. Inefficient cell-surface expression of hybrid complexes formed by the co-assembly of neuronal nicotinic acetylcholine receptor and serotonin receptor subunits. Neuropharmacology. 2001;41:79–87. doi: 10.1016/s0028-3908(01)00042-9. [DOI] [PubMed] [Google Scholar]
- 52.Young GT, et al. Species selectivity of a nicotinic acetylcholine receptor agonist is conferred by two adjacent extracellular β4 amino acids that are implicated in the coupling of binding to channel gating. Mol Pharmacol. 2007;71:389–397. doi: 10.1124/mol.106.030809. [DOI] [PubMed] [Google Scholar]
- 53.Morris GM, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 54.Cheng X, Lu B, Grant B, Law RJ, McCammon JA. Channel opening motion of α7 nicotinic acetylcholine receptor as suggested by normal mode analysis. J Mol Biol. 2006;355:310–324. doi: 10.1016/j.jmb.2005.10.039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.