Abstract
Stroke/brain ischemia is a leading cause of death and long-term disabilities. Increased oxidative stress plays an important role in the pathology of brain ischemia. Hydrogen peroxide (H2O2) is a major oxidant known to cause neuronal injury; however, the detailed mechanism remains unclear. Previous studies have suggested that H2O2-induced injury is associated with increased intracellular Ca2+, mediated by glutamate receptors or voltage-gated Ca2+ channels. Here, we demonstrate that, at concentrations relevant to stroke, H2O2 induces a Ca2+-dependent injury of mouse cortical neurons in the absence of activation of these receptors/channels. With the culture medium containing blockers of glutamate receptors and voltage-gated Ca2+ channels, brief exposure of neurons to H2O2 induced a dose-dependent injury. Reducing [Ca2+]e inhibited whereas increasing [Ca2+]e potentiated the H2O2 injury. Fluorescent Ca2+ imaging confirmed the increase of [Ca2+]i by H2O2 in the presence of the blockers of glutamate receptors and voltage-gated Ca2+ channels. Addition of 2-aminoethoxydiphenyl borate, an inhibitor of transient receptor potential melastatin 7 (TRPM7) channels, or the use of TRPM7-small interference RNA, protected the neurons from H2O2 injury. In contrast, overexpressing TRPM7 channels in human embryonic kidney 293 cells increased H2O2 injury. Our findings indicate that H2O2 can induce Ca2+ toxicity independent of glutamate receptors and voltage-gated Ca2+ channels. Activation of TRPM7 channels is involved in such toxicity. Antioxid. Redox Signal. 14, 1815–1827.
Introduction
Stroke or brain ischemia is a common neurological disorder. Unfortunately, after decades of active research, there is still no effective treatment for stroke patients other than the use of thrombolisis, which has very limited success. Searching for new cell injury mechanisms and therapeutic targets constitutes major challenge for stroke research. Oxidative stress, a cytotoxic consequence of mismatch between production of reactive oxygen species (ROS) and ability of cells to defend against them, has been implicated in neuronal loss associated with a variety of neurological disorders, including brain ischemia (6, 10, 39). ROS include oxygen-centered radicals possessing unpaired electrons such as superoxide anion (O•−2) and hydroxyl radical (OH•), or covalent molecules such as H2O2. O•−2 is generated in mitochondria by electron-transport process, in cytoplasm catalyzed by xanthine oxidase, or at plasma membrane by activation of phospholipase A2 and NADPH oxidase. H2O2 is formed from O•−2 spontaneously or catalyzed by superoxide dismutase. Highly reactive OH• can be formed from H2O2 by interacting with transitional metals.
A large number of studies have focused on the role of H2O2, one of the primary and the most stable ROS in vivo, in cell injury. In various cell culture models, brief incubation with H2O2 has been shown to induce delayed cell injury (15, 18, 46). Although the detailed mechanism is not fully understood, it has been demonstrated that H2O2-induced injury is associated with an increase in the concentration of intracellular Ca2+ ([Ca2+]i) (15, 18, 46), and the increase of [Ca2+]i by H2O2 involves an entry of Ca2+ from the extracellular space (18, 41).
Activation of voltage-gated Ca2+ channels and glutamate receptors is known to cause [Ca2+]i accumulation, and Ca2+-dependent neuronal injury (7, 44). Recent studies have indicated that the activities of voltage-gated Ca2+ channels can be enhanced by ROS (8, 27). For example, bath application of H2O2 increases the currents of cloned neuronal Ca2+ channels (27). Consistent with an involvement of voltage-gated Ca2+ channels in the effects of H2O2, H2O2-induced increase of [Ca2+]i in smooth muscle cells is reduced by the blockers of these channels (37).
In addition to voltage-gated Ca2+ channels, H2O2 may induce its biological effects through the N-methyl-d-aspartate (NMDA) subtype of glutamate receptors. Although free radicals have been shown to directly inhibit the NMDA currents (2), several studies have indicated that they could also facilitate the NMDA-mediated responses, likely through an increased release of glutamate. Two decades ago, Pellegrini-Giampietro and colleagues first demonstrated that ROS can stimulate the release of glutamate in rat hippocampal slices (36). Later on, Avshalumov and Rice showed that H2O2 exposure can cause activation of normally silent NMDA receptors, possibly via inhibition of redox-sensitive glutamate uptake (3). Mailly et al. also reported that H2O2-induced injury of mouse cortical neurons can be inhibited by (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK801), a specific blocker for NMDA receptor-gated channels (31). Taken together, these findings suggest that secondary activation of voltage-gated Ca2+ channels and the glutamate receptors plays a role in H2O2-induced Ca2+ toxicity. It is, however, unclear whether H2O2 can induce Ca2+ toxicity independent of the activation of glutamate receptors and voltage-gated Ca2+ channels. This question is particularly important following the failure of glutamate antagonists for stroke intervention in clinical trials (4).
Transient receptor potential melastatin 7 (TRPM7) channels are Ca2+-permeable nonselective cation channels that belong to the TRP superfamily (11). They are ubiquitously expressed in almost all tissues and cell types. Activation of TRPM7 channels is important for cellular Mg2+ homeostasis (33). However, its activation is also implicated in Ca2+-mediated neuronal injury under ischemic conditions (1, 42). In addition, our recent study suggested a role for TRPM7 channels in Zn2+-mediated neuronal injury associated with brain ischemia (20). Thus, TRPM7 channels represent a novel therapeutic target for ischemic brain injury.
Here, we show that, at clinical relevant concentrations, H2O2 can induce substantial neuronal injury mediated by Ca2+ entry through a distinct, glutamate receptor and voltage-gated Ca2+ channels independent pathway. Activation of TRPM7 channels is responsible, at least partially, for such effect of H2O2.
Materials and Methods
Neuronal culture
Primary neuronal cultures were prepared from embryonic Swiss mice at 16 days of gestation according to previously described techniques (47). The protocol for neuronal culture using prenatal mouse brains was approved by the Institutional Animal Care and Use Committee of Legacy Research. Briefly, time-pregnant mice were anesthetized with halothane followed by cervical dislocation. Fetuses were rapidly removed and placed in cold Hanks' solution. The cerebral cortices from 10 to 12 embryos were dissected and incubated with 0.05% trypsin–ethylene diamine tetraacetic acid for 10 min at 37°C, followed by trituration with fire-polished glass pipettes, and plated on poly-L-ornithine-coated 24-well culture plates or 25 mm glass coverslips at a density of 2.5 × 105 cells per well and 0.5 × 106 cells per coverslip. Neurons were cultured in the Neurobasal medium supplemented with B27 and maintained at 37°C in a humidified 5% CO2 atmosphere incubator. Toxicity studies were performed at 12–14 days after plating; 5 μM 5-fluoro-2-deoxyuridine and 5 μM uridine were normally added to the cultures 72 h after plating for 2 days to suppress the growth of glial cells. This produces cultures in which ∼80% of cells are neurons, as assessed by immunofluorescent staining with the neuron-specific marker neuronal nuclei and the glial-specific marker glial fibrillary acidic protein (not shown).
H2O2 exposure
Neurons were washed three times with fresh, antioxidant-free, Neurobasal medium (Invitrogen), and randomly divided into control and treatment groups. Dilutions of H2O2 were made fresh from 30% stock solution into the Neurobasal medium before each experiment. Exposures to H2O2 were accomplished by incubating cultures with H2O2 for the duration indicated at 37°C in 5% CO2 incubator. Individual wells were then washed three times with the fresh medium and the cultures were incubated at 37°C in an incubator. Unless otherwise specified, the antagonists of glutamate receptors (10 μM MK801, 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione [CNQX]) and the blocker of L-type Ca2+ channels (5 μM nimodipine) were always present in the culture medium during H2O2 exposure. In some cases, they were also present in the medium after H2O2 exposure (see Results).
Measurement of lactate dehydrogenase activity
Quantitative assessment of cell injury was performed by measurement of lactate dehydrogenase (LDH) released into the culture medium as described in our previous studies (47). The LDH value was determined spectrophotometrically using the LDH assay kit (Roche Molecular Biochemicals). Fifty-microliter medium was transferred from each well to a 96-well plate and mixed with 50 μl reaction solution. Optical density at 492 nm was measured 30 min after the mixing utilizing a microplate reader (Spectra Max Plus; Molecular Devices).
Fluorescein-diacetate and propidium iodide staining
Cell viability was determined by simultaneous staining with fluorescein-diacetate (FDA) and propidium iodide (PI) as described previously (47). For staining of live and dead neurons, cultures were incubated in the extracellular solution containing FDA (5 μM) and PI (2 μM) for 30 min, followed by wash three times with dye-free extracellular solution. Live (FDA-positive) and dead (PI-positive) cells were viewed and counted with a fluorescent microscope (Zeiss) at excitation/emission wavelengths of 580 nm/630 nm for PI, and 500 nm/550 nm for FDA. Viable cells fluoresce bright green, whereas nonviable cells (nucli) are bright red. Images were collected using an Optronics DEI-730 3-chip camera equipped with a BQ 8000 sVGA frame grabber and analyzed using computer software (Bioquant).
Ca2+-imaging
Fluorescent Ca2+-imaging was performed as previously described (47). Cortical neurons grown on 25 mm glass coverslips were incubated with 5 μM fura-2-acetoxymethyl ester for 40 min at room temperature, followed by wash three times and incubated in normal extracellular solution for at least 30 min before imaging. Coverslips with fura-2-loaded cells were then transferred to a perfusion chamber on an inverted microscope (TE300; Nikon). Cells were illuminated using a xenon lamp (75 W) and observed with a 40× UV fluor oil-immersion objective lens. Video images were obtained using a cooled charged-couple device camera (Sensys KAF 1401; Photometrics). Digitized images were acquired, stored, and analyzed in a computer controlled by Axon Imaging Workbench software (AIW2.1; Axon Instruments). The shutter and filter wheel (Lambda 10-2) were also controlled by AIW to permit timed illumination of cells at either 340 or 380 nm excitation wavelengths. Fura-2 fluorescence was detected at an emission wavelength of 510 nm. Background-subtracted 340/380 ratio images were analyzed by averaging pixel ratio values in circumscribed regions of cells in the field of view. The values were then exported from AIW to Sigma Plot for further analysis and plotting.
Terminal deoxyribonucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate-biotin nick end labeling
DNA fragmentation in apoptotic cells was observed by terminal deoxyribonucleotidyl transferase-mediated 2′-deoxyuridine 5′-triphosphate (dUTP)-biotin nick end labeling (TUNEL). Cultures were air-dried, fixed with 10% formalin for 15 min, washed three times in PBS, and permeabilized with 1% Triton X-100 for 20 min. Neurons were subsequently incubated with the reaction mixture containing fluorescein isothiocyanate–dUTP and 300 U/ml terminal deoxy-transferase for 90 min at 37°C. Cultures were then mounted with 4′,6′-diamidino-2-phenylindole containing media (Vector Labs) and viewed with fluorescent microscope at an excitation/emission wavelength of 500/550 nm (green) for fluorescein isothiocyanate–TUNEL-labeled cells.
Plasmid construction and transfection
To construct the plasmid for silencing mouse TRPM7, two oligonucleotides were annealed and inserted into pSilencer 1.0-U6 (Ambion) according to manufacturer's instruction. RNA directed to nucleotides 5152–5172 of cording region of TRPM7 (GenBank accession number NM021450) (20). A fragment cut with BamHI was excised and inserted into BamHI site of pCAGGS-enhanced green fluorescent protein (eGFP) (kindly provided by Dr. J. Miyazaki; Division of Stem Cell Regulation Research, Osaka University Medical School, Osaka, Japan) to express both eGFP and shRNA. For the negative control, a fragment cut with BamHI from pSilencer 1.0-U6 was inserted into pCAGGS-eGFP. For transfection, NeuroFect (Genlantis) was used for cortical neurons at DIV 8 in accordance with the manufacturer's instruction.
Chemicals
H2O2, MK801, CNQX, nimodipine, benzamil, dimethyl sulfoxide (DMSO), dithiothreitol (DTT), and 2,2′-dithiobis-5-nitropyridine (DTNP) were purchased from Sigma Chemical Co. 2-(2-(4-(4-Nitrobenzyloxy)phenyl)ethyl)isothiourea (KB-R7943) was purchased from Tocris. H2O2 was freshly prepared from 30% stock just before each experiment. DTNP was dissolved in acetone before it was added into the culture medium. The final concentration of acetone (3%) was tested to be ineffective (see Results).
Statistics
All data are expressed as mean ± SEM. Student's t-test or ANOVA was employed to examine the statistical significance. The criterion of significance was set at p < 0.05.
Results
H2O2 induces substantial neuronal injury independent of glutamate receptors and voltage-gated Ca2+ channels
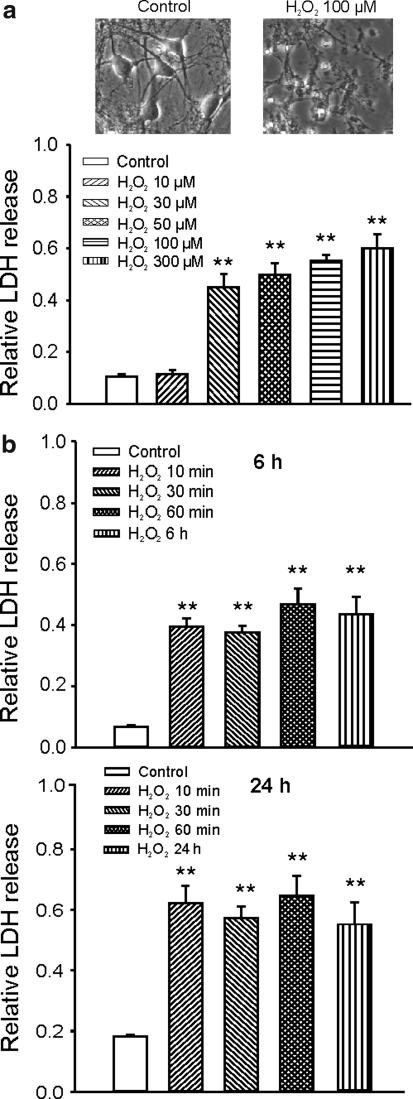
To determine whether H2O2 can induce neuronal injury independent of the activation of glutamate receptors and voltage-gated Ca2+ channels, neurons were treated with H2O2 in the presence of the blockers of glutamate receptors and the voltage-gated Ca2+ channels. Twelve to 14 days after plating, mouse cortical neurons grown in 24-well culture plates were treated with H2O2 in the presence of 10 μM MK801, 20 μM CNQX, and 5 μM nimodipine. In some cases, 3 μM ω-conotoxin MVIIA, a nonselective blocker for N-, P/Q-, and R-type Ca2+ channels, was also added (see below). Cell injury was assayed by the measurement of LDH released into the culture medium at various time points and normalized to the maximal releasable LDH in each well. To obtain the maximal amount of releasable LDH in each well, 1% triton X-100 was added, at the end of each experiment, to permeabilize the cell membrane. Percentage of total LDH release was presented. As shown in Figure 1a, 1 h incubation of neurons with H2O2 in the presence of MK801, CNQX, and nimodipine induced a dose-dependent cell injury, with a threshold concentration of ∼10 μM H2O2. The relative LDH release, measured at 24 h after 1 h H2O2 incubation, was 0.11 ± 0.01, 0.11 ± 0.02, 0.45 ± 0.05, 0.50 ± 0.02, 0.55 ± 0.02, and 0.60 ± 0.05 for 0 μM (control), 10, 30, 50, 100, and 300 μM H2O2, respectively (n = 7–8 wells). These data indicate that H2O2 can induce a significant neuronal injury independent of the activation of glutamate receptors and L-type Ca2+ channels. Addition of high concentration of ω-conotoxin MVIIA (3 μM), a nonselective blocker for N-, P/Q-, and R-type Ca2+ channels, did not affect H2O2-induced LDH release, indicating that activation of these channels is not responsible for H2O2-induced glutamate-independent neuronal injury (n = 4, not shown). Previous studies have shown that, after brain ischemia, the concentration of H2O2 in the brain can reach above 100 μM (19). Therefore, the concentrations used here are pathophysiologically relevant.
FIG. 1.
Concentration- and duration-dependent neuronal injury induced by hydrogen peroxide (H2O2) in the presence of blockers of glutamate receptors and L-type Ca2+ channels. (a) Example phase-contrast images showing neurons before or 24 h after incubation with 100 μM H2O2 (upper panels) and summary bar graph showing concentration-dependent neuronal injury by H2O2 (lower panel). Cultured mouse cortical neurons grown in 24-well plates were treated with different concentration of H2O2 for 1 h in the presence of 10 μM (5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK801), 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 5 μM nimodipine. Neuronal injury was assayed by measurement of the lactate dehydrogenase (LDH) released into the culture medium at 24 h after the H2O2 exposure. The relative LDH release was calculated by normalizing the amount of LDH released at 24 h to the maximal releasable amount of LDH induced by permeabilizing cells with 1% triton X-100. The relative LDH release for control, 10, 30, 50, 100, and 300 μM H2O2 was 0.11 ± 0.01, 0.11 ± 0.02, 0.45 ± 0.05, 0.5 ± 0.05, 0.55 ± 0.02, and 0.60 ± 0.05, respectively (n = 7–8). (b) Summary data demonstrating duration-dependent neuronal injury by 100 μM H2O2. Neurons were treated with 100 μM H2O2 in the presence of MK801, CNQX, and nimodipine for 10 min, 30 min, 60 min, and continuous treatment. Relative LDH release was measured at 6 or 24 h after H2O2 exposure. At 6 h after the exposure of H2O2 for 0 min, 10 min, 30 min, 60 min, and 6 h (continuous), relative LDH release was 0.07 ± 0.01, 0.40 ± 0.03, 0.38 ± 0.02, 0.49 ± 0.06, 0.35 ± 0.05, and 0.46 ± 0.06 (n = 4 wells each). At 24 h after the exposure of H2O2 for 0 min, 10 min, 30 min, 60 min, 120 min, and 24 h (continuous), relative LDH release was 0.017 ± 0.01, 0.62 ± 0.06, 0.57 ± 0.04, 0.65 ± 0.06, and 0.55 ± 0.07 (n = 4 wells each). No statistic significant difference in relative LDH release was detected between the treatment with H2O2 for 10 min, 30 min, 60 min, and 24 h. **p < 0.01 compared with the control group.
In rat hippocampal slice, Avshalumov and Rice have shown that the activity of NMDA receptors is enhanced after washout of H2O2 (3). To exclude the possibility that activation of the NMDA receptors after H2O2 exposure is involved in H2O2-induced injury, in a separate experiment, MK801, CNQX, and nimodipine were present both during and after H2O2 exposure. This measure, however, did not reduce H2O2-induced LDH release (n = 4, not shown), suggesting that a subsequent activation of the glutamate receptors after washout of H2O2 is not responsible for the injury of cultured mouse cortical neurons.
A brief exposure to H2O2 is sufficient to induce glutamate-independent neuronal injury
We then determined the duration of H2O2 incubation required to induce neuronal injury. Neurons were incubated with the medium containing 100 μM H2O2 for 10 min, 30 min, 1 h, 6 h, or 24 h. LDH release was measured at 6 and 24 h after the start of H2O2 treatment. As shown in Figure 1b, 10 min incubation of neurons with 100 μM H2O2 induced a similar proportion of neuronal injury as 1 or 6 h treatment. Six hours after the start of H2O2 incubation, for example, relative LDH release of 0.4 ± 0.03, 0.38 ± 0.02, 0.49 ± 0.06, and 0.46 ± 0.06 were detected for 10 min, 30 min, 1 h, and 6 h H2O2 exposures (n = 4 wells for each group). At 24 h after the start of H2O2 incubation, relative LDH release of 0.62 ± 0.06, 0.57 ± 0.04, 0.65 ± 0.06, and 0.55 ± 0.07 were detected for 10 min, 30 min, 1 h, and 24 h H2O2 exposure (n = 4 wells for each group). No statistically significant differences were detected in LDH release induced by 10 min, 30 min, 1 h, or continuous H2O2 treatment. These data indicate that a brief incubation (e.g., < 10 min) with H2O2 is sufficient to induce substantial delayed, glutamate-independent neuronal injury. This finding is consistent with previous reports that brief (>5 min) incubations with H2O2 caused a significant loss of viability of cultured rat forebrain and cortical neurons (15, 45). Since a substantial neuronal injury is detected at 6 h after H2O2 exposure and the background LDH release is very low at this time point, for the rest of experiments we focused on the effects of H2O2 at 6 h time point.
Involvement of Ca2+ entry in H2O2-induced glutamate receptor and voltage-gated Ca2+ channel-independent neuronal injury
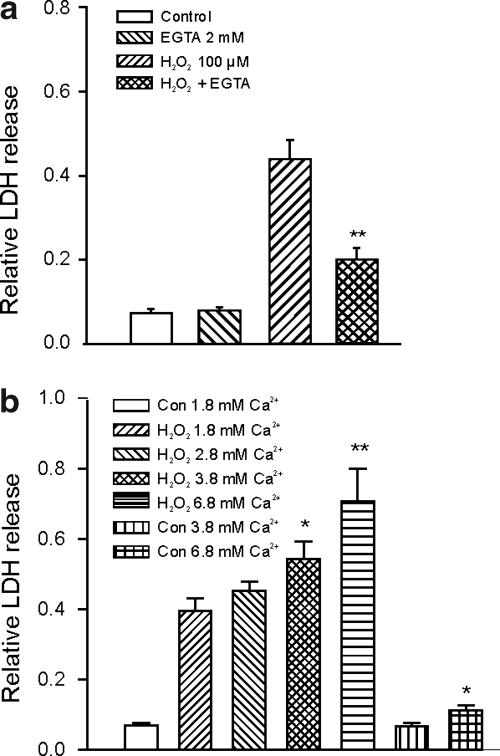
Intracellular Ca2+ accumulation plays an important role in neuronal injury associated with brain ischemia. To determine whether the H2O2-induced glutamate receptor and voltage-gated Ca2+ channel-independent neuronal injury depends on the entry of Ca2+ into neurons, membrane impermeable Ca2+ chelator ethylene glycol tetraacetic acid (EGTA) (2 mM) was added to the culture medium to reduce the concentration of the extracellular Ca2+ in the medium. After the addition of EGTA, which lowers pH, pH was readjusted back to 7.3 by titration with NaOH. With 2 mM EGTA and a total Ca2+ of 1.8 mM in the medium, a free Ca2+ concentration of ∼0.8 μM was calculated (38). To determine the effect of extracellular Ca2+ on H2O2-induced neuronal injury, neurons were exposed to 100 μM H2O2 for 1 h in the absence and presence of EGTA. As shown in Figure 2a, addition of 2 mM EGTA in the culture medium dramatically reduced H2O2-induced relative LDH release from 0.43 ± 0.05 to 0.20 ± 0.03 (n = 4 wells in each group, p < 0.01). Addition of the same concentration of EGTA in the control medium did not affect the background LDH readings (0.075 ± 0.01 vs. 0.080 ± 0.01, n = 4 wells in each group, p > 0.05), suggesting that the decrease of LDH release by EGTA was due to its inhibition of H2O2-induced neuronal injury. In contrast to EGTA, adding Ca2+ to the culture medium potentiated the H2O2-induced neuronal injury (Fig. 2b). In the control medium containing 1.8 mM Ca2+, 1 h incubation with 100 μM H2O2 induced a relative LDH release of 0.39 ± 0.04 (n = 8). With the culture medium containing 2.8, 3.8, or 6.8 mM Ca2+, 1 h incubation with 100 μM H2O2 induced 0.45 ± 0.03, 0.54 ± 0.05, and 0.71 ± 0.09 of relative LDH release (n = 8 for each group, p < 0.05 between control and 3.8 mM Ca2+ group; p < 0.01 between control and 6.8 mM Ca2+ group). The medium with 2.8 or 3.8 mM Ca2+ in the absence of H2O2 did not show different background LDH readings compared with the medium containing 1.8 mM Ca2+, though the medium containing 6.8 mM Ca2+ showed a slightly increased background LDH of 0.11 ± 0.02 (n = 8). Together, these data suggest that H2O2-induced glutamate and voltage-gated Ca2+ channel-independent neuronal injury likely involves an entry of extracellular Ca2+ into neurons. Addition of EGTA did not completely prevent the H2O2 injury (Fig. 2a), indicating that Ca2+ entry-independent mechanism may be partially responsible for H2O2-induced glutamate receptor and voltage-gated Ca2+ channel-independent neuronal injury.
FIG. 2.
Dependence of H2O2-induced glutamate receptor-independent neuronal injury on [Ca2+]e. (a) Summary bar graph demonstrating the protection by 2 mM ethylene glycol tetraacetic acid (EGTA) on neuronal injury induced by H2O2 in the presence of MK801, CNQX, and nimodipine. Neurons were treated with 100 μM H2O2 for 1 h in the presence or absence of 2 mM EGTA and the relative release of LDH was measured at 6 h after the H2O2 exposure. In the absence of EGTA, 1 h exposure to 100 μM H2O2 induced 0.44 ± 0.05 of relative LDH release (n = 4), wherase in the presence of 2 mM EGTA, 1 h treatment with the same concentration of H2O2 only induced 0.20 ± 0.03 of relative LDH release (n = 4, p < 0.01 between H2O2 alone and H2O2 with 2 mM EGTA). Addition of 2 mM EGTA in the control medium without H2O2 did not affect the background LDH release (0.075 ± 0.01 vs. 0.080 ± 0.01, n = 4 wells each, p > 0.05). **p < 0.01 compared with 100 μM H2O2 alone. (b) Summary bar graph showing the dose-dependent increase in H2O2-induced neuronal injury in response to increased [Ca2+]e from 1.8 to 2.8, 3.8, or 6.8 mM. In the control medium containing 1.8 mM Ca2+, 1 h incubation with 100 μM H2O2 induced a relative LDH of 0.39 ± 0.04 at 6 h (n = 8). With the culture medium containing 2.8, 3.8, or 6.8 mM Ca2+, 1 h incubation with the same concentration of H2O2 induced 0.45 ± 0.03, 0.54 ± 0.05, and 0.71 ± 0.09 of relative LDH release (n = 8, p < 0.05 between 1.8 and 3.8 mM Ca2+ group; p < 0.01 between 1.8 and 6.8 mM Ca2+ group). The control medium with 2.8 or 3.8 mM Ca2+ in the absence of H2O2 did not affect the background LDH release, whereas the medium containing 6.8 mM Ca2+ only slightly increased the background LDH from 0.07 ± 0.01 to 0.11 ± 0.02 (n = 8, p < 0.05). *p < 0.05, **p < 0.01.
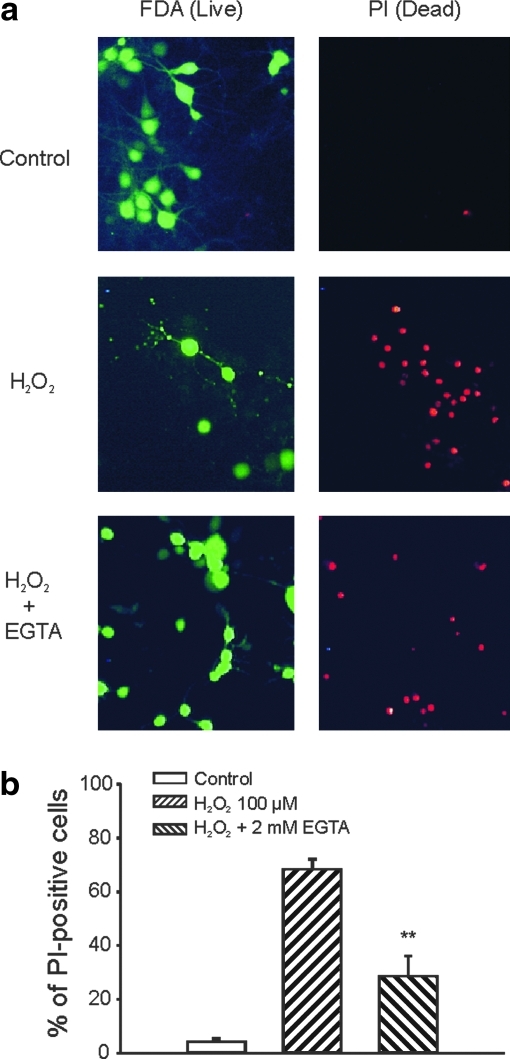
In addition to LDH release, H2O2-induced injury was also studied by fluorescent staining of live (FDA-positive) and dead cells (PI-positive) (21). Neurons were cultured on 25 mm coverslips and used for the studies 12 days after plating. Six hours after H2O2 treatment, PI (2 μM) and FDA (5 μM) were added into the culture medium for 30 min before observation with fluorescent microscope for PI- or FDA-positive cells. As shown in Figure 3, treatment with H2O2 (100 μM, 1 h), in the presence of MK801, CNQX, and nimodipine induced a significant increase in PI-positive cells. Addition of 2 mM EGTA substantially decreased the total number of PI-positive cells (Fig. 3). Six hours after H2O2 incubation, the percentages of PI-positive cells were 4.3% ± 1.0%, 68.2% ± 3.8%, and 28.5% ± 7.6% for control, H2O2 alone, and H2O2 in the presence of 2 mM EGTA (six to eight fields from 2 to 3 coverslips in each group, p < 0.01 between H2O2 alone and H2O2 with 2 mM EGTA).
FIG. 3.
Assessment of H2O2-induced injury by fluorescent staining of live and dead neurons. (a) Example images showing fluorescent staining of live (fluorescein-diacetate [FDA]-positive) and dead (propidium iodide [PI]-positive) cells in various treatment groups. Cultured mouse cortical neurons grown on 25 mm glass coverslips were treated with the control medium, the medium containing 100 μM H2O2, or H2O2 plus 2 mM EGTA for 1 h in the presence of MK801, CNQX, and nimodipine. Six hours after the treatment, neurons were incubated in the extracellular solution containing 5 μM FDA and 2 μM PI for 30 min followed by washing with dye-free solution. Live (FDA-positive) and dead (PI-positive) cells were then viewed with fluorescent microscope at excitation/emission wavelength of 500 nm/550 nm for FDA and 580 nm/630 nm for PI staining. (b) Summary data showing the percentage of dead cells in different treatment groups. Images were collected and analyzed using computer software (Bioquant). Two to three fields of at least 50 cells each from individual coverslip were counted for both FDA and PI-positive staining. Six hours after 1 h treatment, percentage of PI-positive cells were 4.3% ± 1.0%, 68.2% ± 3.8%, and 28.5% ± 7.6% for control, H2O2 alone, and H2O2 in the presence of 2 mM EGTA (n = 6–8 fields from two to three coverslips). **p < 0.01 compared with 100 μM H2O2 alone. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Activation of Na+-Ca2+ exchange is not involved in H2O2-induced glutamate receptor-independent neuronal injury
In L929 cells, H2O2-induced Ca2+ toxicity involves Ca2+ entry through Na+-Ca2+ exchange, operated in the reversed mode (22). To determine whether a similar mechanism is involved in H2O2-induced glutamate receptor-independent injury of cultured mouse cortical neurons, we tested the effect of KB-R7943, a selective blocker for the reversed Na+-Ca2+ exchanger, on H2O2-induced neuronal injury; 10 μM KB-R7943 was added into culture wells 10 min before and during the treatment with 100 μM H2O2. As shown in Supplementary Figure 1 (Supplementary Data are available online at www.liebertonline.com/ars), addition of KB-R7943 had little effect on H2O2-induced neuronal injury. The relative LDH release at 6 h after 1 h H2O2 exposure was 0.41 ± 0.02 in the absence of KB-R7943 and 0.44 ± 0.04 in the presence of 10 μM KB-R7943 (n = 4 and 8, p > 0.05). Similarly, addition of benzamil (100 μM), a common blocker for Na+-Ca2+ and Na+-H+ exchange, did not affect H2O2-induced neuronal injury. In the presence of benzamil, the relative LDH release induced by H2O2 was 0.42 ± 0.03 (n = 4, p > 0.05 compared to H2O2 treatment alone). Together, these data indicate that activation of Na+-Ca2+ exchange system is not responsible for H2O2-induced glutamate receptor-independent injury of cultured mouse cortical neurons.
H2O2-induced glutamate-independent neuronal injury is inhibited by thiol reducing agent
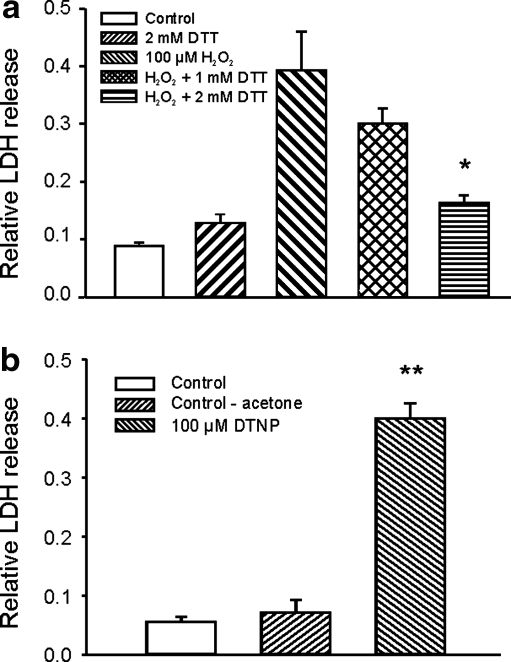
Oxidization of sulfhydryl groups on cysteine residues has been implicated in many physiological or pathological effects of ROS. To know whether H2O2-induced glutamate receptor-independent injury of mouse cortical neurons involves an oxidization of the sulfhydryl groups, we tested the effect of DTT, a thiol-reducing agent (5), on H2O2-induced neuronal injury. As shown in Figure 4a, addition of DTT during H2O2 exposure produced a concentration-dependent reduction of H2O2-induced neuronal injury. In the absence of DTT, 1 h incubation with 100 μM H2O2 induced a relative LDH release of 0.39 ± 0.07 at 6 h. However, in the presence of 1.0 or 2.0 mM DTT, the same concentration of H2O2 induced a relative LDH release of only 0.30 ± 0.03 or 0.16 ± 0.01, respectively (n = 4 wells each, p < 0.05 between H2O2 alone and H2O2 with 2 mM DTT). One hour treatment with either 1.0 or 2.0 mM DTT alone without H2O2 did not affect the background LDH release (n = 4). This finding suggests that H2O2-induced glutamate receptor-independent neuronal injury likely involves the oxidization of sulfhydryl groups on cysteine residues. An alternative explanation could be that DTT reacts with H2O2 directly, thus attenuating the effect of H2O2. To provide further evidence that H2O2-induced glutamate receptor-independent neuronal injury involves the oxidization of sulfhydryl groups, we determined whether incubation of neurons with DTNP, a lipophilic cysteine-specific oxidizing agent, can induce similar injury as H2O2. As shown in Figure 4b, 1 h treatment of neurons with 100 μM DTNP in the presence of MK801, CNQX, and nimodipine induced a 0.39 ± 0.03 of relative LDH release at 6 h (n = 8 wells, p < 0.01 compared with the control group), providing further evidence that oxidization of sulfhydryl groups on cysteine residues might be involved in H2O2-induced glutamate-independent neuronal injury.
FIG. 4.
Evidence that oxidation of sulfhydryl groups on cysteine residues may be involved in H2O2-induced glutamate receptor-independent neuronal injury. (a) Summary bar graph demonstrating the protective effect of thiol-reducing agent dithiothreitol (DTT) on H2O2-induced neuronal injury. Neurons were treated with 100 μM H2O2 in the presence of MK801, CNQX, and nimopidine for 1 h, either in the absence or presence of 1 or 2 mM DTT. Relative LDH release was measured 6 h after H2O2 exposure. One hour treatment with 100 μM H2O2 in the absence of DTT induced 0.39 ± 0.07of relative LDH release. However, in the presence of 1 or 2 mM DTT, relative LDH release by the same concentration of H2O2 was reduced to 0.30 ± 0.03 and 0.16 ± 0.01, respectively (n = 4 wells each, p < 0.05 between H2O2 alone and H2O2 with 2 mM DTT). *p < 0.05 compared with 100 μM H2O2 alone. (b) Summary data showing the relative LDH release induced by cysteine oxidizing agents 2,2′-dithiobis-5-nitropyridine (DTNP) in the presence of MK 801, CNQX, and nimodipine. LDH release was measured 6 h after 1 h exposure to 100 μM DTNP or vehicle control. Relative LDH release induced by DTNP was 0.39 ± 0.03 (n = 8 wells each, p < 0.01 compared with control group). Vehicle control for DTNP (3% acetone) did not induced significant increase in background LDH release (n = 4).
H2O2 may induce its biological effect through a direct oxidation of its substrate, or indirectly through its reactive bi-product OH• (13). To know whether H2O2-induced glutamate receptor-independent neuronal injury involves the formation of OH•, the effect of DMSO, a scavenger for OH• (48), on H2O2-induced neuronal injury was examined. As shown in Supplementary Figure 2a, addition of 0.1%–2.0% DMSO (∼1.5 to 35 mM) to the medium did not affect H2O2-induced glutamate receptor-independent LDH release. In the absence of DMSO, 1 h incubation with 100 μM H2O2 induced a relative LDH release of 0.49 ± 0.09 at 6 h (n = 4). In the presence of 0.1%, 0.5%, or 2% of DMSO, the relative LDH release was 0.46 ± 0.04, 0.47 ± 0.09, or 0.44 ± 0.03, respectively (n = 4 wells in each group, p > 0.05 between control and different DMSO groups).
Since the formation of OH• from H2O2 requires the presence of transition metal iron, we have also examined the effect of iron chelating agent deferoxamine (DFO) on H2O2-induced neuronal injury; 100 μM DFO was added into culture wells 10 min before and during H2O2 incubation. As shown in Supplementary Figure 2b, addition of DFO did not provide any protection against the H2O2 toxicity. In the absence of DFO, 1 h incubation with H2O2 induced a relative LDH release of 0.39 ± 0.03 at 6 h. In the presence of 100 μM DFO, the relative LDH release was 0.45 ± 0.02 (n = 8 in each group, p > 0.05). Together, these data suggest that the formation of OH• is not required for H2O2-induced glutamate receptor-independent neurotoxicity in cultured mouse cortical neurons.
H2O2 induces increase of [Ca2+]i independent of glutamate receptors and voltage-gated Ca2+ channels
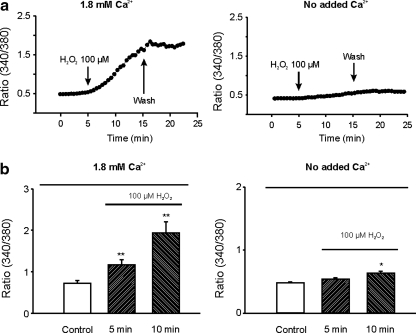
To provide a direct evidence that H2O2 can induce an increase of [Ca2+]i independent of the activation of glutamate receptors and voltage-gated Ca2+ channels, fura-2 fluorescent Ca2+-imaging was performed to determine whether application of H2O2 can produce an increase of [Ca2+]i in the presence of the blockers for glutamate receptors and L-type Ca2+ channels. As shown in Figure 5, in the presence of MK801, CNQX, and nimodipine, perfusion of 100 μM H2O2 increased the 340/380 ratio from 0.73 ± 0.11 to 1.94 ± 0.26 within 10 min (n = 9, p < 0.01). This increase of [Ca2+]i can be inhibited by removing the extracellular Ca2+ (Fig. 5). With no added Ca2+ in the extracellular solution, perfusion of 100 μM H2O2 for 10 min only increased the 340/380 ratio from 0.48 ± 0.02 to 0.63 ± 0.04 (n = 12). The slight increase of [Ca2+]i by H2O2 in the absence of added Ca2+ could be due to residual contaminating Ca2+ in the extracellular solution. Together, these data suggest that a glutamate receptor and voltage-gated Ca2+ channel-independent Ca2+ entry pathway is activated by H2O2.
FIG. 5.
Glutamate receptor and voltage-gated Ca2+ channel-independent increase of [Ca2+]i by H2O2. (a) Time-dependent changes of [Ca2+]i in response to bath perfusion of H2O2 in the presence of MK801, CNQX, and nimopidine. Left panel: with bath solution containing 1.8 mM Ca2+, perfusion of 100 μM H2O2 induced a large increase in [Ca2+]i, as demonstrated by an increase in the intensity of 340/380 ratio image. Right panel: with Ca2+ removed from the bath solution, perfusion of 100 μM H2O2 induced only a small increase in the ratio of 340/380 image. (b) Summary data showing the glutamate receptor and voltage-gated Ca2+ channel-independent increase of [Ca2+]i by H2O2. In the presence of 1.8 mM Ca2+ in the bath solution, perfusion of neurons with 100 μM H2O2 for 10 min increased 340/380 ratio from 0.73 ± 0.11 to 1.94 ± 0.26 (166% increase, n = 9, p < 0.01). In the absence of added Ca2+, perfusion of 100 μM H2O2 for 10 min only increased the 340/380 ratio from 0.48 ± 0.02 to 0.63 ± 0.04 (31% increase, n = 12, p < 0.05). *p < 0.05, **p < 0.01 compared with control.
Involvement of apoptotic cell death by H2O2
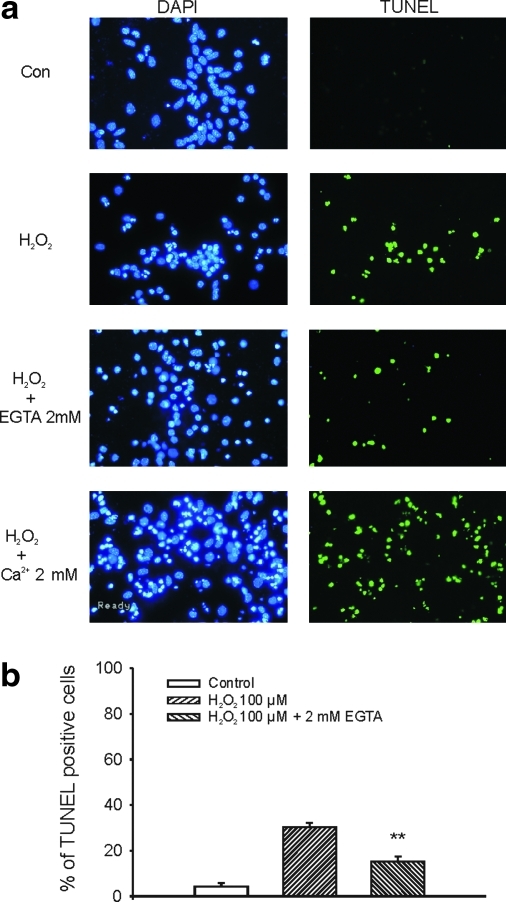
H2O2 treatment, in the absence of glutamate receptor antagonists, has been shown to induce apoptotic cell death (15, 45). Since neuronal injury by activation of glutamate receptors also involves an apoptotic process (26), it is not clear whether H2O2 can also induce apoptotic cell injury when glutamate receptors are blocked. For this reason, we have examined whether H2O2 treatment can induce apoptosis in the presence of the blockers of glutamate receptors and voltage-gated Ca2+ channels. Neurons grown on 25 mm coverslips were treated with H2O2 (100 μM, 1 h) in the presence of 10 μM MK801, 20 μM CNQX, and 5 μM nimodipine. TUNEL staining was performed 6 h after H2O2 exposure. As shown in Figure 6, H2O2 treatment induced a substantial increase in TUNEL-positive cells. Chelating the extracellular Ca2+ by addition of 2 mM EGTA in the medium significantly reduced the total number of TUNEL-positive cells induced by H2O2. The percentage of TUNEL-positive cells was 3.2% ± 1.4%, 30.3% ± 1.9%, and 15.1% ± 2.2% for control, H2O2 alone, and H2O2 in the presence of 2 mM EGTA (n = 6–7 fields from three coverslips in each group, p < 0.01 between control and H2O2 groups, H2O2 alone and H2O2/2 mM EGTA groups). In contrast, adding 2 mM Ca2+ into the medium (final concentration: 3.8 mM) increased the total number of TUNEL-positive staining (data not shown). Together, these data indicate that H2O2 can induce apoptotic cell injury independent of the activation of glutamate receptors, and that the Ca2+ entry from extracellular space is involved in such injury. The finding that the percentage of TUNEL-positive staining (Fig. 6), which is an indication of apoptotic cell injury, is lower than the PI-positive staining (Fig. 3), which detects both necrotic and the late phase of apoptotic cell injury, suggests that both necrotic and apoptotic cell injury processes are involved in H2O2-induced glutamate receptor-independent injury of mouse cortical neurons.
FIG. 6.
Evidence of apoptotic cell injury by H2O2. (a) Six hours after 1 h exposure to H2O2 or the control medium in the presence of MK801, CNQX, and nimodipine, DNA fragmentation in apoptotic cells was observed by terminal deoxyribonucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL, see Materials and Methods). After TUNEL staining, cultures were mounted with 4′,6′-diamidino-2-phenylindole (DAPI) containing media and viewed with fluorescent microscope at an excitation/emission wavelength of 500/550 nm for fluorescein isothiocyanate–TUNEL-labeled cells (green) and 340/425 nm for DAPI staining of all nuclei (blue). One hour exposure of cultured cortical neurons with 100 μM H2O2 induced a significant increase in TUNEL-positive cells. Addition of 2 mM EGTA largely reduced the total number of TUNEL-positive cells, whereas addition of 2 mM Ca2+ (total Ca2+ in the medium: 3.8 mM) increased the total number of TUNEL-positive cells. (b) The percentage of TUNEL-positive staining at 6 h were 3.2% ± 1.4%, 30.3% ± 1.9%, and 15.1% ± 2.2% for control, H2O2 alone, and H2O2 in the presence of 2 mM EGTA (n = 6–7 field from three coverslips in each group, p < 0.01 between control and H2O2 groups, H2O2 alone and H2O2 with 2 mM EGTA groups). **p < 0.01 compared with H2O2 100 μM. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
TRPM7 channels are involved in H2O2-induced glutamate receptor-independent cell injury
Next, we investigated the potential Ca2+-entry pathways responsible for H2O2-induced glutamate receptor-independent cell injury. We have recently demonstrated that activation of TRPM7, a Ca2+-permeable nonselective cation conductance, is involved in prolonged hypoxia-induced glutamate receptor-independent neuronal injury (1). It was also suggested that increased production of reactive nitrogen species by hypoxia, for example, nitric oxide (NO), mediates the activation of TRPM7 channels and the resultant neuronal injury. Unlike NO, however, H2O2 was less involved (1).
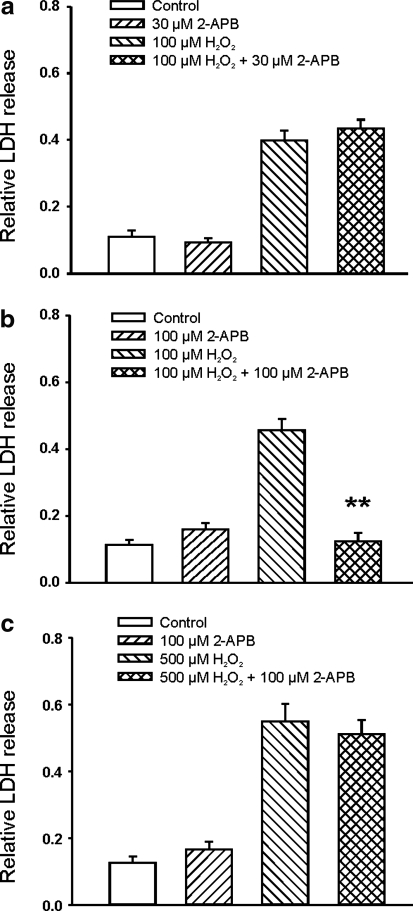
Recent studies also recognized the importance of TRPM2 channels, a nonselective cation channel activated by ADP-ribose, in H2O2-induced responses in some cell types (14). For example, transfection of human embryonic kidney (HEK) cells with rat TRPM2 increased H2O2-induced injury (23). It has also been shown that injury of rat cortical neurons by high concentrations of H2O2 (e.g., 1 mM) is reduced by small interference RNA (siRNA)-TRPM2 (23). To determine whether H2O2-induced glutamate-independent injury of mouse cortical neurons at physiologically relevant concentrations involves the activation of TRPM2 or TRPM7 channels, we first determine whether addition of 2-aminoethoxydiphenyl borate (2-APB), a commonly used nonspecific blocker for TRPM7 (28) and TRPM2 channels (43), can inhibit H2O2-induced injury of mouse cortical neurons. As shown in Figure 7, addition of 30 μM 2-APB, a concentration known to completely inhibit the TRPM2 current (43) but has minor effect on the TRPM7 current (28), did not provide significant protection against H2O2-induced injury. Increasing 2-APB to 100 μM, however, significantly reduced H2O2-induced neuronal injury (Fig. 7), indicating that activation of TRPM7 channels might be involved in H2O2-induced glutamate receptor and voltage-gated Ca2+ channel-independent neuronal injury. Neuronal injury induced by higher concentration of H2O2 (500 μM) was not protected by 100 μM 2-APB, suggesting that nonphysiologically high concentrations of H2O2 may activate additional cell injury mechanisms, for example through nonspecific lipid peroxidation.
FIG. 7.
Effect of 2-aminoethoxydiphenyl borate (2-APB) on H2O2-induced glutamate-independent injury of mouse cortical neurons. (a) Addition of 30 μM 2-APB, a concentration known to completely inhibit the transient receptor potential melastatin 2 (TRPM2) current but has small effect on the TRPM7 current, did not provide protection against H2O2-induced injury (n = 8 wells each). (b) Addition of 100 μM 2-APB, a concentration known to inhibit the TRPM7 channels, significantly reduced H2O2-induced neuronal injury (n = 15–16, **p < 0.01 compared to 100 μM H2O2 group). (c) Neuronal injury induced by higher concentration of H2O2 (500 μM) was not protected by 100 μM 2-APB (n = 11–12).
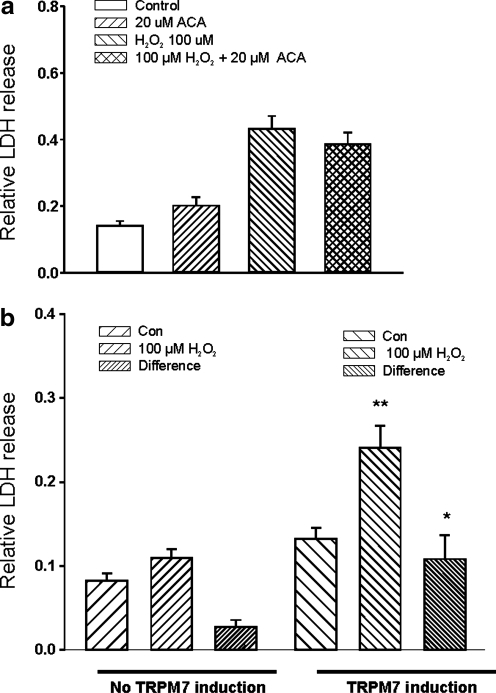
Consistent with a lack of TRPM2 involvement in H2O2-induced injury of mouse cortical neurons, incubation of cells with N-(p-amylcinnamoyl)anthranilic acid, another inhibitor of TRPM2 channels (25), did not have significant effect on H2O2-induced cell injury (Fig. 8a, n = 8 wells).
FIG. 8.
TRPM7 but not TRPM2 channels are involved in H2O2 induced glutamate receptor-independent neuronal injury. (a) Summary data showing the lack of protection of H2O2-induced injury of mouse cortical neurons by N-(p-amylcinnamoyl)anthranilic acid (ACA), an inhibitor of TRPM2 channels (n = 8 wells). (b) Overexpression of TRPM7 channels in human embryonic kidney 293 cells increased their sensitivity to H2O2 injury (n = 15).
To further determine whether activation of TRPM7 channels might be involved in H2O2-mediated cell injury, we questioned whether increasing expression of TRPM7 channels increased the sensitivity of cells to H2O2. Due to limited success in transfecting native neurons with plasmid encoding TRPM7 channels, we used a HEK293 cell line with inducible expression of TRPM7 channels (20, 34). We compared the degree of H2O2-induced injury of HEK293 cells with and without overexpression of TRPM7 channels. HEK293 cells stably transfected with Flag-murine TRPM7/pCDNA4-TO construct (34) were grown in 24-well culture plates with DMEM supplemented with 10% fetal bovine serum, blasticidin (5 μg/ml), and zeocin (0.4 mg/ml). TRPM7 expression was induced by adding 1 μg/ml tetracycline to the culture medium, as described in our previous studies (20). Induced expression of TRPM7 was confirmed by Western blot (20). Forty-eight hours after the induction of TRPM7 expression, H2O2-induced cell injury was analyzed. We expected that, if activation of TRPM7 was involved in H2O2-mediated cell injury, increased expression of these channels would make them more sensitive to H2O2-induced increase of LDH release. As shown in Figure 8b, without overexpression of TRPM7 channels, HEK293 cells were relatively resistant to H2O2 injury as shown by small nonsignificant increase of LDH release. However, after induction of TRPM7 expression, incubation of cells with 100 μM induced large increase of LDH release (n = 15–16). These data further suggest that TRPM7 channels play an important in mediating H2O2-induced glutamate-independent cell injury.
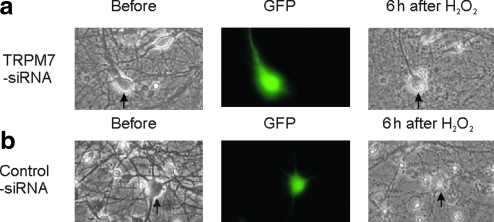
To provide more convincing evidence that TRPM7 channels are required for H2O2-mediated glutamate-independent injury of neurons, we determined whether knockdown the expression of TRPM7 channels with TRPM7-siRNA affects the H2O2-induced neuronal injury. At 8 days after culture, mouse cortical neurons were transfected with TRPM7-siRNA-GFP or control-siRNA-GFP, as described in our previous studies (20). H2O2-induced injury was analyzed 3 days after transfection. Live/dead cells were counted based on the morphology of cells and confirmed by PI staining. To minimize variations, individual cells were followed through before and after H2O2 treatment. In 11 neurons (from three separated cultures) transfected with TRPM7-siRNA-GFP, all of them stayed alive 6 h after 1 h incubation with 100 μM H2O2 (Fig. 9a). In contrast, in 13 neurons transfected with control-siRNA-GFP, only 7 stayed alive (Fig. 9b, p < 0.05, Chi square test).
FIG. 9.
Knockdown of TRPM7 expression with TRPM7-small interference RNA (siRNA) inhibits the H2O2-induced injury of cultured mouse cortical neurons. At 8 days after culture, mouse cortical neurons were transfected with TRPM7-siRNA-green fluorescent protein (GFP) (a) or control-siRNA-GFP (b). H2O2-induced injury was analyzed 3 days after transfection. Alive/dead cells were counted based on the morphology of cells and confirmed by PI staining. In 11 neurons (from three separated cultures) transfected with TRPM7-siRNA-GFP, all of them stayed alive 6 h after 1 h incubation with 100 μM H2O2. In contrast, in 13 neurons transfected with control-siRNA-GFP, only 7 stayed alive (p < 0.05, Chi-square test). Arrows point to the same neurons before and 6 h after 1 h μM H2O2. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Discussion
Intracellular Ca2+ accumulation plays a critical role in the pathology of brain ischemia. Ca2+ entry through glutamate receptors, particularly the NMDA subtype of the glutamate receptors, has been considered as the main source of [Ca2+]i accumulation associated with ischemic brain injury (7). Unfortunately, clinical trials failed to show a satisfactory neuroprotection by the antagonists of glutamate receptors (16). Although multiple factors may have contributed to the failure of the trials, emerging new studies also support an involvement of glutamate receptor-independent Ca2+ entry pathways, for example, TRPM7 channels and acid-sensing ion channels, in ischemic brain injury (1, 42, 47).
The objective of the present study was to determine whether H2O2, the most stable and one of the primary ROS in vivo, induces a glutamate receptor-independent Ca2+ toxicity. Using cultured mouse cortical neurons, we studied the H2O2-induced cell injury in the presence of the blockers of glutamate receptors and voltage-gated Ca2+ channels. We demonstrated that brief exposures to H2O2, at clinical relevant concentrations, induced substantial neuronal injury independent of the activation of glutamate receptors and voltage-gated Ca2+ channels. We further demonstrated that such effect of H2O2 involves an entry of Ca2+ from the extracellular space. This was supported by the finding that chelating the extracellular Ca2+ reduced, whereas increasing the Ca2+ potentiated, neuronal injury. Consistent with activation of a Ca2+ entry pathway, fluorescent Ca2+-imaging demonstrated a H2O2-induced increase of [Ca2+]i in the presence of the blockers of glutamate receptors and voltage-gated Ca2+ channels, and that the increase of [Ca2+]i was diminished with extracellular solutions containing no added Ca2+. More importantly, we demonstrated that H2O2-induced neuronal injury was inhibited by TRPM7 blockade or TRPM7-siRNA, which supports the involvement of TRPM7 channels.
TRPM7 is a member of the large TRP channel superfamily expressed in almost every tissue and cell type (9). The TRP superfamily of ion channels are divided into six subfamilies according to their sequence homology: TRPC, TRPM, TRPV, TRPP, TRPML, and TRPA (9). TRPM subfamily of TRP channels has eight members, TRPM1–8. Different members of the TRPM subfamily appear to have different gating and regulatory mechanisms, along with different ion selectivity and expression patterns. Increasing evidence suggests that activation of TRPM7 channels contributes to various physiological and pathophysiological processes. Notably, we demonstrated that activation of TRPM7 channels by oxygen-free radicals plays a critical role in hypoxia-induced glutamate-independent neuronal injury (1).
Previous studies have indicated activation of nonselective conductance by H2O2 (32, 41). However, the molecular identity of the conductance was unclear. Mendez and Penner demonstrated that conditions that mimic oxidative stress, for example, exposure to ultraviolet light or direct perfusion of H2O2 activated a nonselective cation current in several mammalian cell lines, including RBL, mast, HEK, PC12, and 3T3 cells (32). The H2O2-activated current demonstrated no voltage dependency and little selectivity among monovalent cations with substantial Ca2+ permeability. In rat striatal neurons, Smith and colleagues reported that, at nonphysiological high concentrations (>10 mM), H2O2 activated a Ca2+-permeable nonselective cation channel with a single-channel conductance of 70–90 pS (41). Activation of this conductance was suggested to be responsible for the injury of a sub-population of striatal neurons by H2O2. Since the concentrations of H2O2 used in our studies are ∼100 times lower than that required to activate the cation conductance in striatal neurons (41), it is not clear whether the same channel was activated in both studies.
Recent studies by Kaneko et al. have suggested an involvement of TRPM2 channels in H2O2-induced neuronal injury (23). They demonstrated that, in rat cortical neurons, high concentrations of H2O2 (e.g., 1 mM) induced cell injury that was attenuated by siRNA-TRPM2 (23). Our studies suggest that, at physiologically relevant concentrations, H2O2-induced glutamate-independent injury of mouse cortical neurons does not involve the activation of TRPM2 channels. This was supported by the finding that addition of 30 μM 2-APB, a concentration known to completely inhibit the TRPM2 current (43), did not provide significant protection against H2O2-induced neuronal injury, and that addition of N-(p-amylcinnamoyl)anthranilic acid, another inhibitor of TRPM2 channels (25), had little effect on H2O2-induced neuronal injury.
We have demonstrated previously that activation of TRPM7, a nonselective cation conductance with high Ca2+ permeability, is involved in prolonged hypoxia-induced neuronal injury (1). We further showed that, an increased production of NO was likely responsible for the activation of TRPM7 channels (1). Unlike NO, however, H2O2 was less involved (1). The lack of clear involvement of H2O2 in hypoxia-induced glutamate-independent neuronal injury maybe due to a lack of sufficient production of H2O2 in the cell culture condition. In the present studies, we show that incubation of neurons with exogenous H2O2, at concentrations relevant to brain ischemia (19), produced substantial neuronal injury independent of glutamate receptors and voltage-gated Ca2+ channels, and that such H2O2-induced neuronal injury was inhibited by TRPM7 blockade or TRPM7 knockdown.
TRPM7 channels are highly expressed in brain cells (1, 42). Although a specific agonist for this channel has not been identified, various biochemical changes associated with brain ischemia, for example, reduced extracellular Ca2+, decreased cellular ATP, and increased production of NO, can facilitate the opening of these channels (1, 11). Our present study discloses a new activator for TRPM7 channels. In addition to being activated by these changes, one recent study has shown that expression of TRPM7 channels is also increased after brain ischemia, and that reduced expression is associated with neuroprotection by electric acupuncture (50). Therefore, TRPM7 channels may represent a novel, glutamate receptor-independent, Ca2+ entry pathway responsible for ischemic brain injury.
In addition to Ca2+, TRPM7 channels have substantial Zn2+ permeability (33). Our recent studies suggested that entry of Zn2+ through these channels plays an important role in Zn2+-mediated neuronal cell death (20). Therefore, activation of these channels likely contributes to both Ca2+ and Zn2+ toxicity associated with brain ischemia. A recent study by Hwang and colleagues has shown an increase of intracellular Zn2+ associated with H2O2-mediated neuronal injury (17). Although Zn2+ entry from glutamate receptors and/or release from Zn2+-binding proteins could contribute to the increase of [Zn2+]i, our present studies suggest that activation of TRPM7 channel by H2O2 could be an alternative pathway mediating this H2O2-Zn2+ toxicity.
After brain ischemia, the production of ROS is dramatically enhanced in the central nervous system. Increased production of O•−2 and H2O2, for example, is induced through the action of xanthine oxidase, leakage from mitochondrial electron transport chain, and activation of phospholipase A2 (29). Using microdialysis or electron spin resonance, a number of studies have measured increase of ROS production after ischemia and after reperfusion. Hyslop and colleagues, for example, reported that in the rat brain, a baseline concentration of H2O2 is at low micromolar range, whereas the peak H2O2 concentration after global ischemia is ∼100 μM (19). The concentrations of H2O2 used in our studies are, therefore, close to the in vivo ischemic condition. We show that a threshold concentration for H2O2 to produce glutamate-independent neuronal injury is at ∼10 μM. Thus, this glutamate receptor-independent Ca2+ toxicity by H2O2 should contribute to the overall ischemic brain injury in vivo.
The mechanism of how H2O2 activates TRPM7 channels is not clear and is the subject of future studies. H2O2 may induce its biological effect by a direct oxidation of its substrate, or indirectly through its more reactive by-product OH•. Our studies suggest that H2O2-induced glutamate-independent neuronal injury does not involve OH•. This was based on the following evidence: (a) DMSO, a scavenger for OH•, had no effect on H2O2-induced injury; (b) addition of iron chelator DFO did not affect the H2O2-induced neuronal injury. It is known that generation of OH• from H2O2 requires transition metals such as iron or copper. If the formation of OH• is involved in the effect of H2O2, then the presence of DFO would have attenuated the effect of H2O2. Oxidization of sulfhydryl groups on cysteine residues has been implicated in many physiological or pathological effects of ROS. Our data suggest that such mechanism may be involved in H2O2-induced glutamate receptor-independent neuronal injury. This is supported by the findings that thiol-reducing agent DTT effectively prevented the effect of H2O2, whereas cysteine oxidizing agents DTNP mimicked its effect.
The exact mechanism underlying ROS-mediated cell damage is not fully understood. ROS can markedly alter protein structure and induce protein cross-linking, thereby increase rates of proteolysis (40). Recent studies also suggested important roles of NADPH oxidase and poly(ADP-ribose) polymerase-1 in oxidative stress-mediated cell injury (24). One key link between oxidative stress and cell death is excessive activation of poly(ADP-ribose) polymerase-1, which causes NAD+ depletion and brain damage (35, 49). It has also been postulated that ROS may disrupt the integrity of cell membranes in a nonspecific manner through lipid peroxidation (12). In addition, recent new findings suggest that the majority effects of ROS may be mediated by specific signaling pathways rather than nonspecific damage of cell membrane or intracellular molecules (30). Our present studies also support the involvement of a specific signaling pathway in H2O2-induced cell injury; that is, activation of TRPM7 channels is required for H2O2-induced glutamate receptor-independent Ca2+ toxicity.
Supplementary Material
Abbreviations Used
- 2-APB
2-aminoethoxydiphenyl borate
- ACA
N-(p-amylcinnamoyl)anthranilic acid
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- DAPI
4′,6′-diamidino-2-phenylindole
- DFO
deferoxamine
- DMSO
dimethyl sulfoxide
- DTNP
2,2′-dithiobis-5-nitropyridine
- DTT
dithiothreitol
- dUTP
2′-deoxyuridine 5′-triphosphate
- eGFP
enhanced green fluorescent protein
- EGTA
ethylene glycol tetraacetic acid
- FDA
fluorescein-diacetate
- H2O2
hydrogen peroxide
- HEK293
human embryonic kidney 293
- KB-R7943
2-(2-(4-(4-nitrobenzyloxy)phenylethyl)isothiourea)
- LDH
lactate dehydrogenase
- MK801
(5R,10S)-(+)-5-Methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- NMDA
N-methyl-d-aspartate
- NO
nitric oxide
- O•−2
superoxide anion
- OH•
hydroxyl radical
- PI
propidium iodide
- ROS
reactive oxygen species
- siRNA
small interference RNA
- TRPM7
transient receptor potential melastatin 7
- TUNEL
terminal deoxyribonucleotidyl transferase-mediated dUTP-biotin nick end labeling
Acknowledgments
This work was supported by grants from National Institutes of Health (R01NS47506 and R01NS49470) and American Heart Association (0840132N). We thank Dr. A. Scharenberg (University of Washington) for providing HEK293 cells with inducible expression of TRPM7.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Aarts M. Iihara K. Wei WL. Xiong ZG. Arundine M. Cerwinski W. MacDonald JF. Tymianski M. A key role for TRPM7 channels in anoxic neuronal death. Cell. 2003;115:863–877. doi: 10.1016/s0092-8674(03)01017-1. [DOI] [PubMed] [Google Scholar]
- 2.Aizenman E. Hartnett KA. Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron. 1990;5:841–846. doi: 10.1016/0896-6273(90)90343-e. [DOI] [PubMed] [Google Scholar]
- 3.Avshalumov MV. Rice ME. NMDA receptor activation mediates hydrogen peroxide-induced pathophysiology in rat hippocampal slices. J Neurophysiol. 2002;87:2896–2903. doi: 10.1152/jn.2002.87.6.2896. [DOI] [PubMed] [Google Scholar]
- 4.Birmingham K. Future of neuroprotective drugs in doubt. Nat Med. 2002;8:5. doi: 10.1038/nm0102-5a. [DOI] [PubMed] [Google Scholar]
- 5.Cai S. Sauve R. Effects of thiol-modifying agents on a K(Ca2+) channel of intermediate conductance in bovine aortic endothelial cells. J Membr Biol. 1997;158:147–158. doi: 10.1007/s002329900252. [DOI] [PubMed] [Google Scholar]
- 6.Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- 7.Choi DW. Glutamate neurotoxicity in cortical cell culture is calcium dependent. Neurosci Lett. 1985;58:293–297. doi: 10.1016/0304-3940(85)90069-2. [DOI] [PubMed] [Google Scholar]
- 8.Choi YB. Lipton SA. Redox modulation of the NMDA receptor. Cell Mol Life Sci. 2000;57:1535–1541. doi: 10.1007/PL00000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- 10.Coyle JT. Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 11.Fleig A. Penner R. The TRPM ion channel subfamily: molecular, biophysical and functional features. Trends Pharmacol Sci. 2004;25:633–639. doi: 10.1016/j.tips.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Gutteridge JM. Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990;15:129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B. Oxidants and human disease: some new concepts. FASEB J. 1987;1:358–364. [PubMed] [Google Scholar]
- 14.Hecquet CM. Ahmmed GU. Vogel SM. Malik AB. Role of TRPM2 channel in mediating H2O2-induced Ca2+ entry and endothelial hyperpermeability. Circ Res. 2008;102:347–355. doi: 10.1161/CIRCRESAHA.107.160176. [DOI] [PubMed] [Google Scholar]
- 15.Hoyt KR. Gallagher AJ. Hastings TG. Reynolds IJ. Characterization of hydrogen peroxide toxicity in cultured rat forebrain neurons. Neurochem Res. 1997;22:333–340. doi: 10.1023/a:1022403224901. [DOI] [PubMed] [Google Scholar]
- 16.Hoyte L. Barber PA. Buchan AM. Hill MD. The rise and fall of NMDA antagonists for ischemic stroke. Curr Mol Med. 2004;4:131–136. doi: 10.2174/1566524043479248. [DOI] [PubMed] [Google Scholar]
- 17.Hwang JJ. Lee SJ. Kim TY. Cho JH. Koh JY. Zinc and 4-hydroxy-2-nonenal mediate lysosomal membrane permeabilization induced by H2O2 in cultured hippocampal neurons. J Neurosci. 2008;28:3114–3122. doi: 10.1523/JNEUROSCI.0199-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyslop PA. Hinshaw DB. Schraufstatter IU. Sklar LA. Spragg RG. Cochrane CG. Intracellular calcium homeostasis during hydrogen peroxide injury to cultured P388D1 cells. J Cell Physiol. 1986;129:356–366. doi: 10.1002/jcp.1041290314. [DOI] [PubMed] [Google Scholar]
- 19.Hyslop PA. Zhang Z. Pearson DV. Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- 20.Inoue K. Branigan D. Xiong ZG. Zinc-induced neurotoxicity mediated by transient receptor potential melastatin 7 channels. J Biol Chem. 2010;285:7430–7439. doi: 10.1074/jbc.M109.040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones KH. Senft JA. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985;33:77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- 22.Jussofie A. Kirsch M. de Groot H. Ca2+-dependent cytotoxicity of H2O2 in L929 cells: the role of H2O2-induced Na+-influx. Free Radic Biol Med. 1998;25:712–719. doi: 10.1016/s0891-5849(98)00159-2. [DOI] [PubMed] [Google Scholar]
- 23.Kaneko S. Kawakami S. Hara Y. Wakamori M. Itoh E. Minami T. Takada Y. Kume T. Katsuki H. Mori Y. Akaike A. A critical role of TRPM2 in neuronal cell death by hydrogen peroxide. J Pharmacol Sci. 2006;101:66–76. doi: 10.1254/jphs.fp0060128. [DOI] [PubMed] [Google Scholar]
- 24.Kim GS. Jung JE. Niizuma K. Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraft R. Grimm C. Frenzel H. Harteneck C. Inhibition of TRPM2 cation channels by N-(p-amylcinnamoyl)anthranilic acid. Br J Pharmacol. 2006;148:264–273. doi: 10.1038/sj.bjp.0706739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larm JA. Cheung NS. Beart PM. Apoptosis induced via AMPA-selective glutamate receptors in cultured murine cortical neurons. J Neurochem. 1997;69:617–622. doi: 10.1046/j.1471-4159.1997.69020617.x. [DOI] [PubMed] [Google Scholar]
- 27.Li A. Segui J. Heinemann SH. Hoshi T. Oxidation regulates cloned neuronal voltage-dependent Ca2+ channels expressed in Xenopus oocytes. J Neurosci. 1998;18:6740–6747. doi: 10.1523/JNEUROSCI.18-17-06740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li M. Jiang J. Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher P. Schubert D. Signaling by reactive oxygen species in the nervous system. Cell Mol Life Sci. 2000;57:1287–1305. doi: 10.1007/PL00000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mailly F. Marin P. Israel M. Glowinski J. Premont J. Increase in external glutamate and NMDA receptor activation contribute to H2O2-induced neuronal apoptosis. J Neurochem. 1999;73:1181–1188. doi: 10.1046/j.1471-4159.1999.0731181.x. [DOI] [PubMed] [Google Scholar]
- 32.Mendez F. Penner R. Near-visible ultraviolet light induces a novel ubiquitous calcium- permeable cation current in mammalian cell lines. J Physiol (Lond) 1998;507:365–377. doi: 10.1111/j.1469-7793.1998.365bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Monteilh-Zoller MK. Hermosura MC. Nadler MJ. Scharenberg AM. Penner R. Fleig A. TRPM7 provides an ion channel mechanism for cellular entry of trace metal ions. J Gen Physiol. 2003;121:49–60. doi: 10.1085/jgp.20028740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nadler MJ. Hermosura MC. Inabe K. Perraud AL. Zhu Q. Stokes AJ. Kurosaki T. Kinet JP. Penner R. Scharenberg AM. Fleig A. LTRPC7 is a Mg.ATP-regulated divalent cation channel required for cell viability. Nature. 2001;411:590–595. doi: 10.1038/35079092. [DOI] [PubMed] [Google Scholar]
- 35.Pacher P. Szabo C. Role of the peroxynitrite-poly(ADP-ribose) polymerase pathway in human disease. Am J Pathol. 2008;173:2–13. doi: 10.2353/ajpath.2008.080019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellegrini-Giampietro DE. Cherici G. Alesiani M. Carla V. Moroni F. Excitatory amino acid release from rat hippocampal slices as a consequence of free-radical formation. J Neurochem. 1988;51:1960–1963. doi: 10.1111/j.1471-4159.1988.tb01187.x. [DOI] [PubMed] [Google Scholar]
- 37.Roveri A. Coassin M. Maiorino M. Zamburlini A. van Amsterdam FT. Ratti E. Ursini F. Effect of hydrogen peroxide on calcium homeostasis in smooth muscle cells. Arch Biochem Biophys. 1992;297:265–270. doi: 10.1016/0003-9861(92)90671-i. [DOI] [PubMed] [Google Scholar]
- 38.Schoenmakers TJ. Visser GJ. Flik G. Theuvenet AP. CHELATOR: an improved method for computing metal ion concentrations in physiological solutions. Biotechniques. 1992;12:870–879. [PubMed] [Google Scholar]
- 39.Shi H. Liu KJ. Cerebral tissue oxygenation and oxidative brain injury during ischemia and reperfusion. Front Biosci. 2007;12:1318–1328. doi: 10.2741/2150. [DOI] [PubMed] [Google Scholar]
- 40.Slemmer JE. Shacka JJ. Sweeney MI. Weber JT. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 41.Smith MA. Herson PS. Lee K. Pinnock RD. Ashford ML. Hydrogen-peroxide-induced toxicity of rat striatal neurones involves activation of a non-selective cation channel. J Physiol. 2003;547:417–425. doi: 10.1113/jphysiol.2002.034561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun HS. Jackson MF. Martin LJ. Jansen K. Teves L. Cui H. Kiyonaka S. Mori Y. Jones M. Forder JP. Golde TE. Orser BA. MacDonald JF. Tymianski M. Suppression of hippocampal TRPM7 protein prevents delayed neuronal death in brain ischemia. Nat Neurosci. 2009;12:1300–1307. doi: 10.1038/nn.2395. [DOI] [PubMed] [Google Scholar]
- 43.Togashi K. Inada H. Tominaga M. Inhibition of the transient receptor potential cation channel TRPM2 by 2-aminoethoxydiphenyl borate (2-APB) Br J Pharmacol. 2008;153:1324–1330. doi: 10.1038/sj.bjp.0707675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weiss JH. Hartley DM. Koh J. Choi DW. The calcium channel blocker nifedipine attenuates slow excitatory amino acid neurotoxicity. Science. 1990;247:1474–1477. doi: 10.1126/science.247.4949.1474. [DOI] [PubMed] [Google Scholar]
- 45.Whittemore ER. Loo DT. Cotman CW. Exposure to hydrogen peroxide induces cell death via apoptosis in cultured rat cortical neurons. Neuroreport. 1994;5:1485–1488. doi: 10.1097/00001756-199407000-00019. [DOI] [PubMed] [Google Scholar]
- 46.Whittemore ER. Loo DT. Watt JA. Cotman CW. A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience. 1995;67:921–932. doi: 10.1016/0306-4522(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 47.Xiong ZG. Zhu XM. Chu XP. Minami M. Hey J. Wei WL. MacDonald JF. Wemmie JA. Price MP. Welsh MJ. Simon RP. Neuroprotection in ischemia: blocking calcium-permeable Acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 48.Yildiz G. Demiryurek AT. Ferrous iron-induced luminol chemiluminescence: a method for hydroxyl radical study. J Pharmacol Toxicol Methods. 1998;39:179–184. doi: 10.1016/s1056-8719(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 49.Ying W. Xiong ZG. Oxidative stress and NAD(+) in ischemic brain injury: current advances and future perspectives. Curr Med Chem. 2010;17:2152–2158. doi: 10.2174/092986710791299911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao L. Wang Y. Sun N. Liu X. Li L. Shi J. Electroacupuncture regulates TRPM7 expression through the trkA/PI3K pathway after cerebral ischemia-reperfusion in rats. Life Sci. 2007;81:1211–1222. doi: 10.1016/j.lfs.2007.08.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.